Abstract
Purpose:
To investigate the impact of daily image-guided radiation therapy technique on clinical outcomes in patients with inoperable non-small cell lung cancer treated with definitive chemoradiation therapy.
Methods and Materials:
We compared patients with inoperable non-small cell lung cancer receiving daily cone beam computed tomography (CBCT) after an initial 4-dimensional computed tomography (4DCT) simulation (n = 76) with those receiving daily 2-dimensional orthogonal kilovoltage (kV) imaging (n = 48). The primary endpoint was time to grade ≥2 radiation pneumonitis (RP2), estimated with the cumulative incidence method, compared with Gray’s test, and modeled with the Fine-Gray method.
Results:
Median follow-up was 40.6 months (range, 5.9–58.1 months) for the CBCT group and 75.8 months (range, 9.9–107.8 months) for the orthogonal kV group. Four-dimensional computed tomography simulation was used in 100% (n = 76) of the CBCT group and 56% (n = 27) of the orthogonal kV group (P < .0001). The 1-year cumulative incidence of RP2 was lower in the CBCT group than in the orthogonal kV group (24% vs 44%, P = .020). On multivariate analysis, daily imaging with CBCT after an initial 4DCT simulation was associated with a decreased risk of RP2 (adjusted hazard ratio 0.43, 95% confidence interval 0.22–0.82, P = .011), a finding that persisted among only patients who received 4DCT simulation (adjusted hazard ratio 0.48, 95% confidence interval 0.23–0.98, P = .045). There was no difference in locoregional progression, distant metastasis, any progression, or overall survival between groups.
Conclusions:
Daily image guided radiation therapy with CBCT compared with 2-dimensional orthogonal kV imaging was associated with a decreased risk of RP2. Clinicians could consider the implications of localization methods during curative intent radiation therapy.
Summary
We found a decreased rate of grade ≥2 radiation pneumonitis among inoperable non-small cell lung cancer patients receiving daily image guided radiation therapy (IGRT) with cone beam computed tomography (CBCT) after an initial 4-dimensional computed tomography simulation compared with daily IGRT with 2-dimensional orthogonal kilovoltage imaging. Future studies should consider the ability of CBCT to improve tumor localization and potentially reduce subacute pulmonary toxicity.
Introduction
Delivering tumoricidal doses while avoiding radiation therapy (RT)-related toxicities remains one of the critical challenges in radiation oncology and is particularly meaningful for locally advanced non-small cell lung cancer (NSCLC). Symptomatic radiation pneumonitis occurs in approximately 30% of NSCLC patients in the subacute time frame after RT and is fatal in 2% (1). Tumor and organ motion with respiration, changes in patient anatomy during treatment due to weight loss, and the proximity of tumors to numerous organs at risk pose challenges that, when not adequately considered, potentially lead to insufficient tumor coverage or additional radiation to normal tissues (2–4). One way to increase the precision of RT delivery, with the goal of also reducing RT-related toxicities, derives from the use of on-board image guided radiation therapy (IGRT).
IGRT allows for regular positional adjustments to account for geometric deviations from the simulation session by capturing kilovoltage (kV) or megavoltage (MV) plain films, orthogonal kV films, or cone beam computed tomography (CBCT) images before delivering treatment fractions (5, 6). The benefits of IGRT are well described for many cancer sites (7, 8). For unresectable NSCLC treated with definitive chemoradiation therapy (CRT), several retrospective studies suggest more frequent use of IGRT improves tumor localization and patient outcomes. Higgins et al (9) found that daily versus less than daily use of CBCT decreased the number of fractions incurring residual setup errors ≥5 mm from 20%−43% to 6%. Another series of 168 locally advanced NSCLC patients found a 13% increase in 2-year locoregional failure-free survival with daily CBCT compared with weekly MV portal imaging and a reduction in any late toxicity and acute grade 3 toxicity (10). Furthermore, a study of 91 NSCLC patients found a benefit in overall survival (OS) and a trend toward improved locoregional control with daily orthogonal kV imaging compared with weekly MV portal imaging (11).
