Abstract
Most people with dementia experience neuropsychiatric symptoms (NPS), including anxiety, depression or disinhibition. There is growing interest in the relationship between NPS and cognitive impairment, but data is still limited. This study aimed to investigate the specific associations between NPS and cognition in people with dementia. MEDLINE, EMBASE and PsycINFO were searched for published, peer-reviewed studies of associations between at least one NPS and one cognitive ability in people with dementia. The quality of the studies was assessed with the NIH National Heart, Lung and Blood Institute’s quality assessment tools. A meta-analysis was conducted using Robumeta package for R. Ninety studies were included. We found significant associations between NPS, global cognition and cognitive domains, e.g. apathy was associated with global cognitive and memory impairment; dysphoria was associated with worse attention; delusions with executive dysfunction. Increased NPS in people with dementia are associated with worse cognitive performance. There were few studies looking at associations between some neuropsychiatric clusters and cognitive abilities, and there was little research on causal relationships. Our review was limited by the inclusion of studies that reported associations in specific formats, and most included people with a diagnosis of Alzheimer’s disease (AD). However, given the large number of studies, this is unlikely to have biased results. More research is needed that includes diverse people with different dementia syndromes. Registration: PROSPERO 2020 CRD42020165565.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11065-023-09608-0.
Keywords: Dementia, Neuropsychiatric symptoms, Cognition
Introduction
Dementia is a major cause of disability worldwide and in Australia, the leading cause of death in women (Australian Institute of Health & Welfare, 2022). Globally, an estimated 57.4 million people were living with dementia in 2019, and this number is expected to increase to 152.8 million by 2050 (Nichols et al., 2022).
Neuropsychiatric symptoms (NPS) are behavioural and psychological disturbances highly prevalent in people with dementia (Prince et al., 2014), with around 97% of people with dementia reported experiencing at least one NPS since the onset of their dementia syndrome (Steinberg et al., 2008). Examples of NPS are apathy, irritability, aggression (verbal or physical), anxiety, depression, psychosis, sleep disturbances, eating disturbances, aberrant motor behaviour, wandering and disinhibition. NPS have been associated with worse outcomes (Rabins et al., 2013), lower quality of life (Appelhof et al., 2017) and greater caregiver distress (Millenaar et al., 2016), which is known to lead to higher rates of hospitalisation (de Vugt et al., 2005) and unwanted relocation to long term care (Gaugler et al., 2009).
Because of the adverse effects often associated with pharmacological treatment, current clinical guidelines recommend non-pharmacological interventions as the first-line approach for managing NPS (Livingston et al., 2020). However, no specific non-pharmacological treatment approach has been found to reduce the frequency or severity of these symptoms significantly and consistently. There are multiple theories behind the development of NPS including insufficient understanding of the cognitive and other mechanisms that contribute to NPS. Improving our understanding of the links between specific cognitive processes and specific NPS is important in guiding the development of effective non-pharmacological treatment options.
A considerable amount of literature has been published on the associations between NPS and cognition in people with dementia. However, much of the evidence has come from cross-sectional and longitudinal studies, and to our knowledge, to date, there have been no systematic reviews that have synthesised the evidence. This review addresses this gap, and our overarching goal was to investigate and characterise the associations between neuropsychiatric symptoms (as a group and individually) with overall cognitive function and with specific cognitive abilities in people with dementia, and to explore potential moderators of these relationships (e.g. dementia syndrome, age of symptom onset, severity of dementia).
We aimed to:
Identify whether neuropsychiatric symptoms (e.g. apathy, depression, agitation) are linked with cognitive functioning in people with dementia.
Examine whether such associations differ across symptoms and cognitive domains.
Compare these associations across key dementia syndromes and patient groups.
Identify other potential moderators of such associations.
Methods
This systematic review was prospectively registered on the Prospective Register of Systematic Reviews (CRD42020165565). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 statement provided the framework for reporting.
Eligibility Criteria and Information Sources
MEDLINE, EMBASE and PsycINFO were searched from inception to 14 December 2021 for potentially eligible peer-reviewed papers describing case-series, case–control studies (cases need to be people with dementia with any of the specific NPS) cross-sectional studies, cohort studies and intervention studies that investigated associations between cognition and NPS in people with dementia of any aetiology.
Search Strategy
Search terms were related to dementia syndromes, neuropsychiatric symptoms and cognitive domains. Some of the search terms were as follows: Dement*, Alzheim*, AD, Frontotemporal, FTD, Neurocognitive disorder*, Parkinson*, Huntington*, Lewy Bod*, BPSD, behavior*, psychology*, apath*, depress*, anxi*, aggress*, agitat*, aberrant motor, irritab*, disinhibit*, eat* disturbance*, eat* problem*, sleep* disturbance*, psychotic, psychosis, hallucinat*, delu*, wander*, impuls*, mood, neuropsychology*, cognition, cognitive function*, cognitive skill*, cognitive ability*, cognitive impairment*, cognitive dysfunction*, cognitive problem*, cognitive profile, cognitive deficit*, memory, attention*, executive, speed, language, visuospatial, visuoconstruction*, fluency, learning, associate*, relat* and correlate*.
See the full search strategy (S1) published as supplementary material online attached to the electronic version of this paper.
Selection Process
Studies were independently screened and selected for inclusion using a single Covidence library by two reviewers, with a third reviewer resolving discrepancies.
Studies were eligible if they provided at least one measure of association for the relationship between cognition and neuropsychiatric symptoms. Measures of association could be reported either as correlation coefficients or as mean differences on continuous measures. Authors of studies that did not provide these data were contacted via email. If data were unavailable, the study was excluded from the review.
Only studies conducted in humans were included. Studies published in English or studies for which we were able to request and obtain a manuscript in English were included.
Data Collection Process and Data Items
After the selection of included studies, demographic information was extracted. For intervention or longitudinal studies, only baseline data were extracted since studying longitudinal relationships, which attempts to address issues of a temporal nature (e.g. cause and effect), was beyond the scope of the current review.
Subgroup analysis based on dementia syndrome or diagnosis was conducted.
Outcomes
The main outcome of interest in this review was the association between global cognition or specific cognitive abilities (as measured by cognitive test performance) and overall and specific neuropsychiatric symptoms.
Given the heterogeneous nature of NPS and the lack of a universally accepted classification of these, for the purposes of this review, NPS were grouped in six clinically driven clusters of symptoms and behaviours:
Affect (depression, dysphoria, anxiety, elation/euphoria, apathy, indifference)
Aggression (e.g. agitation, aggression, irritability, lability)
Circadian rhythms (e.g. night-time behaviours, appetite/eating disturbances)
Executive dysfunction (e.g. disinhibition, social inappropriateness)
Motor disturbances (e.g. wandering, repetitive movements)
Psychosis (e.g. delusions, hallucinations)
For the purposes of this review, the following cognitive domains were considered: (1) attention, (2) executive functions, (3) memory, (4) working memory, (5) semantic knowledge, (6) social cognition, (7) speed of information processing, (8) visuospatial skills.
Study Risk of Bias Assessment
Methodological quality of included studies was assessed with the NIH National Heart, Lung and Blood Institute’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, Quality Assessment of Case–Control Studies and Quality Assessment Tool for Case Series Studies (National Institutes of Health, 2014a, b).
These assessments were conducted by two reviewers, with a third reviewer resolving discrepancies.
Synthesis Methods
Outcome data from primary reports were extracted and converted to standardised mean difference (SMD, calculated as Hedges’ g) with 95% confidence interval (CI) of difference in cognitive performance between the exposure groups or correlation coefficient. R package Robumeta was used to synthesise the results.
Pooling of data was conducted for overall as well as symptom- and domain-specific measures. When a study reported both mean differences and correlations for the same measures and population, the correlation scores were used (to avoid double-counting participants).
When a measure was used to evaluate several domains (e.g. NPI Total or Frontal Behavioural Inventory Total score), it was classified under ‘Overall neuropsychiatric symptoms’. If a measure assessed dysphoria and apathy, for example, it was classified under ‘affective’ and used in the meta-analysis of broad domains, but not in the meta-analysis of narrow domains (e.g. dysphoria or apathy).
For the purposes of this review, a negative association was obtained when higher scores on measures of NPS were associated with lower scores on measures of cognitive performance.
Reporting Bias Assessment
R package Publication Bias was used, which allows for visual inspection of funnel plots and testing with an analogue of Egger’s test.
