Abstract
An abdominal aortic aneurysm (AAA) in children is a rare clinical condition, with idiopathic AAAs even more atypical. We report a case of a 19-month-old girl with incidental findings of an infrarenal AAA and right common iliac artery aneurysm during workup for heart failure. Extensive genetic testing was unremarkable for connective tissue disorders. An aortic bi-iliac artery bypass with a Dacron graft from the infrarenal aorta to the right external iliac artery and left common iliac artery was performed. The patient achieved complete recovery and only required one oral hypertensive medication at 30 days of follow-up. Wide patency of the graft was observed on the 3-month follow-up computed tomography angiogram.
Keywords: Abdominal aortic aneurysm, Aortic disease, Congenital, Pediatric aneurysms, Pediatric vascular surgery, Surgical repair
Abdominal aortic aneurysms (AAAs) are an uncommon clinical condition in pediatric patients. Etiologies such as infection, connective tissue disorders, vasculitides, and trauma have been associated with this pathology. Congenital AAAs are even more rare and have an unknown etiology. Neonates and infants have a higher incidence rate compared with young adults and fetuses (57% and 42%, respectively). Clinical manifestations are significantly broad, ranging from asymptomatic presentation to aneurysmal rupture. In the context of rupture, overall mortality rates ascend ≤80%.1, 2, 3 Because of scarce reports and unstandardized approach guidelines for pediatric patients, current clinical decisions on management and treatment have been extrapolated from the adult literature. In the present report, we describe a unique presentation of a pediatric patient with incidental findings of an AAA and right common iliac artery (CIA) aneurysm during workup studies for cardiovascular disease. The patient’s parents provided written informed consent for the report of her case details and imaging studies.
Case report
A 19-month-old girl presented with dyspnea, emesis, and abdominal pain to the emergency department at an outside hospital. She was born at full term without any complications during the pregnancy. Her medical history was remarkable for neonatal jaundice, which was treated with phototherapy, developmental delay, and a poor tolerance of solid food intake at 8 months of age. She was evaluated by a primary care physician 1 month before the admission, with no remarkable vital signs or physical examination findings, and was determined to have incomplete fulfillment of development milestones, raising concern for possible autism. On physical examination on admission, the patient weighed 10.5 kg, measured 2 ft. 7-in., and was tachycardic (163 bpm), hypertensive, and tachypneic, with rhonchi on auscultation. A pulsatile mass was palpable on deep palpation of the abdomen. The laboratory workup revealed anemia (hemoglobin, 6.3 g/dL) with a mean corpuscular volume of 48 fL and elevated C-reactive protein (46.8 mg/L). A respiratory panel for SARS-CoV-2, influenza, and syncytial respiratory virus was negative. A chest radiograph revealed right lower lobe pulmonary effusion and cardiomegaly. The patient was transferred to a pediatric outside hospital and developed respiratory failure with a requirement for mechanical ventilation. Echocardiography studies reported a left ventricular ejection fraction of 20%. Given the occurrence of severe systemic hypertension, treatment with intravenous milrinone 0.75 μg/kg/min and sacubitril/valsartan 1.96 mg twice daily was initiated. Cardiac magnetic resonance imaging and computed tomography angiography (CTA) demonstrated dilated cardiomyopathy with a right lower lobe pulmonary embolus (Fig 1), with subsequent low-molecular-weight heparin initiation. Abdominal CTA was performed to assess the persistent hypertension and revealed right kidney atrophy and grade 1 hydronephrosis, a 4-cm infrarenal AAA with intraluminal thrombus, and a 2.6-cm right CIA aneurysm (Fig 2). Extensive genetic testing for connective tissue disorders, aortopathy, and cardiomyopathy and biochemical panels were performed, with the findings only remarkable for mildly low levels of free and total carnitine, which were considered positive for secondary carnitine deficiency. The patient was referred to our institution for further evaluation and treatment of the AAA and iliac aneurysm. She presented with a palpable aneurysm on deep palpation of the abdomen. After multidisciplinary discussion, a decision was made to defer any intervention until improvement of her heart function and to reevaluate her clinical condition 4 weeks later. A repeat echocardiogram demonstrated stabilization and improvement of ejection fraction to 55.9%. In light of the significant improvement in her clinical condition and left ventricular function, she was deemed stable to proceed with open repair.