Although current evidence indicates that daily IGRT improves outcomes over weekly imaging for unresectable NSCLC, it remains unclear whether one type of imaging results in superior clinical outcomes. At our institution we began implementing daily IGRT with 2-dimensional (2D) orthogonal kV imaging in 2007. In 2009 we gradually transitioned to daily IGRT with CBCT and added 4-dimensional computed tomography (4DCT) simulation. We hypothesized that, within a modern series of inoperable NSCLC patients treated with definitive and concurrent CRT, daily CBCT after an initial 4DCT simulation would decrease radiation pneumonitis compared with daily 2D orthogonal kV imaging.
Methods and Materials
Patients
Using an institutional review board-approved protocol, we conducted a retrospective review of 124 patients with locally advanced or stage IV oligometastatic, biopsyproven, unresectable NSCLC treated with definitive and concurrent CRT and daily image guidance from November 2007 to August 2015. We included patients with stage IV oligometastatic disease, defined as those with a solitary extrathoracic metastasis who received definitive CRT to the primary tumor and metastatic focus, on the basis of survival outcomes approximating those of patients with stage III NSCLC (12, 13). Clinical staging was based on the American Joint Committee on Cancer 7th edition criteria (14).
Treatment
During RT planning, patients were placed in a supine position with arms up in a large rigid mold. The gross tumor volume (GTV) consisted of the primary tumor and clinically positive lymph nodes identified on either planning computed tomography (CT) or pretreatment positron emission tomography imaging. For patients who did not undergo 4DCT simulation (n = 21), motion management with respiratory gating was used. The clinical target volume (CTV) was 5 mm beyond the GTV, and the planning target volume (PTV) was 10 mm beyond the CTV. For patients who underwent 4DCT simulation (n = 103), real-time fluoroscopy with abdominal compression was used before delineation of an internal target volume. The CTV was typically 5 mm beyond the internal target volume, and the PTV was typically 3–5 mm beyond the CTV in the Superior—inferior dimensions and axial plane. Radiation was delivered with 3-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT), or a mix of 3D-CRT and IMRT to a typical dose of 60–66 Gy in 1.8 or 2 Gy per fraction. IMRT was used for patients presenting with bilateral mediastinal lymph node involvement or for the boost/off-cord component. Radiation therapy was delivered with photon beams of 6- or 15-MV energies, and the Varian Eclipse (Varian Medical Systems, Palo Alto, CA) analytic anisotropic algorithm was used with tissue inhomogeneity corrections. Daily IGRT occurred either with orthogonal kV imaging or CBCT. Orthogonal kV imaging was introduced at our institution in November 2007 and routinely used soon thereafter for all patients, until CBCT and 4DCT were introduced in October 2009. From 2010 to 2012 either daily orthogonal kV imaging or daily CBCT was used, with choice of imaging based primarily on physician preference. In some cases CBCT was used for larger tumors exhibiting greater motion on 4DCT. Starting in early 2013, daily CBCT was routinely used for all patients.
The typical chemotherapy regimen consisted of intravenous infusional drug delivery with either paclitaxel (45 mg/m2 per week) and carboplatin (area under the curve = 2 per week) or cisplatin (50 mg/m2 on days 1, 8, 29, and 36) and etoposide (50 mg/m2 on days 1–5 and 29–33).
Follow-up
Patients in both the CBCT and kV groups typically followed up with the radiation and medical oncologists every 1–3 months for the first year after RT, every 3–6 months for the following two years, and every 6–12 months thereafter. Follow-up chest CT scans were generally obtained 6–8 weeks after the completion of RT and then every 3–4 months for the first year, every 6 months for the following 2 years, and yearly thereafter.
Patients were advised about common signs/symptoms of radiation pneumonitis and other toxicities, and monitored more frequently after RT if they reported such symptoms. The Common Terminology Criteria for Adverse Events version 4.03 was used to grade the severity of radiation pneumonitis. A diagnosis of grade ≥2 radiation pneumonitis (RP2) was confirmed by at least 2 treating physicians on the basis of the constellation of patient-reported symptoms and after ruling out other causes of symptoms. All patients with RP2 required treatment with corticosteroids and/or supplemental oxygen.
Study endpoints and statistical analysis
We first assessed the CBCT and orthogonal kV groups for differences in patient, tumor, and treatment-related characteristics. Differences in categorical variables were assessed with the χ2 or Fisher exact test, as appropriate. Differences in continuous variables were assessed with an independent 2-sample t test or Wilcoxon rank-sum test, depending on normality of distribution.
The primary endpoint was time to RP2. Secondary endpoints included time to locoregional progression, distant metastasis, and any progression, and OS. We measured all endpoints from the start of RT to the event of interest.
