Abstract
Background and objectives
The efficacy of rituximab (RTX) in treating steroid-resistant Graves’ orbitopathy (GO) has been limitedly studied in Asians. Moreover, RTX has been considered even less for patients with steroid-resistant dysthyroid optic neuropathy (DON) who failed to undergo orbital decompression surgery for physical or financial reasons, or who responded poorly to the procedure. This study aimed to investigate the efficacy of RTX in treating steroid-resistant active moderate-to-severe and sight-threatening GO in a Chinese population.
Methods
Data from 28 patients with steroid-resistant GO prescribed a single dose of 500 mg RTX were retrospectively retrieved. Treatment responses and contributing factors were analyzed.
Results
The median follow-up time was 22 (8–34) weeks. 23 (82.1 %) patients had a positive objective outcome recommended by the European Group on Graves’ Orbitopathy (EUGOGO), while 25 (92.6 %) had a decrease in 7-item clinical activity score (CAS) by at least 2. Diplopia, visual dysfunction, and MRI-detected T2 relaxation time of the involved extraocular muscles improved significantly at the last follow-up compared to baseline (81.0 % vs. 47.6 %, 38.9 % vs. 16.7 %, and 87.8 (8.64) vs. 75.8 (10.9) ms, respectively; all p values < 0.05). No significant improvement was seen in terms of proptosis and eye muscle duction. Notably, a higher baseline IgG4 to IgG ratio was a predictor for RTX-induced positive EUGOGO outcomes. After RTX treatment, all 8 patients with DON demonstrated inactivation, and 4 improved in visual acuity by ≥ 1 line. No patient with DON experienced obvious deterioration.
Conclusion
A single dose of 500 mg RTX seemed to be an effective and tolerable treatment for steroid-resistant GO. However, larger-scale studies with a control group are required for a more solid conclusion. The role of RTX in steroid-resistant DON management where surgery is unavailable or ineffective should be further explored.
Keywords: Rituximab, Steroid-resistant graves' orbitopathy, Dysthyroid optic neuropathy
Highlights
-
•
First to study the efficacy of rituximab (RTX) exclusively in steroid-resistant Graves' orbitopathy in Asian patients.
-
•
Focused on when orbital decompression surgery is unavailable or ineffective for dysthyroid optic neuropathy.
-
•
Highlighted the effect of RTX on ocular motility and visual dysfunction.
1. Introduction
Graves' orbitopathy (GO), also known as thyroid eye disease or thyroid-associated ophthalmopathy, presents an uncommon [1] yet quality-of-life-threatening autoimmune disorder due to its ocular symptoms and disfiguring effects [2], especially in its active moderate-to-severe [3] or sight-threatening forms. Intravenous glucocorticoids (GCs) are the first-line treatment for active moderate-to-severe and sight-threatening GO, as recommended by the current guidelines of both the European Group on Graves’ Orbitopathy (EUGOGO) and the American Thyroid Association (ATA) and the European Thyroid Association (ETA), and they are administered at different doses under these two conditions [4,5]. However, for active moderate-to-severe GO, 21–40 % of patients who received GCs as initial treatment failed to achieve long-term remission [6], leaving them with choices of second-line therapies because there is an upper limit on the GC dosage [4,7]. Rituximab (RTX) is a human/murine chimeric anti-CD20 monoclonal antibody [8] that leads to B-cell depletion via various mechanisms, including antibody-dependent and complement cytotoxicity, alterations in intracellular calcium and apoptosis [9]. It is mainly used to treat CD20+ B-cell lymphomas but is also involved in the treatment of autoimmune diseases, such as lupus erythematosus or rheumatoid arthritis [10]. RTX is now recommended as a second-line treatment for active moderate-to-severe GO. However, previous studies regarding the efficacy of RTX in treating GO mostly included GC-naïve patients, including one recent pilot study conducted in 7 Chinese patients [11]. Limited studies focusing on the steroid-resistant subpopulation of GO patients have shown inconsistencies in the response to RTX treatment [[12], [13], [14]]. We believe that the evidence for a second-line treatment for GO should come from homogenous steroid-resistant study populations, which provides a rationale for our study in this specific patient subgroup. In addition, the optimal dose of RTX remains controversial and studies on its effect in Asian patients are lacking.
For patients who developed steroid-resistant dysthyroid optic neuropathy (DON), the EUGOGO guidelines urged orbital decompression surgery and recommended against the use of RTX [4]. However, steroid-resistant DON patients are not always able to receive orbital decompression surgery either due to high expenses, especially for those from underdeveloped regions, or poor physical conditions which prevent surgery. Even if DON patients undergo surgery, visual function can sometimes fail to be restored, as reported by a previous review [15]. In contrast, a study by Khanna et al. favored the effect of RTX on visual restoration [16]. A recent literature review comprehensively reported on 13 DON patients who were prescribed RTX, 11 of whom achieved favorable results, but the authors still did not recommend the use of RTX for DON due to conflicting results and potential adverse events [17]. However, these DON patients were heterogeneous in regard to previous treatment (steroid-resistant or not, whether orbital decompression surgery was performed), and the circumstance of DON being unavailable or ineffective has never been addressed. A widely accepted theory of DON development is compression of the optic nerve by enlarged extraocular muscles (EOMs) [5], as a result of autoimmunity-stimulated hyaluronan secretion by orbital fibroblasts and accumulation in between EOM fibers. B lymphocytes and anti-thyrotropin receptor antibodies play important roles in this process [2]. Given the suppressive effect of RTX on B cells and autoimmunity [10], we hypothesized that RTX could be used to treat DON. Therefore, our study aimed to investigate the efficacy of 500 mg RTX as a second-line treatment for steroid-resistant GO in a single Chinese institution, with an emphasis on the treatment responses of patients with DON.
