Abstract
Purpose
The Hepatic Steatosis Index (HSI) is a reliable predictor of non-alcoholic fatty liver disease (NAFLD), which can increase the risk of type 2 diabetes mellitus (T2DM). However, limited research has directly predicted HSI’s association with T2DM occurrence at normal blood glucose levels. Hence, this study aimed to assess the link between baseline HSI and T2DM development under euglycemic conditions while also exploring potential sex differences.
Methods
Using data from the NAGALA cohort study, a Cox regression model analyzed the relationship between HSI and T2DM risk, calculating hazard ratios (HR) and 95% confidence intervals (CI). Subgroup analyses were conducted to investigate factors influencing HSI’s prediction of incident T2DM.
Results
During a mean 6.1-year follow-up, 238 individuals (1.65% of participants) developed T2DM. After adjusting for age, ethanol consumption, smoking status, SBP, DBP, TG, and TC, HSI showed a significant association with incident T2DM in individuals with normal glucose levels, consistent across sexes. Compared to the lowest quartile group (Q1), the HR and 95% CI for Q2, Q3, and Q4 were 1.09 (0.61, 1.93), 1.16 (0.68, 1.98), and 3.30 (2.04, 5.33), respectively (P for trend < 0.001). Subgroup analysis indicated that elevated HSI significantly increased the risk of incident T2DM in individuals with normal TG levels (P for interaction = 0.0170).
Conclusion
This study highlights the significant association between elevated HSI levels and the likelihood of developing incident T2DM in individuals with normal glucose levels. Furthermore, it offers a simple and valuable screening tool for predicting T2DM.
Keywords: Type 2 Diabetes Mellitus, hepatic steatosis index, Non-alcoholic fatty liver disease, risk prediction, longitudinal study
Background
Diabetes mellitus, a metabolic disorder characterized by elevated blood glucose levels, is one of the most prevalent chronic metabolic diseases.1 Type 2 Diabetes Mellitus (T2DM), in particular, accounts for 90% of all diabetes cases.2 Annually, over a million people succumb to diabetes, ranking it as the ninth leading cause of death.3 The cost of diabetes care surpasses per capita healthcare expenditure by a minimum of 3.2 times, escalating to 9.4 times when complications arise.4 T2DM is acknowledged as a significant public health concern, exerting a profound impact on both human lives and healthcare expenses.
The diagnosis and prediction of T2DM involve the utilization of various indicators, as recognized by the American Diabetes Association. These indicators include fasting plasma glucose (FPG), glycated hemoglobin (HbA1c) testing, and oral glucose tolerance testing (OGTT).5 However, research has shown that individuals who have normal levels of these indicators still face the risk of developing T2DM.6,7 For individuals with normal values of traditional markers such as FPG and HbA1c, the risk of T2DM cannot be accurately predicted or intervened early using these conventional indicators, leading to an overlooked risk of developing T2DM. Therefore, identifying an effective predictive marker for individuals with normal blood glucose levels is of significant clinical value and urgently needed.
Existing research suggests that non-alcoholic fatty liver disease (NAFLD) increases the risk of developing T2DM.8 Several factors, including the alanine transaminase-to-aspartate aminotransferase ratio (ALT/AST), sex, and body mass index (BMI), have been identified as indicators associated with the onset of NAFLD.9 A composite index called the Hepatic Steatosis Index (HSI), which incorporates these three factors, has been found to reliably predict NAFLD in previous studies.10 Insulin resistance and metabolic syndrome are also risk factors for T2DM, and the literature shows a strong correlation between HSI and these conditions.11–13 However, limited research exists on using HSI to directly predict incident T2DM, and the existing studies only include individuals with pre-diabetes, which could potentially confound the accuracy of prediction.14 The predictive accuracy of HSI in individuals with normal blood glucose levels has not been validated yet. Therefore, this study aims to assess the association between baseline HSI and incident T2DM in normoglycemic individuals and to examine whether this association differs by sex.
Methods
Population and Study Design
The NAGALA (NAFLD in the Gifu Area, Longitudinal Analysis) cohort study provided the original data for this research. Okamura et al transferred ownership of the study data to the Dryad website (https://datadryad.org/stash/dataset/doi:10.5061/dryad.8q0p192), enabling qualified medical professionals to access the database for secondary research at no cost in order to address newly proposed clinical issues. Initiated by Murakami Memorial Hospital in 1994, the NAGALA study documented potential factors leading to T2DM and NAFLD as endpoint events in the general population. Sixty percent of participants underwent regular medical exams, ensuring a low participant attrition rate and high data reliability. Furthermore, Okamura et al have previously published medical papers based on this database, focusing on the relationship between ectopic adiposity and diabetes.15
By analyzing the NAGALA longitudinal cohort retrospectively, this study aims to explore the predictive value of baseline HSI in individuals with normal blood glucose levels by investigating its association with incident T2DM.
