Abstract
Cytotoxic T-lymphocyte (CTL) responses to Epstein-Barr virus (EBV) tend to focus on a few immunodominant viral epitopes; where these epitope sequences are polymorphic between EBV strains, host CTL specificities should reflect the identity of the resident strain. In studying responses in HLA-B27-positive virus carriers, we identified 2 of 15 individuals who had strong CTL memory to the pan-B27 epitope RRIYDLIEL (RRIY) from nuclear antigen EBNA3C but whose endogenous EBV strain, isolated in vitro, encoded a variant sequence RKIYDLIEL (RKIY) which did not form stable complexes with B27 molecules and which was poorly recognized by RRIY-specific CTLs. To check if such individuals were also carrying an epitope-positive strain (either related to or distinct from the in vitro isolate), we screened DNA from freshly isolated peripheral blood mononuclear cells for amplifiable virus sequences across the EBNA3C epitope, across a different region of EBNA3C with type 1-type 2 sequence divergence, and across a polymorphic region of EBNA1. This showed that one of the unexplained RRIY responders carried two distinct type 1 strains, one with an RKIY and one with an RRIY epitope sequence. The other responder carried an RKIY-positive type 1 strain and a type 2 virus whose epitope sequence of RRIFDLIEL was antigenically cross-reactive with RRIY. Of 15 EBV-seropositive donors analyzed by such assays, 12 appeared to be carrying a single virus strain, one was coinfected with distinct type 1 strains, and two were carrying both type 1 and type 2 viruses. This implies that a small but significant percentage of healthy virus carriers harbor multiple, perhaps sequentially acquired, EBV strains.
Epstein-Barr virus (EBV), a human gammaherpesvirus, persists in vivo as a latent infection of the B-lymphocyte pool from which the virus can reactivate to establish foci of lytic virus replication in the oropharynx (25). Virus loads at both sites remain low in immunocompetent individuals but are elevated dramatically in T-cell-immunocompromised patients. This is one of several pieces of evidence suggesting that T-cell responses, in particular cytotoxic T lymphocytes (CTLs) recognizing immunodominant viral epitopes from latent and lytic cycle antigens, play an important role in controlling a resident EBV infection (26). What remains in doubt is the extent to which these responses can protect the virus-carrying host from infection with additional exogenously acquired EBV strains.
One approach to this question has again been to study EBV carriage in T-cell-immunocompromised individuals, now focusing not on overall viral load but on the number of resident virus strains. In that context, there are two categories of virus genome polymorphisms capable of detecting coresident strains, firstly the linked polymorphisms in the EBNA2, -3A, -3B, and -3C genes that discriminate between EBV types 1 and 2 (10, 27) and secondly a series of markers, many within latent genes, that do not segregate by virus type but which can distinguish between individual virus strains (1, 2, 6, 11, 15, 17, 20, 30, 31, 34). By these criteria, some 25 to 50% of T-cell-immunocompromised individuals in the Western world, especially AIDS patients, are overtly infected with multiple EBV strains. These could be detected both by rescuing the viruses in vitro as EBV-transformed lymphoblastoid cell lines (LCLs) (9, 14, 28, 36, 37) and by the direct amplification of different allelic sequences from peripheral blood B cells or oropharyngeal samples (4, 18, 32, 33). In many cases, the coresident strains were all of type 1, mirroring the apparent prevalence of type 1 viruses in the general Caucasian population (14, 35, 38). However, certain immunocompromised groups, in particular male homosexual AIDS cohorts, showed an unusually high incidence (>25%) of coinfection with type 1 and type 2 strains (28, 32, 36).
It is not clear whether frequent coinfection is a specific feature of the immunocompromised state or actually reflects the situation that exists, albeit covertly, in immunocompetent virus carriers. In that context, in vitro isolates from healthy Caucasian donors have, in the great majority of cases, yielded only a single isolate, usually of type 1 (14, 35), suggesting that coinfections are relatively rare. However, because viral loads are generally lower in such donors, it could be argued that in vitro isolation will not detect minor coresident strains, particularly if, as is the case with type 2 viruses (24), these have less potent B-cell-transforming ability in culture. Indeed, some studies using direct amplification of viral DNA from blood or throat washings have implied frequencies of coinfection of up to 30% based on codetection of type 1 and type 2 sequences (4, 29) and of up to 60% based on codetection of allelic sequences at polymorphic sites in the BamHI F region (20) and in the EBNA1 gene (6).
Here we have approached this question by another route, using the host CTL response to a polymorphic EBV epitope as an indicator of the virus strain(s) resident in vivo. Because CTLs recognize short viral peptides (usually 8 to 11 amino acids long) presented in the peptide binding groove of major histocompatibility complex class I molecules (12), they can be exquisitely sensitive to single amino acid changes in viral proteins if these alter the ability of the relevant peptide either to bind the major histocompatibility complex class I molecule of choice or to interact as bound peptide with the T-cell receptor (3, 5, 11, 13, 21, 23). The present work developed from our interest in CTL responses restricted through the HLA-B27 family of class I alleles, particularly the B*2702, B*2704, and B*2705 subtypes which tend to mediate strong EBV latent antigen-specific responses and for which the immunodominant target epitopes have been identified (7, 8, 26). Interestingly, the one epitope shared by all three subtypes (EBNA3C 258-266) is reported to be polymorphic (17, 27). We therefore asked whether the strength of this epitope-specific response in B27-positive donors directly reflected the particular epitope sequence present in the virus rescued from these donors in vitro.
MATERIALS AND METHODS
Donors and virus isolates.
All donors studied were healthy adults with serological evidence of prior EBV infection. The main study group was comprised of 15 HLA-B27-positive donors (seven Caucasians positive for B*2705, five Caucasians positive for B*2702, and three Southeast Asians positive for B*2704), all of whom were analyzed for EBV-specific CTL epitope choice. For several of these individuals, virus isolates (1 to 30 independent isolates/donor) were rescued as EBV-transformed LCLs in vitro either from peripheral blood mononuclear cell (PBMC) preparations by spontaneous outgrowth or from throat washings by transformation of indicator B cells from adult EBV-seronegative donors. The methods used for in vitro virus isolation have been fully described elsewhere (36). Experiments involving direct amplification of viral DNA from PBMCs also included samples from seven HLA-B27-negative donors.
Generation and functional analysis of EBV-specific CTLs.
