Abstract
Background and aims
Peroral endoscopic myotomy (POEM) is a standard treatment option for achalasia patients. Treatment response varies due to factors such as achalasia type, degree of dilatation, pressure and distensibility indices. We present an innovative approach for treatment response prediction based on an automatic three-dimensional (3-D) reconstruction of the tubular oesophagus (TE) and the lower oesophageal sphincter (LES) in patients undergoing POEM for achalasia.
Methods
A software was developed, integrating data from high-resolution manometry, timed barium oesophagogram and endoscopic images to automatically generate 3-D reconstructions of the TE and LES. Novel normative indices for TE (volume×pressure) and LES (volume/pressure) were automatically integrated, facilitating pre-POEM and post-POEM comparisons. Treatment response was evaluated by changes in volumetric and pressure indices for the TE and the LES before as well as 3 and 12 months after POEM. In addition, these values were compared with normal value indices of non-achalasia patients.
Results
50 treatment-naive achalasia patients were enrolled prospectively. The mean TE index decreased significantly (p<0.0001) and the mean LES index increased significantly 3 months post-POEM (p<0.0001). In the 12-month follow-up, no further significant change of value indices between 3 and 12 months post-POEM was seen. 3 months post-POEM mean LES index approached the mean LES of the healthy control group (p=0.077).
Conclusion
3-D reconstruction provides an interactive, dynamic visualisation of the oesophagus, serving as a comprehensive tool for evaluating treatment response. It may contribute to refining our approach to achalasia treatment and optimising treatment outcomes.
Trial registration number
22-0149.
Keywords: ACHALASIA, OESOPHAGEAL MOTILITY, MOTILITY DISORDERS
WHAT IS ALREADY KNOWN ON THIS TOPIC
Achalasia is a chronic oesophageal motility disorder. Treatment response varies due to factors such as achalasia type, degree of dilatation, pressure and distensibility indices.
WHAT THIS STUDY ADDS
This study presents an innovative approach for treatment response prediction based on an automatic three-dimensional (3-D) reconstruction of the oesophagus in patients undergoing Peroral endoscopic myotomy (POEM) for achalasia using a multimodal dataset.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This innovative 3-D reconstruction approach offers an interactive, dynamic visualisation of the oesophagus, potentially transforming achalasia management with POEM and improving treatment outcomes.
Introduction
Achalasia represents a rare chronic motility disorder characterised by the absence of deglutitive lower oesophageal sphincter (LES) relaxation and impaired propulsive tubular peristalsis.1 The therapeutic approach for achalasia patients focuses on alleviating the obstruction at the oesophagogastric junction (OGJ) by pneumatic dilatation (PD), peroral endoscopic myotomy (POEM) or laparoscopic Heller myotomy (LHM). Response to treatment can be influenced by factors such as type of achalasia, degree of dilatation, kinking of the tubular oesophagus (TE), pressure at the LES and its distensibility. In recent years, POEM has emerged as a first-line treatment option for achalasia, demonstrating clinical efficacy ranging from 80% to 95%.2–4 Clinical trials have primarily focused on assessing the clinical efficacy of treatment modalities, with less emphasis on understanding the pathophysiological changes that result from these therapeutic modifications in achalasia patients. The evaluation of symptoms in achalasia patients is highly subjective and often does not correlate with disease severity, making the Eckardt score (ES) an imperfect parameter for objective symptom assessment. Several studies have investigated high-resolution manometry (HRM), timed barium oesophagogram (TBE) and impedance planimetry (EndoFlip, functional Lumen Imaging Probe), to predict clinical responses post-POEM and showed mostly moderate predictive power.5–12 These measures do not comprehensively capture the multifaceted changes in geometry, pressure, volume and distensibility that occur following achalasia therapy. As up to 20% of achalasia patients experience symptom recurrence over time,4 13 there is a need to shift our therapeutic focus from solely evaluating clinical responses to analysing the underlying pathophysiological changes and remodelling processes induced by treatment.
In this paper, we propose an innovative methodology for the prediction of treatment response, which holds the potential to offer further insights into the remodelling and pathophysiological changes following achalasia treatment. Our approach involves the automatic three-dimensional (3-D) reconstruction of the oesophageal lumen and the LES using a multimodal dataset derived from oesophagogastroduodenoscopy (OGD), HRM and TBE. We aimed to evaluate this 3-D model using data from patients undergoing POEM for achalasia and compare it to a control group of healthy individuals.
Methods
Study design and setting
This study was conducted at the University Hospital Augsburg, a tertiary referral centre for POEM, in collaboration with the Faculty of Applied Computer Science at the University of Augsburg in Augsburg, Germany. The study enrolled patients with confirmed achalasia who presented for diagnostic evaluations and/or endoscopic treatment. There were no modifications to the study protocol following its initiation. Prior to inclusion in the study, all patients provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Development of the oesophageal 3-D reconstruction
In the initial phase of this study, a combination of inputs, including data from HRM, TBE and endoscopic images of the oesophagus from 80 treatment-naive achalasia patients, was retrospectively collected to create a software that automatically reconstructs the oesophageal lumen and the LES three-dimensionally.
The 3-D reconstruction of the oesophagus involves the import of a single TBE image and a variable number of OGD images in JPEG format, along with a representative HRM swallow sequence in CSV format. The TBE and OGD images are used to extract the oesophagus’ shape and diameter at predefined positions. The software ensures consistent mapping of all data sources to a shared scale.
The final software allows the interactive and dynamic 3-D reconstruction of the TE and the LES and displays the lengths of the TE and the LES. Finally, the calculation of numerical value indices was integrated. These indices are calculated independently for the TE and the LES and are derived from volumetric data and HRM pressure values extracted from the reconstructed 3-D images along the HRM sequence.
