Key Points
Question
How does youth-onset prediabetes prevalence differ by exposure to adverse social determinants of health (SDOH), independent of race and ethnicity?
Findings
In this cross-sectional study including a nationally representative sample of 1563 adolescents aged 12 to 18 years with obesity, the SDOH categories of food insecurity, lack of private health insurance, and lower household income were associated with higher prediabetes prevalence, independent of race and ethnicity.
Meaning
These findings suggest that adverse SDOH should be recognized in clinical settings and used to guide efforts to reduce risk of youth-onset type 2 diabetes.
This cross-sectional study evaluates differences in prediabetes prevalence by intersectional social determinants of health (SDOH) and race or ethnicity categories in a nationally representative sample of US adolescents eligible for type 2 diabetes screening based on age and body mass index–based overweight or obesity.
Abstract
Importance
Several clinical practice guidelines advise race- and ethnicity-based screening for youth-onset type 2 diabetes (T2D) due to a higher prevalence among American Indian and Alaska Native, Asian, Black, and Hispanic youths compared with White youths. However, rather than a biological risk, this disparity likely reflects the inequitable distribution of adverse social determinants of health (SDOH), a product of interpersonal and structural racism.
Objective
To evaluate prediabetes prevalence by presence or absence of adverse SDOH in adolescents eligible for T2D screening based on weight status.
Design, Setting, and Participants
This cross-sectional study and analysis used data from the 2011 to 2018 cycles of the National Health and Nutrition Examination Survey. Data were analyzed from June 1, 2023, to April 5, 2024. Participants included youths aged 12 to 18 years with body mass index (BMI) at or above the 85th percentile without known diabetes.
Main Outcomes and Measures
The main outcome consisted of an elevated hemoglobin A1c (HbA1c) level greater than or equal to 5.7% (prediabetes or undiagnosed presumed T2D). Independent variables included race, ethnicity, and adverse SDOH (food insecurity, nonprivate health insurance, and household income <130% of federal poverty level). Survey-weighted logistic regression was used to adjust for confounders of age, sex, and BMI z score and to determine adjusted marginal prediabetes prevalence by race, ethnicity, and adverse SDOH.
Results
The sample included 1563 individuals representing 10 178 400 US youths aged 12 to 18 years (mean age, 15.5 [95% CI, 15.3-15.6] years; 50.5% [95% CI, 47.1%-53.9%] female; Asian, 3.0% [95% CI, 2.2%-3.9%]; Black, 14.9% [95% CI, 11.6%-19.1%]; Mexican American, 18.8% [95% CI, 15.4%-22.9%]; Other Hispanic, 8.1% [95% CI, 6.5%-10.1%]; White, 49.1% [95% CI, 43.2%-55.0%]; and >1 or other race, 6.1% [95% CI, 4.6%-8.0%]). Food insecurity (4.1% [95% CI, 0.7%-7.5%]), public insurance (5.3% [95% CI, 1.6%-9.1%]), and low income (5.7% [95% CI, 3.0%-8.3%]) were each independently associated with higher prediabetes prevalence after adjustment for race, ethnicity, and BMI z score. While Asian, Black, and Hispanic youths had higher prediabetes prevalence overall, increasing number of adverse SDOH was associated with higher prevalence among White youths (8.3% [95% CI, 4.9%-11.8%] for 3 vs 0.6% [95% CI, −0.7% to 2.0%] for 0 adverse SDOH).
Conclusions and Relevance
Adverse SDOH were associated with higher prediabetes prevalence, across and within racial and ethnic categories. Consideration of adverse SDOH may offer a more actionable alternative to race- and ethnicity-based screening to evaluate T2D risk in youth.
Introduction
Type 2 diabetes (T2D) is a disease strongly influenced by poverty and structural racism.1,2 For the past 2 decades, a rapidly rising incidence in youth-onset T2D, particularly among American Indian or Alaska Native, Asian, Black, and Hispanic youths,3 has highlighted the urgent need to prevent the onset of this severe disease, which leads to at least 1 microvascular complication by the third to fourth decades of life in 80% of individuals.4 One potential strategy to stem the tide of youth-onset T2D is to limit the development of youth-onset prediabetes, an intermediate glycemic state that is also associated with cardiometabolic comorbidities and obesity.5,6 Youth-onset prediabetes has more than doubled in prevalence in the past 2 decades and is now present in 28% of US adolescents overall and 40% of adolescents with obesity.7 Like youth-onset T2D, prediabetes is more common among racial and ethnic minority youths,6 and it may be more likely to progress to T2D in non-Hispanic Black youths than in Hispanic or non-Hispanic White youths.8
However, these epidemiological observations may be driven by social determinants of health (SDOH), which are conditions in which people grow, live, and work that are shaped by the distribution of money, power, and resources at global, national, and local levels.2 These conditions can be divided into 5 broad categories: educational access and quality, health care access and quality, neighborhood and built environments, economic stability, and social and community context.2 Exposure to adverse SDOH differs by the social, nonbiological constructs of race and ethnicity.2,9 Although previous studies have demonstrated associations between adult-onset T2D and adverse SDOH,2,10 the potential interactive roles of race, ethnicity, and SDOH in youth-onset prediabetes have not been evaluated.11,12 Racial and ethnic minority youths are more likely to experience adverse SDOH related to income inequality,13 so understanding the intersection between race and ethnicity and SDOH is critical to reduce T2D risk. This is particularly true for youths with overweight or obesity, who are at the highest risk for prediabetes and T2D.14
To address this gap, we evaluated differences in prediabetes prevalence by intersectional SDOH and race or ethnicity categories in a nationally representative sample of American adolescents who would be eligible for T2D screening based on age and body mass index (BMI)–based classification of overweight or obesity.5 We used the Healthy People 2030 SDOH domains15 to guide our selection of measures available in the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2018. We hypothesized that adverse SDOH (food insecurity, lack of private health insurance, and lower household income) would be associated with higher prediabetes prevalence, both within and across racial and ethnic groups, partially explaining the observed racial disparities in prevalence due to higher rates of adverse SDOH among minoritized populations.
