Abstract
Earlier reports have shown that herpes simplex virus 1 (HSV-1) mutants induce programmed cell death and that wild-type virus blocks the execution of the cell death program triggered by expression of viral genes, by the Fas and tumor necrosis factor pathways, or by nonspecific stress agents. In particular, an earlier report from this laboratory showed that the mutant virus d120 lacking the genes encoding infected cell protein 4 (ICP4), the major regulatory protein of the virus, induces a caspase-3-independent pathway of apoptosis in human SK-N-SH cells. Here we report that the pathway of apoptosis induced by the d120 mutant in human HEp-2 cells is caspase dependent. Specifically, in HEp-2 cells infected with d120, (i) a broad-range inhibitor of caspase activity, z-vad-FMK, efficiently blocked DNA fragmentation, (ii) cytochrome c was released into the cytoplasm, (iii) caspase-3 was activated inasmuch as poly(ADP-ribose) polymerase was cleaved, and (iv) chromatin condensation and fragmentation of cellular DNA were observed. In parallel studies, HEp-2 cells were transfected with a plasmid encoding human Bcl-2 and a clone (VAX-3) expressing high levels of Bcl-2 was selected. This report shows that Bcl-2 blocked all of the manifestations associated with programmed cell death caused by infection with the d120 mutant. Consistent with their resistance to programmed cell death, VAX-3 cells overproduced infected cell protein 0 (ICP0). An unexpected observation was that ICP0 encoded by the d120 mutant accumulated late in infection in small, quasi-uniform vesicle-like structures in all cell lines tested. Immunofluorescence-based colocalization studies indicated that these structures were not mitochondria or components of the endoplasmic reticulum or the late endosomal compartment. These studies affirm the conclusion that HSV can induce programmed cell death at multiple steps in the course of its replication, that the d120 mutant can induce both caspase-dependent and -independent pathways of programmed cell death, and that virus-induced stimuli of programmed cell death may differ with respect to the pathway that they activate.
Earlier, this laboratory reported that herpes simplex virus 1 (HSV-1) mutants induce morphologic and biochemical changes characteristic of apoptosis whereas wild-type viruses do not. Of particular interest are studies on two mutants. The first mutant, designated HSV-1(HFEM)tsB7, infects cells at 39°C and its capsid is transported to the nuclear pore but is unable to release its DNA into the nucleus (2, 3). This mutant at the nonpermissive temperature induces apoptosis in Vero cells but not in human SK-N-SH cells (8). In contrast, the second mutant, HSV-1(KOS)d120, induces apoptosis in all cell lines tested (7, 8, 12). This mutant lacks the major regulatory gene α4, and consequently its replication is impaired at the very early stages of infection (5). These studies led to the conclusion that (i) induction of apoptosis by HSV-1 takes place at multiple steps in the course of viral replication, (ii) the consequences of induction are cell type dependent, and (iii) wild-type virus blocks not only the insults to the cell resulting from the action of viral gene products but also those resulting from exposure to Fas ligand, tumor necrosis factor alpha (TNF-α), or osmotic shock induced by exposure to sorbitol.
In this report, we show that d120 infection can induce fundamentally different pathways of apoptosis in different cell lines. Briefly, in an earlier report, this laboratory showed that in the human cell line SK-N-SH the pathway of apoptosis induced by the d120 mutant is caspase-3 independent (7). Specifically, SK-N-SH cells exposed to d120 virus showed no caspase-3 activity or proteolytic activity on caspase-3 substrates such as poly(ADP-ribose) polymerase (PARP). The same cells, however, exhibited typical caspase-3-dependent apoptosis following osmotic shock by incubation in sorbitol, and, moreover, wild-type HSV-1 blocked DNA fragmentation induced by this treatment. The surprising finding reported here is that apoptosis induced by d120 in another human cell line, HEp-2, is a caspase-dependent process. Consistent with this, overexertion of Bcl-2 in HEp-2 cells blocked apoptosis induced by the mutant virus.
Relevant to this report are the following: (i) The pathways of programmed cell death comprise at least two effector branches that converge upon the activation of caspases, a family of cysteine proteases. Central to this process is the proteolytic cleavage of the proenzymes into their active, catalytic forms. The zymogens of different caspases have been shown to be the substrates for other members of the family and to be able to cleave their own precursors. Thus, upon receipt of a death signal, a cascade of proteolytic cleavages results in the activation of the preexisting inactive caspases that ultimately destroy the cell (reviewed in reference 4). For example, ligand-induced clustering of death receptors, such as Fas and TNF receptors, initiates the execution of one of these pathways, in which the caspase machinery is directly engaged through the activation of caspase-8. Active caspase-8 cleaves and activates the downstream effectors caspase-3, -6, and -7 (15, 16, 18). On the other hand, stress-inducing stimuli (e.g., gamma or UV light irradiation, inhibitors of RNA polymerase, or withdrawal of growth factors) have been shown to activate a pathway to cell death that is regulated by the Bcl-2 family of proteins (1) and involves mitochondrial events. Release of cytochrome c from mitochondria initiates the formation of a multiprotein complex of cytochrome c, the CED-4 homologue APAF-1, and procaspase-9. This complex activates caspase-9, which in turn cleaves and activates caspase-3 (22). Inhibition of caspase activity downstream of the release of cytochrome c in the cytoplasm blocks all nuclear manifestations of apoptosis in most systems.
(ii) Inhibition of caspases does not block death induced by proapoptotic stimuli; cells that have committed themselves to death will do so even in the absence of caspase activity (14, 21). The decision whether to live or die is thought to be taken at a step upstream of the activation of caspases and involves the function of the Bcl-2 family. Death antagonist members of this family, such as Bcl-2 or Bcl-XL, may allow cells that have received a proapoptotic stimulus to survive and maintain their clonogenic potential (reviewed in reference 11). On the other hand, death agonist members such as BAX and Bak can induce mitochondrial damage and cell death even in the absence of caspase activity (10, 14, 21). In all cases, Bcl-2 family members reside in or are translocated to mitochondria, where there are thought to exert their effects by modulating the mitochondrial events associated with apoptosis.