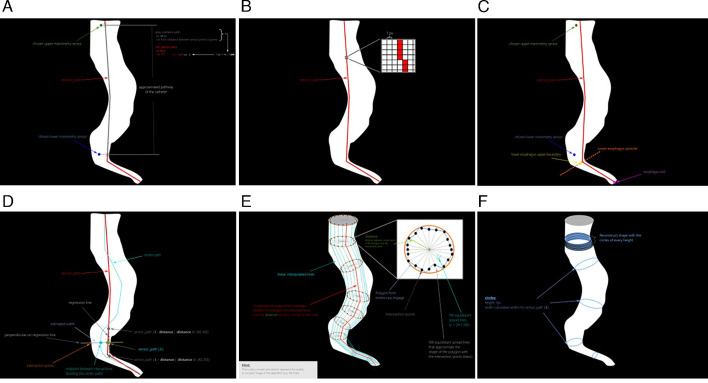
For further information on the specific details of the 3-D reconstruction software, please refer to online supplemental text 1 and figure 1A–F.
Figure 1.
(A–F). Steps of the oesophageal 3-D reconstruction software development. 3-D, three-dimensional.
bmjgast-2024-001396supp001.pdf (141.1KB, pdf)
Pilot-test phase
Prior to the automated numerical value indices software calculations, we delineated distinct scenarios for the TE and the LES and substantiated our hypotheses in a pilot-test phase. Our assumption for the calculation of our indices was based on the individual observed changes in each dataset following POEM. Concerning the TE, our observations indicated a predominant reduction in its volume post-POEM, contrasting with an increase in volume at the LES. On analysing the sole manometry data of achalasia patients before and after POEM, we noted a slight decrease in pressure values across the entire TE following the procedure. Conversely, a significant decrease in pressure was observed at the level of the LES post-POEM. Thus, for the entire cohort of achalasia patients, we hypothesised that in the TE, volume experiences a significant reduction following POEM while total pressure in the TE undergoes minimal reduction. Consequently, for the TE the formula volume (V)×pressure (P)=‘TE’ was formulated. We assumed that the V×P index exhibits a reduction post-POEM compared with pre-POEM. To obviate the potential error of yielding the same overall value from a reversal of multipliers, volume and pressure values were assessed independently, considering various constellations. In this pilot-test phase in 10 cases, minimum and maximum volume and pressure values were individually considered, and a hypothetical minimum and maximum combination were calculated for the specific subgroups of types I, II and III achalasia. Subsequently, pre-POEM and post-POEM indices were juxtaposed. In the rare, although sporadically reported occurrence of tubular motility restoration in achalasia patients following LES myotomy, we conducted fictive index calculations using a comparative group of healthy subjects exhibiting normal tubular peristalsis. This measure was undertaken to ensure the continued validity of our index calculations even in this infrequent scenario. Volume and pressure values were individually determined, and their maximum combination was calculated for comparison before and after POEM. For the LES, we presumed that volume undergoes a significant increase after POEM, concomitant with a decrease in pressure. Accordingly, the formula V/P=‘LES’ was derived for the LES. Predicated on alterations in individual values, we hypothesised that the V/P index significantly increases post-POEM. In 10 cases, minimum and maximum volume and pressure values for the LES were also individually considered and compared in their minimum and maximum hypothetical combinations with the pre-POEM and post-POEM indices.
Study population
Between June 2022 and September 2023, we prospectively collected multimodal datasets of HRM, TBE and OGD images of treatment-naive achalasia patients undergoing POEM. Subsequently, we employed the previously described software for 3-D reconstructions of the oesophagus at three distinct time points: prior to POEM (as an integral component of routine achalasia diagnosis), and at 3 and 12 months post-POEM. The time frame between prediagnostic assessment and POEM did not exceed 3 months. In addition to various other parameters, we prospectively documented the following parameters relevant to this paper: achalasia subtype, duration of disease (from the onset of symptoms to time of initial diagnosis), ES, location and length of the POEM myotomy, total or selective myotomy.
To establish normative indices using the proposed 3-D reconstruction software for the oesophagus, we retrospectively collected multimodal datasets from HRM, TBE and OGD images from a cohort of healthy controls. These individuals underwent diagnostic assessments with the aforementioned diagnostic modalities due to other symptoms, such as bloating or early satiety. However, subsequent manometric evaluation, following the criteria outlined in the Chicago 4 classification,14 confirmed the absence of major or minor motility disorders in this control group.
Prospective data collection
All OGD procedures were conducted using a high-definition video gastroscope (Olympus CF—HQ 190 video gastroscope, Olympus, Japan). Starting at the OGJ, OGD images were acquired at 1 cm intervals while maintaining maximal insufflation. The endoscope was positioned centrally within the oesophagus and oriented perpendicularly to it. The acquired images were stored in jpeg format. A minimum of 10 images per OGD were employed for prospective 3-D reconstruction.
TBE was performed in a fasting state and with ingestion of 150 mL of a 45% w/v barium suspension (Microbar barium Suspension). Static images were captured while the patient assumed a left posterior oblique standing position at 1, 2 and 5 min after swallowing barium suspension.15 All images were saved in jpeg format. By default, the TBE image captured at the 5 min mark was used for the 3-D reconstruction process. This same protocol was consistently applied during repeat TBE examinations conducted at the 3-month and 12-month follow-up intervals.
Following a minimum 6-hour fasting period, HRM studies were conducted using a 24-channel water-perfused catheter (Laborie Medical Technologies, Portsmouth, USA). Side holes connected to pressure transducers were positioned at 2 cm intervals for pressure analysis, spanning from the pharynx to the LES. Within the LES area, holes were spaced at 5 mm intervals and oriented at 120° angles in a spiral arrangement. The transnasally inserted HRM catheter was accurately positioned to record pressure measurements from the hypopharynx to the stomach, including approximately three intragastric pressure sensors. Manometry data were subsequently analysed in accordance with the Chicago classification V.4.0 (14). Following a 2 min baseline recording, the HRM protocol was performed, involving 10 liquid swallows of 5 mL while the patient assumed a 45° supine position. For 3-D reconstruction, the HRM swallow data were stored in accordance with the previously described methodology.
OGD and HRM were consistently conducted on the same day while TBE was performed on the subsequent day.
