Abstract
Background:
Wilson disease (WD) is a chronic disorder of copper metabolism which may affect patient’s quality of life (QOL).
Objective:
Our aim was to assess the relationship between mental QOL (M-QOL) and physical QOL (P-QOL) and severity of the liver, neurological disease and mental health in patients with WD.
Methods:
At enrollment into our multisite international WD registry, adults (n = 62) were administered examinations assessing QOL (Short-Form 12-Item Health Survey), cognition, and mood. Patients also underwent hepatology and neurological assessments.
Results:
Patients had lower M-QOL than P-QOL scores, P = 0.0006. Patients with major depressive disorder (n = 22) had worse M-QOL scores, P = 0.0017 but not P-QOL. We found no association with impaired cognition (n = 37) and QOL. The P-QOL scores have a moderate negative association with neurological disease severity based on the Unified Wilson Disease Rating Scale score (total [r = −0.38, P < 0.003], part 2 [r = −0.50, P < 0.0001], and part 3 [r = −0.37, P = 0.004]). M-QOL was not associated with Unified Wilson Disease Rating Scale scores. Worse P-QOL, but not M-QOL, was found in higher cirrhosis severity indicated by Child-Pugh (r = −0.80, P = 0.002) and Model for End Stage Liver Disease scores (r= −0.64, P = 0.03).
Conclusions:
M-QOL was associated with depression but not cognitive impairment, neurological disease, or liver disease severity, suggesting that mental health issues may affect overall QOL independent of the degree of liver or neurological disease. P-QOL was affected by the severity of neurological and liver disease but not mental health but also contributes to overall QOL in WD. An appreciation of the range of problems that affect QOL in adults with WD will help health care providers address issues that could improve overall well-being. The Short-Form 12-Item Health Survey may provide a useful instrument for QOL surveillance in WD.
Keywords: quality of life, genetic, rare, copper, screening
BACKGROUND AND AIMS
Wilson disease (WD) is a genetic autosomal recessive condition caused by mutations in the WD gene ATP7B. Mutations causing defective copper metabolism lead to abnormal biliary excretion of copper with accumulation in the liver and brain. Symptoms occur because of the toxic accumulation of copper causing hepatic disease, neurological impairment, and/or mental health issues. Medical treatments focused on reducing the copper burden are administered lifelong and include the chelating drugs D-penicillamine and trientine or zinc salts. Liver transplantation may be indicated in those presenting with acute liver failure or develop liver failure owing to the late initiation of treatment or lack of adherence to treatment. With effective treatment life expectancy has improved, however, it is no longer acceptable to look at survival as the sole outcome measure. In chronic lifelong diseases, health-related quality of life (HRQOL) that evaluates the consequences of health status on physical function and psychological health is an important marker of treatment effectiveness. Quality of life (QOL) in patients with WD has been analyzed in only a few small studies.1-4 Recent studies have suggested that presence of psychiatric symptoms may have a significant role in HRQOL in patients with WD.5 Because patients with WD may develop a variety of physical and mental health issues, our aim was to assess the QOL of patients with WD and the relationship between mental and physical health QOL and severity of liver disease, neurological disease, and the most common psychiatric problems in patients with WD: major depressive disorder (MDD) and cognitive impairment.
METHODS
The WD registry was initiated at Yale University with the aim of studying the natural history, current treatment patterns, and reasons for change in treatment and clinical status and aid in the development of tools for monitoring treatment effectiveness. The registry was formed through the collaboration of WD specialist centers in the United States and Europe. All centers complied with their local institutional review boards’ requirements and the Health Insurance Portability and Accountability Act. Biospecimens are collected alongside data on background medical history, treatments, family history, and assessments to evaluate liver, neurological, and psychiatric disease status. Data collection is being conducted prospectively over a 5-year period. Psychiatric measures are administered at enrollment and yearly afterward. Written informed consent was obtained from patients enrolled in the registry.
We performed cross-sectional analysis of data collected at enrollment of adults in our multisite international WD registry study beginning in December 2017 over the first 2.5 years.
Participants
There were 62 adults (36 men) enrolled in the study in the first 2.5 years. The majority of patients (86.71%) in the cohort were white in ethnicity. The median age at study enrollment was 41 years of age (interquartile range [IQR] 30–56).
Medical Therapy for Registry Patients
In line with current treatment guidelines (American Association for the Study of Liver Diseases Wilson Disease Guidelines 2003,6 The European Association for the Study of the Liver Wilson Disease Guidelines 20127 symptomatic patients in the registry were mostly treated with chelation therapy. Patients that received zinc salts were generally asymptomatic or presented with compensated liver disease. The choice of therapy was determined by the treating physician, and patients on any therapy were included in the registry.
