Abstract
Murine gammaherpesvirus 68 (γHV68, or MHV-68) is a genetically tractable, small animal model for the analysis of gammaherpesvirus pathogenesis. The γHV68 genome is colinear with the genomes of other sequence gammaherpesviruses, containing large blocks of conserved genes interspersed by a number of putative genes without clear homologs in the other gammaherpesviruses. One of these putative unique genes, the M1 open reading frame (ORF), exhibits sequence homology to a poxvirus serine protease inhibitor, SPI-1, as well as to another γHV68 gene, M3, which we have recently shown encodes an abundantly secreted chemokine binding protein. To assess the contribution of the M1 ORF to γHV68 pathogenesis, we have generated a recombinant γHV68 in which the M1 ORF has been disrupted through targeted insertion of a lacZ expression cassette (M1.LacZ). Although M1.LacZ replicated normally in tissue culture, it exhibited decreased splenic titers at days 4 and 9 postinfection in both immunocompetent and immunodeficient mice. Despite decreased levels of acute virus replication, M1.LacZ established a latent infection comparable to wild-type (wt) γHV68, but exhibited an approximately fivefold increase in efficiency of reactivation from latency. M1.LacZ also caused severe vasculitis of the great elastic arteries in gamma interferon receptor (IFN-γR)-deficient mice with a frequency comparable to wt γHV68, but did not cause the mortality or splenic pathology observed with wt γHV68 infection of IFN-γR-deficient mice. Restoration of M1 ORF sequences into M1.LacZ (M1 marker rescue, or M1.MR) demonstrated that M1.LacZ phenotypic alterations in growth in vivo and latency were not due to the presence of additional mutations located elsewhere in the M1.LacZ genome. Generation of a second M1 mutant virus containing a deletion at the 5′ end of the M1 ORF (M1Δ511), but lacking the LacZ expression cassette, revealed the same latency phenotype observed with the M1.LacZ mutant. However, M1Δ511 was not attenuated for acute virus replication in the spleen. We conclude that (i) the induction of arteritis in γHV68-infected IFN-γR-deficient mice can occur in the absence of splenic pathology and mortality, (ii) replication during acute infection is not the primary determinant for the establishment of latent infection, and (iii) the M1 ORF, or a closely linked gene, encodes a gene product that functions to suppress virus reactivation.
The gammaherpesviruses include the human pathogens Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV, or HHV-8) (for review, see references 10 and 18). These viruses establish lifelong infection of the host and are associated with a number of malignancies. To better understand gammaherpesvirus pathogenesis, we and others have begun to utilize infection of mice with murine gammaherpesvirus 68 (γHV68, also referred to as MHV-68) (23, 37). γHV68 is a member of the gamma2-herpesvirus subfamily based on genome sequence (7, 8, 13, 35).
The pathogenesis of γHV68 has been reviewed recently (21, 23, 26, 37). Briefly, γHV68 infection of inbred mice results in an acute, productive infection of multiple organs and a CD4+ T-cell-dependent splenomegaly (9, 25, 30, 33). Acute virus replication is largely cleared by 2 to 3 weeks postinfection (30, 39). Subsequently, γHV68 is present in its persistent, latent form, during which time, the γHV68 genome is maintained in infected cells in the absence of detectable preformed infectious virus (30, 36, 38, 40, 41). γHV68 establishes a latent infection in B cells and macrophages and persists in lung epithelial cells (27, 31, 40).
Chronic γHV68 infection is associated with several pathologies. γHV68 infection of some inbred strains of mice has been shown to result in a significant incidence of lymphoproliferative disease (29). Infection of gamma interferon (IFN-γ)-unresponsive mice leads to significant mortality and the development of two pathologies: (i) a severe vasculitis of the great elastic arteries and (ii) a T-cell-dependent splenic fibrosis or atrophy (6, 39). Both major histocompatibility complex class II-deficient mice, devoid of CD4+ T cells, and B-cell-deficient mice develop vasculitis of the great elastic arteries and die during chronic γHV68 infection (5, 39, 41). The precise mechanisms responsible for these pathologies are not clear, although in both class II-deficient mice and B-cell-deficient mice, the host is unable to normally control latent infection (5, 41).
Sequence analysis of γHV68 identified 80 ATG-initiated open reading frames (ORFs) predicted to encode proteins at least 100 amino acids in length (35). The majority of these ORFs were homologous to known genes present in other gammaherpesviruses. In addition, all of the sequenced gammaherpesviruses encode a limited number of ORFs, with no clear homology to genes present in the other gammaherpesviruses. Virus-specific ORFs are located in similar regions of the γHV68, EBV, KSHV, and Herpesvirus saimiri (HVS) genomes (23, 35, 37). In EBV, KSHV and HVS, many of the virus-specific genes appear to be involved in either latency or transformation (see references 23 and 35–37 for further discussion). Based on this association of gammaherpesvirus-specific genes with latency, we have begun to characterize the unique candidate genes encoded by γHV68. This paper presents characterization of a viral mutation in one γHV68-specific gene, the M1 ORF.
The M1 ORF exhibits sequence homology to a poxvirus serine protease inhibitor (3, 35) previously implicated in the regulation of apoptosis (1, 4, 12). Previously, Simas et al. generated a recombinant γHV68 in which they deleted the M1 ORF and the first four viral tRNA genes (20). These investigators demonstrated that this region of the viral genome was nonessential for virus replication in vitro or the establishment of latency in vivo (20). However, this analysis did not measure acute virus replication, nor did it provide a quantitative analysis of virus reactivation and latent viral genome load. Through the independent generation of an M1-disrupted γHV68, we have confirmed the published observations of Simas et al. (20). This report further defines the contribution of the M1 ORF to γHV68 pathogenesis, providing genetic evidence that the M1 ORF, or a closely linked gene, is involved in regulating reactivation from latency.
MATERIALS AND METHODS
Viruses and tissue culture.
γHV68 WUMS (ATCC VR1465) was used for all infections and is designated as wild-type (wt) γHV68. γHV68 was passaged on NIH 3T12 cells for amplification and was propagated as previously described (38). NIH 3T12 cells and mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal calf serum, 100 U of penicillin per ml, 100 mg of streptomycin per ml, and 2 mM l-glutamine (complete DMEM). Cells were maintained in a 5% CO2 tissue culture incubator at 37°C. MEFs were obtained from either BALB/c or C57BL/6 mouse embryos as described previously (38).
Generation of virus mutants.
All recombinant viruses were generated by calcium phosphate cotransfection of γHV68 genomic DNA (35) with the appropriate gene-targeting plasmid. Plasmid constructs were purified on cesium chloride gradients, unless noted otherwise. Recombinant viruses were purified from infected NIH 3T12 monolayers overlaid with methylcellulose, with LacZ-expressing recombinants identified as blue plaques after X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) staining of infected monolayers (17).
Three plasmids were used in the generation of γHV68 recombinants. pBlu-M1 contained a γHV68 genomic subclone, which included the viral tRNA genes 1 to 6 and the M1 and M2 ORFs (bp 107 to 4635; WUMS sequence). This plasmid was generated by cloning the 4.5-kb HindIII-SpeI fragment of γHV68 genomic DNA containing the M1 ORF into a pBluScriptKS+ vector lacking the NgoM I restriction site. The second plasmid, pBlu-M1-LacZ, contained a human cytomegalovirus (HCMV) immediate-early promoter and enhancer-driven LacZ expression cassette cloned into pBlu-M1. The insertion of the LacZ expression cassette deleted γHV68 sequences between bp 1892 and 2403 (35). This deletion removes sequence from 131 bp upstream of the M1 ORF putative ATG through amino acid 126 (of 420) of the M1 ORF. pBlu-M1-LacZ was generated through ligation of a 4.3-kb fragment containing the HCMV promoter-lacZ gene (obtained from the plasmid pHCMV-MP1-LacZ, a gift of Paul Olivo and David Leib), obtained by digestion with HindIII (Klenow blunted) and XmaI, into pBlu-M1 digested with StuI and NgoMI. The third plasmid, pBlu-M1-Δ511, is a derivative of pBlu-M1 and contains a deletion between bp 1892 and 2403 of the γHV68 genome. This deletion is the exact same deletion present in pBlu-M1-LacZ. pBlu-M1-Δ511 was generated through self-ligation of a 6.8-kb fragment from pBlu-M1, obtained by digestion with StuI and NgoMI, followed by Klenow blunting. All cloning was done according to standard protocols (19).
