ABSTRACT
We examined the frequency of iron and iodine deficiencies and associations of iron and iodine deficiencies with common diseases among under-2 children, adolescent girls, and pregnant women of Bangladesh. We assayed the blood hemoglobin concentration in 395 under-2 children, 355 adolescent girls, and 263 pregnant women, the urinary iodine concentration of those adolescent girls and pregnant women, and the iodine level of all household salt specimens. The history of common diseases within their previous 2 weeks were also obtained from recall to explore the associations of iron and iodine deficiencies with common diseases. Anemia was found in 49.1% of children, 24.8% of adolescent girls, and 44.4% of pregnant women using defined cut-off values (Hb<11.0 g/dL for under-2 children and pregnant women; <12.0 g/dL for adolescent girls). Prevalence of iodine deficiencies (urinary iodine <100 μg/L) was 38.4% in adolescent girls and 39.4% in pregnant women, and 39.4% of salt specimens had inadequate iodine (<15 ppm). The relative risk (RR) and 95% confidence intervals (CI) were estimated and adjusted for age, sex, and gestational age to explore the associations of iron and iodine deficiencies with common diseases. The RR of anemia was increased for fever (RR=1.7, 95% CI=1.3–2.3), ear infection (RR=3.4, 95% CI=1.3–8.5), skin disease (RR=1.4, 95% CI=0.9–2.2), and pneumonia (RR=3.7, 95% CI=0.7–19.5). The RR of iodine deficiency was elevated for diarrhea/dysentery (RR=2.2, 95% CI=1.1–4.4) and eye infection (RR=2.1, 95% CI=0.5–9.4). We concluded that iron and iodine deficiencies are quite high among the Bangladeshi population. Observed associations of iron and iodine deficiencies with common diseases indicated the necessity of eliminating iron and iodine deficiencies from this vulnerable population through strengthening of iron and iodine supplementation, in order to prevent diseases and promote health conditions.
Key Words: Iron, Iodine, Children, Adolescent girls, Pregnant women, Bangladesh
INTRODUCTION
Iron deficiency remains one of the most frequent nutritional disorders worldwide. Anemia affects more than a billion people globally and is recognized as a major public health problem in developing countries.1) Iodine deficiency is the world’s leading cause of mental retardation, and more than two billion children suffer from lowered IQ and retardation due to iodine deficiency.2)
Iron deficiency is considered to be the main cause of anemia,1) and has a negative impact on human health and productivity. It impairs immune functions and reduces work output.3) Recent evidence indicates that iron deficiency during pregnancy negatively affects fetal growth4) and increases the risk of infant iron deficiency,5,6) which is associated with lower Apgar scores6) and potentially irreversible delays in cognitive and psychomotor development.7)
Iodine deficiency, in its most extreme form, results in cretinism. Of much greater public health importance, however, are more subtle degrees of brain damage and reduced cognitive capacity, which could affect the entire population.8) People of all ages suffer from the consequences of iodine deficiency; ranging from abortion/stillbirth of fetus to goiter/hypothyroidism at all ages.
In Bangladesh, iron and iodine deficiencies seriously impact the morbidity and mortality of children, women of child-bearing age, and pregnant women. Normal birth-weight infants are usually born with adequate reserves of nutrients for the first six months of life.9) Nutrient reserves tend to decline because of improper breast feeding and complementary feeding, which starts at seven months of age. Even infants with adequate complementary feeding until two years of age, would not substantially improve iron and iodine intake because of the poor nutrient density and microbiological quality of complementary foods.10) Women of child-bearing age, especially pregnant women, are particularly vulnerable to iron and iodine deficiencies because of increased metabolic demands imposed by pregnancy coupled with associated dietary risks.
According to the report of anemia prevalence survey of urban Bangladesh and rural Chittagong Hill Tracts-2003, 63.9% of children aged 6–23 months, 29.9% of adolescent girls, and 45.5% of pregnant women were anemic in Bangladesh.11) Nearly 69% of the Bangladeshi population had a biochemical iodine deficiency (urinary iodine excretion [UIE] < 10 μg/dl) (hilly areas, 84.4%; flood-prone, 67.1%; and plains, 60.4%). Women and children are more affected than men, in terms of both goiter prevalence and UIE.12) The Bangladesh Iodine Deficiency Disorder (IDD) survey conducted in 1993 reported that 47.1% of the population had either visible or palpable goiter (visible, 38.3% and palpable, 8.8%), and 0.5% of the children were cretins.13) According to the report of UNICEF (State of the World’s Children, 2001), only 55% of households were using iodized salt in Bangladesh.14) Salt iodine concentration is used as a proxy indicator for iodine status of a population.
