Abstract
Background
Surgery is sometimes recommended for femoroacetabular impingement where non‐operative interventions have failed.
Objectives
To determine the benefits and safety of surgery for femoroacetabular impingement.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11); MEDLINE (Ovid) (1946 to 19 November 2013); and EMBASE (Ovid) (1980 to 19 November 2013) for studies, unrestricted by language.
Selection criteria
Randomised and quasi‐randomised clinical trials assessing surgical intervention compared with placebo treatment, non‐operative treatment or no treatment in adults with femoroacetabular impingement.
Data collection and analysis
Two authors independently selected trials for inclusion, assessed risk of bias and extracted data.
Main results
There were no studies that met the inclusion criteria, with 11 studies that were excluded following detailed review. There were four ongoing studies identified that may meet the inclusion criteria when they are completed; the results from these ongoing studies may begin to become available within the next five years. Three of the four ongoing studies are comparing hip arthroscopy versus non‐operative care. The fourth study is comparing hip arthroscopy versus a sham arthroscopic hip procedure. All of the ongoing studies are recording at least one of our preferred clinical outcome measures for benefit and safety.
Authors' conclusions
There is no high quality evidence examining the effectiveness of surgery for femoroacetabular impingement. There are four ongoing studies, which may provide evidence for the benefit and safety of this type of surgery in the future.
Keywords: Humans, Femoracetabular Impingement, Femoracetabular Impingement/surgery
Plain language summary
Surgery for hip impingement (femoroacetabular impingement)
This summary of a Cochrane review presents what we know from research about the effectiveness and safety of surgery for hip impingement (also called femoroacetabular impingement). A search for relevant studies was done on 19th November 2013. The review of the results from this search showed the following.
1. No research studies have been completed that are of sufficient quality to accurately determine the benefit and safety of surgery for femoroacetabular impingement.
2. Four ongoing research studies may help to determine the benefit and safety of femoroacetabular impingement surgery when they are completed.
All of the ongoing research studies are recruiting adult patients with femoroacetabular impingement. Three of these studies are comparing hip arthroscopy versus non‐operative care. The fourth study is comparing hip arthroscopy versus a sham arthroscopic hip procedure.
What is femoroacetabular impingement and what is surgery? Femoroacetabular impingement, or hip impingement, occurs because of subtle abnormalities of hip shape. The abnormalities of hip shape can cause damage to soft tissues around the hip including the cartilage (on the surfaces of the joint), which allows the joint to move freely. Femoroacetabular impingement can cause pain and restrict hip function. Surgery for femoroacetabular impingement can be either arthroscopic (keyhole) or open surgery. The aim of surgery is to correct the hip shape abnormalities and to repair damaged soft tissues and cartilage.
Best estimate of what happens to people with femoroacetabular impingement who have surgery
Until any high quality research studies are available for review it is not possible to provide any estimate of whether surgery helps people with femoroacetabular impingement or not.
Background
Description of the condition
In the last few years there has been increasing recognition of the syndrome of femoroacetabular impingement (FAI), sometimes also called hip impingement, which seems to account for a large proportion of previously undiagnosed cases of hip pain and restricted range of motion in young adults (Lavigne 2004).
Subtle shape abnormalities of the hip (a ball and socket joint) combine to cause impingement between the femoral neck and the head (ball) and anterior rim of the acetabulum (socket), most often in flexion and with internal rotation (Lavigne 2004). Three types of deformities have been recognised:
1. cam type, asphericity of the femoral head and widening of the femoral neck (abnormalities of shape, which typically bump around the ball of the joint);
2. pincer type, over coverage of the antero‐superior acetabular wall, and abnormal version of the femur or acetabulum (irregularities of shape, typically a socket that is too deep or pointing in an abnormal direction); or
3. mixed type, a combination of the two (Ganz 2003).
Excess contact forces between the proximal femur and the acetabular rim during the end range of motion of the hip results in soft tissue lesions of the acetabular labrum (the soft cushion around the socket) and the adjacent acetabular cartilage leading to pain and restricted range of movement (Beck 2004). FAI seems to be associated with progressive articular degeneration of the acetabulum (usually starting from the antero‐superior rim and extending medially and posteriorly) and femoral head (Beck 2004).
The cause of FAI shape malformations is likely to be multifactorial but may include slipped capital femoral epiphysis (SCFE), which is thought to lead to cam‐type FAI (Leunig 2000; Mamisch 2009; Millis 2011). However, other authors now suggest that FAI shape malformations are actually part of a hominid evolutionary process (Hogervorst 2011) whereby cam‐type morphology, so called cox recta, is an adaptive morphology to enable faster running. So called coxa profunda, consistent with pincer‐type FAI (more common in females), has enabled the female human pelvis to accommodate child birth and the relatively large fetal head. An evolutionary explanation for FAI would help to explain the relatively high prevalence of FAI shape malformations in the general population and the differences in prevalence of the FAI subtypes between females and males (Gosvig 2010; Reichenbach 2010; Leunig 2013). Despite equivocal information about the causes of FAI, there is growing evidence that the shape malformations consistent with FAI are associated with the development of hip osteoarthritis (Ganz 2003; Kim 2006; Gregory 2007; Harris‐Hayes 2011).
Description of the intervention
Numerous surgical techniques have been described as treatment for FAI, which is a reflection of the number of shape abnormalities that can constitute FAI. However, the underlying principle for all surgery is to correct the perceived abnormality in bony shape in order to prevent impingement between the femoral neck and rim of the acetabulum. In the case of cam‐type FAI this would typically involve osteochondroplasty (removal of bone at the femoral head‐neck junction) and improving the offset between the femoral head and neck. In the case of pincer type FAI it may involve removal of bone at the rim of the acetabulum. Reorientation osteotomies (realignment of the bones) around the hip may also be appropriate. At the same time as bony shape corrective surgery, any soft tissue damage to the cartilage or labrum as a result of the FAI is typically either debrided, repaired or reconstructed. Surgical approaches are either open or arthroscopic.
Open surgery
a. Safe surgical dislocation approach. In 2001, Ganz 2001 described a surgical technique to dislocate the hip joint without damaging the blood supply to the femoral head. This allowed surgeons circumferential access to the hip joint to undertake shape corrective surgery. The Ganz technique is a substantial operation, which requires a part of the femur (the trochanter, a prominence of the femur) to be cut and reattached at the end of the operation (trochanteric osteotomy). Therefore a period of partial or non‐weight bearing is required post‐operatively while the cut bone (osteotomy) heals.
b. Mini‐open anterior approach. This approach does not involve dislocating the hip joint but provides only limited access to the anterior aspect of the hip joint. The anterior aspect of the hip is where FAI typically occurs. The access provided by the mini‐open approach is limited and therefore can be assisted with arthroscopy to improve the visibility (Hartmann 2009).
c. Osteotomy (femoral or acetabular side, or both) allows the femur or acetabulum to be re orientated in such a way as to reduce or prevent FAI.
Arthroscopic surgery
Arthroscopic (keyhole) surgery can be used to treat FAI (Murphy 2004). Traction is applied to the leg in order to distract the joint and allow access to the hip joint. Arthroscopic portals are inserted that run from the skin surface though the soft tissues (skin, fat, muscle and hip capsule) and into the hip joint. An arthroscope is passed through one of the portals and provides visualisation of the hip joint and areas of FAI. Instruments are passed into the hip joint through other portals. Burrs are typically used to undertake osteochondroplasty (reshaping of the hip bones, the femoral head‐neck and acetabulum).
How the intervention might work
In flexion and internal rotation the hip joint would naturally be restricted at some point by contact between the femoral neck and the rim of the acetabulum. The concept of FAI is that premature contact occurs between the femoral head‐neck junction and the anterior rim of the acetabulum, usually as the hip is flexed and internally rotated (Ganz 2003). This restricts range of motion of the joint and causes soft tissue damage leading to pain.
The principle of FAI surgery is that this premature contact is prevented by bony surgery to change the shape or orientation of the hip joint. The surgery also addresses damage that has been caused by impingement, particularly to the labrum (fibrocartilage rim of the acetabulum) and the articular cartilage which lines the acetabulum. The labrum may be debrided, repaired or reconstructed, and the articular cartilage may be repaired by a variety of techniques.