Measures
Quality of Life
The Medical Outcomes Study Short-Form 12-Item Health Survey (SF-12) (RAND Cooperation) is a widely used outcome measure for QOL. The SF-12 screening tool allowed us to look at both mental health (M-QOL) and physical health QOL (P-QOL) outcomes. Patients self-administer this form during the registry study visit. Higher M-QOL and P-QOL scores indicate better QOL. Questionnaires used for measuring HRQOL include the Short Form-36 Health Survey (SF-36)8 and the World Health Organization Quality of Life (WHO-BREF).1 Comparative SF-36 results are available for healthy populations9 and a variety of medical conditions including neurological and chronic liver disease.10 The SF-12 was developed as an abbreviated alternative to the SF-36 for use in situations with constraints on questionnaire length with the objective of reproducing SF-36–derived physical and mental health component scores.11 The resulting SF-12 has been found to explain around 90% of the variance in PCS-36 and MCS-36 scores.11-15 The shorter QOL survey, the SF-12 has been shown to correlate well with the SF-36 scores in other diseases14,16 and has been used in other chronic liver diseases17,18; however, it has not previously been studied in WD. We selected to use the SF-12 because its ease of administration may be particularly useful for future screening in a clinical setting.
Psychiatric Measures
Mini-International Neuropsychiatric Interview is a clinician-administered structured interview that assesses major psychiatric diseases as per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.19 The questions assess psychiatric symptoms as per Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria and can be answered “yes” or “no.” The Mini-International Neuropsychiatric Interview does not quantify the severity of psychiatric symptoms but leads to psychiatric diagnosis and allows for most of the Fifth-Edition Diagnostic and Statistical Manual of Mental Disorders specifiers. We used the most recent version available at the start of the WD registry, Mini-International Neuropsychiatric Interview 7. For this manuscript, we focused the analysis on the results of the depressive disorder module only. Patients with bipolar disorder who also had a history of MDD episodes (n = 5) were also included in the analysis.
Patient Health Questionnaire 9 is a self-administered questionnaire that evaluates the presence of depressive symptoms in the 2 weeks prior. It is a widely used instrument to measure depression in medically ill individuals.20,21 A higher score is indicative of more depression symptomology (normal score, 0–4; mild, 5–9; moderate, 10–14; moderately severe, 15–19; severe, >20).
The Montreal Cognitive Assessment is a widely used cognitive screening tool that allows assessment of a patient’s cognitive health.22 The test was carried out by a trained professional. A score of <26 was considered suggestive of cognitive impairment, the lower the score the worse the impairment.
Neurological Disease Assessment
Neurological assessment was made using the United Wilson Disease Rating Scale (UWDRS) and was performed by a movement disorder specialist as per protocol. The UWDRS assessment has been previously validated in patients with WD.23-25 We reviewed cross-sectional data at enrollment to assess neurological disease severity. A UWDRS score of >0 was used to determine the presence of signs or symptoms of neurological disease, and the total UWDRS score was used to assess neurological disease severity, a high score indicating severe disease.
Liver Disease Assessment
Hepatological assessments were performed by a hepatologist as per protocol. We assessed liver disease severity and cirrhosis by reviewing retrospective data on liver imaging for evidence of portal hypertension and histology of liver biopsy for fibrosis and performed analysis of serological markers of fibrosis, aspartate aminotransferase-to-platelet ratio index, and Fibrosis-4 scores, using specimens obtained at the time of enrollment into the registry. A hepatologist on the study protocol determined cirrhosis status of research subjects based on data review. The algorithm that was used for defining the presence or absence of cirrhosis is provided in Appendix.
Medical Therapy for Registry Patients
In line with current treatment guidelines,6,7 symptomatic patients in the registry were generally being treated with chelation therapy. Patients that received zinc salts were generally asymptomatic or presented with compensated liver disease. The choice of therapy was determined by the treating physician. Patients on any therapy were included in the registry. Patients were on maintenance therapy for WD (d-penicillamine n = 8, trientine n = 20, zinc n = 27, chelator and zinc n = 5, investigational 1). The median treatment duration (time from diagnosis to study enrollment) was 19.5 years (IQR 7–35).
Statistical Considerations
Patient characteristics were summarized using frequencies and percentages for categorical data and medians and IQRs for continuous data owing to small, skewed sample. Analysis included Wilcoxon rank sum or signed rank tests for testing differences in numerical data, Fisher’s exact test for differences in categorical data, and Spearman’s correlation coefficients for correlations between continuous variables. All analyses were performed using SAS/STAT Software, version 9.4. (2012 SAS Institute, INC., Cary, NC USA)26 A P-value of 0.05 was considered statistically significant.
RESULTS
There were 62 adults enrolled in the registry. Participant characteristics are shown in Table 1.
TABLE 1.
