Abstract
Background:
Maternal exposure to environmental chemicals can cause adverse health effects in offspring. Mounting evidence supports that these effects are influenced, at least in part, by epigenetic modifications. It is unknown whether epigenetic changes in surrogate tissues such as the blood are reflective of similar changes in target tissues such as cortex or liver.
Objective:
We examined tissue- and sex-specific changes in DNA methylation (DNAm) associated with human-relevant lead (Pb) and di(2-ethylhexyl) phthalate (DEHP) exposure during perinatal development in cerebral cortex, blood, and liver.
Methods:
Female mice were exposed to human relevant doses of either Pb () via drinking water or DEHP () via chow for 2 weeks prior to mating through offspring weaning. Whole genome bisulfite sequencing (WGBS) was utilized to examine DNAm changes in offspring cortex, blood, and liver at 5 months of age. Metilene and methylSig were used to identify differentially methylated regions (DMRs). Annotatr and ChIP-enrich were used for genomic annotations and gene set enrichment tests of DMRs, respectively.
Results:
The cortex contained the majority of DMRs associated with Pb (66%) and DEHP (57%) exposure. The cortex also contained the greatest degree of overlap in DMR signatures between sexes ( and 8 DMRs with Pb and DEHP exposure, respectively) and exposure types ( and 39 DMRs in males and females, respectively). In all tissues, detected DMRs were preferentially found at genomic regions associated with gene expression regulation (e.g., CpG islands and shores, 5′ UTRs, promoters, and exons). An analysis of GO terms associated with DMR-containing genes identified imprinted genes to be impacted by both Pb and DEHP exposure. Of these, Gnas and Grb10 contained DMRs across tissues, sexes, and exposures, with some signatures replicated between target and surrogate tissues. DMRs were enriched in the imprinting control regions (ICRs) of Gnas and Grb10, and we again observed a replication of DMR signatures between blood and target tissues. Specifically, we observed hypermethylation of the Grb10 ICR in both blood and liver of Pb-exposed male animals.
Conclusions:
These data provide preliminary evidence that imprinted genes may be viable candidates in the search for epigenetic biomarkers of toxicant exposure in target tissues. Additional research is needed on allele- and developmental stage–specific effects, as well as whether other imprinted genes provide additional examples of this relationship. https://doi.org/10.1289/EHP14074
Introduction
The health impacts of toxicant exposures during early life, such as lead (Pb) and phthalates [e.g., di(2-ethylhexyl) phthalate (DEHP)] can be framed within the Developmental Origins of Health and Disease (DOHaD) hypothesis.1 This hypothesis postulates that exposures during sensitive periods of development alter an organism’s normal developmental programming, triggering a myriad of effects on growth and maturation that can persist into adulthood. Developmental exposures can impact gene expression long term by altering the epigenome, which can have significant repercussions for health and disease.2 Epigenetics refers to mitotically heritable and potentially reversible mechanisms modulating gene expression that are independent of the DNA sequence,3 with the most abundantly studied mechanism being DNA methylation (DNAm). DNAm entails the addition of a methyl group to the fifth position of cytosine base adjacent to a guanine (CpG, in the majority of cases), generating what are commonly referred to as methylated cytosines (), by DNA methyltransferases (DNMTs).4 Increased levels of within promoters and enhancers are typically associated with decreased transcription factor binding and subsequent decreases in gene expression.5 Patterns of undergo waves of reprogramming (i.e., global demethylation and remethylation) during critical windows of in utero development, making these periods susceptible targets of developmental exposures.6
One prominent class of genes regulated by DNAm is imprinted genes. Imprinted genes are expressed in a mono-allelic and parent-of-origin-specific manner.7 These genes have important functions in growth and development, and their precise epigenetic regulation is critical for healthy and viable offspring.8,9 Imprinted genes are typically found in clusters in the genome, which are regulated by a differentially methylated imprinting control region (ICR). A classic example of an imprinted locus is the Igf2-H19 cluster, where H19 is expressed from the maternal allele, Igf2 is expressed from the paternal allele, and they are regulated by a ICR that resides between the genes. The ICR is methylated on the paternal allele and binds to the insulator protein CTCF on the maternal allele. This differential epigenetic regulation mediates the appropriate imprinting of the cluster, with dysregulated DNAm associated with human imprinting disorders.10 Notably, other imprinted clusters harbor ICRs, although the location and functions vary, including cases where the unmethylated ICRs serve as promoters for long noncoding RNAs.11 DNAm patterns at ICRs are typically established in the gametes and are maintained through fertilization and the extensive epigenetic reprogramming events that occur in the embryo.12 Because of this requirement for DNAm maintenance at these reprogramming times, gestational periods are particularly sensitive to environmental exposures. Environmentally induced disruption of epigenetic processes during early development have been associated with changes in imprinted gene regulation13 and adverse health outcomes.14
A variety of environmental exposures, including Pb15 and DEHP,16 have been associated with altered patterns of DNAm in humans and mice. Pb is a known neurotoxicant, with developmental exposures linked to neurological damage and cognition deficits in early life, as well as with increased risk of degenerative neurological disease in adulthood.15 Although blood lead levels (BLLs) within the US population have fallen dramatically, nearly 94% between 1976–1980 and 2015–2016, there is still concern regarding chronic low levels of Pb exposure.17 This concern is especially true for early life exposures, as the developing brain and other organ systems are particularly susceptible to the toxic effects of Pb.18 Common sources of Pb exposure continue to be contaminated drinking water from leaded pipes19 as well as dust and chipping paint in older homes.20 Exposure to DEHP, a phthalate commonly used as a plasticizer, has become ubiquitous, with most US adults having detectable levels of DEHP metabolites in their urine.21 DEHP is a known endocrine disruptor, with developmental exposures associated with altered metabolic function.16 Common routes of DEHP exposure include personal care products, food and beverage containers, and medical equipment, making gestational and developmental exposures common.22 Despite great progress over the years, gaps in knowledge remain as to whether perinatal Pb or DEHP exposure–mediated changes in DNAm have implications for the status of the epigenome, whether there are sex-specific effects, and if these changes are conserved among tissues.
As a part of the Toxicant Exposures and Responses by Genomic and Epigenomic Regulators of Transcription (TaRGET II) Consortium,23 we used a mouse model of human-relevant perinatal Pb and DEHP exposures to investigate genome-wide tissue- and sex-specific associations with changes in DNAm. Whole genome bisulfite sequencing (WGBS) quantified DNAm changes in blood (an easily accessible and therefore considered a “surrogate” tissue) as well as cortex and liver (two tissues often difficult to access, representing “target” tissues) collected from male and female 5-month-old mice, with and without perinatal Pb or DEHP exposures. We assessed whether perinatal Pb- or DEHP-exposed mice displayed changes in DNAm across the genome and identified imprinted genes as a relevant gene class common to these two exposures. It is a long-standing goal of many in toxicology and public health to identify biomarkers of exposures, because it would greatly add to the interpretation of epidemiological findings, which often have access only to surrogate tissues.24 As such, the objective of this work was to examine whether toxicant-induced changes in the epigenome of blood were replicated in the tissues of the cortex and liver, two commonly studied organs that are nearly always inaccessible in epidemiological work.
Methods
Animal Exposure Paradigm and Tissue Collection
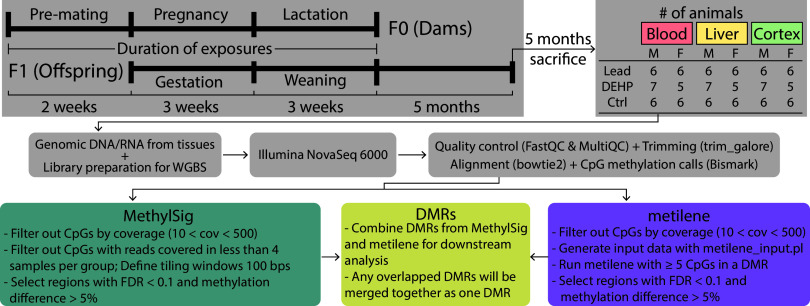
Wild-type nonagouti a/a mice were obtained from an over 230-generation long-term breeding colony of viable yellow agouti () mice,25 which are genetically invariant and 93% identical to the C57BL/6J strain.26,27 Virgin a/a females (6–8 wk old) were randomly assigned to control, Pb-acetate water, or DEHP chow 2 wk prior to mating with virgin a/a males (7–9 wk old). Pb exposure and DEHP exposure were conducted ad libitum via distilled drinking water mixed with Pb-acetate or 7% corn oil chow mixed with DEHP. The Pb-acetate concentration was set as to model human relevant perinatal exposure. We have previously demonstrated that Pb delivered via drinking water results in maternal BLLs of () in mice28 and represents an environmentally relevant dose that, when detected in pregnant humans, would initiate treatment for a high-risk pregnancy because of Pb toxicity.29 DEHP was dissolved in corn oil from Envigo to create a customized stock solution, to produce 7% corn oil chow for experimentation. The DEHP exposure level was selected based on a target maternal dose of and assumes that a pregnant and nursing female mouse weighs and ingests roughly of chow per day. This target dose was selected because previous literature demonstrated obesity-related phenotypes in offspring exposed to DEHP during early development,30 and this dosage falls within the range of exposures previously documented in humans.31 All animals were maintained on a phytoestrogen-free modified AIN-93 G diet (Td.95092, 7% corn oil diet; Envigo) while housed in polycarbonate-free cages. Animal exposure to Pb or DEHP continued through gestation and lactation until weaning at postnatal day 21 (PND21), when pups were switched to Pb-free drinking water or DEHP-free chow, respectively. We defined this exposure period as perinatal, as described by the National Institute of Environmental Health Sciences (NIEHS) TaRGET II Consortium,23 with exposure in the offspring occurring 2 wk prior to mating and through the time of conception, gestation, and the first few weeks of life. Offspring were maintained until 5 months of age. This study included six Pb-exposed, seven DEHP-exposed, and six control litters, with one male and one female used from each litter in each group, except for the DEHP-exposed litters, wherein one male was used from each of the seven litters, but only five litters produced females for use in this study. This selection process resulted in a total animal sample size of . Blood, liver, and cortex tissues were collected from all animals, for a final sample size of (Figure 1). All animals and collected tissues were included in subsequent analyses, with no exclusions necessary. Prior to euthanasia, mice were fasted for 4 h during the light cycle beginning in the morning, with euthanasia and tissue collection occurring in the afternoon. Tissue processing followed previously published protocols, with no necessary deviations.32 Immediately following mouse euthanasia with asphyxiation, blood was collected through cardiac puncture, followed by dissection of the cortex and liver, which were immediately flash frozen in liquid nitrogen and stored at . Blood was collected in EDTA-coated tubes (Cat. no. 365974; Fisher), and plasma was removed following centrifugation for 10 min at and 4°C. Red blood cells were lysed (erythrocyte lysis solution; Cat. no. 79217; Qiagen), and the remaining white blood cells were washed in PBS and resuspended in Buffer RLT (Cat. no. 79216; Qiagen) for later use in DNA extraction. Animal collection was standardized to between 1300 hours to 1500 hours (1 P.M. to 3 P.M.), and collection order was randomized daily. For each mouse, one investigator (K.N.) administered the treatment and was therefore aware of the treatment group allocation. All investigators completing subsequent molecular assays were blinded to treatment group, until treatment group was analyzed during bioinformatic analyses. All mouse procedures were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC) to ensure animals were treated humanely and with respect. All experiments were conducted according to experimental procedures outlined by the NIEHS TaRGET II Consortium.23 In drafting this manuscript, ARRIVE reporting guidelines were used to ensure quality and transparency of reported work.33
Figure 1.