Methods
Study Population
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. This cross-sectional analysis included youths aged 12 to 18 years with BMI at or above the 85th percentile for age and sex and with available hemoglobin A1c (HbA1c) level measurements in 2-year survey cycle waves from 2011 to 2018 of NHANES. NHANES is a large program conducted by the National Center for Health Statistics that collects demographic, socioeconomic, and health-related surveys as well as physical measurements and clinical laboratory evaluations in a selected population that is designed to be representative of the US population after survey weighting. Administration of each NHANES questionnaire is based on participant age and sex, as relevant. Participants 16 years or older are interviewed directly, while an adult proxy (eg, parent or guardian) provided information on behalf of the survey participant for those younger than 16 years or who cannot answer questions themselves.
We began with data from the 2011-2012 cycle due to addition of a non-Hispanic Asian race and ethnicity category that began in that cycle, as Asian youth are at significantly increased risk of T2D.3 More recent data from the 2019-2020 cycle are not considered to be nationally representative due to limitations in data collection during the COVID-19 pandemic, so were not included in this analysis. We chose an age range of 12 to 18 years to be inclusive of a youth population that would be eligible for screening for T2D based on American Diabetes Association guidelines, as well as the availability of HbA1c levels in the dataset (beginning at 12 years of age). Participants with known diabetes based on response to diabetes risk-related survey questions were excluded (eFigure in Supplement 1). All NHANES survey cycles were approved by the National Center for Health Statistics Research Ethics Review Board. Informed consent was obtained from parents or legal guardians or assent from youths 16 years or older prior to participation in NHANES. Survey question numbers and phrasing are listed in the eTable in Supplement 1.
Independent Variables
Social Determinants of Health
We included 3 SDOH measures consistent with Healthy People 2030 domains of economic stability and health care access: food security, household income, and health insurance. These domains are also highly relevant to prediabetes management. Families with food insecurity are less likely to have access to nutrient-rich food to prevent prediabetes; those without private insurance may have more limited access to care; and those living in poverty may have both food insecurity and lack of private insurance, as well as fewer opportunities to engage in physical activity due to neighborhood-level barriers. We used the Food Security Questionnaire to determine the household food security level, defined by number of affirmative responses to the US Food Security Survey Module questions (0 indicates full security; 1-2, marginal; 3-7, low; and 8-18, very low); this was dichotomized to full vs not full (marginal, low, or very low) food security. We chose to include marginal with food insecurity based on evidence that marginal food security is associated with adverse child health outcomes16 as well as with poor glycemic control in adult NHANES participants.17 To assess health care access, we used the Health Insurance Questionnaire to determine whether each individual had public, private, other, or no insurance. This variable was analyzed as any private insurance, only public insurance (or other, including single-service plans), and no insurance. The family poverty-income ratio, reported on the Demographic Questionnaire, was categorized by below 130% of the federal poverty level for that year (ie, <30% above the poverty level) or at or above 130%, consistent with eligibility for governmental assistance including the Supplemental Nutrition Assistance Program.18
Race and Hispanic Ethnicity Categories
In NHANES, participants are given the option of self-identifying as Hispanic or Latino, including country of self or ancestral origin. Participants then self-identify race and are allowed to select multiple; if other race is selected, additional subcategories are presented, including Asian subcategories.19 The publicly available aggregated racial categories included non-Hispanic Asian, Black, and White, and more than 1 or other race (which could include American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and other), while ethnicity categories included Mexican American or Other Hispanic.
Outcomes: Adjusted Prediabetes Prevalence and Prevalence Ratios
Prediabetes was defined only by HbA1c level due to the markedly smaller sample of adolescents with available fasting glucose levels and potential nonrepresentativeness of this small subpopulation. Using American Diabetes Association criteria,20 HbA1c levels of 5.7% to 6.4% were considered consistent with prediabetes; 6.5% or greater, T2D (to convert to mmol/mol, multiply by 10.93 and subtract by 23.50).
Potential Confounders
We adjusted for age, sex assigned at birth, and relative obesity (BMI z score). The BMI z score was calculated using US Centers for Disease Control and Prevention pediatric growth curves21 and the zanthro command in Stata, version 17 (StataCorp LLC).
Statistical Analysis
Demographic and clinical characteristics are reported using summary statistics and corresponding 95% CIs. We estimated associations between each SDOH and prediabetes prevalence using multivariable logistic regression models, adjusted for confounders. We included interaction terms for race and ethnicity and each SDOH. White race was used as the reference group due to being the largest racial or ethnic group. We calculated point estimates and 95% CIs of adjusted prediabetes prevalence by presence or absence of each adverse SDOH category (eg, food insecurity vs food security [reference group]; any vs no adverse SDOH) within racial and ethnic groups.
