Abstract
The T-BOX transcription factor TBX1 is essential for the development of the pharyngeal apparatus and it is haploinsufficient in DiGeorge syndrome (DGS), a developmental anomaly associated with congenital heart disease and other abnormalities. The murine model recapitulates the heart phenotype and showed collagen accumulation.
We first used a cellular model to study gene expression during cardiogenic differentiation of WT and Tbx1−/− mouse embryonic stem cells. Then we used a mouse model of DGS to test whether interfering with collagen accumulation using an inhibitor of lysyl hydroxylase would modify the cardiac phenotype of the mutant.
We found that loss of Tbx1 in a precardiac differentiation model was associated with up regulation of a subset of ECM-related genes, including several collagen genes. In the in vivo model, early prenatal treatment with Minoxidil, a lysyl hydroxylase inhibitor, ameliorated the cardiac outflow tract septation phenotype in Tbx1 mutant fetuses, but it had no effect on septation in WT fetuses. We conclude that TBX1 suppresses a defined subset of ECM-related genes. This function is critical for OFT septation because the inhibition of collagen cross-linking in the mutant reduces significantly the penetrance of septation defects.
Keywords: DiGeorge syndrome model, Tbx1, Cardiac outflow tract, Phenotypic rescue
Highlights
-
•
DiGeorge syndrome is a multi-system developmental anomaly associated with cardiac outflow tract defects
-
•
Tbx1 is the gene primarily responsible for the cardiovascular phenotype.
-
•
Here we used a differentiation cellular model to identify a set of extra cellular matrix-related genes up regulated, including a subset of collagen-encoding genes.
-
•
We show that in vivo, prenatal treatment with a lysyl hydroxylase inhibitor reduces significantly the penetrance of cardiac defects.
-
•
Results strongly suggest that collagen accumulation may be part of the pathogenetic process leading to heart defects in this model.
1. Introduction
Rescue experiments of disease gene phenotypes are important not only because they offer a potential avenue for treatment but also because they provide critical information about the importance of certain processes during normal development and the pathogenesis of the disease. However, phenotypic rescue may occur for different reasons including, but not limited to compensation for perturbed molecular pathways. Tbx1 is a T-box transcription factor that is required for the development of the pharyngeal apparatus [1] and in humans its haploinsufficiency causes a phenotypic spectrum known as DiGeorge syndrome, which is part of a broader clinical entity defined by a segmental aneuploidy known as 22q11.2 deletion syndrome [2,3]. In the mouse, the Tbx1 mutant phenotype, and particularly the cardiac phenotype, can be partially rescued using different drug-based and genetic approaches [[4], [5], [6], [7]]. The diversity of the approaches that have been at least partially successful suggests that there are a number of processes involved, correction of each of which individually is insufficient to resolve the developmental anomaly. In this study, we followed an alternative avenue and leveraged the findings of excess collagen that has been found in Tbx1−/− embryos [8].
We first used transcriptional analysis of a cell differentiation model and observed the upregulation of multiple collagen-encoding genes. Next, we used a drug-based approach in vivo to counterbalance the excessive collagen deposition observed in Tbx1 mutant embryos [8]. A significant improvement in cardiac septation was observed, highlighting the importance of the ECM in this key developmental process and thereby justifying a more systematic dissection of the molecular pathways involved. Minoxidil, an inhibitor of lysyl hydroxylase, reduces collagen cross-linking [9] and it has been used successfully to ameliorate the thymic phenotype in an in vitro model of thymic hypoplasia caused by Tbx1 mutation [10]. Therefore, we tested in vivo treatment of Tbx1 mutants with Minoxidil and found a significant improvement in cardiac septation.
