Abstract
Varicella-zoster virus (VZV) disseminates in the body in peripheral blood mononuclear cells during chickenpox. Up to 1 in 10,000 mononuclear cells are infected during the viremic phase of the disease. We developed an in vitro system to infect human mononuclear cells with VZV by using umbilical cord blood. In this system, 3 to 4% of T cells were infected with VZV. VZV mutants unable to express certain genes, such as open reading frame 47 (ORF47) or ORF66, were impaired for growth in T cells, while other mutants showed little difference from parental virus. VZV unable to express ORF47 was even more impaired for spread from umbilical cord blood cells to melanoma cells in vitro. Early-passage clinical isolates of VZV infected T cells at a similar rate to the Oka vaccine strain; however, the clinical isolates were more efficient in spreading from infected T cells to melanoma cells. This in vitro system for infecting human T cells with VZV should be useful for identifying cellular and viral proteins that are important for virus replication in T cells and for the spread of virus from T cells to other cells.
Varicella-zoster virus (VZV) is the etiologic agent of chickenpox and zoster. Humans are infected when the virus contacts the mucosa of the upper respiratory tract or conjunctiva. The virus then disseminates in the bloodstream in peripheral blood mononuclear cells (PBMC). Virus is transferred from PBMC to epithelial cells, resulting in infection of the skin and the characteristic rash of varicella (2). Virus also disseminates in PBMC to other organs including the nervous system, which results in a lifelong latent infection.
Using in situ hybridization, it has been estimated that 1 to 10 per 105 PBMC from healthy persons with acute varicella contain VZV DNA or RNA (14). The cells containing VZV nucleic acid were thought to be lymphocytes but could not be identified definitively. VZV has also been cultured from PBMC, including monocytes and lymphocytes, from 5 days before to 1 day after the onset of the rash of varicella (3, 19). VZV was isolated from 1 to 83 per 107 PBMC from healthy children with varicella before the onset of their rash (4). Virus was isolated from T cells in 43% of the patients and from B cells in 33% of the patients.
PCR has been used to detect VZV in PBMC and polymorphonuclear leukocytes during varicella (13, 21). More recent studies have quantitated the PBMC infected with VZV during varicella by using immunofluorescence with monoclonal antibody to glycoprotein E (gE) and confocal microscopy. From 1 to 10 in 105 PBMC expressed VZV gE on their surface (15). About two-thirds of the cells were T cells and one-third were B cells and monocytes.
Previous attempts to infect primary lymphocytes in vitro have had limited success. Infection of primary B cells did not result in detectable VZV replication (14). Only 0.01% of T cells were productively infected with VZV in vitro when assayed by in situ hybridization. We have developed an in vitro system to infect mononuclear cells obtained from human umbilical cord blood with VZV. Human umbilical cord blood cells were chosen because they should not contain VZV-specific cytotoxic T cells and because they are preferred over PBMC for infection by certain other herpesviruses, such as Epstein-Barr virus and human herpesvirus 6 (1, 5). With this system, we show that 3 to 4% of human T cells can be infected and that VZV can spread from these cells to melanoma cells. We have used this system to analyze the ability of specific VZV mutants to infect T cells, and to compare infection of low-passage isolates of VZV with that of the vaccine strain.
MATERIALS AND METHODS
Cells and viruses.
Human melanoma cells (MeWo) were used for growth and preparation of virus stocks. Mononuclear cells were obtained from human cord blood after two successive Ficoll-Paque (Pharmacia Biotech, Uppsula, Sweden) gradients. Recombinant viruses were derived from cosmids corresponding to the Oka vaccine strain of VZV. VZV with stop codons in open reading frame 1 (ORF1) (ROka1S) (7), ORF13 (ROka13S) (6), ORF47 (ROka47S) (11), or ORF66 (ROka66S) (12) or with a deletion in ORF32 (ROka32D) (20) or ORF57 (ROka57D) (9) have been previously described. ROka47SR contains stop codons in ORF47 but has an additional intact copy of ORF47 elsewhere in the genome (16). Vaccine strain Oka was a gift from M. Takahashi. VZV Molly and Emily are low-passage varicella isolates used between passages 4 and 7 in melanoma cells.
Infection of human umbilical cord blood.