The M1.LacZ recombinant was generated by calcium phosphate cotransfection of γHV68 genomic DNA (2.5 μg) with HindIII-linearized pBlu-M1-LacZ (2.5 μg) into NIH 3T12 cells in a six-well plate (Costar). The M1.MR recombinant was generated by cotransfection of M1.LacZ genomic DNA (1.5 μg) with HindIII-linearized pBlu-M1 (2.0 μg). The M1Δ511 recombinant virus was generated by cotransfection of M1.LacZ genomic DNA (1.5 μg) with HindIII-linearized pBlu-M1-Δ511 (2.0 μg) purified by the alkaline lysis method (19). Virus was harvested 5 to 8 days posttransfection and stored at −80°C. Subsequently, 10-fold dilutions of the virus recovered from the transfected cells were plated on NIH 3T12 monolayers, and after an hour of infection, the monolayers were overlaid with methylcellulose (2% methylcellulose in DMEM supplemented with 5% fetal calf serum, antibiotics, and l-glutamine as noted above). Recombinants were identified following X-Gal staining (10 to 18 h) to identify a blue plaque morphology (X-Gal stain: 15 mg of X-Gal in 0.3 ml of dimethyl formamide dissolved in a total volume of 50 ml of 0.008% neutral red in DMEM). Blue plaques were chosen on the basis of physical isolation from adjacent plaques, harvested, and frozen at −80°C. Further plaque purification followed the above protocol. We observed approximately a 3% frequency of recombinant viruses harvested from cotransfection of viral DNA with targeting plasmids.
Viruses were plaque purified until homogeneous by blue or white plaque morphology, at which time, small viral stocks were prepared by infecting NIH 3T12 cells with one-half of each plaque isolate in a Costar six-well plate. Virus was harvested when the monolayers exhibited a 30 to 50% cytopathic effect (CPE) and stored at −80°C. DNA was isolated from infected cells following three freeze-thaw cycles by centrifugation of the sample at 4°C for 30 min at 12,000 rpm in a Sorvall microcentrifuge. Samples were then resuspended in Tris-EDTA (TE) containing 0.5% sodium dodecyl sulfate and 0.5 mg of proteinase K per ml (TESP buffer) and incubated at 37°C for at least 8 h. Samples were subjected to at least three phenol-chloroform-isoamyl alcohol (25:24:1) extractions followed by one chloroform-isoamyl alcohol (24:1) extraction. DNA was precipitated with 3 M sodium acetate and 2 to 3 volumes of 100% ethanol. Southern analysis was performed with a diagnostic EcoRV restriction digest and a 32P-labeled M1 locus probe (bp 1702 to 4308 of γHV68 WUMS) (35).
Viral stocks were generated on NIH 3T12 monolayers infected with approximately 0.05 PFU per cell, based on an estimated titer of 108 PFU per ml for small viral stocks. Viruses were harvested at 50% CPE, 4 to 5 days postinfection. Samples were homogenized, clarified, and aliquoted for storage at −80°C. Titers of stocks were independently determined by plaque assay at least twice.
Mice, infections, and organ harvests.
C57BL/6J-Rag1tm1Mom (B6.Rag1 deficient) mice, and IFN-γ receptor (IFN-γR)-deficient mice (on a 129 background) were obtained from Jackson Laboratories and Michel Aguet, respectively, and were used for experiments between 7 and 11 weeks of age (14, 15). Mice were bred and maintained at Washington University, St. Louis, Mo., in accordance with all university and federal guidelines. C57BL/6 mice were purchased from the National Cancer Institute and used between 7 and 17 weeks of age. C.B-17 SCID mice were obtained from Emil Unanue and used between 3 and 5 months of age (described in reference 2). Mice were placed under metofane anesthesia prior to infection, as well as before sacrifice by cervical dislocation. Unless stated otherwise, all mice were infected with 106 PFU by intraperitoneal (i.p.) injection in 0.5 ml of complete DMEM. Upon sacrifice, organs were placed in 1 ml of complete DMEM on ice and frozen at −80°C. Resident peritoneal exudate cells (PECs) were harvested by peritoneal lavage with 10 ml of complete DMEM. Sentinel mice were assayed every 3 months and were negative for adventitious mouse pathogens by serology.
Plaque assay.
Plaque assays were performed with NIH 3T12 monolayers under noble agar overlay as described previously (38), with the following alterations. NIH 3T12 cells were plated in six-well plates at 3 × 105 cells per well the day prior to infection or at 1.2 × 105 cells per well 2 days prior to infection. Organs to be titered were thawed and homogenized with a Ten broeck tissue grinder, and then 10-fold dilutions were made in complete DMEM and plated onto NIH 3T12 cell monolayers. Infections were performed in a 200-μl volume, and plates were rocked every 15 min for 1 h at 37°C. Samples were overlaid with 3 ml of a 1:1 mixture of 1% noble agar and 2× minimal essential medium (MEM) supplemented with 10% fetal calf serum and a 2× concentration of antibiotic and l-glutamine. An additional 2 ml of noble agar–2× MEM mixture was added between days 3 and 6. Monolayers were stained between days 6 and 8 by the addition of 2 ml of neutral red overlay (0.01% neutral red in 2× DMEM diluted 1:1 with 1% Noble agar). After 18 to 24 h, plaques were counted. Titers of all samples were determined in parallel with a laboratory standard virus stock of known titer, and data were used only if the laboratory standard virus stock was within threefold of its known titer. The limit of detection for this assay is 50 PFU per organ.
Aortitis scoring.
Aortitis analysis was carried out as previously published (39). Briefly, IFN-γR-deficient mice between 7 and 8½ weeks of age were infected with 5 × 106 PFU i.p. At the time of death, or at sacrifice between 57 to 100 days postinfection, the heart and lungs were removed en bloc. Spleen, liver, and kidney tissues were also harvested at this time. Samples were stored in 10% phosphate-buffered formalin and embedded in paraffin, and serial sections were stained with hematoxylin and eosin (H&E). Slides were read blind independently by H.W.V., E.T.C., and A. Dal Canto, at which time, samples were scored positive or negative for aortitis as defined previously (39). Splenic pathology was examined microscopically, and samples were scored for the presence of fibrosis or atrophy and disrupted splenic architecture as previously described (6).
Limiting dilution ex vivo reactivation assay.
Detection of γHV68 reactivation from latency was performed as previously described (38, 41). Briefly, PECs and splenocytes were harvested from infected mice between days 42 and 50 postinfection, and single-cell suspensions were generated. Erythrocytes were lysed with ammonium chloride, resuspended in complete DMEM, and plated in a series of twofold dilutions, starting at 105 cells per well with splenocytes and 104 cells per well for PECs onto MEF monolayers in 96-well tissue culture plates. After 21 days, wells were scored microscopically for the presence of CPE. In some cases, samples were replated on fresh MEF monolayers to confirm the presence of infectious virus. Twenty-four wells were plated per dilution, with a total of 12 dilutions per sample per experiment. To detect preformed infectious virus, parallel samples were resuspended in 1/3× DMEM in the presence of 0.5-mm-diameter silica beads and subjected to four rounds of 1-min mechanical disruption in a Mini-Beadbeater-8 (Biospec Products, Bartlesville, Okla.) as previously described (38). This disruption procedure kills >99% of cells, with at most a twofold effect on the titer of preformed infectious virus (38, 41). Disrupted cells were plated in a similar series of twofold dilutions. The frequencies of reactivation were identical when samples were plated onto MEFs derived from BALB/c or C57BL/6 mice.
Limiting dilution nested-PCR detection of γHV68 genome-positive cells.
We determined the frequency of cells containing γHV68 genome by using a previously described nested-PCR assay with ca.-single-copy sensitivity to detect gene 50 of γHV68 (40, 41). PECs were harvested from latently infected mice and frozen in 10% DMSO at −80°C, thawed, counted, resuspended in an isotonic solution, and diluted in a background of 104 uninfected NIH 3T12 cells (40, 41). After overnight lysis of cells with proteinase K, PCR was performed with the samples (40, 41). Subsequently, 1 μl of this reaction mixture was used for a second round of PCR with nested primers (40, 41). Products were analyzed by ethidium bromide staining of a 1.5% agarose gel. For all manipulations subsequent to cell counts, a positive displacement Micromen and tips were used (Rainin, Emeryville, Calif.). Twelve PCRs were performed for each cell dilution, with 6 dilutions per sample per experiment. With each set of samples, 12 water controls and 12 controls for PCR sensitivity were included. There was a single false positive in a total of 70 water controls. To quantitate PCR sensitivity, 10, 1, or 0.1 copy of a gene 50-containing plasmid (pBamHIN) was diluted into a background of 104 uninfected cells. This PCR protocol detected 1 copy of a gene 50-containing plasmid (pBamHIN) diluted in a background of carrier DNA and 104 uninfected cells with ∼50% frequency (40, 41).