3-D reconstruction and numerical value indices calculations
For each enrolled patient, a 3-D reconstruction of the oesophagus was conducted at three distinct time points. Each oesophageal 3-D reconstruction was played back over the duration of a single swallowing cycle. For outcome calculations, the individual numerical value indices were documented for the TE and the LES, respectively.
In assessing normative value indices for both the LES and TE, 3-D reconstructions were conducted on a cohort of healthy controls. Numeric value indices were recorded and calculated in the same manner and referred to as ‘TE(N)’ for the TE and ‘LES(N)’ for the LES.
Additionally, for visual assessment, 3-D reconstruction images captured before and after the POEM procedure were saved for each patient, corresponding to the same time point of HRM.
Outcomes
The primary outcome of this study was to assess volumetric and pressure indices derived from interactive 3-D visualisation of the oesophagus in achalasia patients undergoing POEM and compare them to corresponding normal value 3-D reconstruction indices. Numeric value indices were collected separately for the TE and the LES at three distinct time points: prior to POEM (designated as ‘TE(0)’ and ‘LES(0)’), at 3 months post-POEM (‘TE(3)’ and ‘LES(3)’), and at 12 months post-POEM (‘TE(3)’ and ‘LES(12)’). Mean and median numeric value indices were compared in two stages: before and 3 months after POEM, as well as 3 months and 12 months after POEM. A good clinical response was defined as an ES of ≤3.
Secondary outcomes encompassed the assessment of geometric alterations and remodelling following POEM, based on visual analysis of volumetric and pressure changes observed in the 3-D reconstruction of the oesophagus.
Statistical analysis
In the absence of reference data from previous studies regarding numeric value indices derived from oesophageal 3-D reconstructions, formal sample size calculations were not conducted. All statistical analyses were carried out using SPSS V.28.0 (SPSS) and were performed on the full analysis dataset. Categorical outcomes were presented as ‘N’ or percentages, continuous outcomes as mean±SD or median (IQR). Outcomes were analysed using the Fisher’s exact test and Mann-Whitney U test for paired samples. A p<0.05 (two sided) was considered statistically significant.
Results
Baseline clinical characteristics and procedure details
Between June 2022 and September 2023, a total of 50 patients diagnosed with treatment-naive achalasia who possessed a full data set of HRM, OGD and TBE images, before POEM were prospectively enrolled in this study. All 50 patients (100%) presented with the full data set 3 months post-POEM. 16 patients (32.0%) from this cohort with a complete dataset at the 12 months follow-up, were included for secondary assessment. Baseline patient characteristics and procedural details are presented in table 1. 60.0% were male, with a mean age of 55.48±18.48 years. Mean duration of disease was 2.77±2.1 years. Based on the Chicago classification v4,14 the distribution of achalasia types included 26.0% type I, 66.0% type II and 8.0% type III. All POEMs were conducted as selective circular myotomy with a median tubular myotomy length of 10±1.50 cm. Median gastric myotomy lengths were 2±0.50 cm. 64.0% of the POEM procedures were performed via an anterior (2 o’clock) tunnel approach. 92.0% of patients showed a good clinical response to POEM according to an ES≤3, resulting in a significant reduction in mean ES (6.53 to 1.1; p<0.001).
Table 1.
Baseline clinical characteristics and procedure details of the study participants
| Parameters | 3-D reconstruction cohort (N=50) |
| Age (years) | |
| Mean (SD) | 55.48±18.48 |
| Median (IQR) | 55.5 (18–89) |
| Sex, n (%) | |
| Male | 30/50 (60.0%) |
| Female | 20/50 (40.0%) |
| Type of achalasia, n (%) | |
| Type I | 13/50 (26.0%) |
| Type II | 33/50 (66.0%) |
| Type III | 4/50 (8.0%) |
| Duration of symptoms, years | |
| Mean (SD) | 2.77±2.1 |
| Median (IQR) | 2 (1–4) |
| Eckardt score ≤3 after POEM, n (%) | 46/50 (92.0%) |
| POEM myotomy, n (%) | |
| Selective myotomy | 50/50 (100%) |
| Full thickness myotomy | 0/50 (0%) |
| POEM tubular myotomy length (cm) | |
| Mean (SD) | 10±1.50 |
| Median (IQR) | 10 (8–15) |
| POEM gastric myotomy length (cm) | |
| Mean (SD) | 2±0.50 |
| Median (IQR) | 2 (1–3) |
| POEM orientation of myotomy, n (%) | |
| Anterior (1–2 o′clock) | 32 (64.0%) |
| Posterior (5 o′clock) | 18 (36.0%) |
3-D, three-dimensional; POEM, peroral endoscopic myotomy.
Pilot-test phase calculations
In the pretest, we documented the minimum and maximum volumes, as well as pressure values, for the TE and the LES in patients with achalasia types I, II and III, both before and after POEM. With the underlying hypothesis of a significant reduction in the VxP index for the TE post-POEM and a corresponding significant increase in the V/P index for the LES after POEM, we conducted index calculations.
In cases where tubular motility was restored post-POEM, we incorporated the minimum and maximum pressure values from our comparative group of healthy subjects, performing additional combination index calculations. The computation of minimum and maximum combination indices consistently revealed a reduction in the V×P index for the TE after a successful POEM, regardless of the subgroup. Similarly, for the LES, there was a consistent and significant increase in the V/P index post-POEM in the calculation of minimum and maximum combination indices. Our pretest findings affirmed the realistic nature of our combination indices for both the TE and the LES.
Normal value indices
To establish normal value indices, 3-D reconstructions of the oesophagus were performed on nine non-achalasia controls and are presented in table 2. Mean TE(N) index was 340.39±396.7 cm³×mm Hg, while mean LES(N) index was 0.5269±0.733 cm³/mm Hg.
Table 2.