It is believed that the pain associated with FAI is due to labral and cartilage injury. Once these have been repaired, and further damage prevented by correcting impingement, symptoms may improve.
Why it is important to do this review
FAI surgery has evolved rapidly and at a pace far quicker than our understanding about the natural history and epidemiological characteristics of the condition (Allen 2009; Takeyama 2009; Clohisy 2010; Gosvig 2010; Hack 2010). Although some evidence exists to suggest that abnormal hip shape morphology is associated with both pain and osteoarthritis, a true causal effect relationship has yet to be proven. In light of this, it is not clear whether surgically correcting shape will have any true beneficial effect on symptoms such as pain or reduce the risk of osteoarthritis. Establishing the true effect of surgery in terms of benefits and harms would help guide both clinicians and patients when considering treatment. It has also been suggested that randomised controlled trials (RCTs) are feasible in this field of research (Palmer 2013). Several authors have sought to review the range of adverse events resulting from FAI surgery based on data from case series (Harris 2013; Kowalczuk 2013). However, they acknowledge that more robust adverse event reporting and safety profiling for FAI surgery could be elicited from RCT data.
Objectives
To determine the benefits and safety of surgical interventions for treating FAI.
Methods
Criteria for considering studies for this review
Types of studies
Only studies where participants were either randomised or quasi‐randomised (method of allocating participants to a treatment which is not strictly random, for example by date of birth, hospital record number or alternation) into intervention groups were to be included in this review.
Types of participants
There are no established criteria for FAI (Ayeni 2013a). The diagnosis is generally made on the basis of symptoms of hip or groin pain, or both; restricted range of motion and a positive anterior impingement test (when the hip joint is placed in a position of flexion, adduction and internal rotation pain may be experienced); and the presence of abnormal hip shape morphology and abnormalities of the adjacent labrum and cartilage on imaging. The hip shape imaging should include cross‐sectional studies. These may be: computed tomography (CT), magnetic resonance imaging (MRI), or magnetic resonance arthrography (MRA) (Ganz 2003; Beall 2005).
Trials that included participants with FAI as determined by the trial authors were to be included provided they conformed to the above criteria. We planned to systematically review the definitions of FAI used in any included trials. If definitions of FAI differed we planned to consider the appropriateness of pooling data. We planned to explore whether differences in how FAI was defined in the trials (inclusion criteria) made any difference to the results.
If the trials included people with established osteoarthritis (OA) then we planned to include the trials only if data for people without established OA were presented separately, or if people with OA were 10% or less of the study population. We planned to include definitions of OA as per the included trials. Although current evidence suggests that FAI is a risk factor for subsequent OA (Gosvig 2010), there is no suggestion in the literature that surgery for FAI is a treatment for established OA.
We planned to exclude studies that had more than 10% of participants with evidence of OA.
Types of interventions
Studies of all types of FAI surgery were to be included. Surgery could be performed using open, mini‐open, arthroscopic, assisted mini‐open or arthroscopic approaches; and the interventions could consist of:
1. reshaping of the hip joint by removing bone or cartilage, or both (osteoplasty, osteochondroplasty) from either the femoral head‐neck junction or rim of the acetabulum;
2. reorientating the hip joint by cutting the bones around the hip joint (osteotomy) and refixing the bones in a new orientation. The new orientation of the hip should reduce the risk of future FAI. The bony reorientation can be done for the femur or acetabulum, or both.
Comparators could be:
1. placebo (sham surgery);
2. no treatment;
3. non‐operative treatment (e.g. physical therapy, analgesia, glucocorticoid injection, activity modification).
Types of outcome measures
We planned to not exclude studies on the basis of outcome measures. We did plan to use a hierarchy if multiple tools were used to measure the same functional outcomes. This hierarchy was based upon recent work and evidence supporting the use of a set of core outcomes for painful musculoskeletal conditions (Eccleston 2010; Lodhia 2010).
Major outcomes
1. Proportion of participants with 30% reduction in pain or greater.
2. Pain reported in the following hierarchy of preferred measures:
a. proportion with 50% reduction in pain or greater, or if unavailable
b. proportion below 30/100 mm on the visual analogue scale (VAS), or if unavailable
c. mean change or mean absolute pain score on a VAS or numerical rating scale, or
d. if pain is not reported on its own but as a hip‐specific multidomain outcome assessment, an attempt will be made to obtain the breakdown of the score in order to extract the data on pain.
3. Hip function.
A review of hip‐specific multidomain outcome measures recommended that, of established instruments, the Non‐Arthritic Hip Score and the Hip Outcome Score had the strongest clinimetric evidence to support their use as primary outcome instruments in studies for the measurement of the effectiveness of treatment of FAI (Lodhia 2010). The International Hip Outcome Tool (iHOT‐33 and iHOT‐12) has been recently developed for active patients aged 18 to 60 years who present with a variety of hip conditions (Griffin 2012a ; Mohtadi 2012). Expected measures of hip function include: iHOT‐33; iHOT‐12; Non‐Arthritic Hip Score (NAHS); Hip Outcome Score (HOS); Modified Harris Hip Score (MHHS); Vail Hip Score; Western Ontario and McMaster Universities Arthritis Index (WOMAC).
4. Quality of life, as measured by generic instruments such as the Short Form ‐36 (SF‐36), SF‐12, EuroQol‐5D (EQ‐5D).
5. Participant global assessment of treatment success.
6. The proportion of participants with any adverse events (AEs). AEs are defined as any untoward medical occurrence in a clinical trial patient and which do not necessarily have a causal relationship with the treatment. Possible AEs include death; fluid extravasation; fracture; avascular necrosis; and venous thromboembolism (VTE), numbness, and muscle soreness.
7. Proportion of participants with a serious adverse event (SAE). SAEs are defined as adverse events that are fatal, life‐threatening, or require hospitalisation.
Our research question was on the efficacy and safety of FAI surgery. We regarded an effect on progression to OA to be an important outcome in the longer term (Ganz 2003 ; Beck 2005). However, it was unlikely that if trials existed they would have the long term follow‐up to answer this question. We therefore agreed that the first and most important question to answer was whether surgery in the short to medium term was both effective (relieved pain) and safe.
Timing of outcome assessment
Studies were likely to report the outcomes discussed at several time points. We therefore planned to attempt to group these assessments into three categories: short (up to and including three months), medium (after three months and up to and including 12 months) and long term follow‐up (greater than one year). We planned to extract AEs at the end of the trial.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for trials, unrestricted by date or language:
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 11);
MEDLINE (Ovid) (1946 to 19 November 2013); and
EMBASE (Ovid) (1980 to 19 November 2013).
In MEDLINE, we combined a subject specific search with the Cochrane highly sensitive search strategy for identifying randomised trials, sensitivity‐maximising version (Lefebvre 2011). The strategy was designed in MEDLINE and adapted to the other databases. The search strategies are shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4.
Trial registries (World Health Organization (WHO) International Clinical Trials Registry Platform at http://apps.who.int/trialsearch/; ClinicalTrials register at http://www.clinicaltrials.gov/; Current Controlled Trials register at http://www.controlled‐trials.com/) were also searched to identify trials that are currently underway.
Searching other resources
Reference lists of all primary studies and review articles were searched for additional references. We did not search informally published written material (so called 'grey literature').
Data collection and analysis
Selection of studies
Two authors (PW and JB) independently selected the studies for inclusion in the review. Titles and abstracts obtained from the searches were reviewed to determine potential eligibility and shortlisted if appropriate. The full text of each study in this shortlist was then reviewed to determine which studies, if any, were eligible for inclusion in the review. Any disagreement between the two authors was resolved by consensus or discussion with a third review author (MC or DG). Studies were translated into English where necessary.
