Abstract
Background:
An increasing number of clinical practice guidelines recommend screening children with obesity for non-alcoholic fatty liver disease (NAFLD). However, there is limited evidence regarding what parameters should be used to initiate the screening.
Objective:
The objective of this study was to determine whether obesity class rather than age group can identify a higher percent of children at risk of NAFLD as assessed by abnormal alanine aminotransferase (ALT).
Methods:
This is a cross-sectional study in a regional referral clinic for evaluation of obesity. Children were stratified by age group or by obesity class, and data obtained at first visit were analysed.
Results:
Of the 784 children, 482 were ≥10, 209 were 6 to 9 and 93 were 2 to 5 years of age. Abnormal ALT was observed in 32.1%, 46.9% and 61.0% of children with class I, II or III obesity, respectively (p < 0.001), while the risk of abnormal ALT did not differ in very young (2–5), young (6–9), or children older than 10 years. A multivariable analysis showed that class II and class III obesity were associated with 2.1-fold (1.27–3.72) and 4-fold (2.41–6.96) greater odds of abnormal ALT compared with class I obesity. African-American children had lower risk of abnormal ALT (0.27), whereas Hispanic children had higher risk (2.37). Obesity class was a better predictor of abnormal ALT than age, especially in girls. Furthermore, 66.7% of boys (p = 0.009) and 69% of girls (p < 0.001) with abnormal ALT exhibited additional signs of metabolic dysfunction.
Conclusion:
Obesity class is more strongly associated with abnormal ALT than age.
Keywords: ALT, diabetes, dyslipidaemia, NAFLD, obesity, paediatric
1 |. INTRODUCTION
Non-alcoholic fatty liver diseas e (NAFL D) is the most common cause of elevated liver enzymes in North American children.1,2 An increasing number of medic al societies recommend screening of children with overweight and obesity for NAFLD. 3 The American Academy of Paediatrics Expert Committee on Obesity recommends biannual screening in children with obesity and children with overweight and additional risk factors starting at the age of 10. 4 Similarly, the North American Society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) recommends screening beginning between ages 9 and 11 years in all children with obesity and for children with overweight and metabolic comorbidities. 5 NASPGHAN also suggests to consider earlier screening in younger children with severe obesity, family history of NAFLD or hypopituitarism. 5 The European Society for Paediatric Gastroenterology, Hepatology and Nutrition states that NAFLD typically presents in children over 10 years, but screening should also be performed in children aged 3 to 10 years with obesity. 6 In contrast, the American Association for the Study of Liver Diseases declares that there is insufficient evidence to make a formal recommendation regarding NAFLD screening in children with overweight and obesity. 7 Thus, even though NAFLD is the most common liver disease in childhood, when to initiate the screening is not fully defined.
NAFLD evaluation in adults is stratified based on the likelihood of identifying patients with advanced liver fibrosis. Older age and type 2 diabetes mellitus (T2DM) are important risk factors for advanced NAFLD in adults. Insulin resistance 8 appears to be the fundamental abnormality in pathophysiology of advanced fatty liver disease but other variables such as high dietary fructose intake,9 increased de novo lipogenesis10,11and decreased mitochondrial fatty acid oxidation12 appear to play a role.
The risk factors for advanced NAFLD in children have not been elucidated. NAFLD prevalence increases with age, but the risk of more advanced forms of NAFLD is not increased in older children.13–15 Fortunately, few children with NAFLD have T2DM, even though many have insulin resistance.16,17 Severity of obesity in children is associated with more advanced liver disease,15,18 leading to suggestions that obesity-associated liver disease is a more appropriate name for this condition in children.19,20 Despite the use of different criteria for screening children with obesity, current guidelines do not recommend risk stratification of paediatric NAFLD based on obesity class.
The objectives of our study were to examine whether stratification by obesity class, rather than age, can identify a larger percent of children with abnormal ALT and if these children have an increased risk of other metabolic abnormalities.
2 |. METHODS
This is a retrospective observational study analysing cross-sectional data from Kentucky Children’s Hospital High Body Mass Index Clinic, a regional referral clinic serving children and adolescents with obesity. Subjects included children with obesity (BMI ≥95th % for age and sex) aged 2 to 18 years. The data from the patients’ first visit were analysed. Laboratory values were included only if they were obtained at our centre in a fasted state. Blood pressure values not obtained by auscultation and the laboratory evaluations performed by outside labs were excluded in order to increase accuracy. Children with non-obesity related liver disease, anaemia, hemoglobinopathy or those receiving medication affecting blood pressure, lipid profile, liver enzymes or glucose metabolism were excluded.
During the first visit, we collected the following information: demographic data, anthropometric measurements such as weight (kg), height (cm) and body mass index (BMI), blood pressure (BP) and biomedical markers. The BMI was calculated using the recommended standard formula: BMI (kg/m2) = weight (kg) divided by height in metress quared. A BMI ≥95th percentile for age and sex using the 2000 CDC growth charts indicated obesity.21 The biomedical markers collected included the following: total cholesterol (TCh), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides (TG), fasting glucose (FG), glycosylated haemoglobin A1c (HbA1c), 2-h plasma glucose (2 h glucose) as part of oral glucose tolerance test and liver enzymes.