(iii) The d120 mutant (5) replicates in the Vero cell line E5 expressing the α gene. In cells lacking the complementing gene, the virus expressed predominantly α genes. The strong cytopathic effects observed in noncomplementing cells infected by this mutant led to the initial evidence that the d120 mutant induces apoptosis.
The study reported here shows that infection of HEp-2 cells with the HSV-1 d120 mutant induces a caspase-dependent pathway that can be inhibited by overexpression of Bcl-2. The presence of Bcl-2 is sufficient to block cellular shrinkage, cytochrome c release, chromosomal DNA fragmentation, and chromatin condensation induced by infection of HEp-2 cells with the d120 mutant. These observations, together with our earlier findings that d120 infection induces a caspase-3-independent pathway in SK-N-SH cells, indicates that HSV-1 can induce both caspase-independent and caspase-dependent pathways depending on the cell type it infects.
MATERIALS AND METHODS
Cells.
HEp-2 and SK-N-SH cell lines were obtained from the American Type Culture Collection (Rockville, Md.). Rabbit skin cells (RSC) were originally obtained from John McAllen. All cell lines were grown in Dulbecco's modified Eagle medium supplemented with either 5% (HEp-2 and RSC) or 10% (SK-N-SH) newborn calf serum. Cultures used in all experiments shown here were seeded less than 20 h prior to infection at 60 to 70% confluency.
Viruses.
HSV-1(F) is the prototype HSV-1 strain used in this laboratory (6). The HSV-1(KOS)d120 mutant, a kind gift of N. DeLuca, lacks both copies of the α gene and was grown in a Vero-derived cell line (E5) expressing the α4 gene (8). It also carries a defective US3 gene (12).
Construction of a Bcl-2 stable transfectant.
HEp-2 cells stably transfected with the SFFV.neo vector containing the human Bcl-2 gene (kindly provided by S. Korsmeyer) were selected on the basis of their resistance to neomycin). Neomycin-resistant clones were amplified and screened for Bcl-2 expression by an immunofluorescence assay with 6C8, a hamster monoclonal antibody specific for the human Bcl-2 protein (PharMingen, San Diego, Calif.), conjugated to fluorescein isothiocyanate (FITC). One of the Bcl-2 stable transfectants showing high levels of Bcl-2 expression (VAX-3) was further propagated in Dulbecco's modification of Eagle's minimal essential medium containing 10% newborn calf serum and 400 μg of G418 per ml.
Immunoblot assays.
Protein concentration in whole-cell lysates was determined with the aid of the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) according to the directions provided by the manufacturer. Infected or uninfected cell lysates (60 μg of protein per lane) were electrophoretically separated in a 10% denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with a monoclonal antibody specific for infected cell protein 0 (ICP0; Goodwin Cancer Research Institute) or a rabbit polyclonal antibody specific for PARP; Santa Cruz Biotechnologies, Santa Cruz, Calif.). The protein bands reacting with ICP0 antibody were visualized with alkaline phosphatase. In the case of PARP and in the cytochrome c fractionation studies, the protein bands were visualized by an enhanced chemiluminescent detection (ECL) system (Pierce, Rockford, Ill.) according to the instructions of the manufacturer.
Subcellular fractionation.
A total of 4 × 106 HEp-2 or VAX-3 cells was either mock infected or exposed to 10 PFU of HSV-1(F) or the d120 mutant per cell. At the indicated times after infection, they were collected and resuspended in 0.8 ml of ice-cold buffer A (20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 250 mM sucrose, 0.1 mM TLCK [Nα = p = tosyl = l = lysine chloromethyl ketone], 0.1 mM TPCK [tolylsulfonyl phenylalanyl chloromethyl ketone]). After 15 min on ice, cells were homogenized in a Dounce homogenizer and centrifuged for 10 min at 750 × g, in order to remove unlysed cells and nuclei. The supernatant fluids were transferred to new tubes and centrifuged again at 10,000 × g for 20 min. Supernatant fluids from the second centrifugation represent the cytosolic fractions, whereas the pellets, resuspended in buffer A, represent the mitochondrial fractions.
Localization of cytochrome c.
The protein concentration in mitochondrial and cytosolic fractions was determined by the Bio-Rad protein assay as described above. Equivalent amounts of mitochondrial and cytosolic fractions were subjected to electrophoresis in denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked in phosphate-buffered saline (PBS; 0.14 M NaCl, 3 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4) containing 5% skim milk for 1 h at room temperature or overnight at 4°C, rinsed three times in PBS, and then reacted with the primary antibody against cytochrome c, clone 7H8.2C12 (PharMingen) in a solution of PBS containing 1 μg of the antibody per ml. The nitrocellulose sheets were then rinsed three times with PBS, reacted with a goat anti-mouse antibody conjugated to peroxidase (Sigma, St. Louis, Mo.), rinsed again, reacted with the ECL system according to the protocols provided by the manufacturers, and then exposed to X-Omat AR films (Kodak, Rochester, N.Y.) for detection of specific bands.
DNA fragmentation assay.
Subconfluent HEp-2 or VAX-3 cells were mock infected or exposed to 10 PFU of HSV-1(F) or the d120 mutant per cell. Approximately 106 cells were collected and processed as described elsewhere (8).
Immunofluorescence.