Registry cohort study characteristics
| Gender (n,%) | ||
| Male | 36 (58.06%) | |
| Female | 26 (41.94%) | |
| Age at study enrollment (median, IQR) | 41 (30–56) | |
| Age at diagnosis of WD (median, IQR) | 19 (11–25) | |
| Treatment duration (median, IQR) | 19.5 (7–35) | |
| Major depressive disorder (n) (MINI-7) | 22 (37.29%) | |
| PHQ 9 score (median, IQR) | 3 (1–6) | |
| Cognitive impairment (n) (MOCA <26) | 28 (47.46%) | |
| Neurological symptoms (n) (UWDRS >0) | 50 (80.65%) | |
| Cirrhosis (n)* | 12 (19.67%) | |
| Current WD medication (n) | WD treatment duration median (IQR) | Previous different WD medication regimen (n) |
|---|---|---|
| d-Penicillamine n = 8 | 37 (4,41) | Y = 0, N = 8 |
| Trientine n = 20 | 23.5 (8,43) | Y = 14, N = 6 |
| Zinc n = 27 | 17 (12,30) | Y = 25, N = 10 |
| Investigational n = 1 | 3 (3,3) | Y = 0, N = 1 |
| Chelator and zinc† = 5 | 26 (14,29) | Y = 5, N = 1 |
IQR = interquartile range; MINI = Mini-International Neuropsychiatric Interview; MOCA = Montreal Cognitive Assessment; PHQ = Patient Health Questionnaire; UWDRS = United Wilson Disease Rating Scale; WD = Wilson disease.
See appendix for cirrhosis diagnostic algorithm.
Chelator = d-Penicillamine or Trientine
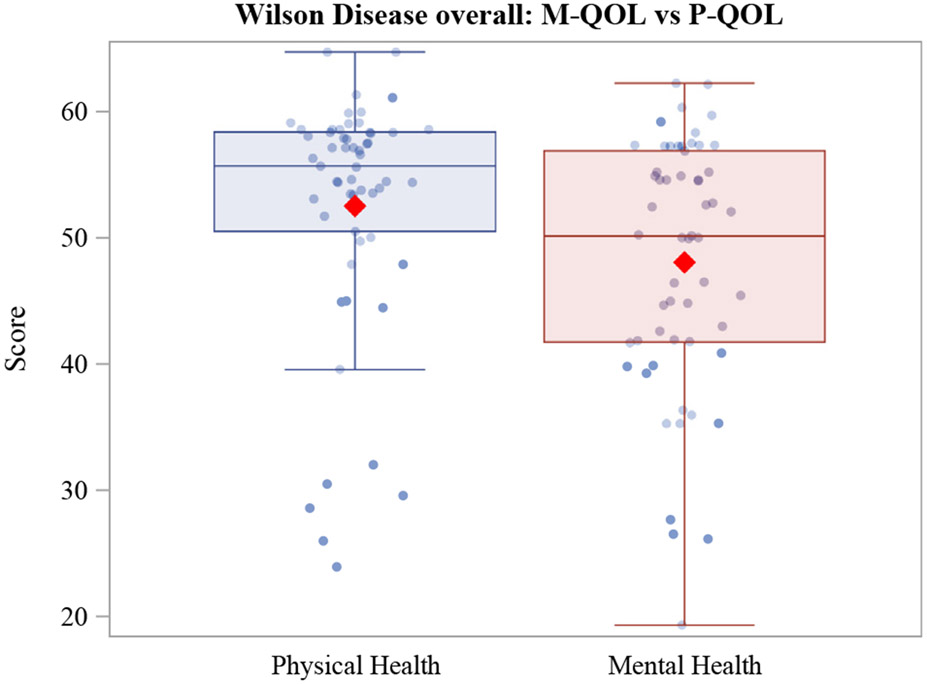
Although the majority of treated patients appear to have a QOL that is similar to the population mean, outliers do exist that fall well below the population mean QOL, as illustrated in Figure 1. It is important to appreciate the factors that adversely impact the QOL in this patient group.
FIGURE 1.
HRQOL Scores. The line in the box corresponds to median while the diamond represents mean. Individual dots show the raw data points. HRQOL = health-related quality of life; M-QOL = mental health quality of life; P-QOL = physical QOL.
There was no significant difference in M-QOL in men versus women (see Table 2). We found no correlation between QOL and age of diagnosis (see Table 2). We found a negative correlation between age at enrollment into the study and P-QOL but not M-QOL (P-QOL r = −0.29, P = 0.025; M-QOL r = −0.15, P = 0.26). We found a positive correlation between disease duration (age at diagnosis and age at study enrollment) and M-QOL (r = 0.30, P = 0.024) but not P-QOL (r = −0.24, P = 0.073).
TABLE 2.
