Abstract
In patients with subacute sclerosing panencephalitis (SSPE), which is associated with persistent measles virus (MV) infection in the brain, little infectious virus can be recovered despite the presence of viral RNA and protein. Based on studies of brain tissue from SSPE patients and our work with MV-infected NSE-CD46+ mice, which express the measles receptor CD46 on neurons, several lines of evidence suggest that the mechanism of viral spread in the central nervous system differs from that in nonneuronal cells. To examine this alternate mechanism of viral spread, as well as the basis for the loss of normal transmission mechanisms, infection and spread of MV Edmonston was evaluated in primary CD46+ neurons from transgenic mice and differentiated human NT2 neurons. As expected, unlike that between fibroblasts, viral spread between neurons occurred in the absence of syncytium formation and with minimal extracellular virus. Electron microscopy analysis showed that viral budding did not occur from the neuronal surface, although nucleocapsids were present in the cytoplasm and aligned at the cell membrane. We observed many examples of nucleocapsids present in the neuronal processes and aligned at presynaptic neuronal membranes. Cocultures of CD46+ and CD46− neurons showed that cell contact but not CD46 expression is required for MV spread between neurons. Collectively, these results suggest that the neuronal environment prevents the normal mechanisms of MV spread between neurons at the level of viral assembly but allows an alternate, CD46-independent mechanism of viral transmission, possibly through the synapse.
After the clearance of acute measles virus (MV) infection in children, a persistent infection of the brain can occur which, after several years, results in the progressive neurological disease subacute sclerosing panencephalitis (SSPE). The symptoms of SSPE consist of gradual deterioration of cognitive and motor functions, and in virtually all cases, the disease is fatal (22). Brain biopsies or postmortem brain analyses from SSPE patients show evidence of astrogliosis, neuronal loss, degeneration of dendrites, demyelination, neurofibrillary tangles, and infiltration of inflammatory cells (2).
Despite the long interval between the acute infection and symptoms of SSPE, there is evidence that MV infection of the brain occurs soon after the acute infection, with subsequent spread throughout the brain (3, 19). However, in contrast to MV infection of nonneuronal cells, which is cytopathic and spreads both by extracellular virus and by cell fusion resulting in multinucleated syncytia, little extracellular infectious virus can be recovered from brains of SSPE patients unless tissues are cocultured with permissive fibroblasts (22). Furthermore, high levels of neutralizing antibody are present in the serum and cerebrospinal fluid of SSPE patients (37). Together, these results suggest that extracellular virus might not be responsible for MV spread in the central nervous system (CNS). In support of this hypothesis, extensive point mutations within envelope-associated viral gene products which affect fusion, viral assembly, and budding have been detected in virus recovered from SSPE brain tissues (4, 8, 10, 40). However, it has not been established whether these mutations are a reflection of a novel mechanism of MV spread in the CNS.
An obstacle to the study of measles pathogenesis has been the lack of a small animal model, because rodents do not express CD46, the human MV receptor used by wild-type and vaccine strains of virus (15, 26, 28). Previous reports of MV infection in mouse, rat, or hamster brains (1, 20, 24) have utilized rodent-adapted measles strains which differ from wild-type and vaccine strains in sequence, receptor usage, and cell tropism (24, 25, 33). In addition, some studies of MV infection in the rodent CNS show evidence of substantial extracellular virus production (24), which is typical of nonneuronal MV infection but contrasts with the findings for human brain infection.
To study neuronal infection that more closely parallels human CNS disease in an animal model system, we have previously utilized transgenic mice which express the human MV receptor, CD46, under the transcriptional control of the neuron-specific enolase (NSE) promoter (23, 31). MV Edmonston infection of NSE-CD46 mice or primary neurons cultured from these mice demonstrated the spread of neuronal infection over time (31), as evidenced by the increased detection of viral RNA and protein in many brain regions, including the hippocampus, cortex, striatum, and thalamus. However, similar to human CNS infection with MV, little infectious virus was recovered from CD46+ brain tissue or from cultured CD46+ hippocampal neurons (31). Thus, in our mouse model, it again seemed possible that MV spread in the CNS occurred via a novel pathway.
To establish the mechanism by which interneuronal MV transmission occurs, we have examined two types of neuronal cultures that can be infected with MV Edmonston: primary hippocampal neurons isolated from embryonic NSE-CD46 transgenic mice and differentiated human NT2 neurons. With these cultures, spread occurred between neurons in direct physical contact but did not require the presence of CD46 or involve the formation of multinucleated syncytia. Moreover, ultrastructural analysis of neurons provided evidence for defective viral budding, which accounted for the lack of extracellular virus in these cultures. Electron microscopy (EM) also showed that nucleocapsids could be found within neuronal processes and aligned with synaptic membranes, suggesting that interneuronal MV spread may occur through synaptic connections. These results are discussed in the context of the pathogenesis of persistent human CNS infections.
MATERIALS AND METHODS
Cells and virus.
Vero and HeLa fibroblasts were maintained in Dulbecco's modified Eagle media (DMEM) (Gibco/BRL, Grand Island, N.Y.) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 ng of streptomycin per ml. Undifferentiated NT2 cells, a generous gift of Robert Doms (University of Pennsylvania), were maintained in Opti-Mem media (Gibco/BRL) with 5% fetal calf serum and the supplements described above. Differentiation of NT2 neuroepithelial cells into the neuronal phenotype followed the protocol of Cook and colleagues (12), involving 5 weeks of culture in supplemented DMEM plus 3 μg of retinoic acid (Sigma Chemical Co., St. Louis, Mo.) per ml, followed by two 10-day steps of replating in supplemented DMEM containing mitotic inhibitors (240 ng each of cytosine β-d-arabinofuranoside, 5-fluoro-2′-deoxyuridine, and uridine [Sigma] per ml). The same compounds were used for mitotic inhibition experiments with Vero, HeLa, and undifferentiated NT2 cells. Primary hippocampal neurons were obtained from day 16 embryonic mice, as described previously (5, 30, 31), except that the cells were maintained in Neurobasal media (Gibco/BRL) containing 4 μg of glutamate per ml, in the absence of an astrocyte feeder layer. MV Edmonston was purchased from the American Type Culture Collection (Manassas, Va.) and was passaged and titered in Vero fibroblasts.
For antibody inhibition experiments, cells grown on coverslips were washed once with phosphate-buffered saline (PBS) immediately after infection, and then media alone or media containing human SSPE immune serum (1:100) was added to appropriate samples for the duration of the experiment. After 1 or 3 days postinfection (d.p.i.) samples were collected and stained by immunohistochemistry (see below) to determine the proportion of infected cells. Data were analyzed by comparison of 95% confidence intervals.
Immunohistochemistry.