Approximately 6.6 × 105 cells were seeded onto glass slides and infected with 5 PFU of HSV-1(F) or 10 PFU of d120 per cell for the experiments involving localization of ICP0 in infected cells. The cells were then incubated at 37°C for 4 or 16 h and fixed in ice-cold methanol for 20 min at −20°C. In the colocalization experiments, the same number of cells were infected with 1 PFU of HSV-1(F) or d120 per cell. The cells were then incubated at 37°C for 16 h and fixed in methanol as described elsewhere (7, 8). The cells were dried and subsequently blocked in PBS containing 1% bovine serum albumin (BSA) at room temperature, rinsed once with PBS, and reacted for 2 h with a 1:1,000 dilution of the mouse monoclonal antibody to ICP0 (clone H1083) in PBS containing 1% BSA. The cells were then rinsed five times in PBS, reacted for 1 h with a 1:64 dilution of a goat anti-mouse immunoglobulin G (IgG) conjugated to FITC (Sigma) in PBS containing 1% BSA, rinsed again five times with PBS, and mounted in 90% glycerol. A 1:1,000 dilution of a rabbit polyclonal antiserum raised against a fusion protein containing the second exon of ICP0 was used in the coimmunofluorescence experiments together with a 1:200 dilution of a monoclonal antibody specific for cathepsin D (Sigma) or a monoclonal antibody specific for proline disulfide isomerase (PDI; Stressgen, Victoria, British Columbia, Canada). The cells were then reacted with a 1:200 dilution of a goat anti-mouse IgG conjugated to Texas red (Molecular Probes, Eugene, Oreg.) with a 1:64 dilution of goat anti-rabbit IgG conjugated to FITC (Sigma). In the case of colocalization studies involving ICP0 and the mitochondrial compartment, infected and uninfected cells were incubated in 100 nmol of the mitochondrion-selective dye MitoTracker CMXRos (Molecular Probes) in accordance with the manufacturer's instructions and then immunostained with a 1:1,000 dilution of the mouse monoclonal antibody to ICP0 (clone H1083). The cells were then reacted with a 1:64 dilution of a goat anti-mouse IgG conjugated to FITC (Sigma) to visualize ICP0. In all experiments, the slides were analyzed with a Zeiss confocal fluorescence microscope. Digitized images of the fluorescent antibody-stained cells were acquired with software provided by the Zeiss confocal microscope and printed with a Tektronics 460 Phaser printer.
Chromatin condensation analysis.
HEp-2 or VAX-3 cells were exposed to 10 PFU of the d120 mutant per cell, incubated at 37°C for 18 h, and then fixed in ice-cold methanol for 20 min at −20°C. The cells were dried and reacted with PBS containing 1% BSA and 10 nM 4′,6′-diamidino-2-phenylindole (DAPI) at room temperature for 15 min, rinsed twice with PBS, and mounted in 90% glycerol. The slides were analyzed in a Zeiss confocal fluorescence microscope.
RESULTS
Chromosomal DNA fragmentation induced in HEp-2 cells by the d120 mutant virus is efficiently blocked by a broad-range caspase inhibitor.
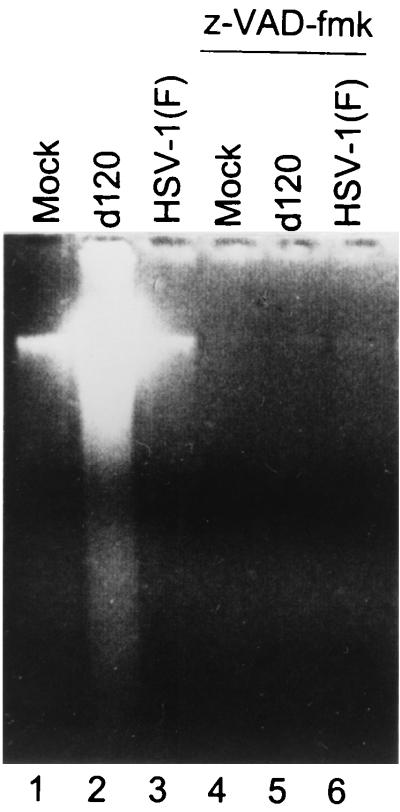
The purpose of the experiments described here was to determine whether the pathway of apoptosis activated by d120 infection in HEp-2 cells was dependent on activation of caspases. HEp-2 cells were either mock infected or infected with 10 PFU of HSV-1(F) or the d120 mutant. Replicate cultures were incubated in the presence of 25 μM z-VAD-fmk for 1 h before, during exposure to 10 PFU of HSV-1(F) or the d120 mutant per cell, and throughout the subsequent incubation of the infected cells at 37°C. At 20 h after infection, the cells were harvested and processed as described in Materials and Methods. Lysates from mock- and HSV-1(F)-infected cells showed little or no degradation of chromosomal DNA (Fig. 1, lanes 1 and 3), whereas lysates from d120-infected cells showed extensive DNA fragmentation (Fig. 1, lane 2). In contrast, the cultures infected with the d120 mutant in the presence of z-VAD-fmk showed no DNA fragmentation (Fig. 1, lane 5). We conclude that z-VAD-fmk effectively blocked DNA degradation induced in HEp-2 cells after infection with the d120 mutant. These results suggest that in HEp-2 cells, the apoptotic pathway induced by d120 is caspase dependent.
FIG. 1.
Photograph of agarose gel containing electrophoretically separated low-molecular-weight DNA fragments. HEp-2 cells were mock infected or exposed to HSV-1(F) or the d120 mutant and were either untreated or incubated in medium containing z-VAD-FMK as described in the text. Cell lysates were collected at 20 h after infection and processed as described in Materials and Methods.
Caspase-3 is activated in d120-infected HEp-2 cells.