2. Materials and methods
2.1. Cell culture and differentiation
The embryonic stem cell line 4D carries a homozygous targeted inactivating mutation of the Tbx1 gene and it was obtained using CRISPR-Cas9 targeting [10]. This cell line was cultured along with the parental mouse ES cell line ES-E14TG2a (ATCC CRL-1821) without feeders, maintaining their undifferentiated state on gelatin-coated dishes. The culture medium consisted of GMEM (Sigma Cat# G5154) supplemented with 103 U/ml ESGRO LIF (Millipore, Cat# ESG1107), 15 % fetal bovine serum (ES Screened Fetal Bovine Serum, US Euroclone Cat# CHA30070L), 0.1 mM non-essential amino acids (Gibco, Cat# 11140-035), 0.1 mM 2-mercaptoethanol (Gibco, Cat# 31350-010), 0.1 mM l-glutamine (Gibco, Cat# 25030081), 0.1 mM Penicillin/Streptomycin (Gibco, Cat# 10378016), and 0.1 mM sodium pyruvate (Gibco, Cat# 11360-070). Cell passaging was performed every 2–3 days using 0.25 % Trypsin-EDTA (1X) (Gibco, Cat# 25200056) as the dissociation buffer.
For differentiation, E14-Tg2a cells (parental line and Tbx1−/− line 4D) were dissociated with Trypsin-EDTA and cultured at a density of 100,000 cells/ml in serum-free differentiation media comprising 75 % Iscove's modified Dulbecco's media (Cellgro Cat# 15-016-CV) and 25 % HAM F12 media (Cellgro #10-080-CV). The media were supplemented with N2 (GIBCO #17502048) and B27 (GIBCO #12587010) supplements, penicillin/streptomycin (GIBCO #10378016), 0.05 % BSA (Invitrogen Cat#. P2489), l-glutamine (GIBCO #25030081), 5 mg/ml ascorbic acid (Sigma A4544), and 4.5 × 10-4 M monothioglycerol (Sigma M − 6145). After 48 h, the embryoid bodies were collected and plated in serum-free differentiation media, supplemented with 1 ng/ml human Activin A (R&D Systems Cat#. 338-AC) and 1 ng/ml human BMP4 (R&D Systems Cat# 314-BP). The media were changed to serum-free differentiation media without additional growth factors after 2 days, and the embryoid bodies were maintained for 96 h, completing the differentiation protocol.
2.2. Gene expression analysis
For RNA-seq/QuantiSeq experiments, RNA was extracted from 4D and parental cell line at d6 of differentiation from 4 independent differentiation experiments (4 biological replicates) and processed for QuantiSeq 3′ mRNA sequencing. Libraries were prepared and sequenced at the Telethon Institute of Genetics and Medicine of Pozzuoli, Italy on a fee-for-service basis. We performed quality control and trimming, including polyA tails and adapter removal, with the bbduck.sh script from the BBTools suite (https://sourceforge.net/projects/bbmap/) with k = 13 ktrim = r useshortkmers = t mink = 5 qtrim = r trimq = 10 minlength = 20. Then, we aligned the sequences to the mouse genome (mm10) using STAR [11], and obtained the gene expression as a raw count matrix using the featureCounts function from the Rsubread package. We filtered out genes with null or very low expression using the filtrered.data function from the NOISeq package [12] using the proportion test as the filtering method and retaining only the genes with counts per million (CPM) above 0.5, as expressed genes. We removed the batch using the ARSyNseq function in the same package with upper quartile normalization and visualized the quality of the samples in the principal component projection. To account for biological variability, we used the noiseqbio function, setting the cutoff for the posterior probability to 0.95, to identify the differentially expressed genes. Gene ontology of the differentially expressed genes was performed with the gprofiler online tool (https://biit.cs.ut.ee/gprofiler/gost) using the entire set of expressed genes as the background.
To collect putative regulatory elements of ECM genes, we downloaded all the cis candidate regulatory elements (cCREs) in mm10 from https://screen.encodeproject.org/of the ENCODE project [13] and considered only distal enhancer-like sequences (dELS) and proximal enhancer-like sequences (pELS). Next, we used the getbioregion function from ChIPseeker (v1.38.0) [14] to obtain the gene body coordinates using the TxDb.Mmusculus.UCSC.mm10.knownGene (v.3.10.0) database and we extended the gene body 50 Kb upstream of the transcription start site (TSS) and 50 Kb downstream of the transcription end site (TES). We intersected the gene coordinates with the selected cCREs regions using the FindOverlaps function from GenomicRanges (v.1.54.1) [15] to retain only regions that overlap with the genes. We filtered the cCREs regions with annotated genes intersecting a list of 21 upregulated genes and we obtained a list of 318 regions (Supplementary Table 3). Finally, we utilized these regions to identify enriched motifs using HOMER (Hypergeometric Optimization of Motif EnRichment) [16], setting as background the annotated cCREs regions that overlap with all the expressed genes (n = 16391, Supplementary Table 3) using the same procedure described above. We repeated the HOMER search using the default background (mouse genome).
