Summary
Encounters with pathogens and other molecules can imprint long-lasting effects on our immune system, influencing future physiological outcomes. Given the wide range of microbes to which humans are exposed, their collective impact on health is not fully understood. To explore relations between exposures and biological aging and inflammation, we profiled an antibody-binding repertoire against 2,815 microbial, viral, and environmental peptides in a population cohort of 1,443 participants. Utilizing antibody-binding as a proxy for past exposures, we investigated their impact on biological aging, cell composition, and inflammation. Immune response against cytomegalovirus (CMV), rhinovirus, and gut bacteria relates with telomere length. Single-cell expression measurements identified an effect of CMV infection on the transcriptional landscape of subpopulations of CD8 and CD4 T-cells. This examination of the relationship between microbial exposures and biological aging and inflammation highlights a role for chronic infections (CMV and Epstein-Barr virus) and common pathogens (rhinoviruses and adenovirus C).
Subject areas: Immunology, Proteomics, Genomics
Graphical abstract
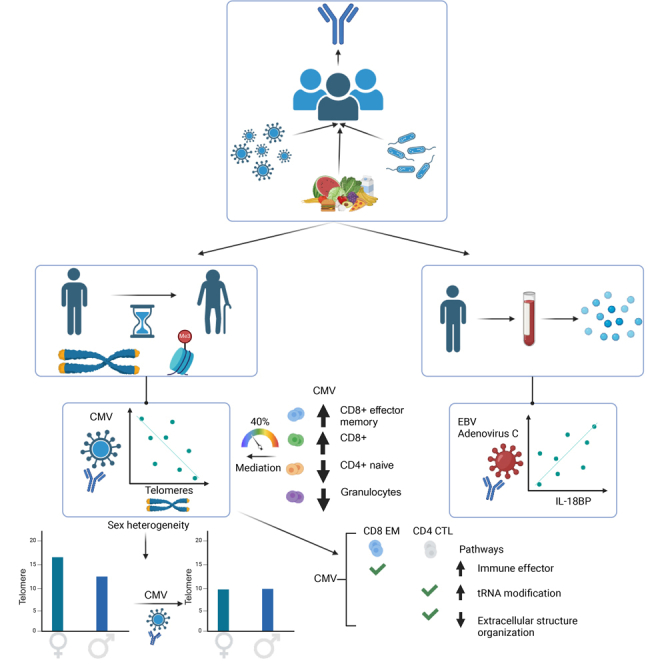
Highlights
-
•
Anti-CMV, rhinovirus, and gut microbial antibodies relate to telomere length
-
•
CMV associates to telomere length of CD45RA + CD57+ cells in a sex-dependent manner
-
•
CMV influences the expression of CD8+ T effector memory and cytotoxic CD4+ cells
-
•
Anti-EBV and anti-adenoviral responses associate with higher IL-18BP
Immunology; Proteomics; Genomics
Introduction
The dramatic increase in human lifespan over the last century has not been accompanied by a similarly rapid increase in health span, leading to a large social and economic burden due to the susceptibility of older people to disease. Aging is associated with an increased incidence of chronic conditions, such as cardiovascular diseases, cancer, diabetes, and neurodegenerative disorders.1,2 Immunosenescence—which represents a series of detrimental changes in the immune system3—is an accelerator of age-related diseases, with known hallmarks of reduced response to new antigens, accumulation of memory T-cells, and low-grade systemic inflammation that is termed “inflammaging”.4
The complex interplay between exposures and the human immune system plays a critical role in determining cellular homeostasis throughout human life. Both genetic and environmental factors influence the behavior of the immune system via molecular recognition and epigenetic imprinting.5 A wide range of exposures, including to environmental factors (e.g., pollen, pollution, and interaction with animals), lifestyle factors (e.g., smoking and diet), and microorganisms such as bacteria, fungi, and viruses, influence the function of the immune system and can have long-term effects on an individual’s health. Previous studies have emphasized the role of latent infections with herpesviruses in immunosenescence via establishment of a life-long persistent infection in the host that may reactivate under certain conditions.6 Common herpesviruses infections include herpes simplex viruses, Epstein-Barr virus (EBV), and human cytomegalovirus (CMV). The significance of these viruses for human health is further highlighted by work showing that presence of EBV antibodies elevates the risk of developing multiple sclerosis (MS), an autoimmune condition affecting the nervous system,7 and by a link between herpes zoster infection and development of Alzheimer’s disease.8 In addition, CMV has been associated to the immune risk profile in the elderly, with CMV associated with an inverted CD8/CD4 ratio, a hallmark of immunosenescence.9 Immunosenescence has also been linked to a reduction of telomere length (TL) in immune cells.10 Telomeres shorten with age, and abnormally short telomeres are hallmarks of premature aging syndromes.11 In the general population, variations in TL has been linked to higher risk of mortality and chronic disorders such as cardiovascular disease, Alzheimer’s disease, and cancer.12 Both genetic determinants13 and lifestyle factors, such as stress, diet, exercise,14 or exposures such as infections10 or smoking,15 have been related with TL variability in the population.
The humoral immune system, which includes antibodies selected to recognize a specific antigen, comprises memory B-cells that continue to produce antibodies even after an immune reaction has ended. Analyzing an individual’s total antibody repertoire can therefore provide insights into the past and current exposures that have triggered an immune response. This study aimed to investigate how such microbial exposures modify immune homeostasis and lead to aging-like features, such as becoming more proinflammatory, and how this is connected with markers of biological aging. To do so, we leveraged antibody epitope repertoires in the Dutch population cohort LifeLines-DEEP (LLD)16 that were profiled using two previously characterized Phage-display Immunoprecipitation Sequencing (PhIP-Seq) libraries.17,18,19 Using large-scale omics datasets available for the same cohort (n = 1,443), including cytokine concentrations, bulk- and single-cell transcriptomics, DNA methylation, blood metabolomics20 and aging biomarkers such as signal joint T cell receptor excision circles (sj-TRECS) and TLs from blood immune cell types,21 we explored the relationships between past exposures and biological aging and systemic inflammation biomarkers. This identified multiple downstream effects of past infections on biological aging, systemic immune responses, and the transcriptional activity of specific immune populations.
Results
TLs of immune cell types associate with specific antibody-bound peptides
To study previous and current exposures to commensal and pathogenic bacteria and viruses, we assessed the reactivity of serum antibodies against two previously developed libraries of phage-displayed peptide antigens. In a previous study in the Dutch LLD cohort19 PhIP-Seq was used to profile two antigen libraries. These libraries consisted of peptides derived from gut microbiota (both commensal species and common pathogens);17 epitopes from common dietary allergens, phages, and viruses; and all B-cell antigens deposited in the immune epitope database (IEDB).18 Extensive IgG antibody responses against both libraries were observed in the study population (n = 1,443),19 and we used the 2,815 responses frequently shared among participants (detectable in 5–95% of individuals) for further analysis19 [Table S1.1].
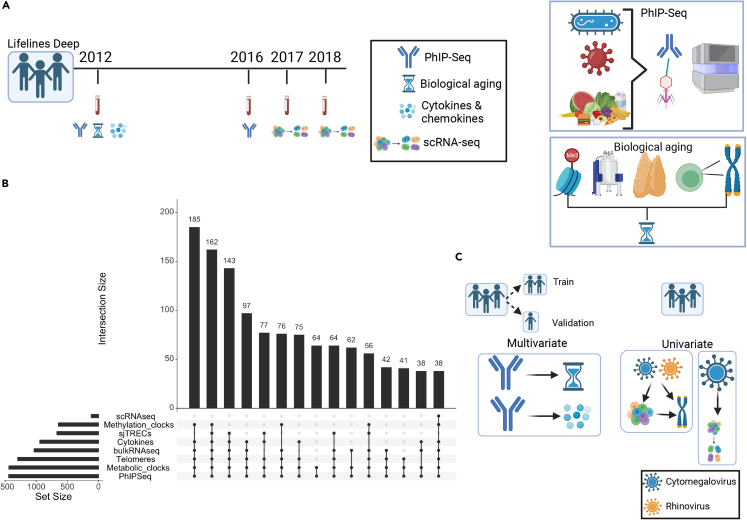
To investigate the relationship between previous and current exposures (which could not be separated in this data) and aging, we explored various subsets of participants using different available biomarker information [Figure 1A]. We explored four types of aging biomarkers. TLs of six different blood cell types were measured by Flow-FISH: granulocytes, lymphocytes, B-cells (CD45RA + CD20+), naive T-cells (CD45RA + CD20−), memory T-cells (CD45RA−) and NK-cells/fully differentiated T-cells (CD45RA + CD57+). DNA methylation age was estimated using Hannum,22 Weidner23 and Horvath24 methods. Metabolic age was calculated from the blood NMR metabolomics profile using two previously published methods: MetaboAge, which predicts chronological age25 and MetaboHealth, which predicts all-cause mortality risk.26 Thymic T cell maturation function was measured by the expression level of its by-products, sj-TRECS. The sample overlap for the different biomarkers is displayed in [Figure 1B]. Demographic summaries are presented in [Table S1.2].
Figure 1.
Data and methodological framework
(A) Data from 1,443 participants of the Dutch cohort Lifelines-DEEP were used to explore the relationship of past and chronic exposures, inflammation and aging. PhIP-Seq was used to profile immune responses against 2,815 peptides. Fourteen circulating cytokines were available for 939 participants for whom we had PhIP-Seq data. Telomere lengths from six blood cell types (n = 1,243), biological aging clocks (methylation clock (n = 641), metabolomic clock (n = 1,437)) and sj-TRECS (n = 633) were used to investigate aging. Bulk blood (n = 1,173) and peripheral blood mononuclear cell (PBMC) single-cell gene expression (n = 119) were also obtained.
(B) Upset plot of common data subsets showing the number of samples with overlapping data layers.
(C) Analysis framework. Participants were split into a training and replication set. Univariate and multivariate techniques were applied to relate exposures (PhIP-Seq) to the other omics-derived aging and immunological biomarkers. Using the whole population, we explored the relationship of cytomegalovirus and rhinoviruses with cell counts and telomere length. Cytomegalovirus, in particular, was related to single-cell gene expression changes measured in samples obtained 1–6 years after collection of blood for PhIP-Seq measurements.
In our previous study,19 we uncovered the widespread association of chronological age with antibody responses, with prevalence of 13% of antibody-bound peptides associated to chronological age. Among these peptides, pathogens showed a wide diversity of age-associated immune responses, including more herpesvirus prevalence with age: CMV (61 associated antibody-bound peptides), EBV (46 associations), and herpesvirus 1 (10 associations). By contrast, other pathogens such as Staphylococcus aureus (22 peptides), Streptococcus pneumoniae (14 peptides), and Influenza A (9 peptides) showed a decrease of prevalence with age. To assess the correlation between antibody repertoires and biomarkers of biological aging (including TLs, metabolic age, methylation age, and sj-TRECs) [Figure 1B], we first removed the variability attributed to chronological aging (see STAR Methods) and then employed a hierarchical approach, ‘High-sensitivity pattern discovery in large, paired multi-omic datasets’ (HAllA)27 that allows for the identification of blocks of highly correlated features while maintaining a low false discovery. We ran HAllA on a ‘training’ set of the data that encompassed 80% of the samples and then validated the significant associations on a separate ‘validation’ test-set of the remaining 20% of samples, applying partial correlations [Figure 1C].
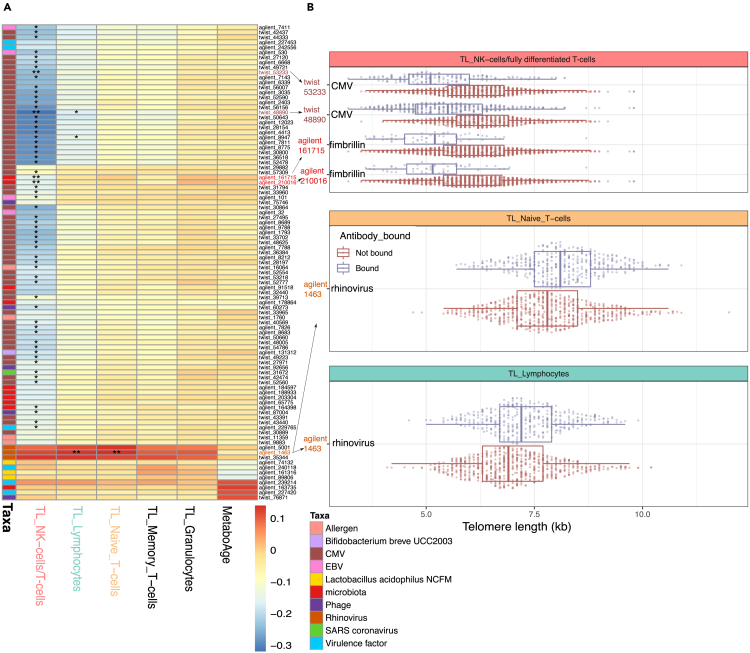
We observed many negative associations between TL and antibody-bound peptides from CMV, gut microbiome, bacteriophages, virulence factors, EBV, an antigen from severe acute respiratory syndrome (SARS) (present at low frequency in the population, ∼5%), and the probiotic strain Bifidobacterium breve (UCC2003) (complete table in Table S2.1). Many of these associations (61/84) were further observed in the validation set (Table S2.2). Of these negative associations, the strongest were between 66 (out of a total of 77 peptides) antibody-bound peptides from CMV and shorter TLs in NK-cells/fully differentiated T-cells and lymphocytes [Figure 2A]. CMV is a common pathogen present in approximately 50% of the Dutch population28 and is known to cause chronic unresolved infections. CMV infection was previously linked to substantial changes in blood cell composition and is considered a hallmark of immunosenescence.29
Figure 2.