2. Materials and methods
2.1. Study population
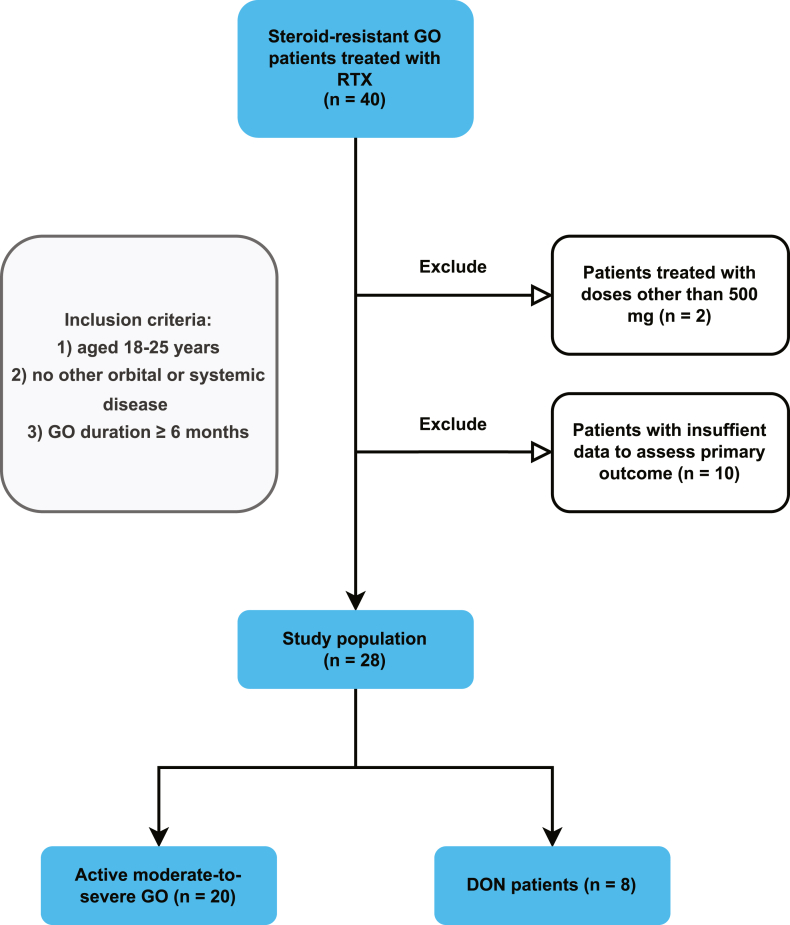
This single-center study first included 40 patients who were hospitalized from May 2018 to March 2023 in the Department of Endocrinology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All patients included in the study had active moderate-to-severe or sight-threatening GO diagnosed according to EUGOGO guidelines [4]. Active moderate-to-severe GO patients were prescribed intravenous RTX injections as second-line treatment due to resistance to intravenous GCs (details are provided in the Definitions section below). Patients were given 5 mg dexamethasone and 50 mg promethazine 30 min before RTX administration as well as 5 mg dexamethasone after RTX to avoid cytokine release syndrome [18] and allergic reactions. The steroid-resistant DON patients included in this study were given RTX either because they failed to undergo orbital decompression surgery due to financial or physical reasons or because visual function was not restored after surgery. The other inclusion criteria were as follows: 1) aged 18–75 years; 2) no known other orbital disease or complex systemic disease; and 3) a GO duration ≥6 months when second-line treatment could be considered. 2 patients who received a RTX dose other than a single dose of 500 mg (both received two doses of 500 mg each) were excluded from the study. As a result, all patients included in the study were treated with a single dose of 500 mg of RTX. 10 patients with missing information to assess the primary outcome (5 missing all primary outcome measures, 3 with only clinical activity, 1 with only clinical activity and duction measure, and 1 without an aperture measure) were also excluded (Fig. 1).
Fig. 1.
Patient selection for the retrospective study.
2.2. Data collection
Data on demographics, including age, sex, and smoking history; GO characteristics, including disease duration, cumulative dose of GCs, exophthalmometric value, diplopia status, duction restriction, visual acuity, and intraocular pressure (IOP); and medical history of thyroid diseases, treatments, comorbidities, biochemical markers, and orbital magnetic resonance imaging (MRI) features were collected and used for analyses. Oculus uterque (OU) means of exophthalmometric value, visual acuity, and IOP were analyzed.
Methods for ophthalmic and biochemical examinations and MRI assessment were as described in our previous study [19], except that we analyzed the MRI features of the involved EOMs reported by the radiologist instead of the four recti [20].
2.3. Definitions
Disease activity was assessed using a 7-item clinical activity score (CAS), which included (1) spontaneous retrobulbar pain, (2) pain on attempted upward or downward gaze, (3) redness of eyelids, (4) redness of conjunctiva, (5) swelling of caruncle or plica, (6) swelling of eyelids, and (7) swelling of conjunctiva (chemosis). 7-item CAS ≥3 indicated active GO, as recommended by the EUGOGO guidelines [4,21]. 7-item CAS <3 but 10-item CAS ≥4 was also considered active GO to cover active GO patients who were excluded by the 7-item CAS criteria but identified by the 10-item CAS criteria. Additional items for 10-item CAS included: (8) increase of at least 2 mm in proptosis, (9) decrease of at least 8° in any duction, and (10) decrease in visual acuity by two lines [5]. Disease severity was evaluated also according to the EUGOGO guidelines [4]. DON diagnosis was made upon the presence of signs of vision loss and optic nerve compression on MRI. GC resistance was defined as a negative primary outcome (the definition is provided below) assessed 6 months after the initiation of standard intravenous GC treatment according to EUGOGO guidelines (4.5 g for 12 weeks) [4,12].
The primary outcome of the study was the EUGOGO-recommended objective outcome, which composed of the following measures: reduction in lid aperture ≥2 mm, reduction in 5-item CAS (excluding subjective, patient-reported spontaneous or gaze-evoked pain) ≥ 1 point, reduction in exophthalmos ≥2 mm, increase in eye muscle duction ≥8°. Improvement in ≥2 features in one eye without deterioration in the other eye might be considered a positive response to treatment [4,22]. The development of DON was considered a negative primary outcome. The secondary outcomes included: a 7-item CAS reduction by at least 2, proptosis reduction by at least 2 mm, diplopia improvement in degree or direction, eye muscle duction improvement in degree or direction, and visual acuity improvement by at least one line, along with other GO characteristics. Visual dysfunction was defined as visual acuity <0.5 [14]. Proptosis was defined as an exophthalmometric value > 19.3 mm, according to a study on normal values of Hertel exophthalmometry in a Chinese Han population [23].