Presently many programs are in effect in Bangladesh for prevention and control of iron and iodine deficiencies among the population. Iron supplementation for adolescent girls and pregnant women is a regular activity to prevent iron deficiency anemia. A universal salt iodization program has also been conducted to prevent and control iodine deficiency disorders. There has been little investigation, however, about the associations of iron and iodine deficiencies with common diseases among under-2 children, adolescent girls, and pregnant women of Bangladesh.
Therefore, the present study in under-2 children, adolescent girls, and pregnant women of Bangladesh was designed to address the following questions: 1) How prevalent and severe are deficiencies of iron and iodine in this population? 2) What are the associations of iron and iodine deficiencies with common diseases? In addition, this study also analysed the salt iodine status at the household level, in order to explore the impact of iodized salt in reducing iodine deficiency in the population.
MATERIALS AND METHODS
Study population and location
A cross-sectional study was conducted among children aged less than 2 years, adolescent girls aged 13–19 years, and pregnant women in Bangladesh between September and December, 2005. A nationally representative study population was selected by the multi-stage cluster sampling technique. Children ≤6 months, adolescent girls during menstrual bleeding phase, and pregnant women in their first trimester of pregnancy (gestational age of ≤12 weeks) were excluded from the study. In total, 395 children, 355 adolescent girls, and 263 pregnant women participated in the study. Information about respondent’s demography and knowledge of salt uses, and common diseases were obtained from recall using an interviewer-administered questionnaire and checklist. The questionnaire and checklist were developed, revised, and finalized after pretesting among guardians of under-2 children, adolescent girls, and pregnant women in Comilla district, Bangladesh.
Methods
Hemoglobin measure is the most widely used indicator of iron deficiency at the population level.15) In this study, the hemoglobin concentration was measured in the field through the HemoCue technique from capillary blood obtained by fingertip puncture from all participants.15) Measurement of urinary iodine excretion provides a direct measure of iodine intake and hence is a useful indicator of the iodine status of a population.15) Urine sample was collected from adolescent girls and pregnant women, in a sterile container and was stored in the refrigerator at –20ºC until assayed for urinary iodine.16) As a proxy indicator, salt sample was collected from every household of respondents to analyse salt iodine concentration. Prior approval from the Ethical Review Committee of the Bangladesh Medical Research Council (BMRC) was obtained for all study procedures. Moreover, written informed consent was obtained from the respondents and guardians of children before interview and specimen collection.
Laboratory analyses
Urinary excretion of iodine concentration was analysed in the Institute of Nutrition and Food Science (INFS) laboratory by the Microplate method of Ohashi et al.8,17) Salt iodine concentration was analyzed in the Institute of Public Health Nutrition (IPHN) laboratory by the Iodometric titration method.8)
Statistical methods
Statistical analyses were performed using Statistical Package for the Social Science® (SPSS) for Windows, version 11.5 software (SPSS Inc., Ill., USA).
Descriptive statistical tests were applied to demographic and biochemical variables. Age of the respondents, gestational age at interview and specimen collection, and biochemical variables were treated as continuous variables; and diseases and category of respondents were treated as categorical variables. Continuous data were presented as mean and standard deviation for normally distributed data; however, mean and percentiles were used for skewed data. Normality of distribution of the biochemical variables was assessed with the Kolmogorov-Smirnov goodness-of-fit test. The prevalence of deficiency for each biochemical index was expressed as the percentage of respondents below the appropriate cut-off value. We computed odds ratio estimates, assumed to provide a valid estimate of the relative risk (RR), and the 95% confidence intervals (CI) of iron and iodine deficiencies to provide an estimate for common diseases. Binary logistic regression was used for estimating RR and all estimates were adjusted for age, sex (under-2 children), and gestational age (pregnant women). Chi-square analyses were used to compare categorical variables. All tests were two-tailed and a p value of <0.05 was considered significant.