Associations between antibody-bound peptides and aging biomarkers
(A) Heatmap showing correlation coefficients in the training dataset. Each row represents a peptide (colored according to taxonomic origin). Each column represents the telomere length (TL) of an immune cell type. ∗ indicates significance (p < 0.05) in both the testing and training datasets when controlling for age and sex using partial correlation. ∗∗ indicates significance (p < 0.05) in both the training and validation datasets with additional control for CMV prediction (see text).
(B) Boxplots display the associations that remained significant in both the training and validation datasets after accounting for CMV serostatus. X axis displays TL measurements determined using Flow-FISH for different cell populations. Y axis indicates the peptides where the association was found. Color indicates whether the PhIP-Seq results predicted antibody-binding against the peptide.
In contrast to these negative associations, antibody-bound peptides from rhinovirus were positively correlated with the TLs of lymphocytes, naive T-cells, and NK-cells/fully differentiated T-cells. Rhinoviruses are respiratory pathogens that are usually responsible for causing the common cold.
In the training set, we further identified negative associations between antibody-binding against peptides and metabolic age. This included peptides belonging to the probiotic strain Lactobacillus acidophilus and to Streptococcus. In addition, we found associations of peptides from virulence factors from Coprococcus and Mycoplasma pneumoniae and of Listeria phage with higher/older metabolic age. However, none of the associations to metabolic age were replicated in the validation set. No antibody responses were significantly associated to methylation age or sj-TRECs.
CMV antibodies tend to co-occur with multiple other antibody responses against bacteria and viruses.19 This signal seems to be largely independent of common motifs for which antibodies cross-react, and it might be related to CMV-seropositive individuals reacting against other non-CMV-related peptides. Thus, the large number of peptides that we observe to negatively correlate with TL (including B. breve and other microbes) might be confounded by a CMV signal. To account for the co-occurrence of antibodies against CMV with multiple other antibody responses,19 we conducted additional analyses by controlling for CMV serostatus. To determine the CMV serostatus of individuals, we trained a prediciton model for which we utilized PhIP-Seq data and leveraged serological results from a subset of participants in a different cohort for which PhIP-Seq information was available30 (see STAR Methods). As prediction of CMV serostatus using this model showed high accuracy (median fold accuracy of 0.966), we applied it to predict CMV serostatus in LLD samples. Using these data, we could test our hypothesis that CMV-seropositive individuals may have more antibody responses than CMV-seronegative individuals. We indeed observed that CMV-seropositive individuals had more antibody responses against non-CMV peptides than CMV-seronegative individuals (reaction against 20 more peptides on average, p = 5x10−3, association controlled for age, sex and sequencing plate ID).
After accounting for predicted CMV serostatus, we re-evaluated our training and validation associations [Figure 2A] (Table S2.2). This revealed two significant associations to shorter telomeres, present in both the train and test sets, of two overlapping peptides belonging to the Bacteroides fimbrillin family protein (known to constitute bacterial pili) and NK-cell/fully differentiated T-cells (CD45RA + CD57+) TL [Figure 2B]. This protein is important for bacterial adhesion to mucosa and is well known for its role in pathogenesis of bacteria such as P. gingivalis,31 although our results point to fimbrillin peptides from commensal microbiota. The two peptides are highly correlated with each other (phi = 0.82), and more often observed in (predicted) CMV-positive individuals (phi = 0.26–0.27). In contrast, antibodies against rhinoviral peptides exhibited a significant association with longer TL in naive T-cells and lymphocytes (as observed prior to CMV serostatus correction) [Figure 2B]. Additionally, even after adjustment for CMV serostatus, two associations with CMV peptides remained significant (peptides with IDs twist_48890 and twist_53233) [Figure 2B]. These peptides belonged to two distinct epitopes from CMV’s ‘phosphoprotein 150 and Large structural phosphoprotein’. There are 35 peptides belonging to those proteins in the library used. The antibody-bound peptide twist_48890 was highly correlated with CMV serostatus prediction (phi = 0.93, 96% of predicted CMV-seropositive individuals had antibody responses against this peptide) and showed a stronger association with TL than CMV serostatus prediction (95% CI effect: twist_48890 [-0.86, −0.62], CMV prediction [-0.83, −0.58]). On the other hand, twist_53233 was less strongly correlated with CMV infection prediction (phi = 0.58, 46% of CMV-seropositive individuals had antibody responses against this peptide) and showed independent effects on TL (multivariable model, pCMV_prediction = 2.63x10−11, ptwist_53233 = 2.21x10−5). This suggests that not all individuals with CMV infection develop an immune reaction against these specific peptides, but those who do tend to have shorter telomeres. This may be related to longer infection time, stronger reactivity to CMV, different CMV variants, or human genetic variability.
CMV serostatus relates to NK-cell/fully differentiated T cell TL and shows sex-specific effects
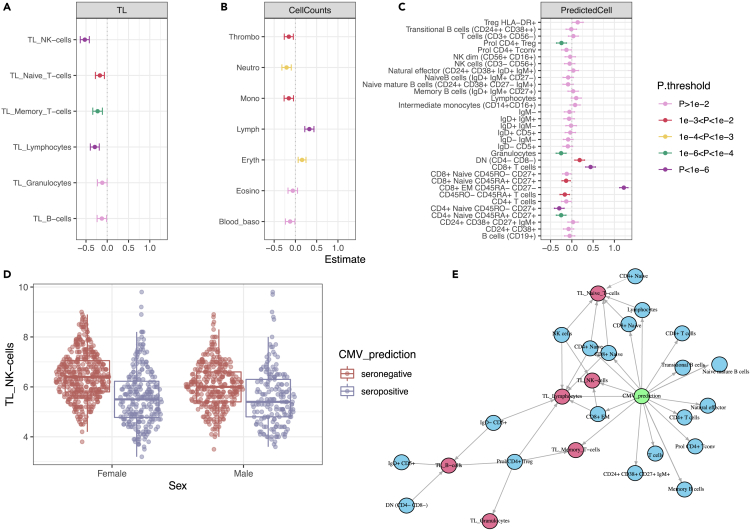
To follow-up the association between antibody-bound peptides from CMV and biological aging, we used the predicted CMV serostatus (rather than the individual antibody-bound peptides) to perform univariate association with TL and other aging markers [Table S3A]. By utilizing the CMV serostatus prediction, we confirmed our previous observation of an association of antibody-bound peptides of CMV with TL [Figure 3A] and a lack of significant associations with other biological aging biomarkers. NK-cells/fully differentiated T-cells (CD45RA + CD57+) had the strongest association with CMV (ordinary least squares (ols), effect = −0.52, p = 3.09x10−22), but we also observed significant associations in naive (ols, effect = −0.17, p = 1.1x10−3) and memory T-cells (ols, effect = −0.22, p = 6.4x10−5) and lymphocytes (ols, effect = −0.29, p = 2.37x10−8). T-cells and NK-cells are known to play an important role in controlling CMV infection.32 Some specific NK-cell populations, such as CD57+NKG2Chi-expressing populations or terminally differentiated CD56dimCD16− cells,33 are known to expand in people with CMV infection. These expanded populations show memory-like features,34 which could potentially lead to a decrease in the average TL of the NK-cell population, providing a potential explanation for the association we observe. On the other hand, senescent T-cells also tend to accumulate with CMV infections.35
Figure 3.
Associations of CMV prediction with cell counts and telomere length
(A–C), Linear association of CMV prediction (binary) with (A) telomere length (TL), (B) measured blood cell types, and (C) RNA-seq deconvoluted predicted cell counts. Estimated effect values, accounting for age and sex, are displayed. Error bars represent the 95% confidence interval of the estimated effect.
(D) TL differences between men and women in the NK-cell/fully differentiated T cell population. CMV interacts significantly with sex.
(E) Mediation network. The CMV infection effect (green circle) on TL (red circles) is partially mediated by changes in predicted cell counts (blue circles). Effects are estimated using lasso regression. Edges represent absolute effect sizes above 0.01.
In addition, we observed that CMV status acts as a confounding factor for correlations between different biological aging biomarkers. In particular, the correlation between the TL of NK-cells/fully differentiated T-cells and all other cell types becomes decoupled in CMV-seropositive individuals [Table S3B], as the TLs of NK-cells/fully differentiated T-cells (CD45RA + CD57+) of CMV-seropositive individuals are the most reduced among all the TLs.
Furthermore, in a subsequent CMV-sex interaction analysis, we observed a significant decrease of TL in NK-cells/fully differentiated T-cells of women infected with CMV as compared to men (ols, effect = −0.28, p = 7.6x10−3) [Table S3C]. This indicates that, on average, CMV infection tends to have a greater impact on TL in women compared to men [Figure 3D]. Previous studies in this cohort have shown differences in NK-cell/fully differentiated T cell TL between men and women (mean difference in women = 0.2, p = 3.45x10−4).21 However, when stratified by CMV infection, the sex differences in TL of CMV-infected individuals are no longer significant (mean difference in infected individuals = 0.018, p = 0.864; mean difference in non-infected individuals = 0.346, p = 3.14x10−8). This observation could not be attributed to the differences in CMV seropositivity prevalence between the sexes as CMV seroprevalence was similar in males and females (Chi-squared test, p = 0.61). In conclusion, while telomeres in women are significantly longer than in men overall, this difference is not significant in CMV-infected individuals due to a larger decrease of TLs in CMV-positive women compared to CMV-positive men.
It was previously reported that women exhibit stronger reactivity against CMV, characterized by increased cytokine secretion,36 and that immune responses against pathogens are, in general, stronger in females.37 This could potentially be linked to a more pronounced reduction in TL among women. To explore this hypothesis, we tested for sex-specific CMV effects in 14 circulating cytokine concentrations; however we did not identify significant associations (FDR1000permutations>0.05) [Table S3D].
To further investigate sex-specific differences related to CMV infection, we examined the antibody titers for CMV peptides by comparing the normalized read counts of immuno-precipitated CMV peptides (as a proxy for antibody titers, see STAR Methods) from CMV-seropositive men and women. Here we observed that women had, on average, a higher number of reads for all CMV peptides (76/77 peptides with higher numbers in women, 45/77 under FDR<0.05) [Table S3E], suggesting a stronger adaptive immune response to CMV. In addition, we observed a similar, albeit not significant, trend in an independent cohort with CMV serology (300BCG, described in STAR Methods) (effectwomen on CMV titers (IU/mL) = 1.08, p = 0.278).
In the same independent 300BCG cohort, we used qPCR measurements of average blood cell TLs to replicate our association with CMV infection. Despite observing an average decrease of TL in individuals with CMV-positive ELISA, this difference did not show strong statistical support (effect CMV seropositive on TL = −0.69, p = 0.3). Sex-specific effects of CMV on TL were also not observed in the 300BCG dataset (interaction effect in women = −0.05, p = 0.96). These results might be related to the cell-population-specific nature of CMV–TL changes: when averaged across different blood cell populations, as is often done through qPCR measurements, the effect that could be observed in NK-cells/fully differentiated T-cells (CD45RA + CD57+) might be masked.
In addition to the associations with CMV, we identified a positive association between anti-rhinoviral responses and TL. While there is a higher anti-rhinoviral prevalence in younger participants,38,39 the positive relationship between rhinovirus and TLs could be replicated in age-matched rhinovirus positive and negative participants (multivariable model with age, CMV and twist_35344 on overall TL, age-matched, effecttwist_35344 = 0.21, ptwist_35344 = 2.5x10−4). Additionally, we observed that having a greater breadth of positive anti-rhinoviral peptides was more strongly associated with longer TL than with individual peptide reactivity [Table S4].