Melanoma cells in six-well plates were infected with cells containing 105 PFU of virus per well. At 6 h after infection, 5 × 106 human umbilical cord blood mononuclear cells were added to each well of VZV-infected melanoma cells. The six-well plates were centrifuged for 45 min at 200 × g at 4°C and incubated at 34°C. After 12 h, the plates were centrifuged again and then incubated at 34°C for an additional 12 h. Cord blood mononuclear cells were harvested by gently washing the melanoma cells. Mononuclear cells were pelleted, washed in RPMI, resuspended in RPMI with 10% fetal bovine serum at 7.5 × 105 cells/ml in cell culture flasks, and incubated at 37°C.
Staining of cells for flow cytometry.
VZV-infected cord blood mononuclear cells were stained with monoclonal antibodies on days 4 to 8 after infection. Monoclonal anti-VZV gE antibody (Chemicon, Temecula, Calif.) was labeled with N-hydroxysuccinimide–fluorescein (Pierce, Rockford, Ill.). Murine anti-human CD3 antibody conjugated to phycoerythrin (Becton Dickinson, San Jose, Calif.) and murine anti-VZV gE conjugated to fluorescein were used for flow cytometery. A total of 106 cells were stained with the antibodies and analyzed by flow cytometry using Cell Quest software (Becton Dickinson).
Staining of cells for immunofluorescence.
Approximately 106 cells were fixed on glass slides in methanol-acetone (1:1, vol/vol) at −20°C for 10 min and air dried. The cells were then stained with murine anti-VZV gE followed by rhodamine-labeled anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.), washed extensively, and then stained with fluorescein isothiocyanate-conjugated murine anti-CD3 (Coulter Corp., Miami, Fla.). Cells were also stained with murine anti-VZV ORF62 protein (Chemicon) followed by fluorescein isothiocyanate-labeled anti-mouse antibody (Jackson Immunoresearch Laboratories). VZV-infected and CD3-positive cells were visualized by fluorescence microscopy.
Infectious-focus assay.
A total of 3 × 106 VZV-infected cord blood mononuclear cells in 2 ml were placed in 3-μm-pore-size transwells (Costar Corp., Cambridge, Mass.) over wells of a six-well plate containing confluent melanoma cells. At 7 to 8 days later, the mononuclear cells were removed and the melanoma cells were fixed and stained with crystal violet. The number of infectious foci was graded from 0 (no plaques) to 4+ (maximum number of plaques).
Transwell assay.
To determine if cell-free virus was released from VZV-infected mononuclear cells, 3 × 106 infected mononuclear cells (in 2 ml) were placed in 0.45-μm-pore-size transwells that were then placed in six-well plates containing confluent melanoma cells. At 7 to 8 days later, the melanoma cells were stained with crystal violet.
Growth studies of VZV isolates.
Growth curves of VZV isolates were performed by infecting melanoma cells with VZV-infected cells containing approximately 100 PFU of VZV. At 1, 2, 3, and 4 days after infection, the cells were harvested and serial dilutions were used to inoculate fresh melanoma cells. Plaques were counted 7 days after infection.
RESULTS
VZV ORF47 is required for efficient growth of virus in human T cells in vitro.
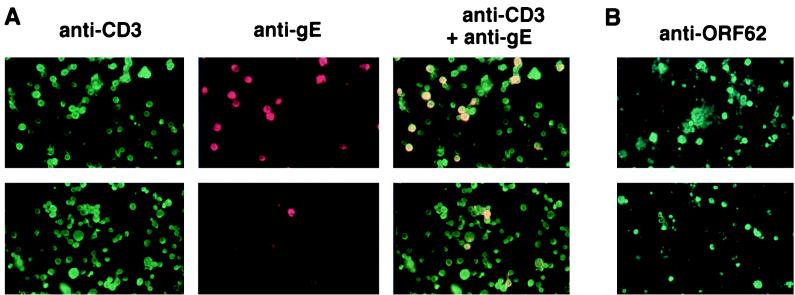
Previous studies (11, 16) showed that while VZV unable to express ORF47 protein kinase grows to similar titers in human fibroblasts and melanoma cells, the ORF47 protein is required for the growth of VZV in human fetal lymphocytes implanted into mice with severe combined immunodeficiency (SCID mice). To verify these results and to analyze additional VZV genes, we developed a system to study infection of human lymphocytes in vitro. Infection of human cord blood mononuclear cells in vitro with recombinant Oka strain VZV (ROka) followed by immunofluorescence microscopy with antibodies to VZV gE and anti-human CD3 showed that nearly all of the infected cells were CD3 positive (Fig. 1A, top panels). In contrast, when the same titer of VZV unable to express ORF47 (ROka47S) was used, only a few cells expressed VZV gE, and these were also CD3 positive (Fig. 1A, bottom panels).