2.4. Statistical analyses
All the statistical analyses and figure drawing were conducted using R version 4.3.0 (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as means (SDs) or medians (IQRs) depending on their distribution. Categorical variables were expressed as numbers (percentages). Differences in continuous variables between groups were analyzed using the Student's t-test or Wilcoxon rank sum test depending on their distribution. Differences in categorical variables were analyzed using the χ2 test or Fisher's exact test depending on expected frequencies. All p values were two-sided, and p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patient demographics and clinical characteristics at baseline
At baseline, the mean age of the patients included was 48.3 (9.59) years, with an equal number of women and men. 9 (32.1 %) were ex- or current smokers. The median duration of GO was 8.00 (6.50–12.0) months and median cumulative dose of glucocorticoids was 4.98 (1.90) g. 10 (62.5 %), 17 (81.0 %), 20 (95.2 %), and 7 (38.9 %) of the patients had proptosis, diplopia, duction restriction, and visual dysfunction at baseline, respectively. 8 (28.6 %) patients had DON and 1 (3.57 %) had corneal breakdown. 3 (10.7 %) patients had history of orbital decompression surgery before RTX injection, of whom 2 had visual acuity ≥0.8 in all 4 eyes and no signs of DON assessed via MRI at baseline, while the other patient did not have restored visual function after surgery (details are provided in the “Outcomes of patients with DON at baseline” section). 6 (21.4 %), 3 (10.7 %), and 19 (67.9 %) patients had hyper-, hypo-, and euthyroidism at baseline, respectively. Other clinical characteristics, such as thyroid diseases, other treatments, biochemical markers, and comorbidities are shown in Table 1.
Table 1.
Demographics and clinical characteristics of patients (all patients, responders, and non-responders) at baseline.
| All | Non-responders | Responders | p | |
|---|---|---|---|---|
| Demographics: | ||||
| Age, years - mean (SD) | 48.3 (9.59) | 42.6 (8.65) | 49.5 (9.51) | 0.160 |
| Female sex - n (%) | 14 (50.0) | 2 (40.0) | 12 (52.2) | 1.000 |
| Smoking history - n (%) | 9 (32.1) | 2 (40.0) | 7 (30.4) | 1.000 |
| GO characteristics: | ||||
| Duration of GO, months - median (IQR) | 8.00 (6.50–12.0) | 8.00 (6.00–11.0) | 8.20 (7.00–12.0) | 0.551 |
| GC cumulative dose, g- mean (SD) | 4.98 (1.90) | 4.50 (0.00) | 5.08 (2.08) | 0.244 |
| 7-item CAS - median (IQR) | 4.0 (3.0–5.0) | 3.0 (2.0a–4.0) | 4.0 (3.0–5.0) | 0.196 |
| Proptosis - n (%) | 10 (62.5) | 2 (100) | 8 (57.1) | 0.500 |
| Exophthalmometric value, mm- median (IQR) | 22.0 (18.8–22.8) | 23.0 (23.0–23.0) | 21.5 (18.6–22.4) | 0.352 |
| Diplopia - n (%) | 17 (81.0) | 4 (100) | 13 (76.5) | 0.546 |
| Duction restriction - n (%) | 21 (95.5) | 3 (100) | 18 (94.7) | 1.000 |
| Visual dysfunction - n (%) | 7 (38.9) | 0 | 7 (41.2) | 1.000 |
| Visual acuity- median (IQR) | 0.70 (0.45–0.88) | 0.70 (0.70–0.70) | 0.70 (0.45–0.90) | 1.000 |
| IOP, mmHg - mean (SD) | 23.3 (6.72) | 19.1 (0.18) | 23.8 (6.96) | 0.013 |
| DON - n (%) | 8 (28.6) | 1 (20.0) | 7 (30.4) | 1.000 |
| Corneal ulcer - n (%) | 1 (3.57) | 0 | 1 (4.35) | 1.000 |
| Thyroid disease - n (%): | ||||
| Graves' disease | 27 (96.4) | 4 (80.0) | 23 (100) | 0.179 |
| Previous radioiodine | 5 (17.9) | 1 (20.0) | 4 (17.4) | 1.000 |
| Hashimoto's disease | 11 (39.3) | 4 (80.0) | 7 (30.4) | 0.062 |
| Thyroid function - n (%) | 0.586 | |||
| Hyperthyroidism | 6 (21.4) | 2 (40.0) | 4 (17.4) | |
| Hypothyroidism | 3 (10.7) | 0 | 3 (13.0) | |
| Euthyroidism | 19 (67.9) | 3 (60.0) | 16 (69.6) | |
| Other treatments - n (%): | ||||
| Methimazole | 18 (64.3) | 3 (60.0) | 15 (65.2) | 1.000 |
| Levothyroxine | 1 (3.57) | 0 | 1 (4.35) | 1.000 |
| Orbital decompression | 3 (10.7) | 0 | 3 (13.0) | 1.000 |
| Mycophenolate Mofetil | 4 (14.3) | 0 | 4 (17.4) | 1.000 |
| Biochemical markers: | ||||
| TSH, μIU/ml - median (IQR) | 1.00 (0.37–2.73) | 0.38 (0.00–0.44) | 1.29 (0.44–2.97) | 0.072 |