Blood cell-type composition is associated with CMV and partially explains the TL changes
Next, we investigated the association between predicted CMV status and various blood cell types from 1,408 participants [Table S3A]. Using clinical measurements of major cell types in these individuals, we found a strong positive association between CMV infection and lymphocytes (ols, effect = 0.32, p = 1.77x10−9), as well as negative associations with neutrophils (effect = −0.208, p = 1.6x10−4) and erythrocytes (effect = 0.156, p = 7.42x10−4) [Figure 3B]. To further explore the specific lymphocyte groups affected by CMV infection, we used deconvoluted cell proportions derived from bulk-blood RNA-seq data in 1,173 participants [Figure 3C]. This deconvoluted dataset includes estimated cell proportions for 32 cell types, to which we applied a log-ratio transformation to account for cell proportion interdependencies introduced by data compositionality. Here we observed strong positive associations between CMV infection and CD8+ TEM CD45RA-CD27− (effect = 1.22, p = 5.66x10−110) and CD8+ T-cells (effect = 0.44, p = 7.38x10−14) and negative associations with the naive subtypes CD45RO-CD27+ (effect = −0.295, p = 2.45x10−7) and proliferative CD4+ T-reg (effect = −0.243, p = 3.73x10−5), as well as with granulocytes (effect = −0.252, p = 1.9x10−5). Positive associations with memory cells and negative associations with naive cell types are generally well-established patterns in CMV infection.40,41,42
Since blood cell type composition might be related to average TLs if the cell populations have heterogeneous TLs, we examined whether the observed association between CMV and TL could be explained by changes in the composition of blood cell type. To investigate this, we conducted a mediation analysis and found that the measured cell populations could be partially mediated by the changes in TL in certain cell types. However, a substantial proportion of the variability remained unexplained, which suggests changes in other unmeasured cell types or mechanisms through which CMV can affect TL independently of changes in (predicted) cell-type composition [Figure 3E]. For example, in the case of NK-cells/fully differentiated T-cells, the major mediator of TL was the abundance of CD8+ EM cells, which accounted for 36% (95% CI, 15.4%–53%) of the CMV effect on the TL of NK-cell/fully differentiated T-cells. This indicates that a significant portion of the CMV effect on TL was not dependent on changes in predicted cell counts.
Interestingly, we observed opposite associations between cell type composition and anti-rhinoviral responses than those observed for CMV [Table S4]. Similar to CMV, such cell changes only partially mediated the rhinoviral effects on TLs [Figure S1]. Specifically, the effect of rhinovirus on TL in naive T-cells was found to be partially mediated by the predicted cell counts of CD8+ naive cells, accounting for 17.1% (95% CI, 0.07–0.35) of its effect on TL.
CMV is associated with gene expression changes in specific cell types
Previously we found an association between CMV seropositivity and predicted cell type composition. However, such analysis may be prone to biases related to cell prediction algorithms using bulk RNA-seq. It also does not identify expansions of specific cellular subpopulations and overlooks transcriptional effects at the cellular level that are unrelated to cell expansions. To shed light on the molecular processes associated to CMV seropositivity in specific blood cell populations, we used 3′-end single-cell RNA-sequencing (scRNA-seq) data previously generated on cryopreserved, unstimulated PBMCs from a subset of the LLD participants, Oelen2022 (n = 94, CMV seronegative = 59, CMV seropositive = 35).43 While the scRNA-seq data collection was posterior to PhIP-Seq (5 years on average), previous literature indicates that infection with CMV late in life is rare.42 This is consistent with our observations [Figure 1A], where only 2 out of 325 individuals with data from two timepoints (3.5-year apart on average) were predicted to be CMV-negative at baseline and CMV seropositive at follow-up. We therefore concluded that we can use CMV status inferred from PhIP-Seq data to label scRNA-seq data from the same donor despite the difference in collection time. In addition, we accounted for potential technical differences between V2 and V3 10X Chromium Single Cell 3′ chemistries in all of our analyses (see STAR Methods).
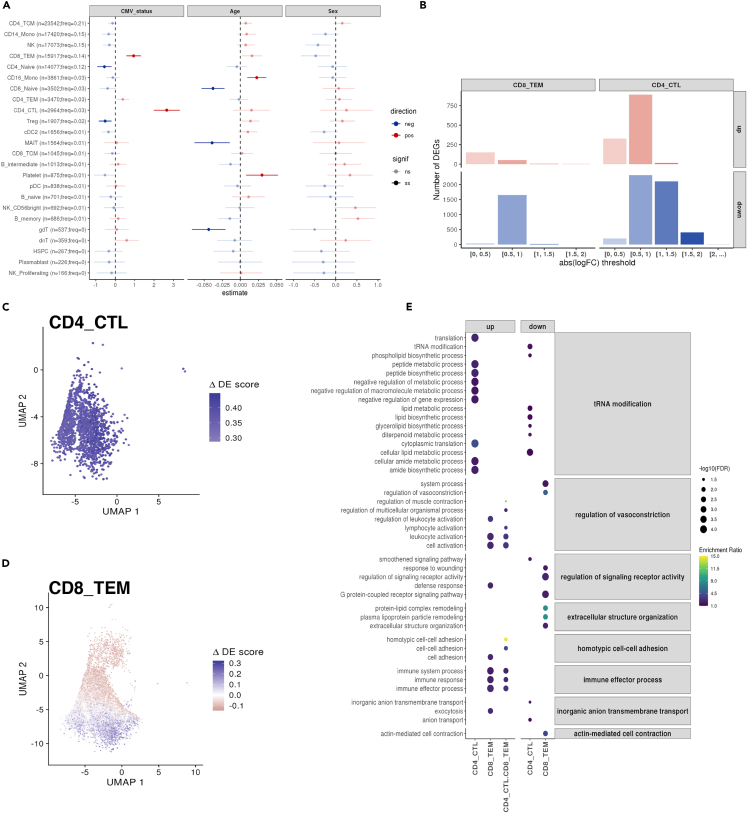
First, we associated CMV serostatus with blood cell composition predicted from scRNA-seq. These results reproduced our previous associations between CMV serostatus and blood cell counts (either measured with EDTA Sysmex [Figure 3A] or deconvoluted from bulk RNA-seq [Figure 3B]), including the positive association between CMV and both CD8+ T-cells and its subpopulation CD8+ TEM and the negative association with CD4+ naive T-cells and a depletion of regulatory T-cells (T-reg). In addition, the single-cell data allowed us to observed an additional expansion of CD4+ cytotoxic T lymphocytes (CTL) in CMV seropositive individuals [Figure 4A] [Table S5A].
Figure 4.
CMV seropositivity associates with both cell composition and cell-type-specific gene expression changes
(A) Forest plot showing the linear association of CMV serostatus, age, and sex with high-level (Azimuth’s l2) cell-type proportions from the Oelen202243 scRNA-seq data. Estimated effect values, accounting for both biological (sex and age) and technical (10X Chromium Single Cell 3′ chemistry and experimental batch) covariates, are displayed. Error bars represent the 95% confidence interval of the estimated effect. The absolute number and relative frequency of each cell type is shown.
(B) Bar plot of the number of CMV-differentially expressed genes (DEGs) across cell types, split by the direction (up- or down-regulated) and magnitude of the effect size (x axis).
(C–D) UMAPs showing the ΔDE (differential expression) scores using data from CD4+ CTL (C) and CD8+ TEM (D).
(E) Enriched gene ontology (GO) terms, using the biological process pathway database, from three different CMV-DEG sets using the Oelen2022 data: CD4+ CTL- DEGs, CD8+ TEM- DEGs, and DEGs shared between CD4+ CTL and CD8+ TEM. GO terms are grouped based on their semantic similarity to simplify the redundancy of GO sets. Dot color indicates enrichment ratio. Dot size indicates statistical significance.
CMV may indirectly affect TL by affecting sub-cell-type composition. Previously, we observed an association between CMV and TLs from mature NK/T-cells (expressing CD45RA+ CD57+). We wondered if different sub-cell-types within CD45RA+ CD57+ may be amplified with CMV infection, thus causing changes in the population’s TL. Investigation of this cell population is not straight-forward, however. While we could use the expression of the CD57-encoding gene (B3GAT1) to determine CD57-positivity, the 3′-biased mRNA capturing of 10X′ scRNA-seq data did not allow us to distinguish the expression of CD45RA/RO [Figure S2B]. We observed enough cells expressing B3GAT1 for analysis in NK-cells, CD8+ TEM, and CD4+CTL [Figure S2C] and saw an increase in the abundance of B3GAT1+ cells (centered-log-ratio (CLR) transformed) in CMV-positive individuals (effect = 0.9, p = 3.75x10−4). However, the association between CMV and different cell populations within B3GAT1+ cells was not homogeneous (likelihood ratio test model with CMV-cell-type interaction vs. base model, p = 6.88x10−4). To understand whether B3GAT1+ cell amplification differs between cell types, we conducted stratified analyses testing for the effect of CMV in each cell type expressing B3GAT1. We found that CMV seropositivity significantly increased CD8+TEM B3GAT1+ (effect = 1.53, FDR = 4.95x10−4) and CD4+ CTL B3GAT1+ (effect = 1.37, FDR = 2.89x10−4). On the other hand, CMV infection showed a non-significant negative trend in NK B3GAT1+ (effect = -0.3, FDR = 0.48) [Table S5A]. These results suggest that, among the cell populations in which TL was greatly influenced by CMV seropositivity, NK-cells were not greatly affected, whereas both CD8+ TEM and CD4+ CTL were increased. This differential amplification of cell populations might be related to the observed TL shortening. However, it is important to highlight that only cells among those that also express CD45RA could be measured by our Flow-FISH probe, and thus the results might misrepresent which populations are expanded in the final measurement. In addition, we checked for expansion of NK-cells expressing NKG2C (i.e., with transcription of the gene KLRC2), a cell population usually associated with CMV infection44 but did not identify a significant increase of this subpopulation in CMV-seropositive individuals (effect = 0.54, p = 0.17).
Next, as we wondered if CMV infection by itself may alter the transcriptional landscape of immune cells, we performed pseudobulk differential gene expression (DGE) analyses between CMV-seropositive and -seronegative individuals for each of the predicted cell types, while controlling for age, sex, and technical factors (see STAR Methods) [Table S5B]. This identified gene expression changes with CMV serostatus in only two T cell subpopulations: CD8+ TEM and CD4+ CTL cells. We found 7,877 unique differentially expressed genes (DEGs) at FDR<0.05, showing a significant predominance (estimate = 0.86, exact binomial test p < 2.2x10−16) of down-regulated genes (n = 6,737) compared to up-regulated genes (n = 1,355) [Figure 4B]. Notably, most of the DEGs were CD8+ TEM- or CD4+ CTL-specific, with only a small fraction of both up- (88 shared-DEGs out of 1,355 up-DEGs; 0.07 Jaccard Index) and down-DEGs (191 shared-DEGs out of 6,546 down-DEGs; 0.03 Jaccard Index) shared among these subpopulations. Interestingly, while the changes in transcription in CD4+ CTL cells were seen in all cells in the population [Figure 4C], the changes in CD8+ TEM cells were attributed to a subpopulation of cells with a higher differential expression score (ΔDE) (this score considers the combined expression pattern of up- and down-regulated DEGs, see STAR Methods) [Figure 4D]. The positive association we had previously observed between the proportion of CD8+ TEM B3GAT1+ and CMV seropositivity made us wonder whether the population of CD8+ TEM cells enriched in ΔDE could contain such a subpopulation [Figures S3A–S3D]. Indeed, we found higher ΔDE scores among CD8+ TEM B3GAT1+ cells compared to the B3GAT1-cells (Wilcoxon rank-sum test p <2.2x10−16) [Figures S3E and S3F]. Thus, we expanded our DGE analysis to the subset of CD8+ TEM and CD4+ CTL that were B3GAT1+ and B3GAT1-in order to define subpopulation-specific transcriptional changes with CMV seropositivity. Although limited by the reduced sample size of the B3GAT1+ subpopulations (375 cells in CD8+ TEM B3GAT1+ vs. 15,917 cells in CD8+ TEM, 85 cells in CD4+ CTL B3GAT1+ vs. 2,964 cells CD4+ CTL), we identified 73 DEGs in CD8+ TEM B3GAT1+ cells [Table S5C]. CD8+ TEM B3GAT1+ DEGs largely overlap with the ones identified in CD4+ CTL cells (40 out of 42 up-regulated CD8+ TEM B3GAT1+ DEGs and 17 out of 31 down-regulated CD8+ TEM B3GAT1+ DEGs). This indicates a cytotoxic-like response of this CD8+ TEM B3GAT1+ subpopulation that is expanded by CMV seropositivity [Figures S3G and S3H].
To follow-up on this, we assessed whether our reported DEGs belonged to similar functional pathways, thereby highlighting the biological interplay between CMV seropositivity and gene expression [Figure 4E]. Here we observed the enrichment of several pathways both for up- and down-DEGs for CD4+ CTL and CD8+ TEM [Table S5D]. This included the enrichment of negative regulation of metabolism and gene expression in CD4+ CTL and an enrichment of leukocyte activation and immune effector process in CD8+ TEM cells, among others.