FIG. 1.
Immunofluorescence microscopy of umbilical cord mononuclear cells infected with ROka and ROka47S VZV. (A) Mononuclear cells were infected with ROka (top panels) and ROka47S (bottom panels) and then incubated with murine antibody to VZV gE followed by rhodamine-labeled anti-mouse antibody and then fluorescein isothiocyanate-labeled anti-human CD3 antibody. Panels (left to right) show cells staining for CD3 (green), VZV gE (red), and both CD3 and VZV gE (yellow). (B) Mononuclear cells infected with VZV ROka (top panel) and ROka47S (bottom panel) were incubated with murine antibody to VZV ORF62 protein followed by fluorescein isothiocyanate-labeled anti-mouse antibody.
To further study the ability of ORF47 mutant virus to infect human cord blood mononuclear cells, we performed immunofluorescence assays with antibody to ORF62 protein, which is encoded by an immediate-early gene. Infection of cells with the ORF47 mutant virus resulted in only a slightly reduced number of infected cord blood mononuclear cells compared with infection by the parental (ROka) virus (Fig. 1B). Therefore, at least one of the blocks in virus replication with the ORF47 mutant virus in these cells occurs between immediate-early- and late-gene expression.
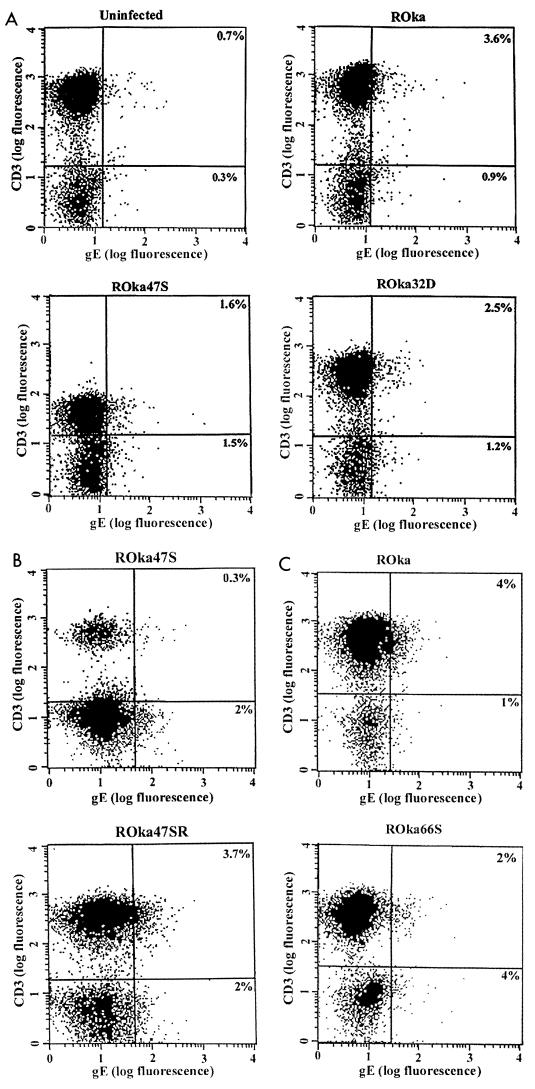
To quantify the VZV-infected T cells, human umbilical cord blood mononuclear cells were infected with cell-associated virus. At 4 to 8 days after infection, the live cells were stained with monoclonal antibodies to VZV gE and human CD3 and analyzed by flow cytometry. Approximately 3 to 4% of cord blood T cells were found to be infected with VZV ROka (Fig. 2A and 3). Most of the VZV ROka-infected cells were CD3 positive (upper right quadrant in Fig. 2A), and only about 1% were CD3 negative (lower right quadrant). Little or no difference was observed in the rate of infection of the cord blood mononuclear cells over an 8-day period of cultivation (W. Soong, J. Schultz, A. Patera, and J. I. Cohen, unpublished data); thereafter the viability of the cells diminished and infection could not be quantified.