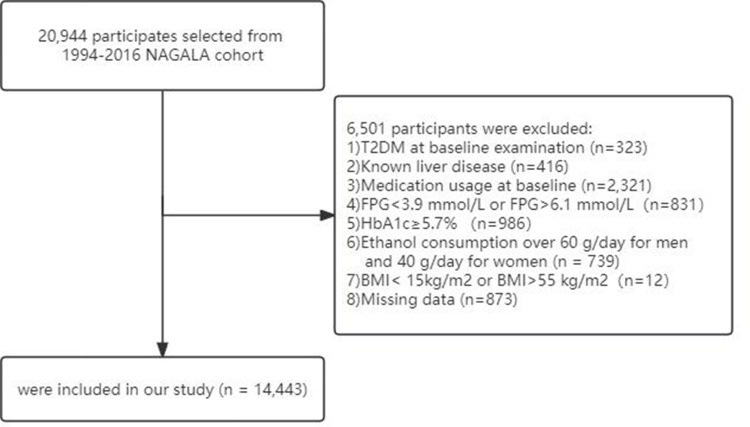
The original study collected data from a total of 20,944 participants. The exclusion criteria were as follows: (1) Participants diagnosed with T2DM at baseline (n = 323); (2) Participants diagnosed with hepatitis (viral/alcoholic) at baseline (n = 416); (3) Participants using medication at baseline (n = 2321); (4) FPG <3.9 mmol/L or FPG >6.1 mmol/L at baseline (n = 831); (5) HbA1c ≥5.7% at baseline (n = 986); (6) Alcohol intake >60g/day (for men) or >40g/day (for women)16 (n = 739); (7) BMI <15kg/m2 or BMI >55 kg/m2 (n = 12); (8) Loss to follow-up (n = 873). All participants in this study provided informed consent for the use of their data. The study protocol was approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University and the Murakami Memorial Hospital Ethics Committee.17
Data Collection and Measurement
The demographic characteristics of all study participants, such as sex, age, weight, BMI, waist circumference (WC), ethanol intake, and smoking habits, were evaluated using standardized questionnaires administered by healthcare professionals with medical expertise. Trained medical personnel measured height, weight, WC, and blood pressure, and BMI was calculated using the formula weight (kg)/[height (m)]2. Ethanol consumption, in terms of average weekly intake, was estimated based on participants’ reported consumption over the previous month. Smoking status led to the categorization of participants into three groups: never smokers, former smokers, and current smokers. Former smokers were defined as individuals who had quit smoking at the time of the baseline survey, while current smokers referred to those who reported smoking during the same period. Regular physical activity was defined as engaging in exercise meeting the intensity and duration guidelines set by the World Health Organization at least once a week.18
During each subsequent visit, it was advised to fast for a minimum of 8 hours before undergoing blood collection. The laboratory parameters that were assessed included blood pressure, gammaglutamyl transferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C). To calculate the HSI, the following formula was utilized: 8 × (ALT/AST ratio) + BMI (+2, if female). This study adhered to the principles stated in the Declaration of Helsinki.
Diagnosis of a Fatter Liver and Incident T2DM
Healthcare professionals with extensive experience have utilized abdominal ultrasound to diagnose fatty livers using four indicators: hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring.19 The diagnostic process adhered to blinding principles. For diabetes diagnosis, the criteria were HbA1c ≥ 6.5% or FPG ≥ 7 mmol/L.20
Statistical Analysis
The participants’ baseline characteristics were compared across quartile groups (Q1–Q4) based on the HSI. Continuous variables were described using mean and standard deviation for normally distributed data or median and range for non-normally distributed data. Categorical variables were presented as the number (%) of cases. The normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Differences between groups were evaluated using a one-way ANOVA for normally distributed continuous variables and the Kruskal–Wallis test for non-normally distributed continuous variables. Inter-group differences in categorical variables were analyzed using the chi-square test.
To investigate the predictive effect of HSI on incident T2DM, Cox proportional hazard regression models were employed. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Covariates, including age,21 ethanol consumption,22 smoking status,23 systolic blood pressure (SBP), diastolic blood pressure (DBP),24 TG,25 and TC,26 were selected based on clinical experience and a literature review to mitigate potential confounding factors. A sex-stratified multivariate Cox regression model, adjusting for covariates, examined the consistency of HSI’s predictive effect on T2DM incidence among different sexes. Categorized HSI variables were used to assess trends, and the P value for each trend was calculated. The area under the receiver operating characteristic (ROC) curve (AUC) was used to validate the usefulness of HSI in predicting incident T2DM. The cumulative hazard of incident T2DM for each HSI quartile was determined using the Kaplan-Meier method, and hazard ratios were compared using the Log rank test. Subgroup analyses using Cox proportional hazard models assessed the consistency of effects across different groups (sex, age, BMI, SBP, and TG).