Viral infections associate with circulating inflammation-related cytokine concentrations
We then wondered if, in addition to affecting biological aging, previous and chronic exposures could be related to systemic inflammation. The mechanisms driving such changes might be related to trained immunity, a set of epigenetic changes in innate immune cells triggered by past immune encounters. To explore this, we utilized the complete dataset of 2,815 peptides (bound in 5–95% of individuals) with antibody information together with measurements of 14 blood cytokine and adipokine concentrations obtained through ELISA from 989 participants.
For the multivariate analysis, we employed sparse canonical correlation analysis (sparse-CCA), a powerful technique that has been successfully utilized to integrate diverse biological layers.45,46 This approach allowed us to identify coordinated patterns of associations between antibody profiles and inflammation-related markers. We also performed univariate analysis using HAllA, as in our previous analysis. This strategy enabled us to explore individual associations between specific antibodies and inflammation markers, while considering both training and validation datasets for robustness and generalizability [Figure 1B].
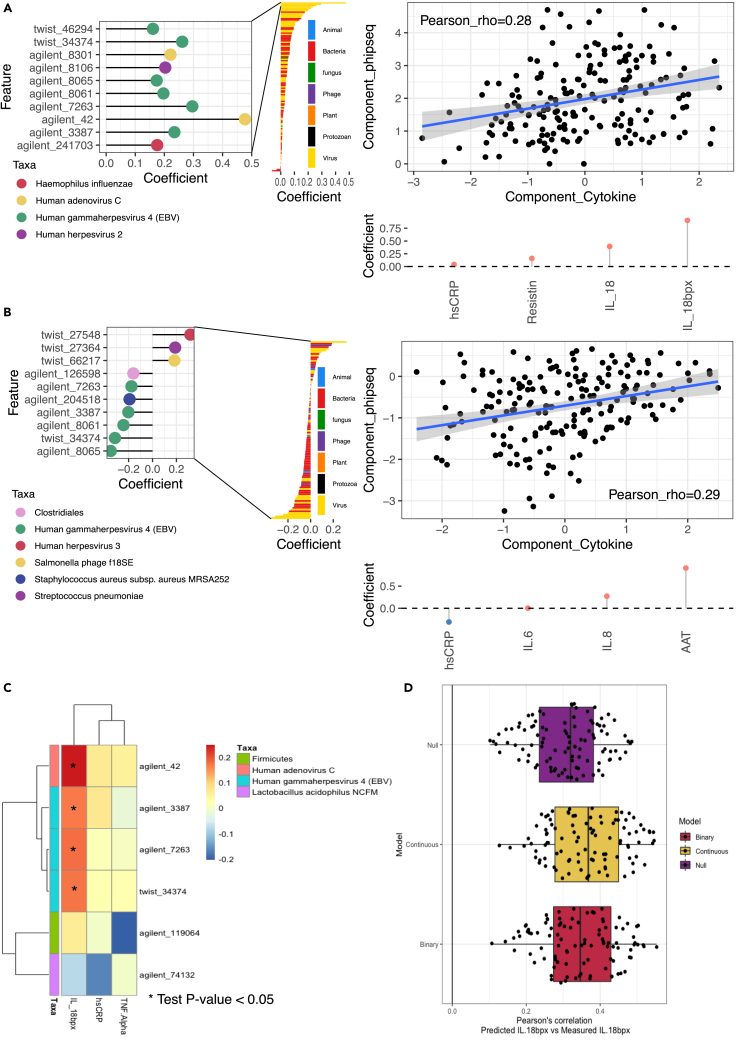
Our analysis revealed a positive association between the anti-inflammatory cytokine Interleukin-18 Binding Protein (IL-18BP) and viral signals that was particularly driven by adenovirus C (Pre-histone-like nucleoprotein, rhopartial_Validation = 0.239) and EBV (two associations with nuclear antigen 1 (rhopartial_Validation = 0.295 and 0.269) and one with nuclear antigen 2 (rhopartial_Validation = 0.187)). These associations were consistently observed using both the sparse-CCA [Tables S6A and S6B] and HAllA [Tables S6C and S6D] methodologies, underscoring their robustness and reliability [Figures 5A and 5C]. Furthermore, prediction models utilizing PhIP-Seq data demonstrated that individual circulating IL-18BP concentrations could be better predicted using antibody responses than by a simple model based on age and sex alone, further supporting the relationship between IL-18BP and antibody profiles [Figure 5D].
Figure 5.
Circulating cytokines and antibody epitope reactivity
(A and B) Sparse-CCA components 1 (A) and 4 (B), which associate a component loaded by presence/absence PhIP-Seq profiles (Y axis) and circulating cytokine concentrations (X axis). Right panel shows the correlation between PhIP-Seq and cytokine component in test data. Left panel shows top antibody-bound peptide loads in the PhIP-Seq component (Y axis in right panel). Bottom panel shows the top cytokine component loads in the cytokine component (X axis in right panel).
(C) HAllA heatmap of associations. X axis shows the cytokines that had at least one significant association in the training data. Color indicates the Spearman’s correlation in the training data. ∗ indicates a partial Spearman’s correlation P-value <0.05 in the validation data. Y axis displays the different peptides with annotation indicating the organism where the peptide originates.
(D) Prediction results for IL-18BP using an L2-regularized linear model with all peptides, using presence-absence scores (binary), normalized read counts (continuous), or prediction using ordinary least squares of age and sex. Each dot represents the Pearson’s correlation of the held-out validation data’s prediction and measurement. Estimations include rho values for 10-fold cross-validation repeated 10 times.
The balance between IL-18BP and IL-18 is important in proinflammatory responses.47 IL-18 stimulates proinflammatory cytokine production in NK-cells and CD4+ T-cells,48 and IL-18BP concentrations have been observed to be negatively correlated with other circulating proinflammatory cytokines in the population.49 While high concentrations of IL-18 have been observed during acute EBV infection,50 we identified that individuals with chronic EBV infection display higher concentrations of IL-18BP. Similarly, individuals with antibodies against adenovirus C also exhibited higher concentrations of IL-18BP. It is worth noting that some viruses are known to express IL-18BP-like molecules as a means to dampen the host immune response,51 and it is possible that similar strategies inducing IL-18BP expression exist during viral infections to promote tolerogenic reactions.
In addition to the associations involving IL-18BP, our analysis identified other signals. In sparse-CCA component 4 (rho = 0.287, p = 7.36x10−5) [Tables S6A and S6B] [Figure 5B], we observed a negative association between EBV and concentrations of α-1-Antitrypsin (AAT), an endogenous protease inhibitor that alleviates tissue damage produced during inflammation, and other proinflammatory cytokines such as IL-6 and IL-8. Conversely, AAT concentrations were positively associated with human herpesvirus 3. On the other hand, high-sensitivity C-reactive protein (hsCRP), a biomarker for inflammation, was increased in individuals showing more EBV antibody-binding. Sparse-CCA component 4 was dominated by EBV peptides but was also influenced by other bacteria and phages, including Staphylococcus aureus subsp. aureus MRSA252 and Streptococcus pneumoniae, as indicated by their loadings (0.192 and 0.189, respectively).
Altogether, these results suggest that chronic infections, such as EBV, may have an effect on circulating cytokine concentrations of the general population.
Discussion
In this study, we performed a comprehensive analysis linking past and current exposures with several markers of biological aging and systemic inflammation. By determining the presence of antibody responses against common pathogens and gut microbiota commensals, we were able to define previous and chronic exposures that were recognized by the immune system and generated an adaptive immune response.
We identified that TLs of immune cells display important relationships with exposures. Most of this signal was driven by a relationship between CMV infection and shorter TLs of NK-cells/fully differentiated T-cells (CD45RA + CD57+). While a relationship between CMV infection and TL reduction has been described previously,52 our cell population–specific approach enabled by Flow-FISH allowed us to fine-map this response to specific cell populations. CD45RA + CD57+ cells might play a role in CMV homeostasis, where CD57+ NK-cells are observed to be an expanded NK-cell memory subtype triggered by CMV infection.34,53 On the other hand, expression of CD45RA + CD57+ is also seen in senescent T-cells.54 CMV is known to drive the expansion and accumulation of memory (known as memory inflation) and senescent cells.42,55 Such cells generally have shorter TLs since they have undergone cell expansion and telomerase activity is progressively lost in differentiated cells.52 We postulated that CMV-induced changes in sub-cell-type composition might drive part of the observed CMV associations with TL, particularly if cell populations in which telomeres were measured are heterogeneous, and our analysis of cell counts using deconvoluted data from bulk-blood RNA-seq supported that CMV was associated with an expansion of memory cells, in particular CD8+ TEM. However, this expansion could only partially explain the observed differences in TL, suggesting that other cell-composition-independent mechanisms might be at work. For instance, in EBV it has been observed that while the expansion due to the infection itself does not result in telomere attrition because of upregulation of telomerase, it does result in reduced telomerase activity and in telomere attrition in subsequent expansions.56 To further explore cell population compositional changes at a higher resolution, we used scRNA-seq data and observed that the populations of CD8+ TEM cells and CD4+ CTL cells expressing CD57+ expanded with CMV infection, while NK-cells expressing CD57+ did not. Previous work has pointed to expansion of CD8+ EMRA T-cells57 (expressing CD45RA+ and commonly CD57+) after CMV infection, which also supports this observation. The differential expansion of senescent T-cells expressing CD57+ and CD45RA + might drive the telomere changes if their average TLs were shorter than those of NK-cells expressing CD57+ CD45RA+. Thus, to fully comprehend if this association is solely driven by that expansion, TLs of CD57+ CD45RA + NK-cells and T-cells ought to be measured separately.
On the other hand, the absence of association between expansion of NK-cells and CMV infection contrasts with previous literature where the specific expansion of the subpopulation NKG2C is commonly observed.58,59 Such a NKG2C population is known to encode CD57+58,60 and should be part of the population analyzed through our single-cell analysis. Interestingly, NKG2C cells have been shown to play a fundamental role in protecting EBV-infected individuals from developing MS.61 CMV infection is believed to have a protective effect on MS, and this mechanism could be mediated by amplification of NKG2C populations that kill T- and B-cells targeting the EBV-EBNA-1 antigen and cross-react with the glial cell adhesion molecule.61
We also identified positive associations between rhinoviral immune responses and TLs. Telomerase is known to be activated during acute infections.56 The fact that no flu-like pathogen other than rhinoviruses is linked with TL might be related to the higher infection rates for rhinoviruses during the sampling seasons. Data from the National Institute for Public Health and the Environment (RIVM) from the Netherlands indicates that the prevalence of rhinovirus and rotavirus were the highest among respiratory viruses during the study sampling period (April-October from 2012) (data not shown). Another possible explanation is that individuals with longer telomeres might have higher potential to produce antibody responses against rhinoviruses. In a previous study, both longer telomeres in PBMCs and antibody titers against rhinovirus were observed to reduce the odds of rhinoviral infection, which might relate to both higher rhinoviral antibodies and TL.62
The associations between infections and telomere shortening might have important implications for public health. Shorter telomeres in immune cells have been associated with mortality and the development of chronic diseases, including hypertension, type 2 diabetes, cardiovascular disease, cancer, and dementia.12,63 However, given the observational nature of this study and the lack of clear long-term consequences of the infection, deeper understanding will be needed to assess the risk associated with CMV infection, in particular the role of telomere loss during infection.
Finally, we uncovered a relationship between EBV and adenovirus C and higher circulating IL-18BP concentrations. Given the anti-inflammatory effect of IL-18BP, these results seem to suggest a tolerogenic response to these infections, which is also supported by the observed negative associations to IL-6, IL-8, and AAT. IL-18BP has been shown to have a role in other viral diseases, for example higher concentrations of IL-18BP are observed with greater severity of both SARS-CoV-264 and dengue65 infections. Those higher concentrations of IL-18BP are normally accompanied by increases in IL-18, but generally similar concentrations of free (not bound to IL-18BP) IL-18. Elevated IL-18BP and IL-18 concentrations are also a hallmark of inflammatory conditions such as inflammatory bowel disease.66 Our results also highlight a sparse CCA component with both IL-18BP and IL-18 that is highly associated with viral peptides from EBV and adenovirus C. Overall, an increase of IL-18BP might be a hallmark of systemic inflammation as a compensatory mechanism by the immune system to avoid overactivity.