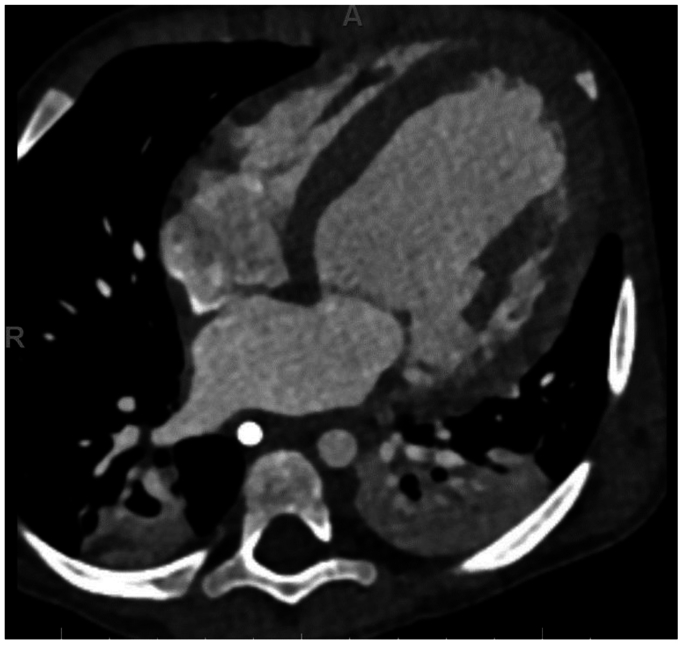
Fig 1.
Initial cardiac computed tomography scan. Axial projection with presence of dilated cardiomyopathy.
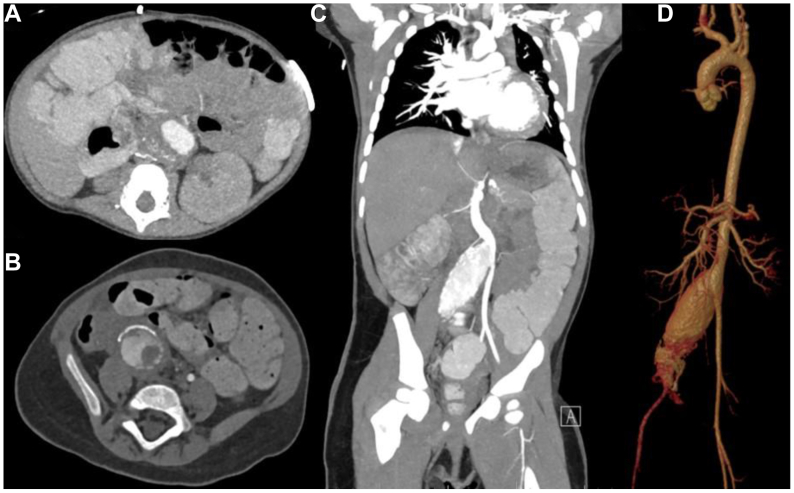
Fig 2.
Preoperative abdominal computed tomography scan. A, Axial projection with evidence of an infrarenal abdominal aortic aneurysm (AAA) with intraluminal thrombus. Axial (B) and coronal (C) projections showing right common iliac artery (CIA) aneurysm. D, Three-dimensional preoperative reconstruction of AAA and CIA aneurysm.