Results
Study Selection
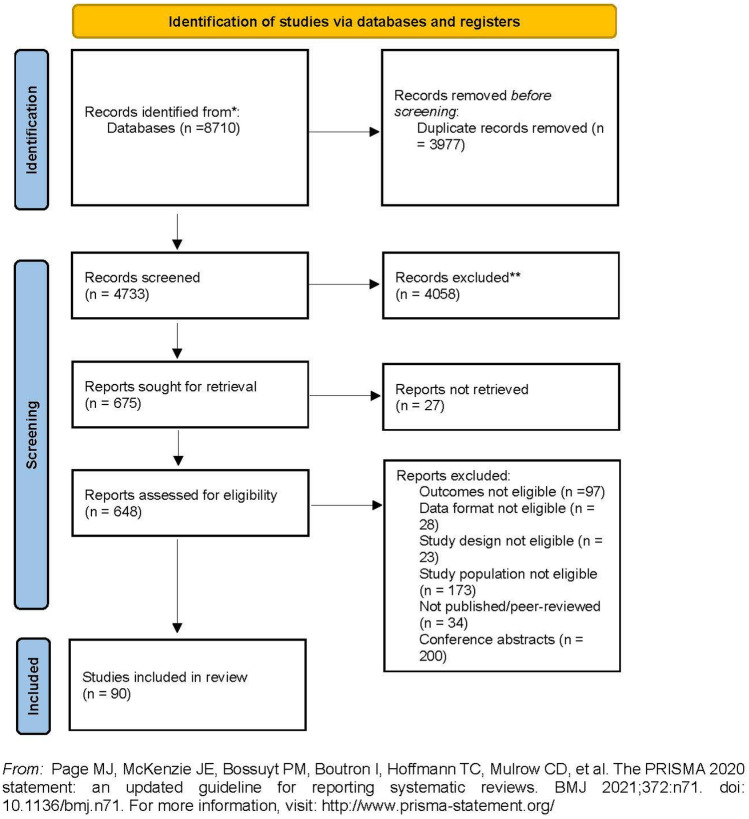
Initial search yielded 8710 items, and after removing duplicates, 4733 records were screened for inclusion. Six hundred and forty-eight studies were assessed for eligibility in the full-text review. Ninety studies met the criteria and were included. Figure 1 presents a flow chart showing the study selection process.
Fig. 1.
Study selection flow chart
Study Characteristics
Ninety studies were included. Of these studies, 64 (71.1%) were cross-sectional design, 16 (17.8%) were longitudinal and 10 (11.1%) were case–control studies. No case-series studies were eligible for this review.
Included studies were published between 1991 and 2021. They were conducted in 23 countries, with the largest number of studies (k = 26) conducted in the USA, followed by Brazil and Italy (k = 8 each).
The total number of participants with dementia included in the studies was 26,893, with sample sizes ranging from 13 to 6265. Participants in all studies had a diagnosis of dementia. In most studies (k = 57, 63.3%), only one suspected clinical dementia syndrome (AD) was included. In 18 studies (20%), participants with dementia were suspected to have AD or another type of dementia (e.g. vascular dementia, frontotemporal dementia or dementia with Lewy bodies (DLB), among other types). There were four studies (4.4%) focusing on people with Parkinson’s disease dementia (PDD), and two (2.2%) on people with DLB. Three studies (3.3%) included people with either of these two types of dementia. Two studies (2.2%) included only people with post-stroke or vascular dementia. One (1.1%) study focused on people with primary progressive aphasia. Three studies did not report dementia syndrome in their included participants.
In 64.4% of the studies (k = 58), at least 51% of participants with dementia were reported as female. The mean age of participants ranged between 58 and 86.3 years old. Most (84.4%) of the studies did not report whether the sample included people with younger-onset or older-onset dementia, that is, the age of onset of the dementia was not reported. They also did not specify whether participants were in residential care or living in the community, or whether they were taking any medications. Participants’ ethnicity was also infrequently reported, with only 22 (24.4%) studies reporting participants’ nationality, race or cultural background.
Sixty-eight studies (75.6%) reported the Mini-Mental State Examination (MMSE) (Folstein et al., 1983) mean scores in participants with dementia, (range 7.8–26.1). Thirty-seven (41.1%) studies used the Neuropsychiatric Inventory (NPI) to measure at least one neuropsychiatric symptom.
A table summarising the characteristics of included studies (Table 1) is presented below. See the full table (S2) published as supplementary material online attached to the electronic version of this paper.
Table 1.
Characteristics of included studies
| Study ID | Country | Sample size (dementia) | Gender ratio (dementia; % female) | Mean age (dementia) | Young vs late onset/age of onset | Dementia syndrome | Severity (CDR)/global cognition (MMSE) |
|---|---|---|---|---|---|---|---|
| Akyol et al. (2020) | Turkey | 106 | 46.23% | 72.25 | NR | AD, VaD, FTD | MMSE = 15.77 |
| Balci et al. (2011) | Turkey | 26 | 80.80% | 76.23 | NR | AD | MMSE = 15.69 |
| Bhat et al. (2021) | India | 76 | 28.90% | 58.05 | NR | VaD | CDR = 1.65 |
| Benedict et al. (1999) | USA | 13 | 92.00% | 73.43 | NR | VaD | MMSE = 22.4 |
| Breitve et al. (2018) | Norway | 196 | 37.00% | 76.06 | NR | AD, DLB | CDR = 0.87 |
| Bronnick et al. (2011) | Norway | 172 | 38.00% | 73.21 | NR | PDD | MMSE = 20.11 |
| Bylsma et al. (1994) | USA | 180 | 58.00% | 72.38 | Late (68.35) | AD | CDR = 1.2 |
| Camargo et al. (2018) | Brazil | 40 | 35.00% | 70.08 | NR | PDD | SCOPA-Cog = 11.25 |
| Chwiszczuk et al. (2017) | Norway | 246 | 56.56% | 75.52 | NR | AD, DLB, PDD | MMSE = 23.64 |
| Contador-Castillo et al. (2009) | Spain | 23 | 65.22% | 75.31 | NR | AD | MMSE = 22.73 |
| D’Antonio et al. (2019) | Italy | 32 | 50.00% | 74.28 | NR | AD | MMSE = 19.63 |
| de Oliveira et al. (2015a) (‘Correlations’) | Brazil | 217 | 67.70% | 78 | Late (73.19) | AD | CDR 1, 2, 3 |
| de Oliveira et al. (2015b)2 ‘contrasts’ | Brazil | 39 | 56.41% | 78 | Late | AD, PDD, LBD | MMSE = 17.52 |
| de Oliveira et al. (2020) | Brazil | 51 | 39.20% | 77.76 | NR | PD, LBD | MMSE = 15.82, CDR = 11.46 |
| DeMichele-Sweet et al. (2011) | USA | 2317 | 57.00% | 78.4 | Late (72.3) | AD | MMSE 17.7 |
| de Paula et al. (2016) | Brazil | 93 | NR | 74.57 | NR | AD | MMSE = 20.59 |
| Drijgers et al. (2011) | The Netherlands | 260 | 56.00% | 74.4 | NR | AD | MMSE 20.3 |
| Eikelboom et al. (2021) | The Netherlands | 1090 | 52.40% | 65.9 | NR | AD | MMSE = 20.3 |
| Eustace et al. (2001) | Ireland | 150 | 69.00% | 76.48 | NR | AD | MMSE = 19.31 |
| Fahlander et al. (1999) | Sweden | 54 | 76.00% | 83.93 | NR | AD | MMSE 19.57 |
| Fernández et al. (2010) | Spain | 1014 | 65.00% | 77.19 | NR | AD | NR |
| Fernandez-Martinez et al. (2010) | Spain | 99 | 56.60% | 75.31 | NR | AD | MMSE = 21.93 |
| Fillit et al. (2021) | USA | 6265 | 69.00% | 80.47 | NR | AD and other aetiologies | NR |
| Fitz and Teri (1994) | USA | 91 | 55.00% | 73.49 | NR | AD | MDRS = 102.52 |
| Flynn et al. (1991) | USA | 33 | 9.00% | 73.36 | NR | AD / MID | MMSE = 16.5 |
| Gallassi et al. (2001) | Italy | 33 | 29.00% | 77.1 | NR | AD | MMSE = 15.4 |
| Gallo et al. (2008) | USA | 48 | 65.00% | 79 | NR | AD, VaD | MMSE = 23 |
| Galynker et al. (1995) | USA | 26 | 57.70% | 78.8 | NR | AD/multi-infarct dementia | MMSE = 16.8 |
| Gilley et al. (1991) | USA | 230 | 67.00% | 71.54 | NR | AD | MMSE 12.57 |
| Grossi et al. (2013) | Italy | 61 | 46.00% | 69.54 | NR | AD, PDD | MMSE = 22.13 |
| Hallikainen et al. (2012) | Finland | 236 | 51.30% | 75.11 | NR | AD | MMSE = 21.5 |
| Harwood et al. (2000) | USA | 114 | 63.00% | 78.8 | NR | AD | MMSE = 17.8 |
| Hopkins and Libon (2005) | USA | 48 | NR | 79.06 | NR | VaD | MMSE = 21/33 |
| Ito et al. (2007) | Japan | 40 | 68.00% | 77.8 | NR | AD | CASI = 25.15 |
| Janzing et al. (2005) | The Netherlands | 60 | 88.30% | 86.3 | NR | NR | NR |
| Keator et al. (2019) | USA | 58 | 50.00% | 69.19 | NR | PPA | NR |
| Kuzis et al. (1999) | Argentina | 184 | NR | 70.88 | NR | AD | MMSE = 22.49 |
| Kwak et al. (2013) | Korea | 230 | 61.73% | 74.66 | Late (71.88) | AD | CDR = 1.06 |
| Lam et al. (2006) | China | 125 | 58.40% | 82.04 | NR | AD, VaD | MMSE = 8.62 |
| Lee et al. (2007) | Japan | 50 | 86.00% | 81.36 | NR | AD | MMSE = 15.58 |
| Lee et al. (2012) | Taiwan | 127 | 40.15% | 77 | NR | PDD | MMSE = 17 |
| Lee et al. (2019) | Korea | 1247 | 56.60% | 72.65 | NR | AD | MMSE = 20.21 |
| Levy et al. (1998) | USA | 28 | 46.43% | 63 | NR | AD, FTD | MMSE = 16.5 |
| Logsdon et al. (1998) | USA | 193 | 49.00% | 76.8 | NR | AD | MMSE = 17.4 |
| Lopez et al. (1991) | USA | 17 | 76.00% | 68 | NR | AD | MMS = 18.7 |
| Machado et al. (2020) | Brazil | 32 | 59.00% | 75.84 | AOO = 71.14 | DLB | MMSE = 17.72 |
| Mariano et al. (2020) | Brazil | 42 | 45.23% | 68.31 | YOD (64.08) | AD, FTD | MMSE = 25.06 |