Overall, in this study, we have explored the links between previous or chronic exposures that induce adaptive immunity and widespread markers of biological aging, blood cell-type composition, and inflammation. Our results highlight the impact of chronic infections in biological aging and systemic inflammation. However, we also found signals related to frequent challenges, including adenovirus and rhinovirus, that might similarly leave an imprint on the immune system (adenoviruses), or conversely be a challenge to which the immune system should be able to respond to well (rhinoviruses). These results provide a comprehensive overview of how immunological challenges may affect healthy aging by modulating systemic inflammation and TL. In particular, we point to CMV as the immune exposure most associated with telomeric changes in blood cells, thereby further contributing to evidence for CMV’s important role in cellular senescence among all other human viruses,55 although other intriguing associations also emerged with responses against rhinoviruses and bacterial fimbrillin that require further validation. At the same time, we identify circulating cytokine signatures related to EBV and adenovirus C immune responses that might point to a mechanism of action of the virus or the host to reduce systemic inflammation. All in all, our study identifies and describes the effect of common commensal and pathogenic bacteria and viral exposures on the immune system of the general population. Further investigation should be conducted to better understand the role of common infections, such as CMV, in long-term human health.
Limitations of the study
This work leveraged computational predictions of antibody responses, using PhIP-Seq as a proxy of past exposures. This proxy might underrepresent past exposures that did not produce long-lasting antibody responses or those where the antibody titers were too low to be identified through the computational workflow applied in PhIP-Seq. The datasets with different biological information were also produced in different subsets of participants that vary in sample sizes and demographic characteristics. In addition, this is an observational cohort study, so the causal relationships identified need to be validated through further investigation. Finally, it is important to acknowledge that while we observed an effect of infections such as CMV in TL of certain immune cells, the short- and long-term consequences of these changes are unknown and require a deeper functional understanding before public health measures can be taken.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| 1,778 serum samples of 1,437 individuals | Tigchelaar EF et al. 201516 | N/A |
| Deposited data | ||
| Raw data for the PhIP-Seq experiments | Andreu-Sanchez et al. 2023 | EGA: EGAS00001006999 |
| Raw data for PhIP-Seq experiment in IBD | Bourgonje et al. 202330 | EGA: EGAD00001010118 |
| Raw data single cell | Wijst et al. 2018, Oelen et al. 202243 | EGA: EGAS00001002560, EGA: EGAS00001005376 |
| Software and algorithms | ||
| Peptide quantification and enrichment determination | Vogl et al. 2021,17 Leviatan et al. 202218 | https://zenodo.org/record/7307894 |
| Statistical analysis | This paper | https://zenodo.org/records/11097418 |
Resource availability
Lead contact
Further information and requests for resources should be directed to the Lead Contact, Alexandra Zhernakova (a.zhernakova@umcg.nl).
Materials availability
Antibody-bound peptides generated are available in the European Genome-Phenome Archive (EGA). Accession number is listed in the key resources table.
Data and code availability
-
•
LLD PhIP-Seq: Raw and processed PhIP-Seq data used for this study are available in the European Genome-Phenome Archive (EGA) and are publicly available from the date of publication. Accession number is listed in the key resources table. LLD single cell RNA-seq data: Raw sequencing reads used for this study are available in the European Genome-Phenome Archive (EGA). Accession number is listed in the key resources table. LLD Phenotypic data: Lifelines is specifically organized to make assessment results available for (re)use by third parties. Phenotypic data can be requested through Lifelines. A research proposal must be submitted for evaluation by the Lifelines Research Office.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Cohort information
We used data from the Lifelines cohort. Lifelines is a population-based cohort study that collects health and health-related behaviors from individuals from the North of the Netherlands.67 For a subcohort of Lifelines, Lifelines-DEEP (LLD), additional molecular information is available. We used data for 1,437 LLD participants (57% women, age=44.6±14 years, 340 with follow-up 4-years apart).For all participants we have self-questionnaires regarding lifestyle and health and anthropometric and blood parameters. For different subsets of this cohort, different molecular data is available.
The Lifelines study was approved by the ethics committee of the University Medical Center Groningen (METc2007/152). All participants signed an informed consent form before enrollment. The LLD study was approved by the Institutional Ethics Review Board of the University Medical Center Groningen (ref. M12.113965), the Netherlands.
For specific analyses, different subsets of the data that differed in age and sex were used. Female proportion varied from 54% to 63%, while average age varied from 39 years (sd 12 years) to 49.7 years (sd 13 years). All analyses were controlled by age and sex.
PhIP-seq libraries and enrichment
PhIP-Seq profiles were previously generated19 as described earlier,17,18 following a previously described protocol.68 Two peptide libraries were profiled. These libraries used different chemistry and contained peptides of different origins: the 244,000 peptide library manufactured by Agilent Technologies, Inc. (here named ‘Agilent’ for brevity) is enriched in bacterial peptides17 and the oligopeptide library of 100,000 variants manufactured by Twist Bioscience (‘Twist’ library) is enriched in known immune epitopes.18 Antibody-bound peptide presence/absence enrichment was previously determined.19
PhIP-Seq data is not directly interpretable using sequencing read counts. On the one hand, raw reads are not comparable between samples as the sequencing experiment yields compositional data. On the other hand, different phages might precipitate without an actual antibody attachment. To account for these possibilities, PhIP-Seq experiments include a sequencing run without immunoprecipitation, which yields the “input levels” for a certain peptide, i.e. the number of reads from a peptide that are obtained without immune-precipitation. Antibody reactivity can then be induced by comparing read levels of peptides with the same input levels within the same individual, for which data compositionality is not an issue. Read numbers from a certain input level are assumed to follow a generalized Poisson distribution, and P-values can be obtained as previously described.17 Bonferroni-corrected P-values below a threshold of 0.05 were used to define antibody-bound peptides in a binary fashion.
In addition, to obtain continuous scores that can act as a proxy for antibody titers, we normalized transcript per million abundances, obtained using Salmon,69 using log-ratios. Log-ratios are commonly used in compositional data analysis.70 The abundances of a specific peptide (plus a pseudocount) are then expressed relative to a denominator that is thought to be constant in different samples. In this case, we used a median-of-ratios strategy to normalize the data. First, we computed the geometric mean among samples in two overlapping Staphylococcus aureus peptides representing protein A (TPA B-domain-containing protein) (agilent_221096 and agilent_133222, respectively). Such protein binds to the fragment crystallizable region of the antibodies, leading to widespread immunoprecipitation events not caused by direct antibody recognition, as previously described.17 Reactivity of these two peptides were predicted in all but three samples, which were removed from the continuous analysis as the denominator is not expected to be constant in such samples. Second, per sample, we divided the values of agilent_221096 and agilent_133222 by their inter-sample peptide-specific geometric mean. Finally, we computed the mean value of the normalized agilent_221096 and agilent_133222 abundances per sample, yielding a sample-specific normalization factor. We used this factor as the denominator in the log-ratio transformation for all other peptides. This data yields a continuous score that is independent from thresholds, but it comes with the caveat that a certain amount of baseline reads are expected even without immunoprecipitation, and this might introduce a certain level of noise into the associations. Due to this issue, read abundances are not directly comparable between peptides.
Peptides for analysis were selected based on the same criteria applied in the original publication of the data.19,30 Peptides with a prevalence of at least 5% and less than 95% in either the 1000IBD30 or LLD cohort19 (excluding follow-up samples) were selected to ensure variability and increase statistical power. In addition, several available peptides with identical amino acid sequences (originating from different nucleotide sequences) were dereplicated by selecting the most prevalent among the group of identical peptides.
Proteomics and cytokine measurements
Proteomics profiles were generated using the Olink® CVD panel III for the 1,433 LLD participants with PhIP-Seq profiles.71 This panel includes 92 proteins previously linked to cardiovascular disease. The protein CCL22 was removed from the analysis based on indications from Olink. Adipokines and cytokines were measured previously in the LLD cohort.72 Cytokines included IL [interleukin]-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α (tumor necrosis factor-α). Leptin, adiponectin, IL-18, IL-18BP, resistin and AAT (alpha-1 antitrypsin) were measured using ELISA kits (R&D Systems, Minneapolis, MN).
Bulk RNA-seq of participant’s blood
Whole-blood RNA-seq data were generated previously.73 Paired-end reads were mapped to the human genome using STAR,74 allowing for a maximum of seven mismatches. HTSeq-count75 was used on uniquely mapping reads to quantify transcripts. We used blood cell proportions previously predicted from whole-blood bulk-RNA profiles using Decon2.76 The main advantage of Decon2 is that the prediction does not depend on measuring gene expression in purified cells to determine gene signatures. Using Decon2, we predicted the proportions of 34 cell populations. In addition to the predicted cell counts, we measured lymphocytes, erythrocytes, monocytes, neutrophils, thrombocytes and eosinophils in participant’s blood with EDTA Sysmex. Additive log-ratio transformation (ALR) was performed on predicted cell proportions, using the proportions of monocytes as denominator.
TLs of immune cells
TL measurements for 1,046 LLD individuals were generated previously using Flow-FISH for six blood cell types: granulocytes, lymphocytes, B-cells (CD45RA+CD20+), naïve T-cells (CD45RA+ CD20-), memory T-cells (CD45RA-), and NK-cells/fully differentiated T-cells (CD45RA+CD57+).21 For some individuals, the TLs of certain cell types were missing (109 missing samples for granulocytes, 9 for B-cells, and 17 for NK-cells, with no samples missing TLs for more than one cell type). Missing points were imputed by building a linear prediction model of the TL based on all other TLs. To assess imputation quality, samples with no missing telomere information were split into training data (80%) and held-out test data (20%). We used a linear model (ols) using a specific TL as dependent variable and including all other TLs as features for prediction. Using the trained-fit model, we computed the R2 of the prediction in the test. The imputation showed high resemblance to the actual held-out values: R2=0.763 for granulocytes, R2=0.881 for B-cells, and R2=0.740 for NK-cells. Thus, we used the model to impute all missing values for the samples not used to build and test the model.
Method details
Residualization of omics layers
Since neither sparse-CCA or HAllA directly account for possible confounders, we performed a residualization procedure for each omic layer to remove the variability attributed to possible confounders. Covariates that might act as confounders, including age, sex, BMI, blood cell counts, smoking habits, and oral contraceptive use, were tested against the distance matrix of each omic layer using permutational analysis of variance (PERMANOVA) implemented in the adonis2 function of R package vegan (v2.6-4). Significant features were then chosen as covariates for residualization. We built independent linear models per feature within an omic layer, including the possible confounders detected by the PERMANOVA analysis as covariates. Residuals of the model were extracted and used as input features for the sparse-CCA and HAllA analyses.
Dataset split
Before the sparse-CCA and HAllA analyses were performed and the datasets were residualized for possible confounders, participants were randomly split into a training dataset (80% of the data) and a held-out validation dataset (20% of the data). Residualization of datasets was then performed independently in the training and validation sets. Multivariate analyses (HAllA and sparse-CCA) were first performed in the training set and subsequently validated in the validation set.
Quantification and statistical analysis
Sparse-CCA
To correlate different omic layers, we used a sparse-CCA framework. PhIP-Seq antibody-bound peptides were filtered for a minimal prevalence of 10% in LLD individuals with matching omic layers. In the training set, we performed a grid search (with size and range changed depending on the molecular type analyzed) to optimize the lasso penalty parameters per dataset. A 5-fold cross-validation was performed (R, caret v6.0-90) using the function CCA (R, PMA v1.2.1), searching for a unique component in the output (K=1) and testing all possible combinations of penalties. We assessed the model’s performance using the 1-fold held-out data, for which we projected both omics layers to the trained-found dimensionality and ran a Pearson correlation analysis. Correlation rho were averaged between folds as an assessment of a specific hyperparameter selection. Hyperparameters that achieved a maximum mean rho were selected. Using the complete test-set and the identified hyperparameters, we ran sparse-CCA searching for 10 new dimensions (K=10). Then, to assess the correlation found in the new dimensionality in an unbiased manner, we projected the 20% held-out validation data to the found space and performed Pearson and Spearman correlations between both dimensional projections. The resulting rho and P-value from the validation set were recorded. A P-value < 0.05 was considered significant and analyzed in more detail.
HAllA analysis
Residualized training omics layers were associated with binary antibody profiles using HAllA v 0.8.20. Spearman correlations were used with thresholds set to --fdr_alpha 0.05 and --fnr_thresh 0.2. We then reproduced the HAllA associations in tests using partial Spearman correlations in non-residualized data while accounting for the covariates of interest.
PhIP-seq predictive models
We used continuous and binary (non-residualized) PhIP-Seq data to predict individual cytokine concentrations and TL. For that, we fitted two linear models, an ols using age and sex to predict the dependent variable and a lasso-regularized model including all peptides on top of covariates (R glmnet, v4.0-2). The strength of the regularization parameter λ was chosen from the default grid search from cv.glmnet using 10-fold cross-validation in bases to the obtained root of the mean squared error. To assess an unbiased model performance, a 10-fold cross-validation framework was used. Test fold was used for prediction, and Pearson’s correlation values between predicted and actual values were used to assess model performance. To estimate the variability of such estimates, cross-validation was repeated 10 times, yielding 100 estimates of performance. We then used a t-test to compare estimated predictability between the base and PhIP-Seq models.