Data extraction and management
Two review authors (PW and JB) planned to independently extract the following data from included trials and enter the data in to RevMan 5:
1. trial characteristics, including size and location of the trial, and source of funding;
2. characteristics of the study population, including age, and characteristics of the FAI including diagnostic criteria, type and duration of symptoms;
3. characteristics of the surgery and comparator treatment, including surgical approach, type of FAI being addressed (cam, pincer or mixed), type of intervention used to correct the FAI (osteochondroplasty or osteotomy);
4. risk of bias domains, as outlined in Assessment of risk of bias in included studies, below;
5. outcome measures as means and standard deviations, number of participants per treatment group for continuous outcomes (pain when reported as either a mean or change in pain score, hip function, and quality of life), and number of events and number of participants per treatment group for dichotomous outcomes (efficacy, pain when reported as a proportion, SAEs, AEs, and participant global assessment of treatment success).
If additional data were required, we planned to contact the trial team to obtain these. Where data were imputed or calculated (Dealing with missing data) we planned to report this in the 'Characteristics of included studies' table. Any disagreements were to be resolved by consensus or discussion with a third review author (RB). Extracted data from the studies were to be managed and collated by the review statistician (NP).
Assessment of risk of bias in included studies
Studies included in the review were to be assessed for risk of bias using the recommended Cochrane Collaboration risk of bias tool (Higgins 2011a). This tool incorporates assessment of randomisation (sequence generation and allocation concealment), blinding (participants, personnel and outcome assessors), completeness of outcome data, selection of outcomes reported, and other sources of bias including any potential conflicts of interest for industry funded research. To determine the risk of bias of a study, we planned to assess each criterion for the presence of sufficient information and the likelihood of potential bias. Each criterion was to be rated as ‘Low risk’ of bias, ‘High risk’ of bias or ‘Unclear risk’ of bias (uncertain of the potential for bias, or insufficient information reported to make an assessment). In a consensus meeting disagreements among the review authors were to be discussed and resolved. A third review author (DG) was available to make the final decision if no consensus could be reached.
Measures of treatment effect
Risk ratios with 95% confidence intervals (CIs) were to be used to express the intervention effect for the following dichotomous outcomes:
pain, when reported as a proportion of participants within defined limits (i.e. reduction in pain of 30% or greater, 30/100 mm or less on VAS);
AEs;
SAEs;
participant global assessment of treatment success.
Where dichotomous data from cross‐over trials were combined with data from parallel‐group trials the odds ratio (OR) with 95% CI was to be calculated, rather than relative risk (RR).
We planned to calculate the mean difference (MD) or, where studies used different measurement tools, standardised mean difference (SMD), both with 95% CIs, for the following continuous outcomes:
pain, when reported as either the mean change in pain scores or mean absolute pain scores;
hip function;
quality of life.
Unit of analysis issues
We expected that all studies would report simple parallel‐group designs. However, if other designs were reported (for example cluster randomised designs), and where appropriate, generic inverse variance methods were to be used to combine data. In the analysis we planned to use details of intra‐class correlation coefficients and cluster sizes (if available) to correct the sample sizes for trials of this type if reported effects had not been adjusted for clustering. For studies containing more than two intervention groups, in making multiple pair‐wise comparisons between all possible pairs of intervention groups possible we planned to include the same group of participants only once in the meta‐analysis.
Where studies included either same day or delayed bilateral FAI hip surgery this information was to be extracted and reported in the summary of findings along with the corresponding unit of analysis (either participants or hips). Studies that used the hip as the unit of analysis were to be regarded as potentially inferior because the outcome variable would no longer be independent.
Dealing with missing data
We planned to seek additional information from the authors of the included studies where the published information or data were incomplete. In cases where individuals were missing from the reported results, we planned to assume that the missing value had a poor outcome. For dichotomous outcomes that measured SAEs and AEs (for example number of SAEs), the number of patients that received the treatment was to be used as the denominator (worst case analysis). For dichotomous outcomes that measured benefits, the worst case analysis was to be calculated using the number of randomised participants as the denominator. For continuous outcomes (for example pain) we planned to calculate the mean difference (MD) or standardised mean difference (SMD) based on the number of patients at the time point. If the numbers of patients were not presented for each time point, the numbers of randomised patients in each group at baseline was to be used. Sensitivity analysis was to be conducted to test the effect of these assumptions.
Where possible, missing standard deviations were to be computed from other statistics such as standard errors, confidence intervals (CI) or P values according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If small amounts of outcome data were missing (for example standard deviations) we planned to consider imputing them (with the appropriate sensitivity analyses) from other studies (Higgins 2011b).
Assessment of heterogeneity
For any studies judged as clinically homogenous, the degree of statistical heterogeneity between studies was first to be assessed graphically using a forest plot and then more formally using the I² statistic (Deeks 2011). The following values were to be used as a rough guide for interpretation: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% considerable heterogeneity. In cases of considerable heterogeneity (defined as I2 ≥ 75%) we planned to explore the data further, including undertaking subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
In order to determine whether reporting bias was present, we planned to determine whether the protocol of the RCT was published before recruitment of patients into the study was started. For studies published after 1 July 2005, we planned to screen the clinical trial register in the WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch) (DeAngelis 2004). We also planned to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
We planned to compare the fixed‐effect model estimate against the random‐effects model to assess the possible presence of small sample bias in the published literature (that is where the intervention effect is more beneficial in smaller studies). In the presence of small sample bias, the random‐effects model estimate of the intervention is more beneficial than the fixed‐effect model estimate (Sterne 2011).
The potential for reporting bias was to be further explored by funnel plots if ≥ 10 studies were available.
Data synthesis
Where studies were sufficiently homogeneous that it was clinically meaningful for them to be pooled, meta‐analysis was to be performed using a random‐effects model regardless of the I2 results. Analysis was to be performed using Review Manager 5 and forest plots produced for all analyses. Where both change and mean pain were reported in studies, we planned to pool the results based upon whichever was reported by the majority of the included studies.
Summary of findings tables
We planned to present the main results of the review in 'Summary of findings' (SoF) tables, which would provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of the available data on the outcomes (efficacy, SAEs, pain, AEs, hip function, participant global assessment, quality of life), as recommended by The Cochrane Collaboration (Schünemann 2011a). The SoF table includes an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2011b).
In addition to the absolute and relative magnitude of effect provided in the SoF table, for dichotomous outcomes the numbers needed to treat to benefit (NNTB) or the numbers needed to treat to harm (NNTH) were to be calculated from the control group event rate (unless the population event rate was known) and the risk ratio using the Visual Rx NNT calculator (Cates 2008). For continuous outcomes the NNT were to be calculated using the Wells calculator software available at the CMSG editorial office (www.cochranemsk.org). The minimal clinically important difference (MCID) for each outcome was to be determined for input into the calculator.
MCIDs have been defined (where known) for each continuous outcome, see below.
Pain
i. VAS: no published references when applied to FAI. However, when used for patients with hip and knee OA treated with non‐steroidal anti‐inflammatory medications an MCID of 15.3 mm has been reported (Tubach 2005).
Hip function
i. NAHS: no published references.
ii. iHOT‐33: no published references when applied to FAI. However, when used for patients undergoing hip arthroscopy an MCID of 6.1 points (100 point scale) is reported in the original paper and more recently < 11 points has been reported (Mohtadi 2012; Kemp 2013).
iii. iHOT‐12: not published but authors suggest similar performance to iHOT‐33 (Griffin 2012a).
iv. HOS: no published references.
v. MHHS: no published references when applied to FAI. However, when used for patients undergoing hip arthroscopy an MCID of < 11 points (100 point scale) is reported (Kemp 2013).
vi. Vail Hip Score: no published references.
vii. WOMAC: no published references when applied to FAI. However, when used for patients undergoing total hip arthroplasty at six months the MCID exceeds 25 points (100 point scale) for all domains (pain, functional limitation, and stiffness) (Quintana 2005).
Quality of life
i. SF‐36: no published references when applied to FAI. However, when applied to patients undergoing total hip arthroplasty the following are reported for each domain, 11 points for physical role and 20 points for physical function (using a 100 point scale) (Keurentjes 2012).
ii. SF‐12: no published references when applied to FAI. However, SF‐12 is a shortened derivative of SF‐36 and as a result correlates closely (Ostendorf 2004). A published report for unrelated surgery (cervical decompression) reports an MCID of 2.5 points for the physical component and 10.1 points for the mental component (Parker 2012).
iii. EQ‐5D: no published references when applied to FAI. However, more empirical analysis across several applications suggests (across a continuous scale from ‐0.59 to 1) a mean of 0.074 (range ‐0.011 to 0.140) (Walters 2005).