Cells grown on glass coverslips were fixed with methanol/acetone (1:1), blocked with 2% (vol/vol) goat serum in PBS, further blocked with avidin and biotin (Vector Laboratories, Burlingame, Calif.), and stained for MV antigens with human SSPE immune serum (1:2,000; Vasquez), followed by incubation with a biotinylated goat anti-human immunoglobulin G (IgG) (1:300; Vector Laboratories), the ABC Elite kit (Vector Laboratories), and diaminobenzidine (0.7 mg/ml in 60 mM Tris) with H2O2 (1.6 mg/ml; Sigma Chemical Co.). Cells were also counterstained with hematoxylin to visualize nuclei. Immunofluorescence detection of MV antigens utilized the same primary antisera but with a fluorescein-conjugated goat anti-human IgG secondary antibody (1:500; Vector Laboratories). Nucleoprotein (NP) of lymphocytic choriomeningitis virus (LCMV) was detected with LCMV ascites (1:2,000; American Type Culture Collection) and rhodamine red-X-conjugated goat anti-mouse IgG (1:1,000; Molecular Probes, Eugene, Oreg.). Fluorescent images were obtained with a Bio-Rad MRC 600 laser scanning confocal microscope paired to a Nikon Optiphot II. The image obtained was a composite of 0.5-μm-thick optical sections taken through a 100× objective with a 2× electronic magnification. Each pixel was 0.072 μm2.
Viral RNA transcript analysis.
Vero fibroblasts and primary CD46+ neurons were infected with MV Edmonston (multiplicity of infection [MOI] = 1) and harvested 2 d.p.i. by scraping into Tri-Reagent (Sigma Chemical Co.). Total RNA was isolated according to manufacturer's instructions and resuspended in diethyl pyrocarbonate-treated water. To determine the proportion of infected cells in some experiments, cells on coverslips included in the cultures were fixed at the time of harvesting and immunostained for the presence of MV antigen, and positive cells were counted. The RNA samples, equivalent to 4,000 infected cells, were added to a final concentration of 48% formamide and 6.6% formaldehyde and then incubated at 68°C for 15 min. Samples were then chilled on ice and diluted twofold with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The RNA samples were applied to a nylon membrane with a slot blot apparatus; the membrane was UV cross-linked and hybridized with a radioactive probe at 68° for 1 h. Probes were DNA-labeled by a random priming kit (Prime-It II; Stratagene, La Jolla, Calif.) in the presence of [32P]dCTP (Dupont NEN, Boston, Mass.). DNA fragments from peN1, peM2, peF1, peH1, and peL-45 (9, 35) were used as templates for hybridization to N, M, F, H, and L, respectively. The signal intensity of radioactive slots was quantified by phosphorimager analysis (Fuji).
Transmission EM.
Vero fibroblasts (300 cells/mm2) or primary neurons (500 cells/mm2) were grown on polycarbonate membranes in cell culture well inserts. The membranes were rinsed with PBS, fixed for 1 h with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), and then removed from the inserts and cut into 1- by 3-mm strips. The strips were postfixed in 1% osmium tetroxide, stained en bloc with uranyl acetate, dehydrated in serial alcohols, and embedded in epon (Embed 812; Electron Microscopy Sciences, Fort Washington, Pa.). Thin sections were cut at 80 nm on a Reichert Ultracut E ultramicrotome, collected on naked copper grids, and stained with lead citrate. Grids were viewed on a Philips 400T electron microscope at 80 kV.
RESULTS
Spread of MV infection in neurons without syncytium formation.
Previously, we showed that spread of MV in neurons occurred over time both in NSE-CD46+ mice and in primary hippocampal neurons isolated from those mice (31). To determine whether interneuronal spread of MV occurs by the same mechanisms as in nonneuronal cells, syncytium formation and infectious virus production were compared in permissive fibroblasts (HeLa or Vero), primary hippocampal neurons from NSE-CD46 mice, and human NT2 neuronal cultures. Cells were plated at the same density on coverslips, infected with MV Edmonston (MOI = 1), collected at various times postinfection, and immunostained for MV antigens.
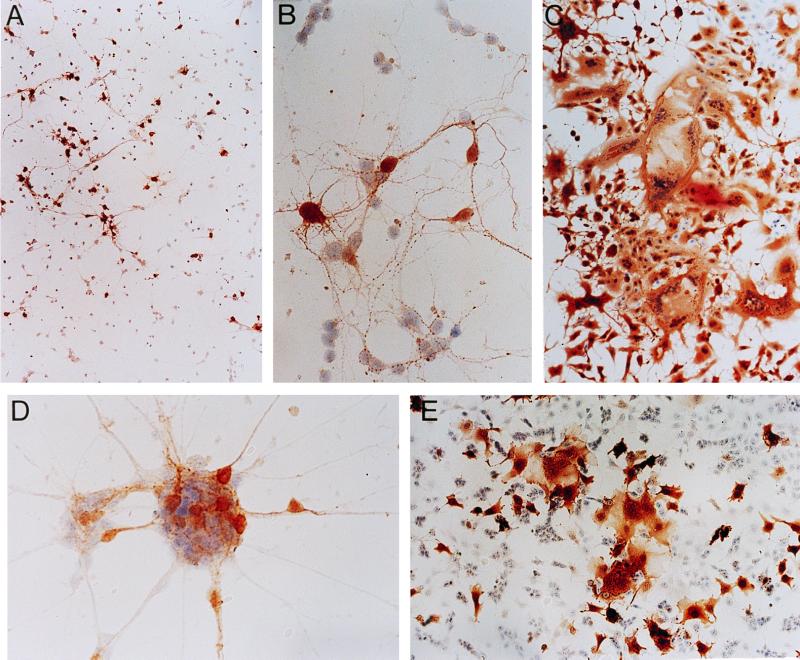
In the primary CD46+ neuronal cultures, the extent of infection increased with time, consistent with previous results (31). At 1 d.p.i. a low proportion of neurons (5% or less) showed evidence of infection, but by 3 d.p.i. the proportion of infected cells averaged 10 to 20%, increasing to 40 to 50% by 5 d.p.i. Nonrandom groups of infected neurons and infected processes connecting them were observed (Fig. 1A), suggesting that the infection was spreading in focal clusters. Infected neurons did not form syncytia in culture; this was particularly evident within clusters of neuronal cell bodies (Fig. 1B). We also did not observe virus-induced cell death within infected primary neurons, as measured by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (data not shown). In contrast, syncytium formation in permissive nonneuronal cells, such as Vero cells (Fig. 1C), was observed as early as 1 to 2 d.p.i. By 3 d.p.i. when 100% of Vero cells were infected, more than 50% of nuclei were contained within syncytia, compared to none from infected neurons.
FIG. 1.
Immunoperoxidase staining of MV antigen in neurons and fibroblasts. (A) MV-infected primary CD46+ neurons at 4 d.p.i. (MOI = 1), showing focal clusters of infection. Magnification, × 70. (B) Contact between infected and uninfected primary neurons in the absence of fusion at 3 d.p.i. (MOI = 1). Magnification, ×280. (C) Syncytium formation in MV-infected Vero fibroblasts at 2 d.p.i. (MOI = 1). Magnification, × 70. (D) MV-infected differentiated NT2 neurons at 1 d.p.i. (MOI = 1), showing contact between infected and uninfected cells in the absence of fusion. Magnification, × 280. (E) Syncytium formation in infected undifferentiated NT2 neuroepithelial cells at 1 d.p.i. (MOI = 1). Magnification, ×70.
Human NT2 neuronal cells, differentiated and separated from their neuroepithelial precursors and grown as more-than-95%-pure cultures (12), were susceptible to infection by MV Edmonston to a much greater degree than the transgenic primary neurons. Approximately 30% of the differentiated NT2 neurons and their processes were infected by 1 d.p.i. and this proportion increased over time to nearly 100% infection by 3 d.p.i. However, as for the primary mouse neurons, syncytium formation was not observed; in clusters of NT2 neurons, individually infected cells were found within groups of uninfected cells (Fig. 1D), again suggesting a lack of membrane fusion. In contrast, the undifferentiated NT2 neuroepithelial progenitors showed marked evidence of syncytium formation as early as 1 d.p.i. (Fig. 1E). Thus, the phenomenon of MV spread without syncytium formation was not restricted to primary mouse neurons and was not explained by a low proportion of infected cells.