The WHO cut-off points for hemoglobin were used to define anemia; <11 g/dL for children under-2 years and pregnant women,18) and <12 g/dL for adolescent girls.1)
Similarly, WHO cut-off points for urinary iodine excretion were used to define iodine status (severe deficiency <20 μg/L, moderate deficiency 20–49 μg/L, mild deficiency 50–99 μg/L, optimal 100–199 μg/L, more than adequate 200–299 μg/L, excessive ≥300 μg/L and particularly high ≥500 μg/L). The WHO cut-off point for inadequate salt iodine, <15 ppm, was used as the proxy indicator.8)
RESULTS
Background characteristics of respondents
Background characteristics of the respondents are summarized in Table 1. The average at interview was 13.9 months for the children, 15.3 years for the adolescent girls, and 25.8 years for the pregnant women. The average period of gestation of the pregnant women was 6.3 months. Among children, the male:female ratio was almost 50:50.
Table 1.
Background characteristics of the respondents
| Factors | Category of respondents | |||||
| Children (n=395) |
Adolescent girls (n=355) |
Pregnant women (n=263) |
All (n=1013) |
|||
| Agea |
13.9±3.9 | 15.3±1.9 | 25.8±4.9 | NAb |
||
| Gestational age in months | NAb |
NAb |
6.3±1.3 | NAb |
||
| Sex | ||||||
| Males Females |
197 (49.9%) 198 (50.1%) |
0 (0.0%) 355 (100.0%) |
0 (0.0%) 263 (100.0%) |
197 (19.4%) 816 (80.6%) |
||
| Information on salt consumed in household | ||||||
| Packaged salt Open salt |
334 (84.6%) 61 (15.4%) |
286 (80.6%) 69 (19.4%) |
216 (82.1%) 47 (17.9%) |
836 (82.5%) 177 (17.5%) |
||
| Iodized salt Non-iodized salt Unknown |
271 (68.6%) 38 (9.6%) 86 (21.8%) |
255 (71.8%) 33 (9.3%) 67 (18.9%) |
177 (67.3%) 18 (6.8%) 68 (25.9%) |
703 (69.4%) 89 (8.8%) 221 (21.8%) |
||
a Age (mean±SD) in ‘months’ for children and in ‘years’ for adolescent girls and pregnant women.
b NA: not applicable
Usually salts are available on the market of Bangladesh in ‘packaged’ form, which presumably means iodized, and ‘open’ form, which is usually non-iodized. Even if open salts are iodized, the level of iodine is decreased because of spontaneous sublimation of iodine in air.19) It was found that 82.5% of households used packaged salt, while 69.4% of them were aware that they used iodized salt, and 8.8% of them thought they used non-iodized salt. Alarmingly, 21.8% of respondents were unaware of what type of salt they consume.
Iron deficiency and associations with common diseases
Hemoglobin concentrations of under-2 children, adolescent girls, and pregnant women are displayed in Table 2. The mean±standard deviation of hemoglobin concentrations among under-2 children, adolescent girls, and pregnant women were 11.0±1.3, 12.7±1.4, and 11.2±1.4 g/dL, respectively. Based on the cut-off value indicative of anemia, 49.1% of under-2 children, 44.4% of pregnant women, and 24.8% of adolescent girls were found to be anemic.
Table 2.
Hemoglobin concentrations and percentage of under-2 children, adolescent girls, and pregnant women with anemia
| Respondents | n | Mean±SD g/dL |
Median g/dL |
Anemiaa % |
| Children | 395 | 11.0±1.3 | 11.0 | 49.1 |
| Adolescent girls | 355 | 12.7±1.4 | 12.8 | 24.8 |
| Pregnant women | 261 | 11.2±1.4 | 11.1 | 44.4 |
| All | 1011 | 11.6±1.6 | 11.6 | 39.4 |
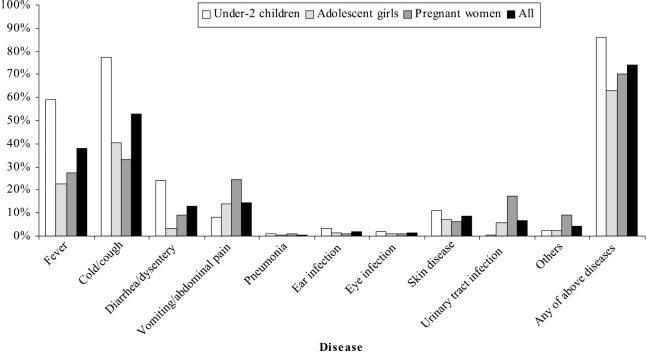
Fig. 1 shows prevalence of diseases among the respondents. Those who suffered from a given disease at least in the most recent 2 weeks were 73.8%. The disease frequency was highest among children (86.1%), followed by pregnant women (70.3%), and adolescent girls (62.8%).