| McPherson et al. (2002) | USA | 44 | 69.00% | 75.72 | NR | AD | MMSE = 22.57 |
| Migliorelli et al. (1995) | Argentina | 103 | 74.00% | 73.58 | Late (69.21) | AD | NR |
| Mizrahi et al. (2006) | Argentina | 771 | NR | 71.1 | NR | AD | MMSE = 21.8 |
| Montagnese et al. (2022) | UK | 284 | 49.00% | 75.42 | NR | DLB, PDD | MMSE = 26.05 |
| Na et al. (2018) | South Korea | 289 | 71.00% | 77.68 | NR | AD | CDR = 1, MMSE = 20.83 |
| Naarding et al. (2007) | The Netherlands | 54 | 46.00% | 73.7 | NR | Post-stroke dementia | MMSE = 19.8 |
| Nagata et al. (2010) | Japan | 50 | 78.00% | 78.16 | NR | AD | MMSE = 19.38 |
| Nagata et al. (2017) | Japan | 421 | 56.00% | 77.9 | NR | AD | MMSE = 15 |
| Nakaaki et al. (2007) | Japan | 42 | 52.38% | 71.4 | NR | AD | MMSE = 19.55 |
| Nakaaki et al. (2008) | Japan | 88 | 55.00% | 71.23 | NR | AD | MMSE = 19.76 |
| Nakatsuka et al. (2014) | Japan | 142 | 66.90% | 80 | NR | AD | MMSE 14.4 |
| Onyike et al. (2007) | USA | 316 | NR | NR | NR | NR | MMSE = 14.32 |
| Pagonabarraga et al. (2008) | Spain | 30 | 43.00% | 77.17 | NR | PDD | MDRS = 102.33 |
| Park et al. (2019) | Korea | 1128 | 62.00% | 73 | NR | AD | MMSE = 20.1 |
| Perneczky et al. (2009) | Germany | 21 | 48.00% | 71.1 | Young (63.1) | DLB | MMSE = 20.8 |
| Perri et al. (2014) | Italy | 86 | 39.53% | 69.06 | NR | AD, frontal variant FTD, SIVD, LBD | CDR = 0.72, MMSE = 22.53 |
| Perri et al. (2018) | Italy | 20 | 55.00% | 76.83 | NR | AD | MMSE = 22.2 |
| Pezzoli et al. (2019) | Italy | 52 | 46.00% | 70.98 | NR | DLB | MMSE = 25.42 |
| Qian et al. (2018) | Canada | 900 | 44.00% | 77.11 | Late | AD | MMSE = 13.17 |
| Quaranta et al. (2015) | Italy | 108 | 64.00% | 73.3 | NR | AD | MMSE = 17.2 |
| Reed et al. (1993) | USA | 57 | NR | 74.43 | NR | AD | MMSE = 19.4 |
| Rochat et al. (2013) | Switzerland | 30 | NR | 72.03 | NR | AD | NR |
| Rolland et al. (2007) | France | 682 | 72.00% | 77.4 | Late (74.1) | AD | MMSE = 20.1 |
| Ross et al. (1998) | USA | 1486 | 69.00% | 77.01 | NR | AD | MMSE = 16.62 |
| Rozum et al. (2019) | USA | 56 | 67.90% | 85.68 | NR | VaD | SCIP total = 154.77 |
| Ruiz et al. (2018) | Spain | 90 | 59.00% | 76 | NR | AD | MMSE = 22.4 |
| Sánchez-Rodríguez (2004) | Spain | 58 | 57.00% | 73.34 | Late | AD | MMSE = 24.3 |
| Senanarong et al. (2005) | Thailand | 73 | 72.60% | 70.28 | NR | AD | MMSE = 18.42 |
| Serra et al. (2010) | Italy | 54 | 66.67% | 73.1 | NR | AD | MMSE = 20.72 |
| Shin et al. (2014) | Korea | 63 | 73.00% | 74.83 | NR | AD | MMSE = 16.73 |
| Soleman Hernandez et al. (2012) | Brazil | 37 | 78.00% | 78.8 | NR | AD | MMSE = 17.2 |
| Starkstein et al. (2005) | Argentina | 150 | 90.00% | 70.49 | NR | AD | MMSE = 23.09 |
| Starr and Lonie (2007) | UK | 556 | 69.96% | 77.3 | NR | AD | MMSE = 19.2 |
| Strauss and Sperry (2002) | USA | 100 | 50.00% | 75 | NR | AD | MMSE = 18.55; CDR = 1.62 |
| Sultzer et al. (1992) | USA | 61 | NR | 73 | NR | AD | MMSE = 10 |
| Sultzer et al. (2014) | USA | 88 | 19.00% | 78 | NR | AD | MMSE = 19.3 |
| Van der Mussele et al. (2013) | Belgium | 402 | 67.00% | 80.1 | Late (76.8) | AD | MMSE = 15.2, Global Deterioration Scale = 5.1 |
| Van der Mussele et al. (2015) | Belgium | 393 | 67.00% | 80.1 | Late (76.9) | AD | MMSE = 15.1 |
| Wagner et al. (1995) | USA | 614 | 69.00% | 79 | NR | Primarily AD | MMSE = 7.8 |
| Welsh et al. (1996) | UK | 18 | 94.40% | NR | NR | AD | NR |
| Wu (2014) | Taiwan | 179 | 39.00% | 78.4 | NR | NR | NR |
| Yeager and Hyer (2008) | USA | 68 | 60.00% | 77 | NR | AD, dementia-NOS | NR |
| Zahodne et al. (2015) | USA | 517 | 57.00% | 74.19 | NR | AD | NR |
AD Alzheimer’s disease, BPSD behavioural and psychological symptoms of dementia, CDR clinical dementia rating, Dementia-NOS dementia not otherwise specified, DLB dementia with Lewy bodies, FTD frontotemporal dementia, MCI mild cognitive impairment, MMSE Mini-Mental State Examination, MID multi-infarct dementia, NPS neuropsychiatric symptoms, NR not reported, PPA primary progressive aphasia, PDD Parkinson’s disease dementia, SCOPA-Cog Scales for Outcomes in PArkinson’s disease-COGnition, SIVD subcortical ischemic vascular dementia, UK United Kingdom, USA United States of America, VaD vascular dementia
Study Quality
Different quality assessment forms were used for cross-sectional/longitudinal studies and for case–control studies. Eight items were selected from the Quality Assessment tool for cross-sectional and longitudinal studies, and nine from the quality assessment tool for case–control studies as being of relevance for this review. The number of ‘Yes’ answers was counted for each study and divided by the total number of relevant items. Studies that had up to 24.9% of positive answers were rated as ‘very low quality’, studies that had between 25 and 49.9% of positive answers were considered ‘low quality’, studies that had between 50% and 74.9% ‘Yes’ answers were considered ‘moderate quality’, and studies that had at least 75% positive answers were considered ‘high quality’.
According to this quality assessment, 60% of the included studies (k = 54) were considered high quality, 33.3% of studies (k = 30) were considered of moderate quality and 6.7% of the included studies (k = 6) were classified as low quality. No studies were considered very low quality.
Reviewers conducting the quality assessment assessed that most of the studies (96.7%; k = 87) clearly stated the research question or objective in the published manuscript; fewer studies (70%; k = 63) clearly specified and defined the study population. Around half of the studies (51.1%, k = 46) measured and adjusted statistically for key potential confounding variables.
Sixty-two out of 80 cross-sectional or longitudinal studies (77.5%) confirmed that all the participants had been selected or recruited from the same of similar populations. In some cases, this was not possible to be determined since authors did not report the time period during which they recruited participants. In these cases, studies received a NR (Not reported) in this item. Among the case–control studies, only 2 out of 10 studies received a Yes in this question (20%). Of the included 90 studies, outcome assessors were reported to have been blinded in only 12.2% (k = 11) of the cases.
See the full quality assessment tables (S3) published as supplementary material online attached to the electronic version of this paper.
Results of Syntheses
Significant associations between NPS and cognition including subgroup analysis based on dementia syndrome are reported below. Results are presented in Hedge’s g. See a table with all results (S4) published as supplementary material online attached to the electronic version of this paper.
Overall NPS
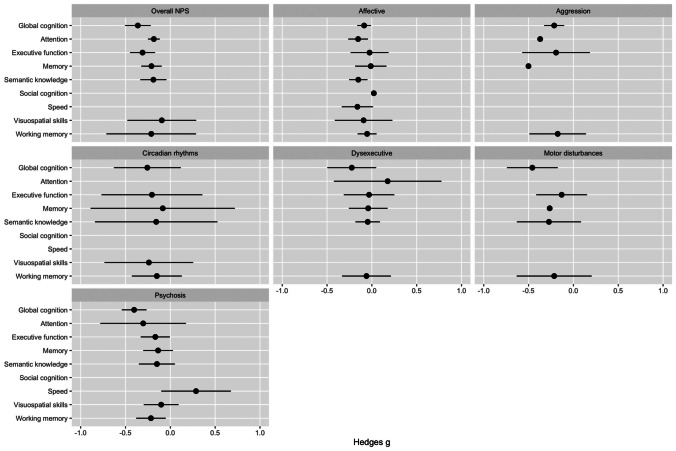
The association between overall NPS and worse global cognition reached statistical significance when the whole sample was analysed (g = − 0.36, 95% CI − 0.51 to − 0.22; k = 22) and remained significant when only participants with AD were included (g = − 0.28, 95% CI − 0.44 to − 0.13; k = 14), but not when only a subsample with PD or DLB were analysed.
