Abstract
To further characterize the nature of proteolytic processing of the astrovirus capsid, we infected Caco-2 cells with a high multiplicity of astrovirus without trypsin in the presence of 5 to 10% fetal calf serum. These infections were characterized by pulse-chase labeling with [35S]methionine, electron microscopy, gel electrophoresis of purified viral particles, and analysis of infectivity of such particles with and without added trypsin. Pulse-chase experiments showed that the astrovirus capsid protein was initially translated as an approximately 87-kDa protein. The 87-kDa capsid protein was rapidly converted intracellularly to a 79-kDa form which was found in smaller amounts in the cell supernatant. Purification by differential centrifugation yielded particles that appeared quite similar to trypsin-grown astrovirus particles by negatively stained electron microscopy. These particles were antigenically distinct from trypsin-treated virions as demonstrated by their various reactions with monoclonal antibodies in a solid-phase immunoassay. The purified trypsin-free particles were mainly composed of the 79-kDa capsid protein which was found to have an amino terminus at residue 71 of the entire open reading frame 2 (ORF2) product. The cleavage site was identified in a highly conserved region of the astrovirus ORF2 product. These trypsin-free particles were minimally infectious in cultured Caco-2 cells but became highly infectious (105-fold increase) after trypsin but not chymotrypsin treatment. This trypsin-enhanced infectivity correlated with conversion of the 79-kDa capsid protein to three smaller peptides of approximately 34, 29, and 26 kDa.
Astroviruses were first identified in the stools of children with gastroenteritis and characterized by electron microscopy (EM) (18). The particles were approximately 28 nm in diameter, and some had a star-like morphology. Since the initial observation in humans, similar viruses have been identified in a variety of other mammalian species. Lee and Kurtz first demonstrated growth of the virus in tissue culture and showed that trypsin is necessary for ongoing astrovirus replication (16). The development of enzyme immunoassays (EIAs) (11, 12) and reverse transcriptase PCR assays has allowed larger numbers of samples to be screened with more-sensitive tests (13, 14, 21). It has become apparent that astroviruses are an important cause of gastroenteritis in a variety of settings, including nosocomial infections, daycare outbreaks, and diarrhea in outpatients (5–10, 19, 20, 24, 26).
The astrovirus genome consists of a single-stranded positive-sense RNA of approximately 7,200 nucleotides with three open reading frames (ORFs). The first, ORF1a, encodes a serine 3C type of viral protease (27). The second, ORF1b, is separated from ORF1a by a frame shift and encodes a viral RNA polymerase (17). The third, ORF2, encodes an 87-kDa polypeptide which functions as the capsid precursor. During infection, a subgenomic RNA that encodes the capsid precursor is found in abundance in the cell cytoplasm (22, 23).
Mature infectious astrovirus virions contain two to five polypeptides with masses of approximately 30 kDa. In human serotype 1 and 2 strains, 26- and 29-kDa peptides have been shown to contain neutralizing epitopes (2, 25). The maturation of the astrovirus capsid from an 86- to 90-kDa precursor to the smaller capsid proteins is poorly understood. Studies examining trypsin-treated cultures have revealed a confusing array of capsid protein intermediates with masses ranging from 74 to 35 kDa (25). Because of the methodology used, it has been impossible to determine whether any viral or cellular proteases participated in the processing. Monroe et al. studied trypsin-free serotype 2 astrovirus infection by EM and found that viral particles accumulated within infected cells rather than in the supernatant, as observed in trypsin-treated infections (23). In those studies, treatment of the trypsin-free material resulted in the generation of three capsid proteins ranging from 20 to 31 kDa.
In order to better understand the morphogenesis of the astrovirus capsid, we have undertaken the study and characterization of immature, noninfectious virions which are the product of trypsin-free astrovirus infection of Caco-2 cells.
MATERIALS AND METHODS
Cells and viruses.