Primary outcomes—comparison of numeric value indices prior and 3 months after POEM in achalasia study patients and in comparison with normal value indices of healthy controls
| Parameters | Prior to POEM (N=50) | 3 months post-POEM (N=50) | P value | 3 months post-POEM (N=50) | Healthy control (N=9) | P value |
| Eckardt score | <0.001* | |||||
| Mean (SD) | 6.53 (2.25) | 1.1 (1.45) | ||||
| Median (IQR) | 6 (3–12) | 0 (0–6) | ||||
| Tubular oesophagus (TE) (V×P), (cm³×mm Hg) | TE (0) | TE (3) | <0 .0001* | TE (3) | TE (N) | 0.010* |
| Mean (SD) | 3438.43 (3805) | 952.27 (1077.7) | 952.27 (1077.7) | 340.39 (396.7) | ||
| Median (IQR) | 2479.5 (164.77–17 879) | 682 (49–5133.5) | 682 (49–5133.5) | 209 (57.5–1337) | ||
| Lower oesophagus sphincter (LES) (V/P), (cm³/mmHg) | LES (0) | LES (3) | <0 .0001* | LES (3) | LES (N) | 0.077* |
| Mean (SD) | 0.1950 (0.3605) | 0.9955 (0.9966) | 0.9955 (0.9966) | 0.5269 (0.733) | ||
| Median (IQR) | 0.0548 (0.0001–2.26) | 0.6372 (0.03–4.51) | 0.6372 (0.03–4.51) | 0.3323 (0.03–2.36) |
*Mann-Whitney U test.
POEM, peroral endoscopic myotomy.
Primary outcomes
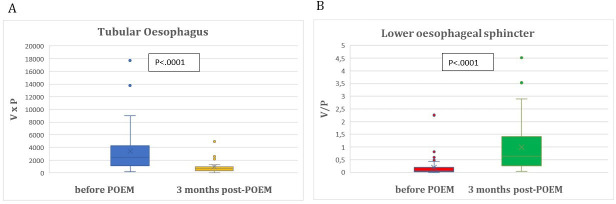
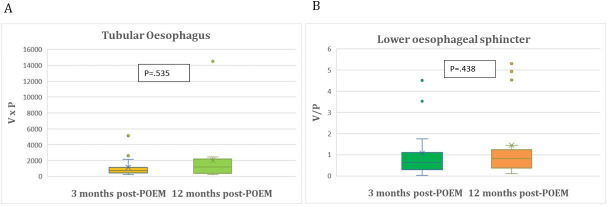
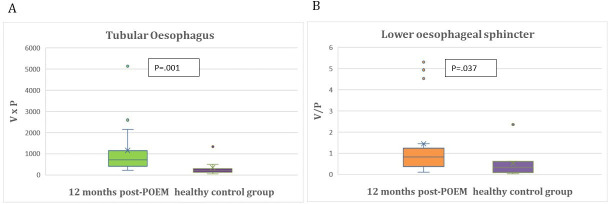
Primary outcomes are detailed in tables 2 and 3. Mean TE(0) index was 3438.43±3805.64 cm³×mm Hg and decreased significantly 3 months after POEM to mean TE(3) index of 952.27±1077.7 cm³×mm Hg (p<0.0001). Mean LES(0) index was 0.1950±0.3605 cm³/mm Hg and increased significantly 3 months post-POEM to mean LES(3) index of 0.9955±0.9966 cm³/mm Hg (p<0.0001) (figure 2A,B). Among the 16 patients who participated in the second follow-up at 12 months, mean TE(3) and mean TE(12) index (p=0.535), as well as mean LES(3) and mean LES(12) index (p=0.438) did not differ significantly (table 3) and (figure 3A,B).
Table 3.
Primary outcomes—comparison of numeric value indices 3 months and 12 months after POEM in achalasia study patients and in comparison with normal value indices of healthy controls
| Parameters | 3 months post-POEM (N=16) | 12 months post-POEM (N=16) | P value | 12 months post- POEM (N=16) |
Healthy control (N=9) |
P value |
| Eckardt score | 0.048* | |||||
| Mean (SD) | 1.69 (1.74) | 2.56 (2.37) | ||||
| Median (IQR) | 2 (0–6) | 3 (0–9) | ||||
| Tubular oesophagus (TE), (V×P), (cm³×mm Hg) | TE (3) | TE (12) | 0.535* | TE (12) | TE (N) | 0.001* |
| Mean (SD) | 1153.22 (1248.28) | 2013.22 (3424.7) | ||||
| Median (IQR) | 721 (212.5–5133.5) | 1185.25 (251–14 521.5) | 2013.22 (3424.7) 1185.25 (251–14 521.5) |
340.39 (396.7) 209 (57.5–1337) |
||
| Lower oesophagus sphincter (LES), (V/P), (cm³/mmHg) | LES (3) | LES (12) | 0.438* | LES (12) | LES (N) | 0.037* |
| Mean (SD) | 1.0593 (1.2609) | 1.4521 (1.7681) | 1.4521 (1.7681) | 0.5269 (0.733) | ||
| Median (IQR) | 0.6387 (0.03–4.51) | 0.8247 (0.11–5.3) | 0.8247 (0.11–5.3) | 0.3323 (0.03–2.36) |
*Mann-Whitney U test.
POEM, peroral endoscopic myotomy.
Figure 2.
(A) Comparison of tubular oesophagus indices prior and 3 months after POEM. (B) Comparison of lower oesophageal sphincter indices prior and 3 months after POEM. POEM, peroral endoscopic myotomy.
Figure 3.
(A) Comparison of tubular oesophagus indices 3 months and 12 months after POEM in achalasia study patients. (B) Comparison of lower oesophageal sphincter indices 3 months and 12 months after POEM in achalasia study patients. POEM, peroral endoscopic myotomy.
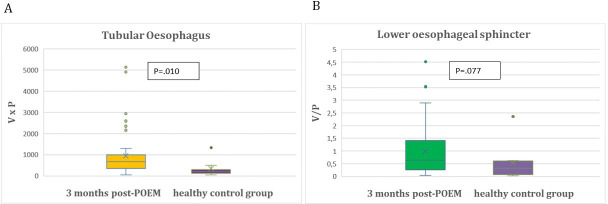
Despite these significant improvements in TE(3) and LES(3) as well as TE(12) and LES(12) indices post-POEM when compared with baseline TE(0) and LES(0) indices before POEM, TE(3) TE(12) and LES(12) still exhibit a statistically significant difference in comparison to those of the healthy control group (p=0.010; p<0.001; p=0.037). However, 3 months post-POEM mean LES(3) index approached the mean LES(N) of the healthy control group (p=0.077) (tables 2 and 3) and (figures 4A,B and 5A,B).