Data management and analysis were performed using R software (version 4.2.0, The R Foundation) (http://www.R-project.org). Statistical significance was set at a two-sided significance level of 0.05.
Result
Baseline Characteristics of Study Participants
The study included a total of 14,443 participants, with 54.96% being male and 45.04% female, who met the screening criteria for FPG and abnormal HbA1c as illustrated in Figure 1. The mean age and BMI of the participants were 43.4±8.8 years and 22.03±3.05 kg/m2, respectively. Over an average follow-up period of 6.1 years, 238 individuals developed T2DM, accounting for 1.65% of all participants.
Figure 1.
Participant flow diagram.
The baseline characteristics of the study subjects are presented in Table 1, where participants were grouped into quartiles based on their HSI values. The HSI quartiles were 26.22±1.47, 29.44±0.75, 32.29±0.95, and 38.09±3.56. Our research findings indicate that as the HSI quartiles increase, there is a statistically significant trend of change in all baseline metrics of the subjects (all P-values <0.001). Individuals in the higher HSI quartiles were more likely to be male, older, have a fatty liver, be current smokers, heavy drinkers, incident T2DM patients, and lack exercise habits. Compared to participants in the lower HSI quartiles (Q1-Q3), those in the top quartile (Q4) had higher body weight, waist circumference, BMI, SBP, DBP, GGT, ALT, AST, TC, TG, and lower HDL-C levels (all P-values <0.001).
Table 1.
Baseline Characteristics of the Participants in Different HSI Groups (n (%) or Mean ± SD or Median [IQR])
| Variables | HSI groups | p value | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| n | 3611 | 3610 | 3611 | 3611 | |
| Male | 1506 (41.71%) | 1644 (45.54%) | 2075 (57.46%) | 2713 (75.13%) | <0.001 |
| HSI | 26.22 ± 1.47 | 29.44 ± 0.75 | 32.29 ± 0.95 | 38.09 ± 3.56 | <0.001 |
| Age(yrs) | 42.34±9.19 | 43.42 ± 8.94 | 44.51 ± 8.70 | 43.21 ± 8.07 | <0.001 |
| Body Weight (kg) | 51.63 ± 7.42 | 56.82 ± 8.05 | 61.87 ± 8.54 | 71.72 ± 10.67 | <0.001 |
| BMI (Kg/m2) | 19.12 ± 1.53 | 20.97 ± 1.58 | 22.59 ± 1.74 | 25.45 ± 2.77 | <0.001 |
| WC (cm) | 68.99 ± 5.86 | 73.33 ± 6.42 | 77.65 ± 6.65 | 85.04 ± 7.85 | <0.001 |
| Ethanol consumption (g/wk) | 42.00 [77.36] | 44.52 [79.88] | 52.21 [84.08] | 56.31 [89.15] | <0.001 |
| SBP (mmHg) | 108.28 ± 13.27 | 111.64 ± 13.61 | 115.29 ± 14.22 | 121.81 ± 14.67 | <0.001 |
| DBP (mmHg) | 67.55 ± 9.31 | 69.67 ± 9.69 | 72.17 ± 10.11 | 76.34 ± 10.58 | <0.001 |
| GGT (IU/L) | 15.01 [13.15] | 16.28 [11.35] | 20.25 [17.83] | 28.88 [23.41] | <0.001 |
| ALT (IU/L) | 13.31 [5.39] | 15.81 [5.80] | 19.03 [7.66] | 30.64 [21.91] | <0.001 |
| AST (IU/L) | 18.11 [6.73] | 17.26 [5.42] | 17.35 [6.06] | 20.08 [13.20] | <0.001 |
| TC (mmol/L) | 4.88 ± 0.82 | 5.02 ± 0.82 | 5.16 ± 0.85 | 5.34 ± 0.86 | <0.001 |
| TG (mmol/L) | 0.64 ± 0.46 | 0.75 ± 0.48 | 0.93 ± 0.61 | 1.27 ± 0.79 | <0.001 |
| HDL-c (mmol/L) | 1.63 ± 0.39 | 1.55 ± 0.40 | 1.43 ± 0.38 | 1.25 ± 0.33 | <0.001 |
| Smoking status | <0.001 | ||||
| Never | 2404 (66.57%) | 2310 (63.99%) | 2049 (56.74%) | 1670 (46.25%) | |
| Past | 509 (14.10%) | 643 (17.81%) | 784 (21.71%) | 825 (22.85%) | |
| Current | 698 (19.33%) | 657 (18.20%) | 778 (21.55%) | 1116 (30.91%) | |
| Habit of exercise | <0.001 | ||||
| No | 2984 (82.64%) | 2916 (80.78%) | 2934 (81.25%) | 3071 (85.05%) | |
| Yes | 627 (17.36%) | 694 (19.22%) | 677 (18.75%) | 540 (14.95%) | |
| Fatty liver | 34 (0.94%) | 170 (4.71%) | 477 (13.21%) | 1691 (46.83%) | <0.001 |
| Incident DM | 21 (0.58%) | 26 (0.72%) | 38 (1.05%) | 153 (4.24%) | <0.001 |
Abbreviations: BMI, body mass index; WC, Waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; GGT, gamma glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, total triglycerides; HDL-c, high-density lipoprotein cholesterol.