Analyses were performed using Stata, version 17. All analyses incorporated appropriate sample weights22 and used a 2-sided α = .05 to indicate statistical significance. Due to repeated testing of the association between SDOH and prediabetes prevalence within multiple racial and ethnic groups (6 groups), false discovery rate–adjusted P values are reported. We used Stata survey procedures to ensure appropriate point and variance estimates for our study’s population. Missing data were not imputed. Relative SEs were calculated to evaluate reliability of estimates; estimates with a relative SE of 30% or greater (less reliable) are reported herein.23 Analyses were performed from June 1, 2023, to April 5, 2024.
Results
Cohort Characteristics
Of the 4522 participants aged 12 to 18 years old with NHANES data available from the 2011-2018 cycles, 1563 had a BMI at or above the 85th percentile for age and sex, an available HbA1c level measurement, and a reported diabetes diagnosis status. The final analytic sample of 1563 participants representing 10 178 400 US youth had a mean age of 15.5 (95% CI, 15.3-15.6) years and BMI z score of 1.77 (95% CI, 1.74-1.80). The sample was evenly distributed between male and female participants (50.5% [95% CI, 47.1%-53.9%] female and 49.5% male [95% CI, 46.1%-52.9%]), and approximately half were of White race (weighted proportion: Asian, 3.0% [95% CI, 2.2%-3.9%]; Black, 14.9% [95% CI, 11.6%-19.1%]; Mexican American, 18.8% [95% CI, 15.4%-22.9%]; Other Hispanic, 8.1% [95% CI, 6.5%-10.1%]; White, 49.1% [95% CI, 43.2%-55.0%]; and >1 or other race, 6.1% [95% CI, 4.6%-8.0%]). Elevated HbA1c level (≥5.7%) was present in 8.5% (95% CI, 6.9%-10.4%) but varied by race and ethnicity, with the largest proportion in Asian (14.3% [95% CI, 8.8%-22.4%]) and Black (24.5% [95% CI, 19.4%-30.7%]) youths, followed by Mexican American (9.7% [95% CI, 5.9%-15.8%]), Other Hispanic (7.9% [95% CI, 4.6%-13.3%]), more than 1 or other race (6.6% [95% CI, 2.9%-14.1%]), and White (3.1% [9% CI, 1.9%-4.9%]) youths. Diabetes-range HbA1c levels were uncommon, occurring in only 3 individuals.
Prevalence of Adverse SDOH
Food insecurity was reported by 41.0% (95% CI, 37.6%-45.5%) of youths overall (available in an unweighted sample of 1534 participants). More than half reported no private health insurance coverage (no insurance, 10.2% [95% CI, 8.4%-12.4%]; public insurance, 43.2% [95% CI, 39.4%-46.9%]; available in an unweighted sample of 1558 participants). Approximately one-third (35.6% [95% CI, 31.6%-39.8%]) reported low household income (income to poverty ratio <130%) (available in an unweighted sample of 1420 participants). When considered jointly (among an unweighted sample of 1416 participants with data for food security, insurance, and income), the experience of at least 1 adverse SDOH was pervasive, with 67.0% (95% CI, 63.0%-70.7%) reporting food insecurity, lack of private insurance, and/or low household income, and 20.5% (95% CI, 17.1%-24.3%) reporting all 3 adverse SDOH. The unadjusted prevalence of each investigated SDOH varied across racial and ethnic groups (P < .001 for each by χ2 test), but adverse SDOH, for example food insecurity, were generally most common among Black (55.7% [95% CI, 50.2%-61.1%]), Mexican American (55.5% [95% CI, 48.4%-62.3%]), and Other Hispanic (51.9% [95% CI, 40.7%-62.9%]) youths and lowest among Asian (26.2% [95% CI, 15.4%-40.8%]) and White (29.6% [95% CI, 23.8%-36.2%) youths (Table 1).
Table 1. Prevalence of Adverse SDOH by Race and Ethnicity.
| Adverse SDOH | Racial and ethnic groups, prevalence (95% CI), %a | |||||
|---|---|---|---|---|---|---|
| Asian | Black | Mexican American | Other Hispanic | White | >1 or Other raceb | |
| Less than full food security | 26.2 (15.4-40.8) | 55.7 (50.2-61.1) | 55.5 (48.4-62.3) | 51.9 (40.7-62.9) | 29.6 (23.8-36.2) | 45.3 (33.5-57.7) |
| Nonprivate insurance | ||||||
| Public | 29.5 (19.7-41.7) | 60.3 (55.7-64.8) | 53.3 (47.1-59.3) | 55.8 (47.2-64.1) | 33.2 (28.7-38.0) | 40.1 (30.3-50.8) |
| None | 6.8 (2.8-15.6)c | 8.6 (6.1-12.1) | 18.7 (14.2-24.0) | 15.1 (9.4-23.4) | 6.7 (4.2-10.5) | 11.8 (5.3-24.3)c |
| Household income <130% poverty level | 29.4 (19.4-41.9) | 49.3 (43.3-55.4) | 51.5 (44.6-58.5) | 51.0 (42.3-59.6) | 24.3 (19.7-29.5) | 33.5 (23.5-45.2) |
| Any adverse SDOH | 52.4 (40.3-64.3) | 85.9 (81.7-89.3) | 83.4 (78.5-87.3) | 82.7 (76.0-87.8) | 54.0 (48.3-59.7) | 67.4 (56.6-76.6) |
| Cumulative adverse SDOH | ||||||
| 1 | 21.1 (13.9-30.8) | 29.0 (23.5-35.2) | 21.7 (17.0-27.4) | 23.0 (15.4-32.9) | 26.6 (21.6-32.3) | 24.2 (14.6-37.3) |
| 2 | 21.0 (12.8-32.4) | 24.8 (20.4-29.9) | 31.0 (25.7-36.8) | 27.7 (20.0-37.1) | 15.2 (11.0-20.6) | 21.8 (12.6-35.0) |
| 3 | 10.3 (4.4-22.3) | 32.0 (26.6-38.0) | 30.7 (24.2-38.0) | 32.0 (23.1-42.4) | 12.2 (8.5-17.3) | 21.4 (12.4-34.5) |
Abbreviation: SDOH, social determinants of health.