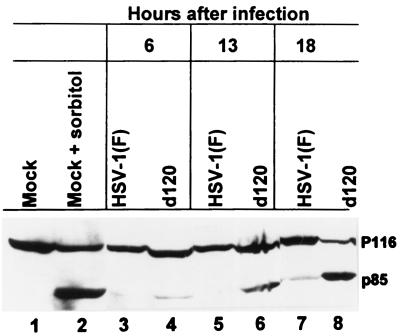
The evidence that z-VAD-fmk blocked HEp-2 cell DNA fragmentation suggested that infection with d120 activated caspase-3 activity. DNA fragmentation has been shown to result from the release of a DNase activity by cleavage of its inhibitory partner by caspase-3 (13, 17). We therefore sought to determine whether active caspase-3 could be detected in extracts from d120-infected HEp-2 cells. A time course analysis was done in which HEp-2 cells were either mock infected or exposed to 10 PFU of HSV-1(F) or the d120 mutant and collected at 6, 13, and 18 h after infection. Cleavage of PARP into its Mr 85,000 product was readily detected in lysates from osmotic shock-treated cells (Fig. 2, lane 2) and in lysates of cells harvested at 13 h after infection with the d120 virus (Fig. 2, lane 6) but not in uninfected or wild-type virus-infected cells (Fig. 2, lanes 1, 3, 5, and 7). The largest accumulation of cleaved PARP was observed at 18 h after infection with d120 (Fig. 2, lane 8). These results, together with the observation that cytochrome c was released from mitochondria in samples harvested at 9 h after d120 infection (unpublished observations) are consistent with a model in which d120 induces a pathway of apoptosis in HEp-2 cells that results in the release of cytochrome c into the cytoplasm and activation of caspases.
FIG. 2.
Photograph of electrophoretically proteins reacted with an antibody to PARP. Lysates of HEp-2 cells mock infected or exposed to HSV-1(F) or the d120 mutant were harvested at the times indicated, denatured, subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with an antibody against PARP.
Construction of stable transfectant that overexpresses Bcl-2.
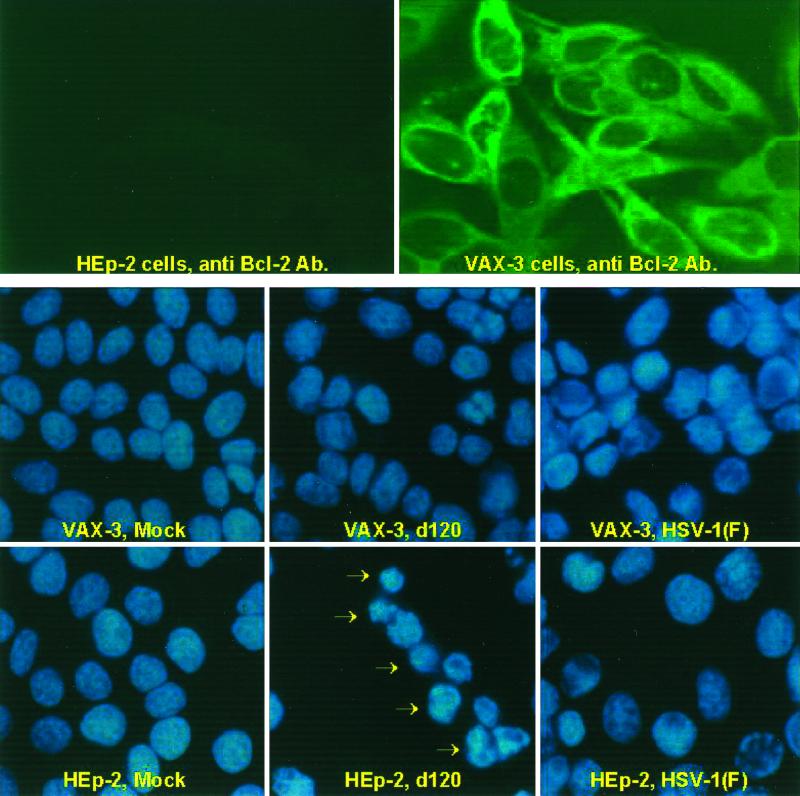
The Bcl-2 protein has been shown to block the execution of stress-induced apoptosis by preventing the release of cytochrome c into the cytoplasm of cells and consequently blocking the activation of the caspase cascade (reviewed in reference 11). If cytochrome c release is a required step in the pathway of apoptosis induced by d120 mutant infection in HEp-2 cells, Bcl-2 should block d120-induced cytochrome c release and apoptosis. To test this hypothesis, an HEp-2-derived, Bcl-2-overexpressing stable transfectant containing the Bcl-2 open reading frame under the control of the SSFV promoter was constructed as noted in Materials and Methods. Individual clones were screened for overexpression of Bcl-2. Of 13 Bcl-2-positive clones, one was selected in which the transgene was expressed to high levels in 99% of the cells (Fig. 3, top row of panels). This clone (VAX-3) was amplified and used in all the experiments described below.
FIG. 3.
Digital images of parental HEp-2 cells and HEp-2 cells expressing the human Bcl-2 protein (VAX-3 cells). (Top row) Cultures of uninfected HEp-2 or VAX-3 cells were reacted with an antibody specific for Bcl-2. (Middle and bottom rows) Mock-infected HEp-2 cells or VAX-3 cells or cells infected with HSV-1(F) or the d120 mutant were stained with DAPI as described in Materials and Methods. The arrows point to cells exhibiting condensation of chromatin typical of cells undergoing apoptosis.
d120 infection does not induce apoptosis in a cell line overexpressing Bcl-2.
To test the hypothesis that overexpression of Bcl-2 blocks apoptosis induced by d120 infection in HEp-2 cells, slide cultures of HEp-2 and VAX-3 cells either mock infected or infected with HSV-1(F) or the d120 mutant were fixed at 24 h after infection and stained with DAPI. The morphology of nuclei was analyzed by fluorescence microscopy. Seventy percent of HEp-2 cells infected with the d120 mutant that remained attached to the slide showed the condensation of chromatin DNA into relatively small bright structures that are indicative of apoptotic death (Figure 3, bottom two rows of panels). d120 mutant-infected VAX-3 cells exhibited uniform DNA staining throughout the nucleus, with a minor proportion (approximately 8%) of nuclei showing chromatin condensation. The same diffuse pattern of DNA staining was found in nuclei from uninfected HEp-2 or VAX-3 cells. HEp-2 or VAX-3 cells infected with wild-type HSV-1 showed the marginalization of chromatin characteristic of late stages of HSV-1 infection (Fig. 3, lower panels).