CMV analysis
Serostatus prediction
To predict CMV seroprevalence from PhIP-Seq antibody-bound peptide data, we first used a cohort of 497 patients with inflammatory bowel disease with PhIP-Seq profiles30 where a subset of participants had ELISA seroprevalence information for CMV (n=294 participants, 114 seropositive, 38%). We tested three algorithms in a 5-times 5-fold cross-validation framework: a logistic regression model using the number of positive antibody-bound peptides as the predictor, a logistic regression model with all CMV peptides using a lasso penalty, and a logistic regression using as regressor the first Redundancy Analysis (RDA) of PhIP-Seq and CMV seroprevalence component. In all models, age and sex were used as covariates. We used complete PhIP-Seq profiles as a predictor for LASSO, and regularization strength parameter λ was optimized using 5-fold cross-validation. We used RDA to identify the linear combination of the peptide-bound antibody data enrichment that better explains CMV ELISA measurements. We then projected the testing data to the trained RDA space and predicted CMV seropositivity using the resulting RDA-1 component as the regressor of logistic regression. Model accuracies were calculated as the proportion of correct predictions out of all predictions. Statistical testing to compare model performances was done using an ANOVA, followed-up by a post-hoc Tukey test. We selected the top-performing model, which was the logistic regression based on the number of positive antibody-bound peptides, and used it for prediction in the whole LLD dataset. A probability of CMV of at least 0.5 was used to predict CMV seropositivity.
CMV associations with cell composition and TLs
CMV predictions were associated with measured blood cell counts, additive log-ratio-transformed predicted blood cell counts (using predicted monocyte proportion as denominator), and TL measurements, all accounting for age and sex. To test for interaction analyses, we included either a CMVxSex or a CMVxAge term in the model. Mediation analysis between CMV (exposure), predicted cell counts (mediators), and TLs (outcome) were run using mvregmed (R regmed v2.0.4), using a grid search of λ parameters between 0.4 and 0.01 (step of 0.01). The best-fitting model was selected based on AIC, and findings were visualized in a network. Individual mediation effects were calculated using mediate v4.5.0.
CMV-stratified correlation analysis
Here we used the 271 samples for which there were TL measurements, methylation age clocks, metabolic age clocks, and qPCR measurements of sj-TRECS. We divided that dataset into participants predicted to be CMV-positive and those predicted to be CMV-negative. We computed the biomarker-to-biomarker Pearson’s correlation coefficient individually in the CMV-positive and CMV-negative samples. To determine whether the observed differences in correlation coefficients were not due to random sampling, we performed Fisher’s Z-transformation by applying the inverse hyperbolic tangent function on the coefficients. Then, we estimated the standard error (SE) for the differences in z-scores using Equation 1.
| (Equation 1) |
SE difference, where n indicates the number of samples used to estimate the correlation coefficient.
Next, we divided the difference of z-scores by the estimated SE difference (Equation 1) to obtain a Z-statistic. Finally, we estimated the P-value of the null hypothesis that the correlation coefficients are the same as the probability lower or equal to achieve the estimated Z-statistic on a standard gaussian distribution.
We repeated the above analysis after removal of the “age” effect from the aging biomarkers by regressing out age using a linear model and taking the resulting residuals for correlation.
CMV and immune single-cell transcriptomics analysis
scRNA-seq datasets
We used two previously processed 10X Genomics’ scRNA-seq datasets on cryopreserved PBMCs from a subset of the LLD participants of the present study (n=119): i) unstimulated data from Oelen202243 generated using 10X Chromium Single Cell 3’ V2 (n=64, CMV seronegative=43, CMV seropositive=21) and V3 (n=30, CMV seronegative=16, CMV seropositive=14) chemistries and ii) unstimulated data from Wijst201877 generated using 10X Chromium Single Cell 3’ V2 (n=25, CMV seronegative=21, CMV seropositive=4). We matched participants from the first and second timepoint at which PhIP-Seq was measured with participants with scRNA-seq data. These scRNA-seq datasets were generated 5 years after the PhIP-Seq measurements, on average. In the original studies, k-nearest neighbor clustering was used to cluster the cells. Here, we performed automated cell type classification using Azimuth to annotate the cells.78 In detail, we conducted a supervised analysis guided by a reference dataset to enumerate cell types that would be challenging to define with an unsupervised approach. We mapped our scRNA-seq query dataset to a recently published CITE-seq reference dataset of 162,000 PBMCs measured with 228 antibodies.78 For this process, we used a set of specific functions from the Seurat R package v4.0.0.78,79 First, we normalized the reference dataset using the SCTransform function. Next, we found anchors between reference and query using a precomputed supervised PCA transformation through the FindTransferAnchors function. We then transferred cell type labels and protein data from the reference to the query and projected the query data onto the UMAP structure of the reference. For these last two steps, we used the FindTransferAnchors function. The Oelen2022 dataset, which has a larger sample size and balanced representation of CMV serostatus, was considered the primary dataset to investigate alterations in cellular composition and gene expression linked to CMV serostatus. It was also used for the CMV serostatus prediction analysis. The Wijst2018 dataset, which has a smaller participant pool and an unbalanced distribution of CMV serostatus, was used only for replication.
We used both the low (l1)- and high (l2)-resolution cell type annotations predicted by Azimuth for the Oelen2022 dataset (n=94) to closely reflect the resolution of the measured blood cell counts and bulk RNA-seq deconvolution-predicted blood cell counts. To calculate cell-type-proportions relative to the total PBMCs, we only considered cell types with >5 cells per donor in at least 5 donors. To account for the compositional nature of the scRNA-seq data, we used the CLR transformation,70 i.e., per donor, we divided cell-type-proportions by their geometric mean. For each cell type, we then fitted a linear mixed model that controls for both biological (sex and age) and technical (10X Chromium Single Cell 3’ chemistry and experimental batch) covariates (Equation 2).
| (Equation 2) |
Cell type proportion model. CLR-transformed cell_type is the CLR-transformed cell-type proportion being tested. CMVserostatus is the CMV serostatus prediction per participant. Chemistry is the 10X Chromium Single Cell 3’ chemistry. Batch is the experimental batch (day of the scRNA-seq library prep) data.
To test for interactions, we included either a CMV serostatus_i x Sex or a CMV serostatus_i x Age term in the model. Cell types were considered to be significantly associated with CMV seropositivity when the cellular composition change was significant at an FDR < 0.05.
We then interrogated whether B3GAT1+ cells from any cell type were increased with CMV seropositivity (Equation 3). We defined B3GAT1+ and B3GAT1- subpopulations using the single-cell expression of the CD57-encoding gene (B3GAT1).
| (Equation 3) |
Analysis of B3GAT1+ cells. An additional random-effect term, (1|Participant), is included into Equation 2 to control for sample origin.
A second model was fitted to determine whether the CMV effect was different in different B3GAT1+ cell populations (Equation 4).
| (Equation 4) |
Analysis of B3GAT1+ cells. An additional fixed-effect term, Cell_type: CMVserostatus, is included into Equation 3 to account for CMV–cell type interactions.
The models in Equation 3 and Equation 4 were then compared using a likelihood ratio test to estimate whether the effect was different from cell type. In a subsequent analysis, we ran Equation 2 per cell type in B3GAT1+ cells separately.
DGE analysis with CMV serostatus
We performed pseudobulk DGE analyses between CMV-seropositive and -seronegative individuals from Oelen2022 (n=94) at the high (l2)-resolution cell-type annotations predicted by Azimuth. Additionally, we defined the B3GAT1+/- subpopulations using the single-cell expression of this CD57-encoding gene (B3GAT1). First, we aggregated (summed) the single-cell gene expression profile per donor and cell-type combination using the sparse_Sums function from the textTinyR R package v1.1.7. Only genes with a minimum expression level (>0.1 counts per million (CPM) in at least 5 donors) were tested in the pseuodbulk DGE analyses.
Next, we performed library size normalization on the pseudobulk gene expression profiles using the DGEList and calcNormFactors from the edgeR R package v3.38.4.80 Afterwards, we used the voomWithDreamWeights function from the variancePartition R package v1.28.9 to transform count data to log2-CPM (logCPM) and estimate the mean-variance relationship, which we used to compute appropriate observation-level weights. Lastly, per cell type, we fitted the following linear mixed model for CMV serostatus–DGE analysis using the dream function from the variancePartition R package v1.28.9 (Equation 5).
| (Equation 5) |
Differential expression analysis. Expr_i is the log-normalized, scaled expression of the gene being tested in donor i. CMV serostatus_i is the CMV serostatus prediction in donor i. Sex and Age are phenotypic variables from the donors. Chemistry is the 10X Chromium Single Cell 3’ chemistry. Batch is the experimental batch (day of the scRNA-seq library prep).
Genes were considered to be differentially expressed with CMV seropositivity when the gene expression change was significant at an FDR < 0.05. A binomial test using the binom.test function from the stats R package v4.3.0 was used to assess whether there was a significant preference for up- or down-regulated DEGs. The Jaccard index was computed to determine sharing among the cell-type-specific DEGs (Equation 6).
| (Equation 6) |
Jaccard index. A and B are the set of cell-type-specific DEGs. |A ∩ B| is the size of the intersection between A and B sets. |A ∪ B| is the size of the union between A and B sets.
Cell-type-specific up- and down-DEGs (with an absolute log-fold-change > 0.5) were used to compute an up- and down-module score using the AddModuleScore function from the Seurat R package v4.0.0,78,79 which calculates the average expression levels of each program (up- and down-DEGs) on single-cell level, subtracted by the aggregated expression of control feature sets. Later, per each single cell type, we combined the two scores by subtracting the down-DEGs scores from the up-DEGs scores (ΔDE score).
Functional enrichment analyses from the CMV serostatus–DGE analysis
We performed a functional enrichment analysis through an over-representation analysis using WebGestalt (WEB-based GEne SeT AnaLysis Toolkit).81 As the input gene list, we used the 6,262 and 1,918 DE genes identified in CD4+ CTL and CD8+ TEM cells, respectively, split by their direction of effect (i.e., positively or negatively associated with CMV seropositivity). As the background gene set, we used the 10,687 and 13,579 expressed genes that were tested in the pseudobulk DGE analyses of the CD4+ CTL and CD8+ TEM cells, respectively. As the functional database, we used the gene ontology (GO) biological process pathway database. Advanced default parameters were used (minimum and maximum number of genes for a category: 5 and 2000, multiple test adjustment: Benjamini-Hochberg, significance level: FDR ≤ 0.05, number of categories expected from set cover: 10). Finally, we used two functions from the rrvgo R package v1.12.2 to simplify the redundancy of GO sets by grouping similar terms based on their semantic similarity. The calculateSimMatrix function allowed us to get the similarity matrix between terms, and the reduceSimMatrix function grouped terms, based on the similarity matrix, with at least a similarity below a specific threshold (i.e., 0.9) and selected the term with the higher score (i.e., score reflecting how unique the term is) within the group as the group representative.
CMV serostatus prediction analysis using single-cell expression
Cellular composition and gene expression profiles from Oelen_2022 (n=94) were used to train a model to predict CMV serostatus. We performed five times a 3-fold cross-validation fitting a logistic regression model with a lasso penalty using the V2 (n=64, CMV seronegative=43, CMV seropositive=21) and V3 (n=30, CMV seronegative=16, CMV seropositive=14) data independently. We used the cv.glmnet function from glmnet v4.1.882 to train and test a lasso regression model (α=1). To determine the hyperparemeter λ, which represents the strength of shrinkage, a 4-fold cross-validation was run on the training data, and the lowest average mean squared error was used for hyperparameter selection. The lasso regression model with best λ was then trained and used to predict response value in the held-out data. For the cellular composition model, we used cell-type proportions as features. We only considered cell types with >5 cells per donor in at least 5 donors. For the gene expression models, we trained a model per l2-subpopulation using all expressed genes (>0.1 CPM in at least 5 donors) as features. To validate our best-performing model (the one based on cellular composition), we used CMV-annotated V2 data from an independent study (Wijst2018) (n=25, CMV seronegative=21, CMV seropositive=4). In this case, we combined the V2 and V3 data from Oelen2022 (n=94, CMV seronegative=59, CMV seropositive=35) to achieve a larger and more balanced training dataset. First, we merged the cell-type-proportions from the three datasets (Oelen2022 V2, Oelen2022 V3 and Wijst2018) and extracted the residuals of Equation 7.
| (Equation 7) |
Batch correction. CLR-transformed cell type is the CLR-transformed cell type proportion being tested. Dataset is V2/V3 Oelen2022 or Wijst2018. Batch is the experimental batch (day of the scRNA-seq library prep).