Relationship with HRQOL*
| Clinical and demographic variable | Physical health QOL Median (IQR) |
P-value | Mental health QOL Median (IQR) |
P-value |
|---|---|---|---|---|
| Neurological impairment | ||||
| Yes (UWDRS > 0) (N = 47) | 55.63 (47.90, 58.38) | 0.24 | 50.04 (41.71, 57.28) | 0.76 |
| No (UWDRS = 0) (N = 11) | 56.90 (54.35, 58.60) | 52.49 (35.28, 55.22) | ||
| Cognitive impairment | ||||
| Yes (MOCA < 26) (N = 27) | 53.78 (49.74, 58.38) | 0.34 | 50.00 (40.86, 56.92) | 0.82 |
| No (MOCA) > 25 (N = 28) | 57.01 (53.02, 58.49) | 51.08 (42.37, 55.06) | ||
| Cirrhosis | ||||
| Yes (N = 12) | 55.66 (53.58, 57.61) | 0.98 | 54.72 (51.23, 58.76) | 0.043 |
| No (N = 46) | 55.48 (47.90, 58.60) | 45.96 (39.89, 55.22) | ||
| Lifetime MDD (MINI) | ||||
| Yes (N = 22) | 54.03 (44.96, 57.48) | 0.36 | 42.85 (36.33, 52.80) | 0.017 |
| No (N = 35) | 56.31 (53.37, 58.38) | 52.65 (44.97, 57.28) | ||
| Gender | ||||
| Male (N = 33) | 55.63 (53.56, 58.38) | 0.36 | 46.52 (36.33, 54.93) | 0.095 |
| Female (N = 25) | 54.59 (44.84, 56.92) | 54.59 (44.84, 56.92) |
| Physical health QOL (correlation, P-value) | Mental health QOL (correlation, P-value) | |
|---|---|---|
| Overall population (N = 58) | ||
| UWDRS II | −0.50 (<0.0001) | −0.26 (0.051) |
| UWDRS III | −0.37 (0.004) | −0.12 (0.367) |
| UWDRS total | −0.38 (0.003) | −0.13 (0.314) |
| Age of diagnosis | −0.02 (0.874) | −0.18 (0.167) |
| Age at enrollment | −0.29 (0.025) | 0.15 (0.26) |
| Treatment duration | −0.24 (0.073) | 0.30 (0.024) |
| Patients with lifetime MDD | ||
| UWDRS II (N = 22) | −0.46 (0.032) | 0.12 (0.63) |
| UWDRS III (N = 22) | −0.52 (0.014) | 0.20 (0.38) |
| UWDRS Total (N = 22) | −0.50 (0.017) | 0.21 (0.35) |
| PHQ raw scores (N = 21) | −0.21 (0.36) | −0.77 (<0.0001) |
| Patients with cirrhosis (N = 12) | ||
| Child-Pugh score | −0.80 (0.002) | 0.02 (0.96) |
| MELD | −0.64 (0.03) | −0.30 (0.34) |
HRQOL = health-related quality of life; IQR = interquartile range; MELD = Model for End Stage Liver Disease; MINI = Mini-International Neuropsychiatric Interview; MOCA = Montreal Cognitive Assessment; PHQ = Patient Health Questionnaire; QOL = quality of life; UWDRS = United Wilson Disease Rating Scale; WD = Wilson disease.
Patients with incomplete assessments were not included.
Adult patients with WD had significantly lower mental health QOL scores than physical health QOL (median 50.1 [IQR 41.7–56.9] versus 55.7 [IQR 50–58.4], P = 0.0006). When comparing the means for MQOL and PQOL in our treated WD cohort, they were not significantly different from the population mean of 50 suggesting that most treated WD patients may have a QOL that is similar to the rest of the US population.27
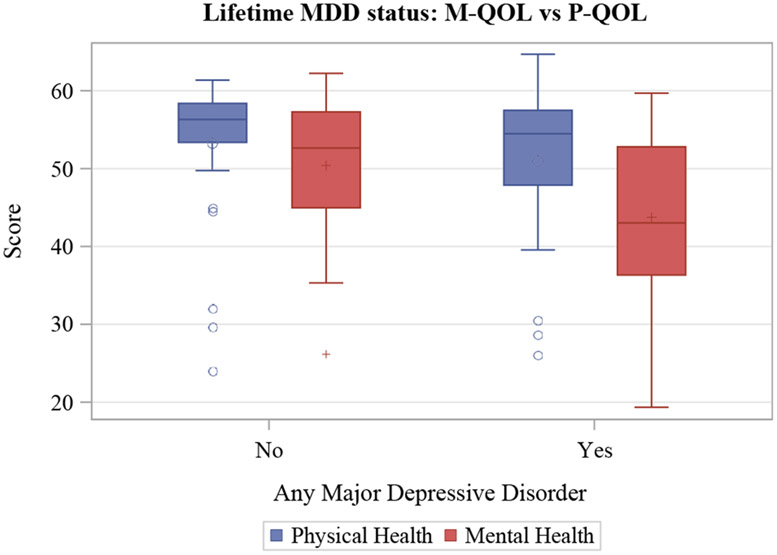
Patients with a lifetime history of MDD (n = 22) had worse M-QOL scores (median 42.85 versus 52.65, P = 0.0017) (illustrated in Figure 2). We found no significant difference for those with MDD compared with those without in P-QOL scores (median 54.03 versus 56.31, P = 0.36) (see Figure 2). There was also a significant difference in M-QOL scores, but not P-QOL scores, in those with higher Patient Health Questionnaire-9 scores indicating that worse depression symptomology may also be associated with worse M-QOL scores (P = 0.032).
FIGURE 2.
Box plot of HRQOL scores in patients with Wilson disease with lifetime MDD and without. The line in the box corresponds to median while the circle in the box represents mean. HRQOL = health-related quality of life; M-QOL = mental health quality of life; P-QOL = physical QOL.
The median Montreal Cognitive Assessment score was 27 (IQR 25–28). There were 27 patients (49%) with cognitive impairment. We did not find an association with impaired cognition and M-QOL nor PQOL scores compared with those without.