EBV-specific CTLs were reactivated from HLA-B27-positive donors by cocultivating PBMCs with γ-irradiated autologous LCL cells (carrying either the standard type 1 EBV strain B95.8 or the donor's endogenous strain) as previously described (19). T-cell clones were generated from these cocultures by seeding them in semisolid agarose on day 4 poststimulation (21) or by limiting dilution cloning on day 14, and the clones were maintained in interleukin 2-supplemented medium as previously described (19). T-cell clones were screened in standard 51chromium release assays either on autologous fibroblast or LCL targets overexpressing the EBNA or latent membrane protein of choice from a recombinant vaccinia virus (rVV) vector or on autologous phytohemagglutinin blast or LCL targets preexposed to the synthetic epitope peptide. Full details of these assays and of relevant controls have been described elsewhere (19). In pulse-chase peptide sensitization assays, 51chromium-labelled target cells were preincubated with peptide at the concentrations stated for 30 min. An aliquot of these cells was then used immediately to provide CTL targets in an assay conducted in the continuing presence of the peptide (pulse). The remaining cells were washed thoroughly to remove unbound peptide and recultured; aliquots of these cells were taken at the indicated times for inclusion as targets in the standard CTL assay (chase).
EBNA3C epitope sequencing and HinfI restriction digest analysis.
To determine the EBNA3C (codons 258 to 266) epitope sequence in EBV isolates rescued in vitro as LCLs, DNA was prepared from LCL cell pellets by standard methods and the relevant region of the EBNA3C gene was amplified by PCR with the primers E3CB27A (5′-GCTGACAGCATCATGTTAACTGCC-3′; B95.8 coordinates, 99045 to 99068) and E3CB27C (5′-GTGCATTCCACGGGTAATATGGCT-3′; B95.8 coordinates, 99409 to 99385) under the following PCR conditions: 94°C for 30 s, 55°C for 60 s, 72°C for 60 s, 35 cycles. The PCR products were gel purified with a QIAquick gel extraction kit (Qiagen, Crawley, West Sussex, United Kingdom) according to the manufacturer's instructions and then sequenced by PCR with an Amplicycle kit (Perkin-Elmer/Applied Biosystems, Warrington, United Kingdom) and 32P-end-labelled E3CB27B primer (5′-GACACCCATGAAACGCACGAAATC-3′; B95.8 coordinates, 99323 to 99299) under the same conditions as described above.
In some cases, the EBNA3C epitope status within LCLs was determined by HinfI restriction digest analysis of the initial PCR product above. Following exposure of the gel-purified DNA to HinfI (Boehringer Mannheim, Roche Diagnostics Ltd., Lewes, East Sussex, United Kingdom) for 1 to 2 h at 37°C, digestion products were separated on a 2% agarose gel, Southern blotted, and probed with a mixture of two 32P-end-labelled probes specific for sequences on either side of the HinfI site: 5′-TGCTGGACCAAGAGAGCAAG-3′ (B95.8 coordinates, 99137 to 99156) and 5′-TGTGTGGCTCTCTGCACCAC-3′ (B95.8 coordinates, 99241 to 99260).
To determine the EBNA3C epitope sequence(s) amplifiable directly from PBMC preparations, DNA was prepared from 107 PBMCs by standard methods and the relevant region of the EBNA3C gene was amplified by a nested PCR with the following primers: round 1, E3CB27A and E3CB27C; round 2, E3CB27A and E3CB27B under PCR conditions of 35 cycles of 94°C for 30 s, 55°C for 60 s, and 72°C for 60 s. The PCR products were gel purified as described above. DNA was ligated into the Pgem T-easy vector system I (Promega, Madison, Wis.) and then used to transform competent XL1 Blue Escherichia coli cells. Colonies were picked and expanded, and the DNA was purified with a Wizard Plus SV Minipreps DNA purification system (Promega). DNA was then sequenced with a T7sequencing kit (Amersham Pharmacia Biotech, Saint Albans, United Kingdom).
EBNA1 gene sequencing.
DNA was prepared from 107 PBMCs, and the relevant region of the EBNA1 gene was amplified by a nested PCR with the following primers: round 1, 5′-GAAAAGAGGCCCAGGAGTCCCAGTAGTCAG-3′ (B95.8 coordinates, 109081 to 109110) and 5′-AACAGCACGCATGATGTCTACTGGGGATTT-3′ (E1 3′; B95.8 coordinates, 109969 to 109940); round 2, 5′-AGAAGGCCCAAGCACTGGAC-3′ (B95.8 coordinates, 109278 to 109297) and E1 3′, under PCR conditions of 35 cycles of 94°C for 60 s, 65°C for 90 s (62°C for nested PCR), and 72°C for 240 s. PCR products were purified and cloned, and multiple clones were sequenced as described above for EBNA3C.
EBV type 1-type 2 analysis.
The type 1 or type 2 status of each in vitro virus isolate was determined by standard typing of the LCL at the relevant EBNA2, -3A, -3B, and -3C loci (27). The type(s) of EBV strain carried by PBMCs was determined by nested PCR amplification across a previously defined type-specific region of the EBNA3C gene (27) with the following primers: round 1, 5′-CACAGAGCACCCCTGAAAGG-3′ (B95.8 coordinates, 99778 to 99797) and 5′-GGCTCGTTTTTGACGTCGGC-3′ (B95.8 coordinates, 100091 to 100072); round 2, 5′-AGAAGGGGAGCGTGTGTTGT-3′ (B95.8 coordinates, 99939 to 99958) and 5′-GTCTTGATGTTTCCGATGTGGCTTA-3′ (B95.8 coordinates, 100055 to 100031) under PCR conditions of 40 cycles of 94°C for 30 s, 45°C for 90 s, and 72°C for 120 s. DNA was purified with a QIAquick kit, separated on a 2% agarose gel, Southern blotted, and probed with the following 32P-end-labelled probes: type 1, 5′-GAAGATTCATCGTCAGTGTC-3′ (B95.8 coordinates, 100002 to 100021; 117-bp product), and type 2, 5′-CCGTGATTTCTACCGGGAGT-3′ (210-bp product).
RESULTS
CTL epitope choice in B27-positive donors.