Statistical analysis.
All data were analyzed with the GraphPad Prism program (GraphPad Software, San Diego, Calif.). Survival data were plotted and statistically analyzed with the Mantel-Haenszel test. To correct for the number of postexperimental comparisons in a conservative way, we multiplied calculated P values by the total number of comparisons between survival curves (P values are indicated in text). Titer data were statistically analyzed with the nonparametric, Mann-Whitney test. Penetrance of aortitis and splenic fibrosis or atrophy were statistically analyzed with a contingency table and Fisher's exact test. The frequencies of reactivation and genome-positive cells were statistically analyzed with the paired t test. To accurately obtain the frequency for each limiting dilution, data were subjected to nonlinear regression (with a sigmoidal dose curve with variable slope to fit the data). Frequencies of reactivation and genome-positive cells were obtained by calculating the cell density at which 63% of the wells scored positive for either reactivating virus or the presence of viral genome based on Poisson distribution.
RESULTS
Targeted disruption of the M1 ORF.
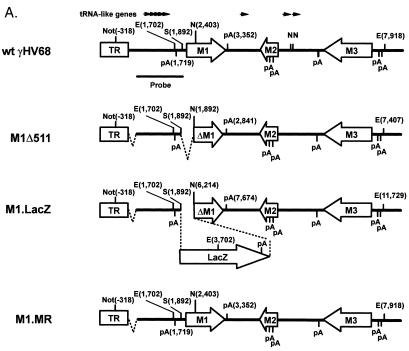
We disrupted the M1 ORF by insertion of an expression cassette containing the lacZ gene under the control of the HCMV immediate-early promoter and enhancer (M1.LacZ virus) (Fig. 1A). This targeted disruption of the M1 ORF deleted the first 480 bp of the 1.26-kb M1 ORF, as well as 131 bp of sequence upstream of the putative ATG for M1 (bp 1892 to 2403; WUMS sequence) (35). This deletion did not remove any of the γHV68 tRNA-like genes, although it is within 173 bp of the first consensus polyadenylation signal downstream of the M2 ORF (Fig. 1A).
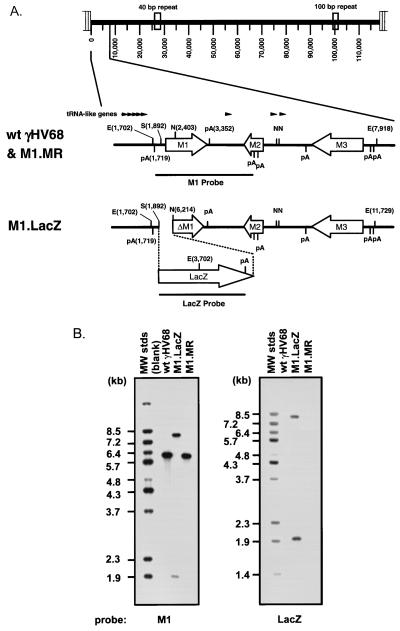
FIG. 1.
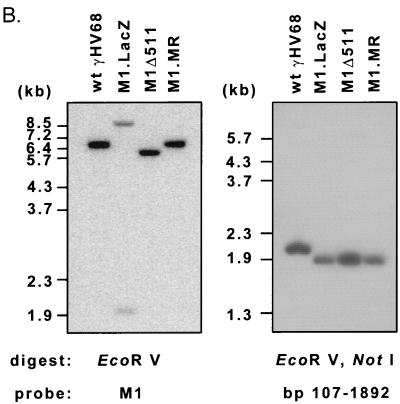
Construction and verification of the M1.LacZ virus. (A) Genomic structure of wt γHV68, M1.LacZ, and M1.MR in the region containing the M1 ORF. In M1.LacZ, the M1 ORF was disrupted through targeted excision of bp 1892 to 2403 of the viral genome by using the restriction enzymes StuI (S) and NgoMI (N). Putative polyadenylation signals are denoted as pA, with poly(A) signals on the top strand indicated by a bar above the horizontal line and poly(A) signals on the bottom strand indicated by a bar below the horizontal line. Genome coordinates of the ORFs in wt γHV68 are as follows: M1, bp 2023 to 3282; M2, bp 4031 to 4627; and M3, bp 6060 to 7277. All genome coordinates are based on the γHV68 WUMS sequence (35). Note that the base pair coordinates for M1.LacZ are calculated based on the inserted mutation. (B) Southern blot analysis of wild-type γHV68, M1.LacZ, and M1.MR viral genomes. Viral DNA was purified from virus stocks and subsequently digested with EcoRV (E), electrophoresed, blotted, and hybridized with either a probe spanning the M1 ORF and flanking sequence (bp 1702 to 4308) or a probe containing the LacZ expression cassette (see panel A for the locations of the probes). 32P-labeled molecular weight standards (MW stds) (Lambda DNA-BstEII digest; New England Biolabs) were included on each Southern blot; the fragment sizes are indicated to the left of each blot.
M1.LacZ was generated as described in Materials and Methods, and after four rounds of plaque purification, M1.LacZ was homogeneous, as determined by 100% blue plaque morphology and by Southern analysis of 20 blue plaques (data not shown). Southern analysis of M1.LacZ by restriction digest with EcoRV, using a 32P-labeled M1 region probe (bp 1702 to 4308), demonstrated, as predicted, the introduction of a new EcoRV restriction site within the M1 ORF (Fig. 1B). The hybridization of the M1 probe to 8.0- and 2.0-kb restriction fragments in M1.LacZ, compared to hybridization with a 6.2-kb EcoRV fragment in wt γHV68, was consistent with targeted insertion of the LacZ expression cassette into the M1 ORF (Fig. 1B). Additionally, a 32P-labeled LacZ probe demonstrated insertion of this cassette into the M1 ORF (Fig. 1B). Overexposure of these Southern analyses failed to reveal any contaminating wt γHV68 DNA in the M1.LacZ virus stock (data not shown).
To rule out the presence of distal mutations in M1.LacZ which might result in phenotypic alterations in M1.LacZ, we constructed a marker rescue virus (referred to as M1.MR) in which M1 ORF sequences were restored in M1.LacZ. M1.MR was purified by white plaque selection, and after two rounds of plaque purification, this virus had a 100% white plaque morphology. Southern analysis of M1.MR upon EcoRV digestion with a 32P-labeled M1 region probe demonstrated hybridization with the expected 6.2-kb fragment and loss of the 8.0- and 2.0-kb fragments present in the M1.LacZ mutant (Fig. 1B). Furthermore, a 32P-labeled LacZ probe failed to hybridize to any sequences present in M1.MR (Fig. 1B), verifying replacement of the LacZ expression cassette with wt M1 ORF sequences.
During the generation of M1.MR, we identified two independent white plaques that failed to hybridize with a 32P-labeled M1 region probe (data not shown). Further genomic analysis of these isolates demonstrated a deletion encompassing the M1, M2, and M3 ORFs (data not shown). Upon overexposure, Southern blot analysis of the M1.LacZ virus stock revealed a minor subpopulation of virus which contained a similar deletion. Based on the minor amount of this deletion virus in the M1.LacZ stock, we believe that this subpopulation cannot account for the observed phenotypes attributed to M1.LacZ (see below). Regardless, the identification of these isolates stresses the importance of generating a marker rescue for study of individual gene mutations in γHV68 and suggests that spontaneous deletions near the left end of the viral genome can occur upon virus passage in tissue culture.
M1.LacZ replicates normally in in vitro replication and exhibits virulence in C.B-17 SCID mice comparable to that of wt γHV68.
Based on our ability to isolate the M1.LacZ mutant, the M1 ORF is nonessential for in vitro replication, consistent with the report by Simas et al. (20). To identify potential alterations in in vitro replication of M1.LacZ, we compared replication of M1.LacZ and wt γHV68 in a single round of replication and in multiple rounds of replication in NIH 3T12 cells. In both single and multiple cycles of replication, M1.LacZ replicated comparably to wt γHV68 (Fig. 2). This demonstrates that the M1 ORF is not required for efficient replication in immortalized murine fibroblasts.
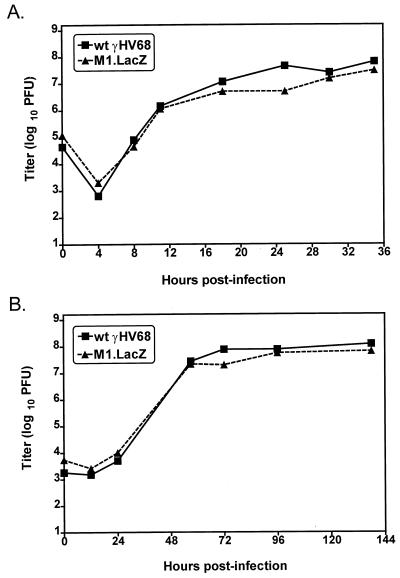
FIG. 2.
