ABSTRACT
The purpose of this paper is to describe the newly-established technique in the field of neurological surgery for fusion imaging of three-dimensional magnetic resonance image (3D-MRI) and/or three-dimensional computed tomography (3D-CT) for brain tumor surgery. Combining neuronavigation technology and intraoperative MRI, this method remarkably demonstrates spatial relationships of neurovascular structures and/or skull base landmarks and is very useful for intraoperative evaluation of completed neurosurgical operations. Using the navigation system and intraoperative MRI during surgery, it is possible to resect the brain tumor maximally and preserve essential neurological functions. Furthermore, advanced multimodal neuroradiological images such as functional MRI (fMRI), diffusion tensor imaging (DTI), MR spectroscopy (MRS), and positron emission tomography (PET) clearly demonstrate the dominant cortex including the speech center, primary motor gyrus, primary sensory gyrus, and support high-quality operation with less invasive surgery. In conclusion, multimodal neuroradiological images are very useful for invasive non-circumscribed brain tumors such as glioma and, in combination with such highly technological analyses, advanced neurosurgical procedures are possible.
Key Words: Intraoperative MRI, Fusion image, Three-dimensional image, Glioma, Neuronavigation
INTRODUCTION
Recent promotion of collaboration between medical science and engineering has brought about significant advancement in the development of diagnostic imaging technology and surgical assisted systems.1) Using a high-resolution microscope, the operation requires high-accuracy technique that refers to the 3D brain image displayed on the neuronavigation robot within a close tolerance of a few millimeters; it is reminiscent of repairs made to a sophisticated electronic circuit.2) On the other hand, the advancement of computer technology makes 3D virtual image technology more efficient, allowing the creation of images analogous to the clinical condition. As a result, it is becoming possible to establish a correct diagnosis of a minute lesion.3) Moreover, “brain shift,” the greatest weakness of a neuronavigative operation, was resolved at once due to the development of image fusion technology which utilizes intraoperative MRI images for visualization of changes in brain morphology so that the navigation map can be adjusted during the surgical procedure. In a neurosurgical operation, this information integration among image, organ and function assures a good balance between maximum tumor resection for overall survival prognosis and provides a functional prognosis even for invasive malignant brain tumors4). Furthermore, this innovation provides the momentum for development of surgical devices applicable even in the microscopic field5). At present, the Department of Neurosurgery, Nagoya University Graduate School of Medicine is working on a project for “development of an intelligent operation system” along with the Department of Mechanical Engineering, Nagoya Institute of Technology and Department of Media Science, Nagoya University Graduate School of Technology. The goal of the project is to create the world’s first intraoperative brain touch sensor and/or microsurgical device for microscopic or endoscopic management.6)
“Brain Theater,” the integrated system of intraoperative MRI and neuronavigation, was set up at Nagoya University Hospital in January of 2006 (Fig. 1). The system features new technology which provides surgical assistance information gathered through intraoperative MRI and networks not only for the operation theater but to other universities and hospitals as well. Briefly, the system works as follows: 1) as a core function, an MRI (Hitachi) is situated at No. 5 operation theater at Nagoya University Hospital. 2) An intraoperative neuronavigation system functions in perfect unison with the operative microscope and peripheral equipment. 3) The secure and high-performance operation theater encourages neurosurgeons to make full use of traditional surgical techniques without qualification (Fig. 2). 4) Awarded the 2007 good design award from the Japan Industrial Design Promotion Organization. Additionally, by making this available on line, it is possible to share the surgical assist system for surgical planning, sharing intraoperative images, supporting tele-surgery, and developing advanced therapy outside the operation theater. With the great technical assistance of the Graduate School of Information Science, Nagoya University and the Department of Radiological Technology, Nagoya University School of Health Science, the system can develop educational and training activities for students and young neurosurgeons in terms of surgical simulation before an operation. Besides, the simulated experience of operations produced by sharing virtual images is useful to decide the strategy for clinical cases.