Reduced extracellular virus production by MV-infected neurons.
Another mechanism of MV spread in nonneuronal cells is the production of extracellular virus, in which the virion acquires its envelope upon budding from the plasma membrane. However, a characteristic of neuronal MV infection in SSPE tissues is the nearly complete lack of infectious virus (22). To determine whether infection of mouse neurons with a CD46-dependent strain of MV results in extracellular progeny, we compared supernatant titers from MV-infected CD46+ primary mouse neurons, CD46+ fibroblasts, NT2 neurons, and undifferentiated NT2 precursors by plaque assay.
As shown in Table 1, primary CD46+ mouse neurons infected with MV Edmonston (MOI = 1) produced at least 1,000-fold fewer PFU per milliliter than Vero cultures and 100-fold less virus than HeLa cultures plated at the same density and infected with the same MOI. Similar results were observed in at least four subsequent experiments. When the data were corrected for both the lower proportion of infected neurons and the increased number of Vero and HeLa cells due to cell division, neuronal virus production per 1,000 infected cells was still substantially lower than that observed for fibroblasts (Table 1). The highest titer detected from primary neurons in any experiment was 400 PFU/ml (equivalent to <10 PFU/1,000 infected cells), after 7 days of infection (data not shown). This small amount of extracellular virus may reflect release of cell-associated virus upon neuronal death, since the viability of our uninfected cultures generally declines by 6 to 7 days postculture.
TABLE 1.
Extracellular virus production from neurons versus that from nonneuronal cellsa
| Type of cell | d.p.i. | % Infected cellsb | PFU/mlc | PFU/1,000 infected cellsd |
|---|---|---|---|---|
| Primary CD46+ mouse neuron | 1 | 5 | NDe | 0 |
| 2 | 5 | 20 | 10 | |
| 3 | 10 | 20 | 6 | |
| Vero | 1 | 80 | ND | 0 |
| 2 | 100 | 6.0 × 104 | 600 | |
| 3 | 100 | 3.2 × 105 | 1,600 | |
| HeLa | 1 | 50 | 360 | 10 |
| 2 | 80 | 7.2 × 103 | 130 | |
| 3 | 100 | 1.6 × 104 | 170 | |
| NT2 | 1 | 50 | 54 | 2 |
| 2 | 85 | 36 | 1 | |
| 3 | 90 | 156 | 3 | |
| Undifferentiated NT2 | 1 | 60 | 40 | 1 |
| 2 | 75 | 6.4 × 103 | 130 | |
| 3 | 95 | 9.2 × 103 | 100 |
Cells plated at a density of 1 × 105 to 2 × 105 cells/coverslip were infected 1 day later with MV Edmonston (MOI = 1). Data represent results from at least two separate experiments, each performed in duplicate.
Percent infected cells was determined by immunohistochemical staining of MV antigen.
Supernatants tested for infectious virus by plaque assay.
PFU/1,000 cell values, used to correct for cell division rates in fibroblast cultures (cell counts determined in parallel uninfected cultures) and the proportion of cells infected at the indicated time, were calculated as {[(PFU/ml) × 4 ml]/(number of cells × proportion infected)} × 1,000.
ND, not detected.
We also compared supernatant viral titers from human NT2-derived neurons and from undifferentiated NT2 progenitor cells. Infection of differentiated NT2 neurons (∼97% pure) also yielded minimal extracellular virus by 2 and 3 d.p.i. compared to the infected neuroepithelial progenitors (Table 1). Collectively, our findings indicate that neither human-derived nor CD46-expressing murine neurons are permissive for the usual mechanisms of MV spread.
Absence of cell division does not explain lack of extracellular virus production and syncytium formation in neurons.
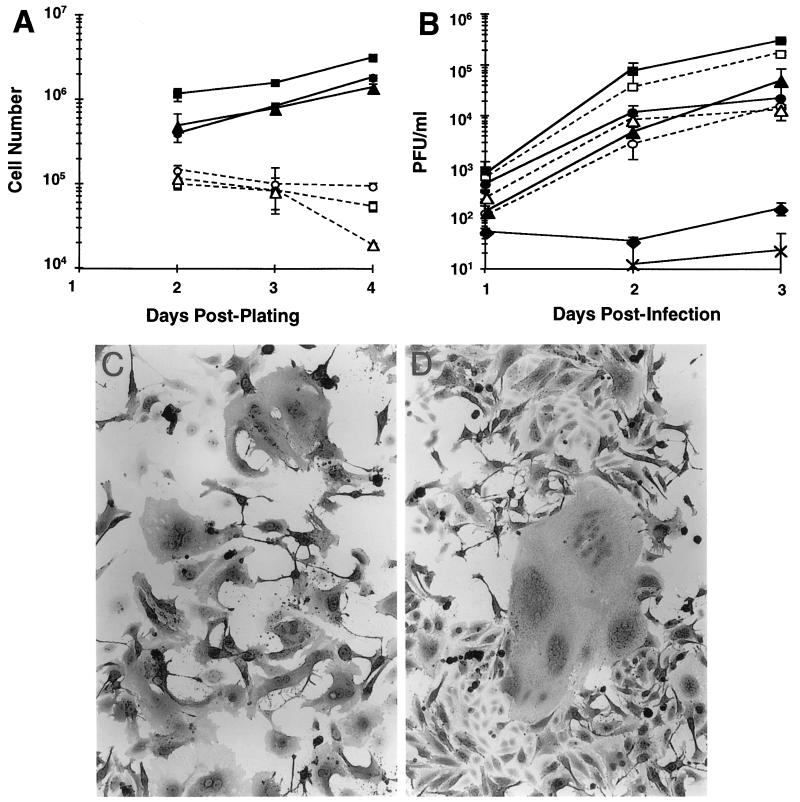
It was possible that the differences we observed between viral infection of neurons and fibroblasts were due to the fact that neurons are terminally differentiated and normally do not divide, either in culture or in vivo. To test whether MV-induced syncytium formation and virus production were affected by a lack of cell division, Vero and HeLa fibroblasts as well as undifferentiated NT2 cells were infected while in the presence of mitotic inhibitors. To confirm that these cultures were not dividing, uninfected cell cultures were plated at 2 × 105 cells/well and counted each day after plating. As expected, the mitotic inhibitors prevented cell division in all three cell lines (Fig. 2A), and no pronounced effects on cell viability were noted except after 4 days, when the number of undifferentiated NT2 cells was reduced fivefold compared to the previous day. In subsequent experiments, cells were plated at 105 cells/coverslip and grown for 24 h in mitotic inhibitors, followed by infection with MV Edmonston (MOI = 1) and continued culturing in mitotic inhibitors. At 1, 2, or 3 d.p.i. supernatants were collected to test infectious virus titers, and coverslips included with the cultures were collected for immunohistochemical staining of MV proteins. Figure 2B shows that for each cell line, the presence of mitotic inhibitors did not significantly affect the levels of extracellular virus production at any time point tested. In addition, syncytia were still present in all cultures with mitotic inhibitors, despite a reduced total cell population (Fig. 2C), compared to standard cultures (Fig. 2D). Thus, the absence of syncytium formation and the reduced infectious virus production by neurons cannot be explained by a lack of cell division.