Figure 4.
(A) Comparison of tubular oesophagus indices of healthy controls and 3 months after POEM in achalasia study patients. (B) Comparison of lower oesophageal sphincter indices of healthy controls and 3 months after POEM in achalasia study patients. POEM, peroral endoscopic myotomy.
Figure 5.
(A) Comparison of tubular oesophagus indices of healthy controls and 12 months after POEM in achalasia study patients. (B) Comparison of lower oesophageal sphincter indices of healthy controls and 12 months after POEM in achalasia study patients. POEM, peroral endoscopic myotomy.
Secondary outcomes
On visual assessment of the 3-D reconstructions before and 3 months after POEM, it was observed that patients with initially narrow tubular oesophagi exhibited widening post-POEM, despite a successful myotomy at the LES, as evidenced by a significant increase in the LES index value (figure 6). Conversely, patients with a wider TE prior to POEM exhibited narrowing post-POEM (figure 7).
Figure 6.
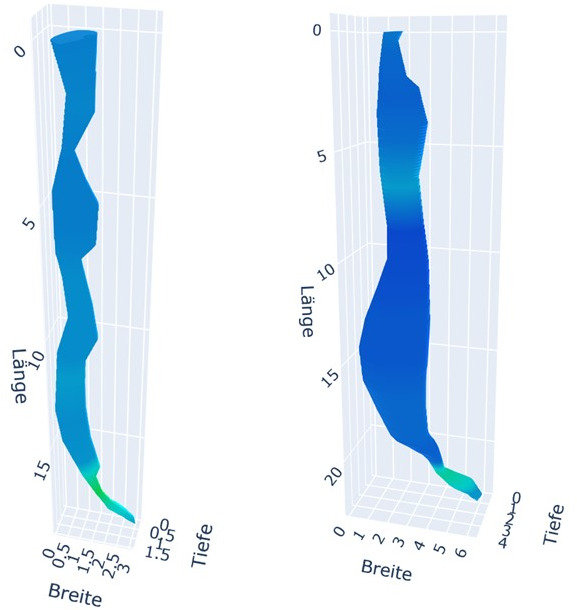
Visual assessment of three-dimensional reconstructions before and 3 months after POEM. Left figure: initial narrow tubular oesophagus before POEM. Right figure: widening of the tubular oesophagus 3 months post-POEM. POEM, peroral endoscopic myotomy.
Figure 7.
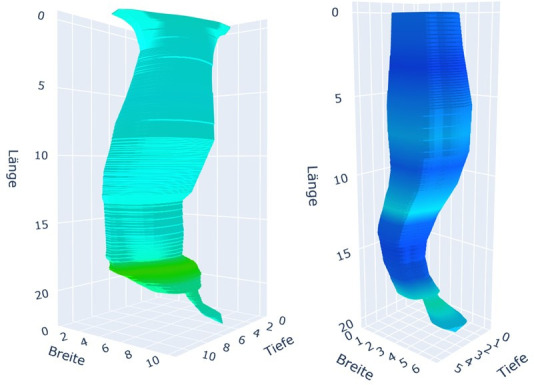
Visual assessment of three-dimensional reconstructions before and 3 months after POEM. Left figure: initial wide tubular oesophagus before POEM. Right figure: narrowing of the tubular oesophagus 3 months post-POEM. POEM, peroral endoscopic myotomy.
Discussion
This is the first study to assess treatment outcomes in achalasia patients following POEM using an interactive and dynamic 3-D visualisation of the oesophagus, incorporating a multimodal data set comprising HRM, TBE and OGD images. Based on the assumption that volume and pressure values change differently for the TE and the LES after POEM, we proposed two novel indices combining volume and pressure values as potential parameters for predicting treatment success after POEM. The realistic nature of these combination indices was confirmed in a pretest, which was regardless of the subgroup type. The indices showed a significant reduction for the TE and a significant increase for the LES in pretreatment versus post-treatment comparisons. Despite the significant improvements observed in the TE and LES volume and pressure indices following POEM, it is notable that TE(3) and TE(12) remain significantly different from the indices observed in healthy controls. The alterations observed in the TE in achalasia patients do not appear to be entirely reversible, even following an appropriate myotomy at the LES. Nevertheless, it is worth highlighting that 3 months post-POEM, the mean LES(3) index approached the mean LES(N) of the healthy control group. Owing to the limited sample size and the high clinical response rate of 92%, the application of logistic regression models or receiver operating characteristic (ROC) calculations for determining the optimal discriminators of a favourable clinical response was not deemed reliable. It was, therefore, not performed but remains subject to further study with a larger population. Additionally, owing to the limited sample size, a reliable correlation between symptom duration and pre-POEM dimensions could not be established. In our cohort of 50 patients, we observed a correlation between symptom response and improvement in both the TE and the LES, with individuals experiencing minimal change in either index demonstrating insignificant symptom alleviation. However, the magnitude of improvement also appears to vary based on the type of achalasia. Given the high clinical response rate of 92% and the overall small sample size of 50 patients encompassing all achalasia subtypes, a statistically significant calculation of ROC or logistic regression was not deemed feasible.