A midline laparotomy incision was performed, and the infrarenal abdominal aorta and bilateral iliac vessels were dissected out. The retroperitoneum was noted to be inflamed, with densely adherent surrounding tissue to the vasculature. The inferior mesenteric artery (IMA) was identified and controlled with a vessel loop (Fig 3, A). Once vessel control was obtained for all arteries, the iliac arteries and infrarenal aorta were clamped. A 12-mm × 6-mm bifurcated Dacron graft (Maquet Cardiovascular, LLC) limb was used. The proximal anastomosis was performed to the infrarenal aorta below the ostia of the IMA and was fashioned with individual interrupted 6-0 Prolene sutures to allow for a greater anastomotic orifice and better pulsatility of the anastomosis. The right CIA aneurysm cavity was debrided and the internal iliac artery ligated (Fig 3, B). Next, the Dacron graft limb was anastomosed to the external iliac artery (EIA) in the same interrupted fashion on the right and to the CIA on the left (Fig 3, C). Doppler ultrasound interrogation of the IMA and iliac arteries demonstrated adequate signals. The retroperitoneum over the repair was closed with running 3-0 Vicryl suture. The patient was extubated and transferred in stable conditions to the cardiovascular intensive care unit. Surgical pathology examination of the excised aortic tissue was negative for active vasculitis, with the presence of fibrotic thickening of the aortic tunica intima, fibrosis of the aortic tunica media, and luminal thrombus with dystrophic calcification. On postoperative day 14, she was deemed stable for discharge with valsartan/sacubitril 2.04 mg twice daily, spironolactone 12.5 mg twice daily, carvedilol 7 mg twice daily, and aspirin 40.5 mg daily. At 30 days of follow-up, she was doing well with ultrasound evidence of a widely patent graft with triphasic waveforms. The 3-month follow-up CTA demonstrated wide patency of the right CIA, EIA, and left CIA graft (Fig 4). The patient will continue to have yearly follow-up with ultrasound imaging.
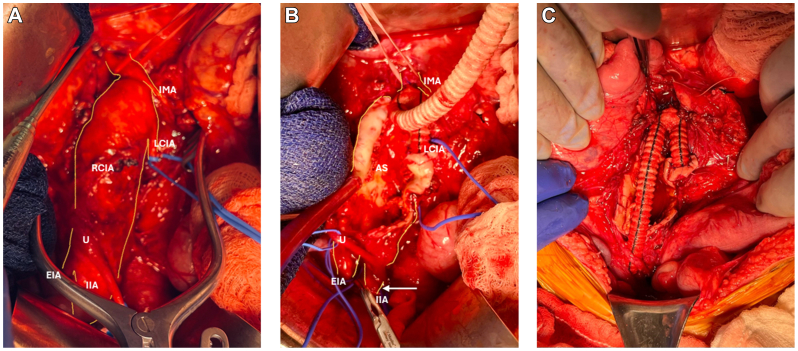
Fig 3.
Intraoperative sequence of open surgical repair with outline of relevant vascular anatomy involved during the procedure. A, Midline laparotomy with dissection of the infrarenal abdominal aorta and bilateral iliac vessels, followed by vessel control with vascular loops. B, Right common iliac artery (CIA) aneurysm debridement and internal iliac artery ligation (arrow). Proximal anastomosis was performed to the infrarenal aorta below the inferior mesenteric artery (IMA) ostia with individual interrupted Prolene sutures and the Dacron graft limb. C, Completion of repair after performing graft anastomosis to the left CIA. AS, Aneurysm sac; EIA, external iliac artery; IIA, internal iliac artery; LCIA, left common iliac artery; RCIA, right common iliac artery; U, ureter.
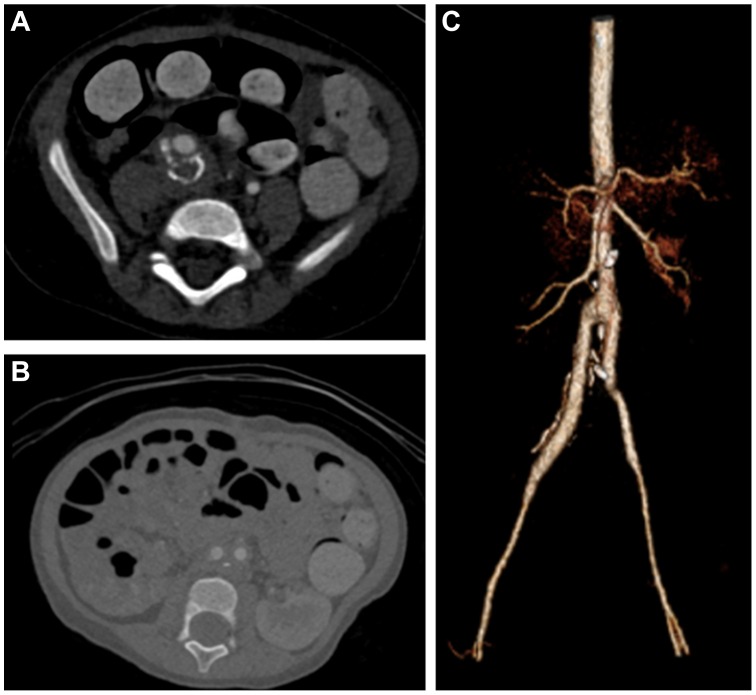
Fig 4.