FIG. 2.
Flow cytometry of umbilical cord blood T cells infected with VZV ROka, ROka47S, ROka47SR, ROka66S, and ROka32D. The cells were incubated with anti-VZV gE antibody (x axis) and anti-human CD3 (y axis). The percentage of VZV-infected T (CD3) cells is shown in the upper right quadrant, while the percentage of virus-infected non-T cells is shown in the lower right quadrant. Results from three independent experiments (A, B, and C) are shown.
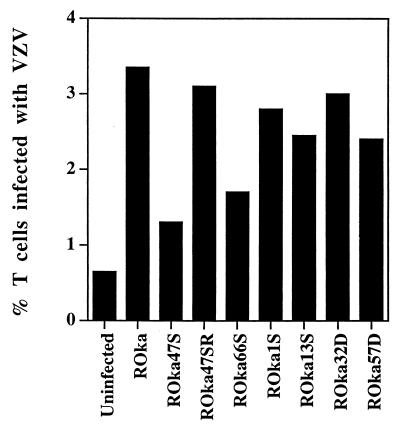
FIG. 3.
Percentage of human T cells infected with VZV mutants determined by flow cytometry. Cells infected with VZV mutants were stained with antibodies as described in the legend to Fig. 2, and the median percentage of VZV-infected T cells was determined. Each median value was derived from at least five independent experiments with samples from different cord blood donors, except VZV ROka1S, which was determined from three experiments.
Infection of human umbilical cord blood mononuclear cells with VZV unable to express ORF47 (ROka47S) resulted in less than 2% of T cells expressing VZV gE (Fig. 2A and B). Using data from 16 separate experiments, the median percentage of T cells infected with ROka47S and displaying gE (1.3%) was significantly lower than the percentage of T cells infected with ROka (3.4%) (P < 0.001, Wilcoxon two-sample test).
To verify that the impairment in infection with ROka47S was due to stop codons in the ORF47 gene and not due to a mutation that occurred elsewhere in the genome during construction of the virus, we infected cord blood mononuclear cells with another virus, ROka47SR, in which an additional intact copy of ORF47 was inserted elsewhere into the genome of ROka47S (16). The median percentage of T cells infected with ROka47SR (3.1%) was not significantly different from the percentage infected with ROka (3.4%), indicating that the reduced ability of ROka47S to replicate in T cells is due to the lack of expression of ORF47 (Fig. 2B and 3).
VZV unable to express the ORF66 putative protein kinase (ROka66S) has an intermediate growth phenotype in human fetal lymphocytes in the SCID-hu mouse (16). Similarly, the median percentage of T cells infected with ROka66S and expressing VZV gE (1.7%) was intermediate between those of ROka and ROka47S (Fig. 2C and 3). We next determined whether VZV unable to express selected other genes could infect cord blood mononuclear cells. VZV ORF1, ORF13, ORF32, and ORF57 do not have homologs in herpes simplex virus type 1 (HSV-1). VZV mutants unable to express each of these viral genes infected and expressed gE on 2.4 to 3% of human T cells (Fig. 2A and 3).
VZV-infected mononuclear cells can transfer infectious virus to melanoma cells.
The above studies measured the ability of VZV to infect human cord blood mononuclear cells and to express gE on their surface; however, they did not show that infectious virus could be released from the cells. To determine if mononuclear cells can release virus in vitro, we used an infectious-focus assay. VZV-infected mononuclear cells were cultured in vitro for 2 days, washed, and then incubated with monolayers of human melanoma cells for 7 to 8 days. The number of infectious foci in the melanoma cells was graded on a scale from 0 to 4+. Each VZV mutant was assayed on at least three separate days with samples from different cord blood donors each day.