We used the cumulative incidence method to estimate RP2, locoregional progression, distant metastasis, and any progression. Gray’s test compared cumulative incidence functions between imaging groups. Death in the absence of the event of interest was considered a competing event. We then used the Fine-Gray method to model the cumulative incidence functions of RP2. Univariate analysis assessed associations between patient, tumor, and treatment-related factors and RP2. We examined the effect of treatment during/after the year 2013 versus before 2013 on RP2, because this is when our institution began routinely using CBCT for all patients. We constructed 2 multivariate models: 1 among all patients, and another among only patients who underwent 4DCT simulation. We included IMRT in both models, regardless of univariate P values, because we believed IMRT could confound the relationship between choice of daily IGRT and RP2 (15). We also considered clinically relevant covariates significant at the P = .2 level in the univariate setting for inclusion in the multivariate models. The number of variables in the multivariate models was determined by the commonly used rule of thumb, 1 variable per 10 events. We reported subdistribution hazard ratios (HRs) along with 95% confidence intervals (CIs). We used the Kaplan-Meier method to estimate OS, and the log-rank test to compare survival distributions between imaging groups. Hypothesis tests were 2-sided, and we considered P < .05 as statistically significant. We performed analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of the 124 patients, 61% (n = 76) received daily CBCT, and 39% (n = 48) received daily orthogonal kV imaging. Median follow-up was 40.6 months (range, 5.9–58.1 months) for the CBCT group and 75.8 months (range, 9.9–107.8 months) for the orthogonal kV group. There were no significant differences in baseline patient characteristics between the groups (Table 1). Table 2 describes radiation and chemotherapy treatment details. All patients in the CBCT group underwent 4DCT simulation, compared with 56% (n = 27) in the orthogonal kV group (P < .0001). Median RT dose was 60 Gy (range, 52–70.2 Gy) in the CBCT group and 63 Gy (range, 50.4–70.2 Gy) in the orthogonal kV group (P = .001). However, there was no difference in the percentage of patients receiving ≥60 Gy between the respective groups (86% vs 85%, P = .99). The CBCT group had a larger proportion of GTVs ≥300 cm3 (29% vs 10%, P = .015). Among those who underwent planning 4DCT, PTV margins were smaller in the CBCT group (median, 3 mm; range, 0–7 mm) than in the orthogonal kV group (median, 5 mm; range, 0–10 mm; P = .0002). Although IMRT was used more often in the CBCT group (P = .0006), the volume of lung receiving at least 20 Gy (lung V20) was higher in the CBCT group (34.6% vs 30.5%, P = .025). There were no differences in chemotherapy schedules or agents.
Table 1.
Baseline patient characteristics
| Characteristic | CBCT (n = 76) | Orthogonal kV (n = 48) | P |
|---|---|---|---|
|
| |||
| Age (y) | |||
| Median | 64 | 66 | .58 |
| Range | 40–83 | 44–89 | |
| Sex | .11 | ||
| Male | 46 (61) | 22 (46) | |
| Female | 30 (39) | 26 (54) | |
| Race | .76 | ||
| Caucasian | 64 (84) | 39 (81) | |
| African American | 6 (8) | 6 (13) | |
| Other | 6 (8) | 3 (6) | |
| ECOG | .22 | ||
| 0 | 45 (59) | 37 (77) | |
| 1 | 22 (29) | 7 (15) | |
| 2 | 7 (9) | 3 (6) | |
| 3 | 2 (3) | 1 (2) | |
| Smoking (pack-years) | |||
| Median | 40 | 39 | .63 |
| Range | 0–168 | 0–125 | |
| Histology | .52 | ||
| Adenocarcinoma | 38 (50) | 28 (58) | |
| Squamous cell carcinoma | 29 (38) | 17 (35) | |
| Poorly differentiated carcinoma | 9 (12) | 3 (6) | |
| Tumor lobe location | .20 | ||
| Upper/middle | 54 (71) | 39 (81) | |
| Lower | 22 (29) | 9 (19) | |
| Clinical stage | .72 | ||
| IIA | 5 (7) | 2 (4) | |
| IIB | 3 (4) | 1 (2) | |
| IIIA | 23 (30) | 17 (35) | |
| IIIB | 35 (46) | 25 (52) | |
| IV | 10 (13) | 3 (6) | |
| T stage | .37 | ||
| X | 2 (3) | 1 (2) | |
| 1 | 17 (22) | 7 (15) | |
| 2 | 16 (21) | 18 (38) | |
| 3 | 16 (21) | 9 (19) | |
| 4 | 26 (34) | 13 (27) | |
| N stage | .71 | ||
| 0 | 5 (7) | 2 (4) | |
| 1 | 11 (14) | 4 (8) | |
| 2 | 32 (42) | 24 (50) | |
| 3 | 28 (37) | 18 (38) | |
Abbreviations: CBCT = cone beam computed tomography; ECOG = Eastern Cooperative Oncology Group; kV = kilovoltage.