M1.LacZ replicates comparably to wt γHV68 in vitro. NIH 3T12 monolayers were infected with either 5 (A) or 0.05 (B) PFU per cell, and samples were harvested at the times indicated. Samples were freeze-thawed four times and subsequently quantitated by plaque assay on NIH 3T12 monolayers. Data are representative of two (A) or three (B) independent experiments. Data are shown as log10 titer. The sensitivity of this plaque assay is 50 PFU, or 1.7 in log10.
To assess the virulence of M1.LacZ, we compared the capacities of M1.LacZ and wt γHV68 to kill C.B-17 SCID mice, which lack mature B and T lymphocytes (2). At 103 and 106 PFU, M1.LacZ killed SCID mice with kinetics comparable to those of wt γHV68 (Fig. 3). However, at 101 PFU, there was a statistically significant 2- to 3-day delay in the kinetics of lethality of M1.LacZ compared to those of wt γHV68-infected SCID mice (P < 0.0005) (Fig. 3). The latter result raised the possibility that M1.LacZ replication is slightly attenuated in vivo.
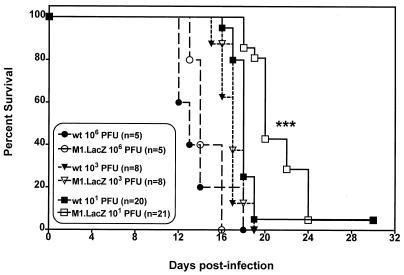
FIG. 3.
M1.LacZ kills C.B-17 SCID mice with kinetics similar to those of wt γHV68. C.B-17 SCID mice were infected by i.p. injection with 106, 103, or 101 PFU. Data were compiled from one (106 PFU), two (103 PFU), or four (101 PFU) independent experiments, with the total number of mice analyzed indicated in parentheses. ∗∗∗, survival (at 101 PFU) of M1.LacZ-infected mice significantly different from that of wt γHV68-infected mice (P < 0.0005).
M1.LacZ-infected mice have decreased acute virus titers in the spleens of immunocompetent and immunodeficient C57BL/6 mice.
To analyze acute virus replication in vivo, we quantitated splenic virus titers in normal C57BL/6 (B6) mice infected with wt γHV68, M1.LacZ, and M1.MR viruses on either day 4 or day 9 postinfection. Notably, compared to wt γHV68- and M1.MR-infected mice, M1.LacZ-infected mice exhibited decreased splenic titers on both day 4 and day 9 (P < 0.0001) (Fig. 4A). Furthermore, on day 9, half of the M1.LacZ-infected animals had titers below the limit of detection for the plaque assay (limit of detection, 101.7 PFU/organ [50 PFU/organ]). Animals infected with M1.MR had titers comparable to that of wt γHV68 (Fig. 4A).
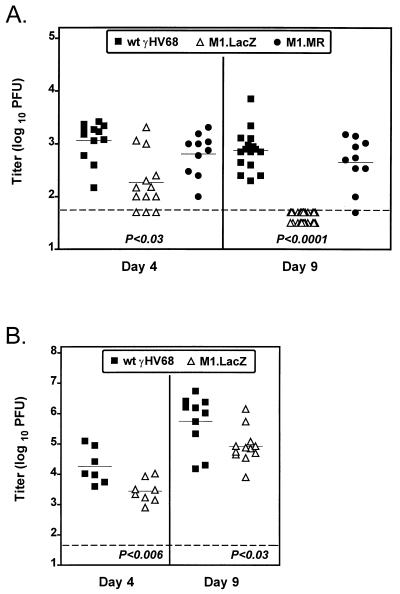
FIG. 4.
M1.LacZ-infected mice have decreased acute viral titers in the spleen compared to wt γHV68-infected mice. C57BL/6 (A) or B6.Rag1-deficient (B) mice were infected with 106 PFU of wt γHV68, M1.LacZ, or M1.MR by i.p. injection. Spleens were harvested at either day 4 or day 9 postinfection. Data for C57BL/6 mice were compiled from two (M1.MR at days 4 and 9), three (wt γHV68, M1.LacZ at day 4), four (wt γHV68 at day 9), or five (M1.LacZ at day 9) independent experiments with a total of 8 to 20 mice per time point. Data for B6.Rag1-deficient mice represent two (day 4) or three (day 9) independent experiments, with 7 to 12 mice total per time point. Each point represents the viral splenic titer of an individual mouse, with the horizontal solid line indicating the mean titer for each group. The horizontal dashed line indicates the level of detection for this assay (50 PFU, or 1.7 in log10). M1.LacZ values which significantly differ from those of the wt γHV68 and M1.MR (A) and values which significantly differ from those of wt γHV68 (B) are indicated below each set of data.
To determine if replication of M1.LacZ is compromised in the absence of antigen-specific immunity, we quantitated acute virus replication in M1.LacZ-infected B6.Rag-1-deficient mice (14). Similar to the phenotype observed in B6 mice, at both days 4 and 9 postinfection, B6.Rag1-deficient mice infected with the M1.LacZ virus had an ∼10-fold reduction in splenic titers compared to wt γHV68-infected mice (P < 0.03 for day 9 titer) (Fig. 4B). These data demonstrate that M1.LacZ has impaired acute virus replication in severely immunocompromised mice compared to wt γHV68. However, M1.LacZ replicated to significantly higher titers in B6.Rag-1-deficient mice compared to that of B6 mice at day 9 (compare Fig. 4A and B), consistent with B- and/or T-cell-dependent control of M1.LacZ replication in wt mice.
M1.LacZ has altered pathogenesis in IFN-γ-unresponsive mice.
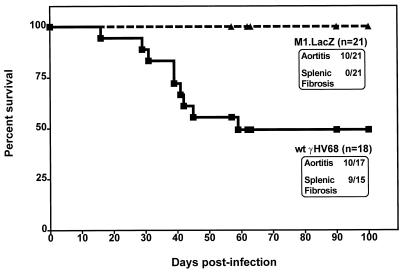
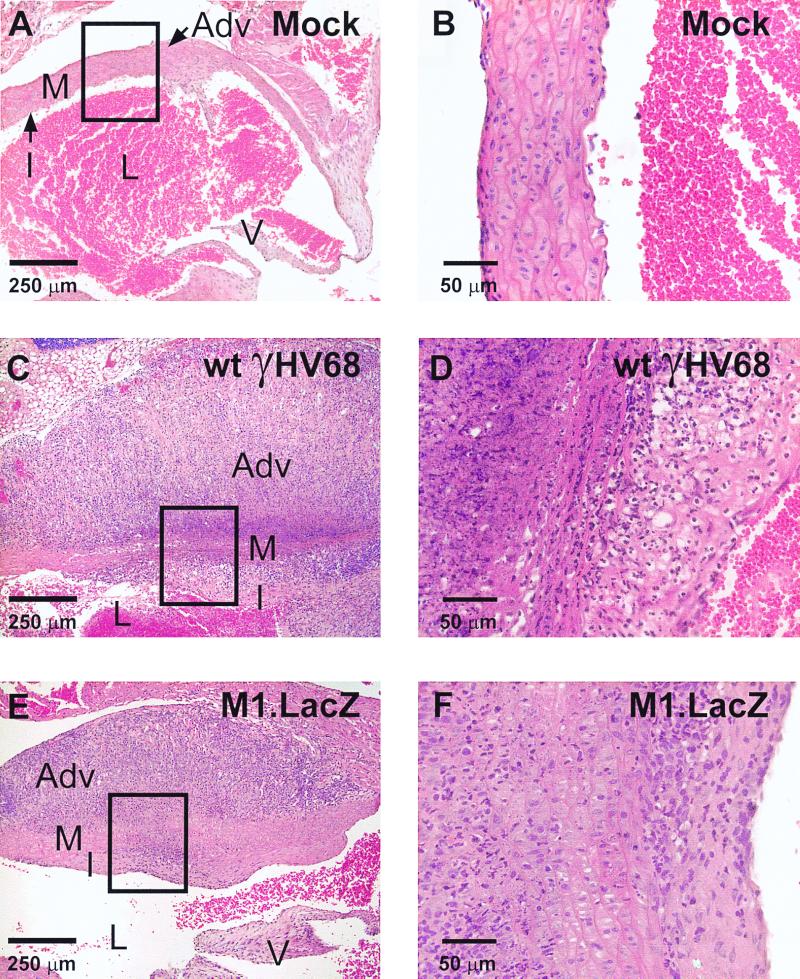
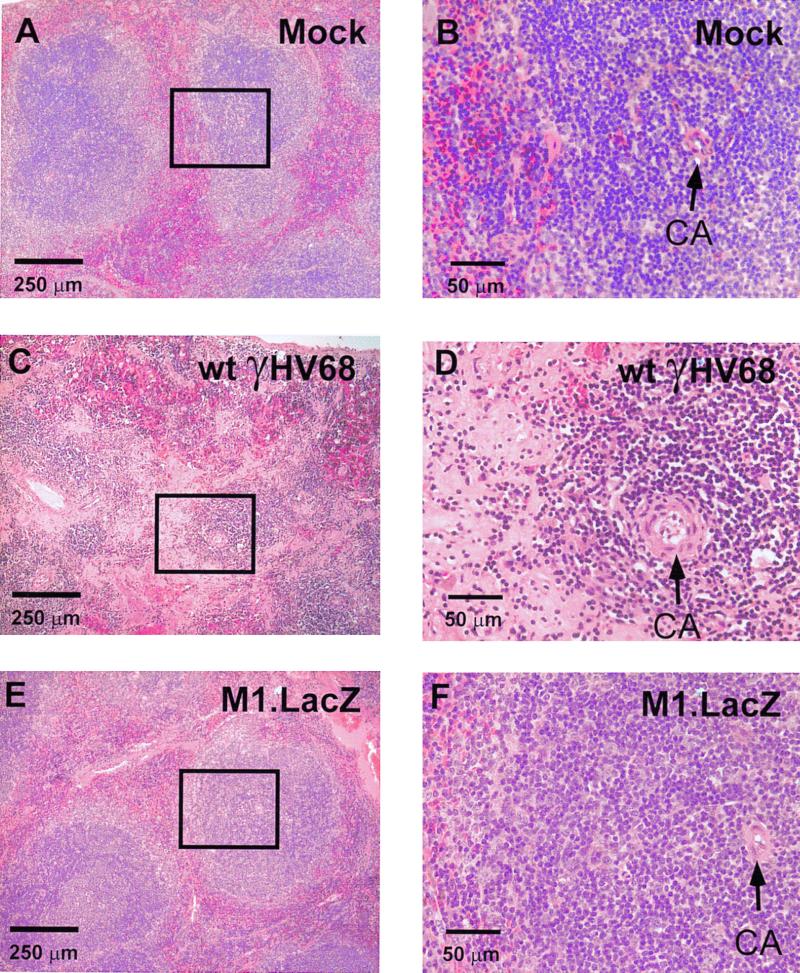