2.3. In vivo treatment with Minoxidil
Mouse lines Tbx1lacZ/+ [13] (also indicated here as Tbx1+/−) and Tbx1neo2/+ [17] have been maintained and used in a C57Bl/6 N genetic background and genotyped according to the original description. Pregnant females were injected intraperitoneally with the vehicle (PBS with 5 % EtOH) or Minoxidil (Sigma-Aldrich M4145). Each injection included 110 μg of Minoxidil or vehicle in 200 μl, at E7.5, E8.5, and E9.5. Fetuses were harvested at E16.5 or E17.5, hearts were dissected, examined and embedded in paraffin for histology after fixation in 4 % paraformaldehyde (PFA). Sections (10 μm) were stained with Hematoxylin and Eosin (Mayer's Hematoxylin Solution MHS16, Eosin Y Solution, Alcoholic HT110116), using standard methods. Images were acquired using a Nikon Eclipse Ni with a 4x objective equipped with Nikon DS-RI1 Camera and software Nis Elements AR 4.20.00.
The rescue of septation defects of the OFT between treated and control Tbx1neo2/lacZ fetuses was tested using the Chi-square test.
3. Results and discussion
3.1. Loss of Tbx1 is associated with up regulation of a subset of ECM-related genes
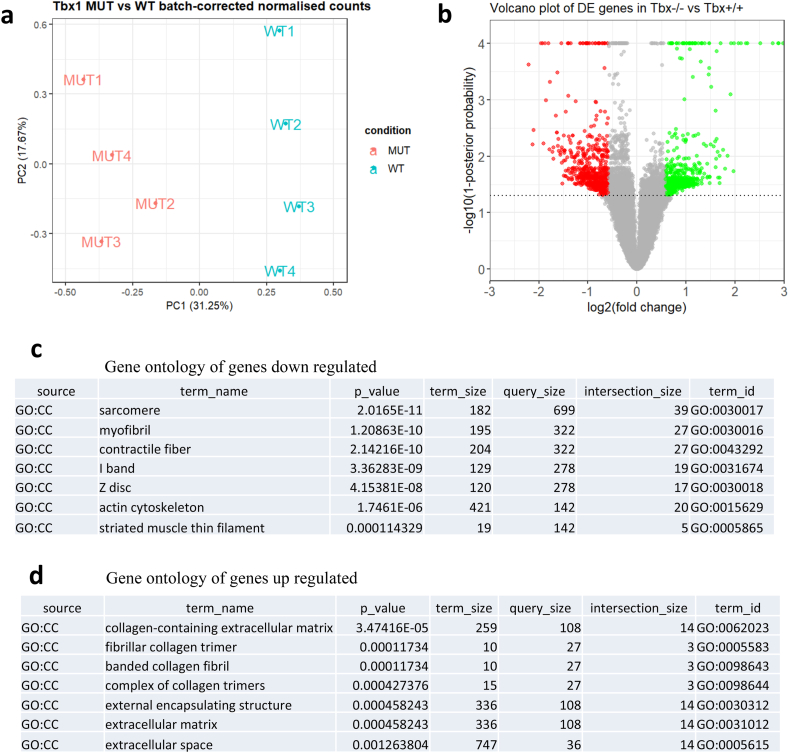
We performed in vitro differentiation of mES cells that have been gene-edited to lack any functional Tbx1 and the parental cell line [18]. We used the protocol published by Andersen et al. [19] with minor modifications because it induces robust expression of the Tbx1 gene at day 6 (d6) of differentiation. Principal component analyses of the transcriptomes of the 4 biological replicates/genotype showed clear separation of WT and mutant samples (Fig. 1a). We generated a list of differentially expressed genes (DEGs) between Tbx1−/− and Tbx1+/+ (parental cell line), calculated using NOISeq [12] and selecting a posterior probability of at least 0.95 and a fold change of at least 1.5 (Fig. 1b). We found 2033 DEGs, of which 1104 were down regulated in mutant cells and 929 were up regulated (genes are listed in the Suppl. Table 1). Gene Ontology analyses using the Cellular Component subset (GO:CC, highlighted in yellow in Suppl. Table 2) indicated that within the down regulated gene set, there was enrichment of terms related to muscle and heart development (Fig. 1c); in contrast, using the up regulated gene set we observed enrichment of terms related ECM (Fig. 1d, top 5; full results shown in Suppl. Table 2), genes related to these terms included several encoding collagens (Suppl. Table 2). Therefore, the results obtained in the cellular model were consistent with the finding of increased collagen expression in Tbx1 mutant embryos [8] and they extended the published observations to additional ECM-related genes, suggesting a broader perturbation of the ECM compartment than previously suspected. We selected a group of 21 up regulated ECM-related genes (suggested by the gene ontology search and listed in Suppl. Table 3) and mapped the ENCODE cis candidate regulatory elements (cCRES) [13] to these genes. In total, we selected 318 regions that are listed in Suppl. Table 3. We next carried out a search for transcription factor (TF) binding motifs using HOMER [16] and the mouse genome as the background. We found a number of motifs (the top 20 are shown in Suppl. Fig. 1), particularly motifs of the AP-1 complex of transcription factors, NF1, and TCF/bHLH factors. However, we did not find T-BOX binding motifs, suggesting that this subset of ECM-related genes may not be direct target of TBX1.