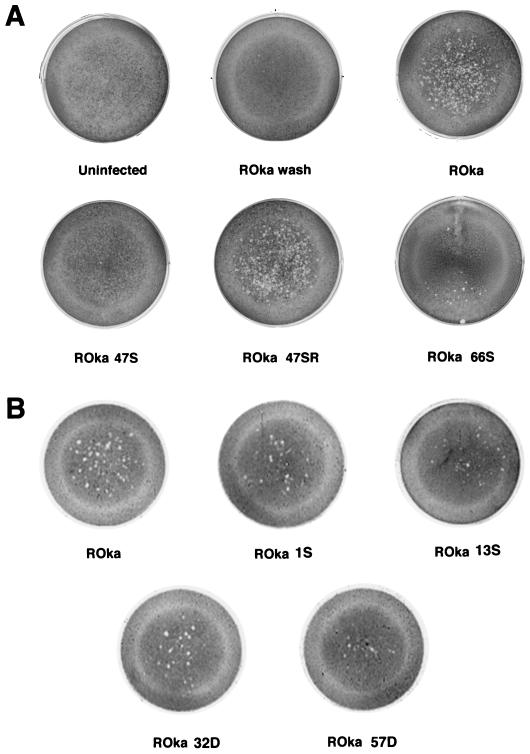
Melanoma cells incubated with 2 ml of VZV ROka-infected cord blood mononuclear cells gave a score of 3+, indicating that VZV-infected mononuclear cells were able to transfer virus to cells in the monolayer (Fig. 4A). Three control experiments were performed to verify that this effect was due to the transfer of virus from mononuclear cells. First, uninfected mononuclear cells were placed in contact with melanoma cells, and no plaques were observed. Second, medium washed from VZV ROka-infected melanoma cells that had been used to infect the mononuclear cells was also used to infect fresh melanoma cells. No infection of the melanoma cells was seen (Fig. 4A, ROka wash). Third, ROka-infected mononuclear cells were placed in the upper well of a 0.45-μm transwell that was placed above a monolayer of melanoma cells. No infection of the melanoma cells was detected (Soong et al., unpublished data), indicating that cell-to-cell contact was required for infection. Thus, the infectious-focus assay measures the ability of mononuclear cells to transfer infectious virus directly to melanoma cells.
FIG. 4.
The infectious-focus assay detects transfer of VZV mutants from umbilical cord mononuclear cells to melanoma cells. VZV-infected human mononuclear cells were incubated with melanoma cells, and 7 to 8 days later the melanoma cells were fixed and stained with crystal violet. Results from two separate experiments (A and B) are shown.
Melanoma cells incubated with 2 ml of ROka47S-infected mononuclear cells gave a score of 0, indicating that little or no virus had been transferred from these cells to melanoma cells in the monolayer (Fig. 4A). In contrast, incubation of the melanoma cells with 2 ml of ROka47SR-infected mononuclear cells gave a score of 3+, indicating that the low infection rate and inability to transfer virus with ROka47S was due to lack of expression of ORF47 and not to a mutation elsewhere in the genome. To verify these results, we incubated melanoma cells with serial dilutions of VZV ROka- or ROka47S-infected mononuclear cells. In three experiments, a mean of 129 ± 6.2 (standard error) plaques were present in melanoma cells incubated with 0.25 ml of ROka-infected mononuclear cells compared with a mean of only 2 ± 0.9 plaques in melanoma cells incubated with 4 ml of ROka47S-infected mononuclear cells. This represents approximately a 1,000-fold decrease in the number of plaques for ROka47S-infected compared with ROka-infected cells. Thus, while ROka47S is partially impaired for infection of mononuclear cells (see above), it is even more impaired for spread of virus from mononuclear to melanoma cells.
Melanoma cells incubated with ROka66S-infected mononuclear cells gave a score of 1+. Melanoma cells incubated with mononuclear cells that were infected with VZV mutants with mutations in ORF1, ORF13, ORF32, or ORF57 gave a score of 1 to 2+ (Fig. 4B). These results indicate that while ORF47 is critical for infection and spread of VZV from infected human mononuclear cells to melanoma cells in vitro, several other ORFs are dispensable for spread of the virus from mononuclear cells.
Low-passage clinical isolates of VZV infect human T cells at a similar rate to the vaccine strain but are more efficient for spread of virus from mononuclear cells.