The analysis also revealed associations between overall NPS and cognitive domains. Specifically, greater/more NPS were shown to be associated with worse attention (g = − 0.18, 95% CI − 0.25 to − 0.12; k = 6), worse executive function (g = − 0.31, 95% CI − 0.45 to − 0.17; k = 12), worse memory (g = − 0.21, 95% CI − 0.32 to − 0.10; k = 5) and worse semantic knowledge (g = − 0.19, 95% CI − 0.33 to − 0.04; k = 8). The associations with global cognition, attention, executive function and memory remained significant when only the subsample of participants with AD was analysed. The same was true for the association between overall NPS and visuospatial skills but, as only two studies contributed data, the results may be unreliable (g = − 0.14, 95% CI − 0.15 to − 0.12; k = 2).
Several significant associations were found between NPS clusters and symptoms and cognition.
Affect Cluster (Depression, Dysphoria, Anxiety, Elation/Euphoria, Apathy, Indifference)
A weak association was found between the affect cluster and worse global cognition (g = − 0.09, 95% CI − 0.16 to − 0.01; k = 46), attention (g = − 0.15, 95% CI − 0.26 to − 0.04; k = 14) and semantic knowledge (g = − 0.15, 95% CI − 0.25 to − 0.05; k = 20). The association with attention was maintained when only a subsample of participants with AD was included in the analysis. However, results were not significant when only participants with DLB or with PPD were included.
Associations between the affect cluster and executive function, memory, social cognition, speed, visuospatial skills and working memory were not significant (p > 0.5) in the whole sample or in the analysed subsamples.
Within the affect cluster, the following significant associations were found:
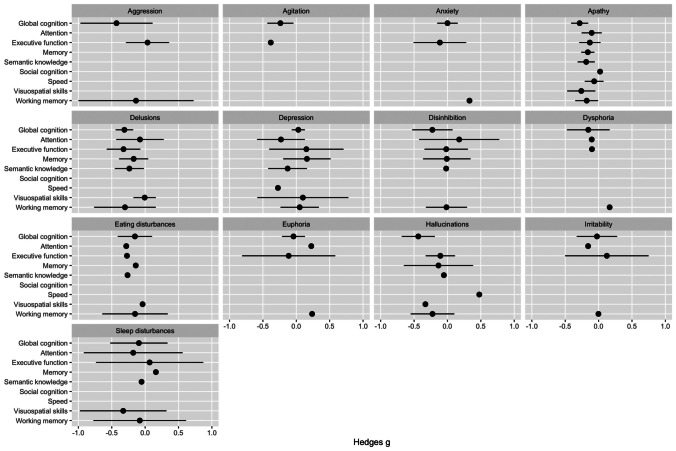
Apathy
Apathy was associated with worse global cognition (g = − 0.28, 95% CI − 0.41 to − 0.16; k = 24), as well as memory, semantic knowledge, visuospatial skills and working memory (g = − 0.28 to − 0.16). The association between apathy and global cognition was maintained when only participants with AD were analysed. No significant links were found between apathy and any cognitive domains when only a subsample with PD or DLB was analysed.
Dysphoria
An association was found between dysphoria and worse attention (g = − 0.10, 95% CI − 0.11 to − 0.09; k = 2). However, only two studies contributed data and therefore results may be unreliable.
Aggression Cluster (Agitation, Aggression, Irritability, Lability)
Elevated symptoms of aggression were associated with lower global cognition (g = − 0.21, 95% CI − 0.33 to − 0.1; k = 16).
Agitation
Agitation was shown to be associated with worse global cognition (g = − 0.24, 95% CI − 0.43 to − 0.04; k = 8), and this remained true when only participants with AD were analysed.
No significant associations were found between irritability, aggression or lability and any cognitive domains.
Circadian Rhythms Cluster (Night-Time Behaviours, Appetite/Eating Disturbances)
No associations were found between the circadian rhythms cluster and any cognitive domains.
Within this cluster, a significant association was found only between eating disturbances and worse visuospatial skills (g = − 0.04, 95% CI − 0.07 to 0; k = 2) in the whole sample. However, only two studies contributed data, therefore results may be unreliable.
Executive Dysfunction Cluster (Disinhibition, Social Inappropriateness)
There were no statistically significant links between the d executive dysfunction cluster or the symptoms within this cluster (disinhibition and social inappropriateness) and any cognitive domains.
Motor Disturbances Cluster (Wandering, Repetitive Movements)
The motor disturbance cluster was associated with impaired global cognition (g = − 0.46, 95% CI − 0.74 to − 0.18; k = 13). This was maintained when only participants with AD were analysed.
An association was also found between this cluster and worse working memory when only participants with AD were included (g = − 0.48, 95% CI − 0.42 to − 0.11; k = 2). However, as only two studies contributed data, results may be unreliable.
No significant links were found between wandering or repetitive movements and cognitive domains.
Psychosis Cluster (Delusions, Hallucinations)
The psychosis cluster showed links with impairment in global cognition (g = − 0.40, 95% CI − 0.54 to − 0.27; k = 36), executive function (g = − 0.17, 95% CI − 0.33 to − 0.01; k = 16), and working memory (g = − 0.22, 95% CI − 0.38 to − 0.05; k = 10).
The association between the psychosis cluster and global cognition was maintained when only participants with AD were included.
Delusions
Delusions were associated with worse global cognition (g = − 0.31, 95% CI − 0.44 to − 0.18; k = 22), as well as with worse executive function, and semantic knowledge (g = − 0.32 to − 0.24), and the association with global cognition was maintained when only a subsample of participants with AD was analysed.
Hallucinations
With regard to hallucinations specifically, an association between them and worse global cognition was found (g = − 0.44, 95% CI − 0.69 to − 0.19; k = 20). When only a subsample with AD was analysed, associations between hallucinations and worse global cognition (g = − 0.44) and working memory (g = − 0.3) were found.
Figure 2 summarises the associations between NPS clusters and cognitive domains. Figure 3 presents the associations between specific NPS and cognitive domains.
Fig. 2.
Forest plot NPS clusters and cognitive domains—all significant associations were negative (i.e. NPS were associated with worse cognitive performance)
Fig. 3.
Forest plots specific NPS and cognitive domains—all significant associations were negative (i.e. NPS were associated with worse cognitive performance
The CI for the following associations were broader than − 1 or 1 and are therefore not showing in the figure: attention and aggression, memory and aggression, memory and motor disturbances.
The CI for the following associations were broader than − 1 or 1 and are therefore not showing in the figure: attention and eating disturbances, semantic knowledge and eating disturbances, working memory and dysphoria, working memory and euphoria, attention and irritability.
Reporting Biases
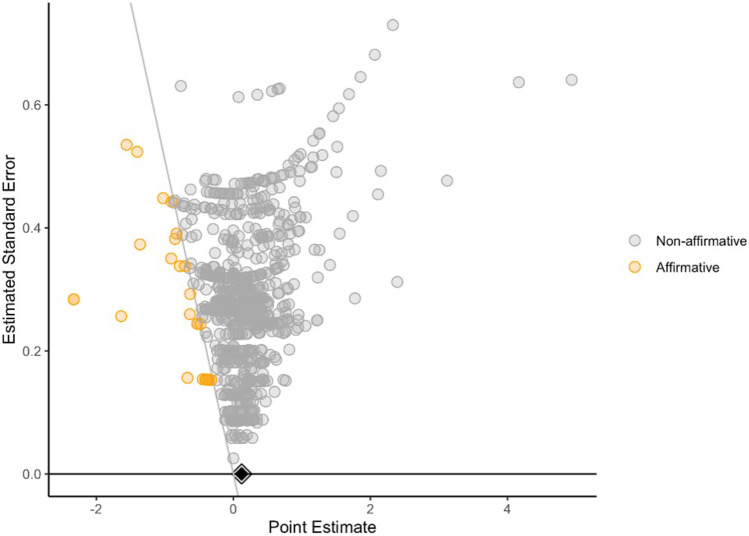
In line with our hypothesis that there was a negative association between the analysed NPS and cognitive domains, we conducted a sensitivity analysis for publication bias favouring negative estimates in our meta-analysis using the ‘Publication Bias’ package in R. The funnel plot (Fig. 4) showed all point estimates from included studies and identified those which were systematically smaller than the entire set of point estimates (i.e. strongly favouring the hypothesised negative direction) and were identified as ‘affirmative’. With visual inspection of the funnel plot, the point estimates from included studies were equally spread across both positive and negative sides of the plot and most point estimates showed a small estimated standard error, suggesting that the meta-analysis may not be affected by publication bias. The funnel plot shows point that fall along distinct curves due to the inclusion of multiple effects based on the same study and, thus, the same sample.
Fig. 4.
Funnel plot—no evidence of publication bias
Following visual inspection of the plot, we conducted a quantitative sensitivity analysis to examine if the affirmative point estimates were affecting the pooled point estimate indicated as a black diamond. The results suggested that the affirmative point estimates as a small part of the meta-analysis (n = 30) were unlikely to shift the pooled point estimate or its confidence intervals derived from a much larger sample size (n = 966), and therefore no further modification, such as removal of outliers, was needed. Both visualisation of funnel plot and severity test of publication bias suggested no clear evidence of publication bias in the current meta-analysis.