The present study focused on responses to five EBV-encoded CTL epitopes restricted through B27 alleles and derived from the latent proteins EBNA3B, EBNA3C, or LMP2 (Table 1). Memory CTLs in the blood of B27-positive EBV-immune donors were reactivated in vitro by stimulating PBMCs with autologous LCL cells carrying the prototype 1 EBV strain B95.8, i.e., a virus strain encoding all five epitopes. The resultant T-cell clones were screened against autologous targets either expressing one of the relevant B95.8 strain latent proteins from an rVV vector or preloaded with one of the relevant epitope peptides. Results from four representative donors are illustrated in Fig. 1. For the B*2705-positive donor RT, the B27-restricted response was dominated by clones specific for the EBNA3C-derived epitope RRIY (30 of 33, represented by RT c11), two further clones from this donor (represented by RT c1) recognized the other EBNA3C-derived epitope FRKA, and one clone (RT c149) recognized the EBNA3B-derived epitope HRCQ. All but one of the B*2705-positive donors resembled donor RT in their spread of epitope choice, the exception being donor ME, who only responded to the HRCQ epitope (11 of 11 clones, represented by ME c24, c67, and c76). Among the B*2702-positive donors analyzed, most resembled donor LY, who yielded responses both to the EBNA3C-derived RRIY epitope (46 of 67 clones, represented by LY c37 and c61) and to the EBNA3B-derived RRAR epitope (21 of 67 clones, represented by LY c77). However, a minority resembled donor NW, who had no detectable RRIY reactivity and yielded only clones specific for the RRAR epitope (44 of 44 clones, represented by NW c75, c88, and c118).
TABLE 1.
EBV-derived CTL epitopes presented by HLA-B27
| Latent antigen | Epitope position | Epitope sequencea | Subtype specificity | Reference |
|---|---|---|---|---|
| EBNA3C | 258–266 | RRIYDLIEL | B*2705, B*2702, B*2704 | 7 |
| EBNA3C | 343–351 | FRKAQIQGL | B*2705 | 26 |
| EBNA3B | 149–157 | HRCQAIRK | B*2705 | 26 |
| EBNA3B | 243–253 | RRARSLSAERY | B*2702 | 8 |
| LMP2b | 236–244 | RRRWRRLTV | B*2704 | 7 |
Epitopes are designated by their first four amino acid residues (shown in italics).
LMP, latent membrane protein.
FIG. 1.
Analysis of EBV target antigens and epitopes recognized by representative HLA-B27-restricted CTL clones from B*2705-positive donors RT and ME and from B*2702-positive donors LY and NW. CTL clones were tested in chromium release assays against autologous target cells either expressing the EBNA3C or EBNA3B latent proteins from rVVs (+ vacc-) or preloaded with individual epitope peptides at a concentration of 10−6 M (+/− peptide). Effector-to-target ratios were between 2:1 and 10:1. Results are expressed as percentage of specific lysis observed in standard 4-h assays.
As summarized in Table 2, 12 of 15 B27-positive donors analyzed, including individuals from each of the three B27 subtype groups, responded to the RRIY epitope. In most cases, the response to this pan-B27 epitope appeared to be immunodominant, but it was always accompanied by additional responses to subtype-specific epitopes, namely FRKA and HRCQ for B*2705 donors, RRAR for B*2702 donors, and RRRW for B*2704 donors. The remaining 3 of the 15 donors yielded no detectable RRIY response yet did respond to type-specific epitopes, HRCQ in the case of B*2705 donor ME and RRAR in the case of B*2702 donors NW and Rov.
TABLE 2.
Summary of CTL responses to EBV-derived epitopes presented by HLA-B27 in a panel of healthy individuals
| Subtype | Donor | Proportion of B27 restricted, EBV-specific CTL clones specific for epitopesa
|
||||
|---|---|---|---|---|---|---|
| RRIY | FRKA | HRCQ | RRAR | RRRW | ||
| B*2705 | RT | +++a | + | + | — | — |
| EN | +++ | NTb | NT | — | — | |
| SB | ++ | + | ++ | — | — | |
| JS | +++ | + | NT | — | — | |
| AL | + | + | +++ | — | — | |
| SC | + | +++ | — | — | — | |
| ME | — | — | +++ | — | — | |
| B*2702 | LY | +++ | — | — | + | — |
| Kor | ++ | — | — | ++ | — | |
| Kla | ++ | — | — | ++ | — | |
| NW | — | — | — | +++ | — | |
| Rov | — | — | — | +++ | — | |
| B*2704 | DH | + | — | — | — | +++ |
| CQN | ++ | — | — | — | ++ | |
| DW | +++ | NT | NT | NT | NT | |
+++, >67%, ++, 33 to 67%, +, <33%, —, no detectable response.
NT, not tested.
Polymorphism of the RRIY epitope sequence.
We therefore set out to determine whether the absence of an RRIY-specific response in certain individuals was associated with any changes in the epitope sequence encoded by their resident EBV strain. LCLs carrying the endogenous EBV isolate (rather than the B95.8 strain LCLs used as stimulators in the above experiments) were available from 8 of 15 of the above-mentioned B27-positive donors, including two RRIY nonresponders, ME and NW. Representative LCLs were sequenced across codons 201 to 293 of the EBNA3C gene encompassing the epitope region (codons 258 to 266). Figure 2A illustrates the range of epitope sequences observed relative to the type 1 (B95.8) and type 2 (Ag876) prototype sequences. Some isolates, for example, from donor RT, were identical to strain B95.8 in this region, whereas others, for example, from donors SC and NW, had a base change in codon 259 which translated to an R→K amino acid substitution at position 2 of the epitope, i.e., RRIY became RKIY. The isolate from donor DH contained Y→F amino acid changes at two positions, immediately outside the epitope at −1 and within the epitope at position 4; i.e., RRIY became RRIF. Note that although one of the nucleotide changes and both of the amino acid changes seen in DH are the same as found in the standard type 2 virus strain Ag876, sequencing further outside the epitope region showed that the DH EBNA3C gene was otherwise very close to the type 1 B95.8 sequence. In fact, all of the LCL-derived isolates from these B27-positive donors proved to be type 1 virus strains when analyzed at the standard EBNA2, EBNA3A, EBNA3B, and EBNA3C typing loci (data not shown).
FIG. 2.
(A) Sequence analysis of EBV strains carried by spontaneous LCLs from B27-positive individuals. The nucleotide and deduced amino acid sequences for EBNA3C codons 254 to 267 are compared with the B95.8 (type 1) and Ag876 (type 2) prototypes, and substitutions relative to the B95.8 sequence are shown in bold. The RRIY epitope region is identified by shading. The recognition sequence for the HinfI restriction enzyme is underlined and the cleavage site is indicated by an arrow. Virus isolates from donors SC and NW contain a G→A substitution (B95.8 coordinate, 99220) which destroys the HinfI site. (B) HinfI restriction analysis of EBV strains carried by spontaneous LCLs from representative donors NW, SB (independently established isolates 1 to 4), and DH and the reference B95.8 cell line. DNA was PCR amplified and digested with HinfI, and digestion products were separated on an agarose gel, Southern blotted, and probed with radiolabelled oligonucleotide probes specific for sequences 5′ and 3′ of the HinfI restriction site. + and −, HinfI was present and absent, respectively, in the restriction digests. Viral isolates from SB and DH all contained the HinfI site, as did B95.8, and therefore retained the R residue at anchor position 2 of the epitope; the HinfI site was lost in the viral isolate NW.