Fig. 1.
Distribution of common diseases over previous 2 weeks.
Table 3 shows the RR of anemia for nine symptoms/diseases. Anemia was associated with increased risk for vomiting/abdominal pain (RR=2.2, 95% CI=1.0–4.8), ear infection (RR=2.3, 95% CI=0.6–7.9), eye infection (RR=1.7, 95% CI=0.4–8.0), and pneumonia (RR=2.5, 95% CI=0.2–26.6) among under-2 children, although not significantly. Among adolescent girls, similar risks were found for fever (RR=1.4, 95% CI=0.8–2.4), diarrhea/dysentery (RR=2.3, 95% CI=0.7–7.7), and ear infection (RR=2.6, 95% CI=0.4–16.6). The risks for skin disease (RR=2.1, 95% CI=0.7–6.3) and urinary tract infection (RR=1.9, 95% CI=1.0–4.0) were higher among pregnant women. Overall, anemic subjects had elevated risks for most of the common diseases with relative risks ranged from 1.1 (95% CI=0.8–1.5) for vomiting/abdominal pain to 3.7 (95% CI=0.7–19.5) for pneumonia.
Table 3.
Relative risk of anemia for common diseases
| Symptom/disease | Under-2 children | Adolescent girls | Pregnant women | All | |||||||||||
| Anemia | RRb |
Anemia | RRb |
Anemia | RRb |
Anemia | RRb |
||||||||
| (+) | (-) | (95% CI) | (+) | (-) | (95% CI) | (+) | (-) | (95% CI) | (+) | (-) | (95% CI) | ||||
| Fever | 60.3% | 57.7% | 1.3 (0.8–1.9) |
27.3% | 21.3% | 1.4 (0.8–2.4) |
29.3% | 25.5% | 1.5 (0.8–2.8) |
44.0% | 34.3% | 1.7c (1.3–2.3) |
|||
| Cold/cough | 79.4% | 75.1% | 1.3 (0.8–2.1) |
30.7% | 43.4% | 0.6c (0.4–1.0) |
31.9% | 34.5% | 0.8 (0.5–1.5) |
54.8% | 51.7% | 1.3 (1.0–1.7) |
|||
| Diarrhea/dysentery | 22.7% | 25.4% | 0.9 (0.6–1.5) |
5.7% | 2.6% | 2.3 (0.7–7.7) |
8.6% | 9.7% | 1.2 (0.5–3.2) |
14.8% | 11.7% | 1.4 (1.0–2.1) |
|||
| Vomiting/abdominal pain | 10.8% | 5.5% | 2.2 (1.0–4.8) |
15.9% | 13.5% | 1.3 (0.6–2.5) |
25.0% | 24.8% | 1.0 (0.5–1.8) |
16.1% | 13.5% | 1.1 (0.8–1.5) |
|||
| Pneumonia | 1.5% | 0.5% | 2.5 (0.2–26.6) |
0.0% | 0.4% | 0.0 (NC)d |
1.7% | 0.0% | ∝ (NC)d |
1.3% | 0.3% | 3.7 0.7–19.5) |
|||
| Ear infection | 4.6% | 2.0% | 2.3 (0.6–7.9) |
2.3% | 1.1% | 2.6 (0.4–16.6) |
1.7% | 0.0% | ∝ (NC)d |
3.3% | 1.1% | 3.4c (1.3–8.5) |
|||
| Eye infection | 2.1% | 1.5% | 1.7 (0.4–8.0) |
1.1% | 1.1% | 0.9 (0.1–8.6) |
0.9% | 1.4% | 1.3 (0.1–17.8) |
1.5% | 1.3% | 1.2 (0.4–3.4) |
|||
| Skin disease | 11.9% | 10.4% | 1.1 (0.6–2.2) |
8.0% | 7.1% | 1.1 (0.4–2.7) |
7.8% | 4.8% | 2.1 (0.7–6.3) |
9.8% | 7.7% | 1.4 (0.9–2.2) |
|||
| Urinary tract infection | 0.5% | 0.0% | ∝ (NC)d |
3.4% | 6.7% | 0.5 (0.1–1.7) |
22.4% | 13.1% | 1.9 (1.0–4.0) |
7.5% | 6.0% | 0.9 (0.6–1.6) |
|||
a: reference category is ‘no deficiency’
b: Adjusted for age and sex in under-2 children, for age in adolescent girls, and for age and gestational age in pregnant women
c: p<0.05
d NC: not calculable
Iodine deficiency and its association with common diseases
Table 4 demonstrates the urinary iodine excretion level of adolescent girls and pregnant women. The mean urinary iodine concentration of adolescent girls was found to be 186.3 μg/L (median, 130.8), and the 20th and 80th percentile range of 45.5–319.7 μg/L, while for pregnant women it was 183.8 μg/L (median, 133.4), and the 20th and 80th percentile range of 54.1–290.1 μg/L. Based on the cut-off of urinary iodine deficiency, 38.4% of adolescent girls and 39.4% of pregnant women were iodine-deficient. However, 20.2% of adolescent girls and pregnant women had excess urinary iodine of ≥300 μg/L.