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, the following subgroup analyses were planned.
1. Cam versus pincer versus mixed‐type FAI. These shape abnormalities arise from different aspects of the hip joint and therefore the results of surgery may differ between the types. Subgroup analysis will measure the results of surgery using pain as the outcome.
2. Open versus arthroscopic surgery. Previous research suggests that the outcome in terms of pain and function is comparable for open and arthroscopic surgery, but that the risk of AEs is greater from open surgery (Botser 2011).
Subgroup analyses were planned to measure the results of surgery using pain and the proportion of participants with any AEs as the outcomes. The subgroup analyses were also planned to informally compare the magnitudes of effect to assess possible differences in response to treatment by considering the overlap of the CIs of the summary estimates in the two subgroups; non‐overlap of the CIs indicating statistical significance.
Sensitivity analysis
If it was deemed necessary to exclude any studies because they appeared to differ markedly (that is if the outcome was different, the effect went in the opposite direction) from the majority of studies then all main analyses were to have been reported with and without these studies.
Where sufficient studies existed, sensitivity analyses were planned to assess the impact on the primary outcome of any bias attributable to inadequate or unclear treatment allocation (including studies with quasi‐randomised designs) and blinding.
Results
Description of studies
Results of the search
A search was completed on 19th November 2013. There were 433 results of which 82 were duplicates. A total of 351 reference titles and abstracts were reviewed for inclusion. We shortlisted 11 primary studies that were reported to describe the effectiveness of surgery for FAI. A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of the search results is shown in Figure 1.
1.
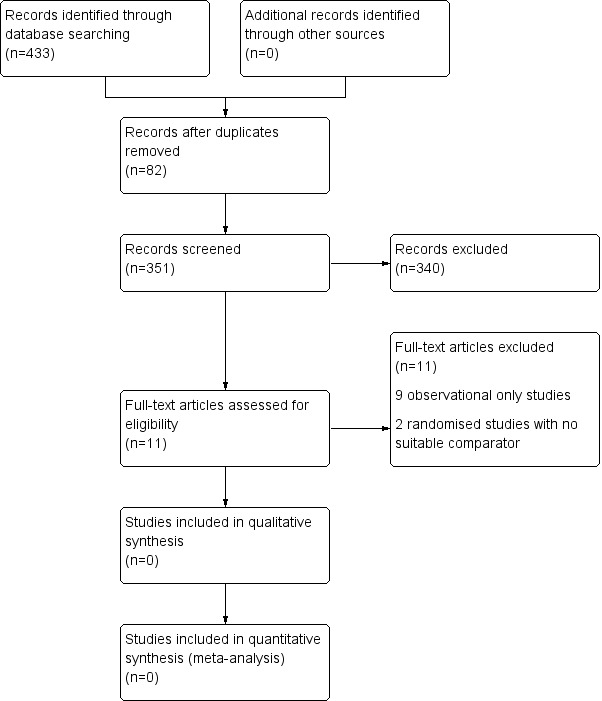
PRISMA flow diagram of search results.
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. For more information, visit www.prisma‐statement.org/
Included studies
None of the shortlisted studies met our inclusion criteria. However, we found four ongoing trials (Naudie 2011 ; Ayeni and Bhandari 2012 ; Griffin 2012; Glynn‐Jones 2013) that may be eligible in future review updates. Naudie 2011 is a single centre RCT comparing arthroscopic surgery versus physiotherapy. The sample size for this RCT is 140 participants. Griffin 2012 is a multicentre (25 sites) RCT comparing arthroscopic surgery versus physiotherapy. The sample size for this RCT is 372 participants. Ayeni and Bhandari 2012 is a multicentre (two sites) RCT comparing arthroscopic surgery with sham arthroscopic surgery. The sample size for this RCT is 220 participants. Glynn‐Jones 2013 is a multicentre (two sites) RCT comparing arthroscopic surgery versus physiotherapy. The sample size for this RCT is 120 participants. A summary of the ongoing studies including number of recruiting centres, target sample size and number recruited up to the end of January 2014, type of surgery and comparator, primary outcome measure and time point post‐randomisation, and projected study completion date is shown in Table 1 and in Characteristics of ongoing studies.
1. Summary of ongoing studies.
| Study | No. of recruiting centres | Sample size | No. recruited to end of January 2014 | Type of surgery | Comparator | Primary outcome measure and time point post‐randomisation | Projected completion date |
| Naudie 2011 | 1 | 140 | na | Arthroscopic surgery | Physiotherapy | HOS at 24 months | May 2014 |
| Griffin 2012 | 25 | 372 | 71 | Arthroscopic surgery | Physiotherapy | IHOT‐33 at 12 months | July 2017 |
| Ayeni and Bhandari 2012 | 2 | 220 | 53 | Arthroscopic surgery | Sham surgery ‐ arthroscopic lavage of the hip joint with 3 litres of normal saline | Change in pain on a VAS at 6 months | June 2017 |
| Glynn‐Jones 2013 | 2 | 120 | na | Arthroscopic surgery | Physiotherapy | HOS at 8 months | July 2017 |
na = not available
Key to outcome measures:
Hip Outcome Score (HOS) ‐ expressed as a score out of 100 (100 being the best outcome);
International Hip Outcome Tool (IHOT‐33) ‐ expressed as a score out of 100 (100 being the best outcome);
Visal Analogue Scale (VAS) for pain ‐ using a 10 cm line and expressed as a score out of 100 (0 being the best outcome).
Excluded studies
Of the 11 shortlisted studies, two were randomised trials (Krych 2013; Zingg 2013). However, both of these studies compared two types of FAI surgery rather than comparing surgery with one of the pre‐determined comparators (placebo, no treatment or non‐operative treatment). The remaining nine studies were excluded because they were observational studies (see Characteristics of excluded studies). A summary of these studies is shown in Table 2.