Discussion
Over the last 30 years, several studies have investigated whether NPS are associated with impairment in cognition in people with dementia. This review examined those relationships by pooling together results from 90 published studies, and this is to the best of our knowledge the first study to systematically review these associations. See the full list of references of the included studies (S5) published as supplementary material online attached to the electronic version of this paper.
Our meta-analysis suggests that various neuropsychiatric clusters and symptoms are associated with impairment in overall and specific cognitive abilities. The main hypothesis in this review was that there would be a negative association between NPS and cognition, i.e. that experiencing the NPS (as opposed to not experiencing it) or experiencing it at a higher frequency or severity is associated with worse cognition. All significant associations found in this review were negative, thus confirming our hypothesis.
Specifically, a negative association was found between overall NPS and overall cognition, which is consistent with previous studies that suggested that NPS were linked to worse cognition in people with mild cognitive impairment (MCI) and dementia, faster cognitive decline and progression from MCI to dementia (Chan et al., 2011; Dietlin et al., 2019; Teng et al., 2007).
The affect cluster was shown to be linked with impairments in global cognition, as well as in attention and semantic knowledge. Within this cluster, apathy was most frequently associated with cognition, including with global cognition and with various cognitive domains. Again, this is consistent with previous research that suggested that apathy was associated with worse cognition and that it is predictive of conversion from MCI to dementia (Fresnais et al., 2022).
No statistically significant associations were found between the executive dysfunction cluster or disinhibition and cognition in this review. A possible explanation for this is that only a few studies (between 2 and 9) investigated these relationships and therefore a significant association could not be found. The same was true for social inappropriateness since there were no studies investigating the relationships between this symptom and cognition.
The aggression and motor disturbances clusters were also found to be associated with global cognition. An association was found between agitation and worse global cognitive performance.
Psychotic symptoms were linked with worse overall cognition and impairment in some cognitive domains, such as executive function and working memory. Within this cluster, hallucinations only showed an association with global cognition, while delusions were found to be associated with several cognitive domains.
Few studies (between 3 and 9) investigated the relationship between circadian rhythms and cognition in dementia, limiting the evidence in this domain, and no significant associations were found in this review in these domains.
This is the first systematic review to synthesise the existing evidence regarding the specific associations between NPS and cognition. Our methodology was robust, including a broad search strategy, which resulted in the retrieval of thousands of items for screening, and all steps of the review process were conducted independently by two or more reviewers.
This study demonstrates that there is an association between overall NPS symptoms and worse cognitive performance, and between specific NPS and worse performance in specific cognitive domains. Despite the large number of studies included in this review, there is still relatively little research looking at the associations between some NPS, particularly those in the executive dysfunction and circadian rhythms clusters, and cognition in people living with dementia. Furthermore, due to the significant heterogeneity that characterised the associations, the relationships we found require further investigation to confirm their validity. This might include the use of standardised measures for various NPS and cognitive domains. Additionally, synthesis of longitudinal results is warranted to detect whether NPS precede or are a consequence of cognitive impairment or whether there is a bidirectional or more complex relationship between them. Moreover, future studies should investigate whether the nature of some of the identified relationships changes over time, depending on the stage of the disease, or following interventions and other treatments.
The aetiology behind NPS is known to be complex and multidimensional (Kálmán et al., 2008), likely with biological, psychological, social and cognitive contributions. This systematic review looked solely at the potential cognitive aspect of this aetiology. However, while this may be considered a limitation, our findings suggest that if we can manage the cognitive deficits using alternative strategies such as biological, psychological and social treatment approaches, we will also address some of the other facets that contribute to the expression of NPS in people with dementia.
Several other limitations warrant consideration in the interpretation of the results of this review. First, for practical reasons, studies for which we were not able to obtain a manuscript in English were excluded. Furthermore, studies that did not present data as Pearson/Spearman correlations or mean differences and for which it was not possible to obtain the data from the authors were excluded, which lessened the scope of our review. However, given the large number of included studies, these exclusions are unlikely to have biased the results. Additionally, due to the large number of studies that were included and practical constraints, a decision was made to focus on the associations between NPS and objective cognition, excluding the investigation of subjective cognition (e.g. perception of one’s memory ability). Moreover, cognitive and NPS were clustered using a classification system created by the authors of this review to account for the lack of a gold standard classification system that could accommodate the various domains reported by the primary studies. We acknowledge that different results may have been found if a different system for clustering domains had been followed. However, the current classification system was developed following the clinical and research expertise of the authors of the review in the fields of neuropsychology and neuropsychiatry.
A further limitation of the review is that the majority of studies focused on people with dementia due to AD and, in most cases, there were not enough data for people with other dementia diagnoses (e.g. DLB or PD). While previous research has shown that different patterns of NPS can be found in different types of dementia (Majer et al., 2019), the relatively little body of work of the associations between NPS and cognition in people with dementia diagnoses other than AD made it difficult to explore whether the relationships are different for people with different types of dementia. Future research should aim to expand the study of these associations to people with a wide range of dementia diagnoses including dementia due to mixed pathologies. It was also not possible to investigate other potential moderators of the identified associations (e.g. age at onset of dementia or severity of dementia) due to poor reporting of these characteristics.
Other potential causes of bias include the heterogeneity between studies in relation to the scales used to measure NPS and tests used to measure cognition and the varying severity of dementia. Although it was not possible to investigate whether the latter moderates the relationships due to insufficient data about participants’ dementia severity, the associations found in our review differ from those found by a recent cross-sectional study that included 7179 cognitively unimpaired older adults (Liampas et al., 2022). For example, Liampas and colleagues found associations between anxiety and impairment in semantic memory and between hallucinations and worse executive functions, both of which were not found in our review of people with dementia. While some of these differences are likely driven by methodological factors, a possible explanation is that the associations between NPS and cognition are not stable throughout the trajectories of cognitive decline. This raises the question of whether the underlying neuroanatomical changes of people with dementia (Wang et al., 2003), that are not present in cognitively unimpaired people, play a role in mediating these associations. While this was beyond the scope of the current review, future studies should explore the associations between NPS and cognition in light of the recent developments in the field of the neurobiological underpinnings of both kinds of symptoms. Furthermore, the construct of dementia itself has suffered several modifications throughout the years, with a shift from diagnostic criteria based primarily on symptomatology towards an approach that includes results from imaging and biomarkers tests. This, coupled with the evolution in the way cognition and NPS are understood and operationalised, could further interfere with the correct interpretation of the associations found. Future reviews should address this heterogeneity controlling for these or other variables. A better understanding of these issues could lead to improvement in early treatment of such symptoms and therefore allow for better clinical outcomes, as well as carer burden relief for family and professional caregivers, and saving of costs associated with care of the person with dementia.
This review demonstrates a link between certain NPS and impairment in specific cognitive skills in people with dementia. Of course, the associations found in the current study do not establish causality. It is not possible to determine, based on results from this meta-analysis, whether NPS precede, result from, or simply coexist with cognitive impairment, or whether there is a complex relationship with overlapping pathologies, interaction with the environment and other health factors and varying depending on the stage of the disease. In any case, these findings should incentivise researchers to further examine co-occurrence of these symptoms and explore novel treatment approaches that target both kinds of symptoms simultaneously, as well as aim to better understand the nature of the relationship between them.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The University of Melbourne Statistical support team assisted in the statistical analysis of the review.
Author Contribution
JS, ABF, AL and HMG formulated the research questions and designed the review. JS, AG, MC, SL and TC screened and selected studies for inclusion. HMG assisted in resolving issues related to study selection. JS and MC extracted the data. AG, SL and TC checked the data. JS, AG and ABF classified the cognitive and neuropsychiatric measures. JS contacted authors for missing relevant information. JS, MC and NL extracted the characteristics of included studies. JS, AG, MC, NL, SL and TC conducted the study quality assessment, with HMG assisting in resolving conflicts. JS and AL supervised the statistical analysis, which was conducted by The University of Melbourne Statistical Support team and MC. JS drafted the manuscript. All authors read and contributed to the writing of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This review is part of Ms Julieta Sabates’s PhD project. Ms Sabates was awarded a Melbourne Research Scholarship by The University of Melbourne.
Availability of Data and Materials
All relevant data are presented in this manuscript and its supporting files.
Declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akyol MA, Küçükgüçlü Ö, Yener G. Investigation of factors affecting apathy in three major types of dementia. Archives of Neuropsychiatry. 2020;57(2):120. doi: 10.29399/npa.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhof B, Bakker C, Zwijsen SA, Smalbrugge M, Verhey FR, de Vugt ME, et al. The determinants of quality of life of nursing home residents with young-onset dementia and the differences between dementia subtypes. Dementia and Geriatric Cognitive Disorders. 2017;43(5–6):320–329. doi: 10.1159/000477087. [DOI] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. (2022). Deaths in Australia, AIHW, Australian Government, accessed 06 July 2022.