Children were grouped into three age categories: very young (2–5 years), young (6–9 years) and children 10 years or older ( ≥10). Subjects were divided into three obesity classes based on age- and sex-specific percentage of the 95th BMI percentile: class I (BMI 95%−119%), class II (120%−139% or BMI ≥35 kg/m2 ) and class III (BMI ≥140% or BMI ≥40 kg/m2). 4,22 Children were classified as White, African-American, Hispanic or Other based on self-identified race and ethnicity. County of residence was referred to as metropolitan, adjacent non-metro and rural. Serum alanine aminotransferase (ALT) >22 U/L for girls and >26 U/L for boys were considered abnormal.5 Risk for NAFLD was diagnosed if the patient had abnormal ALT over 6-month period and other causes of chronic hepatitis had been excluded, as all children had obesity. Chronic hepatitis was excluded by assessing for autoimmune markers, ceruloplasmin and serum copper, alpha 1 antitrypsin level and phenotype and hepatitis B and C. Two times above the upper limit of normal ALT (ALT ≥50 for boys and ≥44 for girls) was used for more stringent diagnosis of NAFLD. These cut-offs have a sensitivity of 88% and a specificity of 26% for diagnosis of NAFLD in children with obesity.3
Dyslipidaemia was defined as one or more of the following: TCh ≥200 mg/dl, HDL <40 mg/dl, LDL ≥130 mg/dl or TG ≥100 mg/dl for 2 t o 9 years and TG ≥130 mg/dl for those ≥10 years.23 Increased BP was defined as having pre-hypertension or hypertension by auscultatory measurements. Pre-hypertension was defined as age-, sex- and height-specific systolic or diastolic BP ≥90th% or ≥120/80 mmHg; hypertension was defined as BP ≥95th% or ≥130/80 mmHg.24 Definitions of pre-diabetes or diabetes were based on the American Diabetes Association25 with pre-diabetes defined as either FG ≥100 mg/dl, HbA1c between 5.7% and 6.4%, or 2-h plasma glucose between ≥140 and <200 mg/dl on oral glucose tolerance test (OGTT). Diabetes was defined as having either FG >125 mg/dl, HbA1c ≥6.5% or 2 h glucose ≥200 mg/dl.25 Metabolic dysfunction was defined as three or more of the following: obesity ≥class II, diabetes risk (pre-diabetes or diabetes), dyslipidaemia (high TGs, low HDL) and/or increased BP (pre-hypertension or hypertension). This definition was adopted with modification from previously published paediatric metabolic syndrome definition26 with waist circumference ≥90th percentile replaced with obesity ≥class II.
Data collection was approved by the Institutional Review Board from the University of Kentucky College of Medicine. Data were prospectively collected on 619 subjects as part of a registry, and the parents and children signed the consent and assent. The data on 165 subjects were collected as a retrospective chart review, and IRB exemption was granted to include these data. Study data were collected and managed using Research Electronic Data Capture (REDCap)27 hosted at the University of Kentucky.
2.1 |. Statistical analysis
For categorical variables, frequencies and column percentages (%) were reported, and p values were calculated using chi-squa re and Fisher’s exact tests as appropriate. Continuous variables were tested for normality using the Shapiro-Wilk normality test along with histograms. Normally distributed continuous variables were reported using means and standard deviations (SD), and p values were calculated using one-way ANOVAs; otherwise, medians and first/third quartiles [Q1,Q3] were reported, and p values were calculated using Kruskal-Wallis tests. Additionally , certain variables such a s age, obesity , TCh , LD L, HDL a nd TG we re teste d for linear trends with age group and obesity class, and p values were calculated using chi-square test for trend. A multivariable binary logistic regression model was used to estimate the effects of obesity class and age group on abnormal ALT while adjusting for race and county. Only weight and height were ana lysed as continuous variables in Tables 1 and 2. Statistical significance was set at p ≤ 0.05 and all tests were two-sided. All analyses were done in R programming language, version 3.6.2. 28 Chi-square tests were conducted using the ‘lbl_test’ function from the R package coin, version 1.3–1.29
TABLE 1.
Metabolic parameters stratified by age group
| Subjects stratified by age group |
|||||
|---|---|---|---|---|---|
| All Patients | 2–5 | 6–9 | ≥10 | p value | |
| Number of Patients | 784 | 93 | 209 | 482 | |
| Male, % (N/T) | 45.2% (354/784) | 50.5% (47/93) | 40.7% (85/209) | 46.1% (222/482) | 0.229 |
| Race, % (N/T) | 0.192 | ||||
| White | 65.8% (516/784) | 63.4% (59/93) | 63.6% (133/209) | 67.2% (324/482) | |
| African American | 17.7% (139/784) | 16.1% (15/93) | 15.3% (32/209) | 19.1% (92/482) | |
| Hispanic | 7.9% (62/784) | 9.7% (9/93) | 12.0% (25/209) | 5.8% (28/482) | |
| Other | 8.5% (67/784) | 10.7% (10/93) | 9.1% (19/209) | 7.9% (37/482) | |
| County, % (N/T) | 0.519 | ||||
| Metro | 51.4% (399/777) | 45.1% (41/91) | 51.5% (106/206) | 52.5% (252/480) | |
| Adjacent | 19.9% (155/777) | 26.4% (24/91) | 20.4% (42/206) | 18.5% (89/480) | |
| Rural | 28.7% (223/777) | 28.6 (26/91) | 28.2% (58/206) | 29.0% (139/480) | |
| Weight, kg ± SD | 79.9 ± 34.1 | 34.2 ± 10.9 | 57.3 ± 14.7 | 98.6 ± 28.3 | <0.001 |
| Height, cm ± SD | 149.0 ± 20.0 | 110.9 ± 9.7 | 137.5 ± 8.9 | 161.3 ± 10.6 | <0.001 |
| Obesity class, % (N/T) | 0.722 | ||||
| Class I, (>95%) | 15.9% (125/784) | 12.9% (12/93) | 16.3% (34/209) | 16.4% (79/482) | |
| Class II, (120–140) | 32.1% (252/784) | 30.1% (28/93) | 30.1% (63/209) | 33.4% (161/482) | |
| Class III, (>140%) | 51.9% (407/784) | 57.0% (53/93) | 53.6% (112/209) | 50.2% (242/482) | |
| Abnormal ALT, % (N/T) | 51.5% (324/629) | 52.6% (40/76) | 54.4% (98/180) | 49.9% (186/373) | 0.588 |
| Dyslipidaemia, % (N/T) | 68.1% (453/665) | 62.3 (48/77) | 70.7% (133/188) | 68.0 (272/400) | 0.410 |
| Increased BP, % (N/T) | 31.1% (187/600) | 27.7% (18/65) | 17.2% (27/157) | 37.6% (142/378) | <0.001 |
| Diabetes risk, % (N/T) | 33.8% (233/690) | 18.2% (14/77) | 30.4% (58/191) | 38.2% (161/422) | 0.002 |
Note: % represents the percent of the patients in the specific group, N represents the number of patients, where T represents total number of patients in that group. SD stands for standard deviation, ALT stands for alanine aminotransferase. Dyslipidaemia is defined as one or more of the following: increased total cholesterol, high LDL cholesterol, elevated triglycerides or low HDL cholesterol. Increased blood pressure was defined as having pre-hypertension or hypertension by auscultatory measurements. Diabetes risk was defined as having pre-diabetes or diabetes.