Caco-2 cells were obtained from the American Type Culture Collection. They were grown in RPMI medium supplemented with penicillin, streptomycin, and 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 incubator. Astrovirus serotype 1 adapted to tissue culture by Kurtz and Lee (8, 9) was the kind gift of S. Matsui, Stanford University. Astroviruses were routinely propagated as previously described in Caco-2 cells in RPMI medium without fetal bovine serum and in the presence of trypsin (type IX; Sigma) at 10 μg/ml (2). Virus was purified by differential centrifugation as previously described (25), followed by cesium chloride gradient centrifugation (2). Positive fractions were identified visually and/or by dot blot immunoassay using 8G4, a previously described group-specific anti-astrovirus monoclonal antibody (MAb) (2). Viral infectivity was titrated on 96-well plates of Caco-2 cells as previously described (2). Briefly, serial dilutions of human astrovirus were added to Caco-2 monolayers for overnight incubation. The following morning, monolayers were washed with phosphate-buffered saline (PBS), fixed with cold methanol, and then immunoperoxidase stained with MAb 8G4.
Immunoaffinity purification of the 79-kDa capsid protein.
Caco-2 monolayers were infected with trypsin-grown serotype 1 astrovirus at a multiplicity of infection of 5 to 10 infectious units/cell for 1 h and then overlaid with RPMI medium containing 5% FBS. The infection was allowed to proceed for 2 days. The supernatant and cell pellet were separated by low-speed centrifugation, and the pellet was homogenized in hypotonic buffer (10 mM NaCl, 50 mM Tris, pH 7.4) containing 1% Nonidet P-40 as well as 1 mM phenylmethylsulfonyl fluoride and 1% aprotinin. Debris was removed from the pellet homogenate by low-speed centrifugation, and the two supernatants were pooled and centrifuged overnight in a Beckman (Palo Alto, Calif.) SW28 rotor at 12,000 rpm overnight. This pellet was resuspended in 5 ml of TNE and extracted with an equal volume of trichlorotriflouroethane. Octyl glucoside was added to the aqueous portion to a final concentration of 0.5%. This was then pelleted through a 30% sucrose cushion in a Beckman SW28 rotor at 28,000 rpm for 2.5 h. The pellet was resuspended in 5 ml of TNE buffer, extracted again with trichlorotriflouroethane, and then preadsorbed with protein A-agarose beads. The resulting supernatant was then adsorbed to protein A-agarose beads which had been cross-linked to MAb 5B7 with dimethylpimelimidate, washed with radioimmunoprecipitation assay buffer, and eluted by boiling in Laemmli sample buffer. Purified protein was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride paper, and sequenced by automated N-terminal Edman degradation on an Applied Biosystems automated Sequenator.
Pulse-chase experiments.
Caco-2 cells were infected with astrovirus at a multiplicity of infection of 5 to 10 for 2 h, washed twice, and then incubated overnight (12 h) in the presence of 10% FBS with no added trypsin. Monolayers were washed several times with methionine-free medium and pulsed for 30 min with [35S]methionine at 1,000 μCi/ml. The monolayers were again washed, and the cells were refed medium containing 10× methionine. At 0, 2, and 4 h, the supernatants were collected and the monolayers were harvested in ice-cold lysis buffer containing 2 mM phenylmethylsulfonyl fluoride, 2% aprotinin, and 5% FBS. After 30 min, the cell lysates were cleared by centrifugation at 10,000 × g for 15 min. Supernatants were immunoprecipitated as previously described with MAb 5B7 and protein A-agarose beads and then analyzed by SDS-PAGE and fluorography.
EIA.
Serotype 1 astrovirus was grown in the absence of trypsin and purified by differential centrifugation as described above. A portion of the virus was then treated with trypsin at 10 μg/ml or mock treated for 1 h at 37°C. The virus preparations were diluted identically to 2 μg/ml and used to coat microtiter plates that were incubated for 3 h at 37°C. The plates were then washed twice with PBS and blocked overnight with 20% FBS in PBS. Plates were washed twice more with PBS and then exposed to a 1:5,000 dilution of MAb ascites or immune rabbit serum. Controls included wells precoated with purified rhesus rotavirus with and without trypsin, as well as astrovirus-coated wells probed with an irrelevant antibody. Plates were developed with peroxidase-conjugated anti-mouse immunoglobulin G (immunoglobulin M for MAb 7C2). TMB substrate from Kirkegaard & Perry (Gaithersburg, Md.) was used, and the plates were read on a Bio-Rad EIA reader at 450 nm after the reaction had been stopped with 1 M phosphoric acid. The reported absorbances were obtained by subtracting the values obtained with negative-control wells.