Fig. 1.
Gene expression analysis of cells differentiated using a cardiogenic protocol.
a) Principal component analysis of QuantSeq data from 4 WT to 4 Tbx1−/− biological replicates (independent differentiation experiments). Samples are clearly separated by genotype.
b) Volcano plot of gene expression values showing populations of differentially expressed genes. The individual values and gene names are listed in Suppl. Table 1
c-d) Top Gene Ontology/Cellular Component (GO:CC) terms identified for down regulated genes (c) and up regulated genes (d). Note the enrichment in ECM terms in the latter group. Full results with gene names are reported in Suppl. Table 2.
3.2. Minoxidil treatment reduces the penetrance of outflow septation defects in Tbx1 mutants
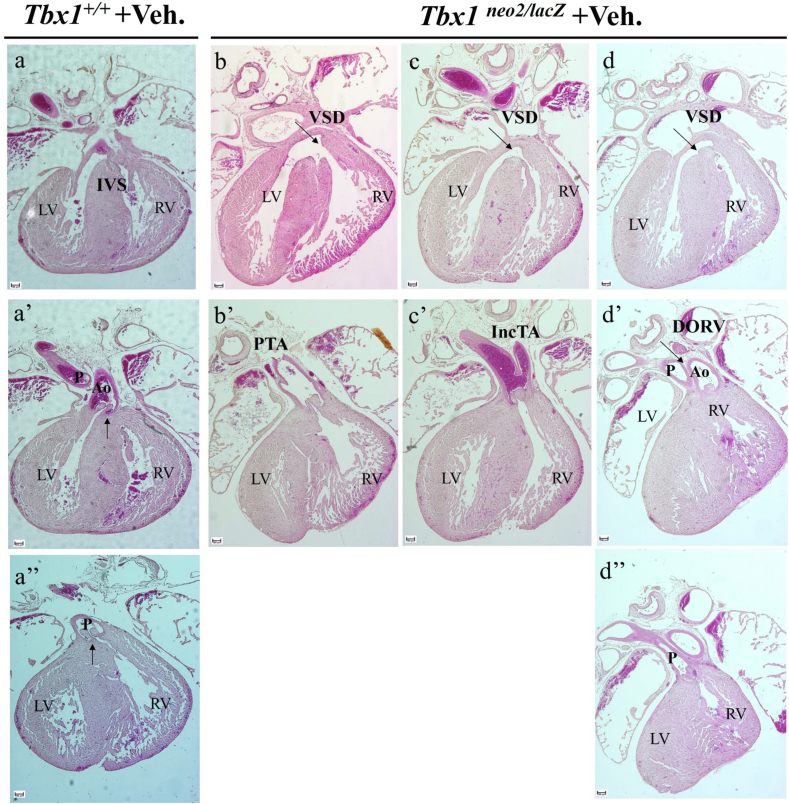
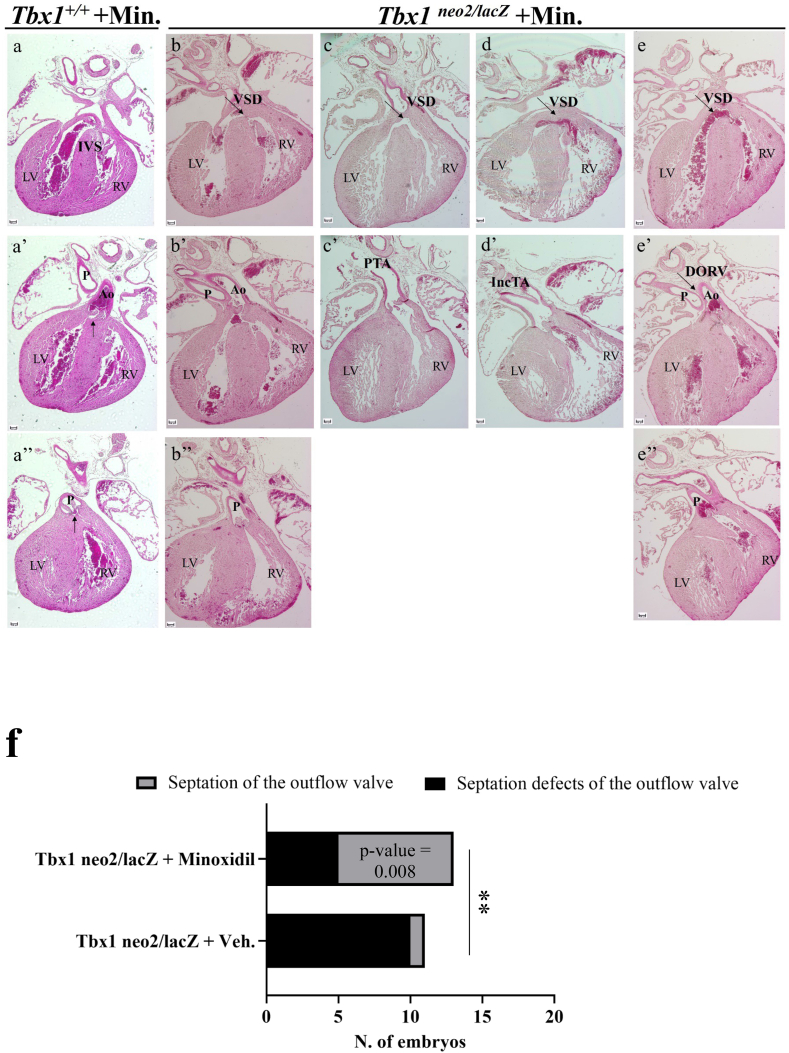
An excess of ECM gene expression might impact cell dynamics/regionalization and the diffusion of signalling molecules, the developmental consequences of which are difficult to predict; cell regionalization abnormalities have been reported in Tbx1 mutants [20]. We asked whether counter-balancing the excess of ECM, particularly collagens, would modify the cardiac phenotype of Tbx1 mutants. Addressing this question through genetic experiments would be very complex, due to the relatively large number of genes involved. Therefore, we opted for a pharmacological approach using a well-tolerated drug, Minoxidil, that inhibits lysyl hydroxylase. This destabilizes collagen by affecting cross-linking and reduces the accumulation of insoluble collagen [21]; this partially compensates for an excess of collagen. To this end, we crossed female mice carrying the hypomorphic Tbx1 allele Tbx1neo2/+ [17] with Tbx1lacZ/+ male mice (the lacZ allele is a null allele [13]). We treated pregnant