TABLE 2.
Metabolic parameters stratified by obesity class
| Subjects stratified by obesity class |
|||||
|---|---|---|---|---|---|
| All Patients | Class I | Class II | Class III | p value | |
| Number of Patients | 784 | 125 | 252 | 407 | |
| Male, % (N/T) | 45.2% (354/784) | 42.4% (53/125) | 40.5% (102/252) | 48.9% (199/407) | 0.086 |
| Race, % (N/T) | <0.001 | ||||
| White | 65.8% (516/784) | 51.2% (64/125) | 67.9% (171/252) | 69.0% (281/407) | |
| African American | 17.7% (139/748) | 20.0% (25/125) | 15.9% (40/252) | 18.3% (74/407) | |
| Hispanic | 7.9% (62/784) | 24.0% (30/125) | 7.1% (18/252) | 3.4% (14/407) | |
| Other | 8.6% (67/784) | 4.8% (6/125) | 9.1% (23/252) | 9.3% (38/407) | |
| County, % (N/T) | <0.001 | ||||
| Metro | 51.4% (399/777) | 77.0% (94/122) | 57.6% (144 /250) | 39.8% (161/405) | |
| Adjacent | 19.9% (155/777) | 11.5% (14/122) | 21.2% (53/250) | 21.7% (88/405) | |
| Rural | 28.7% (223/777) | 11.5% (14/122) | 21.2% (53/250) | 38.5% (156/405) | |
| Weight, kg ± SD | 79.9 ± 34.1 | 59.6 ± 21.5 | 71.0 ± 25.1 | 91.7 ± 37.4 | <0.001 |
| Height, cm ± SD | 149.0 ± 20.0 | 147.0 ± 18.5 | 148.8 ± 19.7 | 149.7 ± 20.7 | 0.424 |
| Age Group, % (N/T) | 0.722 | ||||
| 2–5 | 11.9% (93/784) | 9.6% (12/125) | 11.1% (28/252) | 13.0% (53/407) | |
| 6–9 | 26.7% (209/784) | 27.2% (34/125) | 25.0% (63/252) | 27.5% (112/407) | |
| ≥10 | 61.5% (482/784) | 63.2% (79/125) | 63.9% (161/252) | 59.5% (242/407) | |
| Abnormal ALT, % (N/T) | 51.5% (324/629) | 32.1% (36/112) | 46.9% (91/194) | 61.0% (197/323) | <0.001 |
| Dyslipidaemia, % (N/T) | 68.1% (453/665) | 60.3% (70/116) | 66.7% (140/210) | 71.7% (243/339) | 0.067 |
| Increased BP, % (N/T) | 31.2% (187/600) | 20.9% (18/86) | 25.1% (46/183) | 37.2% (123/331) | 0.002 |
| Diabetes risk, % (N/T) | 33.8% (233/690) | 28.8% (34/118) | 28.1% (62/221) | 39.0% (137/351) | 0.012 |
Note: % represents percent of the patients in the specific group, N represents the number of patients, where T represents total number of patients in that group. SD stands for standard deviation, ALT stands for alanine aminotransferase. Dyslipidaemia is defined as one or more of the following: increased total cholesterol, high LDL cholesterol, elevated triglycerides or low HDL cholesterol. Increased blood pressure was defined as having pre-hypertension or hypertension by auscultatory measurements. Diabetes risk was defined as having pre-diabetes or diabetes.
3 |. RESULTS
3.1 |. Abnormal ALT is equally prevalent in children older or younger than 10 years of age
We evaluated the risk of abnormal ALT in children ≥10 years, who would have been screened following the current paediatric guidelines, and in young children aged 6 to 9 years and very young children 2 to 5 years of age. A total of 784 children with obesity were included in the study: 482 were ≥10 years, 209 children were 6 to 9 years and 93 children were 2 to 5 years (Table 1). There was no difference in sex, race or urbanization of county when subjects were stratified by age. As expected, weight (p < 0.001) and height (p < 0.001) were significantly greater in older compared with younger children. Neither obesity class, risk of abnormal ALT, nor dyslipidaemia increased with age. On the other hand, increased BP and diabetes risk were more prevalent in older children compared with young or very young children. Thus, in children with obesity, the risk of abnormal ALT and dyslipidaemia did not increase with age.
3.2 |. ALT increases with severity of obesity in children
In the subsequent analysis, children were stratified according to obesity class (Table 2). Race and urbanization of county, but not sex, were significantly associated with obesity class. Most White children had obesity class III (69.0%), whereas the highest percent of Hispanic children had class I obesity (24.0%). Children residing in metro counties had the highest prevalence of class I obesity (77.0%), while those from rural counties had high prevalence of class III obesity (38.5%). By definition, weight was significantly increased with higher obesity class (p = 0.001), but height did not increase with obesity class. Age group was not associated with obesity class. Higher obesity class was significantly associated with abnormal ALT (p < 0.001). The percentage of children with abnormal ALT increased from 32.1% in class I to 46.9% in class II to 61.0% in class III obesity. The percent of children with dyslipidaemia also increased with obesity class; however, this finding did not reach statistical significance (p = 0.067). Similar to stratification by age, increased BP and diabetes risk increased with increasing obesity class. In summary, a higher percentage of children with severe obesity had abnormal ALT.
3.3 |. Obesity class, but not age, increases odds of abnormal ALT
In a multiple logistic regression analysis, obesity class was associated with increased odds of abnormal ALT (Table 3). Using class I obesity as the reference group, children with class II obesity had 2.2-fold higher odds of abnormal ALT (p = 0.005). Children with class III obesity had 4.1-fold higher odds of abnormal ALT (p < 0.001) even when controlling for age, race and county. Conversely, age group was not associated with abnormal ALT both with and without adjustment for race, county and obesity class. In agreement with previous studies,30 race was associated with abnormal ALT. African-American children had 72% lower odds (OR = 0.27) (p < 0.001), while Hispanic children had 137% higher odds (OR = 2.37) of abnormal ALT (p = 0.01) relative to White children. County urbanization was not associated with abnormal ALT after adjustment for race, age group and obesity class. In summary, a multiple binary logistic regression analysis demonstrated that obesity class, but not age, was associated with an increased odds of abnormal ALT in children with obesity.