Fig. 1.
Neurosurgical operation room No. 5 called the “Brain Theater,” equipped with open MRI unit (0.4 Tesla HITACHI Aperto) at Nagoya University Hospital. (Low magnetic field and rotation table concept; 1) safer, 2) standard-equipped with all conventional systems, 3) applicable to non-MRI operation, 4) permanent magnet with lower cost (primary as well as running cost), 5) specially designed table for easy patient transportation to scanner, and 6) sufficient image quality).
Fig. 2.
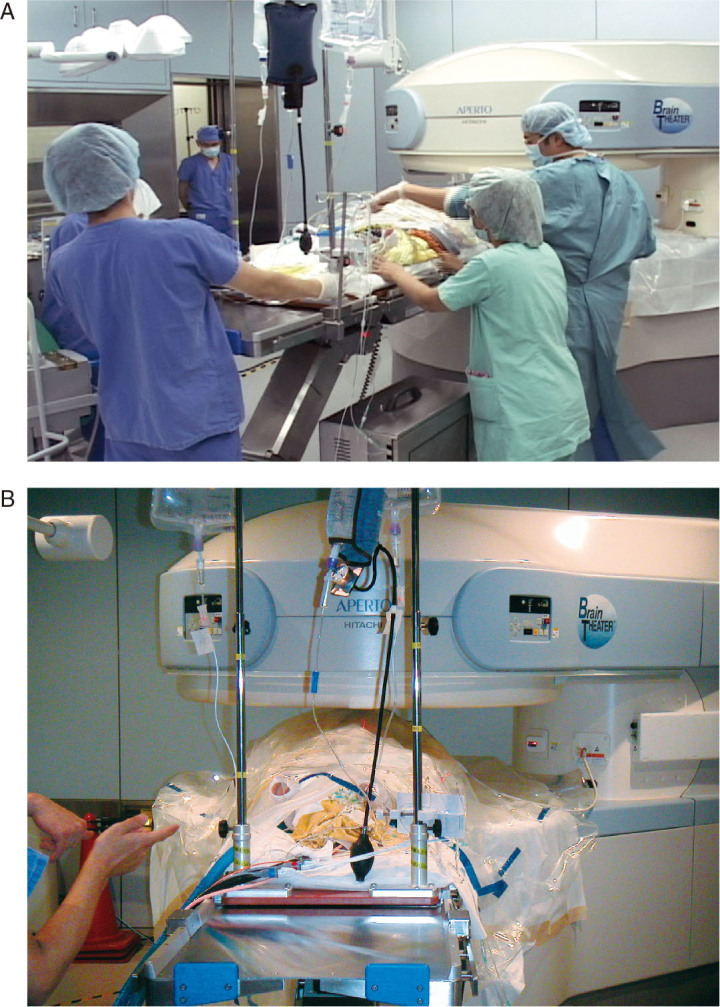
Setup position for intraoperative MRI during neurological surgery in Brain Theater.
A: Transportation of patient to setup. B: Setup position of patient in intraoperative MRI.
In 2007, the “brain SUITE,” operation assistance equipment, similar to the “Brain Theater” made by Siemens, was installed at Nagoya Central Hospital in connection with the relocation of the hospital. Since it is a Nagoya University-affiliated hospital, these two systems are linked by network to exchange information so that it is now possible to assist with difficult operations mutually by remote control. It is easy to imagine that the surgical assist system brought about by 3D virtual images will show rapid progress hand in hand with the advancement of image analysis.