- Balci BD, Yener G, Angin S. The relationship between physical performance, cognition and depression in alzheimer type of dementia. Journal of Neurological Sciences (Turkish) 2011;28(1):051–057. [Google Scholar]
- Benedict RH, Dobraski M, Goldstein MZ. A preliminary study of the association between changes in mood and cognition in a mixed geriatric psychiatry sample. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1999;54(2):P94–P99. doi: 10.1093/geronb/54B.2.P94. [DOI] [PubMed] [Google Scholar]
- Bhat, A., Biswas, A., Das, G., Lahiri, D., Dubey, S., & Mukherjee, A. (2021). Behavioral variations among vascular cognitive impairment subtypes–A comparative study. Applied Neuropsychology: Adult, 1–8. [DOI] [PubMed]
- Breitve MH, Brønnick K, Chwiszczuk LJ, Hynninen MJ, Aarsland D, Rongve A. Apathy is associated with faster global cognitive decline and early nursing home admission in dementia with Lewy bodies. Alzheimer's Research & Therapy. 2018;10(1):1–8. doi: 10.1186/s13195-018-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnick K, Emre M, Tekin S, Haugen SB, Aarsland D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson's disease. Movement Disorders. 2011;26(5):824–829. doi: 10.1002/mds.23525. [DOI] [PubMed] [Google Scholar]
- Bylsma, F. W., Richards, M., & Stern, Y. (1994). Delusions and patterns of cognitive impairment in Alzheimer’s disease.
- Camargo CHF, Serpa RA, Jobbins VA, Berbetz FA, Sabatini JS. Differentiating between apathy and depression in patients with parkinson disease dementia. American Journal of Alzheimer's Disease & Other Dementias. 2018;33(1):30–34. doi: 10.1177/1533317517728333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WC, Lam LC, Tam CW, Lui VW, Leung GT, Lee AT, Chan WM. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: A 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age and Ageing. 2011;40(1):30–35. doi: 10.1093/ageing/afq151. [DOI] [PubMed] [Google Scholar]
- Chwiszczuk L, Breitve MH, Brønnick K, Gjerstad MD, Hynninen M, Aarsland D, Rongve A. REM sleep behavior disorder is not associated with a more rapid cognitive decline in mild dementia. Frontiers in Neurology. 2017;8:375. doi: 10.3389/fneur.2017.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contador-Castillo I, Fernandez-Calvo B, Cacho-Gutierrez LJ, Ramos-Campos F, Hernandez-Martin L. Depression in Alzheimer type-dementia: Is there any effect on memory performance. Revista de Neurología. 2009;49(10):505–510. [PubMed] [Google Scholar]
- D'Antonio F, Di Vita A, Zazzaro G, Brusà E, Trebbastoni A, Campanelli A, Boccia M. Psychosis of Alzheimer's disease: Neuropsychological and neuroimaging longitudinal study. International Journal of Geriatric Psychiatry. 2019;34(11):1689–1697. doi: 10.1002/gps.5183. [DOI] [PubMed] [Google Scholar]
- de Oliveira FF, Wajman JR, Bertolucci PHF, Chen ES, Smith MC. Correlations among cognitive and behavioural assessments in patients with dementia due to Alzheimer's disease. Clinical Neurology and Neurosurgery. 2015;135:27–33. doi: 10.1016/j.clineuro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- de Oliveira FF, Machado FC, Sampaio G, Marin SM, Chen ES, Smith MC, Bertolucci PH. Contrasts between patients with Lewy body dementia syndromes and APOE-ε3/ε3 patients with late-onset Alzheimer disease dementia. The Neurologist. 2015;20(2):35–41. doi: 10.1097/NRL.0000000000000045. [DOI] [PubMed] [Google Scholar]
- de Oliveira, F. F., Machado, F. C., Sampaio, G., Marin, S. D. M. C., da Graça Naffah-Mazzacoratti, M., & Bertolucci, P. H. F. (2020). Neuropsychiatric feature profiles of patients with Lewy body dementia. Clinical Neurology and Neurosurgery,194, 105832. [DOI] [PubMed]
- de Paula JJ, Bicalho MA, Ávila RT, Cintra MT, Diniz BS, Romano-Silva MA, Malloy-Diniz LF. A reanalysis of cognitive-functional performance in older adults: Investigating the interaction between normal aging, mild cognitive impairment, mild Alzheimer's disease dementia, and depression. Frontiers in Psychology. 2016;6:2061. doi: 10.3389/fpsyg.2015.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Verhey FR. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. International Psychogeriatrics. 2005;17(4):577–589. doi: 10.1017/S1041610205002292. [DOI] [PubMed] [Google Scholar]
- DeMichele-Sweet, M. A. A., Lopez, O. L., & Sweet, R. A. (2011). Psychosis in Alzheimer’s disease in the national Alzheimer's disease coordinating center uniform data set: Clinical correlates and association with apolipoprotein e. International Journal of Alzheimer’s Disease, 2011. [DOI] [PMC free article] [PubMed]
- Dietlin S, Soto M, Kiyasova V, Pueyo M, de Mauleon A, Delrieu J, et al. Neuropsychiatric symptoms and risk of progression to Alzheimer’s disease among mild cognitive impairment subjects. Journal of Alzheimer's Disease. 2019;70(1):25–34. doi: 10.3233/JAD-190025. [DOI] [PubMed] [Google Scholar]
- Drijgers RL, Verhey FR, Leentjens AF, Köhler S, Aalten P. Neuropsychological correlates of apathy in mild cognitive impairment and Alzheimer's disease: The role of executive functioning. International Psychogeriatrics. 2011;23(8):1327–1333. doi: 10.1017/S1041610211001037. [DOI] [PubMed] [Google Scholar]
- Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, Papma JM. Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: Cross-sectional and longitudinal associations. Neurology. 2021;97(13):e1276–e1287. doi: 10.1212/WNL.0000000000012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace, A., Kidd, N., Greene, E., Fallon, C., Bhrain, S. N., Cunningham, C., ... & Lawlor, B. A. (2001). Verbal aggression in Alzheimer's disease. Clinical, functional and neuropsychological correlates. International Journal of Geriatric Psychiatry, 16(9), 858–861. [DOI] [PubMed]
- Fahlander K, Berger AK, Wahlin Å, Bäckman L. Depression does not aggravate the episodic memory deficits associated with Alzheimer's disease. Neuropsychology. 1999;13(4):532. doi: 10.1037/0894-4105.13.4.532. [DOI] [PubMed] [Google Scholar]
- Fernández M, Gobartt AL, Balañá M. Behavioural symptoms in patients with Alzheimer's disease and their association with cognitive impairment. BMC Neurology. 2010;10(1):1–9. doi: 10.1186/1471-2377-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martinez M, Molano A, Castro J, J Zarranz J. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer's disease, and its relationship with cognitive impairment. Current Alzheimer Research. 2010;7(6):517–526. doi: 10.2174/156720510792231748. [DOI] [PubMed] [Google Scholar]
- Fillit H, Aigbogun MS, Gagnon-Sanschagrin P, Cloutier M, Davidson M, Serra E, Grossberg G. Impact of agitation in long-term care residents with dementia in the United States. International Journal of Geriatric Psychiatry. 2021;36(12):1959–1969. doi: 10.1002/gps.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz AG, Teri L. Depression, cognition, and functional ability in patients with Alzheimer's disease. Journal of the American Geriatrics Society. 1994;42(2):186–191. doi: 10.1111/j.1532-5415.1994.tb04950.x. [DOI] [PubMed] [Google Scholar]
- Flynn, F. G., Cummings, J. L., & Gornbein, J. (1991). Delusions in dementia syndromes: Investigation of behavioral and neuropsychological correlates. The Journal of Neuropsychiatry and Clinical Neurosciences. [DOI] [PubMed]
- Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Archives of General Psychiatry. 1983;40(7):812–812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- Fresnais, D., Humble, M. B., Bejerot, S., Meehan, A. D., & Fure, B. (2022). Apathy as a predictor for conversion from mild cognitive impairment to dementia: A systematic review and meta-analysis of longitudinal studies. Journal of Geriatric Psychiatry and Neurology, 08919887221093361. [DOI] [PMC free article] [PubMed]
- Gallassi R, Morreale A, Pagni P. The relationship between depression and cognition. Archives of Gerontology and Geriatrics. 2001;33:163–171. doi: 10.1016/S0167-4943(01)00136-4. [DOI] [PubMed] [Google Scholar]
- Gallo JL, Schmidt KS, Libon DJ. Behavioral and psychological symptoms, neurocognitive performance, and functional independence in mild dementia. Dementia. 2008;7(3):397–413. doi: 10.1177/1471301208093291. [DOI] [Google Scholar]
- Galynker II, Roane DM, Miner CR, Feinberg TE, Watts P. Negative symptoms in patients with Alzheimer's disease. The American Journal of Geriatric Psychiatry. 1995;3(1):52–59. doi: 10.1097/00019442-199524310-00007. [DOI] [PubMed] [Google Scholar]
- Gaugler, J. E., Yu, F., Krichbaum, K., & Wyman, J. F. (2009). Predictors of nursing home admission for persons with dementia. Medical Care, 191–198. [DOI] [PubMed]
- Gilley, D. W., Whalen, M. E., Wilson, R. S., & Bennett, D. A. (1991). Hallucinations and associated factors in Alzheimer's disease. The Journal of Neuropsychiatry and Clinical Neurosciences. [DOI] [PubMed]