2. Summary of excluded studies.
| Author | Type of study | Intervention 1 | Intervention 2 or Comparator | Unit of analysis and no. in each group | Outcome | AEs |
| Espinosa 2006 | Observational; comparative case series | 1. Open surgery: osteoplasty + labral resection | 2. Open surgery: osteoplasty + labral refixation | Hips 1. 25 2. 35 |
Mean Merle d’Aubigné score (range) Pre‐operative 1. 12 (8‐13) 2. 12 (5‐16) 24 m post‐operative 1. 15 (10‐18) 2. 17 (13‐18) |
None reported |
| Bardakos 2008 | Observational; comparative case series | 1. Arthroscopic: osteoplasty | 2. Arthroscopic: debridement | Patients 1. 24 2. 47 |
Median HHS (IQR) Pre‐operative 1. 59 (52‐64) 2. 55 (37‐72) 12 m post‐operative 1. 83 (75‐87) 2. 77 (59‐87) |
None reported |
| Larson 2009 | Observational; comparative case series | 1. Arthroscopic: osteoplasty+labral debridement | 2. Arthroscopic: osteoplasty + labral repair | Hips 1. 36 2. 39 |
Mean MHHS 12m post op 1. 88 2. 94 |
1. Revision to THA=1 patient 2. Heterotopic ossification=3 patients 3. Revision osteoplasty=2 patients |
| Gedouin 2010 | Observational; case series | 1. Arthroscopic: osteoplasty | None | Hips 1. 111 |
Mean WOMAC score (SD) Pre‐operative 1. 60 (±14) Post‐operative at mean 10 m 1. 83 (±16) |
1. Revision to THA=5pts 2. Heterotropic ossification=3 patients 3. Neuropraxia=2 patients 4. Skin necrosis=1 patient |
| Schilders 2011 | Observational; comparative case series | 1. Arthroscopic: osteoplasty + labral repair | 2. Arthroscopic: osteoplasty + labral resection | Hips 1. 69 2. 32 |
Mean MHHS (range) Pre‐operative 1. 60 (24‐85) 2. 62 (29‐96) Post‐operative at mean 29 m 1. 93 (55‐100) 2. 88 (35‐100) |
None reported |
| Bulbul 2012 | Observational; case series | 1. Open surgery: osteoplasty | None | Pts 1. 13 |
Mean HHS (range) Pre‐operative 1. 63 (55‐70) Post‐operative 24 m 1. 89 (72‐98) |
None reported |
| Malviya 2012 | Observational; case series | 1. Arthroscopic: osteoplasty | None | Patients 1. 612 |
Mean Rosser Index Matrix‐created QoL score (range) Pre‐operative 1. 0.9 (‐1.4‐0.9) Post‐operative 12 m 1. 0.9 (0.7‐1) |
None reported |
| Palmer 2012 | Observational; case series | 1. Arthroscopic: osteoplasty | None | Hips 1. 201 |
Mean NAHS increased by 24 post‐operative at mean 46 m | None reported |
| Domb 2013 | Observational; comparative case series | 1. Open surgery: osteoplasty | 2. Arthroscopic: osteoplasty | Patients 1. 10 2. 20 |
Mean improvement in MHHS post‐operative at mean 24.8 m (range) 1. 22 (±12) 2. 24 (±11) |
None reported |
| Krych 2013 | Randomised trial comparing two different surgical interventions | 1. Arthroscopic: osteoplasty + labral repair | 2. Arthroscopic: osteoplasty + labral debridement | Patients 1. 18 2. 18 |
Mean HOS‐ADL (range) Pre‐operative 1. 68 (26‐92) 2. 60 (23‐91) Post‐operative at mean 32 m 1. 91 (73‐100) 2. 80 (42‐100) |
None reported |
| Zingg 2013 | Partly* randomised trial comparing two different surgical interventions | 1. Arthroscopic: osteoplasty | 2. Open surgery: osteoplasty | Patients 1. 23 2. 15 |
Mean pain on a VAS at rest (SD) Pre‐operative 1. 15 (21) 2. 18 (13) 12 m post‐operative 1. 5 (12) 2. 15 (22) |
None reported |
m = months, f/u = follow‐up, HHS = Harris Hip Score, IQR = interquartile range, SD = standard deviation, THA = total hip arthroplasty, * = 10 out of 38 patients included were randomised
Key to outcome measures:
Merle d’Aubigné ‐ expressed as a score out of 18 (18 being the best outcome);
Harris Hip Score (HHS) ‐ expressed as a score out of 100 (100 being the best outcome);
Modified Harris Hip Score (MHHS) ‐ expressed as a score out of 100 (100 being the best outcome);
Harris Hip Score Acitivities of Daily Living (HOS‐ADL) ‐ expressed as a score out of 100 (100 being the best outcome);
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) ‐ expressed as a score out of 100 (100 being the best outcome);
Rosser Index Matrix‐created QoL ‐ expressed as a score from ‐1.486 to 1.000 (a score of 1.000 indicates normality and death is given a score of 0.000);
Non Arthritic Hip Score (NAHS) ‐ expressed as a score out of 100 (100 being the best outcome);
Visual Analogue Scale (VAS) at rest for pain ‐ expressed as a score out of 100 (0 being no pain).
Risk of bias in included studies
No eligible studies were identified.
Effects of interventions
No published RCTs were identified, therefore the benefits and safety of surgery to treat FAI can not be reported.
Discussion
Summary of main results
The lack of any randomised or quasi‐randomised controlled trials means there is insufficient evidence to be able to draw conclusions about the benefits and safety of surgery for femoroacetabular impingement. There are, however, four ongoing registered RCTs (Naudie 2011; Ayeni and Bhandari 2012; Griffin 2012; Glynn‐Jones 2013) that are likely to meet the inclusion criteria for this review, and these will be completed within five years. One of these studies (Ayeni and Bhandari 2012) uses sham surgery as a control and can utilise formal participant blinding. The other three ongoing studies have a non‐operative treatment control and are unable to blind participants to their treatment allocation.
There were 11 shortlisted studies of surgery for FAI that were excluded after detailed review. All of these shortlisted but excluded studies reported an improvement in patient‐reported pain, function and quality of life following surgical intervention. However, none of these studies had an appropriate comparator or control group to allow a robust estimate of the true treatment effect of surgery for FAI. Most of these excluded studies (nine out of 11) did not report the frequency of AEs. In the absence of any identifiable published RCTs or quasi‐RCTs meeting the eligibility criteria, this review was not expanded to include non‐randomised studies for the following reasons.
i. None of the studies identified for full text review, which were non‐randomised, had a suitable comparator. In the absence of a suitable comparator the authors decided it was not possible to draw any meaningful inferences about the true benefit or safety of surgery for hip impingement in these studies.
ii. Several ongoing RCTs that may be suitable for future inclusion to help determine the benefit and safety of surgery for hip impingement are likely to begin reporting their findings within the next few years.
Observational studies have been used to support the use of surgery for femoroacetabular impingement. However, these are likely to significantly overestimate any treatment benefit. RCTs are acknowledged to be the most robust design for evaluating the benefit of healthcare interventions (Britton 1998). RCTs in surgery, however, face particular challenges including that many surgeons have limited experience of RCTs; there are often learning curves for particular procedures; surgeons sometimes adopt idiosyncratic individual techniques; and the natural comparator is often very different and more conservative (Ergina 2009 ; McCulloch 2002). Many RCTs measuring the effectiveness of drug therapies use the so‐called 'placebo controlled' design. Undertaking similar RCTs in surgery with a placebo controlled study design is less straightforward (Campbell 2010). A logical placebo for surgical RCTs is a 'sham' operation, however understandably some patients feel uncomfortable with this. Although many healthcare professionals understand the rationale behind 'placebo controlled' design, some remain opposed to taking part on the grounds that it is unethical to subject their patients to such risks (Campbell 2010). Nevertheless, there is some evidence that patients and healthcare professional would be willing to take part in these types of study (Campbell 2011). In order to participate in RCTs, surgeons need to accept at least collective uncertainty or equipoise between treatments, including the possibility that surgery does not work. For patients, the idea that there is uncertainty over the comparative benefits of treatments can be very difficult to accept. These barriers may help to explain why no RCTS examining the benefits and safety of femoroacetabular surgery have yet been completed. The four ongoing studies (Naudie 2011 ; Ayeni and Bhandari 2012 ; Griffin 2012; Glynn‐Jones 2013) will have to effectively negotiate these barriers to be successful.
Potential biases in the review process
We are confident that the broad literature search used in this review has captured relevant literature and minimised the likelihood that any relevant trials were missed. The conclusions we can draw in our systematic review are limited by the lack of any trials that could be included.
Agreements and disagreements with other studies or reviews
Our review is consistent with other recent systematic reviews (Clohisy 2010; Stevens 2010; Ayeni 2013b; Collins 2013; Wall 2013), all of which have highlighted the lack of any RCTs regarding the benefits and safety of FAI surgery.
Authors' conclusions
Implications for practice.
There is a no evidence to either support or discourage the use of surgical interventions for femoroacetabular impingement. Therefore, patients undergoing surgical procedures for this condition should do so in the knowledge that it is still an unproven treatment modality.
Implications for research.
Randomised controlled trials comparing surgical procedures to placebo, no treatment and non‐operative treatments are needed before any conclusions can be made about the role of surgery for femoracetabular impingement. We welcome the ongoing studies highlighted in this review which will help to determine the role of surgery for the treatment of femoroacetabular impingement and we encourage further studies to complement these. In addition, we encourage studies that will help to determine the benefit and safety of surgery in the longer term (that is beyond two years). The CONSORT statement should be used as a guide for both designing and reporting trials (Boutron 2008). Trial reporting should include the method of randomisation and treatment allocation concealment, follow up of all participants who entered the trial, and complete reporting of outcomes. Sample sizes should be reported and have adequate power to answer the research question; ideally trials should assess both the benefits and risks of surgery. We suggest that future trials report means with standard deviations for continuous measures or number of events and total numbers analysed for dichotomous measures. Furthermore, agreement on a standard set of outcome measures and diagnostic criteria for femoracetabular impingement would enhance these research endeavours.