PROGRESS IN DIAGNOSTIC IMAGING IN THE FIELD OF NEUROSURGERY
Progress in neurosurgical diagnostics is supported by advancements in diagnostic radiology imaging. Along with advancements in diagnostic radiology, various kinds of neuroimaging are produced which are useful for preoperative diagnosis, planning operation strategy, intraoperative image assistance, and postoperative follow-up. As for MRI scans, not only standard models such as T1 weighted-image, T2 weighted-image and FLAIR images but also the emergence of new models with diffusion-weighted image, ADC (Apparent Diffusion Coefficient) MAP, MR Angiography have increased the range of qualitative diagnosis and are already used in clinical settings. Such evolution foretells the near future when tissue diagnosis can be done merely by image analysis. In addition, PET as typified by FDG-PET, radionuclide scanning (e.g. SPECT), and magnetic encephalography have made qualitative diagnosis possible.7) Besides, nowadays X-ray computed tomography can be treated as a 3D image since multislice helical CT has become increasingly more widespread. The use of these various technologies for preoperative evaluation and intraoperative assistance has made more accurate neurosurgery feasible. Moreover, progress in computer technology makes it possible to utilize advanced 3D virtual images for more advanced image analysis.8) In particular, the Graduate School of Information Science, Nagoya University (Chief of projects: Dr. Kensaku Mori, PhD, Main Assistance: Dr. Yu-ichiro Hayashi, PhD) has designed the fastest software for image analysis and collaborative research is expected to deliver optimum results.
PROGRESS IN NEUROSURGICAL OPERATION
Neurosurgery has made steady progress, thanks to the introduction of microsurgery in the 1960s, the development of micro-operative devices in the 1970s, the spread of the head computed tomography scans in the 1980s, and the significant improvement of diagnostic techniques provided by the spread of MRI in the 1990s. Meanwhile, it is also obvious that continuous efforts by neurosurgeons have resulted in excellent progress. Brain tumors, particularly in infiltrative intraparenchymal tumors such as glioma, require delicate surgery; however, wide tumor resection might cause brain dysfunction and/or an adverse effect. Therefore, an immediate decision based on both improved resection and avoidance of dysfunction is necessary. Consequently, for the success of these surgical procedures involving tumors, it is essential to combine them with image-guided surgery, a technique based on neuronavigation which first introduced in the 1990s.
The neuronavigation system is a useful surgical-assisted device which can provide accurate information on the surgical site in real time. However, existing navigation functioned based on preoperative imaging has inherent fundamental problems involving the “brain shift.” It means change in the shape of the brain during an operation.9) For example, during surgical procedures, the removal of a space-occupying lesion or drainage of cerebrospinal fluid often causes distortion so that the brain surface sinks several centimeters (Fig. 3). Since this change in brain shape undermines the reliability of navigation which uses a preoperative image as a map, the appropriate adjustment for such brain shifts has become an urgent necessity. In response, using intraoperative MRI to confirm changes in brain morphology, image fusion technology enables a navigation system to adjust the images during an operation so as to resolve the problem (Table 1). Although the advancement in imaging technology is significant, the images of brain function are still not completely reliable; therefore, physiological monitoring such as SEP, MEP and ABP should be used in combination in order to achieve operation safety.10) Also, with tumors near the language area, intraoperative brain function monitoring under awake craniotomy is considered important. Tumor resection is the most important target in future neurosurgical operations. Three kinds of information, image, organ, and function, should be integrated to balance competing goals for maximum resection and ensuring safety (Fig. 4 and Fig. 5).
Fig. 3.
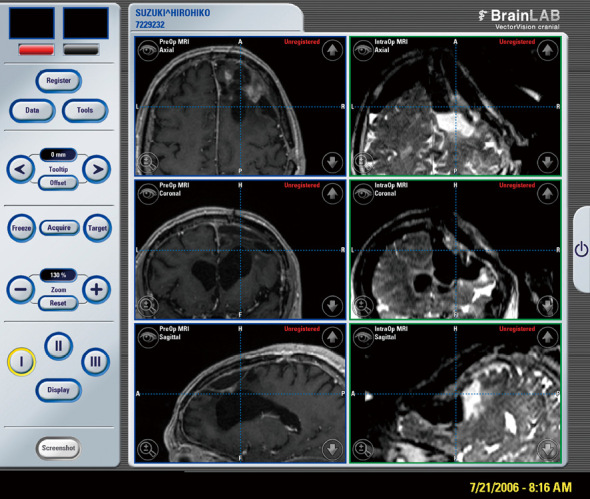
Comparison of MRI image on neuronavigation display (Left: preoperative axial, coronal, and sagittal views on T1WI. Right: intraoperative axial, coronal, and sagittal view on T2WI). Note remarkable “brain shift” after connection of ventricle with tumor removal cavity.