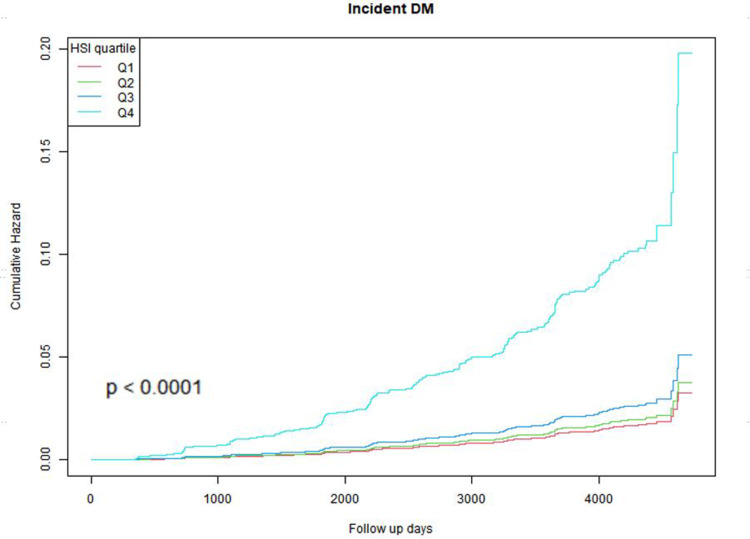
Kaplan-Meier Curve Analysis
The cumulative risk rates of incident T2DM were described using Kaplan-Meier curves according to HSI quartile groups (Figure 2). There was a notable disparity observed in the risk of developing incident T2DM across the four groups (Log rank test, p<0.001). As HSI increased, the cumulative risk rate of incident T2DM also increased. The Q4 group exhibited the highest susceptibility to developing incident T2DM.
Figure 2.
Kaplan–Meier estimation of incident T2DM by HSI quartiles.
Association of HSI with Incident T2DM in Different Sexes
Three Cox models were employed in the multivariate regression analysis to examine the association between HSI and the occurrence of T2DM (Table 2). In the unadjusted model, using the Q1 group as the reference, we observed HR values of 1.17 (95% CI 0.66 to 2.07), 1.58 (95% CI 0.93 to 2.69), and 6.10 (95% CI 3.87 to 9.64) for the Q2, Q3, and Q4 groups, respectively, indicating a significant positive association between HSI and incident T2DM incidence (P for trend <0.001). In Model 2, upon age adjustment, HSI still showed a positive correlation with incident T2DM, although the correlation slightly weakened. In the fully adjusted Model 3, the HR values were 1.09 (95% CI 0.61 to 1.93), 1.16 (95% CI 0.68 to 1.98), and 3.30 (95% CI 2.04 to 5.33), respectively. For each increase of one standard deviation in the three models, the probability of developing incident T2DM increases by 93%, 111%, and 82%, respectively.
Table 2.