Unweighted sample sizes with responses for each measure included 1534 for food security, 1544 for health insurance, 1520 for income, and 1416 youths for any and cumulative SDOH. Data are from the 2011 to 2018 cycles of the National Health and Nutrition Examination Survey including youths aged 12 to 18 years with a body mass index at or above the 85th percentile without known diabetes. P < .001 for all between-group differences.
Other includes American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and other race.
Estimate may be less reliable owing to a relative SE of 30% or greater.
Prevalence of Prediabetes by Adverse SDOH and by Intersectional Racial and Ethnic SDOH Group
Adjusted marginal prevalence of prediabetes by SDOH groups are shown in the Figure. Prediabetes prevalence was 4.1% (95% CI, 0.7%-7.5%) higher among youths from households with food insecurity compared with food security. Similarly, prediabetes prevalence was 5.3% (95% CI, 1.6%-9.1%) higher among youths with public compared with private insurance, though prevalence did not differ significantly between noninsured and privately insured youths (4.6% [95% CI, −1.2% to 10.3%]). Prediabetes prevalence was also 5.7% (95% CI, 3.0%-8.3%) higher among youths with household income at less than 130% of the federal poverty level compared with at least 130%.
Figure. Adjusted Marginal Prediabetes Prevalence Among Youth With Adverse Social Determinants of Health.
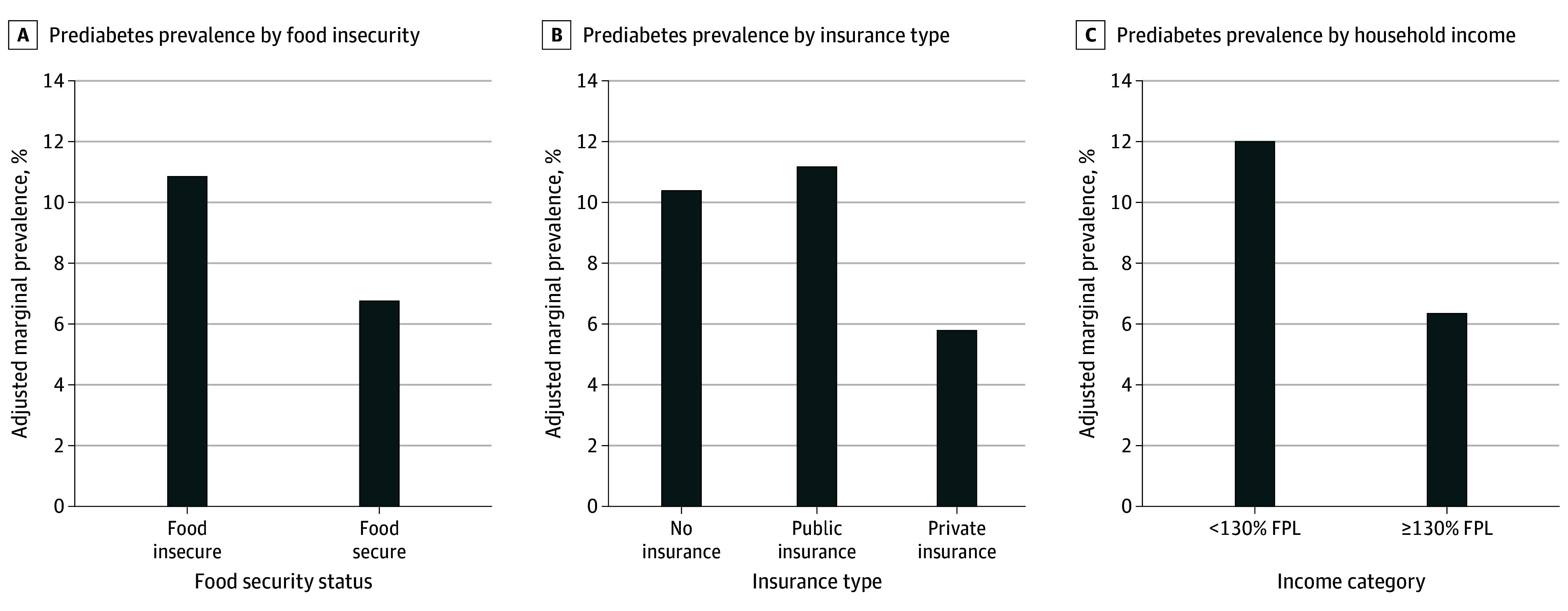
Prediabetes prevalence was higher among youth with food insecurity, public health insurance, and income to poverty ratio less than 130% of the federal poverty level (FPL) (P < .05 for each comparison).