RESULTS
Astrovirus capsid protein is intracellularly postranslationally processed during trypsin-free astrovirus infection.
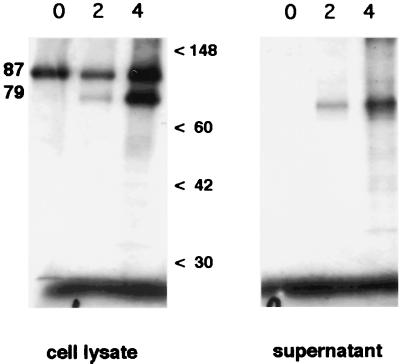
In order to determine whether the capsid precursor underwent any posttranslational modification within cells prior to extracellular proteolysis by trypsin, we performed pulse-chase experiments with astrovirus-infected Caco-2 cells in the absence of trypsin with 5% FBS present as an inhibitor of the trypsin present in the original inoculum. Both the cell supernatant and the lysed cells were analyzed by radioimmunoprecipitation using astrovirus-specific antibodies, SDS-PAGE, and fluorography. The results (Fig. 1) confirm that the initial product of translation is an approximately 87-kDa protein which is observed mainly in the cell lysate throughout the experiment. During the subsequent hours of chase, there is increasing accumulation of an approximately 79-kDa product in both the supernatant and the cellular fraction. Neither protein was observed in mock-infected cells (data not shown). The cell lysates contained much more of the 79-kDa product than did the supernatant, but a longer exposure time was used for the supernatant phase of Fig. 1 for better visualization. Continuation of the chase phase of the experiment to 24 h showed only a slight decrease in the 87-kDa protein compared to the 4-h time point (data not shown).
FIG. 1.
Pulse-chase analysis of processing of the serotype 1 astrovirus capsid in the absence of trypsin. Caco-2 cells in 10% FBS were infected for 12 h with astrovirus, pulsed for 30 min with [35S]methionine, and then chased with 10× methionine. Supernatants and cell lysates were harvested at 0, 2, and 4 h after completion of the pulse and immunoprecipitated with MAb 5B7. The supernatant portion was exposed to film four times longer than the cell lysates were. Molecular size markers (in kilodaltons) are indicated.
Purification of trypsin-free viral particles.
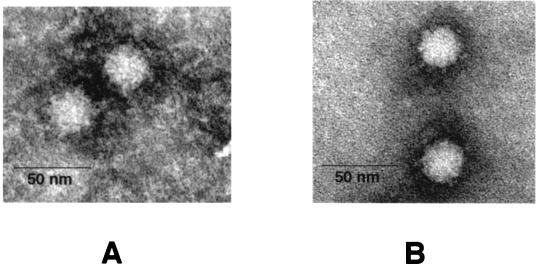
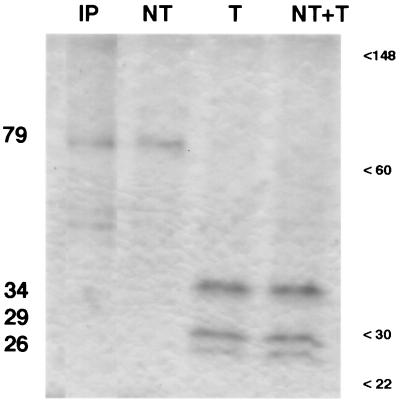
Because Monroe et al. had previously described intracellular viral particles in trypsin-free astrovirus infections (23), we wished to examine their protein composition. Therefore, we used our standard astrovirus purification protocol to purify products of such infections. In CsCl gradients, the particle density was 1.35 g/ml, slightly higher than the 1.34 g/ml obtained with standard trypsin-grown astrovirus. When the resulting material was examined by negatively stained EM, we observed numerous viral particles visually indistinguishable in size and morphology from trypsin-grown astrovirus (Fig. 2). When we measured 150 particles in EM photomicrographs in a blinded fashion, we found that the non-trypsin-treated particles had an average diameter that was slightly larger than that of particles treated with trypsin (40.5 versus 39.0 nm; P < 0.01). Analysis of this material by SDS-PAGE (Fig. 3, lane NT) showed that the particles consisted mainly of a 79-kDa protein analogous to that observed during the chase phase of our pulse-chase experiments as shown in Fig. 1. This 79-kDa band comigrated on SDS-PAGE with material immunoprecipitated from trypsin-free infection (lane IP).