Next, we split the Oelen2022 data into 80% training and 20% testing sets using the createDataPartition function from the caret R package v6.0.94. CMV serostatus was predicted among the 20% testing dataset from Oelen2022 and the independent Wijist2018 dataset.
The performance metrics (ROC AUC, F1 score, Matthews Correlation Coefficient (MCC), and accuracy) were computed using the roc_auc and metrics_set functions from the yardstick R package v1.2.0. Confusion matrices were also calculated using the same R package.
Rhinovirus association analysis
Per TL, we ran a linear regression with lasso penalty using all PhIP-Seq peptides annotated as of rhinoviral origin as covariates and TL as dependent variable, while performing a 5-fold cross-validation for determination of the λ parameter. This procedure was repeated 100 times. We then assessed which peptides were more often selected in each analysis to identify the best peptide for association with TL. Among the most-often-selected peptides, we ran a linear model using all TLs as dependent variables and including a covariate specifying cell type and a random effect on the intercept specifying sample ID. We then compared the fit of models including basic covariates (age, sex), CMV infection, and smoking habits (current smoker and ever smoker). In addition, in a subsequent analysis, we used a binary definition of rhinovirus infection, using the top selected peptide from the lasso regression (twist_35344), and matched samples with and without immune response against this peptide according to their age and sex (R MatchIt v4.3.4, method ‘nearest’, 1:1 ratio).
We performed similar analyses as described for CMV association between the top rhinoviral feature and TLs, cell counts, and predicted cell counts. A mediation analysis was performed using regmed v2.0.4 and mediation v4.5.0.
Assessment of statistical significance
For univariate association models, we used an FDR estimated by calculating the total number of positives and false positives from a null distribution of permuted P-values. For individual terms from a linear model, we permuted the term where the FDR was to be estimated. For interaction effects, we permuted both the interaction terms that make up the interaction. Positives were defined as the number of P-values ≤ a specific threshold in the distribution of non-permuted P-values. False positives were defined as the number of P-values from the null distribution ≤ a given non-permuted P-value, divided by the number of permutations performed (usually 1,000). The FDR for such a threshold was then defined as the ratio of false positives to positives.
We used an FDR_per of at least 0.05 as a significance threshold, but other trends that did not reach this threshold were also analyzed.
In the analyses of single-cell data, we used Benjamini-Hochberg FDR estimation.
Acknowledgments
We thank K. Mc Intyre for English editing. The Lifelines Biobank initiative has been made possible by a subsidy from the Dutch Ministry of Health, Welfare and Sport; the Dutch Ministry of Economic Affairs; the University Medical Center Groningen; the University of Groningen; and the Northern Provinces of the Netherlands. The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants.
Funding:
A.Z.: Netherlands Heart Foundation (IN-CONTROL CVON 2018-27). EU Horizon Europe Program grant INITIALISE (101094099). NWO Gravitation project ExposomeNL (024.004.017) and NWO-VIDI (016.178.056).
J.F.: Netherlands Heart Foundation (IN-CONTROL CVON 2012-03). NWO-VIDI (864.13.013). NWO-VICI (VI.C.202.022). ERC Consolidator Grant (grant agreement no. 101001678).
T.V.: European Union - ERC STG, project number 101075733.
M.M.: MCIN/AEI RYC- 2017-22249 and PID2019-107937GA-I00.
A.R.: MCIN/AEI FPI [grant no. PRE2019-090193].
M.W.: Dutch Research Council (NWO-VENI 192.029 and NWO-VIDI 223.041).
A.R.B.: NWO Rubicon grant [grant no. 452022317].
Author contributions
S.A.-S. designed the project, performed the analyses, supervised the analyses carried out by other team members and wrote the original manuscript.
A.R.-C. designed and performed the single-cell analysis and wrote the original manuscript, M.G.P.W., M.J., and M.M. supervised and designed single-cell analyses. L.F. funded the single cell experiments and analysis.
A.C. partially performed CMV analyses and wrote the draft of the original manuscript.
A.R.B. provided feedback and interpretation to biological findings.
O.B. and M.N. provided replication in 3000BCG.
G.A. and P.L. provided feedback regarding TL measurements and biology in relation to CMV infection.
T.V. provided feedback and developed ideas regarding PhIP-Seq analyses.
D.V.B. provided feedback on the epidemiological implications of CMV and other pathogens.
J.F. and A.Z. provided overall feedback and helped in project design.
All authors read and agreed on the contents of this manuscript.
Declaration of interests
P.L. is a founding shareholder in Repeat Diagnostics, a CLIA certified company specializing in leukocyte telomere length measurements using Flow-FISH, where G.A. is also employed. The other authors declare no competing interests.
Published: May 16, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109981.
Contributor Information
Jingyuan Fu, Email: a.zhernakova@umcg.nl.
Alexandra Zhernakova, Email: j.fu@umcg.n.
Supplemental information
References
- 1.Kotas M.E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pyrkov T.V., Avchaciov K., Tarkhov A.E., Menshikov L.I., Gudkov A.V., Fedichev P.O. Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts human lifespan limit. Nat. Commun. 2021;12:2765. doi: 10.1038/s41467-021-23014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 4.Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M., Ligotti M.E., Zareian N., Accardi G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondilis-Mangum H.D., Wade P.A. Epigenetics and the Adaptive Immune Response. Mol. Aspect. Med. 2013;34:813–825. doi: 10.1016/J.MAM.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fülöp T., Larbi A., Pawelec G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornevik K., Cortese M., Healy B.C., Kuhle J., Mina M.J., Leng Y., Elledge S.J., Niebuhr D.W., Scher A.I., Munger K.L., Ascherio A. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 8.Eyting M., Xie M., Heß S., Heß S., Geldsetzer P. Causal evidence that herpes zoster vaccination prevents a proportion of dementia cases. medRxiv. 2023 doi: 10.1101/2023.05.23.23290253. Preprint at. [DOI] [Google Scholar]
- 9.Strindhall J., Skog M., Ernerudh J., Bengner M., Löfgren S., Matussek A., Nilsson B.O., Wikby A. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age. 2013;35:985–991. doi: 10.1007/S11357-012-9400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellon M., Nicot C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses. 2017;9:289. doi: 10.3390/V9100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armanios M., Blackburn E.H. The telomere syndromes. Nat. Rev. Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossiello F., Jurk D., Passos J.F., d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022;24:135–147. doi: 10.1038/s41556-022-00842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broer L., Codd V., Nyholt D.R., Deelen J., Mangino M., Willemsen G., Albrecht E., Amin N., Beekman M., de Geus E.J.C., et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J., Epel E., Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat. Res. 2012;730:85–89. doi: 10.1016/j.mrfmmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Astuti Y., Wardhana A., Watkins J., Wulaningsih W., PILAR Research Network Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 2017;158:480–489. doi: 10.1016/J.ENVRES.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tigchelaar E.F., Zhernakova A., Dekens J.A.M., Hermes G., Baranska A., Mujagic Z., Swertz M.A., Muñoz A.M., Deelen P., Cénit M.C., et al. Cohort profile: LifeLines DEEP, a prospective, general population cohort study in the northern Netherlands: study design and baseline characteristics. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogl T., Klompus S., Leviatan S., Kalka I.N., Weinberger A., Wijmenga C., Fu J., Zhernakova A., Weersma R.K., Segal E. Population-wide diversity and stability of serum antibody epitope repertoires against human microbiota. Nat. Med. 2021;27:1442–1450. doi: 10.1038/s41591-021-01409-3. [DOI] [PubMed] [Google Scholar]
- 18.Leviatan S., Vogl T., Klompus S., Kalka I.N., Weinberger A., Segal E. Allergenic food protein consumption is associated with systemic IgG antibody responses in non-allergic individuals. Immunity. 2022;55:2454–2469. doi: 10.1016/j.immuni.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Andreu-Sánchez S., Bourgonje A.R., Vogl T., Kurilshikov A., Leviatan S., Ruiz-Moreno A.J., Hu S., Sinha T., Vich Vila A., Klompus S., et al. Phage display sequencing reveals that genetic, environmental, and intrinsic factors influence variation of human antibody epitope repertoire. Immunity. 2023;56:1376–1392. doi: 10.1016/J.IMMUNI.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Kurilshikov A., van den Munckhof I.C.L., Chen L., Bonder M.J., Schraa K., Rutten J.H.W., Riksen N.P., de Graaf J., Oosting M., Sanna S., et al. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk. Circ. Res. 2019;124:1808–1820. doi: 10.1161/CIRCRESAHA.118.314642. [DOI] [PubMed] [Google Scholar]
- 21.Andreu-Sánchez S., Aubert G., Ripoll-Cladellas A., Henkelman S., Zhernakova D.V., Sinha T., Kurilshikov A., Cenit M.C., Jan Bonder M., Franke L., et al. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 2022;5:565. doi: 10.1038/s42003-022-03521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannum G., Guinney J., Zhao L., Zhang L., Hughes G., Sadda S., Klotzle B., Bibikova M., Fan J.-B., Gao Y., et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidner C.I., Lin Q., Koch C.M., Eisele L., Beier F., Ziegler P., Bauerschlag D.O., Jöckel K.-H., Erbel R., Mühleisen T.W., et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15:R24. doi: 10.1186/gb-2014-15-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14 doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Akker E.B., Trompet S., Barkey Wolf J.J.H., Beekman M., Suchiman H.E.D., Deelen J., Asselbergs F.W., Boersma E., Cats D., Elders P.M., et al. Metabolic age based on the BBMRI-NL 1H-NMR metabolomics repository as biomarker of age-related disease. Circ. Genom. Precis. Méd. 2020;13:541–547. doi: 10.1161/CIRCGEN.119.002610. [DOI] [PubMed] [Google Scholar]
- 26.Deelen J., Kettunen J., Fischer K., van der Spek A., Trompet S., Kastenmüller G., Boyd A., Zierer J., van den Akker E.B., Ala-Korpela M., et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019;10:3346. doi: 10.1038/s41467-019-11311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghazi A.R., Sucipto K., Rahnavard A., Franzosa E.A., McIver L.J., Lloyd-Price J., Schwager E., Weingart G., Moon Y.S., Morgan X.C., et al. High-sensitivity pattern discovery in large, paired multiomic datasets. Bioinformatics. 2022;38:i378–i385. doi: 10.1093/bioinformatics/btac232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korndewal M.J., Mollema L., Tcherniaeva I., van der Klis F., Kroes A.C.M., Oudesluys-Murphy A.M., Vossen A.C.T.M., de Melker H.E. Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J. Clin. Virol. 2015;63:53–58. doi: 10.1016/j.jcv.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Solana R., Tarazona R., Aiello A.E., Akbar A.N., Appay V., Beswick M., Bosch J.A., Campos C., Cantisán S., Cicin-Sain L., et al. CMV and Immunosenescence: from basics to clinics. Immun. Ageing. 2012;9:23. doi: 10.1186/1742-4933-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourgonje A.R., Andreu-Sánchez S., Vogl T., Hu S., Vich Vila A., Gacesa R., Leviatan S., Kurilshikov A., Klompus S., Kalka I.N., et al. Phage-display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity. 2023;56:1393–1409. doi: 10.1016/J.IMMUNI.2023.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Lee J.-Y., Sojar H.T., Bedi G.S., Genco R.J. Porphyromonas (Bacteroides) gingivalis fimbrillin: size, amino-terminal sequence, and antigenic heterogeneity. Infect. Immun. 1991;59:383–389. doi: 10.1128/IAI.59.1.383-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picarda G., Benedict C.A. Cytomegalovirus: Shape-Shifting the Immune System. J. Immunol. 2018;200:3881–3889. doi: 10.4049/jimmunol.1800171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodier M.R., Jonjić S., Riley E.M., Juranić Lisnić V. CMV and natural killer cells: shaping the response to vaccination. Eur. J. Immunol. 2018;48:50–65. doi: 10.1002/EJI.201646762. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Vergès S., Milush J.M., Schwartz B.S., Pando M.J., Jarjoura J., York V.A., Houchins J.P., Miller S., Kang S.-M., Norris P.J., et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbar A.N., Fletcher J.M. Memory T cell homeostasis and senescence during aging. Curr. Opin. Immunol. 2005;17:480–485. doi: 10.1016/J.COI.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Villacres M.C., Longmate J., Auge C., Diamond D.J. Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum. Immunol. 2004;65:476–485. doi: 10.1016/j.humimm.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/NRI.2016.90. [DOI] [PubMed] [Google Scholar]