Acknowledgements
We would like to thank Tamara Rader, Cochrane Musculoskeletal Group, Ottawa, Ontario who has helped design and test the search strategy. We would also like to thank the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 10/41/02 and 13/103) who by virtue of an existing funded research project brought together many of the authors of this review.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE®In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE®<1946 to present>
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Femoracetabular Impingement/
2 femoroacetabular.tw.
3 fai.tw.
4 femoro‐acetabular.tw.
5 pincer.mp.
6 (cam adj3 impingement).tw.
7 or/1‐6
8 exp Surgical Procedures, Operative/
9 su.fs.
10 (surger$ or surgical$ or operat$).tw.
11 exp osteotomy/
12 exp osteoplasty/
13 osteochondroplasty.tw.
14 mini‐open.tw.
15 (arthroscopic adj2 assisted).tw.
16 ganz.tw.
17 Arthroscopy/
18 arthroscop$.tw.
19 hueter.tw.
20 (trochanteric adj3 flip).tw.
21 cheilectomy.tw.
22 or/8‐21
23 randomised controlled trial.pt.
24 controlled clinical trial.pt.
25 randomized.ab.
26 placebo.ab.
27 drug therapy.fs.
28 randomly.ab.
29 trial.ab.
30 groups.ab.
31 or/23‐30
32 (animals not (humans and animals)).sh.
33 31 not 32
34 7 and 22
35 33 and 34
Appendix 2. EMBASE search strategy
Database: Embase Classic+Embase <1947 to 2013 November 19>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp femoroacetabular impingement/ (895)
2 femoroacetabular.tw. (887)
3 fai.tw. (1237)
4 femoro‐acetabular.tw. (95)
5 pincer.mp. (908)
6 (cam adj3 impingement).tw. (193)
7 or/1‐6 (2788)
8 exp surgical technique/ (1063185)
9 su.fs. (1718081)
10 (surger$ or surgical$ or operat$).tw. (2386853)
11 exp osteotomy/ (34397)
12 exp hip surgery/ (17380)
13 exp bone remodeling/ (18534)
14 osteochondroplasty.mp. (95)
15 exp orthopedic surgery/ (363517)
16 mini‐open.mp. (449)
17 (arthroscopic adj2 assisted).tw. (245)
18 ganz.tw. (2980)
19 hip arthroscopy/ or arthroscopy/ (13938)
20 arthroscop$.tw. (22671)
21 hueter.tw. (98)
22 (trochanteric adj3 flip).tw. (30)
23 cheilectomy.tw. (106)
24 or/8‐23 (3770913)
25 random$.tw. (855260)
26 factorial$.tw. (22330)
27 crossover$.tw. (48965)
28 cross over.tw. (22241)
29 cross‐over.tw. (22241)
30 placebo$.tw. (202674)
31 (doubl$ adj blind$).tw. (149013)
32 (singl$ adj blind$).tw. (14072)
33 assign$.tw. (235547)
34 allocat$.tw. (80706)
35 volunteer$.tw. (182564)
36 crossover procedure/ (38268)
37 double blind procedure/ (121366)
38 randomized controlled trial/ (354824)
39 single blind procedure/ (18021)
40 or/25‐39 (1406885)
41 7 and 24 and 40 (75)
Appendix 3. The Cochrane Library search strategy
The Cochrane Library search strategy
1 MeSH descriptor: [General Surgery] explode all trees 2 surg*
3 surgery:ti,ab 4 #1 or #2 or #3 5 MeSH descriptor: [Femoracetabular Impingement] explode all trees 6 imping*
7 impingement 8 femoroacetabular impingement:ti,ab
9 #5 or #6 or #7 or #8
10 #4 and #9 from 2013 to 2013
Appendix 4. Clinical Trials.gov search strategy
Basic search screen at clinicaltrials.gov:
femoroacetabular impingement OR hip impingement OR FAI
Advanced search screen at clinicaltrials.gov:
Condition: Femoroacetabular Impingement
Intervention: Surgery
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bardakos 2008 | Observational; comparative case series |
| Bulbul 2012 | Observational; case series |
| Domb 2013 | Observational; comparative case series |
| Espinosa 2006 | Observational; comparative case series |
| Gedouin 2010 | Observational; case series |
| Krych 2013 | Randomised trial comparing two surgical interventions |
| Larson 2009 | Observational; comparative case series |
| Malviya 2012 | Observational; case series |
| Palmer 2012 | Observational; case series |
| Schilders 2011 | Observational; comparative case series |
| Zingg 2013 | Partly (10 out of 38 patients included were randomised) randomised trial comparing two surgical interventions |
Characteristics of ongoing studies [ordered by year of study]
Naudie 2011.
| Trial name or title | Hip Arthroscopy Versus Conservative Management of Femoroacetabular Impingement |
| Methods | Randomized parallel open label efficacy trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures: Hip Outcome Score (HOS) (time frame: 24 months) Secondary outcome measures: Non‐Arthritic Hip Score (time frame: 2 weeks, and at months 3, 6, 12, 18, and 24) Modified Harris Hip Score (time frame: 2 weeks, and at months 3, 6, 12, 18, and 24) SF‐12 (time frame: 2 weeks, and at months 3, 6, 12, 18, and 24) Range of Motion (time frame: 2 weeks, and at months 3, 6, 12, 18, and 24) |
| Starting date | May 2011 |
| Contact information | Stacey Wanlin tel: 519‐661‐2111 ext 80946 email: swanlin@uwo.ca |
| Notes |
Griffin 2012.
| Trial name or title | UK FASHIoN: UK FASHIoN: Feasibility/Full trial of Arthroscopic Surgery for Hip Impingement compared with best coNventional care |
| Methods | Randomised parallel open label efficacy trial with an internal feasibility pilot |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | July 2012 |
| Contact information | Damian Griffin +44 2476 968618 damian.griffin@warwick.ac.uk or Rachel Hobson +44 2476 968629 r.w.hobson@warwick.ac.uk |
| Notes |
Ayeni and Bhandari 2012.
| Trial name or title | Femoroacetabular Impingement Randomized Controlled Trial (FIRST) |
| Methods | Randomised double blind parallel efficacy trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures: Feasibility of definitive trial (time frame: 2 years) Secondary outcome measures: Change in pain scores at 6 months using visual rating scale (time frame: 6 months) Change in heath status and function using SF‐12, NAHS and HOS questionnaires (time frame: 1 year) |
| Starting date | September 2012 |
| Contact information | Nicole Simunovic tel: 905‐527‐4322 ext 44695 email: simunon@mcmaster.ca |
| Notes |
Glynn‐Jones 2013.
| Trial name or title | Trial for Femoroacetabular Impingement Treatment (FAIT) |
| Methods | Randomised parallel open label efficacy trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome measures: Hip outcome score (time frame: 8 months post‐randomisation (approximately 6 months post‐intervention)) Secondary outcome measures: Patient reported outcome measures (time frame: regular intervals up to 38 months post‐randomisation (approximately 3 years post‐intervention)) Morphological and physiological MRI (time frame: regular intervals up to 38 months post‐randomisation (approximately 3 years post‐intervention)) Hip radiographs (time frame: regular intervals up to 38 months post‐randomisation (approximately 3 years post‐intervention)) Serum and urinary biomarkers of osteoarthritis (time frame: regular intervals up to 38 months post‐randomisation (approximately 3 years post‐intervention)) Clinical examination (time frame: regular intervals up to 38 months post‐randomisation (approximately 3 years post‐intervention)) Range of movement Impingement tests |
| Starting date | July 2013 |
| Contact information | Antony Palmer tel: +441865 227374 email: antony.palmer@ndorms.ox.ac.uk |
| Notes |
Differences between protocol and review
1. As there were no published trials that fulfilled our criteria, we have:
a. described the Characteristics of ongoing studies, which included attempting to obtain further details from the named study contact or Chief Investigator;
b. summarised the evidence available from the Excluded studies.