FIG. 2.
Human serotype 1 astrovirus particles grown in the presence (A) or absence (B) of trypsin with negative staining with phosphotungstic acid. Particles were grown and purified as described in Materials and Methods.
FIG. 3.
Protein composition of serotype 1 astrovirus particles by SDS-PAGE. Virus was purified as described in Materials and Methods. Lanes: IP, viral protein immunoprecipitated with MAb 5B7 from trypsin-free astrovirus infection; NT, virus grown in the absence of trypsin; T, virus grown in the presence of trypsin at 10 μg/ml; NT+T, virus grown in the absence of trypsin and subsequently treated for 30 min at 37°C with trypsin at 10 μg/ml. Estimated molecular masses of astrovirus proteins (left) are indicated, as well as molecular size markers, in kilodaltons.
Activation of trypsin-free particles.
Because earlier studies had shown that treatment of crude approximately 90-kDa viral protein from trypsin-free astrovirus-infected cells yielded smaller peptides consistant with astrovirus capsid proteins (23), we treated the purified, trypsin-free particles with trypsin and then assayed them for protein content and infectivity. After 1 h of exposure to trypsin at 10 μg/ml, the 79-kDa protein was converted to three smaller proteins with molecular masses of approximately 34, 29, and 26 kDa which comigrated on SDS-PAGE with the proteins derived from purified trypsin-grown astrovirus (Fig. 3, lanes T and NT+T).
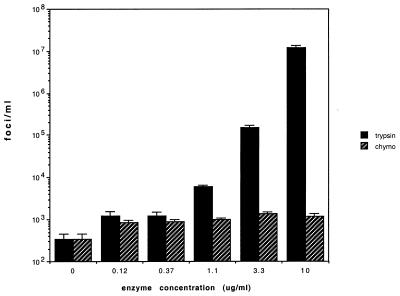
When we compared the infectivity of the 79-kDa particles with that of the trypsin-treated particles by titration on Caco-2 cells, we observed a striking 50,000- to 100,000-fold increase (Fig. 4). A minimal three- to fourfold increase was observed when the trypsin-free virus was treated with chymotrypsin. Chymotrypsin treatment of trypsin-free astrovirus particles resulted in a different pattern of proteins by SDS-PAGE featuring a major band of approximately 38 kDa (data not shown). Indeed, the scant measurable infectivity of the trypsin-free 79-kDa protein (300 foci/ml) might be accounted for by residual trypsin-activated virus which was used as inoculum for the generation trypsin-free particles. Thus, it is likely that the minimally infectious astrovirus particles containing only the 79-kDa protein are the precursors to the infectious virions which have three capsid proteins.
FIG. 4.
Enhancement of astrovirus infectivity by trypsin or chymotrypsin (chymo) treatment. Purified, trypsin-free astrovirus was incubated with the indicated concentrations of trypsin or chymotrypsin (Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK] treated; Sigma-Aldrich, St. Louis, Mo.) at 37°C for 30 min prior to titration on Caco-2 cells by immunoperoxidase focus assay. The experiment was performed three times with three replicate titrations each time. Shown are mean values from a typical experiment. Error bars indicate 1 standard deviation.
Antigenic comparison of trypsin-free astrovirus and trypsin-treated astrovirus.