Values are number (percentage) unless otherwise noted.
Table 2.
Treatment characteristics
| Characteristic | CBCT (n = 76) | Orthogonal kV (n = 48) | P |
|---|---|---|---|
|
| |||
| Treatment year | <.0001 | ||
| 2007–2009 | 1 (1) | 20 (42) | |
| 2010–2012 | 26 (34) | 25 (52) | |
| 2013–2015 | 49 (64) | 3 (6) | |
| Radiation planning | |||
| PET | 76 (100) | 48 (100) | 1 |
| 4DCT | 76 (100) | 27 (56) | <.0001 |
| Radiation delivery | |||
| Dose | |||
| Median (Gy) | 60 | 63 | .001 |
| Range (Gy) | 52–70.2 | 50.4–70.2 | |
| ≥60 Gy | 65 (86) | 41 (85) | .99 |
| GTV | |||
| Median (cm3) | 148.3 | 139.7 | .15 |
| Range (cm3) | 20.5–803.5 | 3.9–347.1 | |
| Distribution | |||
| <100 cm3 | 27 (36) | 16 (33) | |
| 100–200 cm3 | 16 (21) | 17 (35) | |
| 200–300 cm3 | 11 (14) | 10 (21) | |
| 300–400 cm3 | 8 (11) | 5 (10) | |
| ≥400 cm3 | 14 (18) | 0 (0) | |
| PTV margins (4DCT patients, n = 103) | |||
| Median (mm) | 3 | 5 | .0002 |
| Distribution | |||
| <3 mm | 6 (8) | 2 (7) | |
| 3 mm | 33 (43) | 1 (4) | |
| 4 mm | 4 (5) | 0 (0) | |
| 5 mm | 32 (42) | 21 (78) | |
| >5 mm | 1 (1) | 3 (11) | |
| Technique | .0006 | ||
| 3D-CRT only | 38 (50) | 39 (81) | |
| 3D-CRT/IMRT | 15 (20) | 7 (15) | |
| IMRT only | 23 (30) | 2 (4) | |
| MLD, median (Gy) | 19.4 | 18.5 | .18 |
| Lung V20, median (%) | 34.6 | 30.5 | .025 |
| Chemotherapy schedule | |||
| Induction | 16 (21) | 16 (33) | .13 |
| Concurrent | 76 (100) | 48 (100) | 1 |
| Adjuvant | 20 (26) | 9 (19) | .33 |
| Concurrent chemotherapy agent(s) | .30 | ||
| Carboplatin + paclitaxel | 50 (66) | 28 (58) | |
| Cisplatin + etoposide | 12 (16) | 14 (29) | |
| Other doublet | 5 (7) | 3 (6) | |
| Single agent | 9 (12) | 3 (6) | |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; 4DCT = 4-dimensional computed tomography; CBCT = cone beam computed tomography; GTV = gross tumor volume; IMRT = intensity modulated radiation therapy; kV = kilovoltage; MLD = mean lung dose; PET = positron emission tomography; PTV = planning target volume; V20 = volume receiving 20 Gy.
Values are number (percentage) unless otherwise noted.