As achieving clinical success following achalasia therapy is the primary objective, many studies have evaluated treatment efficacy in achalasia patients using clinical measures such as the ES.2 3 16 The ES has shown fair reliability and validity in the assessment of clinical response after treatment17 18; however, the ES is inadequate and insensitive in revealing the causes of insufficient symptom relief or recurrent symptoms and fails to capture the complexity of underlying anatomical and pathophysiological changes in these patients.19 Other studies have focused on objective measures to evaluate and predict treatment outcomes in achalasia patients. In a small patient cohort, changes in integrated relaxation pressure (IRP) following POEM were found to be positively correlated with changes in the ES.20 Ghoshal et al have shown that LES pressure on HRM studies and oesophageal emptying rate on TBE are comparable in assessing the treatment outcome of achalasia patients 1 month after PD.7 Rohof et al, however, demonstrated that oesophageal stasis on TBE serves as a superior predictor of treatment failure in long-standing achalasia compared with LES pressure on HRM (88% vs 20%).11 Measuring the difference in the height of the contrast column in TBE before and after treatment has shown a strong correlation with clinical success following PD and LHM.5 Consequently, TBE is considered a reliable objective measure for predicting treatment outcomes. Sanagapalli et al have further illustrated that the alteration in barium surface area is an even more superior metric for predicting treatment response in achalasia patients compared with the conventional 5 min barium height.21 Impedance measurements using the Endoflip device have demonstrated promise in monitoring and predicting treatment outcomes during POEM or LHM procedures, enabling real-time adjustments to myotomy lengths and depths. EGJ distensibility correlates with symptom severity in achalasia patients9 12 and has proven to be a superior parameter for evaluating treatment efficacy compared with LES pressure10 or the detection of oesophageal retention relative to IRP.5 Percent change (∆%) in distensibility index and cross-sectional area (CSA) with optimal cut-off values of 272% and 360%, respectively, have been identified as reliable parameters for predicting successful treatment outcomes following POEM.8 DeWitt et al have demonstrated equally good to excellent sensitivity in predicting clinical response for HRM and Endoflip.22 While Endoflip has undergone evaluation in studies examining tubular contractility and contraction patterns in response to volume-controlled distension and has been used to map the mechanical work and behaviour of the oesophageal wall in relation to distension and pressure during contractility,23–25 its applicability may be limited in addressing alterations in oesophageal anatomy among achalasia patients, particularly when dysphagia is attributed to kinks or obstructions distant from the LES. In contrast to these measures, our proposed 3-D reconstruction combines the information of anatomical alterations with volumetric and pressure data along the entire TE and the LES. This comprehensive evaluation can be selectively analysed using dynamic and interactive 3-D visualisation, potentially offering more precise predictors of treatment response.
To date, relatively few studies have been published on 3-D reconstruction of the oesophagus. Mittal et al employed a combination of CT scans and 3-D-HRM to evaluate the functional morphology of the LES in 10 healthy adults. Their findings revealed a distinctive circumferentially asymmetric pressure pattern of the LES, which correlated with variations in LES lengths.26 In addition, they investigated the 3-D pressure profiles of the LES in patients with achalasia, also using 3-D-HRM and CT scans for 3-D reconstruction and concluded that specific anatomical alterations in the LES could be discerned in achalasia patients, potentially influencing the optimal lengths and orientation of myotomy procedures for LES pressure reduction.27 Cai et al reported on an innovative methodology for 3-D oesophageal reconstruction in patients with oesophageal cancer, using CT scans in conjunction with an electromechanical rendering approach.28 Halder et al have reported on the 3-D reconstruction of the oesophagus using MRI or fluoroscopy. Based on a convolutional neural network performing segmentation of image sequences, this innovative methodology combines the calculation of anatomical data with fluid velocity and pressure distribution during oesophageal transit, correlating with oesophageal wall stiffness and active relaxation.29 30
Our second outcome entailed identifying anatomical and pressure changes following POEM through visual assessment of 3-D reconstructions. While we acknowledge the inherent subjectivity of visual assessment, 3-D reconstruction may serve as a tool for enhancing our comprehension and visualisation of post-therapy alterations. As anticipated, we observed a reduction in tubular volume following a successful LES myotomy in initially dilated oesophagi, such as type I or II. However, in patients with initially less dilated oesophagi, such as in type III, the tubular volume increased along the entire TE despite sufficient LES myotomy (figures 6 and 7).
This observation aligns with the findings of Kim et al regarding morphological changes in oesophageal body movement during bolus transport following POEM. The study group demonstrated distinct morphological changes 3–6 months post-POEM in type III and type II/I achalasia. In type III achalasia, the main morphological changes post-POEM included a decrease in muscle thickness and muscle cross-sectional area, along with an increase in lumen cross-sectional area (LCSA). In contrast, a significant decrease in LCSA was observed in types I and II achalasia following POEM due to an effective reduction in LES pressure.31 This observation, derived from the visual assessment of 3-D reconstructions, is not captured by ES, HRM, Endoflip or TBE alone. Our findings support the hypothesis that in patients with a less dilated TE, the structural integrity of the oesophagus remains intact. In these patients, tubular myotomy disrupts the oesophageal integrity, leading to an increase in LCSA. Kim et al demonstrated that, despite the LCSA increase, oesophageal body distensibility during bolus transit improves physically at least up to 6 months. In contrast, patients with pre-existing oesophageal dilation or disrupted integrity, such as type I achalasia, cannot experience a significant improvement in lumen-reducing oesophageal contractility after tubular myotomy. This suggests that oesophageal contractility abnormalities in type I achalasia may be irreversible, even following successful LES decompression.31
These observations, gleaned purely through visual assessment of the combined anatomical and pressure-related changes of the 3-D reconstructions before and after POEM, may have the potential to gain insights into achalasia pathophysiology and the pathophysiological changes post-therapy. This aspect is to be further investigated in a future research project involving a larger number of patients, which will also facilitate differentiation between various achalasia subtypes.
Up to this point, our oesophageal 3-D reconstruction has exclusively incorporated the combination of volume and pressure for the entire TE and LES. In the subsequent phase, our objective is to conduct separate analyses for every 1 cm segment of the TE and LES and to incorporate data from impedance planimetry and endosonography to enhance diagnostic accuracy on oesophageal wall thickness and distensibility, allowing for a comprehensive analysis of oesophageal anatomy and pressure distribution for each segment of both the TE and LES during oesophageal transit.