Adjusted marginal prevalences of prediabetes by individual SDOH within racial and ethnic groups are shown in Table 2. Food insecurity (marginal, low, and very low) was associated with significantly higher prediabetes prevalence only among White youths (6.3% [95% CI, 2.8%-9.7%]) (Table 2). Use of public vs private insurance was associated with significantly higher prediabetes prevalence among Asian (32.0% [95% CI, 15.3%-48.6%]) and White (7.1% [95% CI, (3.4%-10.9%]) youths (Table 2). Low income was associated with significantly higher prediabetes prevalence among Black (28.5% [95% CI, 20.4%-36.5%]) and White (6.3% [95% CI, 3.5%-9.2%]) youths (Table 2). When the additive effect of each of the 3 SDOH was evaluated, although point estimates suggested a gradient of risk difference across several groups, prediabetes prevalence was significantly higher only for White youths with 2 (8.1% [95% CI, 1.6%-15.1%]) or 3 (8.3% [95% CI, 4.9%-11.8%]) compared with 0 (0.6% [95% CI, −0.7% to 2.0%]) adverse SDOH (Table 2).
Table 2. Prediabetes Prevalence by Intersectional Adverse SDOH Status and Racial and Ethnic Identity.
| SDOH category | Racial or ethnic groupa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asian | Black | Mexican American | Other Hispanic | White | >1 or Other raceb | |||||||
| Prevalence (95% CI), % | P valuec | Prevalence (95% CI), % | P valuec | Prevalence (95% CI), % | P valuec | Prevalence (95% CI), % | P valuec | Prevalence (95% CI), % | P valuec | Prevalence (95% CI), % | P valuec | |
| Food security status | ||||||||||||
| Marginal, low, or very low | 27.3 (9.6 to 44.9) | .20 | 23.9 (18.0 to 29.8) | .55 | 10.5 (3.5 to 17.5) | .50 | 8.8 (2.2 to 15.5) | .80 | 6.3 (2.8 to 9.7) | .02 | 9.4 (0.4 to 18.4) | .20 |
| Secure (reference) | 12.2 (3.1 to 21.3) | NA | 21.0 (13.1 to 29.0) | NA | 7.6 (3.2 to 12.0) | NA | 7.7 (2.3 to 13.2) | NA | 1.6 (0.2 to 3.0) | NA | 2.8 (−1.0 to 6.7) | NA |
| Insurance | ||||||||||||
| None | 8.6 (−6.9 to 24.0)d | >.99 | 20.5 (6.3 to 34.8) | >.99 | 9.1 (1.0 to 17.2) | .50 | 5.0 (−0.9 to 10.8) | .07 | 6.6 (−3.0 to 16.2) | .20 | 5.9 (−6.7 to 18.5)d | .80 |
| Public | 32 (15.3 to 48.6) | .01 | 23.8 (17.8 to 29.8) | .60 | 10.5 (3.8 to 17.2) | .20 | 6.5 (0.5 to 12.5) | .70 | 7.1 (3.4 to 10.9) | .002 | 7.9 (−0.2 to 16.1) | .40 |
| Private (reference) | 8.6 (1.5 to 15.7) | NA | 20.8 (12.0 to 29.6) | NA | 5.9 (1.3 to 10.4) | NA | 19.6 (4.7 to 34.5) | NA | 0.8 (−0.3 to 1.8) | NA | 4.3 (−0.5 to 9.0) | NA |
| Ratio of income to FPL | ||||||||||||
| <130% | 26.0 (8.7 to 43.4) | .10 | 28.5 (20.4 to 36.5) | .02 | 11.3 (4.9 to 17.8) | .40 | 12.1 (4.5 to 19.7) | .20 | 6.3 (3.5 to 9.2) | .01 | 12.6 (−0.6 to 25.7) | .10 |
| ≥130% (Reference) | 11.5 (3.9 to 19.2) | NA | 18.2 (12.3 to 24.0) | NA | 8.1 (2.7 to 13.5) | NA | 5.3 (0.1 to 10.5) | NA | 2.3 (0.5 to 4.0) | NA | 2.8 (−0.4 to 6.0) | NA |
| No. of adverse SDOH | ||||||||||||
| 3 | 38.2 (9.2 to 67.1) | .08 | 31.3 (22.0 to 40.6) | .70 | 12.2 (2.9 to 21.6) | .20 | 9.8 (1.1 to 18.4) | .20 | 8.3 (4.9 to 11.8) | <.001 | 16.6 (−1.3 to 34.4) | .20 |
| 2 | 22.8 (2.9 to 42.6) | .40 | 19.6 (10.6 to 28.5) | .20 | 9.5 (2.1 to 16.9) | .40 | 13.5 (3.2 to 23.9) | .06 | 8.3 (1.6 to 15.1) | .03 | 1.3 (−1.4 to 3.9) | .40 |
| 1 | 5.6 (−3.1 to 14.4) | .10 | 16.1 (9.0 to 23.1) | .10 | 9 (2.1 to 16.0) | .30 | 6.4 (−2.2 to 15.1) | .40 | 1.9 (−0.5 to 4.3) | .40 | 2.3 (−2.3 to 7.0) | .70 |
| 0 (Reference) | 12.1 (2.4 to 21.8) | NA | 27.9 (14.1 to 41.6) | NA | 5.5 (−0.5 to 11.4) | NA | 2.5 (−2.7 to 7.6) | NA | 0.6 (−0.7 to 2.0) | NA | 4.0 (−1.7 to 9.6) | NA |
Abbreviations: FPL, federal poverty level; NA, not applicable; SDOH, social determinants of health.