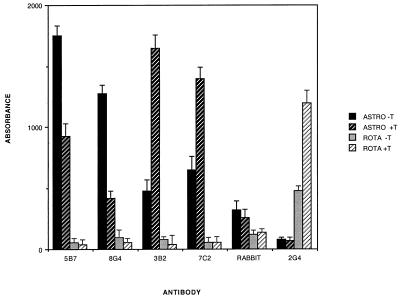
Although we observed no obvious morphologic differences between trypsin-treated virus and untreated virus, we suspected that there would be significant differences in their surface topology given the huge difference in infectivity observed between the two particle types. Therefore, we devised EIAs employing several previously described MAbs (2) directed against astrovirus capsid epitopes to compare the two species of viral particle (Fig. 5). All four MAbs recognized both forms of virus particle, while a control MAb against rotavirus VP5 (2G4) recognized neither form. Two antibodies (5B7 and 8G4) react significantly more readily with trypsin-free particles, while the other two (3B2 and 7C2) react much more strongly with trypsin-treated virions. Rabbit antiserum prepared to trypsin-grown, purified astrovirus particles reacts equally well with both species. Interestingly, 3B2 and 7C2 have previously been shown to react in immunoprecipitation exclusively with the 29-kDa capsid protein found in trypsin-treated virus, in contrast to the broader reactivity of 5B7, which reacts with a variety of precursors, including the 87- and 79-kDa forms. Thus, we conclude that trypsin cleavage of the 79-kDa protein simultaneously activates viral infectivity while unmasking key epitopes involved in viral neutralization.
FIG. 5.
Reactivity of various antibodies with trypsin-treated (ASTRO +T) and non-trypsin-treated (ASTRO −T) astrovirus 1. Plates were precoated with trypsin-treated or trypsin-free astrovirus or rhesus rotavirus (ROTA) and then probed with various antibodies as described in Materials and Methods. Bars indicate the mean A450 of four replicate wells for each condition. Error bars indicate 1 standard deviation.
N-terminal sequence of the 79-kDa capsid protein.
Hoping to gain a better understanding of the genesis the 79-kDa capsid protein, we immunoaffinity purified the latter and submitted it for N-terminal amino acid sequencing via automated Edman degradation. The results (KQGVTGPK) showed that the N terminus of the 79-kDa protein began at residue 71 of the ORF2 product. The cleaved 70 amino acids account for a calculated loss of 8,105 kDa. This change in molecular mass accounts for virtually all of the observed apparent change observed by SDS-PAGE (Fig. 1) and suggests that the amino-terminal cleavage is the only major intracellular modification in the ORF2 product.
DISCUSSION
Although several studies have previously examined proteolytic processing of the astrovirus ORF2 product, the capsid precursor, ours is the first to document trypsin-independent cleavage of the initial 87-kDa translation product. Monroe and coworkers studied trypsin-free human astrovirus infection in LLCMK2 cells and observed only an approximately 90-kDa product by immunoprecipitation using hyperimmune rabbit serum (23). It should be noted that a number of cellular proteins were coprecipitated in their experiments and may have obscured the 79-kDa protein. Sanchez-Fauquier et al. performed pulse-chase experiments with immunoprecipitations similar to our experiments but always included trypsin in the medium (25). They did observe a prominent 74-kDa band during the chase phase which is probably equivalent to our 79-kDa band, but the finding was somewhat obscured by a variety of smaller viral proteins which were coprecipitated. These smaller proteins were most likely the intermediates formed by simultaneous trypsin degradation of the 74-kDa protein. In our experiments, we used MAb 5B7 instead of antiserum and observed minimal coprecipitation of cellular proteins. Furthermore, since the experiments were performed in the presence of FBS, as well as other potent trypsin inhibitors, no trypsin activity was present.
We found that the 87-kDa primary translation product of ORF2 was mainly intracellular (Fig. 1) but that the 79-kDa form appeared in the cell lysates and at lower concentrations in the cell supernatants. It is not surprising that this extracellular form was not observed by Sanchez-Faquier et al., given that their extracellular medium contained a high concentration of trypsin (10 μg/ml). It is possible that Monroe et al. failed to observe the 79-kDa capsid protein in trypsin-free supernatants due to the rather low level of infection they were able to achieve (15 to 25% of cells versus our 90 to 100%) and the fact that their assay for antigen in the supernatant was an EIA which is probably less sensitive than radioimmunoprecipitation.