To assess the capacity of M1.LacZ to induce chronic virus-mediated pathology, we compared lethality, aortic pathology, and splenic fibrosis or atrophy in IFNγR-deficient mice infected with wt γHV68 or M1.LacZ (6, 39). In these experiments, M1.LacZ-infected mice survived out to 100 days postinfection, whereas wt γHV68-infected animals exhibited a 50% mortality over the same time course (P < 0.0003) (Fig. 5). Despite this significant difference in lethality, microscropic analysis of aortic cross-sections revealed that the incidence of aortic inflammation was comparable between M1.LacZ- and wt ΔHV68-infected mice (Fig. 5 and 6). Further analysis identified that aortic inflammation in M1.LacZ-infected mice, while exhibiting pronounced adventitial and intimal thickening, had slightly less cellular infiltrate in the media of the aorta and less destruction of the medial architecture than in wt-infected animals (Fig. 6). This demonstrated that the M1 ORF is dispensable for induction of arteritis, but not lethality, in IFN-γR-deficient mice. While severe arteritis occurred in both M1.LacZ and wt γHV68-infected IFN-γR-deficient mice, none of the M1.LacZ-infected mice demonstrated splenic fibrosis or atrophy whereas approximately 60% of wt γHV68-infected mice exhibited splenic fibrosis or atrophy (P < 0.0001) (Fig. 5 and 7). Thus, disruption of the M1 ORF reveals that induction of arteritis can be unlinked from lethality and splenic fibrosis or atrophy in γHV68-infected IFN-γR-deficient mice.
FIG. 5.
M1.LacZ induces aortitis, but fails to induce either mortality or splenic fibrosis or atrophy in IFN-γR-deficient mice. IFN-γR-deficient mice were infected with 5 × 106 PFU of either wt γHV68 or M1.LacZ, and animals were monitored for the course of infection. During the course of infection, mortality was recorded. Total cumulative pathology is indicated for each group. Animals were sacrificed between days 57 and 100 postinfection, at which time, organs were harvested. Heart and lung sections were analyzed for the presence of aortitis, and spleens were examined for splenic fibrosis or atrophy (assessed independently by three investigators). Data were compiled from five independent experiments, with the total number of mice analyzed indicated. Survival was significantly different between wt γHV68- and M1.LacZ-infected IFN-γR-deficient mice (P < 0.0003), as was the penetrance of splenic fibrosis or atrophy (P < 0.0001). For the wt γHV68-infected group, one mouse was disposed of prior to autopsy, and two mice did not have a spleen at the time of autopsy.
FIG. 6.
M1.LacZ induces aortitis comparable to that induced by wt γHV68 in IFN-γR-deficient mice. Representative cross-sections of aorta from a mock-infected IFN-γR-deficient mouse (A and B), a wt γHV68-infected IFN-γR-deficient mouse (C and D), and an M1.LacZ-infected IFN-γR-deficient mouse (E and F). The wt γHV68- and M1.LacZ-infected mice were sacrificed at 9 weeks postinfection (days 62 and 63 p.i.). All sections are stained with H&E. L, lumen; I, intima; M, media; Adv, adventitia; V, aortic valve. Boxed regions in panels A, C, and E are approximate fields shown at higher magnification in panels B, D, and F, respectively. Mice were from the experimental groups described in the legend to Fig. 5.
FIG. 7.
M1.LacZ fails to induce splenic fibrosis or atrophy in IFN-γR-deficient mice. Representative histology of spleens from either a mock-infected IFN-γR-deficient mouse (A and B), a wt γHV68-infected IFN-γR-deficient mouse (C and D), and an M1.LacZ-infected IFN-γR-deficient mouse (E and F). All sections were stained with H&E. CA, central arteriole. Boxed regions in panels A, C, and E are approximate fields shown at higher magnification in panels B, D, and F, respectively. The wt γHV68-infected spleen was from a mouse that died at day 29 postinfection. The M1.LacZ-infected spleen was from a mouse that was sacrificed at day 62 postinfection. Mice were from the experimental groups described in the legend to Fig. 5.
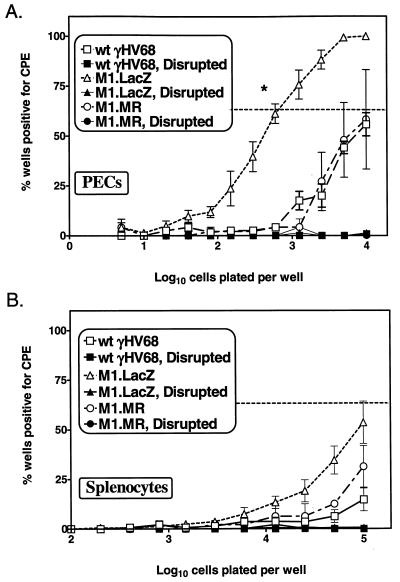
M1.LacZ exhibits an enhanced efficiency of reactivation from latency.
We examined whether M1.LacZ could establish or maintain latency in vivo. At 6 weeks postinfection, M1.LacZ-infected B6 mice had established a latent infection (Fig. 8), consistent with data from Simas et al. (20), suggesting that the M1 ORF is nonessential for the establishment of latency. However, further analysis revealed that PECs recovered from M1.LacZ-infected mice exhibited at least a 10-fold-higher frequency of cells reactivating virus compared to PECs recovered from wt γHV68 (P < 0.0005) or M1.MR-infected mice (P < 0.04) (Fig. 8A). Approximately 1 in 700 PECs reactivated virus from M1.LacZ-infected mice, compared to 1 in 10,000 PECs reactivating virus from wt γHV68-infected mice. In the splenocyte population, the overall frequency of cells reactivating virus was lower, with M1.LacZ-infected mice having, at most, a modest increase in reactivation frequency (Fig. 8B). Significantly, in both M1.LacZ- and wt γHV68-infected mice, there was no detectable preformed infectious virus, as determined by the simultaneous plating of mechanically disrupted cells (Fig. 8). Thus, these data are consistent with the detection and quantitation of reactivation of latent γHV68.