A,B, Axial view of follow-up computed tomography angiography (CTA) with surgical changes of open repair and patent right common iliac artery (CIA), left CIA, and external iliac artery (EIA) graft. C, Three-dimensional postoperative reconstruction of abdominal aortic aneurysm (AAA) and right CIA open repair.
Discussion
Howorth4 described the first case of AAA in a pediatric patient in 1967 after a 3-day-old female newborn presented with a congenital 11-cm saccular infrarenal AAA with an unremarkable medical history. Sarkar et al5 established a classification system for childhood arterial aneurysms according to class, principal compromised artery, and clinical characteristics for true aneurysms (Table). In the context of aneurysmal disease, the most common etiologies are trauma secondary to umbilical arterial catheterization, vasculitis, and connective diseases.5,6 Regarding the anatomical location in infants, 69% of AAA cases have been reported as infrarenal AAAs, followed by juxtarenal and thoracoabdominal aneurysms. Unilateral or bilateral iliac artery involvement can occur, and concomitant aneurysms of other vessels arise in 15% of patients, with involvement of the iliac, renal, or superior mesenteric artery.2,6, 7, 8 The mortality of congenital AAAs is 31%, with aneurysm rupture and renal failure the major causes of death.2
Table.
Classification of childhood arterial aneurysms
| Class | Principal arteries affected | Clinical characteristics |
|---|---|---|
| I: arterial infection | Aorta (particularly thoracic), iliac | Cardiovascular anomalies and umbilical artery catheterization are predisposing factors; dyspnea, cough, chest pain with progression to rupture and death if untreated |
| II: giant cell aortoarteritis | Aorta, (peripheral arteries, rare) | Signs and symptoms vary from being absent to shock; untreated aortic lesions progress to rupture |
| III: Autoimmune vasculitis | Renal, hepatic, and splenic arterial branches | Usually asymptomatic but can cause hematuria, perirenal hematoma, or death with rupture |
| IV: Kawasaki disease | Coronary (20%-30%), axillobrachial, iliofemoral, hepatic | Usually asymptomatic; myocardial infarction or tamponade (coronary), limb ischemia (extremity), and obstructive jaundice (hepatic) can occur |
| V: medial degeneration: Marfan and Ehlers-Danlos syndromes | Aorta | Aortic rupture or dissection common; arteriography and vascular reconstruction hazardous in type IV Ehlers-Danlos syndrome |
| VI: medial degeneration: other forms | Aorta, (peripheral arteries, rare) | Associated with other cardiac (bicuspid aortic valve) and aortic (coarctation) anomalies; often present with aortic dissection or rupture |
| VII: arterial dysplasia | Renal | Usually asymptomatic, detected during arteriography for renovascular hypertension |
| VIII: idiopathic, congenital | Iliofemoral, brachial, aorta | Often asymptomatic but can cause limb ischemia; rupture unreported |
| IX: extravascular causes | Aorta, visceral, and extremity arteries | Protean manifestations; aortic aneurysms often rupture; peripheral lesions often asymptomatic; visceral lesions can cause gastrointestinal bleeding |
Data modified from Sarkar et al.5