- Grossi D, Santangelo G, Barbarulo AM, Vitale C, Castaldo G, Proto MG, Trojano L. Apathy and related executive syndromes in dementia associated with Parkinson's disease and in Alzheimer's disease. Behavioural Neurology. 2013;27(4):515–522. doi: 10.1155/2013/781029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallikainen I, Koivisto AM, Paajanen T, Hiltunen A, Karppi P, Vanhanen M, Hänninen T. Cognitive and neuropsychiatric symptom differences in early stages of Alzheimer’s disease: Kuopio ALSOVA study. Dementia and Geriatric Cognitive Disorders Extra. 2012;2(1):209–218. doi: 10.1159/000338231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood DG, Barker WW, Ownby RL, Duara R. Relationship of behavioral and psychological symptoms to cognitive impairment and functional status in Alzheimer's disease. International Journal of Geriatric Psychiatry. 2000;15(5):393–400. doi: 10.1002/(SICI)1099-1166(200005)15:5<393::AID-GPS120>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hopkins MW, Libon DJ. Neuropsychological functioning of dementia patients with psychosis. Archives of Clinical Neuropsychology. 2005;20(6):771–783. doi: 10.1016/j.acn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Ito T, Meguro K, Akanuma K, Meguro M, Lee E, Kasuya M, Mori E. Behavioral and psychological symptoms assessed with the BEHAVE-AD-FW are differentially associated with cognitive dysfunction in Alzheimer’s disease. Journal of Clinical Neuroscience. 2007;14(9):850–855. doi: 10.1016/j.jocn.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Janzing JG, Naarding P, Eling PA. Depressive symptom quality and neuropsychological performance in dementia. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences. 2005;20(5):479–484. doi: 10.1002/gps.1315. [DOI] [PubMed] [Google Scholar]
- Kálmán, J., Kálmán, S., & Pákáski, M. (2008). Recognition and treatment of behavioral and psychological symptoms of dementias: Lessons from the CATIE-AD study. Neuropsychopharmacologia Hungarica: A Magyar Pszichofarmakologiai Egyesulet Lapja= Official Journal of the Hungarian Association of Psychopharmacology, 10(4), 233–249. [PubMed]
- Keator LM, Wright AE, Saxena S, Kim K, Demsky C, Sebastian R, Tippett DC. Distinguishing logopenic from semantic & nonfluent variant primary progressive aphasia: Patterns of linguistic and behavioral correlations. Neurocase. 2019;25(3–4):98–105. doi: 10.1080/13554794.2019.1625929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzis G, Sabe L, Tiberti C, Dorrego F, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52(7):1403–1403. doi: 10.1212/WNL.52.7.1403. [DOI] [PubMed] [Google Scholar]
- Kwak YT, Yang Y, Kwak SG, Koo MS. Delusions of Korean patients with Alzheimer's disease: Study of drug-naïve patients. Geriatrics & Gerontology International. 2013;13(2):307–313. doi: 10.1111/j.1447-0594.2012.00897.x. [DOI] [PubMed] [Google Scholar]
- Lam CL, Chan WC, Mok CC, Li SW, Lam LC. Validation of the Chinese Challenging Behaviour Scale: Clinical correlates of challenging behaviours in nursing home residents with dementia. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences. 2006;21(8):792–799. doi: 10.1002/gps.1564. [DOI] [PubMed] [Google Scholar]
- Lee CH, Kim DH, Moon YS. Differential associations between depression and cognitive function in MCI and AD: A cross-sectional study. International Psychogeriatrics. 2019;31(8):1151–1158. doi: 10.1017/S1041610218001527. [DOI] [PubMed] [Google Scholar]
- Lee E, Meguro K, Hashimoto R, Meguro M, Ishii H, Yamaguchi S, Mori E. Confabulations in episodic memory are associated with delusions in Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology. 2007;20(1):34–40. doi: 10.1177/0891988706292760. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Tsai CF, Gauthier S, Wang SJ, Fuh JL. The association between cognitive impairment and neuropsychiatric symptoms in patients with Parkinson's disease dementia. International Psychogeriatrics. 2012;24(12):1980–1987. doi: 10.1017/S1041610212001317. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Litvan I. Apathy is not depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(3):314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- Liampas I, Siokas V, Lyketsos CG, Dardiotis E. The relationship between neuropsychiatric symptoms and cognitive performance in older adults with normal cognition. Medicina. 2022;58(11):1586. doi: 10.3390/medicina58111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon RG, Teri L, McCurry SM, Gibbons LE, Kukull WA, Larson EB. Wandering: A significant problem among community residing individuals with Alzheimer's disease. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1998;53(5):P294–P299. doi: 10.1093/geronb/53B.5.P294. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Brenner RP, Rosen J, Bajulaiye OI, Reynolds CF. Alzheimer's disease with delusions and hallucinations: Neuropsychological and electroencephalographic correlates. Neurology. 1991;41(6):906–906. doi: 10.1212/WNL.41.6.906. [DOI] [PubMed] [Google Scholar]
- Machado FC, Oliveira FFD, Marin SDMC, Sampaio G, Bertolucci PHF. Correlates of neuropsychiatric and motor tests with language assessment in patients with Lewy body dementia. Archives of Clinical Psychiatry (São Paulo) 2020;47:75–81. doi: 10.1590/0101-60830000000236. [DOI] [Google Scholar]
- Majer R, Simon V, Csiba L, Kardos L, Frecska E, Hortobágyi T. Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes. Open Medicine. 2019;14(1):307–316. doi: 10.1515/med-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano LI, Caramelli P, Guimarães HC, Gambogi LB, Moura MVB, Yassuda MS, de Souza LC. Can social cognition measurements differentiate behavioral variant frontotemporal dementia from Alzheimer’s disease regardless of apathy? Journal of Alzheimer's Disease. 2020;74(3):817–827. doi: 10.3233/JAD-190861. [DOI] [PubMed] [Google Scholar]
- McPherson S, Fairbanks L, Tiken S, Cummings JL, Back-Madruga C. Apathy and executive function in Alzheimer's disease. Journal of the International Neuropsychological Society. 2002;8(3):373–381. doi: 10.1017/S1355617702813182. [DOI] [PubMed] [Google Scholar]
- Migliorelli R, Petracca G, Teson A, Sabe L, Leiguarda R, Starkstein SE. Neuropsychiatric and neuropsychological correlates of delusions in Alzheimer's disease. Psychological Medicine. 1995;25(3):505–513. doi: 10.1017/S0033291700033420. [DOI] [PubMed] [Google Scholar]
- Millenaar JK, Bakker C, Koopmans RT, Verhey FR, Kurz A, de Vugt ME. The care needs and experiences with the use of services of people with young-onset dementia and their caregivers: A systematic review. International Journal of Geriatric Psychiatry. 2016;31(12):1261–1276. doi: 10.1002/gps.4502. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Starkstein SE, Jorge R, Robinson RG. Phenomenology and clinical correlates of delusions in Alzheimer disease. The American Journal of Geriatric Psychiatry. 2006;14(7):573–581. doi: 10.1097/01.JGP.0000214559.61700.1c. [DOI] [PubMed] [Google Scholar]
- Montagnese M, Vignando M, Collerton D, Ffytche D, Mosimann UP, Taylor JP, Urwyler P. Cognition, hallucination severity and hallucination-specific insight in neurodegenerative disorders and eye disease. Cognitive Neuropsychiatry. 2022;27(2–3):105–121. doi: 10.1080/13546805.2021.1960812. [DOI] [PubMed] [Google Scholar]
- Na HR, Kang DW, Woo YS, Bahk WM, Lee CU, Lim HK. Relationship between delusion of theft and cognitive functions in patients with mild Alzheimer’s disease. Psychiatry Investigation. 2018;15(4):413. doi: 10.30773/pi.2017.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarding P, de Koning I, van Kooten F, Janzing JG, Beekman AT, Koudstaal PJ. Post-stroke dementia and depression: Frontosubcortical dysfunction as missing link? International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences. 2007;22(1):1–8. doi: 10.1002/gps.1599. [DOI] [PubMed] [Google Scholar]
- Nagata T, Shinagawa S, Ochiai Y, Kada H, Kasahara H, Nukariya K, Nakayama K. Relationship of frontal lobe dysfunction and aberrant motor behaviors in patients with Alzheimer's disease. International Psychogeriatrics. 2010;22(3):463–469. doi: 10.1017/S1041610209991323. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nakajima S, Shinagawa S, Plitman E, Graff-Guerrero A, Mimura M, Nakayama K. Psychosocial or clinico-demographic factors related to neuropsychiatric symptoms in patients with Alzheimer's disease needing interventional treatment: Analysis of the CATIE-AD study. International Journal of Geriatric Psychiatry. 2017;32(12):1264–1271. doi: 10.1002/gps.4607. [DOI] [PubMed] [Google Scholar]
- Nakaaki S, Murata Y, Sato J, Shinagawa Y, Tatsumi H, Hirono N, Furukawa TA. Greater impairment of ability in the divided attention task is seen in Alzheimer’s disease patients with depression than in those without depression. Dementia and Geriatric Cognitive Disorders. 2007;23(4):231–240. doi: 10.1159/000099633. [DOI] [PubMed] [Google Scholar]
- Nakaaki S, Murata Y, Sato J, Shinagawa Y, Hongo J, Tatsumi H, Furukawa TA. Association between apathy/depression and executive function in patients with Alzheimer's disease. International Psychogeriatrics. 2008;20(5):964–975. doi: 10.1017/S1041610208007308. [DOI] [PubMed] [Google Scholar]
- Nakatsuka M, Meguro K, Nakamura K, Akanuma K, Yamaguchi S. ‘Residence is not home’ is a particular type of delusion associated with cognitive decline of Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2014;38(1–2):46–54. doi: 10.1159/000357837. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (2014a). National heart, lung and blood Institute quality assessment tool for observational cohort and cross-sectional studies.