Unweighted sample sizes with responses for each measure included 1534 for food security, 1558 for health insurance, 1420 for income, and 1416 youths for cumulative SDOH. Multivariable logistic regression models were adjusted for age, sex, body mass index z score, and survey cycle. Data are from the 2011 to 2018 cycles of the National Health and Nutrition Examination Survey including youths aged 12 to 18 years with a body mass index at or above the 85th percentile without known diabetes.
Other includes American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and other race.
False discovery rate–adjusted P value for comparisons within each of 6 racial and ethnic categories; reference category is the most advantaged group.
Estimate may be less reliable owing to relative SE of 30% or greater.
Discussion
In this cross-sectional study including a nationally representative sample of adolescents with overweight and obesity, prediabetes prevalence was higher in the presence of adverse SDOH for the full subsample, but differing associations were found across racial and ethnic groups. To our knowledge, our study is the first to evaluate prediabetes prevalence in youth using intersectional race, ethnicity, and SDOH categories. This approach allowed us to isolate the association between prediabetes and each adverse SDOH while also highlighting how the association may differ by racial or ethnic identity. Our findings are similar to those from a cross-sectional study by Ogden et al24 that demonstrated differing associations between obesity and household income among youth across race and ethnicity groups, with high household income associated with lower obesity prevalence among Asian and Hispanic youths, but no income-related difference among White and Black youths. Similarly, a scoping review of associations between SDOH and health risk behaviors (eg, substance use, high-risk sexual behavior) among adolescents and young adults aged 10 to 24 years25 found that associations differed across racial and ethnic groups and by SDOH assessed. These findings underscore the complexity of the associations among racial and ethnic identity, exposure to adverse SDOH, and health outcomes in youths.
Differences in T2D risk across and within racial and ethnic groups26 emerge from factors at the individual, interpersonal, community, and societal levels.27 Youth-onset prediabetes results from a complex interplay across these levels, on an intergenerational scale. For example, risk of youth-onset obesity and insulin resistance is significantly higher among offspring of women with diabetes during pregnancy,9,28 but this maternal risk differs by race and ethnicity29 and is driven by exposure to adverse SDOH.30,31 Youth-onset obesity and prediabetes risk are reduced by engagement in health-promoting behaviors, including physical activity and consumption of nutrient-dense foods; unfortunately, these health behaviors are often hardest to achieve for youths from communities which, due to oppressive policies and practices, have concentrated poverty and high rates of community violence.9 We also note that the prevalence of adverse SDOH in this cohort was relatively higher than the general population. Thus, our analytic approach of evaluating within-racial and ethnic group differences minimizes the confounding of associations between SDOH and prediabetes risk that occur due to differences in adverse SDOH exposure across racial and ethnic groups.
Our study also highlights differences in prediabetes prevalence among Asian youths, an understudied population at high risk for T2D.32,33 Asian American individuals are a heterogenous group, with vast differences in socioeconomic status, language identity, and immigration status both between and within cultural subgroups.34 Unfortunately, unrestricted NHANES data do not allow for disaggregation of this heterogenous group, an important limitation given the differing effects of SDOH within Asian subgroups. For example, educational attainment was found to be inversely associated with T2D risk among Filipino American adults, but directly associated with T2D risk among Indian American adults.35 Within Asian adults, those of South Asian extraction tend to be at highest risk, with T2D developing approximately 10 years earlier than among White adults.36 Adverse SDOH further exacerbate this risk: low household income is associated with higher prevalence of obesity among Asian American adolescents37 as well as with prediabetes and T2D among Asian American adults.38 Disaggregating data could allow for better identification of drivers of health disparities within Asian and Pacific Islander communities, supporting the development of effective diabetes prevention interventions.
Strengths and Limitations
A major strength of our study is our evaluation of associations within racial and ethnic groups between adverse SDOH and prediabetes prevalence, which provided a less confounded estimate of associations. We observed consistently direct associations between adverse SDOH and prediabetes prevalence through analyses of individual, any, and cumulative adverse SDOH. Additional strengths include the nationally representative sample of youths with rigorously and prospectively collected clinical and demographic data, including self-reported racial and ethnic identity.
This study also has some limitations. First, we did not include alternate, glucose-based prediabetes definitions. Although HbA1c level has been reported to be higher among certain racial groups,39 we defined prediabetes using HbA1c because (1) an elevated HbA1c level has been associated with development of T2D in diverse populations, including American Indian youth40; (2) it is guideline supported5 and commonly used to screen for, diagnose, and manage T2D in youths41,42; (3) it is not influenced by fasting and reflects longer-term glycemia20; and, (4) our within-group analysis minimizes concerns for between-race differences in glycosylation. Second, detailed family history was not available, so we could not adjust for this potential confounder. Third, we did not evaluate whether prevalence differences were associated with nutrition or physical activity. However, in a study also using NHANES data,43 diet quality was lowest among Black youths, independent of income, suggesting that diet may be an important factor explaining some of the observed racial differences in prediabetes prevalence. Fourth, NHANES aggregates individuals of other race and more than 1 race, an important limitation given the heterogeneity and increasing size of this population in the US.44 Fifth, adverse SDOH may be differentially reported by adolescents and caregivers, potentially leading to underestimation45 or overestimation46 of material needs. Last, this cross-sectional study does not allow us to determine causal relationships between SDOH and prediabetes.