Table 4.
Urinary iodine concentration and percentage of adolescent girls and pregnant women with deficiency and excess
| Respondents | n | Mean±SD μg/L |
Median (20th, 80th percentile) μg/L |
Deficiencya % |
Excessb % |
| Adolescent girls | 354 | 186.3±158.9 | 130.8 (45.5, 319.7) | 38.4 | 21.0 |
| Pregnant women | 259 | 183.8±154.9 | 133.4 (54.1, 290.1) | 39.4 | 19.3 |
| All | 613 | 185.3±157.0 | 132.0 (49.3, 302.2) | 38.9 | 20.2 |
Table 5 shows the RR of iodine deficiency for adolescent girls and pregnant women. There was almost a five-fold risk of iodine deficiency for eye infection (RR=4.8, 95% CI=0.5–47.1) among adolescent girls. Among pregnant women, increased risks were found for diarrhea/dysentery (RR=4.0, 95% CI=1.6–10.3), pneumonia (RR=1.5, 95% CI=0.1–24.7), and ear infection (RR=1.7, 95% CI=0.1–30.4). Overall, diarrhea/dysentery (RR=2.2, 95% CI=1.1–4.4) and eye infection (RR=2.1, 95% CI=0.5–9.4) had elevated risks among iodine-deficient subjects.
Table 5.
Relative risk of iodine deficiency for common disease
| Symptom/ disease |
Adolescent girls | Pregnant women | All | ||||||||
| Iodine deficiency |
RRb |
Iodine deficiency |
RRb |
Iodine deficiency |
RRb |
||||||
| (+) | (-) | (95% CI) | (+) | (-) | (95% CI) | (+) | (-) | (95% CI) | |||
| Fever | 23.5% | 22.5% | 1.1 (0.6–1.8) |
29.8% | 25.5% | 1.3 (0.7–2.2) |
26.3% | 23.7% | 1.1 (0.8–1.7) |
||
| Cold/cough | 41.2% | 39.9% | 1.1 (0.7–1.6) |
34.6% | 32.5% | 1.1 (0.6–1.8) |
38.3% | 36.8% | 1.1 (0.8–1.5) |
||
| Diarrhea/ dysentery |
2.9% | 3.7% | 0.8 (0.2–2.7) |
15.4% | 4.5% | 4.0c (1.6–10.3) |
8.3% | 4.0% | 2.2c (1.1–4.4) |
||
| Vomiting/ abdominal pain |
14.7% | 13.8% | 1.1 (0.6–2.0) |
27.9% | 22.9% | 1.3 (0.7–2.3) |
20.4% | 17.6% | 1.2 (0.8–1.8) |
||
| Pneumonia | 0.0% | 0.5% | 0.0 (NC)d |
1.0% | 0.6% | 1.5 (0.1–24.7) |
0.4% | 0.5% | 0.8 (0.1–8.6) |
||
| Ear infection | 0.7% | 1.8% | 0.40 (0.1–3.4) |
1.0% | 0.6% | 1.7 (0.1–30.4) |
0.8% | 1.3% | 0.6 (0.1–3.4) |
||
| Eye infection | 2.2% | 0.5% | 4.8 (0.5–47.1) |
1.0% | 1.3% | 0.9 (0.1–11.1) |
1.7% | 0.8% | 2.1 (0.5–9.4) |
||
| Skin disease | 4.4% | 9.2% | 0.5 (0.2–1.2) |
4.8% | 7.0% | 0.7 (0.2–2.0) |
4.6% | 8.3% | 0.5 (0.3–1.1) |
||
| Urinary tract infection |
5.1% | 6.4% | 0.8 (0.3–2.0) |
17.3% | 17.2% | 1.0 (0.5–1.9) |
10.4% | 10.9% | 0.9 (0.5–1.6) |
||
a: reference category is ‘no deficiency’
b: Adjusted for age in adolescent girls, and for age and gestational age in pregnant women
c: p<0.05
d NC: not calculable
Salt iodine concentration in household specimens
As shown in Table 6, the mean salt iodine concentration was found to be 25.8 ppm with 20th and 80th percentiles range of 8.5–40.6 ppm in household salt specimens of all respondents. Based on the cut-off for inadequate salt iodine, 39.3% of salt specimens were deficient in iodine.