We noted that the RRIY→RKIY mutation in the SC and NW isolates involved the loss of a HinfI restriction site (GAATC→AAATC) within EBNA3C codons 259 and 260 (Fig. 2A). This provided a rapid method with which to screen all of the multiple independently derived virus isolates available from B27-positive donors. The products from PCR amplification across EBNA3C codons 201 to 293 were digested with HinfI; the digestion products were then separated by gel electrophoresis, Southern blotted, and probed with radiolabelled oligonucleotides specific for sequences 5′ and 3′ of the HinfI restriction site. As shown in Fig. 2B, the endogenous isolate from donor NW (with the RKIY epitope sequence) lacks the HinfI site and so yielded a single band with or without HinfI digestion. By contrast, multiple isolates from donor SB and also the DH isolate (with the RRIF epitope sequence) yielded clear evidence of HinfI digestion, as did the type 1 B95.8 reference strain. The overall results from sequencing and HinfI restriction site analysis are summarized in Table 3. Three important points can be made. First, where multiple independent isolates were available from the same donor, all were identical at the EBNA3C epitope locus. Second, donors ME and NW, who yielded no detectable RRIY-specific CTL response, were indeed carrying EBV strains which had the altered epitope sequence, RKIY. Third, and paradoxically, two other donors, SC and LY, who did yield an RRIY-specific response, nevertheless also appeared to be carrying EBV strains with the RKIY sequence rather than the RRIY sequence.
TABLE 3.
EBNA3C status in LCLs from B27-positive donors
| Subtype | Donor | EBV isolated in vitroa
|
RRIY-specific CTL response | ||
|---|---|---|---|---|---|
| RRIY | RKIY | RRIF | |||
| B*2705 | RT | 1/1 | + | ||
| B*2705 | EN | 12/12 | + | ||
| B*2705 | SB | 30/30 | + | ||
| B*2705 | SC | 7/7 | + | ||
| B*2705 | ME | 18/18 | − | ||
| B*2702 | LY | 1/1 | + | ||
| B*2702 | NW | 1/1 | − | ||
| B*2704 | DH | 1/1 | + | ||
Results are the number of virus isolates carrying the designated sequence out of the total number tested.
Immunogenicity of variant epitope sequences.
The next set of experiments was designed to ask whether the RKIY and RRIF variants of the epitope sequence are themselves immunogenic. In preliminary assays, we noted that both of the above-mentioned sequence variants could be recognized by RRIY-specific CTL clones in peptide sensitization assays, although the RKIY peptide required at least a 10-fold higher concentration compared to that of RRIY or RRIF to achieve 50% maximal lysis (data not shown). These assays were conducted in the continuous presence of the peptide, however, and we therefore went on to test the stability of the relevant B27-peptide complexes at the cell surface in pulse-chase experiments. Autologous target cells were preloaded with the respective peptide at a concentration just sufficient to yield maximal lysis and then either added directly to a CTL assay (pulse) or washed thoroughly to remove unbound peptide and added to the assay at time points between 0 and 4 h later (chase). As shown in Fig. 3A, a B*2705-restricted clone from donor SC recognized all three peptides equally well when they were present throughout the assay (pulse). However, recognition of RKIY-treated targets was immediately lost in the chase period, whereas RRIF- and RRIY-treated targets remained fully sensitive to CTL detection for at least the next 4 h. A similar distinction between RKIY- and RRIY-treated targets was also apparent in assays with B*2702 effectors.
FIG. 3.
CTL recognition of the RRIY epitope and naturally occurring sequence variants. (A) CTL clones were tested in peptide sensitization assays against autologous target cells preloaded with the RRIY or RRIF peptides at a concentration of 10−8 M or the RKIY peptide at a concentration of 10−6 M. Peptide-loaded target cells were either added directly to the assay (pulse) or washed thoroughly to remove unbound peptide before addition to the assay at the indicated time points (chase). Results for one representative clone each from donors SC and LY are shown. The effector-to-target ratio was 4:1 for both clones. (B) CTL clones were screened in chromium release assays against autologous LCLs carrying EBV isolates encoding EBNA3C with either the RRIY, the RKIY, or the RRIF epitope sequence. Results are shown for one representative clone each from donors SC (B*2705) and LY (B*2702). The effector-to-target ratio was 4:1 for both clones. NT, not tested. All results are expressed as the percent specific lysis observed in standard 4-h assays.
In parallel experiments illustrated in Fig. 3B, RRIY-specific CTL clones were tested for their ability to recognize autologous LCL targets transformed with different virus strains encoding RRIY-positive, RKIY-positive, or RRIF-positive versions of the EBNA3C protein. In line with the data from peptide sensitization assays, the B*2705-restricted CTL clone SC c248 could recognize autologous LCL cells expressing either an RRIY-positive or an RRIF-positive EBNA3C protein but not the RKIY-positive protein. The B*2702-restricted CTL clone LY c32 likewise recognized autologous targets carrying an RRIY-positive virus strain but not those carrying an RKIY-positive strain. Finally, we compared these RRIY-positive and RKIY-positive autologous LCLs for their ability to reactivate B27-restricted CTL responses in vitro by the standard protocol of cocultivation with PBMCs. Table 4 summarizes the results of clonal analysis of the responses obtained from donors SC and LY. In both cases, the RRIY-positive LCL elicited a detectable RRIY-specific response whereas the RKIY-positive LCL did not. In contrast, both types of stimulator LCL were capable of eliciting responses to other B27 epitopes, the B*2705 subtype-specific epitope FRKA in the case of donor SC and the B*2702 subtype-specific epitope RRAR in the case of donor LY.
TABLE 4.