FIG. 2.
Extracellular virus production and syncytium formation in nonneuronal cells treated with mitotic inhibitors. Vero, HeLa, and undifferentiated NT2 cells were plated and cultured with or without mitotic inhibitors. Cells were infected with MV Edmonston (MOI = 1) at 1 day postplating. ■, Vero cells; □, Vero cells plus mitotic inhibitors; ●, HeLa cells; ○, HeLa cells plus mitotic inhibitors; ▴, undifferentiated NT2 cells; ▵, undifferentiated NT2 cells plus mitotic inhibitors; ×, primary neurons; ⧫, NT2 neurons. (A) Trypsinized cells were counted by Trypan blue exclusion at the indicated times postplating. Data are the mean cell numbers ± the standard errors of the means from at least two experiments. (B) Infectious virus production from plaque assay of clarified culture supernatants collected at 1, 2, or 3 d.p.i. Data are the mean PFU/ml ± the standard errors of the means from two or three experiments performed in duplicate. (C) Syncytium formation in MV-infected HeLa cells cultured with mitotic inhibitors (2 d.p.i.). Magnification, ×64. (D) Syncytium formation in MV-infected HeLa cells cultured in standard media (2 d.p.i.). Magnification, ×64.
Absence of extracellular virus in neurons is attributed to defects in viral maturation.
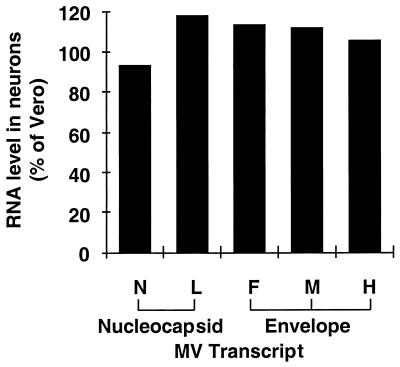
To determine whether the impairment in neuronal virus production was a result of differences in viral gene expression, we next examined MV RNA transcript levels in infected primary neurons and Vero cultures. Total RNA samples from identical numbers of infected cells were hybridized with individual MV gene probes, as described in Materials and Methods. Slot blot analysis showed that both nucleocapsid-specific (N and L) and envelope-specific (F, H, and M) RNA levels were similar in primary neurons and Vero cultures at 2 d.p.i. (Fig. 3), indicating that the reduced viral titers from neurons could not be attributed to reductions in viral gene expression.
FIG. 3.
Viral RNA levels in MV-infected Vero cell and primary neuron cultures. Total RNA samples from equivalent numbers of infected Vero cells or primary CD46+ neurons (2 d.p.i.) were applied to a slot blot and hybridized with DNA probes recognizing MV nucleocapsid transcripts N or L, or envelope transcripts F, M, or H. Data are the percentages of phosphorimager pixel values of each MV transcript from neurons, relative to values obtained from Vero cells (set at 100% for each transcript).
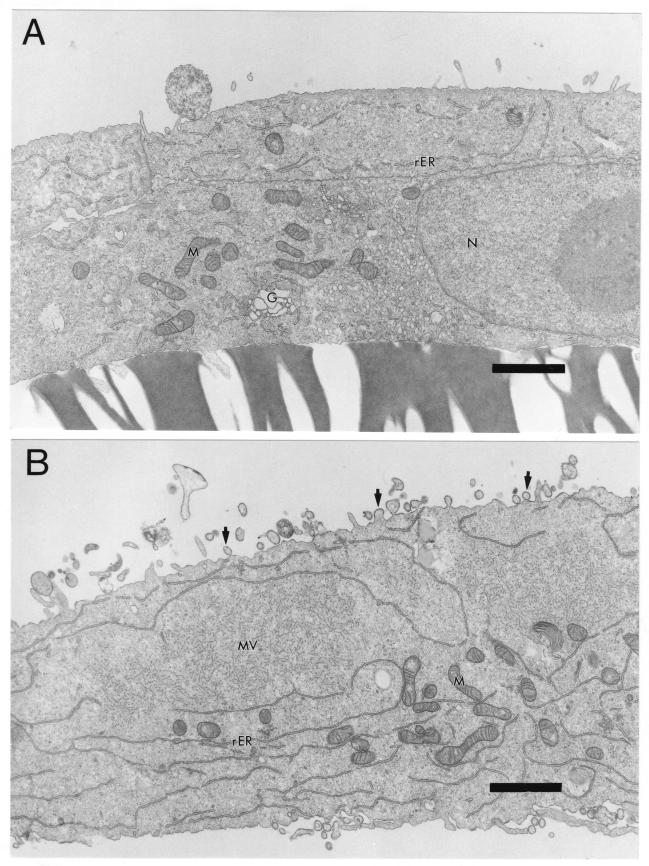
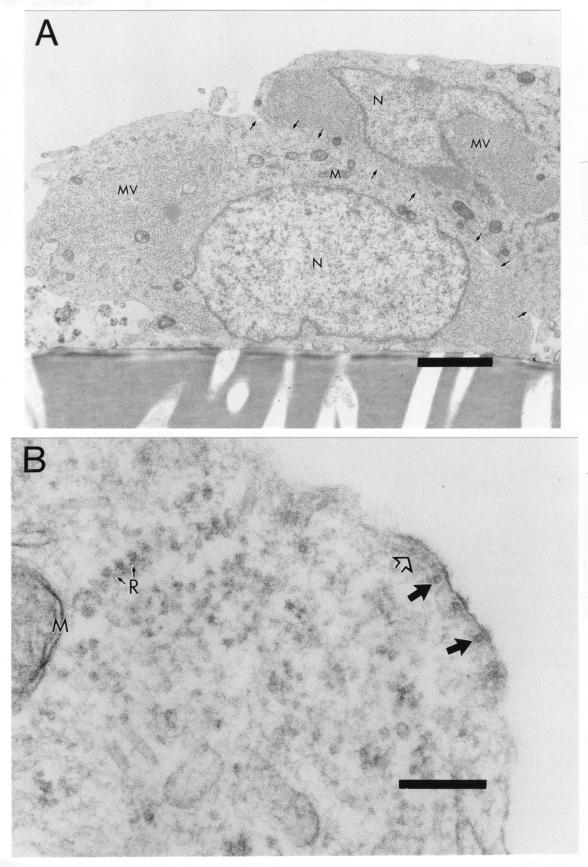
We also used transmission EM to compare viral assembly and budding in primary CD46+ neurons and fibroblasts. Whereas uninfected Vero cells had a smooth surface (Fig. 4A), the surface of MV-infected cells was highly irregular, with viral particles in different stages of budding, from immature bulges to mature buds (100 to 300 nm in diameter) separated from the cell surface, as shown in Fig. 4B. These findings are consistent with classic EM studies of MV infection in Vero cells (16, 32). In addition, within the cytoplasm of infected cells were fuzzy nucleocapsids (Fig. 4B) with a diameter of 25 nm, indicating the presence of P and M viral proteins attached to 17- to 18-nm-diameter viral RNA helices (29). Similarly, within primary transgenic neurons, fuzzy viral nucleocapsids were present in the cytoplasm (Fig. 5A). Unlike fibroblasts, however, MV-infected neurons had a smooth cell surface with little evidence of budding, and cell membranes between infected cells were intact (Fig. 5A). These results were confirmed by evaluation at higher magnification. In addition, at higher magnification there was evidence for nucleocapsid alignment beneath electron-dense regions of the cell surface, but only immature stages of budding were detected as slight bulges of the membrane (Fig. 5B). These findings are consistent with the lack of extracellular virus production and syncytium formation in neurons (Fig. 1; Table 1).