- National Institutes of Health. (2014b). National heart, lung and blood Institute quality assessment tool for controlled intervention studies.
- Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike CU, Sheppard JME, Tschanz JT, Norton MC, Green RC, Steinberg M, Lyketsos CG. Epidemiology of apathy in older adults: The Cache County Study. The American Journal of Geriatric Psychiatry. 2007;15(5):365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Llebaria G, García-Sánchez C, Pascual-Sedano B, Gironell A, Kulisevsky J. A prospective study of delusional misidentification syndromes in Parkinson's disease with dementia. Movement Disorders: Official Journal of the Movement Disorder Society. 2008;23(3):443–448. doi: 10.1002/mds.21864. [DOI] [PubMed] [Google Scholar]
- Park S, Kim DK, Myung W, Yoo JH, Shin SJ, Na DL, Shin J. Risk factors of behavioral and psychological symptoms in patients with Alzheimer disease: The clinical research of dementia of South Korea study. Korean Journal of Family Medicine. 2019;40(1):16. doi: 10.4082/kjfm.17.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Drzezga A, Boecker H, Wagenpfeil S, Förstl H, Kurz A, Häussermann P. Right prefrontal hypometabolism predicts delusions in dementia with Lewy bodies. Neurobiology of Aging. 2009;30(9):1420–1429. doi: 10.1016/j.neurobiolaging.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Perri R, Monaco M, Fadda L, Caltagirone C, Carlesimo GA. Neuropsychological correlates of behavioral symptoms in Alzheimer's disease, frontal variant of frontotemporal, subcortical vascular, and lewy body dementias: A comparative study. Journal of Alzheimer's Disease. 2014;39(3):669–677. doi: 10.3233/JAD-131337. [DOI] [PubMed] [Google Scholar]
- Perri R, Turchetta CS, Caruso G, Fadda L, Caltagirone C, Carlesimo GA. Neuropsychological correlates of cognitive, emotional-affective and auto-activation apathy in Alzheimer's disease. Neuropsychologia. 2018;118:12–21. doi: 10.1016/j.neuropsychologia.2018.01.039. [DOI] [PubMed] [Google Scholar]
- Pezzoli S, Cagnin A, Antonini A, Venneri A. Frontal and subcortical contribution to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease. Postgraduate Medicine. 2019;131(7):509–522. doi: 10.1080/00325481.2019.1656515. [DOI] [PubMed] [Google Scholar]
- Prince, M., Albanese, E., Guerchet, M., & Prina, M. (2014). Dementia and risk reduction: An analysis of protective and modifiable factors. World Alzheimer Report, 66–83.
- Qian W, Fischer CE, Schweizer TA, Munoz DG. Association between psychosis phenotype and APOE genotype on the clinical profiles of Alzheimer's disease. Current Alzheimer Research. 2018;15(2):187–194. doi: 10.2174/1567205014666170829114346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta D, Vita MG, Bizzarro A, Masullo C, Piccininni C, Gainotti G, Marra C. Cognitive and behavioral determinants of psychotic symptoms in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2015;39(3–4):194–206. doi: 10.1159/000369161. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Schwartz S, Black BS, Corcoran C, Fauth E, Mielke M, et al. Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimer's & Dementia. 2013;9(2):204–207. doi: 10.1016/j.jalz.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Jagust WJ, Coulter L. Anosognosia in Alzheimer's disease: Relationships to depression, cognitive function, and cerebral perfusion. Journal of Clinical and Experimental Neuropsychology. 1993;15(2):231–244. doi: 10.1080/01688639308402560. [DOI] [PubMed] [Google Scholar]
- Rochat L, Billieux J, Van der Linden ACJ, Annoni JM, Zekry D, Gold G, Van der Linden M. A multidimensional approach to impulsivity changes in mild Alzheimer’s disease and control participants: Cognitive correlates. Cortex. 2013;49(1):90–100. doi: 10.1016/j.cortex.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Rolland, Y., Andrieu, S., Cantet, C., Morley, J. E., Thomas, D., Nourhashemi, F., & Vellas, B. (2007). Wandering behavior and Alzheimer disease. The REAL. FR prospective study. Alzheimer Disease & Associated Disorders, 21(1), 31–38. [DOI] [PubMed]
- Ross LK, Arnsberger P, Fox PJ. The relationship between cognitive functioning and disease severity with depression in dementia of the Alzheimer's type. Aging & Mental Health. 1998;2(4):319–327. doi: 10.1080/13607869856579. [DOI] [Google Scholar]
- Rozum WJ, Cooley B, Vernon E, Matyi J, Tschanz JT. Neuropsychiatric symptoms in severe dementia: Associations with specific cognitive domains the Cache County Dementia Progression Study. International Journal of Geriatric Psychiatry. 2019;34(7):1087–1094. doi: 10.1002/gps.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M, Arias A, Sánchez-Llanos E, Gil MP, López-Ortega R, Dakterzada F, Piñol-Ripoll G. Minor hallucinations in Alzheimer’s disease. Journal of Alzheimer's Disease. 2018;64(2):543–549. doi: 10.3233/JAD-180234. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez JL. Neuropsychologic performance and depressed mood in sporadic late onset Alzheimer disease. Revista de Neurologia. 2004;38(7):625–630. [PubMed] [Google Scholar]
- Senanarong V, Poungvarin N, Jamjumras P, Sriboonroung A, Danchaivijit C, Udomphanthuruk S, Cummings JL. Neuropsychiatric symptoms, functional impairment and executive ability in Thai patients with Alzheimer's disease. International Psychogeriatrics. 2005;17(1):81–90. doi: 10.1017/S1041610205000980. [DOI] [PubMed] [Google Scholar]
- Serra L, Perri R, Fadda L, Padovani A, Lorusso S, Pettenati C, Carlesimo GA. Relationship between cognitive impairment and behavioural disturbances in Alzheimer's disease patients. Behavioural Neurology. 2010;23(3):123–130. doi: 10.1155/2010/528694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Han HJ, Shin DJ, Park HM, Lee YB, Park KH. Sleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer's disease. Journal of Clinical Neurology. 2014;10(3):203–209. doi: 10.3988/jcn.2014.10.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleman Hernandez SS, Vital TM, Garuffi M, Stein AM, Teixeira CVL, Costa JLR, Stella F. Apathy, cognitive function and motor function in Alzheimer's disease. Dementia & Neuropsychologia. 2012;6:236–243. doi: 10.1590/S1980-57642012DN06040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(8):1070–1074. doi: 10.1136/jnnp.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Lonie J. Relationship between behavioural and psychological symptoms of dementia and cognition in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2007;24(5):343–347. doi: 10.1159/000108632. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. International Journal of Geriatric Psychiatry: A Journal of the Psychiatry of Late Life and Allied Sciences. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, Sperry SD. An informant-based assessment of apathy in Alzheimer disease. Cognitive and Behavioral Neurology. 2002;15(3):176–183. [PubMed] [Google Scholar]
- Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL. Assessment of cognitive, psychiatric, and behavioral disturbances in patients with dementia: The Neurobehavioral Rating Scale. Journal of the American Geriatrics Society. 1992;40(6):549–555. doi: 10.1111/j.1532-5415.1992.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, Leskin LP, Melrose RJ, Harwood DG, Narvaez TA, Ando TK, Mandelkern MA. Neurobiology of delusions, memory, and insight in Alzheimer disease. The American Journal of Geriatric Psychiatry. 2014;22(11):1346–1355. doi: 10.1016/j.jagp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2007;24(4):253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- Van der Mussele S, Bekelaar K, Le Bastard N, Vermeiren Y, Saerens J, Somers N, Engelborghs S. Prevalence and associated behavioral symptoms of depression in mild cognitive impairment and dementia due to Alzheimer's disease. International Journal of Geriatric Psychiatry. 2013;28(9):947–958. doi: 10.1002/gps.3909. [DOI] [PubMed] [Google Scholar]
- Van der Mussele S, Le Bastard N, Saerens J, Somers N, Mariën P, Goeman J, Engelborghs S. Agitation-associated behavioral symptoms in mild cognitive impairment and Alzheimer's dementia. Aging & Mental Health. 2015;19(3):247–257. doi: 10.1080/13607863.2014.924900. [DOI] [PubMed] [Google Scholar]
- Wagner, A. W., Teri, L., & Orr-Rainey, N. (1995). Behavior problems of residents with dementia in special care units. Alzheimer Disease and Associated Disorders. [PubMed]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging☆. NeuroImage. 2003;20(2):667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- Welsh SW, Corrigan FM, Scott M. Language impairment and aggression in Alzheimer's disease. International Journal of Geriatric Psychiatry. 1996;11(3):257–261. doi: 10.1002/(SICI)1099-1166(199603)11:3<257::AID-GPS330>3.0.CO;2-U. [DOI] [Google Scholar]
- Wu, H. S. (2014). Predictors of hyperphagia in institutionalized patients with dementia. Journal of Nursing Research,22(4), 250–258. [DOI] [PubMed]
- Yeager CA, Hyer LEE. Apathy in dementia: Relations with depression, functional competence, and quality of life. Psychological Reports. 2008;102(3):718–722. doi: 10.2466/pr0.102.3.718-722. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. The American Journal of Geriatric Psychiatry. 2015;23(2):130–140. doi: 10.1016/j.jagp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are presented in this manuscript and its supporting files.