Association of imaging technique with RP2
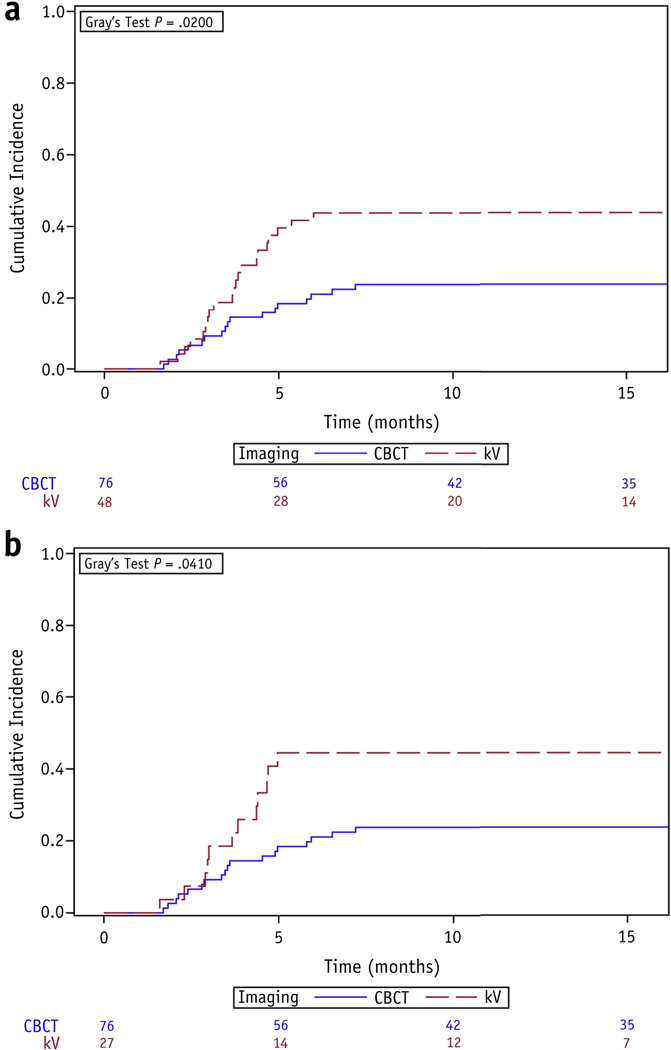
Among all patients, imaging with daily CBCT compared with orthogonal kV was associated with a decreased cumulative incidence of RP2 (1-year rate, 24% vs 44%, P = .020) (Fig. 1a). Median time to diagnosis of RP2 from the start of RT was 3.5 months (range, 1.7–7.2 months) in the CBCT group and 3.8 months (range, 1.6–6.0 months) in the orthogonal kV group. On univariate analysis, CBCT was associated with a decreased risk of RP2 (HR 0.48, 95% CI 0.25–0.89, P = .020), whereas increasing mean lung dose (MLD) was associated with an increased risk (HR 1.12, 95% CI 1.01–1.24, P = .026; Table 3). IMRT (either alone or in combination with 3D-CRT) (HR 0.70, 95% CI 0.36–1.39, P = .31), RT dose (HR 1.01, 95% CI 0.93–1.10, P = .79), and treatment during/after 2013 versus before 2013 (HR 0.74, 95% CI 0.39–1.42, P = .36) were not significantly associated with the development of RP2. On multivariate analysis, after adjusting for IMRT and age, CBCT (adjusted HR 0.43, 95% CI 0.22–0.82, P = .011) and MLD (adjusted HR 1.14, 95% CI 1.03–1.26, P = .011) continued to be the only significant covariates.
Fig. 1.
Cumulative incidence functions for grade ≥2 radiation pneumonitis, stratified by choice of daily imaging, among (a) all patients and (b) only patients who received 4-dimensional computed tomography simulation. Abbreviations: CBCT = cone beam computed tomography; kV = orthogonal kilovoltage imaging.
Table 3.