Table 6.
Salt iodine concentration and percentage of specimens with deficiency
| Category of households |
n | Mean±SD ppma |
Median (20th & 80th percentiles) ppma |
Deficiencyb % |
| Children | 395 | 25.8±25.8 | 20.3 (7.5, 37.4) | 38.7 |
| Adolescent girls | 355 | 25.1±20.0 | 20.3 (7.5, 41.6) | 41.1 |
| Pregnant women | 260 | 26.7±22.1 | 20.3 (8.5, 44.6) | 38.1 |
| All | 1010 | 25.8±22.9 | 20.3 (8.5, 40.6) | 39.3 |
a ppm: parts per million
b Deficiency is defined as salt iodine <15 ppm8)
DISCUSSION
Over 70% of the Bangladeshi population live in rural areas; on average, 50% are illiterate, and one-fourth of the population live below the hard core poverty level (energy intake <1805 kcal/person/day).11) In this study, we found that about 22% of the respondents were unaware of iodized salt, and the level of this lack of awareness was high among pregnant women. Such unawareness also implies that this population is possibly ignorant of other health and nutrition issues. Thus, fostering awareness among them is an important precondition to achieve a desired outcome of any intervention in prevention and control of iron and iodine deficiencies.
The proportion of anemia was significantly higher in pregnant women and children than adolescent girls, which is, according to WHO, a ‘serious’ public health problem. These findings correspond with the assessment that maternal iron deficiency and consequent anemia comprise a major problem in developing countries, affecting >50% of women during pregnancy, which in turn leads to adverse fetal outcomes, such as premature birth and intrauterine growth retardation.20-22) In children, these deficiencies are sustained in their childhood through poor nutrient density as well as poor microbiological quality of complementary foods and displacement of breast milk.10)
The urinary iodine excretion level seems to be adequate for median value (≥100 μg/L), but based on the cut-off value of WHO, a quite high percentage of adolescent girls and pregnant women were living with the curse of iodine deficiency.8) The salt iodine level we found also strongly supports the findings of the urinary iodine excretion level almost homogeneously in all groups of respondents. Though Bangladesh launched an Iodine Deficiency Disorder (IDD) control program in 1994 to eradicate iodine deficiency by the year 2000, led by the International Council for Control of Iodine Deficiency Disorders (ICCIDD), the time has come to explore the root cause of low iodine concentration in consumed salt and also low urinary iodine concentration.8)
A universal salt iodization (USI) survey during 1996 showed that many salt samples from the salt factory had either too low or even dangerously high iodine levels.13) In our study, about one-fifth of adolescent girls and pregnant women had an excess iodine level, possibly due to inappropriate iodization of salt, poor monitoring and excess salt intake, especially among rural people.8) Iodine availability is affected by variability in the amount of iodine added during production, its uneven distribution within the batches or bags produced due to poor mixing, losses at the distribution and retail level, and in the household, during storage and meal preparation.19) These findings agree with the WHO report that with global progress in correcting iodine deficiency, examples of iodine excess are being recognized, particularly when salt iodization is excessive and poorly monitored.23)
Iron deficiency poses a major negative health impact through interfering in many vital activities at cellular level mediated through hemoglobin. It is also associated with reversible abnormalities of immune function24) which can lead an individual prone to more frequent infections including other ailments like fatigue, heart failure or even stroke.25)
In this study, we have found iron deficiency is associated with increased risks for most of the common diseases like fever, diarrhea/dysentery, pneumonia, ear infection and skin disease. It may be due to the disturbed immunity of anemic individuals during iron deficiency states. These findings are in agreement with the findings of Ramakrishnan, who found about 5.75 times more susceptibility of acquiring lower respiratory infections among the anemic population.26)
Iodine plays a major role in proper functioning of almost all cells of the human body. Although the role of thyroid gland for iodine usage is well explained, iodine is needed only in microgram amounts to continue its normal function, whereas the rest of the body needs iodine in milligram amounts to achieve optimum health and prevent diseases.27) Large-scale studies have investigated the consequences of long-term iodine deficiencies on human health; they established the effect of iodine deficiency in terms of lowered IQ, brain damage, and goiter and cretinism, among others. Furthermore, some studies have also found some association of iodine deficiency with cardiovascular diseases, hypertension,28) and cancer through its antioxidant effects.29) But the present study focused on determining the contribution of iodine deficiency to common diseases.