females with i.p. injections at E7.5, E8.5, and E9.5 with Minoxidil at 110μg/injection, or with vehicle (PBS+5 % EtOH). Fetuses were harvested at E16.5 or E17.5, the hearts observed blind to genotype, and then processed for histology. Injection times were selected so as to target early SHF development when Tbx1 is critical for OFT morphogenesis [22]. We used the hypomorphic allele because Tbx1neo2/lacZ fetuses have severe heart abnormalities but a less severe defective development of the pharyngeal apparatus [17]. We harvested a total of 11 Tbx1neo2/lacZ fetuses injected with vehicle (control) and found that 10 out of 11 had conal septation defects, resulting in a single, unseptated outflow valve (Table 1, Fig. 2a–d). Truncal septation was partial in 4 of these embryos as they showed incomplete separation of the aorta and pulmonary trunk (Table 1, Fig. 2c-c'). In only one case did we found double outlet right ventricle (DORV), which was associated with the complete separation of the aorta and pulmonary trunk, including the septation of the outflow valves (Fig. 2d-d''). For purposes of comparison, the sections of a WT heart are shown in Fig. 2a-a''). We harvested 13 Tbx1neo2/lacZ fetuses injected with Minoxidil, of which 5 had septation defects of the outflow valve (Table 1, examples in Fig. 3a–e), while 8 had normally septated outflow valves. Five of the latter had DORV, while 3 had normal OFT morphology but a mild VSD (example in Fig. 3b). Septation defects of the OFT in Tbx1neo2/lacz fetuses were significantly less prevalent after Minoxidil treatment (P = 0.008) (Fig. 3f). We also examined 4 WT fetuses that were treated with Minoxidil and found no morphological anomalies (examples in Supplementary Figs. 2a–c). Thus, Minoxidil treatment as described here does not interfere with OFT development in the WT.