| FT4, ng/L - median (IQR) | 13.2 (10.3–16.1) | 17.7 (14.1–19.9) | 13.1 (10.2–15.1) | 0.082 |
| FT3, pg/ml - median (IQR) | 3.41 (2.72–5.16) | 6.01 (3.88–8.22) | 3.29 (2.62–3.65) | 0.039 |
| A-TG, IU/ml - median (IQR) | 26.9 (16.5–32.5) | 17.8 (14.1–35.0) | 27.3 (19.6–31.5) | 0.943 |
| A-TPO, IU/ml - median (IQR) | 23.8 (11.5–84.3) | 75.5 (40.9–84.2) | 20.1 (11.3–94.5) | 0.406 |
| TG, μg/L- median (IQR) | 54.8 (29.0–110) | 101 (8.82–111) | 53.4 (30.6–91.4) | 0.783 |
| TRAb, IU/L- median (IQR) | 7.15 (3.53–17.0) | 10.5 (6.85–20.4) | 7.03 (3.21–17.0) | 0.356 |
| NLR - median (IQR) | 2.31 (1.81–3.44) | 2.34 (1.97–2.87) | 2.29 (1.59–3.45) | 0.928 |
| IgG4, g/L - mean (SD) | 0.78 (0.57) | 0.54 (0.19) | 0.82 (0.62) | 0.266 |
| IgG, g/L - mean (SD) | 10.5 (3.45) | 14.5 (2.45) | 9.77 (3.14) | 0.162 |
| IgG4 to IgG ratio - mean (SD) | 0.07 (0.05) | 0.04 (0.01) | 0.08 (0.05) | 0.021 |
| Comorbidities - n (%): | ||||
| Thyrotoxic heart disease | 2 (7.14) | 1 (20.0) | 1 (4.35) | 0.331 |
| Hypertension | 5 (17.9) | 2 (40.0) | 3 (13.0) | 0.207 |
| Diabetes or IGR | 6 (21.4) | 0 | 6 (26.1) | 0.553 |
| Fatty liver | 5 (17.9) | 2 (40.0) | 3 (13.0) | 0.207 |
GO, Graves' orbitopathy; GC, glucocorticoids; IOP, intraocular pressure; CAS, clinical activity score; TSH, thyroid stimulating hormones; FT4, free thyroxine; FT3, free triiodothyronine; A-TG, anti-thyroglobulin antibody; A-TPO, anti-thyroid peroxidase antibody; TG, thyroglobulin; TRAb, thyrotropin receptor antibody; NLR, neutrophil to lymphocyte ratio; DON, dysthyroid optic neuropathy; IGR, impaired glucose regulation.
7-item CAS at baseline was <3 in some patients because they had 10-item CAS ≥4 and were also considered with active GO.
3.2. Primary and secondary outcomes of all patients
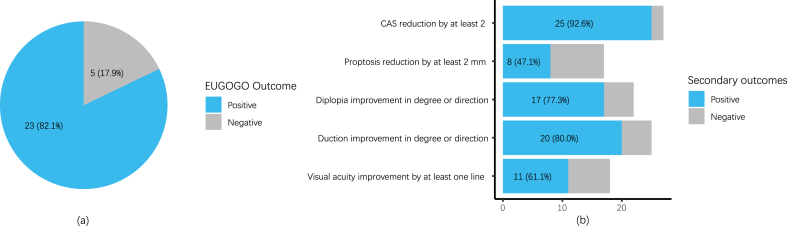
The median follow-up time of the patients was 22 (8–34) weeks. Of all patients included, 23 (82.1 %) had a positive outcome using the EUGOGO-suggested objective composite index [4] after 500 mg RTX treatment. 25 (92.6 %), 8 (47.1 %), 17 (77.3 %), 20 (80.0 %), and 11 (61.1 %) had 7-item CAS reduction by at least 2, proptosis reduction by at least 2 mm, diplopia improvement in degree or direction, duction improvement in degree or direction, and visual acuity improvement by at least one line, respectively (Fig. 2). Compared to those at baseline, the proportions of diplopia, and visual dysfunction, CAS, mean IOP of OU, and mean T2 relaxation time (T2RT) of the involved EOMs showed significant improvements at the last follow-up (p < 0.05). No significant differences were detected between the proportions of duction restriction and proptosis, exophthalmometric value, visual acuity, TRAb, or mean cross-sectional area (CSA) of involved EOMs at baseline and at the last follow-up (Table 2).
Fig. 2.
Primary and major secondary outcomes of all patients.
(a) Primary outcome as suggested by 2021 EUGOGO guidelines; (b) major secondary outcomes.
EUGOGO, European Group on Graves' Orbitopathy.
Data are expressed as numbers (percentages).
Table 2.
GO characteristics of all patients at baseline and at the last follow-up.
| Baseline | Last follow-up | p | |
|---|---|---|---|
| 7-item CAS - median (IQR) | 4.0 (3.0–5.0) | 1.0 (0.0–2.0) | <0.001 |
| Diplopia - n (%) | 17 (81.0) | 10 (47.6) | 0.023 |
| Duction restriction - n (%) | 20 (95.2) | 18 (85.7) | 0.480 |
| Proptosis - n (%) | 10 (62.5) | 6 (37.5) | 0.134 |
| Exophthalmometric value, mm- median (IQR) | 22.0 (18.8–22.8) | 19.5 (17.8–22.8) | 0.540 |
| Visual dysfunction - n (%) | 7 (38.9) | 3 (16.7) | 0.043 |
| Visual acuity - median (IQR) | 0.70 (0.45–0.88) | 0.80 (0.61–0.90) | 0.141 |
| IOP, mmHg - mean (SD) | 23.2 (7.86) | 19.0 (2.97) | 0.018 |
| TRAb, IU/L- median (IQR) | 7.05 (2.92–20.4) | 2.57 (1.84–4.07) | 0.094 |
| Involved EOM T2RT, ms - mean (SD) | 87.8 (8.64) | 75.8 (10.9) | 0.010 |
| Involved EOM CSA, mm2 - mean (SD) | 82.8 (28.5) | 72.9 (24.0) | 0.388 |
GO, Graves' orbitopathy; CAS, clinical activity score; IOP, intraocular pressure; TRAb, thyrotropin receptor antibody; EOM, extraocular muscle; T2RT, T2 relaxation time; CSA, cross-sectional area.