TABLE 3.
Multivariable analysis of obesity class, age, race and county
| Multivariable analysis of abnormal ALT |
|||
|---|---|---|---|
| Odds ratio | 95% confidence interval | p value | |
| Model Intercept | 0.347 | (0.169–0.700) | 0.003 |
| Obesity Class | |||
| Class I | 1.000 | ref. | ref. |
| Class II | 2.153 | (1.271–3.717) | 0.005 |
| Class III | 4.051 | (2.411–6.959) | <0.001 |
| Age Group | |||
| 2–5 | 1.000 | ref. | ref. |
| 6–9 | 1.301 | (0.725–2.330) | 0.377 |
| ≥10 | 1.176 | (0.685–2.018) | 0.554 |
| Race | |||
| White | 1.000 | ref. | ref. |
| African American | 0.277 | (0.161–0.467) | <0.001 |
| Hispanic | 2.373 | (1.241–4.660) | 0.010 |
| Other | 1.449 | (0.298–7.901) | 0.645 |
| County | |||
| Metro | 1.000 | ref. | ref. |
| Adjacent | 1.493 | (0.916–2.447) | 0.109 |
| Rural | 1.301 | (0.828–2.044) | 0.253 |
Note: Obesity class is defined based on age- and sex-specific percentage of the 95th BMI percentile as follows: class I (BMI 95%–119%), class II (120%–139% or BMI ≥35 kg/m2) and class III (BMI ≥140% or BMI ≥40 kg/m2). Race was based on self-reported race and ethnicity. County of residence was referred to as metropolitan, adjacent non-metro and rural.
3.4 |. Obesity class, but not age is associated with abnormal ALT and the metabolic dysfunction, especially in girls
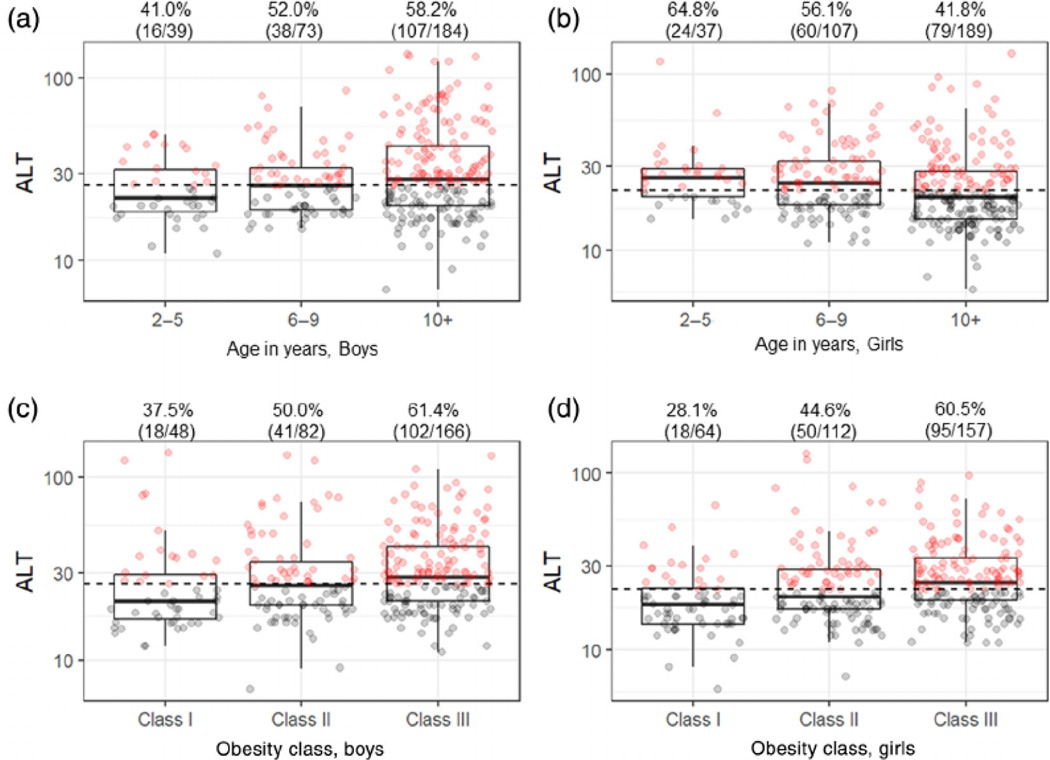
Children with normal and abnormal ALT were sub-analysed by sex (Tables S1 and S2). In boys, abnormal ALT was found in 41.0% of very young, 52.1% in young and 58.2% in boys ≥10 years, but this increase was not statistically significant (p = 0.134) (Figure 1) (Table S1). However, higher obesity class was significantly associated with abnormal ALT in boys (p < 0.001), so that 37.5% of boys with class I, 50.0% of boys with class II and 61.4% of boys with class III obesity had increased ALT. Abnormal ALT was associated with higher obesity class ≥II (p = 0.01) and higher serum TGs (p = 0.02), but not with low HDL (p = 0.08), increased BP (p = 0.90) or diabetes risk (p = 0.05). Moreover, 66.7% of boys with abnormal ALT had three or more signs of metabolic dysfunction compared with 33.3% of boys with normal ALT (p = 0.009). In boys, obesity class was more strongly associated with abnormal ALT than age group.
FIGURE 1.