As a result, 3-D oesophageal reconstruction, characterised by its precise visualisation capabilities, can effectively correlate anatomy, pressure, and, in a subsequent approach, distensibility and wall thickness. This approach may provide specific insights into the post-therapy pathophysiological changes, thereby holding the potential to discern the optimal treatment strategy. By tailoring therapy to each patient’s distinctive geometric and pressure characteristics, including considerations related to myotomy length and orientation, it may become possible to optimise treatment outcomes in the long term and to assist physicians in the optimal treatment choice based on pathophysiological concepts.
Guillaumot et al have suggested that 3-D-HRM could assist in selecting the side and orientation of the myotomy based on the location of the highest-pressure zones. In cases where an asymmetric high-pressure zone is observed, this information could guide the choice of myotomy side. At the same time, a circumferentially uniform pressure distribution might lead to the selection of pneumatic dilation.32 Moreover, 3-D oesophageal reconstruction may aid in identifying the optimal approach for retreatment, the most suitable timing for such interventions and facilitates early identification of treatment response adequacy, such as blown-out myotomy due to oesophageal wall strain in the area weakened by myotomy, for example, due to continued oesophageal outflow obstruction.33 34
Our proposed 3-D reconstruction software currently exhibits several limitations:
The software presently requires manual delineation of the oesophageal lumen and the outline of the oesophageal swallow, and the height of imported oesophageal images must be manually recorded during OGD. This manual input process introduces potential inaccuracies. The inaccuracies in data mapping may be addressed by incorporating new technologies capable of automatically measuring specific objects or lumina.
Another limitation is that the use of retrospective image data in calculating indices for the healthy control group, particularly in OGD images, was not consistently recorded according to the standard data acquisition protocol.
In this initial 3D reconstruction approach, we integrated OGD, HRM and TBE datasets in their standard implementations, despite variations in body positions and diagnostic consistencies (barium vs water vs air), which may have introduced some degree of inaccuracy. OGD and HRM were consistently performed on the same day while TBE was conducted on the subsequent day to mitigate inaccuracies stemming from food retention and potential confounding effects from different time intervals. In our forthcoming evaluation of 3D reconstruction on a larger dataset, we will ensure consistency in body positioning to minimise this source of inaccuracy. Furthermore, ongoing research aims to compare different settings and positions to further enhance accuracy.
The current software is only variable for pressure data but does not account for volumetric changes for each swallow. Future enhancements aim to integrate multiple TBS images captured at various time points and multiple OGD images.
The limited sample size and the lack of assessment of procedural characteristics as outcome parameters impede the ability to comprehensively evaluate different achalasia subtypes and different treatment approaches. Also, the assessment of gastro-oesophageal reflux disease post-POEM based on the 3-D reconstructions was not included.
In the subsequent phase, we intend to incorporate powered sample size calculations for different achalasia subtypes and various outcome parameters (anterior vs posterior, difference in tubular and gastric myotomy lengths). With the refinement of our software, we hope to gain more insights into the most effective treatment strategies, based on information revealed through 3-D oesophageal reconstruction, potentially reshaping our treatment strategies to focus on pathophysiological alterations rather than relying solely on clinical parameters.
Furthermore, this interactive and dynamic 3-D visualisation of the oesophagus can serve as an educational tool not only for medical students but also for patients. It enables physicians to effectively convey complex anatomical concepts to patients, fostering improved understanding and facilitating informed decision-making.
Conclusion
This 3-D reconstruction facilitates an interactive and dynamic 3-D visualisation of the oesophagus, incorporating multiple datasets. It holds the potential to guide and enhance treatment outcomes for achalasia patients while potentially providing valuable insights into post-treatment remodelling and pathophysiological changes. These insights may subsequently influence our approach to treatment. Further research is warranted to comprehensively evaluate the application of 3-D oesophageal reconstruction in achalasia patients across various treatment approaches and modalities.
Footnotes
Contributors: Study concept and design: SN and AE; Acquisition of data: SN, AE, VG, ME, AB, TK, LP, PS, FS and BB. Analysis and interpretation of data: SN and AM; Drafting of the manuscript: SN, VG and AE; Critical revision of manuscript: SN, AE, VG, AM, HM, ME, AB, TK, LP, PS, FS and BB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: HM: Olympus, Satisfai (Grants); Dr. Falk Pharma, Olympus, Norgine, IPSEN, medupdate, Erbe (Speakers fee); Olympus, Ambu, Boston Scientific, Covidien, Takeda (Consultation fees). AE: Olympus Medical, FujiFilm, Pentax, Ambu, Falk Pharma and Medtronic (Lecture fees). SN: Falk Pharma, Pfizer and Sanofi (Lecture fees).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The clinical study was performed in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the medical ethics committee of the Ludwig-Maximilians University of Munich on 27 June 2022 (registration number: 22-0149).