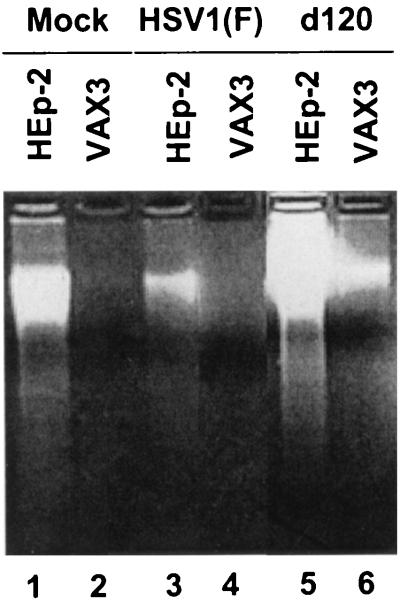
In a concordant experiment, replicate cultures of HEp-2 or VAX-3 cell lines either mock infected or exposed to HSV-1(F) or the d120 mutant virus were harvested 24 h after infection and examined for the presence of oligonucleosomal DNA fragments and chromatin condensation characteristic of apoptotic death. As shown in Fig. 4, at the same time point after infection with wild-type virus or the d120 mutant, lysates from uninfected or d120-infected HEp-2 cells showed the presence of oligonucleosomal DNA fragments whereas no DNA fragmentation could be detected in extracts from uninfected or d120-infected VAX-3 cells (Fig. 4, compare lanes 1 and 5 with lanes 2 and 6) or in extracts from HEp-2 or VAX-3 cells infected by HSV-1(F) (Fig. 4, lanes 3 and 4).
FIG. 4.
Photograph of agarose gel containing electrophoretically separated low-molecular-weight DNA fragments. HEp-2 and VAX-3 cell cultures were mock infected or infected with HSV-1(F) or the d120 mutant. Cell lysates were collected at 24 h after infection and processed as described in Materials and Methods.
Release of cytochrome c induced by d120 infection is blocked in VAX-3 but not in HEp-2 cells.
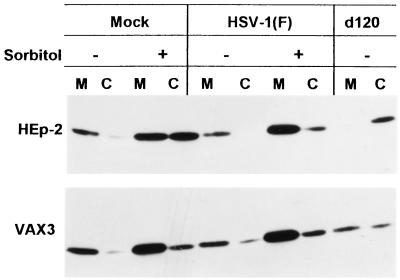
The Bcl-2 protein has been reported to be localized in mitochondria and to block apoptosis through inhibition of release of cytochrome c in the cytoplasm. In an earlier report, it was shown that wild-type HSV-1 infection of HEp-2 cells can block cytochrome c release into the cytoplasm induced by osmotic shock. In contrast, infection with d120 is unable to prevent release of cytochrome c from mitochondria induced by this treatment, and moreover, d120 itself caused the release of cytochrome c into the cytoplasm. To test the effect of overexpression of Bcl-2 on both induction and prevention of apoptosis by HSV-1 infection, replicate cultures of parental HEp-2 cells or VAX-3 cells were mock infected or exposed to 10 PFU of either HSV-1(F) or the d120 mutant per cell. At 14.5 h after infection, duplicate samples of mock- or HSV-1(F)-infected cells were treated with 0.5 M sorbitol for 1.5 h. All of the cultures were harvested 16 h after infection and subjected to subcellular fractionation to separate the mitochondrial and cytoplasmic fractions as described in Materials and Methods. The results (Fig. 5) were as follows:
FIG. 5.
Immunoblot showing cytochrome c distribution in HEp-2 or VAX-3 cells mock infected or exposed to 10 PFU of HSV-1(F) or the d120 mutant per cell and either left untreated or exposed to sorbitol 14.5 h after infection. The cultures were harvested at 16 h after infection. The procedures were as described in Materials and Methods. M, mitochondrial fraction; C, cytosol.
(i) Cytochrome c was present primarily in the mitochondrial fraction of mock-infected cells. After exposure of mock-infected cells to sorbitol, cytochrome c accumulated in significant amounts in the cytoplasm of parental HEp-2 cells and to a much lesser extent in the cytoplasm of VAX-3 cells.
(ii) There was no or minimal accumulation of cytochrome c in the cytoplasm of cells infected with HSV-1(F). Furthermore, HSV-1(F) precluded or reduced the release of cytochrome c from HEp-2 cells exposed to sorbitol. In contrast, there was no significant difference between mock-infected VAX-3 cells exposed to sorbitol and cells infected with wild-type virus and exposed to sorbitol, suggesting that sorbitol had little effect on the release of cytochrome c in VAX-3 cells.
(iii) In d120 mutant-infected HEp-2 cells, virtually all of the cytochrome c was present in the cytoplasmic fraction. The relative amounts of cytochrome c released into the cytoplasm of VAX-3 cells were considerably lower.
These results indicate that (i) Bcl-2 is effective in blocking the release of cytochrome c from mitochondria induced by d120 infection and (ii) wild-type HSV-1 is as effective as Bcl-2 overexpression in its ability to retain cytochrome c in the mitochondria of cells subjected to osmotic shock.
ICP0 is overexpressed in d120-infected cells.