- 38.Winther B., Gwaltney J.M., Jr., Mygind N., Hendley J.O. Viral-induced rhinitis. Am. J. Rhinol. 1998;12:17–20. doi: 10.2500/105065898782102954. [DOI] [PubMed] [Google Scholar]
- 39.Andreu-Sánchez S., Bourgonje A.R., Vogl T., Kurilshikov A., Leviatan S., Moreno A.J.R., Hu S., Sinha T., Vila A.V., Klompus S., et al. Genetic, environmental and intrinsic determinants of the human antibody epitope repertoire. Immunity. 2023;56:1376–1392. doi: 10.1101/2021.12.07.471553. [DOI] [PubMed] [Google Scholar]
- 40.Liechti T., Van Gassen S., Beddall M., Ballard R., Iftikhar Y., Du R., Venkataraman T., Novak D., Mangino M., Perfetto S., et al. A robust pipeline for high-content, high-throughput immunophenotyping reveals age- and genetics-dependent changes in blood leukocytes. Cell Rep. Methods. 2023;3 doi: 10.1016/J.CRMETH.2023.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almanzar G., Schwaiger S., Jenewein B., Keller M., Herndler-Brandstetter D., Würzner R., Schönitzer D., Grubeck-Loebenstein B. Long-Term Cytomegalovirus Infection Leads to Significant Changes in the Composition of the CD8+ T-Cell Repertoire, Which May Be the Basis for an Imbalance in the Cytokine Production Profile in Elderly Persons. J. Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samson L.D., van den Berg S.P., Engelfriet P., Boots A.M., Hendriks M., de Rond L.G., de Zeeuw-Brouwer M.-L., Verschuren W.M., Borghans J.A., Buisman A.-M., van Baarle D. Limited effect of duration of CMV infection on adaptive immunity and frailty: insights from a 27-year-long longitudinal study. Clin. Transl. Immunol. 2020;9 doi: 10.1002/cti2.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oelen R., de Vries D.H., Brugge H., Gordon M.G., Vochteloo M., single-cell eQTLGen consortium. BIOS Consortium. Ye C.J., Westra H.J., Franke L., van der Wijst M.G.P. Single-cell RNA-sequencing of peripheral blood mononuclear cells reveals widespread, context-specific gene expression regulation upon pathogenic exposure. Nat. Commun. 2022;13:3267. doi: 10.1038/s41467-022-30893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Botet M., De Maria A., Muntasell A., Della Chiesa M., Vilches C. Adaptive NK cell response to human cytomegalovirus: Facts and open issues. Semin. Immunol. 2023;65 doi: 10.1016/J.SMIM.2022.101706. [DOI] [PubMed] [Google Scholar]
- 45.Priya S., Burns M.B., Ward T., Mars R.A.T., Adamowicz B., Lock E.F., Kashyap P.C., Knights D., Blekhman R. Identification of shared and disease-specific host gene–microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 2022;7:780–795. doi: 10.1038/s41564-022-01121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu S., Bourgonje A.R., Gacesa R., Jansen B.H., Björk J.R., Bangma A., Hidding I.J., van Dullemen H.M., Visschedijk M.C., Faber K.N., et al. Mucosal host–microbe interactions associate with clinical phenotypes in inflammatory bowel disease. Nat. Commun. 2024;15:1470. doi: 10.1038/s41467-024-45855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinarello C.A., Novick D., Kim S., Kaplanski G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013;4 doi: 10.3389/FIMMU.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamura H., Tsutsui H., Kashiwamura S., Yoshimoto T., Nakanishi K. Interleukin-18: A Novel Cytokine That Augments Both Innate and Acquired Immunity. Adv. Immunol. 1998;70:281–312. doi: 10.1016/S0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 49.Bakker O.B., Aguirre-Gamboa R., Sanna S., Oosting M., Smeekens S.P., Jaeger M., Zorro M., Võsa U., Withoff S., Netea-Maier R.T., et al. Integration of multi-omics data and deep phenotyping enables prediction of cytokine responses. Nat. Immunol. 2018;19:776–786. doi: 10.1038/s41590-018-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Veerdonk F.L., Wever P.C., Hermans M.H.A., Fijnheer R., Joosten L.A.B., van der Meer J.W.M., Netea M.G., Schneeberger P.M. IL-18 serum concentration is markedly elevated in acute EBV infection and can serve as a marker for disease severity. J. Infect. Dis. 2012;206:197–201. doi: 10.1093/infdis/jis335. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello C.A., Fantuzzi G. Interleukin-18 and host defense against infection. J. Infect. Dis. 2003;187:S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 52.van de Berg P.J.E.J., Griffiths S.J., Yong S.-L., Macaulay R., Bemelman F.J., Jackson S., Henson S.M., ten Berge I.J.M., Akbar A.N., van Lier R.A.W. Cytomegalovirus Infection Reduces Telomere Length of the Circulating T Cell Pool. J. Immunol. 2010;184:3417–3423. doi: 10.4049/JIMMUNOL.0903442. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Vergès S., Milush J.M., Pandey S., York V.A., Arakawa-Hoyt J., Pircher H., Norris P.J., Nixon D.F., Lanier L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/BLOOD-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Covre L.P., De Maeyer R.P.H., Gomes D.C.O., Akbar A.N. The role of senescent T cells in immunopathology. Aging Cell. 2020;19 doi: 10.1111/ACEL.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naigeon M., Roulleaux Dugage M., Danlos F.-X., Boselli L., Jouniaux J.-M., de Oliveira C., Ferrara R., Duchemann B., Berthot C., Girard L., et al. Human virome profiling identified CMV as the major viral driver of a high accumulation of senescent CD8+ T cells in patients with advanced NSCLC. Sci. Adv. 2023;9:eadh0708. doi: 10.1126/SCIADV.ADH0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plunkett F.J., Soares M.V., Annels N., Hislop A., Ivory K., Lowdell M., Salmon M., Rickinson A., Akbar A.N. The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein-Barr virus infection. Blood. 2001;97:700–707. doi: 10.1182/BLOOD.V97.3.700. [DOI] [PubMed] [Google Scholar]
- 57.Patin E., Hasan M., Bergstedt J., Rouilly V., Libri V., Urrutia A., Alanio C., Scepanovic P., Hammer C., Jönsson F., et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 2018;19:302–314. doi: 10.1038/s41590-018-0049-7. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z., Sinzger C., Frascaroli G., Reichel J., Bayer C., Wang L., Schirmbeck R., Mertens T. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J. Virol. 2013;87:7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodier M.R., White M.J., Darboe A., Nielsen C.M., Goncalves A., Bottomley C., Moore S.E., Riley E.M. Rapid NK cell differentiation in a population with near-universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood. 2014;124:2213–2222. doi: 10.1182/BLOOD-2014-05-576124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saghafian-Hedengren S., Sohlberg E., Theorell J., Carvalho-Queiroz C., Nagy N., Persson J.-O., Nilsson C., Bryceson Y.T., Sverremark-Ekström E. Epstein-Barr virus coinfection in children boosts cytomegalovirus-induced differentiation of natural killer cells. J. Virol. 2013;87:13446–13455. doi: 10.1128/JVI.02382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vietzen H., Berger S.M., Kühner L.M., Furlano P.L., Bsteh G., Berger T., Rommer P., Puchhammer-Stöckl E. Ineffective control of Epstein-Barr-virus-induced autoimmunity increases the risk for multiple sclerosis. Cell. 2023;186:5705–5718. doi: 10.1016/J.CELL.2023.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Cohen S., Janicki-Deverts D., Turner R.B., Casselbrant M.L., Li-Korotky H.S., Epel E.S., Doyle W.J. Association Between Telomere Length and Experimentally Induced Upper Respiratory Viral Infection in Healthy Adults. JAMA. 2013;309:699–705. doi: 10.1001/JAMA.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber H.J., Semeraro M.D., Renner W., Herrmann M. Telomeres and Age-Related Diseases. Biomedicines. 2021;9 doi: 10.3390/BIOMEDICINES9101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marino L., Criniti A., Guida S., Bucci T., Ballesio L., Suppa M., Galardo G., Vacca A., Santulli M., Angeloni A., et al. Interleukin 18 and IL-18 BP response to Sars-CoV-2 virus infection. Clin. Exp. Med. 2023;23:1243–1250. doi: 10.1007/S10238-022-00943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michels M., De Mast Q., Netea M.G., Joosten L.A., Dinarello C.A., Rudiman P.I.F., Sinarta S., Wisaksana R., Alisjahbana B., Van Der Ven A.J.A.M. Normal free interleukin-18 (IL-18) plasma levels in dengue virus infection and the need to measure both total IL-18 and IL-18 binding protein levels. Clin. Vaccine Immunol. 2015;22:650–655. doi: 10.1128/CVI.00147-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbaz A., ten Hove T., Herren S., Graber P., Schwartsburd B., Belzer I., Harrison J., Plitz T., Kosco-Vilbois M.H., Kim S.-H., et al. IL-18-binding protein expression by endothelial cells and macrophages is up-regulated during active Crohn’s disease. J. Immunol. 2002;168:3608–3616. doi: 10.4049/JIMMUNOL.168.7.3608. [DOI] [PubMed] [Google Scholar]
- 67.Scholtens S., Smidt N., Swertz M.A., Bakker S.J.L., Dotinga A., Vonk J.M., van Dijk F., van Zon S.K.R., Wijmenga C., Wolffenbuttel B.H.R., Stolk R.P. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015;44:1172–1180. doi: 10.1093/ije/dyu229. [DOI] [PubMed] [Google Scholar]
- 68.Larman H.B., Laserson U., Querol L., Verhaeghen K., Solimini N.L., Xu G.J., Klarenbeek P.L., Church G.M., Hafler D.A., Plenge R.M., et al. PhIP-Seq characterization of autoantibodies from patients with multiple sclerosis, type 1 diabetes and rheumatoid arthritis. J. Autoimmun. 2013;43:1–9. doi: 10.1016/j.jaut.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patro R., Duggal G., Love M.I., Irizarry R.A., Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aitchison J. The Statistical Analysis of Compositional Data. J. Roy. Stat. Soc. B. 1982;44:139–160. doi: 10.1111/J.2517-6161.1982.TB01195.X. [DOI] [Google Scholar]
- 71.Zhernakova D.V., Le T.H., Kurilshikov A., Atanasovska B., Bonder M.J., Sanna S., Claringbould A., Võsa U., Deelen P., Franke L., et al. Individual variations in cardiovascular-disease-related protein levels are driven by genetics and gut microbiome. Nat. Genet. 2018;50:1524–1532. doi: 10.1038/s41588-018-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurilshikov A., Van Den Munckhof I.C.L., Chen L., Bonder M.J., Schraa K., Rutten J.H.W., Riksen N.P., De Graaf J., Oosting M., Sanna S., et al. Gut Microbial Associations to Plasma Metabolites Linked to Cardiovascular Phenotypes and Risk. Circ. Res. 2019;124:1808–1820. doi: 10.1161/CIRCRESAHA.118.314642. [DOI] [PubMed] [Google Scholar]
- 73.Zhernakova D.V., Deelen P., Vermaat M., Van Iterson M., Van Galen M., Arindrarto W., Van’t Hof P., Mei H., Van Dijk F., Westra H.J., et al. Identification of context-dependent expression quantitative trait loci in whole blood. Nat. Genet. 2017;49:139–145. doi: 10.1038/NG.3737. [DOI] [PubMed] [Google Scholar]
- 74.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/BIOINFORMATICS/BTS635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anders S., Pyl P.T., Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/BIOINFORMATICS/BTU638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aguirre-Gamboa R., de Klein N., di Tommaso J., Claringbould A., van der Wijst M.G., de Vries D., Brugge H., Oelen R., Võsa U., Zorro M.M., et al. Deconvolution of bulk blood eQTL effects into immune cell subpopulations. BMC Bioinf. 2020;21:243. doi: 10.1186/s12859-020-03576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Wijst M.G.P., Brugge H., de Vries D.H., Deelen P., Swertz M.A., LifeLines Cohort Study. BIOS Consortium. Franke L. Single-cell RNA sequencing identifies celltype-specific cis-eQTLs and co-expression QTLs. Nat. Genet. 2018;50:493–497. doi: 10.1038/s41588-018-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Satija R., Farrell J.A., Gennert D., Schier A.F., Regev A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/BIOINFORMATICS/BTP616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao Y., Wang J., Jaehnig E.J., Shi Z., Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friedman J., Hastie T., Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
LLD PhIP-Seq: Raw and processed PhIP-Seq data used for this study are available in the European Genome-Phenome Archive (EGA) and are publicly available from the date of publication. Accession number is listed in the key resources table. LLD single cell RNA-seq data: Raw sequencing reads used for this study are available in the European Genome-Phenome Archive (EGA). Accession number is listed in the key resources table. LLD Phenotypic data: Lifelines is specifically organized to make assessment results available for (re)use by third parties. Phenotypic data can be requested through Lifelines. A research proposal must be submitted for evaluation by the Lifelines Research Office.
-
•
All original code has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.