References
- 1. Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014;383:83–93. 10.1016/S0140-6736(13)60651-0 [DOI] [PubMed] [Google Scholar]
- 2. Ponds FA, Fockens P, Lei A, et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: a randomized clinical trial. JAMA 2019;322:134–44. 10.1001/jama.2019.8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Werner YB, Hakanson B, Martinek J, et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med 2019;381:2219–29. 10.1056/NEJMoa1905380 [DOI] [PubMed] [Google Scholar]
- 4. Kuipers T, Ponds FA, Fockens P, et al. Peroral endoscopic myotomy versus pneumatic dilation in treatment-naive patients with achalasia: 5-year follow-up of a randomised controlled trial. Lancet Gastroenterol Hepatol 2022;7:1103–11. 10.1016/S2468-1253(22)00300-4 [DOI] [PubMed] [Google Scholar]
- 5. Andersson M, Lundell L, Kostic S, et al. Evaluation of the response to treatment in patients with idiopathic achalasia by the timed barium esophagogram: results from a randomized clinical trial. Dis Esophagus 2009;22:264–73. 10.1111/j.1442-2050.2008.00914.x [DOI] [PubMed] [Google Scholar]
- 6. Carlson DA, Baumann AJ, Prescott JE, et al. Prediction of esophageal retention: a study comparing high-resolution manometry and functional luminal imaging probe panometry. Am J Gastroenterol 2021;116:2032–41. 10.14309/ajg.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghoshal UC, Gupta M, Verma A, et al. High-resolution manometry is comparable to timed barium esophagogram for assessing response to pneumatic dilation in patients with achalasia. Indian J Gastroenterol 2015;34:144–51. 10.1007/s12664-015-0551-x [DOI] [PubMed] [Google Scholar]
- 8. Moran RA, Brewer Gutierrez OI, Rahden B, et al. Impedance planimetry values for predicting clinical response following peroral endoscopic myotomy. Endoscopy 2021;53:570–7. 10.1055/a-1268-7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandolfino JE, de Ruigh A, Nicodème F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25:496–501. 10.1111/nmo.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328–35. 10.1053/j.gastro.2012.04.048 [DOI] [PubMed] [Google Scholar]
- 11. Rohof WO, Lei A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol 2013;108:49–55. 10.1038/ajg.2012.318 [DOI] [PubMed] [Google Scholar]
- 12. Su B, Callahan ZM, Novak S, et al. Using impedance planimetry (Endoflip) to evaluate myotomy and predict outcomes after surgery for achalasia. J Gastrointest Surg 2020;24:964–71. 10.1007/s11605-020-04513-w [DOI] [PubMed] [Google Scholar]
- 13. Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic heller myotomy. Gut 2016;65:732–9. 10.1136/gutjnl-2015-310602 [DOI] [PubMed] [Google Scholar]
- 14. Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: chicago classification version 4.0(©). Neurogastroenterol Motil 2021;33:e14058. 10.1111/nmo.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Oliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997;169:473–9. 10.2214/ajr.169.2.9242756 [DOI] [PubMed] [Google Scholar]
- 16. Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic heller myotomy (LHM) for the treatment of type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015;3:E195–201. 10.1055/s-0034-1391668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil 2018;30:e13287. 10.1111/nmo.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren Y, Tang X, Chen Y, et al. Pre-treatment eckardt score is a simple factor for predicting one-year peroral endoscopic myotomy failure in patients with achalasia. Surg Endosc 2017;31:3234–41. 10.1007/s00464-016-5352-5 [DOI] [PubMed] [Google Scholar]
- 19. Chandrasekhara V, Desilets D, Falk GW, et al. The American society for gastrointestinal endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on peroral endoscopic myotomy. Gastrointest Endosc 2015;81:1087–100. 10.1016/j.gie.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 20. Tang Y, Xie C, Wang M, et al. Association of high-resolution manometry metrics with the symptoms of achalasia and the symptomatic outcomes of peroral esophageal myotomy. PLoS ONE 2015;10:e0139385. 10.1371/journal.pone.0139385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanagapalli S, Plumb A, Maynard J, et al. The timed barium swallow and its relationship to symptoms in achalasia: analysis of surface area and emptying rate. Neurogastroenterology Motil 2020;32. 10.1111/nmo.13928 [DOI] [PubMed] [Google Scholar]
- 22. DeWitt JM, Siwiec R, Kessler WR, et al. Comparison of functional lumen imaging probe and high-resolution manometry to assess response after peroral endoscopic myotomy. Gastrointest Endosc 2022;95:855–63. 10.1016/j.gie.2021.12.029 [DOI] [PubMed] [Google Scholar]
- 23. Acharya S, Halder S, Carlson DA, et al. Assessment of esophageal body peristaltic work using functional lumen imaging probe panometry. Am J Physiol Gastrointest Liver Physiol 2021;320:G217–26. 10.1152/ajpgi.00324.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donnan EN, Pandolfino JE. Endoflip in the esophagus: assessing sphincter function, wall stiffness, and motility to guide treatment. Gastroenterol Clin North Am 2020;49:427–35. 10.1016/j.gtc.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halder S, Yamasaki J, Acharya S, et al. Virtual disease landscape using mechanics-informed machine learning: application to esophageal disorders. Artif Intell Med 2022;134:102435. 10.1016/j.artmed.2022.102435 [DOI] [PubMed] [Google Scholar]
- 26. Mittal RK, Zifan A, Kumar D, et al. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol 2017;313:G212–G219. 10.1152/ajpgi.00130.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mittal RK, Kumar D, Kligerman SJ, et al. Three-dimensional pressure profile of the lower esophageal sphincter and crural diaphragm in patients with achalasia esophagus. Gastroenterology 2020;159:864–72. 10.1053/j.gastro.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai H, Wang R, Li Y, et al. Role of 3D reconstruction in the evaluation of patients with lower segment oesophageal cancer. J Thorac Dis 2018;10:3940–7. 10.21037/jtd.2018.06.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halder S, Acharya S, Kou W, et al. Mechanics informed fluoroscopy of esophageal transport. Biomech Model Mechanobiol 2021;20:925–40. 10.1007/s10237-021-01420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halder S, Johnson EM, Yamasaki J, et al. MRI-MECH: mechanics-informed MRI to estimate esophageal health. Front Physiol 2023;14:1195067. 10.3389/fphys.2023.1195067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim AJS, Ong S, Kim JH, et al. Morphologic changes in esophageal body movement during bolus transport after peroral endoscopic myotomy in type III achalasia. J Neurogastroenterol Motil 2022;28:131–44. 10.5056/jnm21020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guillaumot M-A, Léandri C, Leblanc S, et al. Three-dimensional high-resolution esophageal manometry study of the esophagogastric junction in patients with achalasia. Dig Dis Sci 2020;65:1092–8. 10.1007/s10620-019-05824-y [DOI] [PubMed] [Google Scholar]
- 33. Halder S, Acharya S, Kou W, et al. Myotomy technique and esophageal contractility impact blown-out myotomy formation in achalasia: an in Silico investigation. Am J Physiol Gastrointest Liver Physiol 2022;322:G500–12. 10.1152/ajpgi.00281.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Triggs JR, Krause AJ, Carlson DA, et al. Blown-out myotomy: an adverse event of laparoscopic heller myotomy and peroral endoscopic myotomy for achalasia. Gastrointest Endosc 2021;93:861–8. 10.1016/j.gie.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2024-001396supp001.pdf (141.1KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.