No serious side effects, including cytokine release syndrome, were observed in the patients’ electronic medical history. Only 1 patient experienced diarrhea after RTX, which lasted for one day. However, 2 patients who underwent orbital decompression surgery after RTX were reported, of whom 1 patient already had symptom- and MRI-confirmed DON at baseline, suggesting a poor response to RTX; whereas the other patient had no signs of DON at baseline, suggesting deterioration. The latter patient was a 40-year-old female patient with history of radioiodine treatment. No other patient experienced deterioration in ophthalmological parameters or relapse before the last follow-up.
3.3. Baseline factors contributing to positive EUGOGO outcomes
Compared to patients who did not respond positively to the treatment, those with positive responses had significantly greater mean IOP of OU, while lower FT3 and IgG4 to IgG ratio (p < 0.05). The two groups of patients did not differ significantly in other baseline characteristics (Table 1).
3.4. Outcomes of patients with DON
A total of 8 patients had DON at baseline. 4 (66.7 %) out of 6 patients with available data on visual function improved by at least one Snellen line. 8 (100 %) patients had a CAS reduction by at least 2. Visual acuity, CAS, and IOP went from 0.45 (0.32–0.75), 3.50 (3.00–5.25), and 24.2 (6.10) mmHg at baseline to 0.90 (0.55–0.90), 0.50 (0.00–1.25), and 18.6 (3.48) mmHg at the last follow-up, respectively. Other outcomes are as listed in Table 3, Table 4. However, due to the small sample size, statistical significance was not assessed. These data should be interpreted with caution. Except that visual acuity of one eye mildly decreased from 1.0 to 0.8, no deterioration in visual acuity or other parameters was observed in these patients at the last follow-up.
Table 3.
Outcomes of patients with active moderate-to-severe GO and DON.
| Active moderate-to-severe GO | DON | |
|---|---|---|
| Visual acuity improvement by at least one line | 7 (58.3) | 4 (66.7) |
| EUGOGO outcome | 16 (80.0) | 7 (87.5) |
| CAS reduction by at least 2 | 17 (89.5) | 8 (100) |
| Proptosis reduction by at least 2 mm | 4 (33.3) | 4 (80.0) |
| Diplopia improvement in degree or direction | 13 (86.7) | 4 (57.1) |
| Duction improvement in degree or direction | 15 (83.3) | 5 (71.4) |
EUGOGO, European Group on Graves' Orbitopathy.
Data are expressed as n (%).
Table 4.
GO characteristics of patients with DON at baseline and at the last follow-up.
| Baseline | Last follow-up | |
|---|---|---|
| Visual acuity - median (IQR) | 0.45 (0.32–0.75) | 0.90 (0.55–0.90) |
| Visual dysfunction – n (%) | 3 (60.0) | 1 (20.0) |
| CAS - median (IQR) | 3.50 (3.00–5.25) | 0.50 (0.00–1.25) |
| Diplopia - n (%) | 3 (50.0) | 1 (16.7) |
| Duction restriction - n (%) | 6 (85.7) | 5 (71.4) |
| Proptosis - n (%) | 3 (60.0) | 1 (20.0) |
| Exophthalmometric value, mm - median (IQR) | 22.0 (20.0–22.0) | 18.5 (17.5–19.0) |
| IOP, mmHg - mean (SD) | 24.2 (6.10) | 18.6 (3.48) |
| TRAb, IU/L - median (IQR) | 3.50 (2.82–21.8) | 1.86 (1.33–2.21) |
| Involved EOM T2RT, ms - mean (SD) | 89.8 (7.47) | 78.2 (8.67) |
| Involved EOM CSA, mm2 - mean (SD) | 69.6 (18.6) | 67.3 (26.7) |
GO, Graves' orbitopathy; CAS, clinical activity score; IOP, intraocular pressure; TRAb, thyrotropin receptor antibody; EOM, extraocular muscle; T2RT, T2 relaxation time; CSA, cross-sectional area.
P values were not shown due to small sample sizes.
It is noteworthy that 1 of the patients with DON underwent orbital decompression surgery on the left and right eyes 7 and 6 weeks before RTX, respectively. However, after the surgery, the visual acuity of this patient remained at 0.1 OU and CAS remained at 6, and then RTX was given. At the last follow-up, visual acuity of this patient's right and left eyes was 1.0 and 0.8, respectively; IOP of the right and left eyes improved from 31.0 to 18.0 and from 23.5 to 16.0 mmHg, respectively; CAS reduced from 6 to 3 and the EUGOGO outcome was positive. The outcomes of DON patients who did and did not undergo surgery were shown in Supplementary Table 1. However, statistical analysis of the difference between the two groups could not be conducted since there was only one DON patient who underwent surgery.
3.5. Sensitivity analyses
Due to the potential heterogeneity among patients with active moderate-to-severe GO and DON, we repeated the analyses in the 20 patients with active moderate-to-severe GO (Table 3 & Supplementary Table 2). Due to the lack of long-term follow-up data for some patients, we repeated the analyses in the 17 patients whose follow-up time >12 weeks (Supplementary Tables 3 and 4). The two sets of sensitivity analyses produced results similar to those of the main analyses.
4. Discussion
The current study is, to the best of our knowledge, the first to investigate the efficacy of 500 mg RTX as a second-line treatment for GC-resistant GO in China. In our retrospective study, 23 (82.1 %) patients had a positive response using the 2021 EUGOGO guideline-recommended objective outcome [4]. GO inactivation was demonstrated in 25 (92.6 %) patients by a CAS reduction by at least 2 and objectively confirmed by a significant decrease in the mean T2RTs of the involved EOMs detected by MRI T2 mapping. In terms of ophthalmological parameters, we noted improvements in diplopia, visual dysfunction, and IOP, but not in proptosis or MRI-determined EOM CSA. Exclusively, we identified that a higher IgG4-to-IgG ratio, along with higher IOP and lower FT3 at baseline were predictors for RTX-induced positive EUGOGO responses. As for patients with DON, RTX led to overall inactivation with improvement in visual acuity and other GO parameters in some patients, with no signs of deterioration. 1 of these patients received both RTX and orbital decompression surgery within 2 months and exhibited improvement in visual acuity and clinical activity.