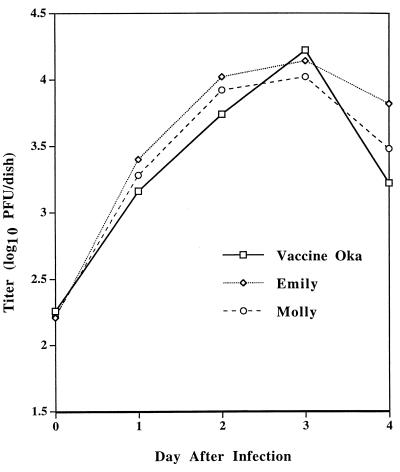
While the Oka vaccine strain is not impaired for growth in human fetal T cells in the SCID-hu mouse, it is partially impaired for growth in fetal human skin compared to low-passage clinical isolates (17). As an initial test to compare the growth of low-passage clinical isolates with the vaccine strain, we measured titers of these viruses in melanoma cells over time. Two low-passage varicella isolates (VZV Molly and Emily) grew to titers similar to those seen with the Oka vaccine strain (Fig. 5).
FIG. 5.
Growth of Oka vaccine virus and early-passage varicella isolates in melanoma cells. Melanoma cells were inoculated with VZV-infected cells, aliquots were harvested on days 1, 2, 3, and 4 after infection, and the titer of virus was determined by plating on melanoma cells. Day 0 indicates the titer of virus in the inocula. The titer (log10 of the mean number of PFU per dish) is indicated on the y axis.
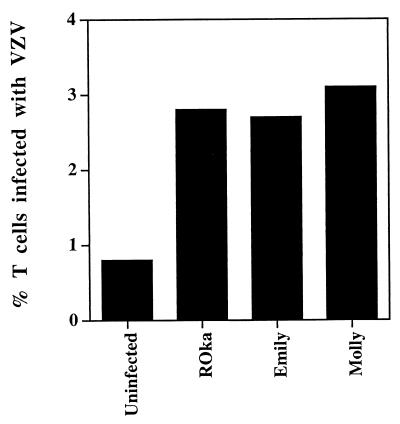
We next compared the ability of the low-passage clinical isolates with the recombinant Oka vaccine virus to infect human umbilical cord blood cells. Flow cytometry showed that about 3% of the cord blood T cells were infected with each of the viruses (Fig. 6). Thus, the vaccine strain was not impaired in its ability to infect human T cells in vitro.
FIG. 6.
Percentage of human T cells infected with VZV ROka and early-passage clinical isolates of VZV. The infected cells were incubated with anti-VZV and anti-human CD3 antibodies (as described in the legend to Fig. 2), and the median percentage of VZV-infected T (CD3) cells was determined. Each median value was derived from at least five independent experiments with samples from different cord blood donors, except VZV Emily, which was determined from three experiments.
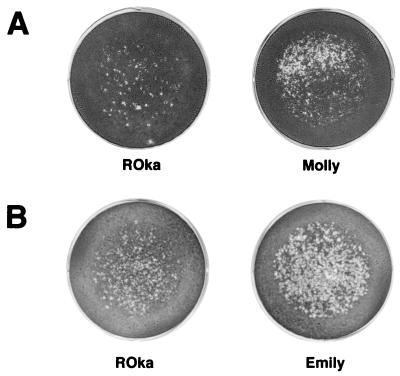
Using the infectious-focus assay, we found that melanoma cells incubated with cord blood mononuclear cells infected with either of two low-passage clinical isolate gave a score of 4+ while melanoma cells incubated with ROka-infected cord blood cells gave a score of 3+ (Fig. 7). In three independent experiments with samples from three different cord blood donors, the mean number of plaques in melanoma cells incubated with ROka-infected cord blood cells (162 plaques) was 1.9-fold lower than the number of plaques in cells incubated with the low-passage clinical isolates (305 plaques). Thus, while the vaccine strain infected T cells at a similar rate to that for the low-passage clinical isolates, the vaccine strain was less efficient in spreading from these cells to melanoma cells.
FIG. 7.
The infectious-focus assay detects increased transfer of infectious virus from human mononuclear cells infected with early-passage clinical isolates (VZV Molly and Emily) compared with VZV ROka. Results from two separate experiments (A and B) are shown.
DISCUSSION
We have shown that human umbilical cord blood mononuclear cells can be infected with VZV and that the majority of the infected cells are T cells. Human fetal lymphocytes have also been infected with VZV after implantation of human fetal thymus and liver into SCID-hu mice (18). Using VZV ROka, the same strain used in the present study, approximately 5% of the human fetal T cells in the mice were VZV positive by flow cytometry (16). This number is similar to the 3 to 4% of VZV-infected cord blood T cells found in our study. Infection of cord blood mononuclear cells allowed us to test the role of individual viral genes for growth of VZV in T cells in vitro. Recombinant VZV unable to express ORF47 was markedly impaired for growth in T cells in vitro. A VZV ORF66 mutant had an intermediate phenotype for growth and virus spread. Similar results with these two mutants were seen previously in the SCID-hu mouse model (16).