We have found risks of iodine deficiency for some common infectious diseases, e.g. diarrhea/dysentery, eye infection, ear infection and pneumonia, among the study population. These findings are in agreement with the concept of the universal anti-infective role of iodine. Eliminating iodine deficiencies from the population, especially from vulnerable groups like children, adolescent girls, and pregnant women can save millions of lives. Assurance of adequate iodine among infants can help reduce infant mortality30) through reducing common diseases such as pneumonia and diarrhea.
Although the present study clearly demonstrated new findings regarding the associations of iron and iodine deficiencies with common diseases of Bangladeshi people, there were some limitations in the study design. In this cross-sectional study, the common diseases two weeks before specimen sampling were asked, not after the sampling. This would indicate that the measured temporality between cause (deficiencies) and outcome (diseases) is reversed. Accordingly, the assumption that the deficiencies observed at specimen collection exist before disease onset is required to draw the conclusion that the deficiencies may be the causes of diseases. This assumption seems plausible, because the deficiencies were generally chronic. Concerning biases on the estimated RRs, the effects did not seem to be serious. The subjects were sampled in a cross-sectional study, and they did not know their deficiency levels at the interview on disease history. However, the disease history was obtained through the interview, not from records such as medical charts at medical facilities; the validity of the history should have been measured at least for a part of the subjects.
In conclusion, given the prevalence of the grave iron and iodine deficiency in Bangladesh, the highly unstable iodine level in salt and urine, and the observed association of these deficiencies with common diseases, our recommendations are to strengthen iron supplementation for the population now at risk along with strong monitoring of salt iodization and assurance of optimally iodized salt at the household level. Elimination of iron and iodine deficiencies can prevent diseases and improve health conditions in this population.
ACKNOWLEDGEMENTS
I am greatly indebted to Prof. Atsuko Aoyama for her generous and insightful review of the manuscript, which has enriched my paper to a great extent. This work was supported in part by a non-profit organization “Epidemiological and Clinical Research Information Network (ECRIN)”. We would also like to thank Alfressa Foundation for their generous financial support as CSR. The authors would like to thank the field and laboratory staffs of IPHN along with all study respondents for their cooperation and assistance in the course of this study. Thanks also to the laboratory staffs of INFS for their assistance in the urinary iodine analysis.
Professor Nobuyuki Hamajima deserves special thanks for his invaluable contribution to this paper.
REFERENCES
- 1).Ahmed F, Khan MR, Akhtaruzzaman M, Karim R, Marks GC, Banu CP, Nahar B, Williams G. Efficacy of twice-weekly multiple micronutrient supplementation for improving the hemoglobin and micronutrient status of anemic adolescent schoolgirls in Bangladesh. Am J Clin Nutr, 2005; 82: 829–835. [DOI] [PubMed]
- 2).Regional Office for South Asia. Iodine deficiency disorders and universal salt iodisation: South Asia priorities. UNICEF. pp. 1–20, 2002, Kathmandu, Nepal.
- 3).Committee on Nutritional Status During Pregnancy and Lactation. Iron nutrition during pregnancy. In: The Nutrition During Pregnancy. Institute of Medicine. pp. 272–298, 1990, National Academy Press, Washington, D.C.
- 4).Singla PN, Tyagi M, Kumar A, Dash D, Shankar R. Fetal growth in maternal anemia. J Trop Pediatr, 1997; 43: 89–92. [DOI] [PubMed]
- 5).Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron deficiency anaemia in infancy. A case-control study in Jordan. Int J Epidemiol, 1999; 28: 461–468. [DOI] [PubMed]
- 6).Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: Consequences for newborns. Am J Clin Nutr, 1997; 66: 1178–1182. [DOI] [PubMed]
- 7).Lozoff B, Wolf AW, Jimenez E. Iron deficiency anemia and infant development: Effects of extended oral iron therapy. J Pediatr, 1996; 129: 382–389. [DOI] [PubMed]
- 8).Department of Nutrition for Health and Development. Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers. WHO. 2001, Geneva, Switzerland.