Overview of experimental workflow. F0 generation females (6–8 wk of age) were exposed to either of Pb via drinking water or of DEHP via food, beginning 2 wk prior to mating using virgin males (8–10 wk of age). Exposure to Pb or DEHP or control continued through gestation and weaning, when F1 mice () were removed from the dams and placed on control water or chow. At 5 months of age, F1 mice were sacrificed, and genomic DNA was extracted from blood, liver, and cortex tissues (). DNA was used to prepare libraries for WGBS. Following initial data processing, DMRs were called using MethylSig and metilene. Note: DEHP, di(2-ethylhexyl) phthalate; DMR, differentially methylated region; Pb, lead; WGBS, whole genome bisulfite sequencing.
DNA Extraction and Whole Genome Bisulfite Sequencing
DNA extraction was performed using the AllPrep DNA/RNA/miRNA Universal Kit (Cat. no. 80224; Qiagen). Genomic DNA (gDNA) was used in the preparation of WGBS libraries at the University of Michigan Epigenomics Core. gDNA was quantified using the Qubit BR dsDNA kit (Cat. no. Q32850; Fisher) and quality assessed using Agilent’s Genomic DNA TapeStation Kit (Cat. no. A63880; Agilent). One WGBS library was prepared for each animal () and identified with a unique dual index identifier for deconvolution. For each sample, of gDNA was spiked with 0.5% of unmethylated lambda DNA (Cat. no. D1521; Promega) and sheared using a Covaris S220 (10% Duty Factor, 140W Peak Incident Power, 200 Cycle/Burst, 55s). A aliquot of processed gDNA was taken to assess shearing using an Agilent High Sensitivity D1000 Kit (Cat. no. G2991AA; Agilent). Once shearing was assessed, the remaining gDNA was concentrated using a Qiagen PCR Purification column and processed for end-repair and A-tailing. Ligation of cytosine-methylated adapters was done overnight at 16°C. Following this, ligation products were cleaned using AMPure XP Beads (Cat. no. NC9933872; Fisher) before processing for bisulfite conversion using the Zymo EZ DNA Methylation Kit (Cat. no. D5001; Zymo), and by amplifying the bisulfite converted products over 55 cycles of 95°C for 30 s followed by 55°C for 15 min, according to the manufacturer’s guidelines. After cleanup of the bisulfite converted products, final libraries were amplified over 10 cycles by polymerase chain reaction (PCR) using KAPA Uracil+ Ready Mix (Cat. no. 501965287; Fisher) and NEB dual indexing primers. Final libraries were cleaned with AMPure XP beads, concentration assessed using the Qubit BR dsDNA Kit and library size assessed on the Agilent High Sensitivity D1000 TapeStation Kit. Prior to pooling, each library was quantified using KAPA Library Quantification Kit (Cat. no. 501965234; Fisher). We constructed two pools of 18 libraries, combining a mix of individuals from different litters, sexes, and exposures, to minimize batch effects, and each pool was sequenced on an Illumina NovaSeq6000 S4 200 cycle flow cell (PE-100) at the University of Michigan Advanced Genomics Core. Unless otherwise stated, all enzymes used in library generation were purchased from New England Biolabs. Adapters with universally methylated cytosines were synthesized by Integrated DNA Technologies (IDT).
Data Processing, Quality Control, and Differential DNA Methylation Analysis
FastQC (version 0.11.5; Babraham Bioinformatics, Babraham Institute) and MultiQC34 (version 1.8) were used to assess the quality of all sequenced samples. Sequencing adapters and low-quality bases were removed by Trim Galore (version 0.4.5; Babraham Bioinformatics, Babraham Institute). After trimming, reads shorter than were removed from further analysis. Bismark35 (version 0.19.0) with Bowtie 236 (version 2.3.4) as backend alignment software were used for read alignment and methylation calling, with Genome Reference Consortium Mouse Build 38 (mm10) as the reference genome. All alignments were performed with zero mismatches and multiseed length of . The bisulfite conversion rates were calculated through the unmethylated lambda phage DNA spike-ins. Metilene37 (version 0.2.8) and R Bioconductor package methylSig38 (version 1.4.0) were used to identify the differentially methylated regions (DMRs) independently. CpG sites with reads or >500 reads were excluded from DMR detection. For methylSig, CpG sites that had reads covered in fewer than four samples within a treatment group were filtered out for DMR identification. Tiling windows were used with methylSig to identify DMRs, with a window size of . For metilene, DMRs were identified de novo with at least five CpGs in a single DMR. For both methods, a false discovery rate (FDR) cutoff of and a DNAm difference of were applied to select significant DMRs. Results were filtered by adjusted -value to correct for multiple comparisons. Given the difference in methods of DMR detection between MethylSig and metilene, both were employed in this study to ensure robust detection of DMRs in the data. All overlapping DMRs from methylSig and metilene were confirmed to be in the same direction and merged for downstream analysis (Excel Table S1). A minimum overlap cutoff of was applied to identify overlapping DMRs between tissues, sexes, and exposures, based on DMR coordinates, with no specification of methylation change direction considered for the purposes of initial comparisons. The annotatr Bioconductor package39 was used to annotate all significant DMRs associated with genes and genomic locations, including CpG islands, CpG shores, CpG shelves, promoters, exons, introns, 5′ UTRs, 3′ UTRs, enhancers, and regions upstream of transcription start sites (TSSs). Random genomic regions were generated and annotated with annotatr for each tissue using the mm10 reference genome. These random regions were used as background information to show the distribution of the genomic annotation of the DMRs if distributed purely by chance. An overview of the complete methods is illustrated in Figure 1.
Gene Set Enrichment Test
R Bioconductor package ChIP-enrich40 (version 2.16.0) was used to perform gene set enrichment testing of gene ontology (GO) terms enriched with significant DMRs. Twelve analyses were performed stratified by each tissue and sex (i.e., male cortex, male blood, male liver, female cortex, female blood, and female liver) across each exposure group (i.e., Pb, DEHP, and control). Gene assignments were determined with the nearest_tss locus definition in the chipenrich function to find all three categories of ontology [i.e., biological process (BP), cellular component (CC), and molecular function (MF)]. An FDR cutoff of was applied for selecting significantly enriched GO terms. GO terms containing fewer than 15 genes or more than 500 genes were removed from analysis.
Mouse Imprinted Genes and Imprinted Control Regions
DMRs were compared to mouse imprinted genes and ICRs. We compiled a reference list of imprinted genes (Excel Table S2) using previously documented efforts9,41,42 and obtained ICR coordinates from Wang et al.43 The valr R package44 (version 0.6.4) was used to identify overlapping regions between the DMRs and ICRs. A binomial test was used to assess whether the DMRs were significantly enriched in ICRs, and an adjusted -value cutoff was used for identifying significant results.
Results
DMRs among Perinatally Pb- and DEHP-Exposed Tissues
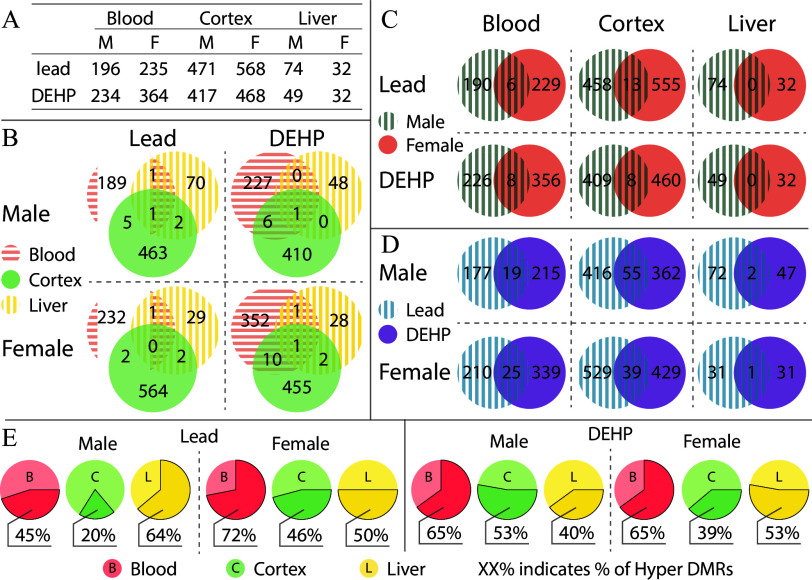
Among Pb-exposed tissues, the majority of the DMRs were detected in the cortex [, ], followed by blood (, ), and liver (, ). A similar pattern was observed in DEHP-exposed tissues, with the majority of DMRs detected in the cortex (, ), followed by blood (, ), and liver (, ) (Figure 2A). Excel Table S1 provides an overview of the overlap and convergence of DMRs detected by MethylSig and metilene. Excel Tables S3–S14 provide a summary of all DMRs detected in this analysis in each tissue, sex, and exposure combination. There was limited overlap in DMRs between each tissue type, relative to the total number detected in each tissue and sex (Figure 2B). For instance, Pb-exposed animals had only a few DMRs appear in multiple tissues. Males had one DMR common among all three tissues, with six DMRs overlapping between cortex and blood, three between cortex and liver, and two between liver and blood. Females had two common DMRs between cortex and blood, two between cortex and liver, and one between liver and blood, with no DMRs detected in all three tissues. Similar patterns were present in DEHP-exposed animals, wherein males had one DMR common to all three tissues and seven detected in cortex and blood, and there was no additional overlap among the remaining tissue pairs. DEHP-exposed females had more overlapping DMRs in comparison with males, with one DMR common to all tissues, 11 in both cortex and blood, 3 in cortex and liver, and 2 in liver and blood (Figure 2B).
Figure 2.