2. We have removed the hierarchy for hip‐specific multidomain outcome measures described in the Secondary outcomes.
3. We have added a comparison of mixed‐type FAI to our proposed subgroup analysis of cam versus pincer‐type FAI.
4. We have edited some of the background text to provide more up to date information.
In accordance with latest Cochrane Musculoskeletal Group recommendations, we replaced the terms 'Primary outcomes' and 'Secondary outcomes' with the heading of 'Major outcomes'. 'Major outcomes' includes the seven most important outcomes that we intend to implement in the GRADE assessment and Summary of findings' tables in future versions of the review; we consider these outcomes are essential for decision‐masking.
Contributions of authors
PDH Wall designed the study, reviewed the abstracts and articles and drafted the review.
JS Brown contributed to the study design, reviewed the abstracts and articles and helped draft the review.
N Parsons is the review statistician and contributed to the study design and helped with data management and the analysis plan.
ML Costa contributed to the study design, and edited the protocol and final review.
R Buchbinder contributed to the study design and edited the protocol and final review.
DR Griffin designed the study, reviewed articles, and edited the protocol and final review. He is the guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
NHR, Health Technology Assessment programme, (project number 10/41/02)
Declarations of interest
DR Griffin is the Chief Investigator, and PDH Wall and ML Costa are co‐Investigators, in UK FASHIoN, a UK NIHR HTA funded RCT of hip arthroscopy compared with best conventional care for FAI.
New
References
References to studies excluded from this review
Bardakos 2008 {published data only}
- Bardakos NV, Vasconcelos JC, Villar RN. Early outcome of hip arthroscopy for femoroacetabular impingement: the role of femoral osteoplasty in symptomatic improvement. The Journal of Bone and Joint Surgery. British volume 2008;90:1570‐5. [DOI] [PubMed] [Google Scholar]
Bulbul 2012 {published data only}
- Bulbul M, Uzun M, Ayanoglu S, Imren Y, Ozturk K, Gurbuz H. Analysis of surgical treatment outcomes in femoroacetabular impingement syndrome. Turkije Klinikleri Journal of Medical Science 2012;32(5):1201‐6. [Google Scholar]
Domb 2013 {published data only}
- Domb BG, Stake CE, Botser IB, Jackson TJ. Surgical dislocation of the hip versus arthroscopic treatment of femoroacetabular impingement: a prospective matched‐pair study with average 2‐year follow‐up. Arthroscopy 2013;29:1506‐13. [DOI] [PubMed] [Google Scholar]
Espinosa 2006 {published data only}
- Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro‐acetabular impingement: preliminary results of labral refixation. The Journal of Bone and Joint Surgery. American volume 2006;88:925‐35. [DOI] [PubMed] [Google Scholar]
Gedouin 2010 {published data only}
- Gedouin JE, May O, Bonin N, Nogier A, Boyer T, Sadri H, et al. Assessment of arthroscopic management of femoroacetabular impingement. A prospective multicenter study. Orthopaedics & Traumatology: Surgery & Research 2010;96(8 Suppl):59‐67. [DOI] [PubMed] [Google Scholar]
Krych 2013 {published data only}
- Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: A prospective randomized study. Arthroscopy ‐ Journal of Arthroscopic and Related Surgery 2013;29:46‐53. [DOI] [PubMed] [Google Scholar]
Larson 2009 {published data only}
- Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy 2009;25:369‐76. [DOI] [PubMed] [Google Scholar]
Malviya 2012 {published data only}
- Malviya A, Stafford GH, Villar RN. Impact of arthroscopy of the hip for femoroacetabular impingement on quality of life at a mean follow‐up of 3.2 years. The Journal of Bone and Joint Surgery. British volume 2012;94(4):466‐70. [DOI] [PubMed] [Google Scholar]
Palmer 2012 {published data only}
- Palmer DH, Ganesh V, Comfort T, Tatman P. Midterm outcomes in patients with cam femoroacetabular impingement treated arthroscopically. Arthroscopy 2012;28:1671‐81. [DOI] [PubMed] [Google Scholar]
Schilders 2011 {published data only}
- Schilders E, Dimitrakopoulou A, Bismil Q, Marchant P, Cooke C. Arthroscopic treatment of labral tears in femoroacetabular impingement: a comparative study of refixation and resection with a minimum two‐year follow‐up. The Journal of Bone and Joint Surgery. British volume 2011;93:1027‐32. [DOI] [PubMed] [Google Scholar]
Zingg 2013 {published data only}
- Zingg PO, Ulbrich EJ, Buehler TC, Kalberer F, Poutawera VR, Dora C. Surgical hip dislocation versus hip arthroscopy for femoroacetabular impingement: Clinical and morphological short‐term results. Archives of Orthopaedic and Trauma Surgery 2013;133:69‐79. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Ayeni and Bhandari 2012 {published data only}
- Femoroacetabular Impingement Randomized Controlled Trial (FIRST). Ongoing study September 2012.
Glynn‐Jones 2013 {published data only}
- Trial for Femoroacetabular Impingement Treatment (FAIT). Ongoing study July 2013.
Griffin 2012 {published data only}
- UK FASHIoN: UK FASHIoN: Feasibility/Full trial of Arthroscopic Surgery for Hip Impingement compared with best coNventional care. Ongoing study July 2012. [DOI] [PMC free article] [PubMed]
Naudie 2011 {published data only}
- Hip Arthroscopy Versus Conservative Management of Femoroacetabular Impingement. Ongoing study May 2011.
Additional references
Allen 2009
- Allen D, Beaule PE, Ramadan O, Doucette S. Prevalence of associated deformities and hip pain in patients with cam‐type femoroacetabular impingement. The Journal of Bone and Joint Surgery. British volume 2009;91:589‐94. [DOI] [PubMed] [Google Scholar]
Ayeni 2013a
Ayeni 2013b
- Ayeni OR, Chan K, Al‐Asiri J, Chien T, Sprague S, Liew S, et al. Sources and quality of literature addressing femoroacetabular impingement. Knee Surgery, Sports Traumatology, Arthroscopy 2013;21:415‐9. [DOI] [PubMed] [Google Scholar]
Beall 2005
- Beall DP, Sweet CF, Martin HD, Lastine CL, Grayson DE, Ly JQ, et al. Imaging findings of femoroacetabular impingement syndrome. Skeletal Radiology 2005;34:691‐701. [DOI] [PubMed] [Google Scholar]
Beck 2004
- Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clinical Orthopaedics and Related Research 2004;418:67‐73. [PubMed] [Google Scholar]
Beck 2005
- Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. The Journal of Bone and Joint Surgery. British volume 2005;87:1012‐8. [DOI] [PubMed] [Google Scholar]
Botser 2011
- Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy 2011;27:270‐8. [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K F, Ravaud P, CONSORT Group. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148:W60‐6. [DOI] [PubMed] [Google Scholar]
Britton 1998
- Britton A, McKee M, Black N, et al. Choosing between randomised and non‐randomised studies: a systematic review. Health Technology Assessment 1998;2:1‐124. [PubMed] [Google Scholar]
Campbell 2010
- Campbell MK, Skea ZC, Sutherland AG, et al. Effectiveness and cost‐effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo‐controlled trial (the KORAL study). Health Technology Assessment 2010. [DOI] [PubMed]
Campbell 2011
- Campbell MK, Entwistle VA, Cuthbertson BH, et al. Developing a placebo‐controlled trial in surgery: issues of design, acceptability and feasibility. Trials 2011; Vol. Feb 21;12:50. [DOI] [PMC free article] [PubMed]
Cates 2008 [Computer program]
- Cates C. Visual Rx, Version 3 http://www.nntonline.net/visualrx/. Dr Christopher Cates, 2008.