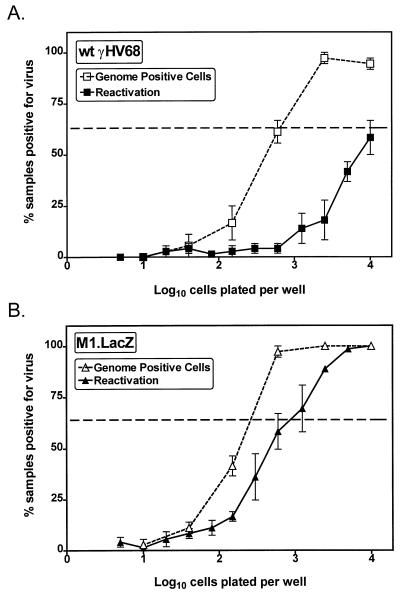
FIG. 8.
M1.LacZ establishes a latent infection characterized by an increased frequency of ex vivo reactivation from latency. B6 mice were infected with 106 PFU of wt γHV68, M1.LacZ, or M1.MR and were harvested between days 42 and 50 postinfection. Samples were tested for ex vivo reactivation with PECs (A) and splenocytes (B). For each cell dilution, 24 wells were analyzed per experiment. The horizontal dashed line indicates 63%, which was used to calculate the frequency of reactivation of cells by Poisson distribution. Mechanically disrupted cells were plated in parallel to identify the presence of preformed infectious virus, as described in Materials and Methods. Data represent two (M1.MR), five (wt γHV68), or six (M1.LacZ) independent experiments, with cells pooled from three to five mice per experiment per group. Data are shown as mean percentage of wells positive for CPE ± standard error of the mean. The M1.LacZ frequency of reactivation from PECs was significantly different from that of wt γHV68 (P < 0.0005)- or M1.MR (P < 0.04)-infected mice (asterisk in panel A).
The increased frequency of cells reactivating γHV68 ex vivo from PECs recovered from M1.LacZ-infected mice could be due to either an increased reactivation efficiency or to a higher frequency of latently infected cells. To address this issue, we quantitated the frequency of virus genome-positive cells in the PEC population by using a nested-PCR protocol to detect the γHV68 genome (40, 41). This assay has been shown to detect a single copy of the viral genome in a background of cellular DNA from 104 uninfected cells (data not shown and references 40 and 41). In this analysis, PECs isolated from M1.LacZ-infected mice had an approximately threefold increase in the frequency of viral genome-positive cells compared with PECs isolated from wt γHV68-infected mice (∼1 in 200 cells in M1.LacZ-infected mice and ∼1 in 600 cells in wt γHV68-infected mice) (P < 0.03) (Fig. 9). By comparing the frequency of cells that reactivate γHV68 to the frequency of virus-genome-positive cells, we determined that approximately 1 in 3 virus genome-positive cells were reactivated in the PEC population harvested from M1.LacZ-infected mice, while only 1 in 15 virus-genome-positive cells were reactivated in the PEC population harvested from wt γHV68-infected animals (Fig. 9).
FIG. 9.
M1.LacZ has an increased efficiency of reactivation from latency. Latently-infected PECs were analyzed for the frequency of viral genome by nested PCR in wt γHV68-infected mice (A) or in M1.LacZ-infected mice (B). Twelve PCRs were performed per cell dilution for each experiment, with the inclusion of PCR specificity controls as discussed in Materials and Methods. The reactivation data shown are from three independent experiments presented in Fig. 8. The horizontal dashed line indicates 63%, which was used to calculate the frequency of genome-positive cells and cells reactivating virus by Poisson distribution. The data represent three independent experiments with cells pooled from three to five mice per group. Data are shown as mean percentage of wells positive for viral genome or CPE ± standard error of the mean. M1.LacZ latently infected PECs had a statistically significant increase in the frequency of reactivation (P < 0.0005) and viral-genome-positive cells (P < 0.03) compared with wt γHV68 latently-infected PECs.
Generation of M1Δ511.
To investigate the contribution of the LacZ expression cassette to the phenotypes observed with M1.LacZ, we generated another viral recombinant (M1Δ511). M1Δ511 contains the same 511-bp deletion (bp 1892 to 2403; WUMS sequence) present in M1.LacZ, which spans the 5′ end of the M1 ORF (Fig. 10A). M1Δ511 was purified by white plaque selection and had 100% white plaque morphology after three rounds of plaque purification. Southern analysis of EcoRV-digested M1Δ511 viral DNA, using a 32P-labeled M1 region probe, demonstrated hybridization with the expected 5.7-kb fragment and loss of the 8.0- and 2.0-kb fragments present in the M1.LacZ mutant (Fig. 10B). Furthermore, a 32P-labeled LacZ probe failed to hybridize to any sequences present in M1Δ511 (data not shown), verifying replacement of the LacZ expression cassette by insertion of the truncated M1 ORF. Notably, further Southern blot analysis of M1Δ511 identified an anomaly in restriction fragments generated following digestion with ApaLI and NgoMIV, in which an ApaLI restriction site present in wt γHV68 (bp 80 in the viral genome) appeared to be absent in M1Δ511, M1.LacZ, and M1.MR (data not shown). Further Southern blot analysis with a NotI-EcoRV restriction digest and a probe corresponding to bp 107 to 1892 of the γHV68 genome identified that wt γHV68 DNA hybridized with the expected 2.0-kb fragment, whereas M1Δ511, M1.LacZ, and M1.MR DNA hybridized with a 1.9-kb fragment (Fig. 10B). Based on this analysis, M1Δ511, M1.LacZ, and M1.MR possess a common deletion spanning approximately the first 100 bp of unique sequence in the γHV68 genome. This deletion was verified with a NotI-BglII restriction digest (data not shown). Genomic restriction mapping with BamHI, HindIII, and EcoRI demonstrated that, with the exception of the ∼100-bp deletion, wt γHV68, M1.LacZ, M1Δ511, and M1.MR exhibited the expected pattern of restriction fragments (data not shown). It is important to note that this deletion is found in each of the viral recombinants (M1Δ511, M1.LacZ, and M1.MR) and that M1.MR has acute replication and reactivation from latency comparable to those of wt γHV68. This result indicates that this 100-bp deletion does not account for the phenotypic alterations observed in M1.LacZ-infected mice.
FIG. 10.
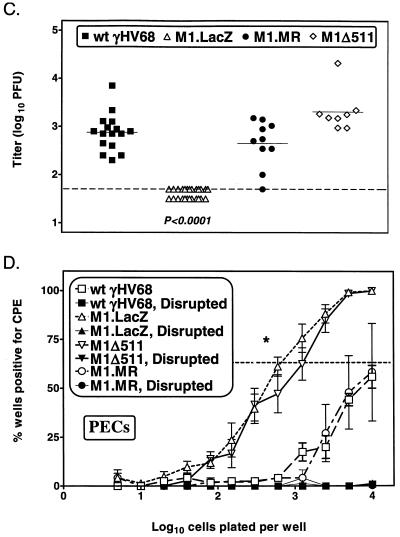
Construction and characterization of M1Δ511. (A) Genomic structure of wt γHV68, M1.LacZ, M1Δ511, and M1.MR in the region containing the M1 ORF. In both M1.LacZ and M1Δ511, the M1 ORF was disrupted through targeted excision of bp 1892 to 2403 of the viral genome by using the restriction enzymes StuI (S) and NgoMI (N). TR indicates terminal repeat sequence, with Not indicating a NotI restriction site. In M1.LacZ, M1Δ511, and M1.MR, there was an ∼100-bp deletion which removed the first 100 bp of γHV68 unique sequence, denoted by a dashed line. Further annotations are the same as those described for Fig. 1A. (B) Southern blot analysis of wt γHV68, M1.LacZ, M1Δ511, and M1.MR viral genomes following either an EcoRV or a NotI-EcoRV restriction enzyme digest followed by the indicated probe (M1 probe [Fig. 1A] or probe specific for bp 107 to 1892 of γHV68]), with molecular size markers indicated to the left. A longer exposure of the NotI-EcoRV Southern blot revealed hybridization with the higher-molecular-weight restriction fragments predicted for each virus (data not shown). (C) Day 9 splenic viral titer of C57BL/6 mice infected with M1Δ511 compared with wt γHV68, M1.LacZ, and M1.MR (106 PFU by i.p. injection). The data include two experiments which contained wt γHV68, M1.LacZ, and M1Δ511 with seven or eight mice total per group, with additional data included from Fig. 4A. M1.LacZ titers significantly differed from wt γHV68, M1Δ511, and M1.MR titers, as indicated. (D) Ex vivo reactivation frequency of PECs from C57BL/6 mice infected with M1Δ511 (106 PFU by i.p. injection) harvested between days 44 and 50 postinfection. The data include three experiments which contained wt γHV68, M1.LacZ, and M1Δ511 with cells pooled from three to five mice per group per experiment, with additional data included from Fig. 8A.