Association Between HSI Groups and Incidence of Incident T2DM
| HSI groups | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR (95% CI) P value | |||
| Male | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.02 (0.55, 1.87) | 1.00 (0.54, 1.84) | 0.95 (0.51, 1.75) |
| Q3 | 1.74 (1.02, 2.99) | 1.80 (1.05, 3.08) | 1.43 (0.83, 2.46) |
| Q4 | 4.54 (2.82, 7.32) | 5.08 (3.14, 8.22) | 3.21 (1.93, 5.33) |
| P for trend | 1.17 (1.13, 1.21) P<0.001 | 1.18 (1.14, 1.23) P<0.001 | 1.13 (1.09, 1.18) P<0.001 |
| per SD increase | 1.93 (1.71, 2.17) | 2.11 (1.86, 2.39) | 1.82 (1.58, 2.10) |
| Female | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.51 (0.36, 6.33) | 1.44 (0.34, 6.04) | 1.29 (0.31, 5.44) |
| Q3 | 3.24 (0.90, 11.62) | 2.99 (0.83, 10.76) | 2.38 (0.66, 8.64) |
| Q4 | 8.89 (2.71, 29.20) | 7.70 (2.33, 25.49) | 4.64 (1.36, 15.81) |
| P for trend | 1.32 (1.19, 1.47) P<0.001 | 1.30 (1.17, 1.45) P<0.001 | 1.21 (1.09, 1.36) P<0.001 |
| per SD increase | 2.19 (1.81, 2.66) | 2.18 (1.79, 2.67) | 1.80 (1.43, 2.27) |
| Total | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.17 (0.66, 2.07) | 1.13 (0.64, 2.01) | 1.09 (0.61, 1.93) |
| Q3 | 1.58 (0.93, 2.69) | 1.46 (0.86, 2.49) | 1.16 (0.68, 1.98) |
| Q4 | 6.10 (3.87, 9.64) | 5.98 (3.79, 9.44) | 3.30 (2.04, 5.33) |
| P for trend | 1.23 (1.18, 1.27) P<0.001 | 1.23 (1.19, 1.28) P<0.001 | 1.15 (1.11, 1.20) P<0.001 |
| per SD increase | 2.06 (1.87, 2.26) | 2.19 (1.98, 2.42) | 1.81 (1.61, 2.03) |
Notes: Model 1 was not adjusted, Model 2 was adjusted for age, Model 3 was adjusted for age, Ethanol consumption, Smoking status, SBP, DBP, TG, TC.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, total triglycerides; TC, total cholesterol.
The research also investigated how the association between HSI and incident T2DM varies across sexes. In the initial analysis, HSI showed a positive correlation with incident T2DM in both males and females. When considering additional factors in Model 2, every standard deviation increase in HSI corresponded to an 82% rise in the risk of incident T2DM in males (HR: 1.82, 95% CI: 1.58, 2.10, P-trend < 0.001) and an 80% increase in females (HR: 1.82, 95% CI: 1.43, 2.27, P-trend < 0.001). Nonetheless, based on the interaction test, no significant difference was found in the relationship between HSI and incident T2DM across sexes (p for interaction = 0.0916).
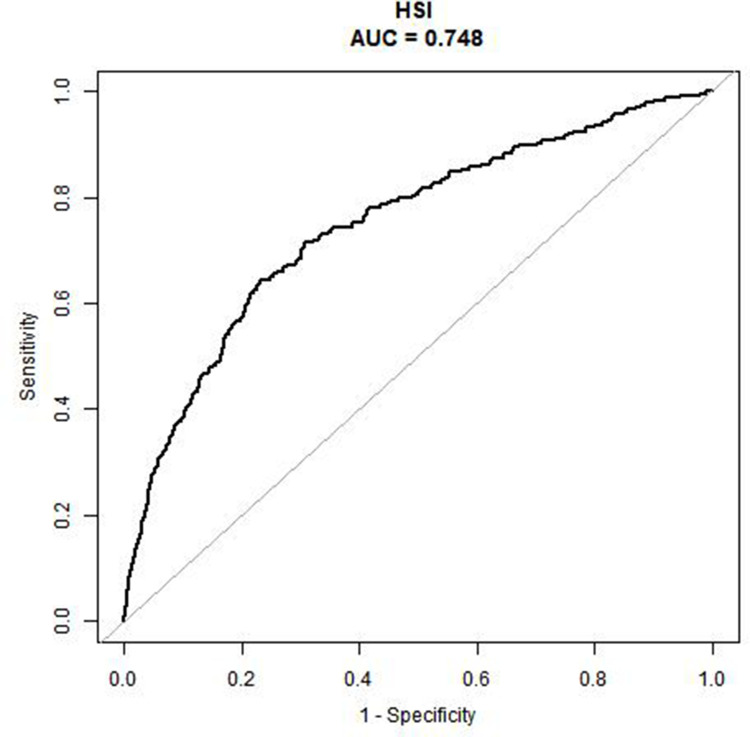
The accuracy of using HSI to predict incident T2DM was evaluated using the ROC curve, and HSI demonstrated a good area under the curve (AUC = 0.748) (Figure 3).
Figure 3.
Receiver operative characteristic curve of HSI for identifying incident T2DM.
Subgroup Analyses
After adjusting for factors such as age, ethanol consumption, smoking status, SBP, DBP, TG, and TC, we performed an interaction analysis to examine the potential influential factors on the prediction of incident T2DM by HSI (Table 3). Taking age, BMI, SBP, and TG as categorical variables, it was found that the HRs for patients with normal TG values and those with abnormal TG values were 1.15 (1.12, 1.19) and 1.09 (1.05, 1.14), respectively. The data revealed a significant interactive role of TG levels in the association between HSI and T2DM, as evidenced by a notable interaction effect (P for interaction = 0.0170). No statistically significant differences were observed in other factors (p > 0.05).