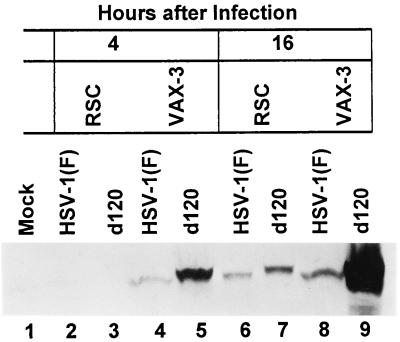
Cell lines in which HSV-1 infection is restricted have been described. For example, it has been shown that infection is blocked in Chinese hamster ovary cells at the entry stage (9). To rule out the possibility that the resistance of VAX-3 cells to d120-induced apoptosis is in fact due to a lack of infection, we sought to determine whether VAX-3 cells were susceptible to HSV-1 by assaying for the presence of ICP0, an early viral transactivator, in wild-type- or d120-infected HEp-2 or VAX-3 cells. HSV-1(F)- or d120-infected HEp-2 and VAX-3 cells were harvested at 4 and 16 h after infection, solubilized, subjected to electrophoresis in denaturing polyacrylamide gels, and reacted with a monoclonal antibody to ICP0. The results shown in Fig. 6 indicate that ICP0 was detected in lysates of HSV-1(F)- or d120-infected HEp-2 or VAX-3 cells as early as 4 h after infection. Both at 4 and 16 h after infection, considerably higher amounts of ICP0 were detected in lysates from cells infected with the d120 mutant than in lysates from wild-type infected cells (Fig. 6, compare lanes 5, 7, and 9 with lanes 4, 6, and 8). These observations, however, are particularly significant. First, there was a significant accumulation of ICP0 in VAX-3 cells that were maintained for 16 h after infection with the d120 mutant. Densitometric measurements of the ICP0 bands showed that d120-infected VAX-3 cells accumulated 15-fold-higher amounts of ICP0 than d120-infected HEp-2 cells. Second, ICP0 in d120-infected RSC and VAX-3 cells migrated more slowly than the corresponding protein accumulating in HSV-1(F)-infected cells. This difference in the electrophoretic mobility could be due to a difference in the posttranslational processing of the ICP0 protein.
FIG. 6.
Photograph of electrophoretically separated cell lysates reacted with an antibody to ICP0. Lysates from uninfected HEp-2 or VAX-3 cells or cells infected with HSV-1(F) or the d120 mutant were harvested at the times indicated in the figure, solubilized, subjected to electrophoresis in a denaturing gel, transferred to a nitrocellulose sheet, and reacted with an antibody against ICP0.
ICP0 localizes to cytoplasmic vesicle-like structures in d120-infected cells.
In the course of these experiments, we noted that the cytoplasmic effects induced by wild-type virus in VAX-3 cells were significantly reduced or delayed relative to that seen in infected HEp-2 cells (data not shown). To test the permissiveness of VAX-3 cells to infection, two series of experiments were done. In the first replicate, 25-cm2 flask cultures of HEp-2 or VAX-3 cells were exposed to 5 PFU of HSV-1(F) per cell and incubated at 37°C for 18 h. The infected cultures were then collected, and the titer of viral progeny released into the medium was determined in Vero cells. The titers of viral progeny from HSV-1(F)-infected VAX-3 cells were not significantly different from those obtained from HSV-1(F)-infected HEp-2 cells (7.5 × 105 and 3 × 105, respectively).
To satisfy ourselves that the VAX-3 cells were infected, slide cultures of VAX-3 cells were exposed to 5 PFU of HSV-1(F) or 10 PFU of d120 mutant per cell. The slide cultures were fixed at 4 or 16 h after infection, reacted with a monoclonal antibody specific for ICP0, and examined with the aid of a Zeiss confocal microscope. The results (Fig. 7) were as follows:
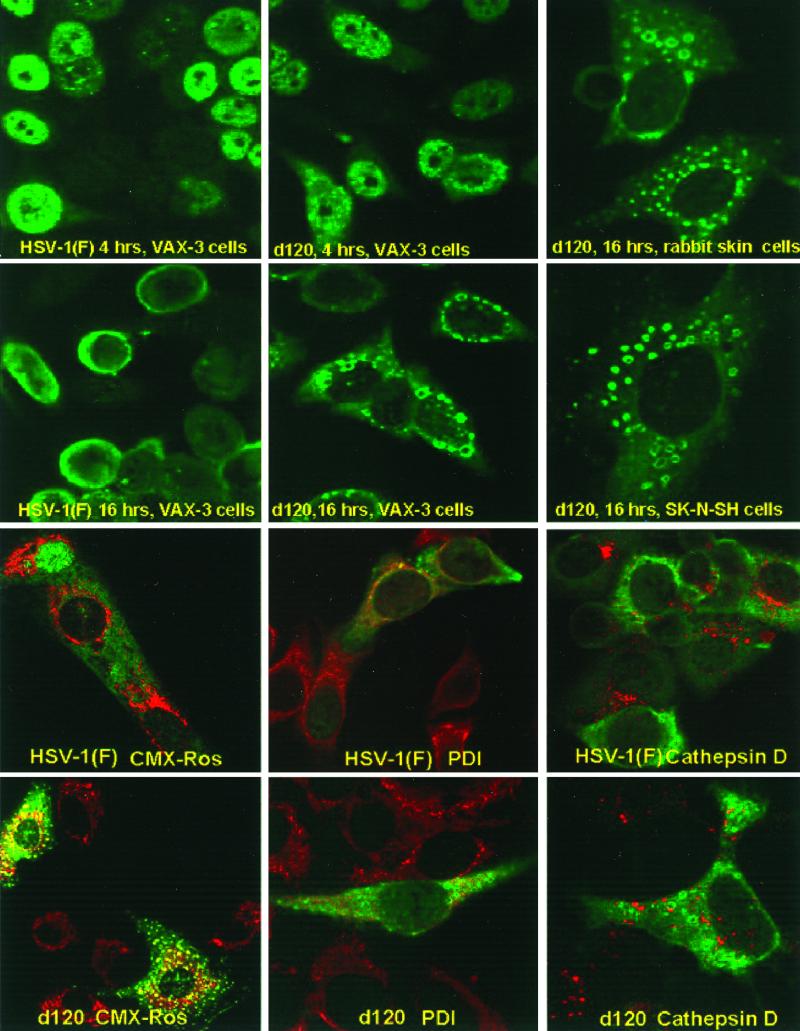
FIG. 7.