Serum ALT stratified by age versus obesity class in boys and girls. ALT values were stratified by age for boys (A) and girls (B) and by obesity class for boys (C) and girls (D). The black dashed lines represent the sex-specific upper normal ALT of 26 U/L for boys and 22 U/L for girls. The dark lines inside of the box plots represent the age group-/obesity class-specific median ALT values. The upper and lower parts of the boxes represent the 75th and 25th percentiles, respectively. The whiskers represent either 1.5-times the interquartile range (75th percentile minus the 25th percentile) from the 25th/75th percentiles or the maximum/minimum values, whichever is smaller. The red dots indicate the subjects with abnormal and the grey dots indicate those with normal ALT. The percentages represent the frequency of children with abnormal ALT in that group. The numbers list the subjects with abnormal ALT and the total number of subjects in that group
This relationship observed in boys was even stronger in girls (Table S2). The percent of girls with abnormal ALT decreased with age such that 64.8% of very young, 56.1% of young and 41.8% of girls ≥10 years had abnormal ALT (p = 0.007) (Figure 1). In contrast, ALT increased with obesity class in girls, so that 28.1% of girls with class I, 44.6% with class II and 60.5% of girls with class III obesity had abnormal ALT (p < 0.001). When assessing the individual components of the metabolic dysfunction, abnormal ALT was associated with increased obesity class ≥II (p < 0.001), low HDL (p = 0.001) and high TGs (p < 0.001), as compared with girls with normal ALT. Moreover, we found that 69.0% of girls with abnormal ALT had three or more signs of metabolic dysfunction compared with only 31.0% of girls with normal AL T (p < 0.001). Thus, obesity class correlates more strongly with abnormal ALT and the metabolic dysfunction than age especially in girls.
While increased ALT was not more prevalent in children ≥10, two times the upper limit of normal ALT did increase with age (p = 0.008) and obesity class (p = 0.048) in boys (Table S3). Similarly, ALT above two times the upper limit of normal also increased with age (p = 0.017) and obesity class (p < 0.001) in girls.
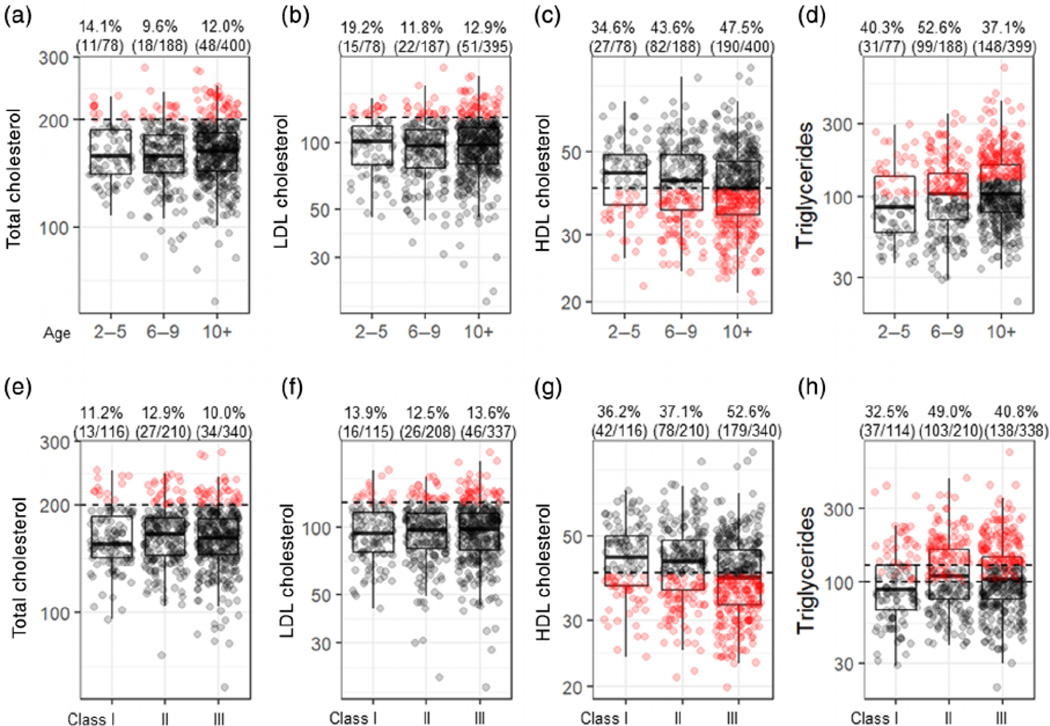
3.5 |. Young and very young children with obesity have similar prevalence of dyslipidaemia as children over 10 years
The most common lipid abnormalities in children with obesity were low HDL cholesterol and high triglycerides (Figure 2), in agreement with prior report. 31 Mean HDL was significantly (p = 0.04) lower in children ≥10 years (40.9 mg/dl) and in young children (42.2 mg/dl) compared with very young c hildren (43.7 mg/dl). Mean serum TGs significantly increased (p = 0.007) with age in very young (102.0 mg/dl) compared with young children (115.4 mg/dl) and children ≥10 years (126.9 mg/dl). However, abnormal serum TGs are defined as values >100 mg/dl in children younger than 10 years an d over 130 mg/dl in children ≥10 years. Thus, hyper-triglyceridaemia was less prevalent in children over 10 years of age compared with those 6 to 9 and 2 to 5 years.
FIGURE 2.
Lipid parameters stratified by age versus obesity class. Total cholesterol (A), LDL cholesterol (B), HDL cholesterol (C), and triglycerides (D) as stratified by age group. Total cholesterol (E), LDL cholesterol (F), HDL cholesterol (G) and triglycerides (H) as stratified by obesity class. The black dashed lines represent the age-specific upper normal for individual lipid parameters. The dark lines inside of the box plots represent the age group-/obesity class-specific median values. The upper and lower parts of the boxes represent the 75th and 25th percentiles, respectively. The whiskers represent either 1.5-times the interquartile range (75th percentile minus the 25th percentile) from the 25th/75th percentiles or the maximum/minimum values, whichever is smaller. The red dots indicate the subjects with abnormal and the grey dots indicate those with normal lipid parameters. The percentages represent the frequency of children with abnormal lipids in that group. The numbers list the subjects with abnormal lipids and the total number of subjects in that group
When stratified by the obesity class, TCh, LDL and TGs did not significantly differ in children with class I, II and III obesity, while an abnormal HDL increased with increasing obesity class (p = 0.0001). In summary, dyslipidaemia was neither more prevalent in children ≥10 than younger children, nor in children with higher obesity class than those with lower obesity class.