In vitro reactivation of B27-restricted, EBV-specific CTL responses using LCLs carrying EBV strains with either the RRIY or RKIY sequence
| Subtype | Donor | Expt. no. | Reactivation of epitope-specific CTL clones bya
|
|||
|---|---|---|---|---|---|---|
| RRIY+ LCL | RKIY+ LCL | |||||
| B*2705 | SC | 1 | RRIY, 5/30 | FRKA, 9/30 | RRIY, 0/15 | FRKA, 4/15 |
| B*2705 | SC | 2 | RRIY, 3/32 | FRKA, 10/32 | RRIY, 0/19 | FRKA, 6/19 |
| B*2702 | LY | 1 | RRIY, 13/36 | RRAR, 4/36 | RRIY, 0/45 | RRAR, 14/45 |
Results are the number of CTL clones specific for the given epitope out of the total number of EBV-specific CTL clones tested.
Coresidence of distinct EBV strains in CTL donors.
The above results strongly suggested that infection with an RKIY-positive virus strain could not explain the presence of RRIY-specific T-cell memory in donors such as SC and LY. The final set of experiments therefore sought to examine the possibility that such individuals were carrying EBV strains in addition to the one isolated in vitro. For this purpose we established sensitive PCR-based assays which were capable of amplifying EBV DNA directly from PBMC preparations and which focused on three different polymorphic loci: (i) across codons 201 to 293 of EBNA3C, encompassing the pan-B27 epitope region, (ii) across codons 499 to 537 of EBNA3C, where there are multiple type-specific sequence changes (27), and (iii) across codons 460 to 510 of EBNA1, where a number of different sequence variants have been reported (6, 15). These were then used to identify the resident EBV genome sequences in PBMCs from eight of the above B27-positive donors (including SC and LY) and also from seven B27-negative donors from whom a resident virus strain had previously been isolated by LCL establishment in vitro; in each case the in vitro isolate was also studied at all three loci. Note that in this panel of additional donors, we deliberately included two individuals (AC and AM) and who consistently yielded type 2 virus-positive LCLs in vitro.
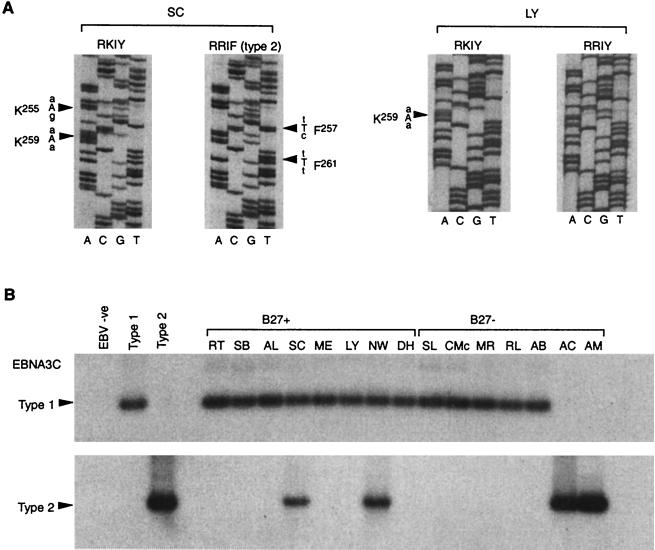
For EBNA3C epitope analysis, the PCR product from nested amplification of PBMC DNA was cloned and 8 to 20 individual clones per donor were sequenced. Five of eight B27-positive donors and seven of seven B27-negative donors thus analyzed yielded only one EBNA3C epitope sequence. The exceptions were the B*2705-positive donor SC and the B*2702-positive donors LY and NW. As illustrated in Fig. 4A, two epitope sequences could be amplified from donor SC. These were the RKIY sequence (with an additional R→K mutation upstream of the epitope at EBNA3C residue 255, which was also present in the in vitro isolate from this donor; cf. Fig. 2A) and an RRIF sequence with nucleotide changes characteristic of a type 2 EBV strain (cf. Fig. 2A, Ag876). Also shown in Fig. 4A are the two epitope sequences which could be amplified from donor LY; these were the RKIY sequence, as found in the one in vitro isolate available from this donor (see Table 3) and a coresident RRIY sequence identical to that seen in B95.8. Interestingly, donor NW yielded evidence of three coresident epitope sequences, RKIY (as seen in the one in vitro isolate available from this donor; Fig. 2A), RRIY, and RRIF.
FIG. 4.
(A) Direct sequence analysis of EBV DNA amplified from PBMCs. Using a nested PCR, DNA was amplified across the region of the EBNA3C gene encoding the RRIY epitope, the products were cloned, and multiple clones were sequenced. Autoradiographs of sequencing gels for two representative clones each from donors SC and LY are shown. Nucleotide changes relative to the B95.8 prototype are shown in capital letters, and the identities and positions of the corresponding amino acid substitutions are indicated. Clones are designated as RRIY, RKIY, or RRIF according to their sequence across the epitope region. (B) Direct PCR typing of EBV strains carried by eight B27-positive and seven B27-negative healthy individuals. DNA extracted from PBMCs was PCR amplified across the type-specific region of EBNA3C with common nested 5′ and 3′ primers. The two panels show autoradiographs of Southern blots probed with type 1- (top) and type 2- (bottom) specific radiolabelled oligonucleotide probes. The type 1 virus control was B95.8 and the type 2 control was Ag876.
Figure 4B shows the results obtained when the same panel of 15 donors was screened for the presence of EBNA3C type 1 and/or EBNA3C type 2 viral sequences by amplifying with common nested primers and screening the product(s) with separate type-specific probes. Here six of eight B27-positive donors and seven of seven B27-negative donors amplified for a single virus type; this was type 1 in every case except for donors AC and AM who, as anticipated from the identity of their in vitro isolates, yielded only a type 2-specific signal. However, the B*2705-positive donor SC and the B*2702-positive donor NW clearly carried both type 1 and type 2 viral sequences. This result was obtained on several occasions of testing and with repeat blood samples from these particular donors.
The same individuals were further analyzed by amplification across a polymorphic region of the EBNA1 gene, of which the two most common allelic variants among Caucasian EBV isolates have been recently described (15). These either accord to the B95.8 prototype sequence conventionally designated 487A from the alanine residue at signature position 487 or show a series of changes in codons 476, 487, 492, and 499, designated 487T from the threonine residue at the signature position. All but three of the donors analyzed yielded a single amplifiable EBNA1 sequence among the 7 to 20 individual clones sequenced per donor. The three exceptions were, again, B*2705 donor SC and the B*2702 donors LY and NW; in each of these cases we identified the coresidence of both 487A and 487T EBNA1 sequences. The overall results of these PCR-based assays on PBMCs are summarized in Table 5.
TABLE 5.