Digital images of cells infected with HSV-1(F) or the d120 mutant. (Top two rows of panels) Uninfected HEp-2, VAX-3, SK-N-SH, or RSC mock infected (HEp-2 or VAX-3) or exposed to 5 PFU of wild-type (HEp-2 or VAX-3) or d120 (all cell lines) virus per cell were fixed at 4 (HEp-2 or VAX-3) or 16 (all cell lines) h after infection and reacted with antibody to ICP0. (Bottom two rows of panels) HEp-2 cells were infected with 1 PFU of either HSV-1(F) or the d120 mutant per cell. The cells were fixed at 16 h after infection and reacted with an antibody specific for ICP0 (green fluorescence) and antibodies specific for PDI (endoplasmic reticulum; red fluorescence) or cathepsin D (late endosomal compartment; red fluorescence) or labeled with the mitochondrion-selective dye CMX-Ros (red fluorescence). The images were acquired with a Zeiss confocal microscope as described in Materials and Methods.
(i) HSV-1(F) ICP0 localized in both discrete structures and diffusely in nuclei of VAX-3 cells infected with HSV-1(F). At 16 h after infection, the HSV-1(F) ICP0 localized in the perinuclear space and in the cytoplasm of infected VAX-3 cells.
(ii) The localization of d120 ICP0 at 4 h after infection of VAX-3 cells could not be differentiated from that of HSV-1(F) ICP0 at 4 h after infection. At 16 h after infection, however, ICP0 localized predominantly in the cytoplasm, in small spherical structures resembling uniformly sized vesicles located at the midline of the cytoplasm.
Intrigued by this observation, we repeated these studies with SK-N-SH cells and RSC. As shown in Fig. 7, d120 ICP0 localized in similar cytoplasmic structures at 16 h after infection of SK-N-SH cells or RSC. These results indicate that the formation of these structures is not dependent on overexpression of Bcl-2.
In an attempt to determine whether the ICP0-containing vesicle-like structures are associated with defined subcellular structures, we carried out colocalization studies with the aid of antibodies to markers for specific subcellular compartments. In these experiments, HEp-2 or RSC were infected with 1 PFU of HSV-1(F) or the d120 mutant per cell and incubated for 16 h. The cultures were then incubated in the presence of an antibody specific for ICP0 and either (i) an antibody specific for PDI as a marker for the endoplasmic reticulum, (ii) an antibody specific for cathespin D as a marker for the late endosomal compartment, and (iii) the cell-permeant CMX-Ros dye as a marker for the mitochondrial compartment. ICP0 does not colocalize with PDI, cathepsin D, or the mitochondrial compartment in wild-type- or d120-infected HEp-2 cells (Fig. 7, bottom two rows of panels).
DISCUSSION
An earlier publication from this laboratory reported that the HSV-1(KOS)d120 mutant activates a caspase-3-independent pathway of apoptosis in SK-N-SH, a human cell line derived from malignant glioma (7). The same cells responded to osmotic shock with the induction of a caspase-3-dependent pathway of apoptosis. It was further reported that wild-type HSV-1 blocked apoptosis induced by osmotic shock. In this report, we have shown the following:
(i) In HEp-2, another human cell line, the same mutant induced caspase-dependent apoptosis. This conclusion was based on several observations. Thus, in HEp-2 cells infected with the d120 mutant, cytochrome c was released from mitochondria, PARP was cleaved into appropriate-size peptides, cellular chromatin was condensed, and chromosomal DNA was fragmented. In specific assays, the latter activity was blocked by a broad-spectrum caspase inhibitor.
(ii) We transformed a cell line with a plasmid encoding Bcl-2 and selected a cell clone (VAX-3) overexpressing the protein. In cells infected with this mutant, DNA was not fragmented, cellular chromatin was not condensed, and the release of cytochrome c was significantly reduced.
(iii) The d120 mutant arrests cells at the α stage of viral gene expression (5). This is reflected in the overexpression of α proteins, particularly ICP0. In VAX-3 cells, ICP0 accumulated to levels many times higher than those accumulating in the parental HEp-2 cell line expressing the same protein. Examination of the infected cells revealed that whereas at 4 h after infection of VAX-3 cells the distribution levels of wild-type virus or d120 ICP0 were nearly identical, at 16 h after infection of VAX-3 cells with d120, ICP0 was concentrated primarily in numerous small spherical structures in the cytoplasm. The same vesicle-like spherical structures appeared in SK-N-SH cells and RSC infected with d120 virus.
(iv) Attempts to identify the nature of the vesicles containing ICP0 were not successful. We have excluded mitochondria, endoplasmic reticulum, and the late endosomal compartment.
The significance of the results we report may be summarized as follows:
(i) In the current models of programmed cell death, apoptosis results from the activation of a family of cysteine proteases known as caspases. The result of the activation is a highly organized dismemberment of the cell (20). Activation itself is controlled by at least two different pathways. The first is activated by signaling through death receptors. In this instance, proteolysis is activated directly. The second pathway is activated by stress. In this instance, the signal to activate the proteolytic cascade is regulated at the level of mitochondria by members of the Bcl-2 family of proteins (reviewed in references 1 and 11). Once launched, both pathways converge by activating the effector caspases (caspase-3, -6, and -7) responsible for most of the manifestations of apoptotic death (15, 16, 18).
Pathways of apoptotic death that do not depend on the activation of the caspase family have also been described. Recently, a mitochondrial protein designated apoptosis-inducing factor (AIF) was shown to translocate to the nucleus in dying cells and to induce the nuclear changes associated with apoptosis (19). Inhibitors of caspase activity could not block the effect of apoptosis-inducing factor on chromatin condensation and fragmentation. Also, the death agonist member of the Bcl-2 family BAX induces mitochondrial damage and cell death in the absence of caspase activity (10, 21). Overexpression of the death antagonist members of the Bcl-2 family, Bcl-2 or Bcl-XL, is sufficient to block all the mitochondrial changes associated with apoptosis induced by caspase-dependent or caspase-independent pathways in most systems (reviewed in references 1 and 11).