Table 1.
Profile of 18 recent operative cases of glioma performed in Brain Theater. Remarkably improved removal rate of tumor on postoperative MRI was achieved. (GBM: glioblastoma multiforme; CN: central neurocytoma; O: oligodendroglioma; AO: anaplastic oligodendroglioma; A: astrocytoma)
| Case | Age | M/F | Histology | Location | Tumor size (ml) | MRI sequence | Resection rate (%) | neurology (postop.) |
| 1 | 20 | F | GBM | rt Th | 60.1 | T1 (E) | 93.9 | Gerstmansyndrome |
| 2 | 20 | M | CN | rt LV | 49.5 | T1 (E) | 100.0 | free |
| 3 | 29 | F | O | rt F | 81.9 | T2 | 83.4 | free |
| 4 | 58 | M | GBM | rt F, M | 20.3 | T1 (E) | 100.0 | no change |
| 5 | 30 | M | AO | lt T | 180.5 | FL | 100.0 | free |
| 6 | 56 | M | AO | lt T | 71.2 | T1 (E) | 100.0 | no change |
| 7 | 67 | F | GBM | rt T | 12.1 | T1 (E) | 100.0 | free |
| 8 | 38 | F | A | rt Ol | 51.5 | T1 (E) | 100.0 | no change |
| 9 | 31 | M | O | lt F | 69.1 | T1 (P) | 100.0 | improved |
| 10 | 72 | M | A | lt F | 25.7 | T2 | 94.3 | transient aphasia |
| 11 | 56 | F | GBM | rt P | 91.2 | T1 (E) | 100.0 | free |
| 12 | 40 | M | A | lt F | 55.3 | T2 | 85.9 | transient aphasia |
| 13 | 55 | M | O | lt P | 71.5 | T2 | 90.3 | transient aphasia |
| 14 | 61 | F | O | rt F | 31.5 | T2 | 80.9 | free |
| 15 | 66 | M | GBM | lt F | 87.4 | T1 (E) | 97.7 | no change |
| 16 | 60 | M | O | rt F | 23.8 | T1 (E) | 95.5 | no change |
| 17 | 66 | M | GBM | rt P | 10.8 | T1 (E) | 100.0 | no change |
| 18 | 50 | M | AO | rt F | 3.9 | T1 (E) | 100.0 | no change |
| MEAN 48.6 | 55.4 | 95.7 | ||||||
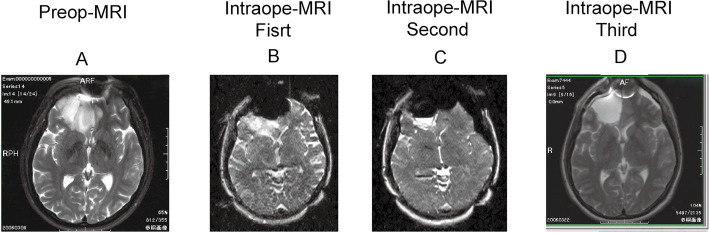
Fig. 4.
Ilustrative case of right frontal oligodendroglioma, primary case. A: Pre-operative MRI (T2 weighted image) before surgery revealed a relatively huge mass at the entire right frontal tip invading the corpus callusum at the bottom of this mass. B: Intraoperative MRI during surgery showed a residual tumor at the bottom of this removal cavity. C: Second intraoperative MRI revealed subtotal removal with still small residual mass at the bottom of this cavity. D: Third intraoperative MRI eventually showed total removal of this mass.