Conclusions
In this cross-sectional study including a nationally representative sample of 1563 adolescents with overweight and obesity aged 12 to 18 years, we found that adverse SDOH, including food insecurity, lack of private health insurance, and low income were differentially associated with prediabetes prevalence across racial and ethnic groups. However, prediabetes prevalence remained high even in the setting of favorable SDOH among Asian, Black, and Hispanic youth. Other social and structural determinants of health, such as racism and ethnic discrimination, should be evaluated to determine whether the remaining disparities in prediabetes prevalence can be further explained to develop targeted approaches to T2D risk reduction. Furthermore, given the higher prevalence of prediabetes in the setting of SDOH, even in White youth who are not considered to be at high risk for T2D,5 pediatric T2D screening guidelines should move beyond use of race and ethnicity and instead critically consider exposure to adverse SDOH.47 Such an approach would be well aligned with recent efforts by many pediatric health care organizations to make screening for health-related social needs standard of care.48 If successfully implemented, approaches that screen for and address adverse SDOH may ultimately reduce the risks associated with youth-onset T2D via prevention as well as early identification and treatment.
eTable. Questions and Questionnaires Used, Target Age Range, and Respondent Detail
eFigure. Consort Diagram
Data Sharing Statement
References
- 1.McGavock J, Wicklow B, Dart AB. Type 2 diabetes in youth is a disease of poverty. Lancet. 2017;390(10105):1829. doi: 10.1016/S0140-6736(17)32461-3 [DOI] [PubMed] [Google Scholar]
- 2.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258-279. doi: 10.2337/dci20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419-1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornstad P, Drews KL, Caprio S, et al. ; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416-426. doi: 10.1056/NEJMoa2100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ElSayed NA, Aleppo G, Aroda VR, et al. ; American Diabetes Association . 14: Children and adolescents: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(suppl 1):S230-S253. doi: 10.2337/dc23-S014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr. 2020;174(2):e194498. doi: 10.1001/jamapediatrics.2019.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Li Y, Zhang D, Yi SS, Liu J. Trends in prediabetes among youths in the US from 1999 through 2018. JAMA Pediatr. 2022;176(6):608-611. doi: 10.1001/jamapediatrics.2022.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Love-Osborne KA, Sheeder JL, Nadeau KJ, Zeitler P. Longitudinal follow up of dysglycemia in overweight and obese pediatric patients. Pediatr Diabetes. 2018;19(2):199-204. doi: 10.1111/pedi.12570 [DOI] [PubMed] [Google Scholar]
- 9.Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-onset type 2 diabetes: the epidemiology of an awakening epidemic. Diabetes Care. 2023;46(3):490-499. doi: 10.2337/dci22-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright L, Stallings-Smith S, Arikawa AY. Associations between food insecurity and prediabetes in a representative sample of US Adults (NHANES 2005-2014). Diabetes Res Clin Pract. 2019;148:130-136. doi: 10.1016/j.diabres.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 11.Lee AM, Scharf RJ, Filipp SL, Gurka MJ, DeBoer MD. Food insecurity is associated with prediabetes risk among US adolescents, NHANES 2003-2014. Metab Syndr Relat Disord. 2019;17(7):347-354. doi: 10.1089/met.2019.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson SL, Yang EC, Zhang Z. Income disparities and cardiovascular risk factors among adolescents. Pediatrics. 2018;142(5):e20181089. doi: 10.1542/peds.2018-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Council on Community Pediatrics; Gitterman BA, Flanagan PJ, Cotton WH, et al. Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339. doi: 10.1542/peds.2016-0339 [DOI] [PubMed] [Google Scholar]
- 14.Twig G, Zucker I, Afek A, et al. Adolescent obesity and early-onset type 2 diabetes. Diabetes Care. 2020;43(7):1487-1495. doi: 10.2337/dc19-1988 [DOI] [PubMed] [Google Scholar]
- 15.Healthy People 2030: social determinants of health. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Accessed September 22, 2023. https://health.gov/healthypeople/priority-areas/social-determinants-health
- 16.Cook JT, Black M, Chilton M, et al. Are food insecurity’s health impacts underestimated in the US population? marginal food security also predicts adverse health outcomes in young US children and mothers. Adv Nutr. 2013;4(1):51-61. doi: 10.3945/an.112.003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casagrande SS, Bullard KM, Siegel KR, Lawrence JM. Food insecurity, diet quality, and suboptimal diabetes management among US adults with diabetes. BMJ Open Diabetes Res Care. 2022;10(5):e003033. doi: 10.1136/bmjdrc-2022-003033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A quick guide to SNAP eligibility and benefits. Center on Budget and Policy Priorities. Updated October 2, 2023. Accessed October 13, 2023. https://www.cbpp.org/research/food-assistance/a-quick-guide-to-snap-eligibility-and-benefits
- 19.National Health and Nutrition Examination Survey (NHANES) Interviewer Procedures Manual. Centers for Disease Control and Prevention. March 2017. Accessed March 30, 2023. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Manuals.aspx?BeginYear=2017
- 20.ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 2: Classification and diagnosis of diabetes: Standards of Care in Diabetes—2023 . Diabetes Care. 2023;46(suppl 1):S19-S40. doi: 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 22.NHANES survey methods and analytic guidelines. National Center for Health Statistics; Centers for Disease Control and Prevention. Accessed March 30, 2023. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#estimation-and-weighting-procedures