Table 1.
Summary of cardiac outflow septation status in control and Minoxidil-treated fetuses.
| Genotype/treatment | N. fetuses | Unseptated OFT (one arterial valve) | Septated OFT (two arterial valves)] | ||
|---|---|---|---|---|---|
| Tbx1neo2/lacZ + Vehicle (control) | 11 | 10 (91 %) | 6 PTA 4 Inc TA |
1 | DORV |
| Tbx1neo2/lacZ + Minoxidil | 13 | 5 (38 %) | 2 PTA 3 Inc TA |
8 | 5 DORV 3 mild VSD |
OFT: cardiac outflow tract. PTA: patent truncus arteriosus. Inc TA: incomplete truncus arteriosus, partially divided aorta and pulmonary trunk. DORV: double outlet right ventricle. VSD: Ventricular septal defect.
Fig. 2.
Cardiac outflow tract phenotype in vehicle-treated Tbx1neo2/lacZ fetuses (E16.5-E17.5). a-a'') H&E stained histological sections of a WT heart as reference. b-d) three examples of vehicle-treated Tbx1neo2/lacZ fetuses. Sections indicated with the same letter are from the same heart. IVS: Interventricular septum; LV: Left ventricle; RV: Right ventricle; VSD: Ventricular septal defect; PTA: Patent truncus arteriosus; IncTA: Incomplete septation of the arterial trunk; Ao: Aorta; P: Pulmonary trunk; DORV: Double outlet right ventricle. Scale bare: 100 μm.
Fig. 3.
Cardiac outflow tract phenotype in Minoxidil-treated Tbx1neo2/lacZ fetuses (E16.5-E17.5). a-a'') H&E stained histological sections of a WT heart as reference. b-e) Examples of hearts from Minoxidil-treated Tbx1neo2/lacZ fetuses. f) the incidence of septation defects (one OFT valve vs. two OFT valves) in Minoxidil-treated individuals is significantly lower than in vehicle-treated individuals (Chi-square test). IVS: Interventricular septum; LV: Left ventricle; RV: Right ventricle; VSD: Ventricular septal defect; PTA: Patent truncus arteriosus; IncTA: Incomplete septation of the arterial trunk; Ao: Aorta; P: Pulmonary trunk; DORV: Double outlet right ventricle. Scale bare: 100 μm.
These results indicate that Minoxidil treatment during SHF development is a significant modifier of the cardiac OFT septation phenotype in Tbx1 mutants. Because the treatment was carried out well before the actual process of septation takes place, and because the drug, under the conditions tested, did not interfere with OFT formation, we propose that the drug interacts with or partially compensates for the alterations caused by Tbx1 mutation. In addition to the cited role of the drug as a lysyl hydroxylase inhibitor, Minoxidil is an anti-hypertensive drug. This effect is thought to be produced through relaxation of vascular smooth muscle by acting on ATP-sensitive potassium (KATP) channels of the sarcolemma [14]. This particular mechanism is unlikely to be relevant in the context of our study because at the time of treatment, there is little or no smooth muscle differentiation in the cardiovascular system. Minoxidil is also used topically to treat androgenetic alopecia, but the mechanism by which it acts in this context is unclear [23]. The finding that Minoxidil ameliorates the thymic phenotype in an in vitro model of thymic hypoplasia caused by Tbx1 mutation [10] supports the hypothesis that it interferes with a pathological process triggered by reduced Tbx1 gene dosage.