There were 50 patients with neurological symptoms (UWDRS score > 0). The P-QOL scores have a moderate negative association with the severity of neurological disease based on total UWDRS score (r = −0.38, P < 0.003) as well as subscores UWDRS part II (self-assessed disability, r = −0.50, P < 0.0001) and part III (neurologist assessed severity score, r = −0.37, P = 0.004). This indicates that patients with more severe neurological disease had a worse physical health QOL scores. There are no associations with M-QOL and UWDRS scores.
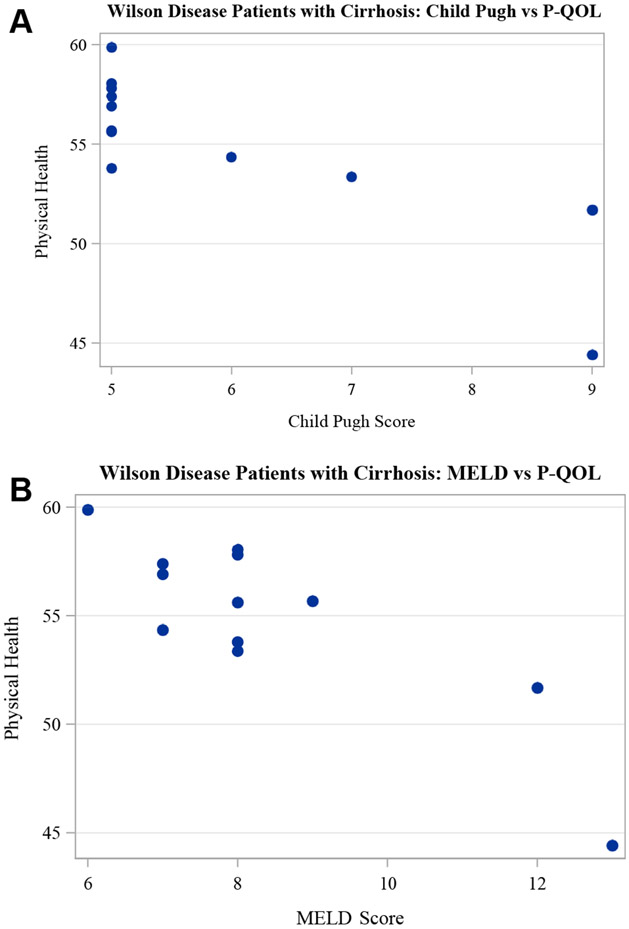
Twelve patients had cirrhosis based on review of imaging, aspartate aminotransferase-to-platelet ratio index, and Fibrosis-4 scores. In those with cirrhosis higher Child-Pugh scores were associated with a worse P-QOL (r = −0.80, P = 0.002) but not M-QOL scores (r = 0.02, P = 0.96) (see Figure 3). Similarly higher Model for End Stage Liver Disease scores were associated with worse P-QOL scores (r = −0.64, P = 0.03) (see Figure 3) but not M-QOL (r = −0.30, P = 0.34). In patients with cirrhosis, 75% were of Child-Pugh A (score 5–6) and 25% were of Child-Pugh B (score 7–9). The median Model for End Stage Liver Disease score was 7 (IQR 6–8; range 6–18).
FIGURE 3.
Scatter plots demonstrating higher Child-Pugh (A) and MELD (B) scores associated with worse P-QOL in patients with cirrhosis. MELD = Model for End Stage Liver Disease; P-QOL = physical quality of life.
DISCUSSION
QOL is an increasingly recognized important clinical outcome measure and maximizing HRQOL is a central aim in managing chronic diseases with a near-normal life expectancy such as treated patients with WD. Our results support that psychiatric symptoms have a major impact on HRQOL in patients with WD and that longer disease duration is associated with worse mental health QOL. Age at diagnosis or study enrollment, however, did not affect QOL. To our knowledge, this is the first study performing a prospective systematic evaluation of psychiatric, neurological, and liver conditions in patients with WD. We reviewed HRQOL in relation to mental health status, in particular how cognition (Montreal Cognitive Assessment) and major depressive disorder (Mini-International Neuropsychiatric Interview-7) affects HRQOL. We also assessed how the severity of neurological and liver disease affects HRQOL in WD using the UWDRS as the neurological rating tool.
We found that QOL is affected by the lifetime prevalence of MDD. Patients with WD with a lifetime diagnosis of MDD had worse mental health QOL scores than those without. Physical health QOL scores were not affected by lifetime MDD. There were 37 patients (62.71%) with cognitive impairment (Montreal Cognitive Assessment <26). We did not find that cognitive impairment was associated with a worse QOL scores. Previous cross-sectional studies have reported an association between psychiatric symptoms and QOL in WD: depression3 and bipolar disorder.5 A study of Svetel et al.3 evaluated QOL and psychiatric symptoms in their cohort of patients with WD using the MMSE and the Hamilton Depression Rating Scale. They found that QOL in patients with WD with psychiatric symptoms was lower than in those without. Our study was the first to evaluate patients with WD using comprehensive diagnostic tools to assess the presence of psychiatric symptoms.