We then purified virions from trypsin-free infections and found that the main protein component was the 79-kDa form of the ORF2 product. When treated in vitro with trypsin, these particles had a dramatic 4- to 5-log10 rise in infectivity (Fig. 4) which correlated with conversion of the 79-kDa protein to three proteins of approximately 34, 29, and 26 kDa (Fig. 3). The increased infectivity is several orders of magnitude higher than that observed with other enteric viruses, such as rotavirus and reovirus, which typically increase by 10- to 100-fold after proteolytic activation. We do not believe that this increase in infectivity is due to disaggregation of virus particles because we did not observe such aggregation in negatively stained electron micrographs of purified trypsin-free virus. Given that the increased infectivity seen in some other enteric viruses after protease treatment is due to enhanced viral penetration of target cells (1, 15), it is tempting to speculate that a similar mechanism is responsible for trypsin activation of astrovirus.
Sanchez-Fauquier et al. have previously shown that the amino termini of the 26- and 29-kDa capsid proteins of trypsin-grown serotype 2 human astrovirus are at amino acids 395 and 362, respectively (25). Both of these amino acids follow arginine residues, lending credence to the notion that they are the result of trypsin cleavage. More recently, Monroe et al. have reported preliminary evidence of the same termini in other human astrovirus serotypes (3). In our laboratory, we have identified residue 395 as the amino terminus of the 29-kDa capsid protein (data not shown). Unfortunately, we have been unable to obtain N-terminal sequence data from the 26- and 34-kDa capsid components, possibly because the N termini are blocked.
Trypsin treatment also produced a shift in the antigenicity of the virus, with epitopes recognized by MAbs 3B2 and 7C2 becoming more reactive while epitopes related to MAbs 5B7 and 8G4 became less reactive (Fig. 5). MAbs 3B2 and 7C2 are directed against capsid-specific epitopes on the 29-kDa protein and fail to immunoprecipitate either higher-molecular-weight precursors or the smaller 26-kDa capsid protein (2). MAb 5B7 immunoprecipitates not only the 26- and 29-kDa capsid components but also a variety of higher-Mr precursors, including the 87-kDa primary ORF2 product and the 79-kDa modification (Fig. 1 and 3).
Our observation of the intracellular modification of the 87-kDa capsid protein precursor led us to wonder what the nature of this processing is. The N-terminal sequence which we obtained from the 79-kDa form suggests that cleavage at amino acid 71 is responsible for the observed shift in molecular weight. The amino-terminal 415 residues of ORF2 are remarkably conserved across serotypes (28), and the sequence surrounding the cleavage site (amino acids 69 to 89) is completely conserved among the eight complete astrovirus capsid sequences available. The cleavage site follows an arginine residue and is a potential trypsin site. Preliminary observations made while expressing astrovirus ORF2 alone in BHK cells show similar processing of the initial 87-kDa product to a 79-kDa form (S. Matsui and T. Chu, personal communication), strongly suggesting that cellular proteases can mediate this change.
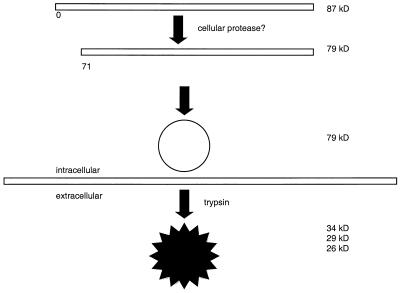
The functional significance of the intracellular capsid protein processing is not known. It is possible that the modification promotes capsid formation and/or facilitates the association of viral genomic RNA with nascent virions. It is noteworthy that we observed no 87-kDa form of the capsid protein when we purified trypsin-free virions by differential centrifugation. This observation supports the notion that the conversion to the 79-kDa form is important for the formation of an intact capsid. The proposed processing of the capsid is depicted in Fig. 6.
FIG. 6.
Schematic model of proposed astrovirus capsid processing. Astrovirus ORF2 is translated as an 87-kDa protein. Within the cell, the 70 amino-terminal amino acid residues are removed, probably by a cellular protease. This may facilitate capsid formation. Outside the cell, trypsin cleavage at sites which include residue 395 results in the mature, infectious virion consisting of three proteins of approximately 26, 29, and 34 kDa.