3.3. Conclusions
We found that in mice, Tbx1 suppresses a group of ECM-related genes including multiple collagen genes; in vivo treatment with a drug that reduces collagen cross-linking leads to significant improvement of cardiac outflow tract development in Tbx1 hypomorphic mutants, suggesting that an excess of collagen may be part of the pathogenetic mechanism or modify significantly the phenotypic outcome of reduced gene dosage. Further work will be necessary to establish the molecular links through which Tbx1 suppresses ECM-related genes.
4. Ethics declaration
All animal procedures were reviewed and approved by the Italian Ministry of Health, protocol 28/2022-2 PR (to AB).
Data availability statement
All relevant data can be found within the article and its supplementary information. RNA-seq data are available through the GEO database, accession number GSE261206. Mouse lines are available through the European public repository EMMA/Infrafrontier.
CRediT authorship contribution statement
Ilaria Aurigemma: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. Rosa Ferrentino: Methodology, Visualization. Varsha Poondi Krishnan: Data curation, Formal analysis. Olga Lanzetta: Data curation, Formal analysis, Visualization. Claudia Angelini: Conceptualization, Formal analysis, Supervision, Writing – review & editing. Elizabeth Illingworth: Resources, Supervision, Writing – review & editing. Antonio Baldini: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Nicolai Van Oers and Pratibha Bhalla for suggestions about Minoxidil treatment; Marchesa Bilio and Lucia Mele for expert technical help. RNA-seq experiments were carried out at the Telethon Institute of Genetics and Medicine, Pozzuoli, Italy on a fee-for-service basis. We also acknowledge the support of the Microscopy core of the Institute of Genetics and Biophysics.
This work was funded in part by grants from Fondazione Telethon GMR22T1012 and the Italian Ministry of University and Research PRIN 2022XFE7M2 (to AB) and P2022ARZ5J (to EI).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2024.150104.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
HOMER output of 318 candidate regulatory elements mapped to the 21 up regulated genes related to extra cellular matrix, using the mouse genome as background.
Minoxidil treatment does not affect normal development of the OFT in WT individuals.
a-c) Hematoxylin & eosin-stained sections of hearts from fetuses WT fetuses treated with Minoxidil. There are no morphological defects.
Sections indicated with the same letter are from the same heart. IVS: Interventricular septum; LV: Left ventricle; RV: Right ventricle; Ao: Aorta; P: Pulmonary trunk; Scale bare: 100 μm.
References
- 1.Baldini A., Fulcoli F.G., Illingworth E. Tbx1: transcriptional and developmental functions. Curr. Top. Dev. Biol. 2017;122:223–243. doi: 10.1016/bs.ctdb.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Paylor R., Glaser B., Mupo A., Ataliotis P., Spencer C., Sobotka A., Sparks C., Choi C.H., Oghalai J., Curran S., Murphy K.C., Monks S., Williams N., O'Donovan M.C., Owen M.J., Scambler P.J., Lindsay E. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagi H., Furutani Y., Hamada H., Sasaki T., Asakawa S., Minoshima S., Ichida F., Joo K., Kimura M., Imamura S., Kamatani N., Momma K., Takao A., Nakazawa M., Shimizu N., Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 4.Caprio C., Baldini A. p53 suppression partially rescues the mutant phenotype in mouse models of DiGeorge syndrome. Proc. Natl. Acad. Sci. U.S.A. 2014;111:13385–13390. doi: 10.1073/pnas.1401923111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulcoli F.G., Franzese M., Liu X., Zhang Z., Angelini C., Baldini A. Rebalancing gene haploinsufficiency in vivo by targeting chromatin. Nat. Commun. 2016;7 doi: 10.1038/ncomms11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lania G., Bresciani A., Bisbocci M., Francone A., Colonna V., Altamura S., Baldini A. Vitamin B12 ameliorates the phenotype of a mouse model of DiGeorge syndrome. Hum. Mol. Genet. 2016;25:4369–4375. doi: 10.1093/hmg/ddw267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Racedo S.E., Hasten E., Lin M., Devakanmalai G.S., Guo T., Ozbudak E.M., Cai C.-L., Zheng D., Morrow B.E. Reduced dosage of β-catenin provides significant rescue of cardiac outflow tract anomalies in a Tbx1 conditional null mouse model of 22q11.2 deletion syndrome. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfano D., Altomonte A., Cortes C., Bilio M., Kelly R.G., Baldini A. Tbx1 regulates extracellular matrix-cell interactions in the second heart field. Hum. Mol. Genet. 2019;28:2295–2308. doi: 10.1093/hmg/ddz058. [DOI] [PubMed] [Google Scholar]