Summary of detected DMRs. (A) DMRs were categorized by tissue (blood, cortex, and liver), sex (F: female, M: male), and exposure group (Pb, DEHP, and control) with a methylation difference of and an FDR cutoff of using MethylSig and metilene, and (B) DMRs found in more than one tissue type were further categorized by sex and exposure. DMRs shared by both sexes (C) and by exposure group (D) were quantified and broken down by tissue type. Proportions of DMR directional changes were generally summarized for each tissue–sex–exposure combination, with %XX designating the percent of DMRs that were hypermethylated (E). A summary of DMRs detected with MethylSig and metilene can be found in Excel Table S1. Exposure, sex, and tissue specific DMRs are summarize in Excel Tables S3–S14. A summary of hypermethylation vs. hypomethylation is included in Excel Table S15. Note: DEHP, di(2-ethylhexyl) phthalate; DMR, differentially methylated region; FDR, false discovery rate; Pb, lead.
Relative to the low overlap in exposure-associated DMRs between tissues, there was more DMR similarity between the sexes when stratified by tissue (Figure 2C), with the exception of the liver. In Pb-exposed animals, 13 and 6 DMRs were common to both males and females in the cortex and blood, respectively. Similarly, in DEHP-exposed animals, eight DMRs were found in males and females in both the cortex and blood, (Figure 2C). Overall, the greatest degree of DMR overlap was found between exposure types. Pb- and DEHP-exposed cortex has the greatest degree of overlap, with 55 and 39 DMRs detected under both exposure conditions in males and females, respectively (Figure 2D). In addition, 19 and 25 DMRs appeared in both exposure conditions in male and female blood, respectively, whereas Pb- and DEHP-exposed liver shared 2 DMRs in males and 1 in females (Figure 2D).
Patterns in the direction of DNA methylation changes (DNA hyper or hypomethylation) were tissue, sex, and exposure specific. In the cortex, the majority of DMRs detected in Pb- exposed males and females and DEHP-exposed females were largely hypomethylated (Pb , Pb , DEHP ), whereas DEHP-exposed males presented slightly more hypermethylated DMRs (53%). Nearly two-thirds of detected DMRs in the blood of DEHP-exposed animals were hypermethylated (65%) in both males and females, and rates were even greater in Pb-exposed females, wherein 72% of DMRs detected in the blood were hypermethylated. Pb-exposed male liver presented a high proportion of hypermethylated DMRs (64%), whereas Pb-exposed female liver had an even presentation of both hyper and hypomethylated DMRs (50% each). Inverse of the trends seen in Pb-exposed males, DEHP-exposed male liver had a greater amount of hypomethylated DMRs (60%), but again directionality was roughly evenly split in DEHP-exposed liver (53% hypermethylated) (Figure 2E; Excel Table S15).
Prevalence of Detected DMRs in Mouse Genomic Regions
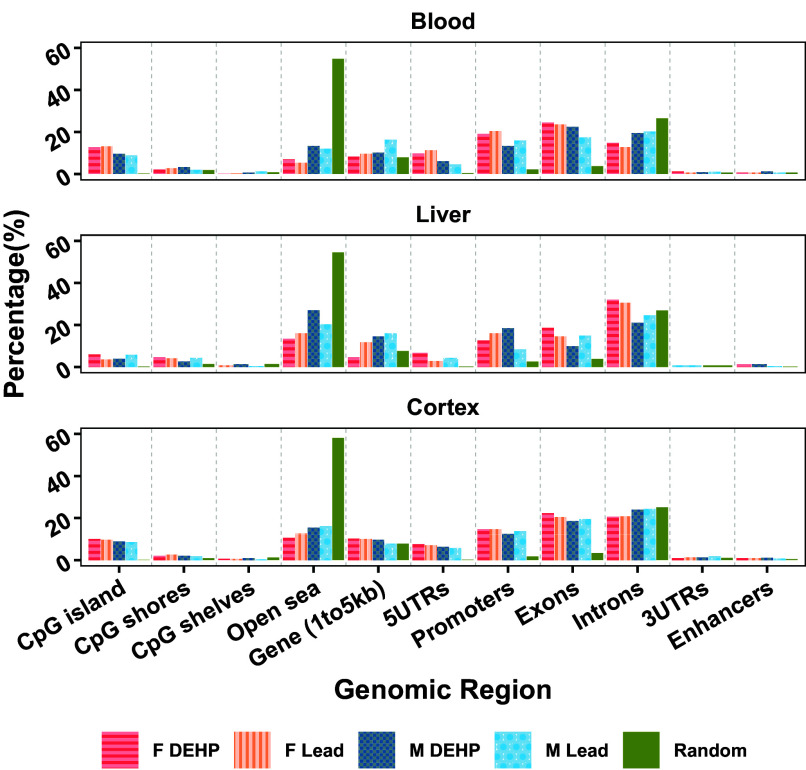
The DMRs detected in this study occurred in specific genomic regions to a greater degree than would have been expected by a random distribution generated for comparison, given known patterns of CpG sites in the mouse genome (mm10). Detected DMRs mapped to CpG islands to a greater degree than would have been expected by chance (3.95%–13.03% of all DMRs across sex, tissues, and exposures, compared with 0.12%–0.29% at random). In blood and cortex across both sexes and exposures, more DMRs were detected in 5′ UTRs than predicted 4.52%–11.24%, in comparison with 0.18%–0.4% under a random distribution, and a similar pattern was observed in liver of Pb-exposed males and females (2.78%–4.21%, in comparison with 0.29% under a random distribution). Several transcriptional regulatory regions demonstrated significant derivation from what would be expected by chance as well. DMRs were present in promoter regions 3.27–9.33 times more than would have been predicted by chance across all conditions (8.43%–20.36%, in comparison with 1.74%–2.58% at random). Exons were another notable location of DMRs, with 2.57–6.52 times more DMRs than what would have been seen under a random distribution (9.87%–24.39%, in comparison with 3.37%–3.84% at random). Conversely, there were fewer DMRs detected in the open sea (5.21%–13.37% in blood, 13.33%–26.97% in liver, and 10.54%–16.15% in cortex) than would be expected by chance (54.56%–58.09%) (Figure 3; Excel Table S16).
Figure 3.
Genomic region of detected DMRs. DMRs were mapped to the mouse reference genome (mm10) and their genomic region annotated as percentage of total DMRs (comparing control and exposed samples) for that sex and exposure within each tissue. This distribution was compared to what would be expected in a random distribution. Annotation of all regions performed using Bioconductor package annotatr. Summary data for this figure can be found in Excel Table S16. Note: DMR, differentially methylated region.
GO Terms Associated with DMR-Containing Genes
DMRs were annotated using annotatr R Bioconductor package, and a summary of the overlap in DMR-containing genes across sexes, tissues, and exposures can be found in Figure S1. ChIP-enrich was used to perform gene set enrichment tests and Gene Ontology (GO) Resource was used to identify DMR-related GO terms. The number of DMR-containing genes associated with each GO result from both Pb- and DEHP-exposed samples are summarized in Excel Table S17 and S18, respectively.
Within Pb-exposed tissues, cortex had the greatest number of gene ontology biological pathway (GOBP)-related DMR-containing genes in both males (25) and females (52). DMR-associated GOBPs in female cortex were dominated by early developmental processes (18 out of 52 genes) and metabolic processes (22 out of 52 genes), whereas male cortex contained an abundance of DMR-containing genes related to gene expression regulation (11 out of 25). In male and female blood, significant () GO terms were related to early embryonic development (9 of 9 DMR-containing genes) and protein regulation (6 of 6 DMR-containing genes), respectively. In male liver, nearly half of DMR-containing genes were significantly associated with molecular function pathways such as DNA binding and regulation (22 of 49). Only one GOBP term was returned for DMR-containing genes in female liver (T-cell apoptotic process, two genes). The most common biological process associated with Pb exposure was genomic imprinting (GO:0071514), which appeared in male cortex and liver (), as well as male blood (although this result was just shy of statistical significance at ). In total, DMRs were detected in 14 genes associated with genomic imprinting in these tissues (Figure 4).
Figure 4.
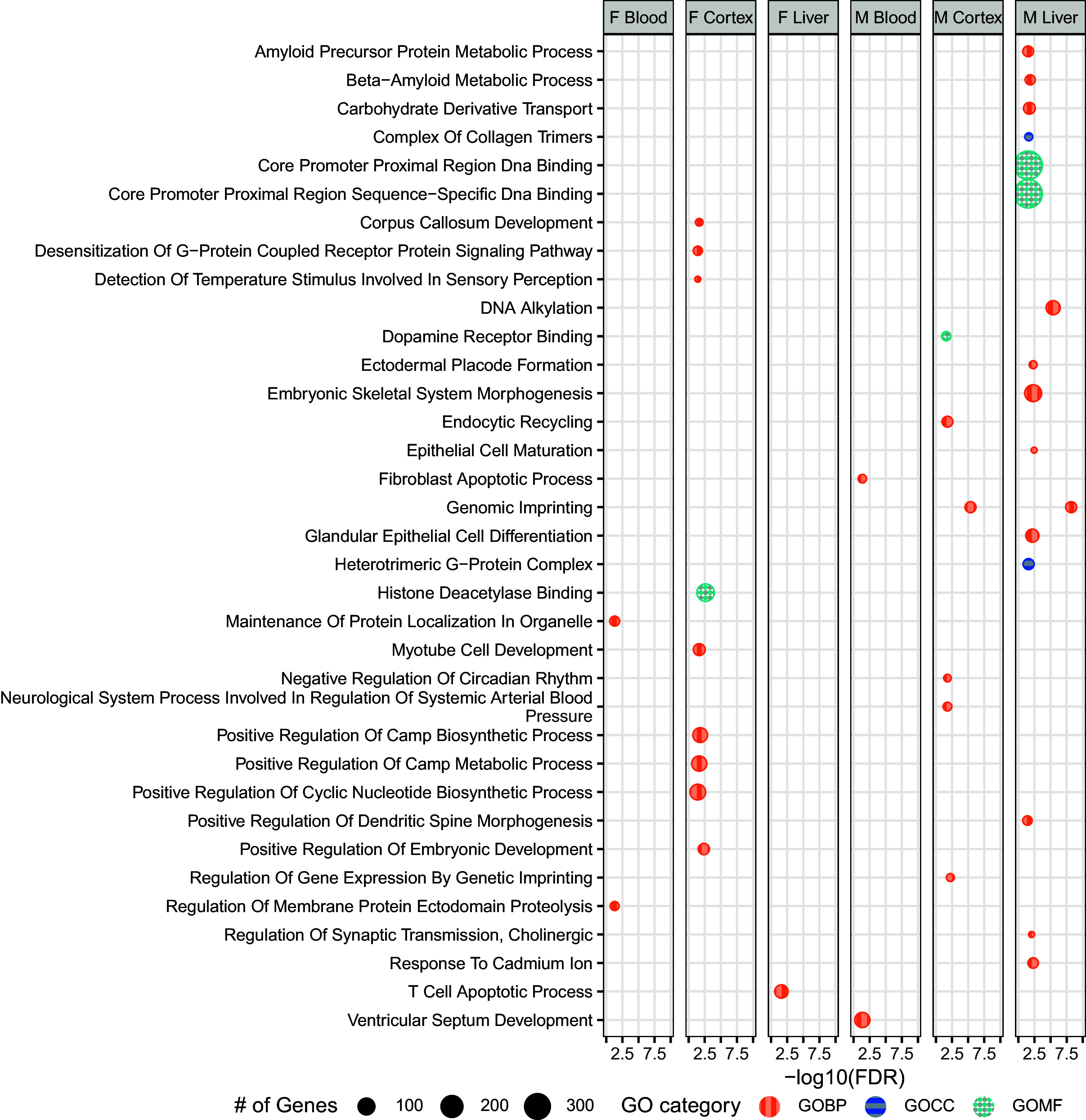
GO-terms associated with DMR-containing genes among Pb-exposed tissues. DMR-containing genes found in Pb-exposed tissues were submitted for GO term analysis across three categories: Biological Process (GOBP), Cellular Component (GOCC), and Molecular Function (GOMF). Bioconductor package ChIP-enrich was used to identify related GO terms, with an FDR cutoff of . Summary data for this figure can be found in Excel Table S17. Note: DMR, differentially methylated region; FDR, false discovery rate; GO, gene ontology; Pb, lead.