FIG. 4.
Ultrastructural comparison of uninfected and MV-infected Vero cells. Cultures grown on coverslips were infected 1 day postplating with MV Edmonston (MOI = 1) or mock infected and then fixed 3 d.p.i. with glutaraldehyde and processed for EM. (A) Uninfected Vero cell, showing smooth cell surface. Magnification, ×7,560. Bar = 2 μm. (B) MV-infected Vero cell, showing several viral buds at cell surface (arrows) and cytoplasmic fuzzy nucleocapsids (MV). Magnification, ×7,560. Bar = 2 μm. N, nucleus; rER, rough endoplasmic reticulum; G, Golgi; M, mitochondria.
FIG. 5.
Incomplete budding at surface of MV-infected neurons. Primary CD46+ neuron cultures were infected with MV Edmonston (MOI = 1) or mock infected, fixed at 3 d.p.i. with glutaraldehyde, and processed for EM. (A) Two adjacent neuronal cell bodies containing cytoplasmic fuzzy nucleocapsids (MV) but few buds at the cell surface. Arrows indicate intact cell membranes separating the two cells. Magnification, ×7,560. Bar = 2 μm. (B) Higher magnification of infected neuron shows smooth nucleocapsid alignment at the cell surface, but only at the immature stage of budding. Magnification, ×96,600. Bar = 200 nm. Closed large arrows, cross-sectional view of nucleocapsid; open large arrow, longitudinal view of nucleocapsid; N, nucleus; M, mitochondria; R with small arrows, ribosomes.
MV spread in neurons is dependent on cell contact but not on CD46 expression.
To determine whether viral spread in neurons required either cell contact or CD46 receptor expression, the following two primary neuron populations were isolated: neurons from NSE-CD46+ mice expressing the MV receptor and neurons from NSE-NP+ mice, which express the NP protein from LCMV but lack CD46. The NP molecule cannot be used for viral entry, but it can be identified by immunohistochemistry, allowing its use as a marker for distinction from the CD46+ cells. CD46 labeling was not used for identification because CD46 levels are typically downregulated by MV infection (36). CD46+ neurons were infected in suspension and washed extensively (four times over a serum cushion), and supernatants from the last wash were subsequently tested by plaque assay to retrospectively confirm that no extracellular virus remained when the cells were plated. As depicted in the experimental outline (Fig. 6A), the infected CD46+ neurons were plated either on coverslips together with uninfected NP+ neurons (ratio = 1:1) or on separate coverslips, sharing the same media but without direct contact. At multiple times postplating (postinfection), coverslips were collected and stained by immunofluorescence, using fluorescein-conjugated antibodies to detect MV proteins and rhodamine-conjugated antibodies to detect the LCMV-NP protein.
FIG. 6.
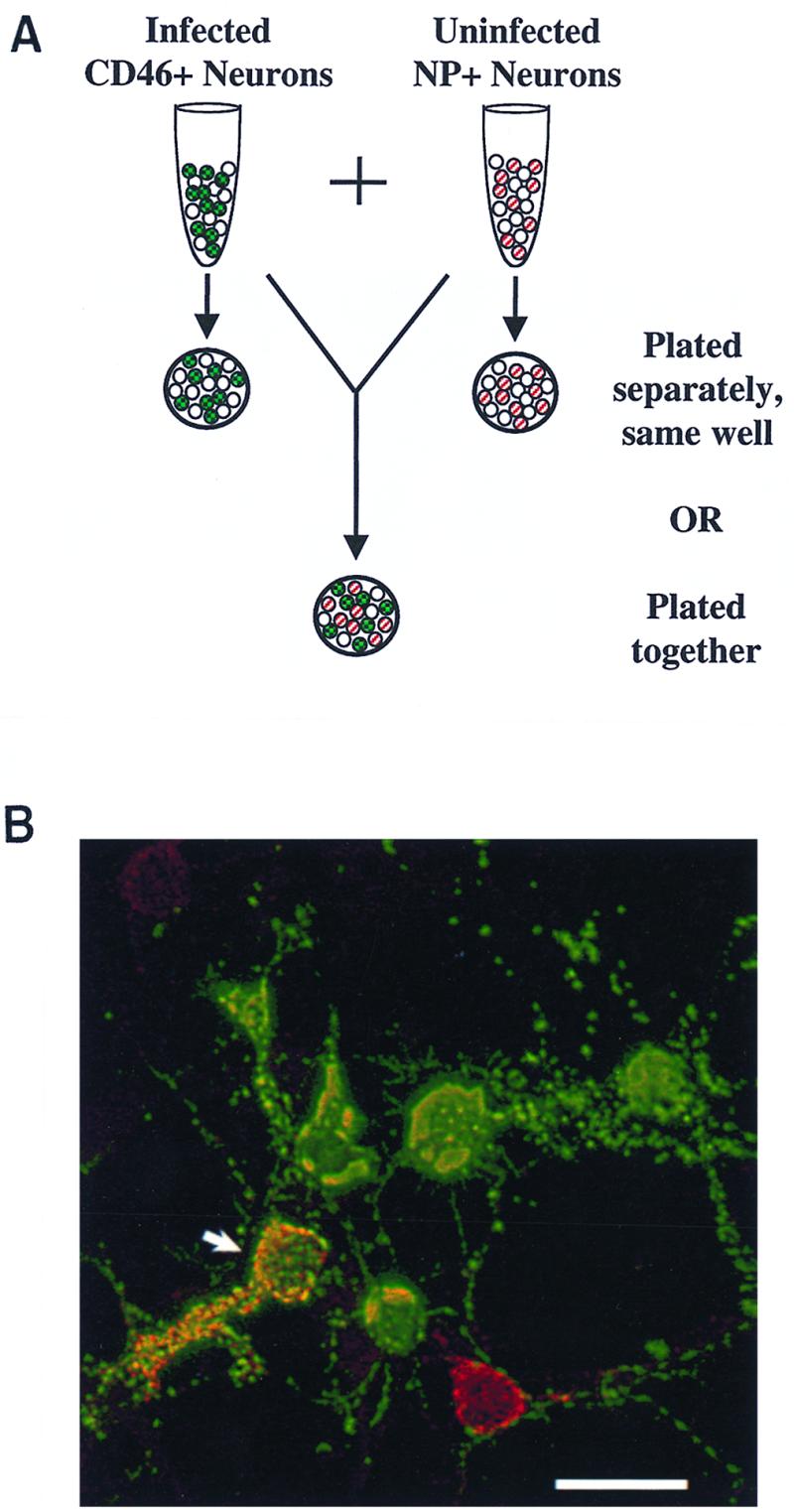
(A) Diagram of neuron coculture experiments, showing infected CD46+ cells (green with checkerboard, indicating staining for MV antigen with fluorescein isothiocyanate) and NP+ cells (red stripe, indicating staining for NP with rhodamine red-X) cultured together or on separate coverslips. The NP transgene was detected only on approximately 50% of transgenic neurons. (B) Mixture of CD46+ and NP+ cells on the same coverslip, 3 d.p.i., fluorescently immunostained for MV antigen with fluorescein isothiocyanate (green) and for NP protein with rhodamine red-X (red). Colocalization, shown by yellow (arrow), indicates MV infection of NP+ cells. Bar = 25 μm.