Fig. 5.
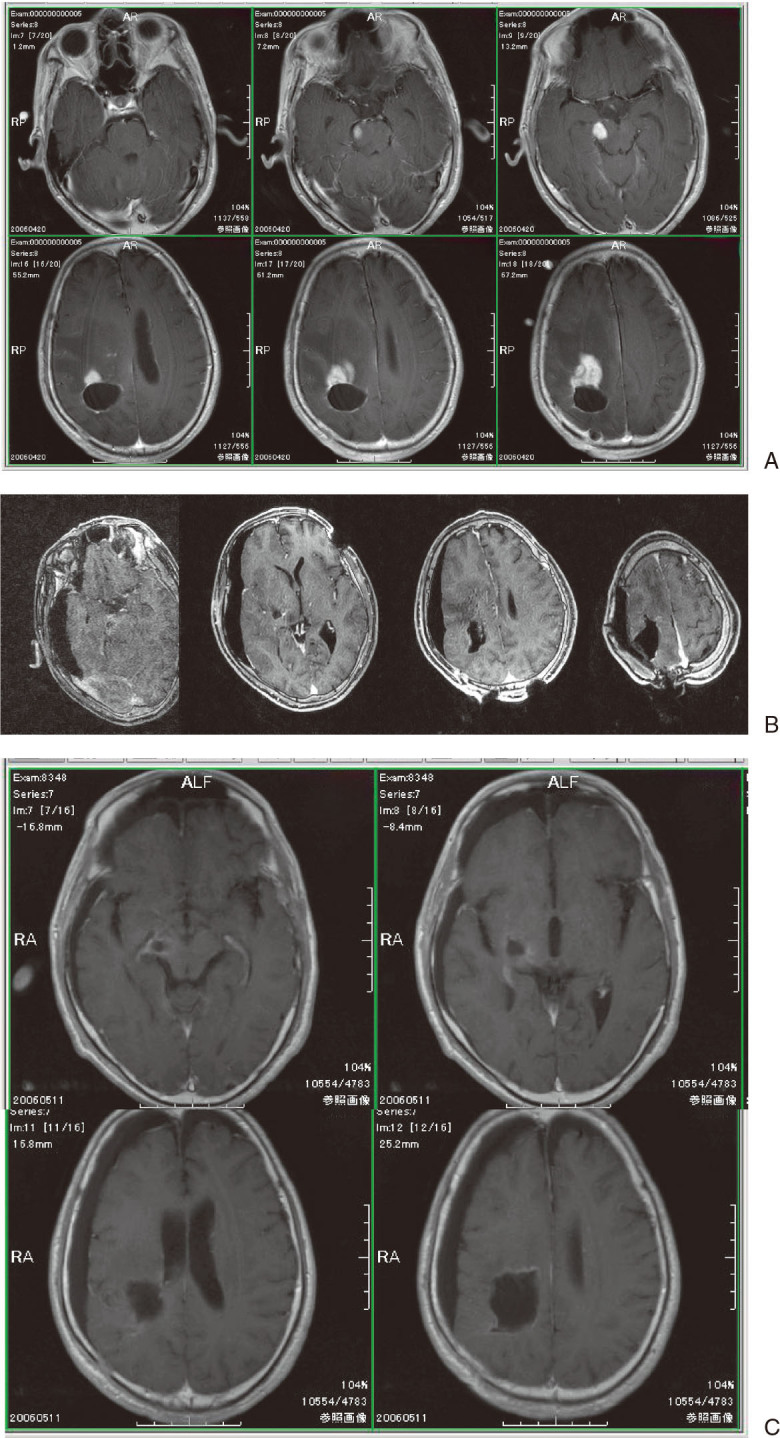
Illustrative case of recurrent glioblastoma occurring primarily at right parietal site. A: Pre-operative MRI (T1WI gadolinium enhanced axial image) revealed multiple recurrent enhanced masses not only in adjacent area of pre-operative space but also in remote area as a part of midbrain. B: Intraoperative MRI during surgery showed removal of almost all mass with a small residual tumor at part of the remote disseminated area on midbrain. C: Final intraoperative MRI indicated complete removal of mass.