In DEHP-exposed samples, a greater number of DMR-containing genes were associated with various GO terms in comparison with Pb-exposed, especially the female cortex, which contained 150 genes associated with various GOBPs, most notably those associated with development (e.g., organ development, differentiation, and morphogenesis) (138 of 150). Male cortex contained far fewer GOBP term-associated DMRs compared with females (46 in comparison with 150), and there was an abundance of genes associated with cellular development and organization (19 out of 46). Male and female blood from DEHP-exposed animals had few genes significantly associated with GO terms, relative to cortex (22 in males and 20 in females). In female blood, a notable number of genes (7 of 20) were associated with GOBP terms related to gene expression regulation (i.e., genomic imprinting and regulation of production of small RNA involved in gene silencing). In male liver, half of DMR-containing genes significantly associated with GOBP terms were involved in epithelial cell development (6 of 12 genes). No DMR-containing genes were significantly associated in the pathway analysis in liver from DEHP-exposed females. With Pb-exposed tissues, the most prevalent GO term among DEHP samples was genomic imprinting, which was associated with DMRs in eight genes across male and female blood (), as well as in male cortex just shy of statistical significance (four genes, ) (Figure 5).
Figure 5.
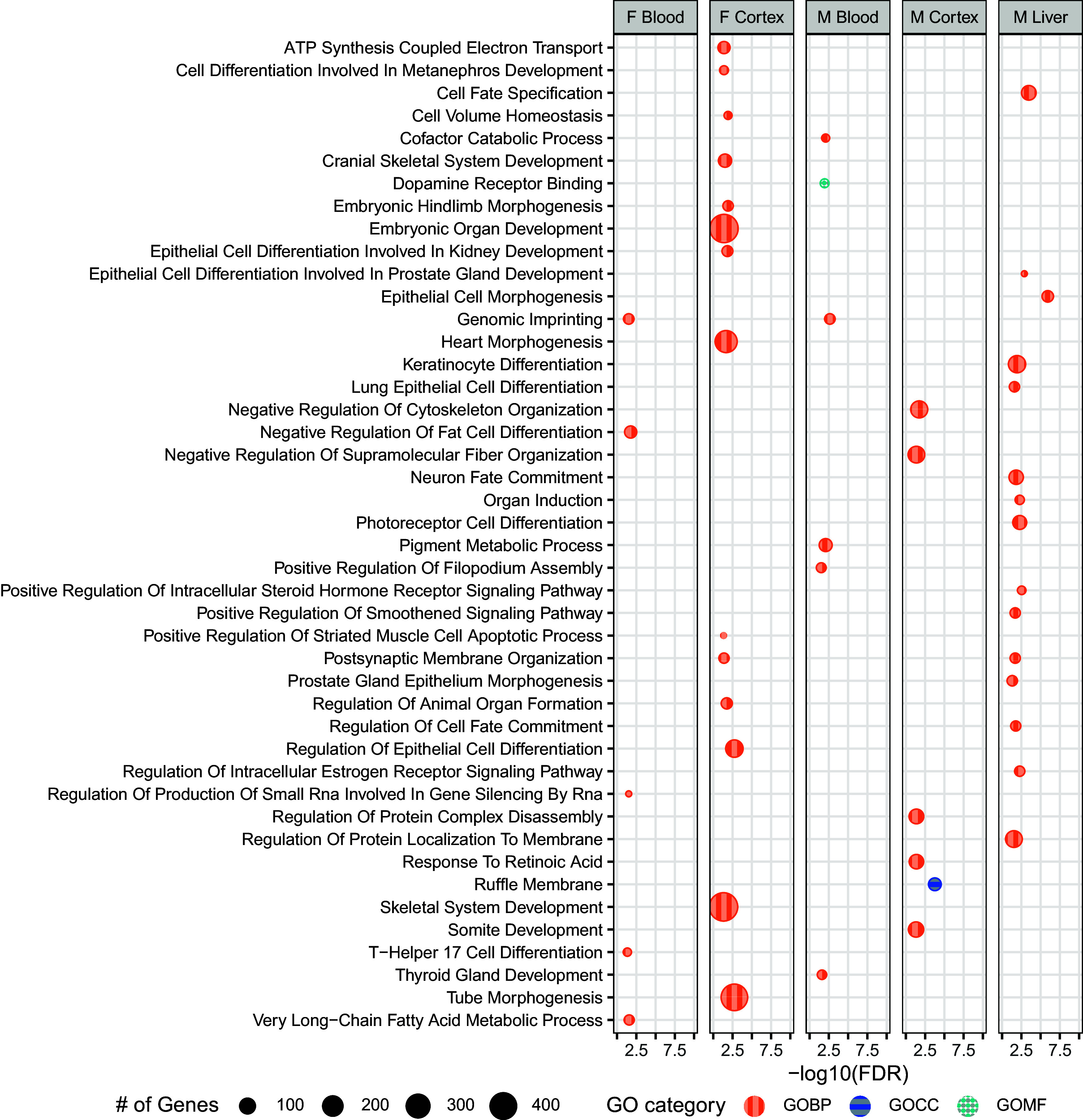
GO-terms associated with DMR-containing genes among DEHP-exposed tissues. DMR-containing genes found in DEHP-exposed tissues were submitted for GO term analysis across three categories: Biological Process (GOBP), Cellular Component (GOCC), and Molecular Function (GOMF). Bioconductor package chipenrich was used to identify related GO terms, with an FDR cutoff of . Summary data for this figure can be found in Excel Table S18. Note: DEHP, di(2-ethylhexyl) phthalate; DMR, differentially methylated region; FDR, false discovery rate; GO, gene ontology.
DNA Methylation Changes at Imprinted Loci
The appearance of imprinted genes in both exposure models during pathway analysis (Figures 4 and 5) was motivation to take a closer look at the effects of Pb and DEHP exposure on imprinted genes. All tissue types, across both sexes and exposures had detectable differences in DNAm within imprinted genes compared with control (Figures S2–S5). A reference list of imprinted genes used in this analysis can be found in Excel Table S2, and genes that did not contain a DMR in any tissue were omitted from the final figure. Cortex had the greatest number of DMRs as well as the greatest magnitude of methylation changes in assessed imprinted genes. A total of 63 Pb-associated DMRs were detected in cortex at imprinted genes (41 in males and 22 in females with magnitude changes of 5.08%–23.77%), and 52 were detected in DEHP-exposed cortex (29 in males and 23 in females with magnitude changes of 5.26%–24.9%). Twenty-seven Pb-associated DMRs were detected in blood at imprinted genes (13 in males and 14 in females with magnitude changes of 5.04%–20.06%), and 55 were detected in DEHP-exposed blood (24 in males and 20 in females with magnitude changes of 5.41%–28.43%). Liver contained fewer changes in DNAm at imprinted genes, in comparison with blood and cortex, for each sex–exposure combination, with 7 DMRs in Pb-exposed liver (6 in males and 1 in females with magnitude changes of 6.83%–19.85%) and 10 DMRs in DEHP-exposed liver (2 in males and 8 in females with magnitude changes of 8.88%–16.39%). Blood from Pb-exposed females largely contained hypermethylated sites at imprinted genes (10 of 14 DMRs), whereas cortex from the same animals was largely hypomethylated in the same gene class (17 of 22 DMRs). A similar pattern was seen in DEHP- and Pb-male tissues, with the bulk of detectable differences found in the blood and cortex, with the former being largely hypermethylated (21 of 24 DMRs in DEHP blood and 10 of 13 in Pb blood) and the latter hypomethylated (19 of 29 DMRs in DEHP cortex and 29 of 41 DMRs in Pb cortex). A summary of all DMRs detected within assessed imprinted genes can be found in Excel Tables S19 and S20 for Pb and DEHP exposure, respectively.
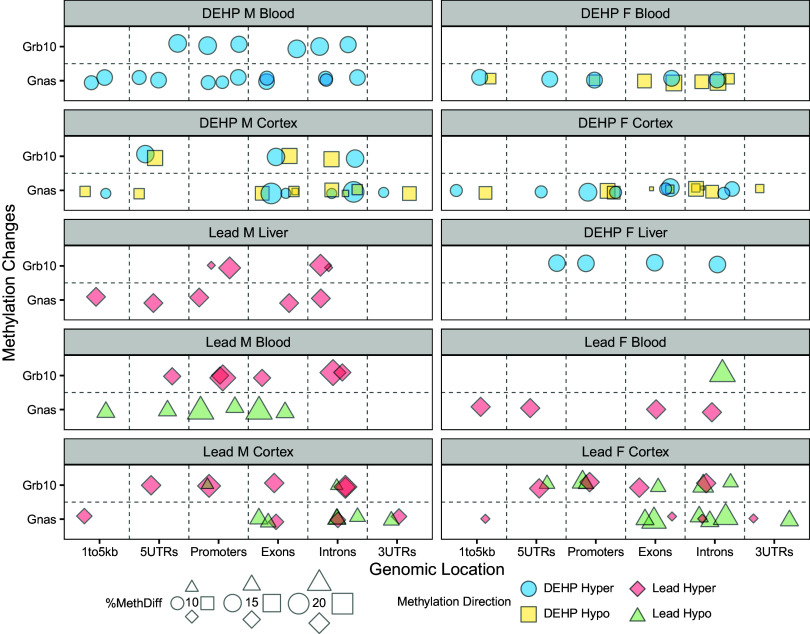
Two imprinted genes, Gnas and Grb10, contained a notable number of exposure-associated DMRs. A complete overview of these DMRs is summarized in Figure 6 and included in Excel Tables S21 and S22. Among Pb-exposed cortex samples, 60% of DMRs in the Gnas locus were hypomethylated in both males and females. In Pb-exposed blood, DMRs within the Gnas locus were entirely hypermethylated in females and hypomethylated in males. In Pb-exposed liver, Gnas DMRs were entirely hypermethylated in males; however, there were no DMRs detected in Gnas in the liver of Pb-exposed females. Among Pb-exposed cortex, DMRs within the Grb10 locus were largely hypermethylated in males (71%) and hypomethylated in females (60%). A similar pattern presented in Pb-exposed blood, wherein the entirety of Grb10 DMRs in males were hypermethylated, whereas those in females were hypomethylated. Male liver contained only hypermethylated sites within the Grb10 locus, and no DMRs were detected in this tissue in Pb-exposed females.