(ii) Evidence in support of the hypothesis that HSV-1 infection could induce multiple, cell-type-specific pathways of apoptosis has already emerged from earlier studies. Thus, infection with the d120 mutant induces apoptosis in all cell lines tested (7, 8, 12). Induction of apoptosis by another HSV-1 mutant that is impaired at the entry step [HSV-1(HFEM)tsB7], however, is cell type dependent: tsB7, at the nonpermissive temperature, induces apoptosis in Vero but not in SK-N-SH cells (8). On the other hand, wild-type virus efficiently blocks both the caspase-3-independent pathway activated by infection in SK-N-SH cells and the caspase-dependent pathways activated by signaling through the Fas or TNF receptors (7, 8). Nevertheless, the ability of wild-type HSV-1 to block the caspase-dependent pathways is also dependent on cell-type-specific factors. SK-N-SH cells infected with wild-type virus are protected from a variety of exogenous inducers of apoptosis, whereas HeLa cells treated in the same way are not (8). The data presented in this report extend these observations further. In effect, we show that the same virus infecting two different human cell lines activates entirely different pathways that lead to essentially identical outcomes. On the basis of the evidence presented in the earlier publication that SK-N-SH cells can respond with either caspase-3-dependent or -independent apoptosis (7), these results indicate that the d120 stimuli that led to apoptosis in HEp-2 and SK-N-SH cells are different. A corollary of this conclusion, subject to experimental verification, is that different apoptosis-inducing viral stimuli may differ with respect to the pathways that they activate.
(ii) A striking feature of wild-type virus- or d120 mutant-infected VAX-3 cells was a striking reduction in cytopathic effects, so much so that we were concerned that the cells resist viral infection. In the course of these studies, we found that wild-type virus replicates equally well in the parental HEp-2 and VAX-3 cell lines. Inasmuch as d120 mutant assays of infectious progeny would not be indicative of infection, we used ICP0 as a marker of infection. This led to the discovery of the overproduction of ICP0 and the localization of ICP0 in structures resembling cytoplasmic vesicles of unknown origin. These observations, however, raise several questions, not all of which are currently resolved.
Current evidence suggests that Bcl-2 acts at the level of mitochondria to block apoptosis (reviewed in references 1 and 11). Our data are consistent with this hypothesis since in VAX-3 cells all prognosticators of apoptosis were reduced or suppressed. The evidence that cytopathic effects were reduced even in cells infected with wild-type virus suggested that the origin of the CPE reflects a potential mitochondrial malfunction that is retarded by Bcl-2. It should be pointed out that the cytopathic effects have been attributed to two manifestations: exhaustion of cellular resources which lead to failure to maintain the integrity of the cell and direct effects of the expression of viral gene products. We may add to this a host response to infection regulated by Bcl-2 and not blocked by viral gene expression.
The roles of ICP0 and that of other α gene products in the induction of apoptosis are unclear. The data presented in this report do provide some clues to the behavior and the role of ICP0. First, wild-type ICP0 and ICP0 expressed by d120 behave differently. Late in infection, ICP0 produced by d120 is sequestered in small bodies resembling vesicles, a distribution not previously reported. The data lead to two conclusions. Since the same vesicles were observed in cells undergoing apoptosis (SK-N-SH cells and RSC) and in VAX-3 cells, the mere formation of such structures is not indicative or predictive of apoptosis. We cannot exclude the possibility that such structures would induce apoptosis were it not for the overexpression of Bcl-2. The second conclusion is that the fate of ICP0 is dependent on genes expressed later (e.g., β or γ) in infection. This conclusion is based on the observation that the distribution of HSV-1(F) ICP0 and that of d120 protein in VAX-3 cells were similar at 4 h after infection but differed thereafter.
The second observation of interest is that accumulation of d120-encoded ICP0 was higher in VAX-3 cells than in HEp-2 cells. The simplest explanation of this observation is that HEp-2 cells are in the throes of apoptosis and have curtailed production of ICP0, a conclusion that remains to be verified experimentally. What is clear is that the mere overproduction of ICP0 is neither indicative nor predictive of apoptosis.
In essence, the perception of the role of HSV-1 in cellular apoptosis is that of a game in which four teams play simultaneously: viral events which induce apoptosis, the cellular pathways activated by the viral stimuli, viral gene functions that block apoptosis, and cellular functions that normally regulate apoptosis. Dissection of the role of the various players in each team is a formidable challenge. The return may be equal to the challenge because such studies may be the only avenue to uncover the function and regulation of each participant.
ACKNOWLEDGMENTS
This study was aided by grants from the National Cancer Institute (CA47451, CA71933, and CA78766), U.S. Public Health Service. J.M. is a predoctoral trainee, with support from NIH Molecular and Cell Biology training grant T32GM01797.
REFERENCES
- 1.Adams J, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;54:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of α genes. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 5.DeLuca N A, McCarth A, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;58:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 7.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang A S, Palma E L, Hewlett N, Roizman B. Pseudotype formation between enveloped RNA and DNA viruses. Nature. 1974;252:743–745. doi: 10.1038/252743a0. [DOI] [PubMed] [Google Scholar]
- 10.Jurgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Bax directly induces release of cytochrome C from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 12.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy N J, Whyte M K B, Gilbert C S, Evan G I. Inhibition of Ced3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevshenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 16.Reed J. Cytochrome C: can't live with it, can't live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 17.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 18.Salvesen G S, Dixit V. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 19.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Constantini P, Loeffler M, Larochette N, Goodlett D R, Aebersold R, Siderovski D P, Penninger J M, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 20.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 21.Xiang J, Chao D T, Korsmeyer S J. Bax-induced cell death may not require interleukin 1 α converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]