P-QOL scores were affected by the severity of neurological disease; however, mental health QOL scores did not appear to be affected in our study. Previous studies in WD have examined QOL using tools including the SF-3623 and the WHO-BREF.1 These studies have indicated that the severity of neurological disability in patients is related to a worse QOL, although few have reported on how it affects physical health QOL compared with M-QOL. A study by Svetel et al. in 2011 evaluated 60 treated clinically stable patients with WD with the SF-36 QOL outcome measure. They assessed the neurological status of patients using the Global Assessment Scale for WD. Lower HRQOL scores were found in patients with neurological WD compared with those with a predominately hepatic form of WD. The finding that neurological WD patients had lower total SF-36 scores has also been demonstrated by Schaefer et al.2 A study by Kumar et al. in 2008 where 30 adult patients with WD were assessed using the neurological symptom score and WHO-BREF for QOL also found that the neurological symptom score correlated inversely with the physical domain of the WHO-BREF QOL (P < 0.02).1 QOL scores are also affected in other chronic neurological conditions including multiple sclerosis,28 epilepsy,29 and Parkinson’s disease.30 Several neurological conditions have disease-specific QOL measures to determine outcomes in these disease groups. Given the heterogeneity of presentation of WD and the potential effect of symptoms on mental and physical health, a disease-specific tool may be useful for measuring HRQOL outcomes.
The severity of liver disease in subjects in our study affected their physical health QOL scores. In patients with WD with cirrhosis, higher Child-Pugh and Model for End Stage Liver Disease scores indicating worse prognosis in patients with liver cirrhosis were associated with a worse P-QOL but not M-QOL. Studies in other chronic liver diseases have demonstrated a similar effect of liver disease severity on QOL, although data on the effect of liver disease on HRQOL in patients with WD are limited.31-34
The impact of liver disease upon HRQOL may vary by the etiology of liver disease. For viral liver disease, a previous study found that presence of liver disease in particular cirrhosis was associated with a lower QOL in comparison with a healthy population; however, the physical component was more impaired than the mental health component.17 Other studies have demonstrated differences in the pattern of QOL impairment measured in patients with different liver disease aetiologies. A German study of patients without cirrhosis noted that patients with primary biliary cholangitis scored lowest on the P-QOL component score of the SF-36, whereas patients with hepatitis C had lower M-QOL scores.10 This may be related to fatigue being a key factor in primary biliary cholangitis,35 while HCV has been shown to be associated with depression.36 Similarly, mental health issues, including depression, are highly prevalent in WD which may be related to copper toxicity affecting the brain. A study by Younossi et al.33 showed that in patients with cirrhosis, P-QOL scores were similar between cholestatic diseases and viral hepatitis but poorer in other hepatocellular diseases. Another large study of patients with cirrhosis found that patients with NAFLD had lower P-QOL scores than those with ALD, cholestatic liver disease, and viral hepatitis.31 We found that patients with WD in our study have worse median M-QOL scores than P-QOL scores and mean M-QOL scores from our cohort were lower than the population mean M-QOL score of 50. This suggests that the burden of mental health issues in WD may be greater than that of physical health issues on QOL. However, the difference between the mean M-QOL and P-QOL summary scores and the population mean of each score was not significantly different indicating that most treated patients with WD may have a QOL that is similar to the rest of the US population.27 Despite this, outliers do exist that fall well below the population mean QOL score. Our study provides important insight into the range of potentially unrecognized impairment in both physical and mental health that negatively impacts QOL in adults with WD. A greater appreciation of how QOL is impacted on adults with WD and the factors that contribute to this will help health care professionals take a holistic approach to treating patients with WD and address issues that could improve their patient’s overall well-being.
Our study has several limitations that must be considered. The median treatment duration of the group is 19.5 years. Factors that impact M-QOL may be different for the newly diagnosed patients compared with stable patients and those who have lived with the disease for longer. As the registry cohort increases over time, this may allow these groups to be examined separately in the future. There are currently no disease-specific QOL-screening tools available for WD, and therefore, the SF-12 may not be as accurate at detecting issues that affect QOL in the WD population as a tool developed specifically for WD. Furthermore, we only examine the impact of depression and cognitive impairment on QOL and other psychiatric factors such as impulsivity and personality traits are not analyzed. In addition, in the majority of patients enrolled in our registry, cognitive impairment was mild in severity which may be why it was not found to be associated with worse QOL. Similarly, although there was a range in severity in those with cirrhosis and neurological disease, the majority were not highly symptomatic (Child-Pugh Score Median 5 [5–9] UWDRS total score median 5 [0–73]). Owing to the rarity of the condition, our sample size is relatively small, and the present analysis focuses on cross-sectional enrollment data. Owing to the small sample size, multifactorial analysis to assess the impact of liver disease, neurological disease, and psychiatric symptoms for our group was not performed. Further prospective data collection in our multisite registry will help determine whether the conclusions from our study are maintained at enrollment and longitudinally in studied patients.