Figure 6.
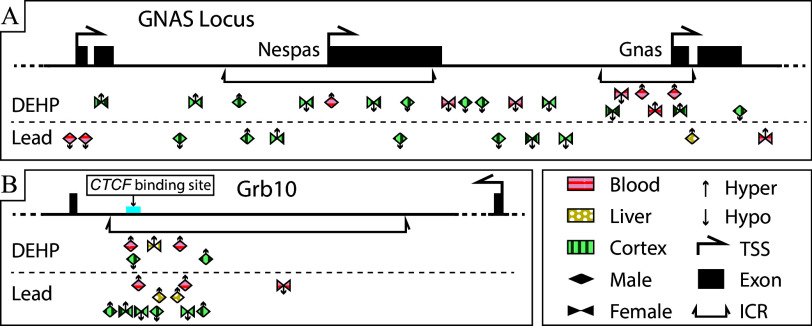
Genomic location and direction of Pb and DEHP-associated DMRs in the Gnas and Grb10 loci. DMRs detected in the Gnas and Grb10 loci were classified as to their genomic location within each gene. Percentage change in methylation is denoted by size and direction of methylation change by color (circles: hypermethylated DMRs among DEHP samples; squares: hypomethylation among DEHP samples; diamonds: hypermethylation among Pb samples; triangles: among hypomethylation among Pb samples). Bioconductor package annotatr was used to annotate the genomic locations of these DMRs. Summary data for this figure can be found in Excel Table S21 and S22 for Pb and DEHP exposure, respectively. Note: DEHP, di(2-ethylhexyl) phthalate; DMR, differentially methylated region; Pb, lead.
DEHP exposure was associated with more hypomethylated DMRs at the Gnas locus in both male (60%) and female cortex (55%). In blood, DEHP exposure was associated with slightly more hypomethylated DMRs in females (58%), but DMRs associated with this exposure in male blood were entirely hypermethylated. No DMRs in Gnas were detected in the liver for any DEHP-exposed animals. Regarding Grb10, 50% of DMRs identified in male cortex were hypomethylated, whereas all DMRs identified in male blood were hypermethylated. No DMRs in Grb10 were detected in male liver or in female cortex or blood.
Exposure-Associated Changes in Imprinting Control Regions
Imprinted genes are regulated in part through ICRs, which are elements whose methylation is set up in the germline and that regulate gene expression and subsequent functions of imprinted gene clusters.45 Changes in the DNAm status of these regions can impact the expression of imprinted and nonimprinted genes within a given cluster, thus magnifying the regulatory effects of what would otherwise be a single-gene effect.35 Gnas contains two ICRs, the Gnas ICR and the Nespas ICR, whereas Grb10 contains one ICR.43 The current analysis identified multiple DMRs within the ICRs of both Gnas (7 in ICR Gnas and 8 in ICR Nespas) and Grb10 (16 in the Grb10 ICR) across exposure and tissue types (Figure 7). A binomial test was conducted to assess whether exposure-associated DMRs occurred in these ICRs to a greater degree than would have been expected to occur by random chance. Both the Gnas and Grb10 ICRs contained more DMRs than would have been expected to occur by chance in multiple sex–exposure–tissue combinations. A summary of these findings for Pb and DEHP exposure can be found in Excel Table S23 and S24, respectively.
Figure 7.
DMRs detected within Gnas and Grb10 ICRs among Pb- and DEHP-exposed tissues. (A) DMRs detected within Gnas ICRs Nespas and Gnas. (B) DMRs detected within Grb10. DMRs only represents the related genomic locations corresponding to the genomic coordinates of ICRs. The genomic coordinates of these DMRs can be found in Excel Table S25 and S26 for Pb and DEHP exposure, respectively. Note: DEHP, di(2-ethylhexyl) phthalate; DMR, differentially methylated region; ICR, imprinting control region; Pb, lead; TSS, transcription start site.
Pb exposure was associated with relatively limited changes in DNAm in Gnas ICRs (4 DMRs across both ICRs) when compared to Grb10 (11 DMRs). In the Nespas ICR, Pb exposure was associated with hypermethylation in female cortex (1 of 1 DMR) and a mix of hyper- (1 of 2 DMRs) and hypomethylation (1 of 2 DMRs) in male cortex. In the Gnas ICR, Pb exposure was associated only with hypermethylation in male liver (1 of 1 DMR) (Figure 7A; Excel Table S25). In the Grb10 ICR, Pb exposure was associated more hypermethylated DMRs (2 of 3 DMRs) in male cortex and more hypomethylated DMRs (2 of 3) in female cortex. Pb exposure was entirely associated with hypermethylation in both male blood (2 of 2 DMRs) and liver (2 of 2 DMRs) but was associated with hypomethylation in female blood (1 of 1 DMR) (Figure 7B; Excel Table S25).
There were comparatively more differences in DNAm in the Gnas ICRs associated with DEHP exposure. In male cortex there was again a mix of hyper- (1 of 2) and hypomethylated (1 of 2) DMRs in the Nespas ICR. Unlike Pb exposure, DEHP was associated only with hypomethylated DMRs (2 of 2) in female cortex in the Nespas ICR. In male blood there were 1 and 2 hypermethylated DEHP-associated DMRs within the Nespas and Gnas ICRs, respectively. Female cortex and blood both contained a mix of hyper- (1 of 2) and hypomethylated (1 of 2) DMRs in the Gnas ICR associated with DEHP exposure (Figure 7A; Excel Table S26). Within the Grb10 ICR, DEHP exposure was associated with a mix of hyper- (1 of 2) and hypomethylated (1 of 2) DMRs in male cortex, hypermethylated (2 of 2) DMRs in male blood, and 1 hypermethylated DMR in female liver (Figure 7B; Excel Table S26).
Discussion
Toxicant exposures that occur during critical periods of development can have ramifications for health and well-being throughout the life course.46 Perinatal Pb and DEHP exposures have been linked to aberrant brain development47 and metabolic function,48 respectively, at environmentally relevant doses. With regard to epigenetic mechanisms governing gene expression, Pb49 and DEHP50 exposures have both been associated with differential DNAm in human populations. Although an important goal of such toxicological work is to assess the functional health outcomes of such exposures on epigenetic regulation of gene expression, there are additional opportunities in such studies to evaluate the use of epigenetic signatures in more accessible tissues as a predictor of effects seen in ones harder to access, thus supporting future epidemiological work that must often rely on surrogate tissues such as blood. It is currently unknown whether toxicant-induced changes in difficult-to-access tissues, such as brain and liver, are reflected in surrogate tissues, such as blood, and the objective of this study was to address this knowledge gap. Here, we examined how two prominent developmental exposures, Pb and DEHP, affect DNAm in these target and surrogate tissues to assess whether this epigenetic mark could be used as a potential biomarker of changes in more difficult to access tissues, as is being evaluated in the TaRGET II Consortium.23
Pb and DEHP Exposures Were Associated with Sex, Tissue, and Exposure-Specific General Changes in DNA Methylation
Overall, Pb and DEHP exposures resulted in a similar number of DMRs between the sexes for each of the three tissues assessed (Figure 2A). The cortex contained the greatest number of DMRs for each exposure, followed by blood and liver. Between the sexes, females had more DMRs across both exposures in cortex and blood, whereas males had more DMRs in the liver (Figure 2A). This overall DNAm pattern is consistent with previous reports, which have shown significant changes in DNAm in female brain following gestational Pb exposure as well as in male liver following DEHP exposure.51,52 There was minimal overlap in DMRs between cortex-blood among DEHP-exposed animals, with 1.70% and 2.36% of DMRs detected in cortex also detected in blood from the same animals, in males and females, respectively. This pattern was replicated in the liver of DEHP-exposed animals, with 2.0% and 6.66% of DMRs detected in that target tissue also appearing in blood from the same animals, in males and females, respectively. Results from Pb-exposed animals further supported the idea that general trends in DMRs provided little opportunity for target–surrogate measures. In the cortex, 1.28% and 0.35% of DMRs were replicated in blood, in males and females, respectively, and results were only slightly better when examining how many DMRs in the liver were also detected in blood (2.78% and 3.33% in males and females, respectively). (Figure 2B).
The number of DMRs in common between the sexes did not exceed 2% of the total DMRs detected in any tissue-exposure combination (Figure 2C). These findings highlight the need to evaluate sex-specific effects in toxicoepigenetic studies.53,54 General trends in DMR directionality were also not conserved across tissue types (Figure 2E), adding complexity to comparisons of changes in DNAm patterns between target and surrogate tissues. Although the majority of DMRs in blood from DEHP-exposed male and female mice was largely hypermethylated (65% in both cases), DMRs in the corresponding target tissues displayed either an approximate even distribution of hyper- and hypomethylated DMRs (as in male cortex and female liver) or with a majority of DMRs that were hypomethylated (as in female cortex and male liver). These results are somewhat consistent with previous reports on DEHP, which have observed a mix of differential methylation direction in female liver at 5 months of age.52 However, that work also explores changes in methylation at an earlier time point (PND21), wherein dramatically different patterns were seen. For example, male liver at PND21 contained many more changes in DNAm compared with 5-month-old animals, suggesting some epigenetic effects may be modified with age, as has been previously discussed,55 and emphasizes the need to expand this research objective to other life stages as well.