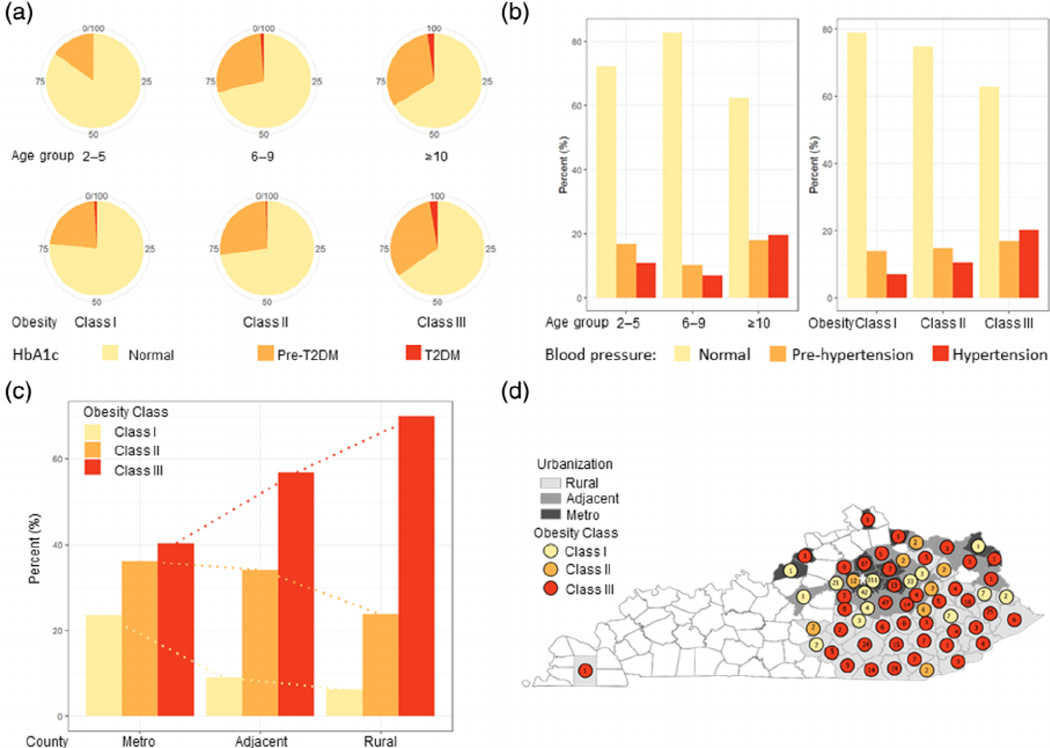
3.6 |. Additional components of metabolic dysfunction stratified by age or obesity class
The other components of metabolic dysfunction were also analysed by age or obesity class (Figure 3). Glucose handling was assessed by measuring either HbA1c, FG and/or 2-h OGTT. HbA1c was measured in 658 children, of whom 458 had normal readings, 189 were in pre-diabetic range and 11 were in diabetic range (Figure 3A). FG was measured in 671 children, of which 631 had normal readings, 39 were in pre-diabetic and one was in diabetic range. OGTT was performed in 217 children, of which 186 had normal readings, 30 were in pre-diabetic and one was in diabetic rage. In summary, a third of children with obesity had pre-diabetes, but very few presented with diabetes. HgA1c was the most commonly abnormal test, as compared with the FG or OGTT.
FIGURE 3.
Additional metabolic risks stratified by age versus obesity class. The pie charts (A) represent the percent of children in age groups and obesity classes who were normal (yellow) or diagnosed with pre-diabetes, HbA1c 5.7% to 6.4% (orange) and diabetes HbA1c ≥6.5% (red). The bar graphs (B) represent the frequency of normal (yellow), pre-hypertension systolic or diastolic BP ≥90th% (orange) or hypertension systolic or diastolic BP ≥95th% (red) in each age group and obesity class. The bar graphs (C) illustrate the percent of children in each obesity class living in metro, adjacent to metro and rural counties. The map of Kentucky (D) shows the counties, metro (dark grey), adjacent (grey) and rural (light grey) and mean obesity class (yellow class I, orange class II and red class III obesity) of subjects from that county. Numbers inside the circles represent the number of patients from that county. Star represents the location of our centre
Systolic and diastolic BP was measured in 599 children with obesity. Systolic BP was increased in 134 children and diastolic BP was abnormal in 120 children. Abnormal systolic BP, defined as having either pre-hypertension or hypertension, was more prevalent with both increasing age (p < 0.001) and obesity class (p = 0.006) (Figure 3B). Abnormal diastolic BP also increased with age (p = 0.04) and obesity class (p = 0.01).
County urbanization was associated with obesity class (p = 0.001), as children with more severe obesity resided in rural counties over metro counties (Figure 3C). This is not entirely accounted for by a referral bias, as children with obesity class III did not live further away from our centre compared with children with obesity class I (Figure 3D).
4 |. DISCUSSION
NAFLD is the most common cause of abnormal ALT in American children,1–3 and is associated with increased liver-related and all-cause mortality in both children32 and adults.33,34 Early identification and successful treatment aimed at prevention of liver fibrosis and obesity-associated comorbidities can improve NAFLD-associated morbidity and mortality. Screening for NAFLD by measuring serum ALT is recommended in children who are overweight or have obesity beginning at 9 to 11 years of age. We undertook this study to assess the risk of abnormal ALT in children with obesity when stratified by age group or obesity class. Additionally, we compared the prevalence of abnormal ALT in young and very young children, as compared with children older than age 10, who would have been screened for NAFLD per current guidelines. We found that older and younger children with obesity have equivalent risk of abnormal ALT. Interestingly, obesity class, rather than age group, is more frequently associated with abnormal ALT. In a multivariable analysis, higher obesity class was associated with increased odds of abnormal ALT, whereas older age was not. Further, abnormal ALT was associated with increased risk of metabolic dysfunction. In summary, higher obesity class can identify a larger percentage of children with abnormal ALT than age group.
When stratified by age group, children ≥10 years with obesity have similar risk of abnormal ALT as younger children with obesity. In contrast, children with more severe obesity have higher prevalence of abnormal ALT than those with less severe obesity. Risk of abnormal ALT in younger and older children is analogous to risk of dyslipidaemia in our study in younger and older children with obesity. Screening for dyslipidaemia is universally recommended in all children 9 to 11 years23 and in child ren with obesity over 2 years. 22,23,35 With additional paediatric data on how best to identify children at risk, recommendations for NAFLD screening may parallel the evolution of screening guidelines for dyslipidaemia, to include younger children with obesity.