Ever since a pilot study in 2006 [24], research into the role of RTX in GO treatment has been plentiful, and the most well-known of these studies are the two randomized controlled trials by Salvi et al. and Stan et al. [18,25]. Currently RTX appears in guidelines as one of the second-line therapies for GO, with an emphasis on its inactivation effect [4,5]. However, much of the existing evidence comes from studies involving patients who were given RTX as first-line treatment. In effect, the number of RTX studies focusing on steroid-resistant GO is limited [[12], [13], [14],16,26], with two being case series of fewer than 10 patients. These studies generally agreed on the effect of RTX on GO inactivation, which is in line with our study. Nevertheless, analyses on the effects of RTX on ophthalmological parameters of GO, including proptosis, eye motility, and visual acuity, produced inconsistent results. Bennedjaï et al. saw no improvement in proptosis, diplopia, or visual acuity [12]. Du Pasquier-Fediaevsky et al. reported no improvement in proptosis or diplopia without stressing the changes in visual acuity [13]. Deltour et al. reported improvement in visual dysfunction, similar to our study, while no significant change in eye motility [14]. In contrast to these studies, our results showed significant improvement in diplopia rates, which is in accordance with the RCT by Salvi et al. [18]. A possible explanation for the divergence in eye motility improvement might be the shorter mean duration of GO in the latter two studies than in the former three (11.2 (8.09) and 4.5 (2.9) vs 27 (1.9), 17.1 (17.8), and 19.1 (27) months).
Another factor that might have affected the efficacy of RTX treatment is the dose of RTX. Our study used a dose of 500 mg, as is deemed optimal by the ATA-ETA guidelines [5], while two of the studies mentioned above prescribed lower doses (200 mg and 100–400 mg) to steroid-resistant patients [12,13], which had a compromised effect on diplopia as discussed above. However, whether this inconsistency results from their lower dose or longer GO duration is unclear. Moreover, several studies applied doses as low as 100 mg [[27], [28], [29]], all of which reported B-cell depletion and effective disease inactivation. However, the outcomes of ophthalmological parameters were not comparable due to the different evaluation criteria applied. In addition, the study populations of none of these three studies were restricted to steroid-resistant GO patients, limiting the generalization of their conclusions to this specific patient subgroup. Moreover, although a dose of 100 mg RTX was shown to adequately deplete B-cells, higher doses of RTX were reported to prolong B cell depletion [18]. Whether steroid-resistant GO patients can further benefit from higher doses of RTX is not clear and more comparative studies using same evaluation criteria are required.
RTX was associated with various adverse effects seemingly in a dose-independent manner: in some studies, a dose of 100 mg was followed by major adverse effects such as cytokine release syndrome and DON [18,27] which resembled the adverse effects of doses as high as 2000 mg [14,25]; while in other studies, doses of 500 mg or lower were only associated with minor side effects [13,28,29]. In rheumatoid arthritis and multiple sclerosis populations, repeated doses of 1000 mg or 500 mg RTX were proven to be safe [30,31]. In our study, we only recorded short-lasting diarrhea in one patient, probably due to the anti-allergic and -infusion reaction measures that we used. Although EUGOGO recommends RTX doses of 500 mg or 100 mg for second-line GO treatment, there seems to be no established safety range for RTX. The safety profile of RTX in GO treatment remains elusive.
We identified a higher baseline IgG4-to-IgG ratio as a predictor of RTX-induced positive EUGOGO outcomes. IgG4-related thyroid diseases have been proposed as a distinct entity in the thyroid disease spectrum [32]. Our team previously found that this subgroup of GO patients with elevated serum IgG4 levels had a more severe form of GO but a better response to GC treatment than did those with normal IgG4 levels [19]. Other studies reported that RTX is an effective treatment for IgG4-related diseases, even without concomitant GC therapy [33]. Our study further suggested that RTX might be more effective for treating IgG4-elevated GO, but further studies involving in this subgroup of patients with GO are warranted.
A lower baseline FT3 was also found to be a predictor of positive EUGOGO outcomes, suggesting that effective control of hyperthyroidism might be essential to GO prognosis, as is already suggested in EUGOGO guidelines [4,34]. The higher IOP in positive responders is in contrast to that reported by Deltour et al. [14], who reported greater IOP in non-responders. This might have resulted from their different alternative of the primary outcome compared to ours. Additionally, the significance of the difference in IOP between the responder and the non-responder groups in the Deltour et al. study was blunted in a multivariate analysis. An explanation for this finding is that higher baseline IOP saved room for improvement, which was reflected in positive outcomes. However, the underlying reason for elevated IOP in positive responders is unclear and should be further investigated.
Finally, we analyzed the efficacy of RTX in steroid-resistant patients with DON who either received no orbital decompression surgery for financial or physical reasons, or who responded poorly to the surgery. We showed that RTX induced inactivation in all patients, and improved visual acuity and IOP in part, with no obvious deterioration. Notably, the outcomes of the only DON patient who received both RTX and orbital decompression surgery were satisfactory in terms of both visual function and inactivation. Khanna et al. studied the efficacy of RTX in 4 DON patients and reported improved visual acuity in all 4 patients [16]. However, among these 4 patients, 1 underwent orbital decompression surgery before RTX treatment and the other received both surgery and orbital irradiation before RTX. Furthermore, a previous review showed that RTX treatment in 13 DON patients was successful in 11 according to Pelewicz-Sowa et al. [17]. However, the surgical history of these patients was inconsistent. The other 2 patients who failed to respond, 1 who used RTX as the first-line treatment and the other who used RTX after steroids experienced DON recurrence after improvement in visual acuity for 2–4 months. These authors disagreed with the use of RTX in the case of DON mainly due to conflicting results and adverse events (cytokine release syndrome in 9.1 % [17]). In summary, the efficacy of RTX in DON patients with or without orbital decompression surgery remains unclear. However, our results supported the effect of RTX on clinical activity and visual dysfunction, while adverse events or deterioration were not seen in our DON patients. Therefore, we believe that the role of RTX in DON treatment has yet to be determined, especially where orbital decompression is not available or successful.