In this case, we highlight the presence of an infrarenal AAA, with a concomitant CIA aneurysm as an uncommon clinical presentation. To the best of our knowledge, to date, only seven cases with this clinical vignette have been described in the literature for AAAs. A diagnostic challenge was observed given the negative results for genetic mutations related to connective tissue disorders associated with aneurysmal diseases and vascular and organ anomalies. Likewise, the surgical pathology examination did not suggest active vasculitis. Because scar tissue was observed in the postoperative excision sample, we suspect the possibility of the occurrence of retroperitoneal fibrosis (RPF) as a potential cause. RPF involves inflammation and progressive development of a fibrotic mass around the aorta and other large vessels in the retroperitoneal space. The most common location involves the infrarenal abdominal aorta, with possible involvement of the iliac arteries and veins, inferior vena cava, pelvic plexuses, and retroperitoneal organs.9 The etiology for this clinical condition can be idiopathic or secondary (ie, infection, malignancy, radiotherapy). The potential pathogenesis for RPF includes a local inflammatory response to atherosclerotic plaque antigens and a local autoimmune response linked to IgG4-related disease.9 The diagnostic criteria for IgG4-related disease RPF include characteristic/diffuse localized swelling or masses in single or multiple organs on clinical examination, an increased serum IgG4 concentration (≥135 mg/dL), and histopathologic examination findings of marked lymphocyte and plasmacyte infiltration, fibrosis, and infiltration of IgG4 plasma cells (ratio of IgG4/IgG+ cells >40% and >10 IgG4+ plasma cells/high power field).10
Two subtypes have been related to vascular disease: idiopathic RPF, which consists of periaortic tissue fibrosis without aortic dilation, and inflammatory AAAs, with aneurysmal aortic dilatation with inflammatory involvement of the aortic wall. The latter has incidence rates from 2.3% to 10% of all AAAs.11,12 Additionally, renoureteral compromise has been described, with occurrence of uni- or bilateral hydronephrosis secondary to obstructive uropathy as the most frequent complication. If one of the kidneys demonstrates hypoplasia at diagnosis, it could suggest a chronic disease process. De novo hypertension can develop in cases of renal vein and artery compression.13 Considering the clinical presentation and course of our patient, it is possible that asymptomatic idiopathic RPF was present before admission, with renal compromise as the potential origin of the kidney atrophy, subsequent hypertension onset, and later heart failure. Aortic wall stress could have been increased due to an increased hemodynamic burden, further leading to aneurysm development. Based on this clinical presentation and childhood arterial aneurysm classification, this case might correspond to a type VIII aneurysm, given the presence of retroperitoneal scarring. Most cases have been described in adult patients.14 An interesting feature was the presence of aneurysm calcification, which commonly occurs in the setting of concomitant abdominal aortic atherosclerotic disease.15 However, recent evidence for the clinicopathological features of RPF has shown aortoiliac vascular calcifications with a higher frequency in the lower abdominal aortic and iliac vessels.15 Although the etiology is unclear in the atypical scenario of this case, this could be a potential pathological mechanism that can explain the patient's clinical findings. A paucity of case reports of children with similar clinical vignettes exists. Thus, we consider RPF as the potential etiology after correlation of the intraoperative findings and an extensive workup. To the best of our knowledge, this is the first report of an inflammatory AAA with a concomitant CIA aneurysm and idiopathic RPF in a pediatric patient.