Generation of M1Δ511 allowed us to examine acute virus replication and reactivation from latency employing an M1 mutant virus lacking the LacZ expression cassette. As previously observed, mice infected with M1.LacZ had an ∼10-fold decrease in splenic viral titer at day 9 postinfection (Fig. 10C). Unexpectedly, mice infected with M1Δ511 had titers comparable to those of wt γHV68 and M1.MR at day 9 postinfection (Fig. 10C). This result indicated that deletion of the 5′ end of the M1 ORF was not sufficient to result in decreased acute replication in immunocompetent mice. We extended the analysis of M1Δ511 by analyzing the frequency of cells reactivating virus by using PECs recovered from mice infected with M1Δ511. B6 mice were infected with either wt γHV68, M1.LacZ, M1.MR, or M1Δ511, and PECs were harvested between days 44 and 50 postinfection. Both M1.LacZ and M1Δ511 exhibited the previously observed increased frequency of reactivation compared to that of wt γHV68-infected mice (Fig. 10D), indicating that deletion of the 5′ end of the M1 ORF is sufficient to result in an increased frequency of reactivation from latency. Southern blot analysis demonstrated that M1Δ511 was recovered from M1Δ511-infected mice harvested at both day 9 and day 44 postinfection (data not shown). Experiments are currently in progress to examine the outcome of M1.MR and M1Δ511 infection in IFN-γR-deficient mice.
DISCUSSION
The data presented here provide genetic evidence for the M1 ORF and/or a closely linked gene(s) as a determinant of γHV68 pathogenesis. While our data are consistent with those of Simas et al. (20) in demonstrating that the M1 ORF is nonessential for in vitro replication and establishment of latency in vivo, further analysis revealed that the M1 ORF and/or a closely linked gene or genes play a critical role in the regulation of reactivation from latency. Through restoration of M1 ORF sequences into M1.LacZ and generation of M1Δ511, we have demonstrated that the enhanced reactivation phenotype of M1.LacZ is not due to additional mutations located elsewhere within M1.LacZ or insertion of the LacZ expression cassette.
The putative M1 ORF-encoded protein.
This analysis has focused on characterizing the role of the M1 ORF in γHV68 pathogenesis. At this time, nothing is known about the putative protein(s) encoded by the M1 ORF or possible function(s) of this molecule(s). Previously, BLASTP analysis of the putative M1 protein identified sequence homology to two viral gene products: (i) the poxvirus serine proteinase inhibitor (serpin), SPI-1; and (ii) the M3 protein of γHV68 (3, 34, 35). Of the SPI-1 genes present in the orthopoxviruses, M1 has greatest sequence homology to the Rabbitpox virus (RPV) SPI-1 gene product. In RPV, SPI-1 determines host range, and an SPI-1-deficient RPV exhibits premature apoptosis in certain cell types (1, 4). In addition, RPV SPI-1 has been implicated in regulating target cell lysis by cytotoxic T lymphocytes in coordination with another poxvirus serpin, SPI-2 (12). Although M1 and SPI-1 are homologous, M1 does not have clear sequence homology to the highly conserved, functionally important hinge domain and reactive site loop present in SPI-1 and other inhibitory serpins (3, 16, 32; P. C. Hopkins and J. Whisstock, Letter, Science 265:1893–1894, 1994; unpublished observations). Thus, the functional significance of the homology between M1 and SPI-1 is unclear.
The γHV68 M1 and M3 ORFs exhibit ∼25% sequence homology over the length of their protein sequences (34). Previously, the M3-encoded protein was identified as an abundantly secreted protein in γHV68-infected cultures (34). Recently, the M3 protein has been demonstrated to function as a chemokine-binding protein, suggesting a potentially important role for M3 in γHV68 pathogenesis through the subversion of host inflammatory and immune responses (V. van Berkel, S. H. Speck, and H. W. Virgin IV, unpublished data). Based on this information, it is interesting to speculate that M1 might also be involved in chemokine binding, acting together with M3 to subvert the appropriate host responses. However, despite the homology between M1 and M3, it is clear that M1 and M3 are not redundant, since disruption of the M1 ORF is sufficient to result in enhanced reactivation from latency.
While the data presented here are consistent with disruption of the M1 ORF resulting in enhanced reactivation from latency, it is formally possible that the mutations present in M1.LacZ and M1Δ511 alter expression of another gene product (see below) or alter important genomic structure in this region of the viral genome. Future studies will elucidate the contribution of the M1 protein to these phenotypic alterations.
Decreased lytic virus in the spleens of M1.LacZ-infected B6 and B6.Rag1-deficient mice.
We have demonstrated that the M1.LacZ mutant exhibits decreased acute virus replication in the spleens of both immunocompetent and immunodeficient mice on the B6 background. However, the M1Δ511 mutant, in which the first 480 bp of the M1 ORF is deleted, does not recapitulate this phenotype. Thus, this suggests that the presence of the LacZ expression cassette is required for the attenuation of acute virus replication (Fig. 10C). Significantly, the M1.LacZ defect in acute replication does not appear to result from immunogenicity of the LacZ protein, since M1.LacZ has decreased acute replication in immunodeficient mice (Fig. 4B). Furthermore, we have characterized another γHV68 mutant harboring the LacZ expression cassette within gene 72 of γHV68, and this mutant exhibits normal acute virus replication in the liver, lung, and spleen (L. van Dyk, S. H. Speck, and H. W. Virgin IV, unpublished data). Thus, this suggests that the decreased acute virus replication observed with M1.LacZ arises from a position-dependent effect of the LacZ gene cassette. Previously, another group has identified LacZ+ murine cytomegalovirus recombinants which have decreased replication in the salivary gland (28). However, this defect appears to be independent of the site of LacZ insertion. To our knowledge, this is the first evidence of a position-dependent effect of the LacZ gene cassette on herpesvirus pathogenesis. Whether this phenotype is due to alterations in transcription within this region, altered genomic stability, or some other effect remains unclear. It is possible, for example, that a partially functional truncated M1-encoded protein may be produced with the M1Δ511 mutant that is not expressed with the M1.LacZ mutant virus. Further analysis of this possibility will require generation of appropriate reagents for detection of the putative M1 ORF-encoded protein. Nonetheless, the difference in the acute replication levels observed with the M1.LacZ and M1Δ511 mutants stresses that LacZ mutations may not be sufficient to characterize viral mutations in γHV68.
Altered pathogenesis in IFN-γR-deficient mice.
The pathogenesis of the M1.LacZ mutant in IFN-γR-deficient mice revealed an unusual combination of phenotypes. First, M1.LacZ-infected animals developed aortic inflammatory lesions very similar to those observed in wt γHV68-infected mice. Despite this severe vascular pathology, M1.LacZ-infected animals did not die, even after 100 days of infection. This was in striking contrast to the 50% mortality observed in wt γHV68-infected IFN-γR-deficient mice over the same time course. While the severity of the arteritic lesions caused by wt γHV68 in IFN-γR-deficient mice was previously considered to be the cause of mortality in IFN-γR-deficient mice (39), data from M1.LacZ-infected mice indicate that the observed severe aortic pathology may not be the cause of death in wt γHV68-infected IFN-γR-deficient mice. This provides an interesting example of how genetic analysis of viral mutants can contribute to an understanding of the relationship between pathology and disease outcome. Analysis of further mutants with various models of γHV68 pathogenesis may further refine our understanding of how virus-induced pathology relates to disease outcome during chronic infection.
A second disparity between M1.LacZ- and wt γHV68-infected IFN-γR-deficient mice was the induction of splenic pathology. In contrast to wt γHV68-infected IFN-γR-deficient mice, none of the M1.LacZ-infected IFN-γR-deficient mice developed splenic fibrosis or atrophy. These data raise the question of whether death in chronically infected IFN-γR-deficient mice is related to the splenic fibrosis or atrophy. Previously, Dutia et al. demonstrated that this splenic pathology was ameliorated when either CD4+ or CD8+ T cells were depleted (6). Based on this observation (6), it is possible that a T-cell-dependent process may contribute to mortality in γHV68-infected IFN-γR-deficient mice and that M1.LacZ may be deficient in inducing this response. To better understand the alteration in M1.LacZ which results in the absence of splenic pathology, experiments are currently in progress to characterize the pathogenesis of M1Δ511 in IFN-γR-deficient mice. This analysis may help identify the genetic basis of the M1.LacZ phenotypic alterations in these mice, as well as begin to identify the requirement for acute replication to induce splenic pathology and mortality in IFN-γR-deficient mice.