CONCLUSION
We conclude that overall QOL in WD is affected by both mental and physical health. We found that mental health issues such as depression (but not cognitive impairment) may affect M-QOL of patients with WD independent of their degree of liver and/or neurological disease, while physical health QOL scores were affected by the severity of liver and neurological disease but not by mental health issues. Our study is the first to use the SF-12 to assess QOL in WD. It has shown a good correlation with the SF-36 scores in other diseases. As the questionnaire is shorter, we suggest that it is a useful screening tool that can be applied in clinical settings. Given the heterogeneity of WD and the demonstrated effect of symptoms on mental and physical health, further research into developing a more disease-specific tool may be useful for assessing outcomes in this patient population.
Supplementary Material
Funding:
Funding was provided by the Wilson Disease Association, Paula C. Zimbrean, 20 York st., New Haven, CT, 06510.
Footnotes
CRediT AUTHORSHIP CONTRIBUTION STATEMENT
Michelle A. Camarata: Writing - original draft, Investigation. Aftab Ala: Investigation. Ayse K. Coskun: Methodology. Yanhong Deng: Formal analysis. Regino P. Gonzalez-Peralta: Investigation. Kaitlin R. Maciejewski: Formal analysis. Amar Patel: Investigation. Susan Rubman: Investigation. Uyen To: Investigation. Ricarda Tomlin: Methodology. Michael L. Schilsky: Investigation, Methodology, Writing - review & editing. Paula C. Zimbrean: Methodology, Writing - review & editing.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaclp.2021.04.004.
Submission Declaration: The work described has not been published previously except in the form of an abstract, and is not under consideration for publication elsewhere. The publication is approved by all authors and by the responsible authorities where work was carried out and if accepted it will not be published elsewhere in the same form including electronically without the written consent of the copyright-holder.
Conflicts of Interest: Paula Zimbrean received consultant fees from Wilson Therapeutics, Alexion and Univar. Michael L. Schilsky is the Chair of the Medical Advisory Committee of the Wilson Disease Association and has received grant support for studies from Alexion, GMPO and the Wilson Disease Association. Aftab Ala is on the advisory boards of Wilson Therapeutics (now Alexion), Vivet, Univar, Ultragenyx. He has received unrestricted educational grants from Alexion, Bayer, Wilson Therapeutics, Intercept and the Wilson Disease Association. He is the holder of National Institute for Health and Research (NIHR) and UKRI awards.
Ethical Approval: All centers complied with their local institutional review boards’ requirements and the Health Insurance Portability and Accountability Act (HIPAA). Written informed consent was obtained from patients enrolled in the registry.
Availability of data and materials: The data sets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Contributor Information
Michelle A. Camarata, Yale University, Digestive Diseases, Transplantation and Immunology, New Haven, United States; Royal Surrey County Hospital, Gastroenterology and Hepatology, Guilford, United Kingdom; Department of Clinical and Experimental Medicine, University of Surrey, Guilford, United Kingdom
Aftab Ala, Royal Surrey County Hospital, Gastroenterology and Hepatology, Guilford, United Kingdom; Department of Clinical and Experimental Medicine, University of Surrey, Guilford, United Kingdom; King’s College Hospital, Institute of Liver Studies, London, United Kingdom
Ayse K. Coskun, Yale University, Digestive diseases, transplantation and immunology, New Haven, United States
Yanhong Deng, Yale Center for Analytical Sciences, Yale University, New Haven, United States
Regino P. Gonzalez-Peralta, AdventHealth Transplant Institute, Orlando, Florida, United States
Kaitlin R. Maciejewski, Yale Center for Analytical Sciences, Yale University, New Haven, United States
Amar Patel, Yale University, Neurology, New Haven, United States
Susan Rubman, Yale University, Psychiatry, New Haven, United States
Uyen To, Yale University, Digestive diseases, transplantation and immunology, New Haven, United States
Ricarda Tomlin, Yale University, Digestive diseases, transplantation and immunology, New Haven, United States
Michael L. Schilsky, Yale University, Digestive diseases, transplantation and immunology, New Haven, United States
Paula C. Zimbrean, Yale University, Psychiatry, New Haven, United States
References
- 1.Komal Kumar RN, Taly AB, Nair KP, et al. : Quality of life in Wilson’s disease. Ann Indian Acad Neurol 2008; 11:37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer M, Gotthardt DN, Ganion N, et al. : Wilson disease: health-related quality of life and risk for depression. Clin Res Hepatol Gastroenterol 2016; 40:349–356 [DOI] [PubMed] [Google Scholar]
- 3.Svetel M, Pekmezović T, Tomić A, et al. : Quality of life in patients with treated and clinically stable Wilson’s disease. Mov Disord 2011; 26:1503–1508 [DOI] [PubMed] [Google Scholar]
- 4.Sutcliffe RP, Maguire DD, Muiesan P, et al. : Liver transplantation for Wilson’s disease: long-term results and quality-of-life assessment. Transplantation 2003; 75:1003–1006 [DOI] [PubMed] [Google Scholar]