While VZV unable to express ORF47 was impaired for growth in T cells, it was even more impaired for cell-to-cell spread. After subtracting out the background staining for VZV gE in uninfected cells from the staining for gE in VZV ROka- or ROka47S-infected cells (Fig. 3), we found that there were about fourfold more CD3 cells expressing gE with VZV ROka (2.8%) than with ROka47S (0.7%). However, when VZV-infected mononuclear cells were incubated with melanoma cells, the differences were more striking, with a 1,000-fold increase in virus spread for VZV ROka-infected mononuclear cells compared with ROka47S-infected cells.
While VZV grows in human fetal lymphocytes in the SCID-hu mouse, HSV grows in human fetal epithelial cells but not lymphocytes in these animals (17). While nearly all of the VZV genes have homologs in the HSV genome, five VZV genes are not present in the HSV genome (8). Therefore, we determined whether four of these genes might be important for the ability of VZV to grow in lymphocytes. Analysis of VZV mutants unable to express these genes, i.e., ORF1, ORF13, ORF32, and ORF57, indicated that they play little or no role in T-cell infection or spread from infected human mononuclear cells to melanoma cells in vitro. Previously we showed that ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase (20). While ORF47 was required for efficient infection of human T cells in vitro, ORF32 was dispensable. Thus, the marked impairment of the ORF47 mutant to grow in T cells was not due to its role in processing of the ORF32 protein.
Our assay differs from the SCID-hu mouse model (18) in several respects. First, we infected only mononuclear cells with the virus. In the SCID-hu model, fetal thymus and liver, which contain other cell types including epithelial cells, are inoculated with virus. Second, we infected cells on the same day that they were isolated from umbilical cord blood without additional culturing; in the animal model the implants mature for 1 to 6 months before inoculation. Third, we used only human cells (mononuclear and melanoma cells), while the SCID-hu model uses human cells (lymphocytes), mouse cells (that encapsulate the implants), and simian cells (for the infectious-focus assay).
While prior experiments with the SCID-hu mouse indicated that human skin implants release cell-free virus and suggested that human lymphocytes released cell-free VZV (18), we did not observe the release of cell-free virus, as evidenced by our failure to detect virus in the supernatant passing through a 0.45-μm-pore-size transwell. The prior studies used a 3-μm-pore-size transwell to detect the release of virus from infected lymphocytes; however, we found that human umbilical cord blood mononuclear cells could readily pass through this size of transwell.
Low-passage varicella isolates and the Oka vaccine strain of VZV infected human T cells at similar rates; however, the low-passage isolates spread to uninfected melanoma cells more efficiently. In contrast, comparison of the growth of the low-passage clinical isolates and the vaccine strain in melanoma cells showed no differences in the titers of these viruses over time. Prior studies have shown that the Oka vaccine strain can be distinguished from wild-type isolates by growth at different temperatures and by growth in guinea pig embryo fibroblasts compared to human embryo fibroblasts (10). Thus, we have developed an additional in vitro assay, measuring the spread of virus from T cells to melanoma cells, that distinguishes the Oka vaccine strain from clinical isolates.
In summary, we have developed a system to rapidly test the growth of VZV mutants in primary human mononuclear cells in vitro. This method gives similar results to those seen in the SCID-hu mouse but without the need for using animals and waiting for thymus and liver implants to mature. Future candidate VZV vaccine viruses could be tested in this system to determine if the virus can replicate in lymphocytes and spread from these cells to other cells. Since VZV is transferred from PBMC to epithelial cells during acute varicella, our in vitro model of virus transfer should be helpful in identifying viral and cellular genes that are critical in this process.
ACKNOWLEDGMENTS
Weily Soong and Julie Schultz contributed equally to this work.
We thank Molly Cohen and Emily Heineman for contributing viruses to this study, Claire Hallahan for statistical analysis, and Stephen E. Straus for reviewing the manuscript.
Julie Schultz and Weily Soong are Howard Hughes Medical Institute-National Institutes of Health Research Scholars.