Clohisy 2010
- Clohisy JC, John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clinical Orthopaedics and Related Research 2010;468:555‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2013
- Collins JA, Ward JP, Youm T. Is prophylactic surgery for femoroacetabular impingement indicated?: A systematic review. American Journal of Sports Medicine 2013;[Epub ahead of print]:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
DeAngelis 2004
- DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292:1363‐4. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Deeks JJ, Higgins JPT, Altman (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Eccleston 2010
- Eccleston C, Moore RA, Derry S, Bell RF, McQuay H. Improving the quality and reporting of systematic reviews. European Journal of Pain 2010;14:667‐9. [DOI] [PubMed] [Google Scholar]
Ergina 2009
- Ergina PL, Cook JA, Blazeby JM, et al. Challenges in evaluating surgical innovation. Lancet 2009;374:1097‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2001
- Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. The Journal of Bone and Joint Surgery. British volume 2001;83:1119‐24. [DOI] [PubMed] [Google Scholar]
Ganz 2003
- Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clinical Orthopaedics and Related Research 2003;417:112‐20. [DOI] [PubMed] [Google Scholar]
Gosvig 2010
- Gosvig KK, Jacobsen S, Sonne‐Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population‐based survey. The Journal of Bone and Joint Surgery. American volume 2010;92:1162‐9. [DOI] [PubMed] [Google Scholar]
Gregory 2007
- Gregory JS, Waarsing JH, Day J, Pols HA, Reijman M, Weinans H, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis?. Arthritis and Rheumatism 2007;56:3634‐43. [DOI] [PubMed] [Google Scholar]
Griffin 2012a
- Griffin DR, Parsons N, Mohtadi NG, Safran MR. A short version of the International Hip Outcome Tool (iHOT‐12) for use in routine clinical practice. Arthroscopy: the Journal of Arthroscopic & Related Research 2012;28:611‐6; quiz 616‐8. [DOI] [PubMed] [Google Scholar]
Hack 2010
- Hack K, Primio G, Rakhra K, Beaule PE. Prevalence of cam‐type femoroacetabular impingement morphology in asymptomatic volunteers. The Journal of Bone and Joint Surgery. American volume 2010;92:2436‐44. [DOI] [PubMed] [Google Scholar]
Harris 2013
- Harris JD, McCormick FM, Abrams GD, Gupta AK, Ellis TJ, Bach BR Jr, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy 2013;29:589‐95. [DOI] [PubMed] [Google Scholar]
Harris‐Hayes 2011
- Harris‐Hayes M, Royer NK. Relationship of acetabular dysplasia and femoroacetabular impingement to hip osteoarthritis: a focused review. PM&R 2011;3:1055‐67 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2009
- Hartmann A, Gunther KP. Arthroscopically assisted anterior decompression for femoroacetabular impingement: technique and early clinical results. Archives of Orthopaedic and Trauma Surgery 2009;129:1001‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topic in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogervorst 2011
- Hogervorst T, Bouma H, Boer SF, Vos J. Human hip impingement morphology: an evolutionary explanation. The Journal of Bone and Joint Surgery. British volume 2011;93:769‐76. [DOI] [PubMed] [Google Scholar]
Kemp 2013
- Kemp JL, Collins NJ, Roos EM, Crossley KM. Psychometric properties of patient‐reported outcome measures for hip arthroscopic surgery. American Journal of Sports Medicine 2013;4(9):2065‐73. [DOI] [PubMed] [Google Scholar]
Keurentjes 2012
- Keurentjes JC, Tol FR, Fiocco M, Schoones JW, Nelissen RG. Minimal clinically important differences in health‐related quality of life after total hip or knee replacement: A systematic review. Bone and Joint Research 2012;1:71‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2006
- Kim WY, Hutchinson CE, Andrew JG, Allen PD. The relationship between acetabular retroversion and osteoarthritis of the hip. The Journal of Bone and Joint Surgery. British volume 2006;88:727‐9. [DOI] [PubMed] [Google Scholar]
Kowalczuk 2013
- Kowalczuk M, Bhandari M, Farrokhyar F, Wong I, Chahal M, Neely S, et al. Complications following hip arthroscopy: a systematic review and meta‐analysis. Knee Surgery, Sports Traumatology, Arthroscopy 2013;21:1669‐75. [DOI] [PubMed] [Google Scholar]
Lavigne 2004
- Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M. Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clinical Orthopaedics and Related Research 2004;418:61‐6. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Lefebvre C, Manheimer E, Glanville J (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Leunig 2000
- Leunig M, Casillas MM, Hamlet M, Hersche O, Notzli H, Slongo T, et al. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthopaedica Scandinavica 2000;71:370‐5. [DOI] [PubMed] [Google Scholar]
Leunig 2013
- Leunig M, Juni P, Werlen S, Limacher A, Nuesch E, Pfirrmann CW, et al. Prevalence of cam and pincer‐type deformities on hip MRI in an asymptomatic young Swiss female population: a cross‐sectional study. Osteoarthritis and Cartilage 2013;21:544‐50. [DOI] [PubMed] [Google Scholar]
Lodhia 2010
- Lodhia P, Slobogean GP, Noonan VK, Gilbart MK. Patient‐reported outcome instruments for femoroacetabular impingement and hip labral pathology: A systematic review of the clinimetric evidence. Arthroscopy: the Journal of Arthroscopic & Related Research 2010;27:279‐86. [DOI] [PubMed] [Google Scholar]
Mamisch 2009
- Mamisch TC, Kim YJ, Richolt JA, Millis MB, Kordelle J. Femoral morphology due to impingement influences the range of motion in slipped capital femoral epiphysis. Clinical Orthopaedics and Related Research 2009;467:692‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCulloch 2002
- McCulloch P, Taylor I, Sasako M. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millis 2011
- Millis MB, Novais EN. In situ fixation for slipped capital femoral epiphysis: perspectives in 2011. The Journal of Bone and Joint Surgery. American volume 2011;93 Suppl 2:46‐51. [DOI] [PubMed] [Google Scholar]
Mohtadi 2012
- Mohtadi NG, Griffin DR, Pedersen ME, Chan D, Safran MR, Parsons N, et al. The development and validation of a self‐administered quality‐of‐life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT‐33). Arthroscopy: the Journal of Arthroscopic & Related Research 2012;28:595‐605; quiz 606‐10 e1. [DOI] [PubMed] [Google Scholar]
Murphy 2004
- Murphy S, Tannast M, Kim YJ, Buly R, Millis MB. Debridement of the adult hip for femoroacetabular impingement: indications and preliminary clinical results. Clinical Orthopaedics and Related Research 2004;429:178‐81. [DOI] [PubMed] [Google Scholar]
Ostendorf 2004
- Ostendorf M, Stel HF, Buskens E, Schrijvers AJ, Marting LN, Verbout AJ, et al. Patient‐reported outcome in total hip replacement. A comparison of five instruments of health status. The Journal of Bone and Joint Surgery. British volume 2004;86:801‐8. [DOI] [PubMed] [Google Scholar]
Palmer 2013
- Palmer AJ, Thomas GE, Pollard TC, Rombach I, Taylor A, Arden N, et al. The feasibility of performing a randomised controlled trial for femoroacetabular impingement surgery. Bone and Joint Research 2013;2:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2012
- Parker SL, Mendenhall SK, Shau DN, Adogwa O, Anderson WN, Devin CJ, et al. Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same‐level recurrent lumbar stenosis: understanding clinical versus statistical significance. Journal of Neurosurgery. Spine 2012;16:471‐8. [DOI] [PubMed] [Google Scholar]
Quintana 2005
- Quintana JM, Escobar A, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF‐36 after hip joint replacement. Osteoarthritis and Cartilage 2005;13:1076‐83. [DOI] [PubMed] [Google Scholar]
Reichenbach 2010
- Reichenbach S, Juni P, Werlen S, Nuesch E, Pfirrmann CW, Trelle S, et al. Prevalence of cam‐type deformity on hip magnetic resonance imaging in young males: a cross‐sectional study. Arthritis Care and Research (Hoboken) 2010;62:1319‐27. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. Higgins JPT, Green S,editors. Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1 (updated September 2011).
Stevens 2010
- Stevens MS, Legay DA, Glazebrook MA, Amirault D. The evidence for hip arthroscopy: grading the current indications. Arthroscopy 2010;26:1370‐83. [DOI] [PubMed] [Google Scholar]
Takeyama 2009
- Takeyama A, Naito M, Shiramizu K, Kiyama T. Prevalence of femoroacetabular impingement in Asian patients with osteoarthritis of the hip. International Orthopedics 2009;33:1229‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tubach 2005
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Annals of Rheumatological Disease 2005;64(1):29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 2013
- Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM&R 2013;5:418‐26. [DOI] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Quality of Life Research 2005;14:1523‐32. [DOI] [PubMed] [Google Scholar]