The standard of care has not been established for the treatment of AAAs in the pediatric population. Conservative treatment can be considered if the patient has a high surgical mortality risk, and it is unlikely to improve life expectancy. Medical treatment includes antiplatelet therapy, beta-receptor blockers, angiotensin-converting enzyme inhibitors, and ultrasound follow-up. The objective is to reduce aortic wall stress, prevent aneurysm progression and rupture, reduce the risk of cardiovascular events, and reduce the long-term requirement for surgery.16,17 During the clinical course of an AAA, mural thrombosis can arise and is considered a stronger predictor for rupture compared with the arterial diameter in adults. Considering the scarce body of literature for this parameter in children, we consider this scenario requires closer monitoring with multimodal imaging until the patient is considered fit for surgery or requires urgent surgical repair.18,19
Surgical interventions for AAAs include open repair and endovascular aneurysm repair. Multiple factors must be considered before performing procedures in pediatric patients.20 Due to the lack of reports for endovascular techniques and obvious size mismatch concerns, open repair has been the mainstay of treatment. A series by Dueppers et al21 with inclusion of 11 pediatric patients reported favorable outcomes of surgical repair, with 7 year of follow-up. They reported one proximal aortic graft anastomosis dehiscence at 15 months postoperatively and survival rate of 84%.21 Techniques to perform open repair include autologous grafts and end-to-end anastomosis with Dacron, polytetrafluoroethylene, or cadaveric grafts.22, 23, 24, 25 In the case of using synthetic grafts, oversizing and interrupted anastomosis is essential to avoid strictures and allow for a greater anastomotic orifice and better pulsatility. Multiple factors must be considered for procedures in pediatric patients given their smaller anatomy, distinct physiological parameters, and the potential for surgical revisions due to child development.19 Thus, an adequate preoperative comorbidity assessment, surgical planning, and multidisciplinary evaluation are paramount to ensure superior clinical outcomes. Additionally, it is important to discuss with the patient and family members the likelihood of further interventions in the future. Close follow-up with vascular surgery is essential.
Conclusions
Aortic aneurysms and, especially, iliac aneurysms in pediatric patients can be not only a diagnostic but also a treatment challenge in cases of incidental findings or presentations. The diagnosis should be considered in patients with underlying cardiac disease. To the best of our knowledge, this is the first case report of a concomitant AAA and iliac aneurysm in a pediatric patient with idiopathic RPF.
Disclosures
M.L.K. is a consultant for W.L. Gore & Associates. C.H.T. has been a consultant for, and received research support from, Cook Medical Inc, W.L. Gore & Associates, and Phillips Healthcare.
From the Southern Association for Vascular Surgery
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Millar A.J., Gilbert R.D., Brown R.A., Immelman E.J., Burkimsher D.A., Cywes S. Abdominal aortic aneurysms in children. J Pediatr Surg. 1996;31:1624–1628. doi: 10.1016/s0022-3468(96)90034-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Tao Y. Diagnosis and treatment of congenital abdominal aortic aneurysm: a systematic review of reported cases. Orphanet J Rare Dis. 2015;10:4. doi: 10.1186/s13023-015-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAteer J., Ricca R., Johansen K.H., Goldin A.B. Extensive congenital abdominal aortic aneurysm and renovascular disease in the neonate. J Vasc Surg. 2012;55:1762–1765. doi: 10.1016/j.jvs.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Howorth M.B. Aneurysm of abdominal aorta in the newborn infant. Report of case. N Engl J Med. 1967;276:1133–1134. doi: 10.1056/NEJM196705182762007. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar R., Coran A.G., Cilley R.E., Lindenauer S.M., Stanley J.C. Arterial aneurysms in children: clinicopathologic classification. J Vasc Surg. 1991;13:47–56. discussion: 56-7. [PubMed] [Google Scholar]