- 23.Klein R, Proctor S, Boudreault M, Turczyn K. Healthy People 2010 criteria for data suppression. Statistical Notes. Centers for Disease Control and Prevention. July 2002. Accessed April 5, 2023. https://www.cdc.gov/nchs/data/statnt/statnt24.pdf [PubMed]
- 24.Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of obesity among youths by household income and education level of head of household—United States 2011-2014. MMWR Morb Mortal Wkly Rep. 2018;67(6):186-189. doi: 10.15585/mmwr.mm6706a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroe P, Campbell JA, Harris M, Egede LE. Racial/ethnic differences in social determinants of health and health outcomes among adolescents and youth ages 10-24 years old: a scoping review. BMC Public Health. 2023;23(1):410. doi: 10.1186/s12889-023-15274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan S, Gujral UP, Quarells RC, et al. Disparities in diabetes prevalence and management by race and ethnicity in the USA: defining a path forward. Lancet Diabetes Endocrinol. 2023;11(7):509-524. doi: 10.1016/S2213-8587(23)00129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker AF, Graham S, Maple-Brown L, et al. Interventions to address global inequity in diabetes: international progress. Lancet. 2023;402(10397):250-264. doi: 10.1016/S0140-6736(23)00914-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang X, Ma H, Wang Y, Liu Y. Early life factors and type 2 diabetes mellitus. J Diabetes Res. 2013;2013:485082. doi: 10.1155/2013/485082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326(7):660-669. doi: 10.1001/jama.2021.7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez H, DiTosto JD, Niznik CM, Yee LM. Understanding food security as a social determinant of diabetes-related health during pregnancy. Am J Perinatol. 2023;40(8):825-832. doi: 10.1055/s-0041-1740194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pu J, Zhao B, Wang EJ, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol. 2015;29(5):436-443. doi: 10.1111/ppe.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabelea D, Mayer-Davis EJ, Saydah S, et al. ; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence JM, Divers J, Isom S, et al. ; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA. 2021;326(8):717-727. doi: 10.1001/jama.2021.11165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budiman A, Ruiz N. Key facts about Asian origin groups in the US. Pew Research. April 29, 2021. Accessed September 22, 2023. https://www.pewresearch.org/short-reads/2021/04/29/key-facts-about-asian-origin-groups-in-the-u-s/
- 35.Shah MK, Gandrakota N, Gujral UP, Islam N, Narayan KMV, Ali MK. Cardiometabolic risk in Asian Americans by social determinants of health: serial cross-sectional analyses of the NHIS, 1999-2003 to 2014-2018. J Gen Intern Med. 2023;38(3):571-581. doi: 10.1007/s11606-022-07933-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becerra MB, Becerra BJ. Disparities in age at diabetes diagnosis among Asian Americans: implications for early preventive measures. Prev Chronic Dis. 2015;12:E146. doi: 10.5888/pcd12.150006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook WK, Tseng W, Bautista R, John I. Ethnicity, socioeconomic status, and overweight in Asian American adolescents. Prev Med Rep. 2016;4:233-237. doi: 10.1016/j.pmedr.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah AD, Vittinghoff E, Kandula NR, Srivastava S, Kanaya AM. Correlates of prediabetes and type II diabetes in US South Asians: findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Ann Epidemiol. 2015;25(2):77-83. doi: 10.1016/j.annepidem.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin E. Are there clinical implications of racial differences in HbA1c? a difference, to be a difference, must make a difference. Diabetes Care. 2016;39(8):1462-1467. doi: 10.2337/dc16-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijayakumar P, Nelson RG, Hanson RL, Knowler WC, Sinha M. HbA1c and the prediction of type 2 diabetes in children and adults. Diabetes Care. 2017;40(1):16-21. doi: 10.2337/dc16-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vajravelu ME, Lee JM, Amaral S, Kelly A. Sex-based differences in screening and recognition of pre-diabetes and type 2 diabetes in pediatric primary care. Pediatr Obes. 2021;16(2):e12699. doi: 10.1111/ijpo.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011;158(6):947-952.e3. doi: 10.1016/j.jpeds.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson JL, Tussing-Humphreys LM, Goodman MH, Landry AS. Diet quality in a nationally representative sample of American children by sociodemographic characteristics. Am J Clin Nutr. 2019;109(1):127-138. doi: 10.1093/ajcn/nqy284 [DOI] [PubMed] [Google Scholar]
- 44.Jones N, Marks R, Ramirez R, Rios-Vargas M. 2020 Census illuminates racial and ethnic composition of the country. August 12, 2021. Accessed September 2, 2022. https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html
- 45.Landry MJ, van den Berg AE, Asigbee FM, Vandyousefi S, Ghaddar R, Davis JN. Child compared with parent perceptions of child-level food security. Curr Dev Nutr. 2019;3(10):nzz106. doi: 10.1093/cdn/nzz106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokol RL, Clift J, Martínez JJ, Goodwin B, Rusnak C, Garza L. Concordance in adolescent and caregiver report of social determinants of health. Am J Prev Med. 2022;63(5):708-716. doi: 10.1016/j.amepre.2022.06.004 [DOI] [PubMed] [Google Scholar]
- 47.Vajravelu ME, Okah E, Bensignor M, Karbeah J. A call to replace race- and ethnicity-based screening with health-related social needs evaluation when assessing risk of type 2 diabetes in youth. J Adolesc Health. 2023;73(4):620-621. doi: 10.1016/j.jadohealth.2023.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragavan MI, Garg A, Raphael JL. Creating healing-centered health systems by reimagining social needs screening and supports. JAMA Pediatr. 2023;177(6):555-556. doi: 10.1001/jamapediatrics.2023.0343 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Questions and Questionnaires Used, Target Age Range, and Respondent Detail
eFigure. Consort Diagram
Data Sharing Statement