Trends in DMR directionality between target and surrogate pairs differed even more among Pb-exposed animals. Male blood contained slightly more hypomethylated DMRs, whereas the cortex contained significantly more hypomethylated regions (80%) and the liver contained a majority of hypermethylated DMRs (64%). In Pb-exposed females, nearly three-quarters of DMRs detected in blood were hypermethylated, whereas the distribution in the cortex and liver was close to evenly split (Figure 2E). These results are consistent with a previous report looking at DMRs in the liver following developmental Pb exposure, with a mix of hyper- and hypomethylation in males and females. Additionally, this report found a significant amount of hypomethylated DMRs in Pb-exposed male blood, as reported in our data.56 Taken together, this broad analysis of differences in DNAm following Pb and DEHP exposure in comparison with controls does not support the idea of using general trends in changes in DNAm in blood following a toxicant exposure to predict how that exposure may be influencing the same epigenetic patterns in cortex or liver. However, it should be noted that our results are limited to the tissue pairings discussed here, and this conclusion may not be accurate for other target–surrogate tissue combinations or at other time points.
Exposure-Associated DMRs Occurred to a Notable Degree in Imprinted Genes in Mice Exposed to Pb or DEHP
An analysis of GO terms associated with DMR-containing genes identified genomic imprinting as a common category for half of the tissue–sex–exposure combinations (DEHP-exposed female and male blood, as well as male cortex, and in all examined tissues from Pb-exposed males) (Figures 4 and 5). Imprinted genes are an important class with regard to early growth and development, and their epigenetically controlled mono-allelic parent-of-origin expression may confer particular susceptibility to the impacts of environmental exposures.57,58 Early disruption of imprinted gene expression and function can result in developmental disorders (e.g., pseudohypoparathyroidism type 1B and Silver-Russell syndrome), for which perturbations in gene expression regulation of Gnas59 and Grb10,60 respectively, have been implicated. The DNAm and hydroxymethylation status of imprinted genes is particularly susceptible to environmental exposures during early development, including Pb61 and DEHP.62 The biology of imprinted genes makes them a promising candidate in the search for epigenetic biomarkers of toxicant effects in difficult to access tissues, because many are thought to be exempt from postfertilization epigenetic reprogramming.63 As such, it may be that the effects of an exposure present during this fertilization window on the epigenetic regulation of imprinted genes would be maintained, despite postfertilization reprogramming,64 and propagated in multiple tissues subsequent to germ layer specification (such as brain from the ectoderm and blood from the mesoderm).65 This biology and potential maintenance of epigenetic signatures of the effects of toxicant exposures present during fertilization and germ layer differentiation made imprinted genes an interesting gene class to explore further as a potential candidate for target–surrogate biomarkers.
Imprinted Genes Gnas and Grb10 Contained Some Patterns of Differential Methylation Replicated between Target and Surrogate Tissues
Gnas encodes for the G-protein alpha-subunit protein, which contributes to signal transduction via cAMP generation,66 and its imprinting dysregulation has been associated with neural tube defects67 and hypothyroidism.68 The imprinted expression of Gnas is complex, because this gene gives rise to several maternal- and paternal-allele–encoded gene products, and these patterns of expression are highly tissue-specific in mice and humans.69–71 It is therefore important to note that this work captured changes in DNAm in 5-month-old (e.g., adulthood) animals that were exposed during a finite window of development (fertilization through 3 wk of age), and it is possible that the effects of exposure on this imprinted gene are varied across the lifespan, given the dynamic nature of Gnas regulation.72 Here, Gnas contained a mix of hyper- and hypomethylated DMRs in the cortex, under both exposure conditions and in both sexes, making the prediction of the observed effects difficult to ascertain, especially without allele-specific measures.73 There were few patterns of DEHP- or Pb-associated changes in DNAm throughout Gnas that were replicated between a target and surrogate tissue. For example, both DEHP- and Pb-exposed male blood contained entirely hypomethylated DMRs, but DEHP-exposed male liver contained no DMRs in Gnas and Pb-exposed male liver contained entirely hypermethylated DMRs in the same gene. The one instance of pattern replication between a target and surrogate tissue at this level of analysis in Gnas was in DEHP-exposed blood and cortex in females, which both presented with a combination of hyper- and hypomethylated DMRs throughout the gene, including the region, promoters, exons, and introns, and uniform hypermethylation in the 5′ UTR of Gnas. Given these results, but their consistency between blood and cortex, it would be pertinent to perform allele-specific analysis of changes in DNAm to ascertain whether there are paternal- or maternal-specific alterations that are replicated between these two tissue types in females, because this added layer of specificity may provide stronger evidence of epigenetic signatures.73
Grb10 encodes a signal adaptor protein involved in growth control, cellular differentiation, and insulin response signaling74 and is imprinted in a tissue- and cell type–specific manner.75 For example, Grb10 expression is cell-type specific during early brain development, as it is paternally expressed in cortical neurons and maternally expressed in glial cells, and this distinction appears to disappear in adulthood.75 Grb10 expression also changes significantly in the liver during development, because maternal expression is high during fetal development,76 but nearly all Grb10 expression is silenced in the liver in adulthood.77 Grb10 contained DMRs in a limited subset of sex and tissue combinations in the DEHP-exposed animals. The gene was entirely hypermethylated in male blood and female liver and contained a mix of hyper and hypomethylated DMRs in male cortex, resulting in no consistent patterns between blood and a target tissue in either sex. Given the known cell type–specific patterns of Grb10 expression,78 it may be beneficial to examine cell type–specific changes in DNAm following DEHP exposure, particularly during an earlier developmental time point, because it may be that the uniform surrogate measure detected here (i.e., hypomethylation in male blood) may better correlate with changes occurring in a particular cell type in the brain, rather than a bulk tissue.79 Grb10 contained more DMRs in the Pb-exposed animals and revealed one potential signature replicated between a target and surrogate tissue. Pb-exposed male blood and liver both contained only hypermethylated DMRs in Grb10, and this signature was specifically replicated in the promoters and introns of this gene. The male cortex also contained entirely hypermethylated DMRs in the 5′ UTR and exons of Grb10, and this pattern was seen in the blood from these animals as well; however, other regions of Grb10 (promoter and introns) contained a mix of DMR directionality in the cortex, limiting our confidence in the use of epigenetic changes in this gene as a potential biomarker of the effects of Pb exposure in this target tissue.
Together, evaluation of differences in DNAm across both Gnas and Grb10 provided preliminary evidence that imprinted genes may be candidates for biomarkers of exposure effects in a surrogate tissue. It is important to consider that these results are exposure- and sex-specific, with a target–surrogate association seen in Gnas only in the DEHP-exposed female tissues and in Grb10 only in the Pb-exposed male tissues. Given the fluidity of gene regulation for some imprinted genes throughout life, it is likely that these associations may change with time as well, and it would be pertinent to consider age and developmental stage when continuing the search for epigenetic biomarkers of exposure, particularly when examining imprinted genes as a candidate class.
Imprinting Control Regions Provided Evidence of Exposure Signatures in Target–Surrogate Pairs in Mice Exposed to Pb or DEHP
ICRs are environmentally sensitive regulatory regions, and changes to their DNAm can have consequences for all genes within a cluster.80 The ICRs of both Gnas and Grb10 contained numerous DEHP- and Pb-associated DMRs, and potential overlap in these signatures between either cortex or liver and the blood was reminiscent of patterns observed at the whole gene level, as described above. For example, in the DEHP-exposed female mice, Gnas contained a mix of hyper- and hypomethylated DMRs in one of its ICRs (Gnas) in blood (two hypermethylated, two hypomethylated DMRs) and cortex (two hypermethylated, two hypomethylated DMRs). As mentioned, it would be prudent to examine whether this ICR has allele-specific alterations that may be contributing to these mixed results (such as hypermethylation of the paternal allele and hypomethylation of the maternal allele) and whether they are replicated between the cortex and blood.
This observation of DNAm signatures observed in the whole gene being maintained in the ICR was also replicated in the case of Grb10 under Pb-exposure conditions, as the Grb10 ICR contained entirely hypermethylated DMRs in both the male blood and liver. This target–surrogate pattern was also accompanied by potential additional relationships in this same gene and exposure type, because the Grb10 ICR was largely hypermethylated (2 of 3 DMRs) in the male cortex. In the case of female tissues, Grb10 ICRs in blood were only hypomethylated, whereas those in the cortex from the same animals were also largely hypomethylated (2 of 3 DMRs). It may again be the case that allele-specific evaluation of changes in DNAm may provide more concrete signals of changes replicated between tissue types. The current study is one of few reports that examine the effects of environmental exposures on the Grb10 ICR, with a previous report highlighting the effects on hydroxymethylation,81 though more exist pertaining to changes throughout the gene.82,83 Much of this published work is restricted to germ cells, so additional work is needed to assess whether Grb10 regulation and function are impacted by the environment in the soma.
These results suggest imprinted genes and their ICRs may be viable candidates in the search for epigenetic biomarkers of toxicant exposures. To maximize the utility of this research objective, future work aimed at evaluating the impacts on gene expression would expand our appreciation of the health effects of an exposure on a target tissue such as the brain or liver. It is one thing to establish imprinted genes as a class suitable for use as a biomarker of exposure effects on the epigenome; it is another to say that changes observed in the blood correlate or even predict health effects linked to those same genes in a target tissue. This work has highlighted the potential of imprinted genes in this endeavor. Future work on allele-specific effects, the role of developmental stage, and what these epigenetic changes mean for functional health outcomes are now needed.
Limitations
DNAm patterns vary across cell types within a given tissue.84,85 This study was unable to account for cell type, and therefore changes in DNAm resulting from Pb or DEHP exposure may be due to exposure-induced changes in cell populations.86 In addition, we were not able to evaluate changes in DNA hydroxymethylation (5hmC) in these samples. This study was conducted using bisulfite conversion, which does not distinguish and 5hmC, and the resulting data are unable to differentiate between these two signatures.81 Imprinted genes are typically 50% methylated (accounting for mono-allelic expression or repression), and these data represent DNAm averages for both alleles. Thus, any allele-specific changes in DNAm associated with Pb or DEHP cannot be detected.
Conclusion
This study systematically evaluated changes in DNAm for cortex, blood, and liver collected from mice at 5 months of age following perinatal exposure to either Pb or DEHP. Genomic imprinting was impacted by Pb and DEHP exposure, as determined by GO term analysis, and imprinted genes Gnas and Grb10 provided preliminary evidence of consistent changes in DNAm in a surrogate and target tissue in an analysis of both the whole gene as well as their ICRs, suggesting they may be useful candidates when exploring DNAm-based biomarkers of environmental exposures.
Supplementary Material
Acknowledgments
The authors would like to acknowledge members of the University of Michigan Epigenomics Core and the Advanced Genomics Core as well as the Michigan Lifestage Environmental Exposures and Disease Center (M-LEEaD), which facilitated the generation and analysis of WGBS data.