Table 3.
Subgroup Analysis of the Impact of HSI on T2DM Incidence
| Variables | HSI continue variable | ||
|---|---|---|---|
| HR, 95% CI | P value | P for interaction | |
| Sex | 0.0916 | ||
| Female | 1.18 (1.12, 1.24) | <0.0001 | |
| Male | 1.12 (1.09, 1.15) | <0.0001 | |
| Age | 0.3195 | ||
| <60 | 1.11 (1.09, 1.14) | <0.0001 | |
| ≥60 | 1.20 (1.04, 1.40) | 0.0156 | |
| BMI | 0.6508 | ||
| <24 | 1.12 (1.07, 1.18) | <0.0001 | |
| ≥24 | 1.14 (1.10, 1.18) | <0.0001 | |
| SBP | 0.1930 | ||
| <140 | 1.14 (1.11, 1.17) | <0.01 | |
| ≥140 | 1.10 (1.04, 1.16) | 0.0012 | |
| TG | 0.0170 | ||
| <1.7 | 1.15 (1.12, 1.19) | <0.0001 | |
| ≥1.7 | 1.09 (1.05, 1.14) | <0.0001 | |
Notes: The model adjusted for the following variables: age, ethanol consumption, smoking status, SBP, DBP, TG and TC excluding itself.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, total triglycerides; TC, total cholesterol.
Discussion
In this longitudinal cohort study, we observed a significant predictive role of HSI in the development of incident T2DM among individuals with normal blood glucose levels, irrespective of age, ethanol consumption, smoking status, SBP, DBP, TG, and TC. The probability of developing incident T2DM is directly proportional to the HSI value. This trend remains consistent across different sexes and is not influenced by age or SBP. In subgroup analyses, we observed a significant association between elevated HSI and an increased risk of developing incident T2DM in individuals with normal TG levels. This study presents novel evidence linking HSI to the development of incident T2DM in individuals with normal baseline blood glucose levels, thereby offering a straightforward yet valuable screening tool for predicting T2DM in this specific population.
In order to simplify the screening of NAFLD, HSI is a new screening tool proposed by scholars in recent years and has been widely used in clinical practice.27 In a cross-sectional study, Lee employed multifactorial analysis to identify independent risk factors associated with NAFLD and established a calculation formula for HSI using logistic regression. Lee defined HSI > 36 as indicative of NAFLD and HSI < 30 as non-indicative of NAFLD.10 It has been verified that HSI has high sensitivity and specificity. In a study involving 14,281 participants, researchers discovered a robust correlation between the HSI and NAFLD,28 while another study of 1866 individuals found that the area under the receiver operator characteristic (AUROC) curve for the HSI was 0.803, demonstrating its good diagnostic performance.29 In addition, compared with measurement tools such as liver biopsy, ultrasound, computed tomography (CT), and chemical shift-encoded magnetic resonance imaging (MRI), HSI has the advantages of simplicity and efficiency, making it suitable for large-scale screening.30,31
In recent years, scholars have also discovered the value of HSI in other chronic diseases. In a cross-sectional study involving 768 participants, Wang32 discovered that HSI was independently linked to carotid atherosclerosis and could serve as an indicator of cardiovascular complications in patients with T2DM. A comprehensive study conducted in Japan, involving a total of 94,893 individuals, investigated the association between HSI and chronic kidney disease (CKD), revealing a clear dose-response relationship that suggests HSI’s potential as an effective tool for early CKD detection.33 Consistent with prior research, our study also identified an independent association between HSI and incident T2DM, a prevalent chronic condition. Moreover, this study focused on individuals with normal glucose levels, who are often overlooked as a population at risk for T2DM.
The predictive role of HSI in the development of T2DM may involve multiple underlying mechanisms. The study revealed that, even after adjusting for age, sex, and hepatocellular lipid content, HSI showed independent correlations with insulin sensitivity and b-cell function.34 Both factors are crucial for maintaining normal glucose levels, and their dysregulation can contribute to diabetes.35,36 HSI can also reflect the level of insulin-like growth factor 1 (IGF-1) in the body, and the IGF-1 signaling pathway plays a vital role in preserving the b-cell differentiation phenotype. Failure in b-cell function can lead to T2DM development.37,38 Additionally, studies utilizing bidirectional Mendelian randomization analysis have identified a significant association between genetically determined NAFLD and an increased risk of T2DM. Given HSI’s high specificity and sensitivity in detecting NAFLD, it may serve as an indirect mechanism for predicting T2DM occurrence.39
In subgroup analyses, we found a positive correlation between elevated HSI and an increased risk of incident T2DM in individuals with normal TG levels. In order to explore this observation, we conducted additional analysis on the baseline data. Among participants with normal TG levels, those who developed diabetes exhibited higher age, weight, BMI, WC, SBP, DBP, AST, TC, and a higher proportion of smokers, as well as lower HDL-c levels and less physical exercise (all P values<0.001) (Supplement Table 1). These factors are recognized as known risk factors for T2DM. Additionally, individuals with higher TG levels demonstrated poorer baseline health status, which contributed to an elevated risk of developing T2DM and subsequently led to a decrease in the predictive accuracy of HSI for T2DM.