Regulation of latency by M1 or a closely linked gene.
The increased reactivation efficiency of M1.LacZ and M1Δ511 is the first demonstration of viral mutations in γHV68 which result in altered regulation of latency. This alteration upon disruption of the M1 ORF was unexpected. Although both the M2 and M3 ORFs have been identified as regions of the genome that are transcriptionally active during latent infection, the M1 ORF has not been previously identified as a candidate latency-associated gene (11, 22, 36). The failure to detect M1 transcripts in the previous analyses may reflect the insufficient sensitivity of the assays employed. Furthermore, none of the screens for latent transcripts were designed to identify genes involved in controlling reactivation. It is possible that the M1 ORF is intricately involved in regulating the reactivation process from latency. Alternatively, it is possible that transcription of the M2 ORF, or another unidentified transcript in this region, might be altered by disruption of the M1 ORF (specifically by disruption of bp 1892 to 2403).
Recently, analysis of an M2 cDNA clone was published (11). This transcript, isolated from the γHV68 latently-infected S11 cell line, identified an M2 transcript which used a noncanonical polyadenylation signal located immediately downstream of the M1 ORF. Thus, expression of this M2 transcript would not be disrupted by the M1.LacZ or M1Δ511 mutations. However, it is possible that there are additional spliced forms of the M2 transcript which may be influenced by disruption of the 5′ end of the M1 ORF. Further analysis is in progress to identify the genetic alteration responsible for the enhanced reactivation frequency observed with the M1.LacZ and M1Δ511 mutants.
Curiously, increased reactivation efficiency has also been observed in B-cell-deficient mice infected with wt γHV68 (41). Although increased reactivation efficiency may be a common phenotypic alteration which identifies multiple independent mechanisms of controlling γHV68 reactivation, it is possible that enhanced reactivation of M1.LacZ may reflect a common pathway of regulation. For example, the M1-encoded gene product (or the product of a closely linked gene) might be essential for the effect of a B-cell-dependent signal that impairs viral reactivation. In this scenario, M1.LacZ would be unresponsive to this B-cell-dependent signal, and as a result, M1.LacZ would have an increased reactivation efficiency from latency.
Conclusions.
Based on the current analysis, four important conclusions can be made. First, disruption of the M1 ORF and/or a closely linked gene or genes results in enhanced reactivation from latency, providing the first genetic identification of a γHV68 gene which regulates latency and reactivation. Second, our analysis clearly demonstrates that alterations in acute virus replication do not directly correlate with alterations in the establishment of latency, consistent with a recent publication by Stevenson et al. (24). Third, this analysis shows that the induction of arteritis can be uncoupled from the induction of splenic pathology and mortality in IFN-γ-unresponsive mice. Finally, while genetic manipulation of γHV68 is tractable, our analysis stresses the importance of generating a marker rescue virus as well as mutations beyond LacZ+ recombinants to fully characterize the genetic basis of phenotypic alterations in viral recombinants.
ACKNOWLEDGMENTS
This research was supported by NIH grants CA74730 and HL60090 to H.W.V. and S.H.S.; NIH grants CA43143, CA52004, and CA58524 to S.H.S.; NIH grant AI39616 to H.W.V.; and ACS grant RP6-97-134-01-MBC to H.W.V. E.T.C. was supported by a Lucille P. Markey Pathway predoctoral fellowship and NIH grant CA43143.
We thank Paul Olivo for reagents, David Leib and Nat Moorman for technical assistance in establishing recombinant technology for γHV68, Joy Loh for assistance in generation of the M1.LacZ virus, and Albert dal Canto for assistance with reading slides for histopathology. We also acknowledge helpful discussions with members of the Speck and Virgin laboratories, as well as discussions during joint laboratory meetings with members of the laboratories of David Leib and Lynda Morrison.
REFERENCES
- 1.Ali A N, Turner P C, Brooks M A, Moyer R W. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology. 1994;202:305–314. doi: 10.1006/viro.1994.1347. [DOI] [PubMed] [Google Scholar]
- 2.Bosma M J. The scid mutation: occurrence and effect. Curr Top Microbiol Immunol. 1989;152:3–9. doi: 10.1007/978-3-642-74974-2_1. [DOI] [PubMed] [Google Scholar]
- 3.Bowden R J, Simas J P, Davis A J, Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- 4.Brooks M A, Ali A N, Turner P C, Moyer R W. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J Virol. 1995;69:7688–7698. doi: 10.1128/jvi.69.12.7688-7698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutia B M, Clarke C J, Allen D J, Nash A A. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou S, Ho Y M, Hall S, Styles C J, Scott S D, Gompels U A. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 8.Efstathiou S, Ho Y M, Minson A C. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 9.Ehtisham S, Sunil-Chandra N P, Nash A A. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganem D. KSHV and Kaposi's sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 11.Husain S M, Usherwood E J, Dyson H, Coleclough C, Coppola M A, Woodland D L, Blackman M A, Stewart J P, Sample J T. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:7508–7513. doi: 10.1073/pnas.96.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macen J L, Garner R S, Musy P Y, Brooks M A, Turner P C, Moyer R W, McFadden G, Bleackley R C. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc Natl Acad Sci USA. 1996;93:9108–9113. doi: 10.1073/pnas.93.17.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackett M, Stewart J P, Pepper S de V, Chee M, Efstathiou S, Nash A, Arrand J R. Genetic content and preliminary transcriptional analysis of a representative region of murine gammaherpesvirus 68. J Gen Virol. 1997;78:1425–1433. doi: 10.1099/0022-1317-78-6-1425. [DOI] [PubMed] [Google Scholar]
- 14.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 15.Muller U, Steinhoff S, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 16.Potema J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 17.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 18.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields' virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Simas J P, Bowden R J, Paige V, Efstathiou S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J Gen Virol. 1998;79:149–153. doi: 10.1099/0022-1317-79-1-149. [DOI] [PubMed] [Google Scholar]
- 21.Simas J P, Efstathiou S. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- 22.Simas J P, Swann D, Bowden R, Efstathiou S. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J Gen Virol. 1999;80:75–82. doi: 10.1099/0022-1317-80-1-75. [DOI] [PubMed] [Google Scholar]
- 23.Speck S H, Virgin H W. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol. 1999;2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson P G, Belz G T, Castrucci M R, Altman J D, Doherty P C. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc Natl Acad Sci USA. 1999;96:9281–9286. doi: 10.1073/pnas.96.16.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson P G, Doherty P C. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J Virol. 1999;73:1075–1079. doi: 10.1128/jvi.73.2.1075-1079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart J P. Of mice and men: murine gammaherpesvirus 68 as a model. Epstein-Barr Virus Rep. 1999;6:31–35. [Google Scholar]
- 27.Stewart J P, Usherwood E J, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoddart C A, Cardin R D, Boname J M, Manning W C, Abenes G B, Mocarski E S. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunil-Chandra N P, Arno J, Fazakerley J, Nash A A. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am J Pathol. 1994;145:818–826. [PMC free article] [PubMed] [Google Scholar]
- 30.Sunil-Chandra N P, Efstathiou S, Arno J, Nash A A. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 31.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 32.Turner P C, Musy P Y, Moyer R W. Poxvirus serpins. In: McFadden G, editor. Viroreceptors, virokines, and related immune modulators encoded by DNA viruses. R. G. Austin, Tex: Landes Company; 1995. pp. 67–88. [Google Scholar]
- 33.Usherwood E J, Ross A J, Allen D J, Nash A A. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol. 1996;77:627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 34.van Berkel V, Preiter K, Virgin IV H W, Speck S H. Identification and initial characterization of the murine gammaherpesvirus 68 gene M3, encoding an abundantly secreted protein. J Virol. 1999;73:4524–4529. doi: 10.1128/jvi.73.5.4524-4529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virgin H W, IV, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virgin H W, IV, Speck S H. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 38.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weck K E, Dal Canto A J, Gould J D, O'Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W. Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 40.Weck K E, Kim S S, Virgin IV H W, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weck K E, Kim S S, Virgin IV H W, Speck S H. B cells regulate murine gammaherpesvirus 68 latency. J Virol. 1999;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]