The advantages of our study include the relatively larger sample size compared to other related studies on this rare disorder as well as the in-depth examinations, such as IgG4 measurements and MRI T2 mapping. However, the limitations are evident. First, our study was not prospective, which limits the integrity and accuracy of follow-up data, including important parameters such as lid apertures and subjective questionnaires; second, as the activity and severity of GO could be relieved in its natural history [35], we were unable to rule out the effects of the natural history of GO without a control group.
5. Conclusions
In summary, a single dose of 500 mg RTX seemed to show noticeable efficacy, notably in diplopia, with acceptable tolerance as second-line treatment for steroid-resistant GO, which appears to be a clinically tricky condition. This efficacy seemed compromised in studies including patients with longer durations, calling for early detection and decision-making in this subpopulation of patients with GO. However, larger-scale studies with a control group are needed to reach a more solid conclusion. Additionally, the role of RTX in steroid-resistant DON management where surgery is unavailable or ineffective is worthy of further discussion.
Ethics declarations
The China Ethics Committee of Registering Clinical Trials approved the study protocol (ChiECRCT- 20170087). Informed consent was not required for this study because personal information of the patients included was not retrieved.
Consent for publication
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82270855 G Y).
Data availability statement
Data associated with this study have not been deposited into a publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Ziyin Zhang: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Xiaohui Feng: Data curation. Yaoyao Guo: Data curation. Xiaonan Kang: Data curation. Dan Wang: Data curation. Jing Zhang: Resources, Data curation. Zhixuan Zeng: Writing – review & editing, Supervision, Conceptualization. Gang Yuan: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all patients for their cooperation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31932.
Contributor Information
Zhixuan Zeng, Email: 985801524@qq.com.
Gang Yuan, Email: gangyuan@tjh.tjmu.edu.cn.
List of abbreviations
- GO
Graves' orbitopathy
- GC
glucocorticoids
- IOP
intraocular pressure
- OU
Oculus Uterque
- CAS
clinical activity score
- TSH
thyroid stimulating hormone
- FT4
free thyroxine
- FT3
free triiodothyronine
- A-TG
anti-thyroglobulin antibody
- A-TPO
anti-thyroid peroxidase antibody
- TG
thyroglobulin
- TRAb
thyrotropin receptor antibody
- NLR
neutrophil to lymphocyte ratio
- DON
dysthyroid optic neuropathy
- IGR
impaired glucose regulation
- EUGOGO
European Group on Graves' Orbitopathy
- EOM
extraocular muscle
- T2RT
T2 relaxation time
- CSA
cross-sectional area
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bartalena L., Piantanida E., Gallo D., Lai A., Tanda M.L. Epidemiology, natural history, risk factors, and prevention of Graves' orbitopathy. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.615993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahn R.S. Graves' ophthalmopathy. N. Engl. J. Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurberg P., Berman D.C., Bülow Pedersen I., Andersen S., Carlé A. Incidence and clinical presentation of moderate to severe Graves' orbitopathy in a Danish population before and after iodine fortification of salt. J. Clin. Endocrinol. Metabol. 2012;97:2325–2332. doi: 10.1210/jc.2012-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartalena L., Kahaly G.J., Baldeschi L., Dayan C.M., Eckstein A., Marcocci C., et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur. J. Endocrinol. 2021;185:G43–G67. doi: 10.1530/EJE-21-0479. [DOI] [PubMed] [Google Scholar]
- 5.Burch H.B., Perros P., Bednarczuk T., Cooper D.S., Dolman P.J., Leung A.M., et al. Management of thyroid eye disease: a consensus statement by the American thyroid association and the European thyroid association. Thyroid. 2022;32:1439–1470. doi: 10.1089/thy.2022.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartalena L., Krassas G.E., Wiersinga W., Marcocci C., Salvi M., Daumerie C., et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J. Clin. Endocrinol. Metabol. 2012;97:4454–4463. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 7.Marcocci C., Watt T., Altea M.A., Rasmussen A.K., Feldt-Rasmussen U., Orgiazzi J., et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves' orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur. J. Endocrinol. 2012;166:247–253. doi: 10.1530/EJE-11-0779. [DOI] [PubMed] [Google Scholar]
- 8.Reff M.E., Carner K., Chambers K.S., Chinn P.C., Leonard J.E., Raab R., et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 9.Eisenberg R., Looney R.J. The therapeutic potential of anti-CD20 ‘what do B-cells do? Clin. Immunol. 2005;117:207–213. doi: 10.1016/j.clim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Supronik J., Szelachowska M., Kretowski A., Siewko K. Rituximab in the treatment of Graves’ orbitopathy: latest updates and perspectives. Endocrine Connections. 2022;11 doi: 10.1530/EC-22-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Hu H., Chen L., Zhang H., Yang T., Xu X., et al. Observation study of using a small dose of rituximab treatment for thyroid-associated ophthalmopathy in seven Chinese patients: one pilot study. Front. Endocrinol. 2023;13 doi: 10.3389/fendo.2022.1079852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennedjaï A., Bouheraoua N., Gatfossé M., Dupasquier-Fediaevsky L., Errera M.-H., Tazartes M., et al. Tocilizumab versus rituximab in patients with moderate to severe steroid-resistant Graves' orbitopathy. Ocul. Immunol. Inflamm. 2022;30:500–505. doi: 10.1080/09273948.2020.1808688. [DOI] [PubMed] [Google Scholar]
- 13.Du Pasquier-Fediaevsky L., Andrei S., Berche M., Leenhardt L., Héron E., Rivière S. Low-dose rituximab for active moderate to severe Graves' orbitopathy resistant to conventional treatment. Ocul. Immunol. Inflamm. 2019;27:844–850. doi: 10.1080/09273948.2018.1453078. [DOI] [PubMed] [Google Scholar]