Summary of EBV sequences amplifiable across three polymorphic loci from healthy donor PBMCs
| Subtype | Donor | EBNA3C epitopea
|
EBNA3C typeb
|
EBNA1a
|
Multiple infectionb | ||||
|---|---|---|---|---|---|---|---|---|---|
| RRIY | RKIY | RRIF | 1 | 2 | 487A | 487T | |||
| B*2705 | RT | 10/10 | + | − | 10/10 | − | |||
| B*2705 | SB | 10/10 | + | − | 9/9 | − | |||
| B*2705 | AL | 10/10 | + | − | 9/9 | − | |||
| B*2705 | SC | 15/18 | 3/18 | + | + | 1/8 | 7/8 | + | |
| B*2705 | ME | 10/10 | + | − | 10/10 | − | |||
| B*2702 | LY | 12/17 | 5/17 | − | + | − | 5/20 | 15/20 | + |
| B*2702 | NW | 4/20 | 15/20 | 1/20 | + | + | 6/7 | 1/7 | + |
| B*2704 | DH | 10/10 | + | − | NTc | NT | − | ||
| B27− | SL | 10/10 | + | − | 10/10 | − | |||
| B27− | CMc | 10/10 | + | − | 12/12 | − | |||
| B27− | MR | 8/8 | + | − | 11/11 | − | |||
| B27− | RL | 9/9 | + | − | 8/8 | − | |||
| B27− | AB | 10/10 | + | − | 10/10 | − | |||
| B27− | AC | 10/10 | − | + | 12/12 | − | |||
| B27− | AM | 10/10 | − | + | 10/10 | − | |||
Results are the number of DNA clones carrying the specific sequence out of the total number sequenced.
+, present. −, not present.
NT, not tested.
DISCUSSION
The present work developed from earlier studies in which donors with either the B*2705, the B*2702, or the B*2704 alleles all showed strong memory CTL responses to the pan-B27 RRIY epitope from the EBV latent cycle antigen EBNA3C (7). We were therefore surprised to identify 3 of 15 B27-positive individuals with no evidence of RRIY reactivity yet with responses to the relevant subtype-specific epitopes. A likely explanation arose when we isolated LCLs carrying the endogenous EBV strain from two of these individuals and found by sequencing that in both cases the resident virus encoded a variant of the epitope, RKIY. A similar variant sequence has also been reported among Caucasian virus strains in a previous study (17). The consensus-binding motif for all B27 subtypes (16) includes a critical R residue at anchor position 2, and so we considered it unlikely that the RKIY sequence would be immunogenic. Subsequent results (see below) strongly supported this. However, further analysis of in vitro virus isolates revealed two donors, SC and LY, who also carried an RKIY-positive virus yet did mount an RRIY-specific response and another donor, DH, who responded to RRIY yet carried an RRIF-positive virus.
This prompted us to examine the immunogenicity of the RKIY and RRIF variant epitope sequences in a series of assays involving (i) the stability of the B27-peptide complex following exogenous loading of the peptide on the target cell surface, (ii) the ability of RRIY-specific effectors to recognize LCLs infected with either RKIY-positive or RRIF-positive virus strains, and (iii) the ability of the RKIY-positive LCL to stimulate epitope-specific memory CTLs from the blood of the autologous donor. These experiments strongly indicated that while the RRIF sequence could effectively serve as an epitope, the RKIY sequence could not. This conclusion is further supported by observations in the human immunodeficiency virus system, in which an R→K mutation at position 2 of a B27-restricted CTL epitope effectively abrogated its antigenicity (13). Hence, for donor DH the existence of RRIY epitope-reactive memory probably reflects this individual's colonization by an RRIF epitope-positive virus strain. However, for donors SC and LY, their RRIY-specific memory was very unlikely to have resulted from infection with their resident RKIY-positive strain.
We considered the possibility that a cross-reactive epitope from another commensal organism might have elicited an apparent RRIY-specific response in donors SC and LY. Database searches, however, identified only one close predicted match to the RRIY sequence, namely IRGYDLIEL from the citrate synthase gene of Bacillus subtilis. This peptide, though differing from the EBV epitope sequence only at positions 1 and 3, was nevertheless not recognized by RRIY-specific CTLs (J. M. Brooks, unpublished data). We therefore considered the other possibility, that donors SC and LY were in fact carrying more than one resident EBV strain and that the additional strain(s) was positive for the RRIY or RRIF epitope sequences. If true, it would then be important to know whether the coresident strains showed sequence identity at other polymorphic loci (in which case the RKIY-positive virus might have arisen de novo as an immune escape variant [13, 23]) or were distinct from one another at these other loci (in which case the coresident viruses would represent independent viral strains). We therefore sought to amplify EBV DNA sequences directly from PBMCs, focusing not just on the EBNA3C epitope but also on two other informative loci, namely, a type-specific region of EBNA3C (27) and a recently characterized region of EBNA1 (6, 15). This work involved the establishment of PCR amplification protocols that were sufficiently sensitive firstly to detect EBV sequences in aliquots of 107 PBMCs from healthy virus carriers and secondly to amplify coresident strain-specific sequences where these exist. Once established, these assays were used to screen members of the panel of B27-positive CTL donors and also a number of healthy B27-negative donors from whom endogenous EBV strains had already been rescued in vitro.
The assays clearly showed that donor SC, who yielded dual signals at all three loci, was carrying two independent virus strains. One of these, represented by the existing in vitro isolate from this donor, was EBNA3C type 1 and had an RKIY sequence at the EBNA3C epitope locus (Fig. 2A) and a 487T allelic sequence at the EBNA1 locus (15). The second strain, which had not been rescued in vitro, could therefore be assigned as type 2, RRIF at the epitope locus and 487A at the EBNA1 locus. The inability to rescue this coresident type 2 virus could reflect its lower abundance in vivo and/or the weaker in vitro transforming efficiency of type 2 EBV strains (24). The in vitro response of donor SC to RRIY epitope challenge (Table 2) probably reflects CTL memory induced by this RRIF-positive type 2 virus since the RRIF epitope was recognized as efficiently as RRIY in the present CTL assays and, from the example of donor DH, clearly can be immunogenic in vivo. Donor LY was likewise shown to be carrying two independent virus strains, in this case, both of type 1. One strain, represented by the existing in vitro isolate, had an RKIY sequence at the epitope locus (Table 3) and had a 487T EBNA1 allele (data not shown); the other strain therefore appears to have RRIY at the epitope locus and 487A at EBNA1. It is infection with this second strain that explains the presence of RRIY epitope-specific memory in this donor. The third donor from whom multiple EBV sequences were detected, NW, appeared to be carrying three different virus strains. One of these was the type 1, RKIY-positive strain originally isolated in vitro (Table 3); the others appear to be a type 1 RRIY-positive strain and a type 2 RRIF-positive strain. Unexpectedly, neither of these two additional viruses has induced a detectable RRIY-specific response. The reason for this is not known but may be related to the host genotype. Thus, donor NW has yielded atypical CTL responses in other viral systems (22), and a family member with the same B*2702 allele has also failed to develop an RRIY-specific response following EBV infection (J. M. Brooks, unpublished data).