- 9).Brown K, Dewey K, Allen L. Complementary feeding of young children in developing countries: A review of current scientific knowledge. WHO, 1998, Geneva, Switzerland.
- 10).Kimmons JE, Dewey KG, Haque E, Chakraborty J, Osendarp SJM, Brown KH. Low nutrient intakes among infants in rural Bangladesh are attributable to low intake and micronutrient density of complementary foods. J Nutr, 2005; 135: 444–451. [DOI] [PubMed]
- 11).Bangladesh Bureau of Statistics. Statistical Pocketbook of Bangladesh-2004. 2006, Dhaka, Bangladesh.
- 12).Yusuf HK, Quazi S, Kahn MR, Mohiduzzaman M, Nahar B, Rahman MM, Islam MN, Khan MA, Shahidullah M, Hoque T, Baquer M, Pandav CS. Iodine deficiency disorders in Bangladesh. Indian J Pediatr, 1996; 63: 105–110. [DOI] [PubMed]
- 13). Shahjahan M, Baquer M, Schaetzel TT. Possible barriers to quality assurance of iodized salt in Bangladesh. In: Proceedings of the Eighth World Salt Symposium, 2000, The Hague, The Netherlands.
- 14).The United Nations Children’s Fund. The State of the World’s Children 2001. UNICEF. pp. 82, 2001, Geneva, Switzerland.
- 15). Trowbridge FL. Surveillance of micronutrient deficiency. Food Nutr Bull, 1994; 15: 290–294.
- 16).El-Mougi FA, Abd-El-Ghaffar S, Fayek NAF, Mohammad MS. Urinary iodine and other iodine deficiency indicators in a sample of school-age children in Egypt. East Mediterr Health J, 2004; 10: 863–870. [PubMed]
- 17). Ohashi T, Yamaki M, Pandav CS, Karmakar MG, Irie M. Simple microplate method for determination of urinary iodine. Clin Chem, 2000; 46: 529–536. [PubMed]
- 18). Gueri M, Pena M. Maternal and child nutrition. In: The Maternal and Child Health Activities at the Local Level: Towards the Goals of the World Summit for Children. pp. 266–282, 1998, Washington D.C.
- 19).Diosady LL, Venkatesh Mannar MG. Stability of iodine in iodized salt. In: Proceedings of the Eighth World Salt Symposium, 2000, Amsterdam, The Netherlands.
- 20).Huffman SL, Baker J, Shuman J, Zehner ER. The case for promoting multiple vitamin/mineral supplements for women of reproductive age in developing countries. Academy for Educational Development. 1998, Washington D.C.
- 21). Ramakrishman U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev, 2002; 60: S46–S52. [DOI] [PubMed]
- 22). Seshadri S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br J Nutr, 2001; 85: S87–S92. [PubMed]
- 23). Delange F, de Benoist B, Alnwick D. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency disorders by iodized salt. Thyroid, 1999; 9: 545–556. [DOI] [PubMed]
- 24). Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr, 2001; 131: 616S–633S. [DOI] [PubMed]
- 25).Maguire JL, deVeber G, Parkin PC. Association between iron-deficiency anemia and stroke in young children. Pediatrics, 2007; 120: 1053–1057. [DOI] [PubMed]
- 26).Ramakrishnan K, Harish PS. Hemoglobin level as a risk factor for lower respiratory tract infections. Indian J Pediatr, 2006; 73: 881–883. [DOI] [PubMed]
- 27).Miller DW. Iodine in health and civil defense. In: Proceeding of the Twenty-fourth Annual Meeting of Doctors for Disaster Preparedness. 2006, Portland, Oregon.
- 28).Hoption Cann SA. Hypothesis: dietary iodine intake in the etiology of cardiovascular disease. J Am Coll Nutr, 2006; 25: 1–11. [DOI] [PubMed]
- 29).Patrick L. Iodine: deficiency and therapeutic considerations. Altern Med Rev, 2008; 13: 116–127. [PubMed]
- 30).Cobra C, Muhilal, Rusmil K, Rustama D, Djatnika, Suwardi SS, Permaesih D, Muherdiyantiningsih, Martuti S, Semba RD. Infant survival is improved by oral iodine supplementation. J Nutr, 1997; 127: 574–578. [DOI] [PubMed]