Our results showed that when infected CD46+ neurons and uninfected NP+ neurons were grown on the same coverslip, colocalization of MV (green) and NP (red) occurred, but only after 3 days of culture (Fig. 6B), consistent with the kinetics of spread reported in Fig. 1. In each of four experiments, between 2 and 5% of neurons stained for NP were also positively stained for MV; the number of infected NP neurons was always greater at 4 d.p.i. than at 3 d.p.i. Importantly, MV-infected NP+ neuronal cell bodies did not appear to be in direct contact with other infected cells, although in most cases, there were infected processes that appeared to link the infected NP+ neurons to other infected neurons. When the two cell populations were grown on separate coverslips, colocalization was never detected (data not shown), indicating that the infection did not spread through the media. However, when infected CD46+ neurons and uninfected Vero cells were cultured separately, occasional clusters and syncytia of infected Vero cells were detected (data not shown), consistent with the presence of a small amount of infectious extracellular virus production by neurons. The fact that this amount was unable to infect the NP+ neurons indicated a need for cell contact to achieve CD46-independent infection. In addition, when uninfected CD46+ neurons were grown separately but sharing the same media with infected fibroblasts, neurons did become infected (data not shown). Thus, because NP+ neurons only became infected when in direct contact with infected CD46+ neurons on the same coverslip, but in the apparent absence of syncytium formation, we hypothesized that viral spread in neurons occurs by MV traveling through the axon and across synaptic connections rather than through extracellular virus-cell interactions and that this mechanism of spread can occur in the absence of CD46.
This hypothesis was confirmed by the addition of neutralizing antibody to cultures of infected neurons and fibroblasts. As shown in Table 2, viral spread (determined as the increase in the proportion of infected neurons from 1 to 3 d.p.i.) was not significantly affected when anti-MV antibody was added to the culture media immediately following inoculation. This antibody treatment was sufficient to completely inhibit viral spread in the infected HeLa cells. This result indicates that extracellular virus was not responsible for the spread of neuronal infection.
TABLE 2.
Effect of neutralizing antibody on MV spread in neurons versus nonneuronal cells
| Culture | % Infected cellsa
|
||
|---|---|---|---|
| Initial (1 d.p.i.) | Final (3 d.p.i.) | Final with neutralizing antibody (3 d.p.i.) | |
| HeLa | 9.6 ± 1.3 | 69.7 ± 1.8 | 13.3 ± 1.1b |
| Neuron | 2.6 ± 0.5 | 13.8 ± 1.0 | 15.9 ± 1.1c |
Cells were infected with MV Edmonston (MOI = 1), and immediately after infection, neutralizing antibody was added to appropriate wells, as described in Materials and Methods. Immunostained samples were evaluated for the number of infected cells and the number of total counterstained nuclei per field, using a 40× objective; data are the proportions of infected cells ± standard errors, pooled over 10 fields.
The 95% confidence interval for antibody treatment overlaps that of the initial value but not that of the final value, showing that neutralizing antibody inhibited spread in HeLa cultures.
The 95% confidence interval for antibody treatment overlaps that of the final value but not that of the initial value, indicating that spread was not affected by neutralizing antibody in neuron cultures.
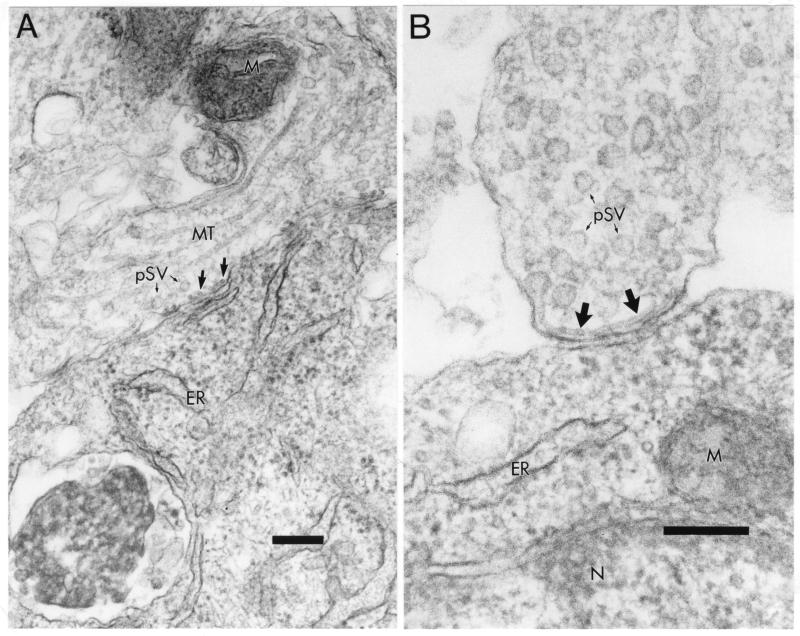
Consequently, we used EM to determine if viral nucleocapsids could be detected at neuronal termini. Figure 7A shows a presynaptic terminal, defined by the presence of synaptic vesicles of 50 to 75 nm in diameter, and the electron-dense material in the synaptic cleft, containing multiple synapses with the postsynaptic cell. As indicated by the larger arrows, there were several end-on nucleocapsids visible, aligned inside the presynaptic membrane. These nucleocapsids were identified by having a diameter of 18 nm and a hollow core of approximately 5 nm, consistent with other reports of isolated MV nucleocapsids (17, 41). In the synaptic contact shown in Fig. 7B, a longitudinal view of a MV nucleocapsid was visualized along the presynaptic membrane. The nucleocapsid helix again had a diameter of 18 nm, a pitch (periodicity) of 5.5 nm, and a length of approximately 400 nm. These values are consistent with classic studies of isolated MV nucleocapsids, reported to be 1 to 2 μM in length (11, 17, 29, 41). Taken together, these findings provide evidence that MV nucleocapsids can travel from the cell body to align with synaptic membranes, suggesting that there may be specific mechanisms that allow nucleocapsid transport across the synapse.
FIG. 7.
Viral nucleocapsid alignment at the presynaptic membrane. (A) Smooth nucleocapsids in cross section (large arrows) along the membrane within a presynaptic neuronal process (top), characterized by the presence of presynaptic vesicles (pSV with small arrows). Several synapses with the adjacent cell body (bottom) are visible. Magnification, ×56,280. Bar = 200 nm. (B) Higher magnification of presynaptic neuron terminal (top) showing a longitudinal view of a smooth nucleocapsid (large arrows) aligned with the presynaptic membrane. Magnification, ×92,460. Bar = 200 nm. pSV and small arrows, presynaptic vesicles; MT, microtubules; M, mitochondria; ER, endoplasmic reticulum; N, nucleus.