Limitations
Our study has some limitations. First, it is based on data from participants in the Japanese NAGALA project, so it is not yet clear whether the conclusions are generalizable, and further extensive research is needed for validation. Second, in our study, which involves a secondary analysis of previously published research data, the diagnostic criteria for baseline-diagnosed fatty liver, for instance, still lack clarity. Third, despite rigorous adjustment for all known risk factors, there is still some unavoidable, unmeasured, and unobtainable covariate information.
Conclusions
To sum up, our research uncovered a notable association between elevated HSI levels and the likelihood of developing incident T2DM in people with normal blood glucose levels. This finding is consistent for both sexes. Within this cohort, HSI can function as an innovative and economical means of foretelling the onset of T2DM.
Funding Statement
This work was supported by the Beijing Natural Science Foundation (Grant No. 7232042) for the project “The Role of Cytoskeletal Protein Tmod1 in Macrophage Lipid Uptake and Atherosclerosis and Its Mechanisms”. We appreciate the support from the funding agency that made this research possible.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006(1084):1–29. [DOI] [PubMed] [Google Scholar]
- 2.Gvazava IG, Karimova MV, Vasiliev AV, et al. Type 2 diabetes mellitus: pathogenic features and experimental models in rodents. Acta Naturae. 2022;14(3):57–68. doi: 10.32607/actanaturae.11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M, Hashim M J, King J K, et al. Epidemiology of Type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Maskari F, El-Sadig M, Nagelkerke N. Assessment of the direct medical costs of diabetes mellitus and its complications in the United Arab Emirates[J]. BMC Public Health. 2010;679. doi: 10.1186/1471-2458-10-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 6.Song B, Wang K, Lu W, et al. A U-shaped association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of incident type 2 diabetes mellitus in Japanese men with normal glycemic levels: a population-based longitudinal cohort study. Front Endocrinol;2023. 1180910. doi: 10.3389/fendo.2023.1180910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie X, Liao J, Huang C, et al. U-shaped association between triglyceride and risk of incident diabetes in normoglycemic males with NAFLD: a population-base cohort study. Int J Med Sci. 2023;20(11):1417–1424. doi: 10.7150/ijms.83371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Targher G, Corey K E, Byrne C D, et al. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi: 10.1038/s41575-021-00448-y [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine J E, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 10.Lee J H, Kim D, Kim H J, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease[J]. Dig Liver Dis. 2010;42(7):503–508. doi: 10.1016/j.dld.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 11.Chung J, Park H S, Kim Y J, et al. Association of hepatic steatosis index with nonalcoholic fatty liver disease diagnosed by non-enhanced ct in a screening population. Diagnostics. 2021(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: New insights and potential new treatments. Nutrients. 2017(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.F CA, S D, Reggi A, et al. Hepatic steatosis index and lipid accumulation product as middle-term predictors of incident metabolic syndrome in a large population sample: data from the Brisighella Heart Study. Intern Emerg Med. 2013;8(3):265–267. doi: 10.1007/s11739-012-0875-9 [DOI] [PubMed] [Google Scholar]
- 14.Cai X, Gao J, Liu S, et al. Hepatic steatosis index and the risk of type 2 diabetes mellitus in china: insights from a general population-based cohort study. Dis Markers. 2022;3150380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamura T, Hashimoto Y, Hamaguchi M, et al. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes Lond. 2019;43(1):139–148. doi: 10.1038/s41366-018-0076-3 [DOI] [PubMed] [Google Scholar]
- 16.Chitturi S, Farrell G C, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22(6):778–787. doi: 10.1111/j.1440-1746.2007.05001.x [DOI] [PubMed] [Google Scholar]
- 17.Okamura T. Data From: Ectopic Fat Obesity Presents the Greatest Risk for Incident Type 2 Diabetes: A Population-Based Longitudinal Study. Dryad; 2019. Dataset][J. [DOI] [PubMed] [Google Scholar]