- 6.Mehall J.R., Saltzman D.A., Chandler J.C., Kidd J.N., Wells T., Smith S.D. Congenital abdominal aortic aneurysm in the infant: case report and review of the literature. J Pediatr Surg. 2001;36:657–658. doi: 10.1053/jpsu.2001.22314. [DOI] [PubMed] [Google Scholar]
- 7.Kim E.S., Caiati J.M., Tu J., Nowygrod R., Stolar C.J. Congenital abdominal aortic aneurysm causing renovascular hypertension, cardiomyopathy, and death in a 19-day-old neonate. J Pediatr Surg. 2001;36:1445–1449. doi: 10.1053/jpsu.2001.26394. [DOI] [PubMed] [Google Scholar]
- 8.Cheung S.C., Khong P.L., Chiu W., Metreweli C. Congenital abdominal aortic aneurysm and renal dysplasia. Pediatr Radiol. 2004;34:827–830. doi: 10.1007/s00247-004-1215-7. [DOI] [PubMed] [Google Scholar]
- 9.Łoń I., Wieliczko M., Lewandowski J., Małyszko J. Retroperitoneal fibrosis is still an underdiagnosed entity with poor prognosis. Kidney Blood Press Res. 2022;47:151–162. doi: 10.1159/000521423. [DOI] [PubMed] [Google Scholar]
- 10.Umehara H., Okazaki K., Masaki Y., et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 11.Dalainas I., Nano G., Ranucci M., et al. Inflammatory abdominal aortic aneurysms. A 20-year experience. J Cardiovasc Surg. 2007;48:305–308. [PubMed] [Google Scholar]
- 12.Rasmussen T.E., Hallett J.W. Inflammatory aortic aneurysms. A clinical review with new perspectives in pathogenesis. Ann Surg. 1997;225:155–164. doi: 10.1097/00000658-199702000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bommel E.F.H., Jansen I., Hendriksz T.R., Aarnoudse A. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine (Baltim) 2009;88:19–201. doi: 10.1097/MD.0b013e3181afc420. [DOI] [PubMed] [Google Scholar]
- 14.Fatima J., Gota C., Clair D.G., Billings S.D., Gornik H.L. Inflammatory abdominal aortic aneurysm with retroperitoneal fibrosis. Circulation. 2014;130:1300–1302. doi: 10.1161/CIRCULATIONAHA.114.010173. [DOI] [PubMed] [Google Scholar]
- 15.Corradi D., Maestri R., Palmisano A., et al. Idiopathic retroperitoneal fibrosis: clinicopathologic features and differential diagnosis. Kidney Int. 2007;72:742–753. doi: 10.1038/sj.ki.5002427. [DOI] [PubMed] [Google Scholar]
- 16.Golledge J., Norman P.E. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217:57–63. doi: 10.1016/j.atherosclerosis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Truijers M., Pol J.A., Schultzekool L.J., van Sterkenburg S.M., Fillinger M.F., Blankensteijn J.D. Wall stress analysis in small asymptomatic, symptomatic and ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2007;33:401–407. doi: 10.1016/j.ejvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Moxon J.V., Parr A., Emeto T.I., Walker P., Norman P.E., Golledge J. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. 2010;35:512–548. doi: 10.1016/j.cpcardiol.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenbaek J., Kalin B., Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20:466–469. doi: 10.1053/ejvs.2000.1217. [DOI] [PubMed] [Google Scholar]
- 20.Chadi S.A., Rowe B.W., Vogt K.N., et al. Trends in management of abdominal aortic aneurysms. J Vasc Surg. 2012;55:924–928. doi: 10.1016/j.jvs.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 21.Dueppers P., Duran M., Grabitz K., Schelzig H. Open repair for abdominal aortic aneurysm in a young boy with tuberous sclerosis and review of the literature. Ann Vasc Surg. 2017;39:286.e1–286.e5. doi: 10.1016/j.avsg.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Sawan E.B., Henaine R., Daou L., Jebara V. Successful operation for thoracoabdominal aortic aneurysm in a 5-year-old boy with tuberous sclerosis. Ann Thorac Surg. 2015;100:e119–e120. doi: 10.1016/j.athoracsur.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 23.Salerno A.E., Marsenic O., Meyers K.E., Kaplan B.S., Hellinger J.C. Vascular involvement in tuberous sclerosis. Pediatr Nephrol. 2010;25:1555–1561. doi: 10.1007/s00467-010-1466-5. [DOI] [PubMed] [Google Scholar]
- 24.Moon S.B., Shin W.Y., Park Y.J., Kim S.J. An abdominal aortic aneurysm in an 8-month-old girl with tuberous sclerosis. Eur J Vasc Endovasc Surg. 2009;37:569–571. doi: 10.1016/j.ejvs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Kaye A.J., Slemp A.E., Chang B., Mattei P., Fairman R., Velazquez O.C. Complex vascular reconstruction of abdominal aorta and its branches in the pediatric population. J Pediatr Surg. 2008;43:1082–1088. doi: 10.1016/j.jpedsurg.2008.02.035. [DOI] [PubMed] [Google Scholar]