DISCUSSION
In this report, we demonstrate that efficient MV Edmonston spread in viral receptor-positive neurons occurs independently of syncytium formation and extracellular virus production, implicating an alternative mechanism of transmission between neurons. The fact that neurons are nondividing did not account for the 1,000-fold lower levels of virus production or the lack of syncytium formation compared to nonneuronal cells, because treatment of Vero, HeLa, or undifferentiated NT2 cells with mitotic inhibitors did not reduce either viral yields or cell fusion. The neuronal restriction of these classical mechanisms of MV spread was supported by EM analysis, which showed a lack of viral budding from the neuronal cell surface. In addition, the presence of neutralizing antibody did not inhibit the spread of infection in primary neuron cultures, confirming that MV budding does not contribute to interneuronal viral spread. Coculture experiments combining infected CD46+ neurons with uninfected neurons lacking CD46 indicated that cell contact was required to allow viral spread between neurons but that the CD46 receptor for MV was dispensable. EM analysis of neurons showed no evidence of fusion between infected cell bodies, but there were several examples of presynaptic nucleocapsid localization; thus, it is likely that MV can spread between neurons via synaptic connections in the absence of cell fusion.
Our finding that neuronal MV infection could spread without a requirement for extracellular virus production and cell fusion leading to syncytium formation implied that two events were occurring. First, the virus-host cell interactions that allow cell fusion and synthesis of infectious virus must be inhibited or absent in neurons. Second, the virus must overcome this block by using a unique mechanism that allows interneuronal spread.
To address the first issue of how normal mechanisms of MV spread are inhibited in neurons, we compared infected neurons and fibroblasts for differences in the viral life cycle, including the viral replication-transcription stage, as well as the later stages of assembly and budding. Cattaneo and colleagues (7) have reported that reduced levels of MV transcription in SSPE patients may account for the lack of viral budding in neurons. In our study, levels of viral RNAs encoding both envelope and nucleocapsid proteins were not reduced, and comparative transmission EM analysis showed similar cytoplasmic nucleocapsid accumulation in both neurons and Vero cells, indicating that replication was normal. However, our EM studies indicated that the reduced yield of infectious virus was due to a block in viral budding from the neuron. While infected Vero cells contained smooth nucleocapsids aligned with the cell membrane in various stages of budding, consistent with early EM studies of infection with Halle and Edmonston strains of MV (16, 32), CD46+ neurons showed minimal evidence of cell-surface budding or cell fusion. Nucleocapsids were aligned with electron-dense areas of the cell membrane in the cell body as well as in axonal and dendritic processes, but only immature stages of budding were detected as slight bulges at the surface. This finding is consistent with EM studies of brain samples from SSPE patients (6, 21) and of persistent infection in human prostate cells by an SSPE strain of MV (Mantooth) (17). Our current efforts are aimed at determining whether viral protein synthesis is intact in infected neurons and whether viral budding is blocked because of defective envelope protein expression at the neuronal cell surface.
Our results with NT2 cultures are in contrast to those reported by McQuaid and colleagues (27), who reported that infection of differentiated neurons with the CAM strain of MV occurred only when these cells were in direct contact with infected undifferentiated precursors in mixed cultures. Furthermore, no infectious virus was detected in these cultures, suggesting an inability of both the undifferentiated and the differentiated NT2 cells to produce extracellular virus. In our study, both differentiated and undifferentiated NT2 cells could be infected when grown separately, although the undifferentiated cells produced titers equivalent to 130 PFU/1,000 infected cells after 2 d.p.i., while differentiated neurons produced approximately 1 infectious particle/1,000 infected cells at the same time postinfection. The basis for the differences between our study and that of McQuaid et al. could be attributable to differences in the clone of NT2 cells used or to differences in the strain of virus used. Interestingly, it was also found that CD46 was downregulated on differentiated neurons (27), which lends support to the hypothesis that interneuronal MV spread occurs independently of the expression of CD46.
Our conclusion that the neuronal environment alters the mechanism of viral spread is one that has been reached with other virus model systems. For example, LCMV, which normally buds from the cell surface during an acute infection (13, 39), is associated only with ribosomes and not cell membranes during a persistent neuronal infection in vivo (34). In nerve growth factor-differentiated PC12 neuronal cells a 1,000-fold reduction in infectious LCMV yield compared to that in undifferentiated PC12 chromaffin cells was also found (14), and in persistently infected cell lines, a reduction in LCMV NP protein production was associated with reduced virus production (42). The fact that other neurotropic viruses result in persistent, noncytolytic infections lends support to the speculation that neurons, which are an essential nonrenewable cell population, may have evolved ways to block the devastating consequences of cytolytic infections.
To address the second issue of cell fusion- and extracellular virus-independent viral transmission, we examined whether MV could spread to neurons that lacked CD46 receptor expression. Our results showed that CD46− neurons could be infected, but only when in contact with CD46+ neurons, indicating that the mechanism of MV spread in neurons depends on cell contact, but not on receptor expression. A possible explanation for contact-dependent viral spread in the apparent absence of syncytium formation is that infectious particles or nucleocapsids travel across synaptic connections. In an EM study of persistent mouse brain infection with the hamster-adapted HNT strain of measles, nucleocapsids were detected in postsynaptic regions (38). This finding, combined with our observations that nucleocapsids are aligned at the presynaptic membrane, lends support to the hypothesis that interneuronal viral transmission occurs via the synapse.
If viral particles or nucleocapsids alone can migrate across the synapse, either they might utilize existing synaptic machinery to be secreted into the synaptic cleft and taken up by the postsynaptic cell or they may travel passively between cells by localized fusion at the point of synaptic contact. A recent study (18) showed that MV can spread from a persistently infected cell line to HeLa fibroblasts in a contact-dependent manner, although an inhibitor of MV fusion did not completely block transfer of viral proteins. The authors proposed that “microfusion events” at sites of cell-cell contact, possibly involving viral glycoprotein interaction with CD46 on target cells, allowed virus release and entry (18). It is possible that in our system, similar focal fusion events occur independently of CD46 expression to allow nucleocapsid transfer across the synapse. Regardless of the precise manner by which MV traverses the synapse, we hypothesize that the primary route of interneuronal transmission of MV in neurons is transsynaptic, which may account for the absence of extracellular (infectious) virus and the apparent slow rate of transmission in brains of SSPE patients. Moreover, we do not detect increased neuronal cell death in our primary cultures following MV infection, which suggests that this mechanism of viral transmission may be a strategy by the neuron to protect itself from cytolytic MV replication.
In conclusion, our results suggest that the outcome of an infection is predicated on both viral and host determinants. In a fibroblast environment, MV results in a highly productive infection that causes cytolysis, giant cell formation, and high titers of extracellular virus. In the neuron, despite the spread of virus, there is little evidence of MV-induced cell death, syncytium formation, or infectious virus production. Given the immediate appearance of this modification in our neuron cultures, the dramatic change in the mechanism of viral spread is most likely due to a property of the neurons rather than to the accumulation of viral mutations. In summary, an alternate mechanism of spread, requiring cell contact but not the MV receptor, is used by MV to maintain a persistent neuronal infection. The consequence of this phenomenon, which may be the case for many neurotropic infections, is to protect the host from critical damage resulting from neuronal death.
ACKNOWLEDGMENTS
We thank Michal Jarnik, director of the EM core facility, for assistance with ultrastructural analysis and Jonathan Boyd of the cell imaging facility for assistance with the confocal microscopy experiments. We also thank the laboratory of Robert Doms (University of Pennsylvania) for assistance with NT2 cell culture techniques. Finally, we are most appreciative of the constructive comments made by Bill Mason and Kerry Campbell regarding the manuscript.