- 5.Carta M, Mura G, Sorbello O, Farina G, Demelia L: Quality of life and psychiatric symptoms in Wilson’s disease: the Relevance of bipolar disorders. Clin Pract Epidemiol Ment Health 2012; 8:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts EA, Schilsky ML: A practice guideline on Wilson disease. Hepatology 2003. Jun; 37:1475–1492 [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver: EASL clinical practice guidelines: Wilson’s disease. J Hepatol 2012. Mar 1; 56:671–685 [DOI] [PubMed] [Google Scholar]
- 8.Ware JE: How to score the revised MOS Short Form Health Scale (SF-36). Boston: Health Institute, New England Medical Center Hospitals; 1988 [Google Scholar]
- 9.Jenkinson C, Coulter A, Wright L: Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993; 306:1437–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tillmann HL, Wiese M, Braun Y, et al. : Quality of life in patients with various liver diseases: patients with HCV show greater mental impairment, while patients with PBC have greater physical impairment. J Viral Hepat 2011; 18:252–261 [DOI] [PubMed] [Google Scholar]
- 11.Ware J Jr, Kosinski M, Keller SD: A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–233 [DOI] [PubMed] [Google Scholar]
- 12.Gandek B, Ware JE, Aaronson NK, et al. : Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA Project. International quality of life assessment. J Clin Epidemiol 1998; 51:1171–1178 [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson C, Layte R: Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997; 2:14–18 [DOI] [PubMed] [Google Scholar]
- 14.Pickard AS, Johnson JA, Penn A, Lau F, Noseworthy T: Replicability of SF-36 summary scores by the SF-12 in stroke patients. Stroke 1999; 30:1213–1217 [DOI] [PubMed] [Google Scholar]
- 15.Huo T, Guo Y, Shenkman E, Muller K: Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. Health Qual Life Outcomes 2018; 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pezzilli R, Morselli-Labate AM, Frulloni L, et al. : The quality of life in patients with chronic pancreatitis evaluated using the SF-12 questionnaire: a comparative study with the SF-36 questionnaire. Dig Liver Dis 2006; 38:109–115 [DOI] [PubMed] [Google Scholar]
- 17.Svirtlih N, Pavic S, Terzic D, et al. : Reduced quality of life in patients with chronic viral liver disease as assessed by SF12 questionnaire. J Gastrointestin Liver Dis 2008; 17:405–409 [PubMed] [Google Scholar]
- 18.Untas A, Boujut E, Corpechot C, et al. : Quality of life and illness perception in primary biliary cirrhosis: a controlled cross-sectional study. Clin Res Hepatol Gastroenterol 2015; 39:52–58 [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. : The MINI-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–23 [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL: The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002; 32:509–515 [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bédirian V, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699 [DOI] [PubMed] [Google Scholar]
- 23.Leinweber B, Möller JC, Scherag A, et al. : Evaluation of the Unified Wilson’s disease rating Scale (UWDRS) in German patients with treated Wilson’s disease. Movement Disord 2008; 23:54–62 [DOI] [PubMed] [Google Scholar]
- 24.Czlonkowska A, Tarnacka B, Moller JC, et al. : Unified Wilson’s Disease Rating Scale-a proposal for the neurological scoring of Wilson’s disease patients. Neurologia i neurochirurgia polska 2007; 41:1. [PubMed] [Google Scholar]
- 25.Członkowska A, Litwin T, Dzieżyc K, Karliński M, Bring J, Bjartmar C: Characteristics of a newly diagnosed Polish cohort of patients with neurological manifestations of Wilson disease evaluated with the Unified Wilson’s Disease Rating Scale. BMC Neurol 2018; 18:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SAS/STAT® Software, version 9.4 (©2012. SAS Institute, INC., Cary, NC, USA: ). [Google Scholar]
- 27.Ware JE, Kosinski M, Keller SD: SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA, ed. New England Medical Center: The Health Institute; 1995 [Google Scholar]
- 28.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L: Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler 2001; 7:340–344 [DOI] [PubMed] [Google Scholar]
- 29.Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK: Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology 1999; 53:162–166 [DOI] [PubMed] [Google Scholar]
- 30.Schrag A, Jahanshahi M, Quinn N: How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 2000; 15:1112–1118 [DOI] [PubMed] [Google Scholar]
- 31.Afendy A, Kallman JB, Stepanova M, et al. : Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther 2009; 30:469–476 [DOI] [PubMed] [Google Scholar]
- 32.Marchesini G, Bianchi G, Amodio P, et al. : Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001; 120:170–178 [DOI] [PubMed] [Google Scholar]
- 33.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G: Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol 2001; 96:2199–2205 [DOI] [PubMed] [Google Scholar]
- 34.Kanwal F, Hays RD, Kilbourne AM, Dulai GS, Gralnek IM: Are physician-derived disease severity indices associated with health-related quality of life in patients with end-stage liver disease? Am J Gastroenterol 2004; 99:1726–1732 [DOI] [PubMed] [Google Scholar]
- 35.Newton JL, Bhala N, Burt J, Jones DE: Characterisation of the associations and impact of symptoms in primary biliary cirrhosis using a disease specific quality of life measure. J Hepatol 2006; 44:776–783 [DOI] [PubMed] [Google Scholar]
- 36.Schaefer M, Capuron L, Friebe A, et al. : Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol 2012; 57:1379–1390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.