Obesity class is even more strongly associated with abnormal ALT in girls than boys. The percentage of girls with abnormal ALT actually decreased with age, whereas the prevalence of abnormal ALT increased with higher obesity class. While we did not record pubertal status, it is likely that girls with obesity older than 10 years are more likely to have entered puberty compared with young and very young girls. Oestrogen may have beneficial effects on NAFLD36,37 while oestrogen deficiency worsens NAFLD.38,39 Another possibility is that an ALT decrease in young girls may represent a natural history of ALT regression in young children. 40 However, a decrease in ALT was much greater in girls over 10 years of age than in younger girls. In our study a decrease in ALT with age was not observed in boys, pointing to a hormonal effect.
While the risk of abnormal ALT did not differ in younger children and children ≥10, most patients with ALT elevation above twice the upper limit of normal were older than 10 years of age. The guidelines recommend that diagnosis of NAFLD should be considered in children with ALT values above two times the sex-specific cut-offs.4 Since abnormal ALT values in younger children have not been clearly established in terms of NAFLD risk, applying these stringent cut-offs to young and very young children may overlook a significant number of children at risk. Indeed, a more recent study of 1156 participants reported a median ALT of 12 in children at 9 years of age. 41 Moreover, over-relying on ALT to diagnose NAFLD may be problematic as up to 24% of children with NAFLD activity score (NAS >3) have been reported to have normal ALT.42 Assessing the severity of obesity and additional metabolic risk factors may improve the accuracy of diagnosing NAFLD in children older and younger than 10 years of age.
In agreement with our finding that obesity class is more strongly associated with abnormal ALT, Sethet al., found that the odds of having ALT>80 U/L was the greatest in children with class III obesity. 43 Furthermore, the children with class III obesity had more advanced liver disease as assessed by higher liver stiffness on magnetic resonance elastography and greater percent of patients with NAS >5 on liver histology. However, the study by Sethet al., did not include many young and very young children and thus did not stratify their results by age group.
Several factors might explain why obesity class rather than age can more accurately identify children at risk of severe NAFLD compared with the adults. The short time span of paediatric NAFLD might be one reason why age is not a pivotal factor in paediatrics. Age range for paediatric NAFLD is already tightly defined (2–18 years), whereas NAFLD in adults commonly spans over several decades. In adults, T2DM is a fundamental risk factor for severe NAFLD.44,45 A third of the children in our study had pre-diabetes, but only a few had diabetes, yielding more importance to obesity class as a predictor of NAFLD severity. Lastly, paediatric NAFLD is likely a more severe form of disease than adult NAFLD, as it develops early in life and can progress to liver failure in a relatively short period of time. 32,46,47 Indeed, adolescents with severe obesity were found to have more pronounced balloon degeneration and higher incidence of liver fibrosis compared with adults with similar level of obesity. 18
In adults, the risk of advanced NAFLD increases with BMI and age. 48,49 Whereas severe obesity correlates with increased risk of liver fibrosis,50 obesity status by itself is a poor predictor of NAFLD severity in adults. Up to 50% of adults with obesity might not even develop NAFLD.51,52 On the other hand, age is a much stronger predictor of NAFLD severity in adults. Age is used in non-invasive disease scoring models, such as i n the NAFLD fibrosis score (NFS)53 and fibrosis-4 index (FIB-4),54 which are used clinically to stratify the risk of advanced NAFLD in adult patients. Moreover, the results of another commonly used non-invasive test, enhanced liver fibrosis (ELF),55 are affected by the patient’s age. The use of these non-invasive tests is encouraged by the clinical practice guidelines,7,56,57 as they can stratify the risk of advanced NAFLD in patients between 35 and 65 years of age. These measures do not perform as well at extremes of ages. FIB-4 was found not to be reliable in patients younger than 35 years 58 and children.59 Thus, age is an important variable in adults but not in children with NAFLD.
Identifying a larger percentage of children with abnormal ALT is clinically significant as these children have increased risk of metabolic dysfunction. Our findings are in agreement with a recent study in school-age children showing that children with NAFLD have higher prevalence of metabolic risk factors. 60 Identifying a greater number of children with abnormal ALT has the potential to select patients in need of intensive lifestyle interventions aimed at avoiding further metabolic complications. The first step in identifying more children with abnormal ALT is to determine age- and sex-specific upper limits of normal for young and very young children. Appropriate ALT cut-offs for children with obesity or overweight in terms of NAFLD risk must be determined in future longitudinal studies.
The strengths of our study are that it includes a large number of children with obesity, 482 of whom were older and 302 were younger than 10 years. When stratified by obesity class, but not by age group, we found that African-American race was associated with decreased odds of abnormal ALT, while Hispanic race with increased odds of an abnormal ALT. The effect of race on NAFLD risk when stratified by obesity class is consistent with other reports in the literature. 30,61 Thus, stratification by obesity class more accurately identifies NAFLD risk factors than stratification by age. The main limitation of our study is that presence of NAFLD was not confirmed by imaging or liver biopsy, although other causes of chronic hepatitis were excluded.
In summary, we found that stratification of children by obesity class identifies a greater proportion of children with abnormal ALT than stratification by age. Obesity class may be readily assessed during routine well-child visits to identify those children at greater risk to develop obesity-associated complications. Future studies are needed to determine whether guidelines to screen for NAFLD in children should be based on age group and/or the severity of obesity.
Supplementary Material
ACKNOWLEDGEMENTS
AR and SS contributed to conceptualization, methodology, investigating and wrote the manuscript. AJD preformed data curation and analysis. MK contributed to investigation and data curation. All authors helped with the investigation, writing the manuscript and had final approval of the submitted and published versions.
We would like to thank Jungjun Bae and Christian Eisinger from the Institute for Pharmaceutical Outcomes & Policy, at University of Kentucky (UKY) College of Pharmacy for mapping assistance. SS was supported by the Department of Paediatrics at UKY and the Department of Pharmacology and Nutritional Sciences startup funds, as well as by P30 GM127211 and NASPGHAN Foundation Young Investigator Award. MK was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant UL1TR0001198 and the Professional Student Medical Research Fellowship (PSMRF).