FUTURE PROGRESS IN NEUROSURGERY
To achieve minimally invasive and accurate neurosurgical operations, the development of more sophisticated diagnostic devices and surgical-assist devices is required. Present advances in prevention medicine enhance the opportunities for early detection by brain medical checkups and rapid cures of neurosurgical disorders, that might otherwise cause serious disability. Now we are at a major turning point for neurosurgical treatment since safer and more secure ways of providing it have been established as prevention measures in recent years. Now there is a pressing need to create a support system by promoting expertise and innovations to meet the increasing demand for neurosurgical treatment.11)
In conclusion, multimodal neuroradiological images are very useful for invasive non-circumscribed brain tumors such as glioma and further progress in the field of medical technology may bring about a next-generation neurosurgical world.
REFERENCES
- 1).Darakchiev BJ, Tew JM Jr, Bohinski RJ, Warhick RE. Adaptation of a standard low-field (0.3-T) system to the operating room: focus on pituitary adenomas. Neurosurg Clin N Am, 2005; 16: 155–164. [DOI] [PubMed]
- 2).Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimnsky C. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg, 2001; 95: 381–390. [DOI] [PubMed]
- 3).Kelly JJ, Hader WJ, Myles ST, Sutherland GR. Epilepsy surgery with intraoperative MRI at 1.5 T. Neurosurg Clin N Am, 2005; 16: 173–183. [DOI] [PubMed]
- 4).Matsumae M, Koizumi J, Fukuyama H, Ishizaka H, Mizokami Y, Baba T, Atsumi H, Tsugu A, Oda S, Tanaka Y, Osada T, Imai M, Ishiguro T, Yamamoto M, Tominaga J, Shimoda M, Imai Y. World’s first magnetic resonance imaging/X-ray/operating room suite: a significant milestone in the improvement of neurosurgical diagnosis and treatment. J Neurosurg, 2007; 107: 266–273. [DOI] [PubMed]
- 5).Nimnsky C, Ganslandt O, Fahlbusch R. 1.5-T intraoperative imaging beyond standard anatomic imaging. Neurosurg Clin N Am, 2005; 16: 185–200. [DOI] [PubMed]
- 6).Oh DS, Black PM. A low-field intraoperative MRI system for glioma surgery: is it worthwhile? Neurosurg Clin N Am, 2005; 16: 105–114. [DOI] [PubMed]
- 7).Schulder M, Catrambone J, Carmel PW. Intraoperative magnetic resonance imaging at 0.12 T. Is it enough? Neurosurg Clin N Am, 2005; 16: 143–154. [DOI] [PubMed]
- 8).Bello L, Gambini A, Castellano A. Motor and language DTI fiber tracking combied with intraoperative subcortical mapping for surgical removal of gliomas. Neuro-image, 2008; 39: 369–382. [DOI] [PubMed]
- 9).Bermann JI, Berger MS, Chung SW. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg, 2007; 107: 488–494. [DOI] [PubMed]
- 10).Roessler K, Gatterbauer B, Becherer A. Surgical target selection in cerebral glioma surgery: linking methionine (MET) PET image fusion and neuronvigation. Minim Invasive Neurosurg, 2007; 50: 273–280. [DOI] [PubMed]
- 11).Tanaka Y, Nariai T, Momose T. Glioma surgery using a multimodal navigation system with integrated metabolic images. J Neurosurg, 2009; 110: 163–172. [DOI] [PubMed]