REFERENCES
- 1.Andiman W A. Epstein Barr virus. In: Schmidt N J, Emmons R W, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. Washington, D.C.: American Public Health Association; 1989. pp. 407–452. [Google Scholar]
- 2.Arvin A, Moffat J F, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res. 1996;46:263–309. doi: 10.1016/s0065-3527(08)60074-3. [DOI] [PubMed] [Google Scholar]
- 3.Asano Y, Itakura N, Hiroshi Y, Hirose S, Osaki T, Yazaki T, Yamanishi K, Takahashi M. Viremia is present in incubation period in nonimmunocompromised children with varicella. J Pediatr. 1985;106:69–71. doi: 10.1016/s0022-3476(85)80468-6. [DOI] [PubMed] [Google Scholar]
- 4.Asano Y, Itakura N, Kajita Y, Suga S, Yoshikawa T, Yazaki T, Ozaki T, Yamanishi K, Takahashi M. Severity of viremia and clinical findings in children with varicella. J Infect Dis. 1990;161:1095–1098. doi: 10.1093/infdis/161.6.1095. [DOI] [PubMed] [Google Scholar]
- 5.Black J B, Sanderlin K C, Goldsmith C S, Gary H E, Lopez C, Pellett P E. Growth properties of human herpesvirus-6 strain Z29. J Virol Methods. 1989;26:133–146. doi: 10.1016/0166-0934(89)90143-2. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J I, Seidel K E. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J I, Seidel K E. Varicella-zoster virus open reading frame 1 encodes a membrane protein that is dispensable for growth of VZV in vitro. Virology. 1995;206:835–842. doi: 10.1006/viro.1995.1006. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J I, Straus S E. Varicella-zoster virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2525–2545. [Google Scholar]
- 9.Cox E, Reddy S, Iofin I, Cohen J I. Varicella-zoster virus ORF57, unlike its pseudorabies virus UL3.5 homolog, is dispensable for viral replication in cell culture. Virology. 1998;250:205–209. doi: 10.1006/viro.1998.9349. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa Y, Torigoe S, Shiraki K, Yamanishi K, Takahashi M. Biologic and biophysical markers of a live varicella vaccine strain (Oka): identification of clinical isolates from vaccine recipients. J Infect Dis. 1984;149:956–963. doi: 10.1093/infdis/149.6.956. [DOI] [PubMed] [Google Scholar]
- 11.Heineman T C, Cohen J I. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J Virol. 1995;69:7367–7370. doi: 10.1128/jvi.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heineman T C, Seidel K E, Cohen J I. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J Virol. 1996;70:7312–7317. doi: 10.1128/jvi.70.10.7312-7317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koropchak C M, Graham G, Palmer J, Winsberg M, Ting S F, Wallace M, Prober C G, Arvin A M. Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis. 1991;163:1016–1022. doi: 10.1093/infdis/163.5.1016. [DOI] [PubMed] [Google Scholar]
- 14.Koropchak C M, Solem S M, Diaz P S, Arvin A M. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mainka C, Fub B, Geiger H, Hofelmayr H, Wolff M H. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:91–98. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Moffat J F, Zerboni L, Sommer M H, Heineman T C, Cohen J I, Kaneshima H, Arvin A M. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffat J F, Zerboni L, Kinchington P R, Grose C, Kaneshima H, Arvin A M. Attenuation of vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffat J F, Stein M D, Kaneshima H, Arvin A M. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaki T, Ichikawa T, Matsui Y, Kondo H, Nagai T, Asano Y, Yamanishi K, Takahashi M. Lymphocyte-associated viremia in varicella. J Med Virol. 1986;19:249–253. doi: 10.1002/jmv.1890190307. [DOI] [PubMed] [Google Scholar]
- 20.Reddy S M, Cox E, Iofin I, Soong W, Cohen J I. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J Virol. 1998;72:8083–8088. doi: 10.1128/jvi.72.10.8083-8088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawyer M H, Wu Y N, Chamberlin C J, Burgos C, Brodine S K, Bowler W A, LaRocca A, Oldfield E O, Wallace M R. Detection of varicella-zoster virus DNA in oropharynx and blood of patients with varicella. J Infect Dis. 1992;166:885–888. doi: 10.1093/infdis/166.4.885. [DOI] [PubMed] [Google Scholar]