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Lavine JE, Schwimmer JB. Nona lcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549–558. viii-ix. [DOI] [PubMed] [Google Scholar]
- 2.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 5.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North America n Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–713. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 8.Softic S, Kirby M, Berger NG, Shroyer NF, Woods SC, Kohli R. Insulin concentration modulates hepatic lipid accumulation in mice in part via transcriptional regulation of fatty acid transport protein s. PLoS One. 2012;7:e38952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Softic S, Stanhope KL, Boucher J, et al. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 2020;57(5):308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Softic S, Gupta MK, Wang GX, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017;127:4059–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic De novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Softic S, Meyer JG, Wang GX, et al. Dietary sugars Alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019;30:735–753. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton HM, Lavine JE, Van Natta ML, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54–61. [DOI] [PubMed] [Google Scholar]
- 15.Alqahtani A, Elahmedi M, Alswat K, Arafah M, Fagih M, Lee J. Features of nonalcoholic steatohepatitis in severely obese children and adolescents undergoing sleeve gastrectomy. Surg Obes Relat Dis. 2017;13:1599–1609. [DOI] [PubMed] [Google Scholar]
- 16.Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170:e16197 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinic opathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003; 143:500–505. [DOI] [PubMed] [Google Scholar]
- 18.Holterman AX, Guzman G, Fantuzzi G, et al. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity (Silver Spring). 2013;21:591–597. [DOI] [PubMed] [Google Scholar]
- 19.Softic S, Kahn CR. Fatty liver disease: is it nonalcoholic fatty liver disease or obesity-associated fatty liver disease? Eur J Gastroenterol Hepatol. 2019;31:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchesini G, Moscatiello S, Di Domizio S, et al. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93:S74–S80. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 22.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Integrated Guidelines for Cardio vascular H, Risk Reduction in C, Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn JT, Kaelber DC, Baker-S mith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes A. 2. Classi fication and diagnosis of diabetes: standards of medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. [DOI] [PubMed] [Google Scholar]
- 26.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the third National Health and nutrition examination survey. Circulation. 2004;110 :2494–2497. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Team RC. R: A Language and Environment for Statistical Computing. 2020. https://www.r-project.org/ [Google Scholar]
- 29.Torsten Hothorn KH, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Softw. 2008;28(8) :1–13.27774042 [Google Scholar]
- 30.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. [DOI] [PubMed] [Google Scholar]
- 31.Corey KE, Vuppalanchi R, Vos M, et al. Improvement in liver histology is associated with reduction in dyslipidemia in children with non-alcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2015;60:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009; 58:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obesity Angulo P. and nonalcoholic fatty liver disease. Nutr Rev. 2007;65:S57–S63. [DOI] [PubMed] [Google Scholar]
- 34.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873. [DOI] [PubMed] [Google Scholar]
- 35.Daniels SR, Greer FR, Committee on N. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. [DOI] [PubMed] [Google Scholar]
- 36.Ponnusamy S, Tran QT, Thiyagarajan T, Miller DD, Bridges D, Narayanan R. An estrogen receptor beta-selective agonist inhibits non-alcoholic steatohepatitis in preclinical models by regulating bile acid and xenobiotic receptors. Exp Biol Med (Maywood). 2017;242:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart-Unger S, Arao Y, Hamilton KJ, et al. Hormone signaling and fatty liver in females: analysis of estrogen receptor alpha mutant mice. Int J Obes (Lond). 2017;41:94 5–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venetsanaki V, Polyzos SA. Menopause and non-alcoholic fatty liver disease: a review focusing on therapeutic perspectives. Curr Vasc Pharmacol. 2019;17:546–555. [DOI] [PubMed] [Google Scholar]
- 39.Klair JS, Yang JD, Abdelmalek MF, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.England K, Thorne C, Pembrey L, Tovo PA, Newell ML. Age- and sex-related reference ranges of alanine aminotransferase levels in children: European paediatric HCV network. J Pediatr Gastroenterol Nutr. 2009;49:71–77. [DOI] [PubMed] [Google Scholar]
- 41.Sekkarie A, Welsh JA, Northstone K, et al. ALT trends through childhood and adolescence associated with hepatic steatosis at 24years: a population-based UK cohort study. Children (Basel). 2020;7(9):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molleston JP, Schwimm er JB, Yates KP, et al. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707–713. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seth A, Orkin S, Yodoshi T, et al. Severe obesity is associated with liver disease severity in pediatric non-alcoholic fatty liver disease. Pediatr Obes. 2020;15:e12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bian H, Zhu X, Xia M, et al. Impact of type 2 diabetes on nonalcoholic steatohepatitis and advanced fibrosis in patients with nonalcoholic fatty liver disease. Endocr Pract. 2020;26:444–453. [DOI] [PubMed] [Google Scholar]
- 45.Lee BW, Lee YH, Park CY, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research Group of the Korean Diabetes Association. Diabetes Metab J. 2020;44:382–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohli R, Boyd T, Lake K, et al. Rapid progression of NASH in childhood. J Pediatr Gastroenterol Nutr. 2010;50:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molleston JP, White F, Teckman J, et al. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2462. [DOI] [PubMed] [Google Scholar]
- 48.Church TS, Kuk JL, Ross R, Priest EL, Biltoff E, Blair SN. Association of cardiorespiratory fitness , body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. [DOI] [PubMed] [Google Scholar]
- 49.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 50.Loomis AK, Kabadi S, Preiss D, et al. Body mass index and risk of non-alcoholic fatty liver disease: two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes P, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis. 2004;8:535–547. viii. [DOI] [PubMed] [Google Scholar]
- 52.Amarapurkar D, Kamani P, Patel N, et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 53.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:84 6–854. [DOI] [PubMed] [Google Scholar]
- 54.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The enhanced liver fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–242. [DOI] [PubMed] [Google Scholar]
- 56.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:20 05–2023. [DOI] [PubMed] [Google Scholar]
- 57.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marches ini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. [DOI] [PubMed] [Google Scholar]
- 58.McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson JA, Konomi JV, Mendoza MV, et al. Performance of fibrosis prediction scores in paediatric non-alcoholic fatty liver disease. J Paediatr Child Health. 2018;54:172–176. [DOI] [PubMed] [Google Scholar]
- 60.Geurtsen ML, Santos S, Felix JF, et al. Liver fat and Cardiometabolic risk factors among school-age children. Hepatology. 2020;72:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagi R, Reif S, Neuman G, Webb M, Phillip M, Shalitin S. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209–1213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.