These findings make it clear that direct PCR amplification, cloning, and sequencing of PBMC DNA can provide a more sensitive method of detecting coresident EBV strains than in vitro virus isolation. Recent work monitoring the incidence of type 1-type 2 coinfections in immunocompromised patients has led to a similar conclusion (32). Clearly, both the sensitivity and reliability of such PCR-based approaches will be increased by analysis at multiple polymorphic loci rather than the single site screening employed in many studies to date. Even in the present study, based on analysis at three sites, it is possible that some instances of coinfection have not been detected. We nevertheless conclude, on the basis of current assays, that the majority of immunocompetent individuals studied (12 of 15) still appear to carry only a single EBV strain. Furthermore, in all these cases the virus strain detected by direct PCR amplification and sequencing of PBMC DNA was identical at the EBNA3C and EBNA1 loci to that isolated in vitro (reference 15 and data not shown).
At the same time, the PCR-based assays did identify a minority of healthy donors who were carrying more than one virus strain. In such cases, one does not know whether the coresident strains were transmitted together at the time of primary infection or were acquired sequentially. If the latter proves correct, it would imply that the immune response induced by the first viral encounter sometimes cannot protect the host from subsequent exogenously transmitted strains. Might such incidences of secondary infection be explained by an absence of sufficiently strong CTL cross-reactivity in the primed host? For donor SC in the present work, one can speculate that primary infection with an RKIY-positive type 1 virus strain resulted in CTL responses focused on the B*2705 subtype-specific FRKA epitope. Such responses would not be protective against subsequent infection with a type 2 virus strain because there would be no preexisting immunity against the RRIF epitope and because the type 2 version of the FRKA epitope (27) shows several sequence changes, including an R→L substitution at anchor position 2, which will almost certainly destroy its antigenicity. The circumstances predisposing B*2702-positive donors LY and NW to multiple infection are more difficult to explain, however, since both donors respond to the subtype-specific RRAR epitope and this sequence is conserved not only within all type 1 isolates that we have studied to date (data not shown) but also in type 2 virus strains (27). Ultimately, we will need to conduct long-term prospective studies on donors from the time of primary infection if we are to understand when and how coinfections are established.
ACKNOWLEDGMENTS
This work was supported by the Medical Research Council and the Cancer Research Campaign, United Kingdom.
REFERENCES
- 1.Abdel-Hamid M, Chen J J, Constantine N, Massoud M, Raab-Traub N. EBV strain variation: geographical distribution and relation to disease state. Virology. 1992;190:168–175. doi: 10.1016/0042-6822(92)91202-6. [DOI] [PubMed] [Google Scholar]
- 2.Aitken C, Sengupta S K, Aedes C, Moss D J, Sculley T B. Heterogeneity within the Epstein-Barr virus nuclear antigen 2 gene in different strains of Epstein-Barr virus. J Gen Virol. 1994;75:95–100. doi: 10.1099/0022-1317-75-1-95. [DOI] [PubMed] [Google Scholar]
- 3.Apolloni A, Moss D, Stumm R, Burrows S, Suhrbier A, Misko I, Schmidt C, Sculley T. Sequence variation of cytotoxic T cell epitopes in different isolates of Epstein-Barr virus. Eur J Immunol. 1992;22:183–189. doi: 10.1002/eji.1830220127. [DOI] [PubMed] [Google Scholar]
- 4.Apolloni A, Sculley T B. Detection of A-type and B-type Epstein-Barr virus in throat washings and lymphocytes. Virology. 1994;202:978–981. doi: 10.1006/viro.1994.1422. [DOI] [PubMed] [Google Scholar]
- 5.Bertoletti A, Sette A, Chisari F V, Penna A, Levrero M, De Carli M, Fiaccardori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia K, Raj A, Gutierrez M I, Judde J-G, Spangler G, Venkatesh H, Magrath I T. Variation in the sequence of Epstein-Barr virus nuclear antigen 1 in normal peripheral blood lymphocytes and in Burkitt's lymphomas. Oncogene. 1996;13:177–181. [PubMed] [Google Scholar]
- 7.Brooks J M, Murray R, Thomas W A, Kurilla M G, Rickinson A B. Different HLA-B27 subtypes present the same immunodominant Epstein-Barr virus peptide. J Exp Med. 1993;178:879–887. doi: 10.1084/jem.178.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks J M, Colbert R A, Mear J P, Leese A M, Rickinson A B. HLA-B27 subtype polymorphism and CTL epitope choice: studies with EBV peptides link immunogenicity with stability of the B27:peptide complex. J Immunol. 1998;161:5252–5259. [PubMed] [Google Scholar]
- 9.Buisson M, Morand P, Genoulaz O, Bourgeat M-J, Micoud M, Seigneurin J-M. Changes in the dominant Epstein-Barr virus type during human immunodeficiency virus infection. J Gen Virol. 1994;75:431–437. doi: 10.1099/0022-1317-75-2-431. [DOI] [PubMed] [Google Scholar]
- 10.Dambaugh T, Hennessy K, Chamnankit L, Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci USA. 1984;81:7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Campos-Lima P O, Levitsky V, Brooks J, Lee S P, Hu L F, Rickinson A B, Masucci M G. T cell responses and virus evolution: loss of HLA A11-restricted CTL epitopes in Epstein-Barr virus isolates from highly A11-positive populations by selective mutation of anchor residues. J Exp Med. 1994;179:1297–1305. doi: 10.1084/jem.179.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 13.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 14.Gratama J W, Oosterveer M A P, Weimar W, Sintnicolaas K, Sizoo W, Bolhuis R L H, Ernberg I. Detection of multiple ‘Ebnotypes’ in individual Epstein-Barr virus carriers following lymphocyte transformation by virus derived from peripheral blood and oropharynx. J Gen Virol. 1994;75:85–94. doi: 10.1099/0022-1317-75-1-85. [DOI] [PubMed] [Google Scholar]