The fate of the cleaved 8-kDa portion of the ORF2 product is not clear from our studies. We have not observed the protein in purified virus or immunoprecipitation experiments using 4 to 20% acrylamide gradient gels, but that may reflect the fact that this peptide does not react with any of our MAbs or polyclonal antibodies. If the peptide noncovalently associates with the nascent viral particles, it may be eluted by conditions used during our viral purification. Similar-size capsid components have been reported in some, but not all, descriptions of purified astrovirus particles, raising the possibility that this portion could remain associated with the virion.
Further studies are necessary to completely map the main constituents of the mature trypsin-treated viral capsid within the initial ORF2 product. It is possible that some elements of ORF2 play a role in viral assembly and are not present in the final capsid structure.
ACKNOWLEDGMENTS
We thank Suzanne Matsui for viral stocks and helpful discussion. We also thank Usha Upadhyayula for technical assistance and Nafissa Ghori for excellent assistance with EM.
This work was supported by Public Health Service grant DK52389 from the National Institutes of Health.
REFERENCES
- 1.Bass D M, Bodkin D, Dambrauskas R, Trier J S, Fields B N, Wolf J L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J Virol. 1990;64:1830–1833. doi: 10.1128/jvi.64.4.1830-1833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass D M, Upadhyayula U. Characterization of human serotype 1 astrovirus-neutralizing epitopes. J Virol. 1997;71:8666–8671. doi: 10.1128/jvi.71.11.8666-8671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belliot G, Laveran H, Monroe S S. Capsid protein composition of reference strains and wild isolates of human astroviruses. Virus Res. 1997;49:49–57. doi: 10.1016/s0168-1702(97)01457-3. [DOI] [PubMed] [Google Scholar]
- 4.Blom N, Hansen J, Blaas D, Brunak S. Cleavage site analysis in picornaviral polyproteins: discovering cellular targets by neural networks. Protein Sci. 1996;5:2203–2216. doi: 10.1002/pro.5560051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz J R, Bartlett A V, Herrmann J E, Cáceres P, Blacklow N R, Cano F. Astrovirus-associated diarrhea among Guatemalan ambulatory rural children. J Clin Microbiol. 1992;30:1140–1144. doi: 10.1128/jcm.30.5.1140-1144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echeverria P, Hoge C W, Bodhidatta L, Tungtaem C, Herrmann J, Imlarp S, Tamura K. Etiology of diarrhea in a rural community in western Thailand: importance of enteric viruses and enterovirulent Escherichia coli. J Infect Dis. 1994;169:916–919. doi: 10.1093/infdis/169.4.916. [DOI] [PubMed] [Google Scholar]
- 7.Esahli H, Breback K, Bennet R, Ehrnst A, Eriksson M, Hedlund K O. Astroviruses as a cause of nosocomial outbreaks of infant diarrhea. Pediatr Infect Dis J. 1991;10:511–515. doi: 10.1097/00006454-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gaggero A, O'Ryan M, Noel J S, Glass R I, Monroe S S, Mamani N, Prado V, Avendano L F. Prevalence of astrovirus infection among Chilean children with acute gastroenteritis. J Clin Microbiol. 1998;36:3691–3693. doi: 10.1128/jcm.36.12.3691-3693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass R I, Noel J, Mitchell D, Herrmann J E, Blacklow N R, Pickering L K, Dennehy P, Ruiz-Palacios G, de Guerrero M L, Monroe S S. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch Virol Suppl. 1996;12:287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero M L, Noel J S, Mitchell D K, Calva J J, Morrow A L, Martinez J, Rosales G, Velazquez F R, Monroe S S, Glass R I, Pickering L K, Ruiz-Palacios G M. A prospective study of astrovirus diarrhea of infancy in Mexico City. Pediatr Infect Dis J. 1998;17:723–727. doi: 10.1097/00006454-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J E, Hudson R W, Perron-Henry D M, Kurtz J B, Blacklow N R. Antigenic characterization of cell-cultivated astrovirus serotypes and development of astrovirus-specific monoclonal antibodies. J Infect Dis. 1988;158:182–185. doi: 10.1093/infdis/158.1.182. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann J E, Nowak N A, Perron-Henry D M, Hudson R W, Cubitt W D, Blacklow N R. Diagnosis of astrovirus gastroenteritis by antigen detection with monoclonal antibodies. J Infect Dis. 1990;161:226–229. doi: 10.1093/infdis/161.2.226. [DOI] [PubMed] [Google Scholar]