This work was supported by funding from the following sources: NIEHS TaRGET II Consortium (ES026697), NIEHS Grant R35 (ES031686), NIEHS Grant K01 (ES032048), NIEHS Grant R01 (ES028802), the Michigan Lifestage Environmental Exposures and Disease (M-LEEaD) NIEHS Core Center (P30 ES017885), Institutional Training Grant T32 (ES007062), Institutional Training Grant T32 (HD079342), NIEHS Grant K99 (ES034429), and National Institute on Aging (NIA) Grant R01 (AG072396).
Data used in the preparation of this manuscript are available through the TaRGET Data Portal (https://data.targetepigenomics.org/), supported by the University of Washington in St. Louis, Missouri. Additional data that support the findings of this study are available from the corresponding author, DCD, upon reasonable request.
Work outlined in this manuscript was approved by the University of Michigan IACUC and conducted in accordance with the highest animal welfare standards.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Gillman MW. 2005. Developmental origins of health and disease. N Engl J Med 353(17):1848–1850, PMID: 16251542, 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal AJ, Jirtle RL. 2010. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol 88(10):938–944, PMID: 20568270, 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollati V, Baccarelli A. 2010. Environmental epigenetics. Heredity (Edinb) 105(1):105–112, PMID: 20179736, 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyko F. 2018. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet 19(2):81–92, PMID: 29033456, 10.1038/nrg.2017.80. [DOI] [PubMed] [Google Scholar]
- 5.Siegfried Z, Simon I. 2010. DNA methylation and gene expression. Wiley Interdiscip Rev Syst Biol Med 2(3):362–371, PMID: 20836034, 10.1002/wsbm.64. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Y, Chen T. 2019. DNA methylation reprogramming during mammalian development. Genes 10(4):257, 10.3390/genes10040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinoy DC, Das R, Weidman JR, Jirtle RL. 2007. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res 61(5 pt 2):30R–37R, PMID: 17413847, 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]
- 8.SanMiguel JM, Bartolomei MS. 2018. DNA methylation dynamics of genomic imprinting in mouse development. Biol Reprod 99(1):252–262, PMID: 29462489, 10.1093/biolre/ioy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucci V, Isles AR, Kelsey G, Ferguson-Smith AC, Erice Imprinting Group. 2019. Genomic imprinting and physiological processes in mammals. Cell 176(5):952–965, PMID: 30794780, 10.1016/j.cell.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Chang S, Bartolomei MS. 2020. Modeling human epigenetic disorders in mice: Beckwith-Wiedemann syndrome and Silver-Russell syndrome. Dis Model Mech 13(5):dmm044123, PMID: 32424032, 10.1242/dmm.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henckel A, Chebli K, Kota SK, Arnaud P, Feil R. 2012. Transcription and histone methylation changes correlate with imprint acquisition in male germ cells. Embo J 31(3):606–615, PMID: 22117218, 10.1038/emboj.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jima DD, Skaar DA, Planchart A, Motsinger-Reif A, Cevik SE, Park SS, et al. 2022. Genomic map of candidate human imprint control regions: the imprintome. Epigenetics 17(13):1920–1943, 10.1080/15592294.2022.2091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faulk C, Dolinoy DC. 2011. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics 6(7):791–797, PMID: 21636976, 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argyraki M, Damdimopoulou P, Chatzimeletiou K, Grimbizis GF, Tarlatzis BC, Syrrou M, et al. 2019. In-utero stress and mode of conception: impact on regulation of imprinted genes, fetal development and future health. Hum Reprod Update 25(6):777–801, PMID: 31633761, 10.1093/humupd/dmz025. [DOI] [PubMed] [Google Scholar]
- 15.Senut MC, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, et al. 2012. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4(6):665–674, PMID: 23244311, 10.2217/epi.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsanathan R, Karundevi B. 2014. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol 223:47–66, PMID: 25232145, 10.1530/JOE-14-0111. [DOI] [PubMed] [Google Scholar]
- 17.Dignam T, Kaufmann RB, LeStourgeon L, Brown MJ. 2019. Control of lead sources in the United States, 1970–2017: public health progress and current challenges to eliminating lead exposure. J Public Health Manag Pract 25(suppl 1):S13–S22, PMID: 30507765, 10.1097/PHH.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yin G, Gao Y, Liu D, Xie J, Ouyang L, et al. 2019. Toxicity assessment due to prenatal and lactational exposure to lead, cadmium and mercury mixtures. Environ Int 133(pt B):105192, PMID: 31639605, 10.1016/j.envint.2019.105192. [DOI] [PubMed] [Google Scholar]
- 19.Kamenov GD, Swaringen BF, Cornwell DA, McTigue NE, Roberts SM, Bonzongo JJ. 2023. High-precision Pb isotopes of drinking water lead pipes: implications for human exposure to industrial Pb in the United States. Sci Total Environ 871:162067, PMID: 36758690, 10.1016/j.scitotenv.2023.162067. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich M, Barlow CF, Entwistle JA, Meza-Figueroa D, Dong C, Gunkel-Grillon P, et al. 2023. Predictive modeling of indoor dust lead concentrations: sources, risks, and benefits of intervention. Environ Pollut 319:121039, PMID: 36627044, 10.1016/j.envpol.2023.121039. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Qian H. 2021. Phthalates and their impacts on human health. Healthcare (Basel) 9(5):603, PMID: 34069956, 10.3390/healthcare9050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V. 2014. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol 98(24):9967–9981, PMID: 25376446, 10.1007/s00253-014-6183-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Pehrsson EC, Purushotham D, Li D, Zhuo X, Zhang B, et al. 2018. The NIEHS TaRGET II Consortium and environmental epigenomics. Nat Biotechnol 36(3):225–227, PMID: 29509741, 10.1038/nbt.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakulski KM, Halladay A, Hu VW, Mill J, Fallin MD. 2016. Epigenetic research in neuropsychiatric disorders: the “tissue issue”. Curr Behav Neurosci Rep 3(3):264–274, PMID: 28093577, 10.1007/s40473-016-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galbraith DB, Wolff GL. 1974. Aberrant regulation of the Agouti pigment pattern in the viable yellow mouse. J Hered 65(3):137–140, PMID: 4847754, 10.1093/oxfordjournals.jhered.a108484. [DOI] [PubMed] [Google Scholar]
- 26.Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, et al. 2014. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect 122(5):485–491, PMID: 24487385, 10.1289/ehp.1307449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulk C, Barks A, Sánchez BN, Zhang Z, Anderson OS, Peterson KE, et al. 2014. Perinatal lead (Pb) exposure results in sex-specific effects on food intake, fat, weight, and insulin response across the murine life-course. PLoS One 9(8):e104273, PMID: 25105421, 10.1371/journal.pone.0104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faulk C, Barks A, Liu K, Goodrich JM, Dolinoy DC. 2013. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics 5(5):487–500, PMID: 24059796, 10.2217/epi.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The American College of Obstetricians and Gynecologists. 2012. Lead Screening During Pregnancy and Lactation. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2012/08/lead-screening-during-pregnancy-and-lactation [accessed 9 January 2024].
- 30.Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, et al. 2011. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab 301(3):E527–E538, PMID: 21673306, 10.1152/ajpendo.00233.2011. [DOI] [PubMed] [Google Scholar]
- 31.Neier K, Cheatham D, Bedrosian LD, Dolinoy DC. 2019. Perinatal exposures to phthalates and phthalate mixtures result in sex-specific effects on body weight, organ weights and intracisternal A-particle (IAP) DNA methylation in weanling mice. J Dev Orig Health Dis 10(2):176–187, PMID: 29991372, 10.1017/S2040174418000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svoboda LK, Neier K, Wang K, Cavalcante RG, Rygiel CA, Tsai Z, et al. 2021. Tissue and sex-specific programming of DNA methylation by perinatal lead exposure: implications for environmental epigenetics studies. Epigenetics 16(10):1102–1122, PMID: 33164632, 10.1080/15592294.2020.1841872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. 2020. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 18(7):e3000410, PMID: 32663219, 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewels P, Magnusson M, Lundin S, Käller M. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32(19):3047–3048, PMID: 27312411, 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger F, Andrews SR. 2011. Bismark: a flexible aligner and methylation caller for bisulfite-seq applications. Bioinformatics 27(11):1571–1572, PMID: 21493656, 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359, PMID: 22388286, 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jühling F, Kretzmer H, Bernhart SH, Otto C, Stadler PF, Hoffmann S. 2016. Metilene: fast and sensitive calling of differentially methylated regions from bisulfite sequencing data. Genome Res 26(2):256–262, PMID: 26631489, 10.1101/gr.196394.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y, Figueroa ME, Rozek LS, Sartor MA. 2014. MethylSig: a whole genome DNA methylation analysis pipeline. Bioinformatics 30(17):2414–2422, PMID: 24836530, 10.1093/bioinformatics/btu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalcante RG, Sartor MA. 2017. Annotatr: genomic regions in context. Bioinformatics 33(15):2381–2383, PMID: 28369316, 10.1093/bioinformatics/btx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch RP, Lee C, Imbriano PM, Patil S, Weymouth TE, Smith RA, et al. 2014. ChIP-enrich: gene set enrichment testing for ChIP-seq data. Nucleic Acids Res 42(13):e105, PMID: 24878920, 10.1093/nar/gku463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson CM, Blake A, Thomas S, Beechey CV, Hancock J, Cattanach BM, et al. 2013. World Wide Web Site - Mouse Imprinting Data and References. Oxfordshire, UK: MRC Hartwell. [Google Scholar]