- 14.Deltour J.-B., d'Assigny Flamen M., Ladsous M., Giovansili L., Cariou B., Caron P., et al. Efficacy of rituximab in patients with Graves' orbitopathy: a retrospective multicenter nationwide study. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:2013–2021. doi: 10.1007/s00417-020-04651-6. [DOI] [PubMed] [Google Scholar]
- 15.Currò N., Covelli D., Vannucchi G., Campi I., Pirola G., Simonetta S., et al. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24:897–905. doi: 10.1089/thy.2013.0445. [DOI] [PubMed] [Google Scholar]
- 16.Khanna D., Chong K.K.L., Afifiyan N.F., Hwang C.J., Lee D.K., Garneau H.C., et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology. 2010;117:133–139.e2. doi: 10.1016/j.ophtha.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelewicz-Sowa M., Miśkiewicz P. Dysthyroid optic neuropathy: emerging treatment strategies. J. Endocrinol. Invest. 2023;46:1305–1316. doi: 10.1007/s40618-023-02036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvi M., Vannucchi G., Currò N., Campi I., Covelli D., Dazzi D., et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J. Clin. Endocrinol. Metabol. 2015;100:422–431. doi: 10.1210/jc.2014-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Luo B., Zhang J., Zhou X., Shao S., Xu W., et al. Clinical relevance of serum immunoglobulin G4 in glucocorticoid therapy of Graves' ophthalmopathy. Clin. Endocrinol. 2021;95:657–667. doi: 10.1111/cen.14493. [DOI] [PubMed] [Google Scholar]
- 20.Politi L.S., Godi C., Cammarata G., Ambrosi A., Iadanza A., Lanzi R., et al. Magnetic resonance imaging with diffusion-weighted imaging in the evaluation of thyroid-associated orbitopathy: getting below the tip of the iceberg. Eur. Radiol. 2014;24:1118–1126. doi: 10.1007/s00330-014-3103-3. [DOI] [PubMed] [Google Scholar]
- 21.The European Group on Graves’ Orbitopathy (EUGOGO) Wiersinga W.M., Perros P., Kahaly G.J., Mourits M.P., Baldeschi L., et al. Clinical assessment of patients with Graves' orbitopathy: the European Group on Graves' Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur. J. Endocrinol. 2006;155:387–389. doi: 10.1530/eje.1.02230. [DOI] [PubMed] [Google Scholar]
- 22.Perros P., Dayan C.M., Dickinson A.J., Ezra D., Estcourt S., Foley P., et al. Management of patients with Graves' orbitopathy: initial assessment, management outside specialised centres and referral pathways. Clin. Med. 2015;15:173–178. doi: 10.7861/clinmedicine.15-2-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D., Liu X., Wu D., Di X., Guan H., Shan Z., et al. Normal values of Hertel exophthalmometry in a Chinese han population from shenyang, Northeast China. Sci. Rep. 2015;5:8526. doi: 10.1038/srep08526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvi M., Vannucchi G., Campi I., Rossi S., Bonara P., Sbrozzi F., et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur. J. Endocrinol. 2006;154:511–517. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 25.Stan M.N., Garrity J.A., Carranza Leon B.G., Prabin T., Bradley E.A., Bahn R.S. Randomized controlled trial of rituximab in patients with Graves' orbitopathy. J. Clin. Endocrinol. Metabol. 2015;100:432–441. doi: 10.1210/jc.2014-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell A.L., Gan E.H., Morris M., Johnson K., Neoh C., Dickinson A.J., et al. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves' orbitopathy. Clin. Endocrinol. 2013;79:437–442. doi: 10.1111/cen.12141. [DOI] [PubMed] [Google Scholar]
- 27.Karasek D., Cibickova L., Karhanova M., Kalitova J., Schovanek J., Frysak Z. Clinical and immunological changes in patients with active moderate-to-severe Graves orbitopathy treated with very low-dose rituximab. Endokrynol. Pol. 2015 doi: 10.5603/EP.a2017.0040. VM/OJS/J/49066. [DOI] [PubMed] [Google Scholar]
- 28.Vannucchi G., Campi I., Covelli D., Currò N., Lazzaroni E., Palomba A., et al. Efficacy profile and safety of very low-dose rituximab in patients with Graves' orbitopathy. Thyroid. 2021;31:821–828. doi: 10.1089/thy.2020.0269. [DOI] [PubMed] [Google Scholar]
- 29.Insull E.A., Sipkova Z., David J., Turner H.E., Norris J.H. Early low‐dose rituximab for active thyroid eye disease: an effective and well‐tolerated treatment. Clin. Endocrinol. 2019 doi: 10.1111/cen.13970. cen. [DOI] [PubMed] [Google Scholar]
- 30.Van Vollenhoven R.F., Emery P., Bingham C.O., Keystone E.C., Fleischmann R.M., Furst D.E., et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 2013;72:1496–1502. doi: 10.1136/annrheumdis-2012-201956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torgauten H.M., Myhr K.-M., Wergeland S., Bø L., Aarseth J.H., Torkildsen Ø. Safety and efficacy of rituximab as first- and second line treatment in multiple sclerosis – a cohort study. Multiple Sclerosis Journal - Experimental, Translational and Clinical. 2021;7 doi: 10.1177/2055217320973049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotondi M., Carbone A., Coperchini F., Fonte R., Chiovato L. Diagnosis of endocrine disease: IgG4-related thyroid autoimmune disease. Eur. J. Endocrinol. 2019;180:R175–R183. doi: 10.1530/EJE-18-1024. [DOI] [PubMed] [Google Scholar]
- 33.Carruthers M.N., Topazian M.D., Khosroshahi A., Witzig T.E., Wallace Z.S., Hart P.A., et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann. Rheum. Dis. 2015;74:1171–1177. doi: 10.1136/annrheumdis-2014-206605. [DOI] [PubMed] [Google Scholar]
- 34.Prummel M.F., Wiersinga W.M., Mourits M.P., Koornneef L., Berghout A., van der Gaag R. Effect of abnormal thyroid function on the severity of Graves' ophthalmopathy. Arch. Intern. Med. 1990;150:1098–1101. [PubMed] [Google Scholar]
- 35.Piantanida E., Tanda M.L., Lai A., Sassi L., Bartalena L. Prevalence and natural history of Graves' orbitopathy in the XXI century. J. Endocrinol. Invest. 2013;36 doi: 10.3275/8937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study have not been deposited into a publicly available repository. Data will be made available on request.