- 13.Jonassen T O, Kjeldsberg E, Grinde B. Detection of human astrovirus serotype 1 by the polymerase chain reaction. J Virol Methods. 1993;44:83–88. doi: 10.1016/0166-0934(93)90010-o. [DOI] [PubMed] [Google Scholar]
- 14.Jonassen T O, Monceyron C, Lee T W, Kurtz J B, Grinde B. Detection of all serotypes of human astrovirus by the polymerase chain reaction. J Virol Methods. 1995;52:327–334. doi: 10.1016/0166-0934(94)00168-g. [DOI] [PubMed] [Google Scholar]
- 15.Kaljot K T, Shaw R D, Rubin D H, Greenberg H B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee T W, Kurtz J B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J Gen Virol. 1981;57:421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- 17.Lewis T L, Greenberg H B, Herrmann J E, Smith L S, Matsui S M. Analysis of astrovirus serotype 1 RNA, identification of the viral RNA-dependent RNA polymerase motif, and expression of a viral structural protein. J Virol. 1994;68:77–83. doi: 10.1128/jvi.68.1.77-83.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madeley C R, Cosgrove B P. Viruses in infantile gastroenteritis. Lancet. 1975;ii:124. doi: 10.1016/s0140-6736(75)90020-3. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado Y, Cantwell M, Old M, Hill D, Sanchez M L, Logan L, Millan-Velasco F, Valdespino J L, Sepulveda J, Matsui S. Population-based prevalence of symptomatic and asymptomatic astrovirus infection in rural Mayan infants. J Infect Dis. 1998;178:334–339. doi: 10.1086/515625. [DOI] [PubMed] [Google Scholar]
- 20.Midthun K, Greenberg H B, Kurtz J B, Gary G W, Lin F-Y C, Kapikian A Z. Characterization and seroepidemiology of a type 5 astrovirus associated with an outbreak of gastroenteritis in Marin County, California. J Clin Microbiol. 1993;31:955–962. doi: 10.1128/jcm.31.4.955-962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell D K, Van R, Morrow A L, Monroe S S, Glass R I, Pickering L K. Outbreaks of astrovirus gastroenteritis in day care centers. J Pediatr. 1993;123:725–732. doi: 10.1016/s0022-3476(05)80846-7. [DOI] [PubMed] [Google Scholar]
- 22.Monroe S S, Jiang B, Stine S E, Koopmans M, Glass R I. Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. J Virol. 1993;67:3611–3614. doi: 10.1128/jvi.67.6.3611-3614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monroe S S, Stine S E, Gorelkin L, Herrmann J E, Blacklow N R, Glass R I. Temporal synthesis of proteins and RNAs during human astrovirus infection of cultured cells. J Virol. 1991;65:641–648. doi: 10.1128/jvi.65.2.641-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palombo E A, Bishop R F. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J Clin Microbiol. 1996;34:1750–1753. doi: 10.1128/jcm.34.7.1750-1753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Fauquier A, Carrascosa A L, Carrascosa J L, Otero A, Glass R I, Lopez J A, San Martin C, Melero J A. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology. 1994;201:312–320. doi: 10.1006/viro.1994.1296. [DOI] [PubMed] [Google Scholar]
- 26.Shastri S, Doane A M, Gonzales J, Upadhyayula U, Bass D M. Prevalence of astroviruses in a children's hospital. J Clin Microbiol. 1998;36:2571–2574. doi: 10.1128/jcm.36.9.2571-2574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willcocks M M, Brown T D, Madeley C R, Carter M J. The complete sequence of a human astrovirus. J Gen Virol. 1994;75:1785–1788. doi: 10.1099/0022-1317-75-7-1785. [DOI] [PubMed] [Google Scholar]
- 28.Willcocks M M, Kurtz J B, Lee T W, Carter M J. Prevalence of human astrovirus serotype 4: capsid protein sequence and comparison with other strains. Epidemiol Infect. 1995;114:385–391. doi: 10.1017/s0950268800058015. [DOI] [PMC free article] [PubMed] [Google Scholar]