- 15.Habeshaw G, Yao Q Y, Bell A I, Morton D, Rickinson A B. Epstein-Barr virus nuclear antigen 1 sequences in endemic and sporadic Burkitt's lymphoma reflect virus strains prevalent in different geographic areas. J Virol. 1999;73:965–975. doi: 10.1128/jvi.73.2.965-975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jardetsky T S, Lane W S, Robinson R A, Madden D R, Wiley D C. Identification of self-peptides bound to purified HLA-B27. Nature. 1991;353:326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- 17.Khanna R, Slade R W, Poulsen L, Moss D J, Burrows S R, Nicholls J, Burrows J M. Evolutionary dynamics of genetic variation in Epstein-Barr virus isolates of diverse geographical origins: evidence for immune pressure-independent genetic drift. J Virol. 1997;71:8340–8346. doi: 10.1128/jvi.71.11.8340-8346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyaw M T, Hurren L, Evans L, Moss D J, Cooper D A, Benson E, Esmore E, Sculley T B. Expression of B-type Epstein-Barr virus in HIV-infected patients and cardiac transplant recipients. AIDS Res Hum Retrovir. 1992;8:1869–1874. doi: 10.1089/aid.1992.8.1869. [DOI] [PubMed] [Google Scholar]
- 19.Lee S P, Tierney R J, Thomas W A, Brooks J M, Rickinson A B. Conserved CTL epitopes within EBV latent membrane protein 2. A potential target for CTL-based tumor therapy. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 20.Lung M L, Lam W P, Chan K H, Li S, Sham J, Choy D. Direct detection of Epstein-Barr virus in peripheral blood and comparison of Epstein-Barr virus genotypes present in direct specimens and lymphoblastoid cell lines established from nasopharyngeal carcinoma patients and healthy carriers in Hong Kong. Int J Cancer. 1992;52:174–177. doi: 10.1002/ijc.2910520203. [DOI] [PubMed] [Google Scholar]
- 21.Moss D J, Misko I S, Burrows S R, Burman K, McCarthy R, Sculley T B. Cytotoxic T-cell clones discriminate between A- and B-type transformants. Nature. 1988;331:719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- 22.Pazmany L, Rowland-Jones S, Huet S, Hill A, Sutton J, Murray R, Brooks J, McMichael A. Genetic modulation of antigen presentation by HLA-B27 molecules. J Exp Med. 1992;175:361–369. doi: 10.1084/jem.175.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips R E, Rowland-Jones S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R M, Rizza C R, McMichael A J. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 24.Rickinson A B, Young L S, Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA2 on the growth phenotype of virus-transformed B cells. J Virol. 1987;61:1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 26.Rickinson A B, Moss D J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 27.Sample J, Young L, Martin B, Chatman T, Kieff E, Rickinson A, Kieff E. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J Virol. 1990;64:4084–4092. doi: 10.1128/jvi.64.9.4084-4092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sculley T B, Apolloni A, Hurren L, Moss D J, Cooper D A. Co-infection with A- and B-type Epstein-Barr virus in human immunodeficiency virus-positive subjects. J Infect Dis. 1990;162:643–648. doi: 10.1093/infdis/162.3.642. [DOI] [PubMed] [Google Scholar]
- 29.Sixbey J W, Shirley P, Chesney P J, Buntin D M, Resnick L. Detection of a second widespread strain of Epstein-Barr virus. Lancet. 1989;ii:761–765. doi: 10.1016/s0140-6736(89)90829-5. [DOI] [PubMed] [Google Scholar]
- 30.Snudden D K, Smith P R, Lai D, Ng M H, Griffin B. Alterations in the structure of the EBV nuclear antigen, EBNA1, in epithelial cell tumours. Oncogene. 1995;10:1545–1552. [PubMed] [Google Scholar]
- 31.Sung N S, Edwards R H, Seillier-Moiseiwitsch F, Perkins A G, Zeng Y, Raab-Traub N. EBV strain variation in nasopharyngeal carcinoma from the endemic and non-endemic regions of China. Int J Cancer. 1998;76:207–215. doi: 10.1002/(sici)1097-0215(19980413)76:2<207::aid-ijc7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.van Baarle D, Hovenkamp E, Kersten M J, Klein M R, Miedema F, van Oers M H J. Direct Epstein-Barr virus (EBV) typing on peripheral blood mononuclear cells: no association between EBV type 2 infection or superinfection and the development of acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Blood. 1999;93:3949–3955. [PubMed] [Google Scholar]
- 33.Walling D M, Edmistan S N, Sixbey J W, Abdel-Hamid M, Resnick L, Raab-Traub N. Co-infection of multiple strains of the Epstein-Barr virus in human immunodeficiency virus-associated hairy leukoplakia. Proc Natl Acad Sci USA. 1992;89:6560–6564. doi: 10.1073/pnas.89.14.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrightham M N, Stewart J P, Janjua N J, Pepper S D V, Sample C, Rooney C M, Arrand J R. Antigenic and sequence variation in the C-terminal unique domain of the Epstein-Barr virus nuclear antigen EBNA-1. Virology. 1995;208:521–530. doi: 10.1006/viro.1995.1183. [DOI] [PubMed] [Google Scholar]
- 35.Yao Q Y, Rowe M, Martin B, Young L S, Rickinson A B. The Epstein-Barr virus carrier state: dominance of a single growth-transforming isolate in the blood and in the oropharynx of healthy virus carriers. J Gen Virol. 1991;72:1579–1590. doi: 10.1099/0022-1317-72-7-1579. [DOI] [PubMed] [Google Scholar]
- 36.Yao Q Y, Tierney R J, Croom-Carter D, Dukers D, Cooper G M, Ellis C J, Rowe M, Rickinson A B. Frequency of multiple Epstein-Barr virus infections in T-cell immunocompromised individuals. J Virol. 1996;70:4884–4894. doi: 10.1128/jvi.70.8.4884-4894.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Q Y, Croom-Carter D S G, Tierney R J, Habeshaw G, Wilde J T, Hill F G H, Conlon C, Rickinson A B. Epidemiology of infection with Epstein-Barr virus types 1 and 2: lessons from the study of a T-cell immunocompromised hemophiliac cohort. J Virol. 1998;72:4352–4363. doi: 10.1128/jvi.72.5.4352-4363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimber U, Adldinger H K, Lenoir G M, Vuillaume M, Knebel-Doeberitz M V, Laux G, Desgranges C, Wittman P, Freese U K, Schneider U, Bornkamm G W. Geographic prevalence of two Epstein-Barr virus types. Virology. 1986;154:56–66. doi: 10.1016/0042-6822(86)90429-0. [DOI] [PubMed] [Google Scholar]