- 18.Bull F C, Al-Ansari S S, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sed entary behaviour. Br J Sports Med. 54(24):1451–1462. doi: 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x [DOI] [PubMed] [Google Scholar]
- 20.Standards of medical care in diabetes--2011. Diabetes Care. 2011;1(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverman-Retana O, Hulman A, Nielsen J, et al. Effect of familial diabetes status and age at diagnosis on type 2 diabetes risk: a nation-wide register-based study from Denmark. Diabetologia. 2020;63(5):934–943. doi: 10.1007/s00125-020-05113-8 [DOI] [PubMed] [Google Scholar]
- 22.van de Wiel A. Diabetes mellitus and alcohol. Diabetes Metab Res Rev. 2004;20(4):263–267. [DOI] [PubMed] [Google Scholar]
- 23.Ye Z, Li J, Gu P, et al. Early-life tobacco smoke exposure, genetic susceptibility and the risk of type 2 diabetes in adulthood: a large prospective cohort study. Sci Total Environ. 2023;893:164698. doi: 10.1016/j.scitotenv.2023.164698 [DOI] [PubMed] [Google Scholar]
- 24.Ohishi M. Hypertension with diabetes mellitus: physiology and pathology[J]. Hypertens Res. 2018;41(6):389–393. doi: 10.1038/s41440-018-0034-4 [DOI] [PubMed] [Google Scholar]
- 25.Ahmad S, Mora S, Ridker P M, et al. Gene-based elevated triglycerides and type 2 diabetes mellitus risk in the women’s genome health study. Arterioscler Thromb Vasc Biol. 2019;39(1):97–106. doi: 10.1161/ATVBAHA.118.311562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontopantelis E, Springate D A, Reeves D, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study[J]. Diabetologia. 2015;58(3):505–518. doi: 10.1007/s00125-014-3473-8 [DOI] [PubMed] [Google Scholar]
- 27.Sviklāne L, Olmane E, Dzērve Z, et al. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33(1):270–276. doi: 10.1111/jgh.13814 [DOI] [PubMed] [Google Scholar]
- 28.Sheng G, Lu S, Xie Q, et al. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20(1):134. doi: 10.1186/s12944-021-01561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Mao X, Deng M, et al. Validation of nonalcoholic fatty liver disease (NAFLD) related steatosis indices in metabolic associated fatty liver disease (MAFLD) and comparison of the diagnostic accuracy between NAFLD and MAFLD. Eur J Gastroenterol Hepatol. 2023;35(4):394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papatheodoridi M, Cholongitas E. Diagnosis of non-alcoholic fatty liver disease (NAFLD): Current concepts. Curr Pharm Des. 2018;24(38):4574–4586. doi: 10.2174/1381612825666190117102111 [DOI] [PubMed] [Google Scholar]
- 31.Starekova J, Hernando D, Pickhardt P J, et al. Quantification of liver fat content with ct and mri: state of the art. Radiology. 2021;301(2):250–262. doi: 10.1148/radiol.2021204288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Cai Z, Deng X, et al. Association of hepatic steatosis index and fatty liver index with carotid atherosclerosis in type 2 diabetes. Int J Med Sci. 2021;18(14):3280–3289. doi: 10.7150/ijms.62010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochiai H, Shirasawa T, Yoshimoto T, et al. Hepatic steatosis index and chronic kidney disease among middle-aged individuals: A large-scale study in Japan. Dis Markers. 2021;2021:9941834. doi: 10.1155/2021/9941834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahl S, Straßburger K, Nowotny B, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. 2014;9(4):e94059. doi: 10.1371/journal.pone.0094059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: implications for the pathogenesis and treatment of diabetes mellitus[J]. Biochem Pharmacol. 2018;149:60–76. doi: 10.1016/j.bcp.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 36.Kowluru A. Regulatory roles of CARD9-BCL10-Rac1 (CBR) signalome in islet β-cell function in health and metabolic stress: Is there room for MALT1?. Biochem Pharmacol. 2023;218:115889. doi: 10.1016/j.bcp.2023.115889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciresi A, Guarnotta V, Campo D, et al. Hepatic steatosis index in acromegaly: Correlation with insulin resistance regardless of the disease control. Int J Endocrinol;2018. 5421961. doi: 10.1155/2018/5421961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rachdaoui N. Insulin: The friend and the foe in the development of type 2 diabetes mellitus. Int J Mol Sci. 2020;2020(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Yu Y, Wang Y, et al. Nonalcoholic fatty liver disease and type 2 diabetes: an observational and Mendelian randomization study. Front Endocrinol. 2023;14:1156381. doi: 10.3389/fendo.2023.1156381 [DOI] [PMC free article] [PubMed] [Google Scholar]