- 42.Juan AM, Foong YH, Thorvaldsen JL, Lan Y, Leu NA, Rurik JG, et al. 2022. Tissue-specific Grb10/Ddc insulator drives allelic architecture for cardiac development. Mol Cell 82(19):3613–3631.e7, PMID: 36108632, 10.1016/j.molcel.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, et al. 2014. Programming and inheritance of parental DNA methylomes in mammals. Cell 157(4):979–991, PMID: 24813617, 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riemondy KA, Sheridan RM, Gillen A, Yu Y, Bennett CG, Hesselberth JR. 2017. Valr: reproducible genome interval analysis in R. F1000Res 6:1025, PMID: 28751969, 10.12688/f1000research.11997.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, et al. 2006. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc Natl Acad Sci USA 103(28):10684–10689, PMID: 16815976, 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolinoy DC, Weidman JR, Jirtle RL. 2007. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol 23(3):297–307, PMID: 17046196, 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Thomason ME, Hect JL, Rauh VA, Trentacosta C, Wheelock MD, Eggebrecht AT, et al. 2019. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage 191:186–192, PMID: 30739062, 10.1016/j.neuroimage.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neier K, Montrose L, Chen K, Malloy MA, Jones TR, Svoboda LK, et al. 2020. Short- and long-term effects of perinatal phthalate exposures on metabolic pathways in the mouse liver. Environ Epigenet 6(1):dvaa017, PMID: 33391822, 10.1093/eep/dvaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rygiel CA, Goodrich JM, Solano-González M, Mercado-García A, Hu H, Téllez-Rojo MM, et al. 2021. Prenatal lead (Pb) exposure and peripheral blood DNA methylation (5mC) and hydroxymethylation (5hmC) in Mexican adolescents from the ELEMENT birth cohort. Environ Health Perspect 129(6):67002, PMID: 34152198, 10.1289/EHP8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C-H, Jiang SS, Chang I-S, Wen H-J, Sun C-W, Wang S-L. 2018. Association between fetal exposure to phthalate endocrine disruptor and genome-wide DNA methylation at birth. Environ Res 162:261–270, PMID: 29367177, 10.1016/j.envres.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Sobolewski M, Varma G, Adams B, Anderson DW, Schneider JS, Cory-Slechta DA. 2018. Developmental lead exposure and prenatal stress result in sex-specific reprograming of adult stress physiology and epigenetic profiles in brain. Toxicol Sci 163(2):478–489, PMID: 29481626, 10.1093/toxsci/kfy046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Wang K, Svoboda LK, Rygiel CA, Neier K, Jones TR, et al. 2021. Perinatal DEHP exposure induces sex- and tissue-specific DNA methylation changes in both juvenile and adult mice. Environ Epigenet 7(1):dvab004, PMID: 33986952, 10.1093/eep/dvab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svoboda LK, Ishikawa T, Dolinoy DC. 2022. Developmental toxicant exposures and sex-specific effects on epigenetic programming and cardiovascular health across generations. Environ Epigenet 8(1):dvac017, PMID: 36325489, 10.1093/eep/dvac017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh G, Singh V, Sobolewski M, Cory-Slechta DA, Schneider JS. 2018. Sex-dependent effects of developmental lead exposure on the brain. Front Genet 9:89, PMID: 29662502, 10.3389/fgene.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrere-Cain R, Allard P. 2020. An understudied dimension: why age needs to be considered when studying epigenetic-environment interactions. Epigenet Insights 13:2516865720947014, PMID: 32864568, 10.1177/2516865720947014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K, Liu S, Svoboda LK, Rygiel CA, Neier K, Jones TR, et al. 2020. Tissue- and sex-specific DNA methylation changes in mice perinatally exposed to lead (Pb). Front Genet 11:840, PMID: 32973866, 10.3389/fgene.2020.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang E-R, Iqbal K, Tran DA, Rivas GE, Singh P, Pfeifer GP, et al. 2011. Effects of endocrine disruptors on imprinted gene expression in the mouse embryo. Epigenetics 6(7):937–950, PMID: 21636974, 10.4161/epi.6.7.16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnamoorthy M, Gerwe BA, Scharer CD, Heimburg-Molinaro J, Gregory F, Nash RJ, et al. 2011. GABRB3 gene expression increases upon ethanol exposure in human embryonic stem cells. J Recept Signal Transduct Res 31(3):206–213, PMID: 21619448, 10.3109/10799893.2011.569723. [DOI] [PubMed] [Google Scholar]
- 59.Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, et al. 2003. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112(8):1255–1263, PMID: 14561710, 10.1172/JCI19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggermann T, Begemann M, Kurth I, Elbracht M. 2019. Contribution of GRB10 to the prenatal phenotype in Silver-Russell syndrome? Lessons from 7p12 copy number variations. Eur J Med Genet 62(7):103671, PMID: 31100449, 10.1016/j.ejmg.2019.103671. [DOI] [PubMed] [Google Scholar]
- 61.Nye MD, King KE, Darrah TH, Maguire R, Jima DD, Huang Z, et al. 2016. Maternal blood lead concentrations, DNA methylation of MEG3 DMR regulating the DLK1/MEG3 imprinted domain and early growth in a multiethnic cohort. Environ Epigenet 2(1):dvv009, PMID: 28123784, 10.1093/eep/dvv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Zhang T, Qin X-S, Ge W, Ma H-G, Sun L-L, et al. 2014. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep 41(3):1227–1235, PMID: 24390239, 10.1007/s11033-013-2967-7. [DOI] [PubMed] [Google Scholar]
- 63.Kalish JM, Jiang C, Bartolomei MS. 2014. Epigenetics and imprinting in human disease. Int J Dev Biol 58(2–4):291–298, PMID: 25023695, 10.1387/ijdb.140077mb. [DOI] [PubMed] [Google Scholar]
- 64.Reik W, Dean W, Walter J. 2001. Epigenetic reprogramming in mammalian development. Science 293(5532):1089–1093, PMID: 11498579, 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 65.Muhr J, Arbor TC, Ackerman KM. 2023. Embryology, Gastrulation. StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- 66.Weinstein LS, Xie T, Zhang Q-H, Chen M. 2007. Studies of the regulation and function of the Gs alpha gene Gnas using gene targeting technology. Pharmacol Ther 115(2):271–291, PMID: 17588669, 10.1016/j.pharmthera.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Chang S, Wang Z, Wang S, Huo J, Ding G, et al. 2017. Altered GNAS imprinting due to folic acid deficiency contributes to poor embryo development and may lead to neural tube defects. Oncotarget 8(67):110797–110810, PMID: 29340017, 10.18632/oncotarget.22731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanna P, Francou B, Delemer B, Jüppner H, Linglart A. 2021. A novel familial PHP1B variant with incomplete loss of methylation at GNAS-A/B and enhanced methylation at GNAS-AS2. J Clin Endocrinol Metab 106(9):2779–2787, PMID: 33677588, 10.1210/clinem/dgab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turan S, Bastepe M. 2013. The GNAS complex locus and human diseases associated with loss-of-function mutations or epimutations within this imprinted gene. Horm Res Paediatr 80(4):229–241, PMID: 24107509, 10.1159/000355384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wroe SF, Kelsey G, Skinner JA, Bodle D, Ball ST, Beechey CV, et al. 2000. An imprinted transcript, antisense to Nesp, adds complexity to the cluster of imprinted genes at the mouse Gnas locus. Proc Natl Acad Sci USA 97(7):3342–3346, PMID: 10716699, 10.1073/pnas.97.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayward BE, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, et al. 1998. The human GNAS1 gene is imprinted and encodes distinct paternally and biallelically expressed G proteins. Proc Natl Acad Sci USA 95(17):10038–10043, PMID: 9707596, 10.1073/pnas.95.17.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie T, Plagge A, Gavrilova O, Pack S, Jou W, Lai EW, et al. 2006. The alternative stimulatory G protein alpha-subunit XLalphas is a critical regulator of energy and glucose metabolism and sympathetic nerve activity in adult mice. J Biol Chem 281(28):18989–18999, PMID: 16672216, 10.1074/jbc.M511752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tycko B. 2010. Allele-specific DNA methylation: beyond imprinting. Hum Mol Genet 19(R2):R210–R220, PMID: 20855472, 10.1093/hmg/ddq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desbuquois B, Carré N, Burnol A-F. 2013. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J 280(3):794–816, PMID: 23190452, 10.1111/febs.12080. [DOI] [PubMed] [Google Scholar]
- 75.Plasschaert RN, Bartolomei MS. 2015. Tissue-specific regulation and function of Grb10 during growth and neuronal commitment. Proc Natl Acad Sci USA 112(22):6841–6847, PMID: 25368187, 10.1073/pnas.1411254111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo L, Jiang W, Liu H, Bu J, Tang P, Du C, et al. 2018. De-silencing Grb10 contributes to acute ER stress-induced steatosis in mouse liver. J Mol Endocrinol 60(4):285–297, PMID: 29555819, 10.1530/JME-18-0018. [DOI] [PubMed] [Google Scholar]
- 77.Blagitko N, Mergenthaler S, Schulz U, Wollmann HA, Craigen W, Eggermann T, et al. 2000. Human GRB10 is imprinted and expressed from the paternal and maternal allele in a highly tissue- and isoform-specific fashion. Hum Mol Genet 9(11):1587–1595, PMID: 10861285, 10.1093/hmg/9.11.1587. [DOI] [PubMed] [Google Scholar]
- 78.Yamasaki-Ishizaki Y, Kayashima T, Mapendano CK, Soejima H, Ohta T, Masuzaki H, et al. 2007. Role of DNA methylation and histone H3 lysine 27 methylation in tissue-specific imprinting of mouse Grb10. Mol Cell Biol 27(2):732–742, PMID: 17101788, 10.1128/MCB.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bakulski KM, Blostein F, London SJ. 2023. Linking prenatal environmental exposures to lifetime health with epigenome-wide association studies: state-of-the-science review and future recommendations. Environ Health Perspect 131(12):126001, PMID: 38048101, 10.1289/EHP12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doshi T, D’souza C, Vanage G. 2013. Aberrant DNA methylation at Igf2-H19 imprinting control region in spermatozoa upon neonatal exposure to bisphenol A and its association with post implantation loss. Mol Biol Rep 40(8):4747–4757, PMID: 23653003, 10.1007/s11033-013-2571-x. [DOI] [PubMed] [Google Scholar]
- 81.Kochmanski JJ, Marchlewicz EH, Cavalcante RG, Perera BPU, Sartor MA, Dolinoy DC. 2018. Longitudinal effects of developmental bisphenol A exposure on epigenome-wide DNA hydroxymethylation at imprinted loci in mouse blood. Environ Health Perspect 126(7):077006, PMID: 30044229, 10.1289/EHP3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schrott R, Greeson KW, King D, Symosko Crow KM, Easley CA, Murphy SK. 2022. Cannabis alters DNA methylation at maternally imprinted and autism candidate genes in spermatogenic cells. Syst Biol Reprod Med 68(5–6):357–369, PMID: 35687495, 10.1080/19396368.2022.2073292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soubry A, Hoyo C, Butt CM, Fieuws S, Price TM, Murphy SK, et al. 2017. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ Epigenet 3(1):dvx003, PMID: 29492305, 10.1093/eep/dvx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakulski KM, Feinberg JI, Andrews SV, Yang J, Brown S, McKenney SL, et al. 2016. DNA methylation of cord blood cell types: applications for mixed cell birth studies. Epigenetics 11(5):354–362, PMID: 27019159, 10.1080/15592294.2016.1161875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armand EJ, Li J, Xie F, Luo C, Mukamel EA. 2021. Single-cell sequencing of brain cell transcriptomes and epigenomes. Neuron 109(1):11–26, PMID: 33412093, 10.1016/j.neuron.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campbell KA, Colacino JA, Park SK, Bakulski KM. 2020. Cell types in environmental epigenetic studies: biological and epidemiological frameworks. Curr Environ Health Rep 7(3):185–197, PMID: 32794033, 10.1007/s40572-020-00287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.