This work was supported by Public Health Service grants MH56951, AI07429, and CA06927, as well as by a grant from the Kirby Foundation.
REFERENCES
- 1.Albrecht P, Schumacher H P. Neurotropic properties of measles virus in hamsters and mice. J Infect Dis. 1971;124:86–93. doi: 10.1093/infdis/124.1.86. [DOI] [PubMed] [Google Scholar]
- 2.Anlar B. Subacute sclerosing panencephalitis: diagnosis and drug treatment options. CNS Drugs. 1997;7:111–120. doi: 10.2165/00023210-199707020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Baczko K, Lampe J, Liebert U G, Brinckmann U, ter Meulen V, Pardowitz I, Budka H, Cosby S L, Isserte S, Rima B K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993;197:188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- 4.Baczko K, Liebert U G, Billeter M, Cattaneo R, Budka H, ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with SSPE. J Virol. 1986;59:472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banker G, Goslin K. Culturing nerve cells. Cambridge, Mass: MIT Press; 1991. [Google Scholar]
- 6.Barbosa M M, Cruz C. Nerve cell fusion in a case of subacute sclerosing panencephalitis. Ann Neurol. 1981;9:400–403. doi: 10.1002/ana.410090414. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo R, Rebmann G, Backzo K, ter Meulen V, Billeter M A. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987;160:523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo R, Rose J K. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993;67:1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattaneo R, Schmid A, Billeter M A, Sheppard R D, Udem S A. Multiple viral mutations rather than host factors cause defective measles virus gene expression in a subacute sclerosing panencephalitis cell line. J Virol. 1988;62:1388–1397. doi: 10.1128/jvi.62.4.1388-1397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter M A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compans R W, Choppin P W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci USA. 1967;57:949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook D G, Lee V M-Y, Doms R W. Expression of foreign proteins in a human neuronal system. Methods Cell Biol. 1994;43:289–303. doi: 10.1016/s0091-679x(08)60609-3. [DOI] [PubMed] [Google Scholar]
- 13.Dalton A J, Rowe W P, Smith G H, Wilsnack R E, Pugh W E. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968;2:1465–1478. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Torre J C, Rall G, Oldstone C, Sanna P P, Borrow P, Oldstone M B A. Replication of LCMV is restricted in terminally differentiated neurons. J Virol. 1993;67:7350–7359. doi: 10.1128/jvi.67.12.7350-7359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 16.Dubois-Dalcq M, Reese T S. Structural changes in the membrane of Vero cells infected with a paramyxovirus. J Cell Biol. 1975;67:551–565. doi: 10.1083/jcb.67.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois-Dalcq M, Reese T S, Murphy M, Fuccillo D. Defective bud formation in human cells chronically infected with subacute sclerosing panencephalitis virus. J Virol. 1976;19:579–593. doi: 10.1128/jvi.19.2.579-593.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firsching R, Buchholz C J, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. Measles virus spread by cell-cell contacts: uncoupling of contact-mediated receptor (CD46) downregulation from virus uptake. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 20.Griffin D E, Mullinix J, Narayan O, Johnson R T. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974;9:690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki Y, Koprowski H. Cell to cell transmission of virus in the central nervous system I: subacute sclerosing panencephalitis. Lab Investig. 1974;31:187–196. [PubMed] [Google Scholar]
- 22.Katz M. Clinical spectrum of measles. Curr Top Microbiol Immunol. 1995;191:1–12. doi: 10.1007/978-3-642-78621-1_1. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence D M P, Vaughn M M, Belman A R, Cole J S, Rall G F. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J Virol. 1999;73:1795–1801. doi: 10.1128/jvi.73.3.1795-1801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebert U G, Finke D. Measles virus infections in rodents. Curr Top Microbiol Immunol. 1995;191:149–166. doi: 10.1007/978-3-642-78621-1_10. [DOI] [PubMed] [Google Scholar]
- 25.Liebert U G, Flanagan S G, Loffler S, Baczko K, ter Meulen V, Rima B K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994;68:1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McQuaid S, Campbell S, Wallace I J C, Kirk J, Cosby S L. Measles virus infection and replication in undifferentiated and differentiated human neuronal cells in culture. J Virol. 1998;72:5245–5250. doi: 10.1128/jvi.72.6.5245-5250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norrby E, Oxman M N. Measles virus. In: Fields B N, Knipe D M, editors. Fields virology. 2nd ed. Vol. 1. New York, N.Y: Raven Press; 1990. pp. 1013–1044. [Google Scholar]
- 30.Pasick J M M, Kalicharran K, Dales S. Distribution of trafficking of JHM coronavirus structural proteins of virions in primary neurons and the OBL-21 neuronal cell line. J Virol. 1994;68:2915–2928. doi: 10.1128/jvi.68.5.2915-2928.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rall G F, Manchester M, Daniels L R, Callahan E M, Belman A R, Oldstone M B A. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad of Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rentier B, Hooghe-Peters E L, Dubois-Dalcq M. Electron microscopic study of measles virus infection: cell fusion and hemadsorption. J Virol. 1978;28:567–577. doi: 10.1128/jvi.28.2.567-577.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rima B K, Earle J A P, Baczko K, Rota P A, Bellini W J. Measles virus strain variations. Curr Top Microbiol Immunol. 1995;191:65–83. doi: 10.1007/978-3-642-78621-1_5. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez M, Buchmeier M J, Oldstone M B A, Lampert P W. Ultrastructural localization of viral antigens in the CNS of mice persistently infected with lymphocytic choriomeningitis virus. Am J Pathol. 1983;110:95–100. [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid A, Cattaneo R, Billeter M A. A procedure for selective full length cDNA cloning of specific RNA species. Nucleic Acids Res. 1987;15:3987–3996. doi: 10.1093/nar/15.10.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider-Schaulies J, Schnorr J J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tourtellotte W W, Parker J A, Herndon R M, Cuadros C V. Subacute sclerosing panencephalitis: brain immunoglobulin-G, measles antibody and albumin. Neurology. 1968;18:117–121. doi: 10.1212/wnl.18.1_part_2.117. [DOI] [PubMed] [Google Scholar]
- 38.Van Pottelsberghe C, Rammohan K W, McFarland H F, Dubois-Dalcq M. Selective neuronal, dendritic, and postsynaptic localization of viral antigen in measles-infected mice. Lab Investig. 1979;40:99–108. [PubMed] [Google Scholar]
- 39.Walker D H, Murphy F A, Whitfield S G, Bauer S P. Lymphocytic choriomeningitis: ultrastructural pathology. Exp Mol Pathol. 1975;23:245–265. doi: 10.1016/0014-4800(75)90022-2. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe M, Wang A, Sheng J, Gombart A F, Ayata M, Ueda S, Hirano A, Wong T C. Delayed activation of altered fusion glycoprotein in a chronic measles virus variant that causes subacute sclerosing panencephalitis. J Neurovirol. 1995;1:412–423. doi: 10.3109/13550289509111034. [DOI] [PubMed] [Google Scholar]
- 41.Waters D J, Bussell R H. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology. 1974;61:64–79. [PubMed] [Google Scholar]
- 42.Welsh R M, Buchmeier M J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979;96:503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]