- 9.Murad S., Pinnell S.R. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J. Biol. Chem. 1987;262:11973–11978. [PubMed] [Google Scholar]
- 10.Bhalla P., Du Q., Kumar A., Xing C., Moses A., Dozmorov I., Wysocki C.A., Cleaver O.B., Pirolli T.J., Markert M.L., de la Morena M.T., Baldini A., van Oers N.S. Mesenchymal cell replacement corrects thymic hypoplasia in murine models of 22q11.2 deletion syndrome. J. Clin. Invest. 2022;132 doi: 10.1172/JCI160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarazona S., Furió-Tarí P., Turrà D., Pietro A.D., Nueda M.J., Ferrer A., Conesa A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015;43:e140. doi: 10.1093/nar/gkv711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsay E.A., Vitelli F., Su H., Morishima M., Huynh T., Pramparo T., Jurecic V., Ogunrinu G., Sutherland H.F., Scambler P.J., Bradley A., Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- 14.Wang T. The effects of the potassium channel opener minoxidil on renal electrolytes transport in the loop of henle. J Pharmacol Exp Ther. 2003;304:833–840. doi: 10.1124/jpet.102.043380. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence M., Huber W., Pagès H., Aboyoun P., Carlson M., Gentleman R., Morgan M.T., Carey V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013;9 doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinz S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., Glass C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum. Mol. Genet. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- 18.Cirino A., Aurigemma I., Franzese M., Lania G., Righelli D., Ferrentino R., Illingworth E., Angelini C., Baldini A. Chromatin and transcriptional response to loss of TBX1 in early differentiation of mouse cells. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.571501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen P., Tampakakis E., Jimenez D.V., Kannan S., Miyamoto M., Shin H.K., Saberi A., Murphy S., Sulistio E., Chelko S.P., Kwon C. Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat. Commun. 2018;9:3140. doi: 10.1038/s41467-018-05604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lania G., Franzese M., Adachi N., Bilio M., Flore G., Russo A., D'Agostino E., Angelini C., Kelly R.G., Baldini A. A phenotypic rescue approach identifies lineage regionalization defects in a mouse model of DiGeorge syndrome. Dis Model Mech. 2022;15 doi: 10.1242/dmm.049415. dmm049415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosell-García T., Palomo-Álvarez O., Rodríguez-Pascual F. A hierarchical network of hypoxia-inducible factor and SMAD proteins governs procollagen lysyl hydroxylase 2 induction by hypoxia and transforming growth factor β1. J. Biol. Chem. 2019;294:14308–14318. doi: 10.1074/jbc.RA119.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H., Cerrato F., Baldini A. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development. 2005;132:4387–4395. doi: 10.1242/dev.02018. [DOI] [PubMed] [Google Scholar]
- 23.Suchonwanit P., Thammarucha S., Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777–2786. doi: 10.2147/DDDT.S214907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HOMER output of 318 candidate regulatory elements mapped to the 21 up regulated genes related to extra cellular matrix, using the mouse genome as background.
Minoxidil treatment does not affect normal development of the OFT in WT individuals.
a-c) Hematoxylin & eosin-stained sections of hearts from fetuses WT fetuses treated with Minoxidil. There are no morphological defects.
Sections indicated with the same letter are from the same heart. IVS: Interventricular septum; LV: Left ventricle; RV: Right ventricle; Ao: Aorta; P: Pulmonary trunk; Scale bare: 100 μm.
Data Availability Statement
All relevant data can be found within the article and its supplementary information. RNA-seq data are available through the GEO database, accession number GSE261206. Mouse lines are available through the European public repository EMMA/Infrafrontier.