Risk factors for grade ≥2 radiation pneumonitis
| Factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR | 95% CI | P | aHR | 95% CI | P | |
|
| ||||||
| All patients (n = 124) | ||||||
| Age (≥60 vs <60 y) | 1.95 | 0.93–4.09 | .079 | 1.92 | 0.90–4.09 | .091 |
| Sex (male vs female) | 0.95 | 0.51–1.77 | .87 | |||
| Smoking (≥30 vs <30 pack-years) | 1.17 | 0.58–2.36 | .66 | |||
| Histology (AC vs all else) | 1.41 | 0.74–2.70 | .30 | |||
| Tumor location (lower vs upper/middle) | 1.28 | 0.60–2.32 | .64 | |||
| Clinical stage (IIIB/IV vs all else) | 0.77 | 0.42–1.45 | .42 | |||
| CBCT (vs orthogonal kV) | 0.48 | 0.25–0.89 | .020 | 0.43 | 0.22–0.82 | .011 |
| Treatment year (≥2013 vs <2013) | 0.74 | 0.39–1.42 | .36 | |||
| 4DCT | 0.62 | 0.30–1.30 | .21 | |||
| Dose (Gy) | 1.01 | 0.93–1.10 | .79 | |||
| GTV (cm3) | 1.00 | 1.00–1.00 | .20 | |||
| IMRT only (vs all else) | 1.04 | 0.48–2.24 | .93 | |||
| IMRT only + IMRT/3D-CRT (vs 3D-CRT only) | 0.70 | 0.36–1.39 | .31 | 0.96 | 0.47–1.95 | .91 |
| MLD (Gy) | 1.12 | 1.01–1.24 | .026 | 1.14 | 1.03–1.26 | .011 |
| Lung V20 (%) | 1.03 | 0.99–1.08 | .15 | |||
| Concurrent chemo (carbo/taxol vs all else) | 1.10 | 0.58–2.10 | .77 | |||
| Induction chemo | 1.04 | 0.50–2.15 | .93 | |||
| Adjuvant chemo | 1.06 | 0.54–2.10 | .86 | |||
| 4DCT patients (n = 103) | ||||||
| Age (≥60 vs <60 y) | 1.92 | 0.83–4.45 | .13 | |||
| Sex (male vs female) | 1.33 | 0.64–2.79 | .45 | |||
| Smoking (≥30 vs <30 pack-years) | 1.12 | 0.52–2.43 | .77 | |||
| Histology (AC vs all else) | 0.99 | 0.49–2.02 | .99 | |||
| Tumor location (lower vs upper/middle) | 1.33 | 0.64–2.76 | .45 | |||
| Clinical stage (IIIB/IV vs all else) | 0.75 | 0.37–1.53 | .44 | |||
| CBCT (vs orthogonal kV) | 0.47 | 0.23–0.96 | .038 | 0.48 | 0.23–0.98 | .045 |
| Treatment year (≥2013 vs <2013) | 0.92 | 0.45–1.86 | .81 | |||
| Dose (Gy) | 1.01 | 0.92–1.10 | .90 | |||
| GTV (cm3) | 1.00 | 1.00–1.00 | .26 | |||
| PTV margins (mm) | 0.96 | 0.71–1.30 | .79 | |||
| IMRT only (vs all else) | 1.24 | 0.56–2.78 | .59 | |||
| IMRT only + IMRT/3D-CRT (vs 3D-CRT only) | 0.76 | 0.36–1.58 | .46 | 0.86 | 0.41–1.80 | .69 |
| MLD (Gy) | 1.10 | 0.98–1.24 | .10 | 1.11 | 0.98–1.25 | .09 |
| Lung V20 (%) | 1.03 | 0.97–1.08 | .36 | |||
| Concurrent chemo (carbo/taxol vs all else) | 1.13 | 0.54–2.37 | .75 | |||
| Induction chemo | 0.81 | 0.32–2.03 | .65 | |||
| Adjuvant chemo | 0.97 | 0.44–2.15 | .94 | |||
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; 4DCT = 4-dimensional computed tomography; AC = adenocarcinoma; aHR = adjusted subdistribution hazard ratio; carbo = carboplatin; CBCT = cone beam computed tomography; chemo = chemotherapy; CI = confidence interval; GTV = gross tumor volume; HR = subdistribution hazard ratio; IMRT = intensity modulated radiation therapy; kV = kilovoltage; MLD = mean lung dose; PTV = planning target volume; V20 = volume receiving 20 Gy; taxol = paclitaxel.
Because 4DCT simulation could confound the relationship between choice of daily IGRT and RP2, we performed a separate subgroup analysis of only 4DCT patients (n = 103). Within this subgroup there were no additional differences between patients who received CBCT and those who received orthogonal kV imaging (Tables E1 and E2; available online at www.redjournal.org). Again, the cumulative incidence functions for RP2 differed significantly between imaging groups (Fig. 1b; P = .041). There was no association between PTV margins and risk of RP2 (HR 0.96, 95% CI 0.71–1.30, P = .79). After adjusting for MLD and IMRT, CBCT remained the only factor associated with a reduced risk of RP2 (adjusted HR 0.48, 95% CI 0.23–0.98, P = .045).
Association of imaging technique with locoregional progression, distant metastasis, any progression, and OS
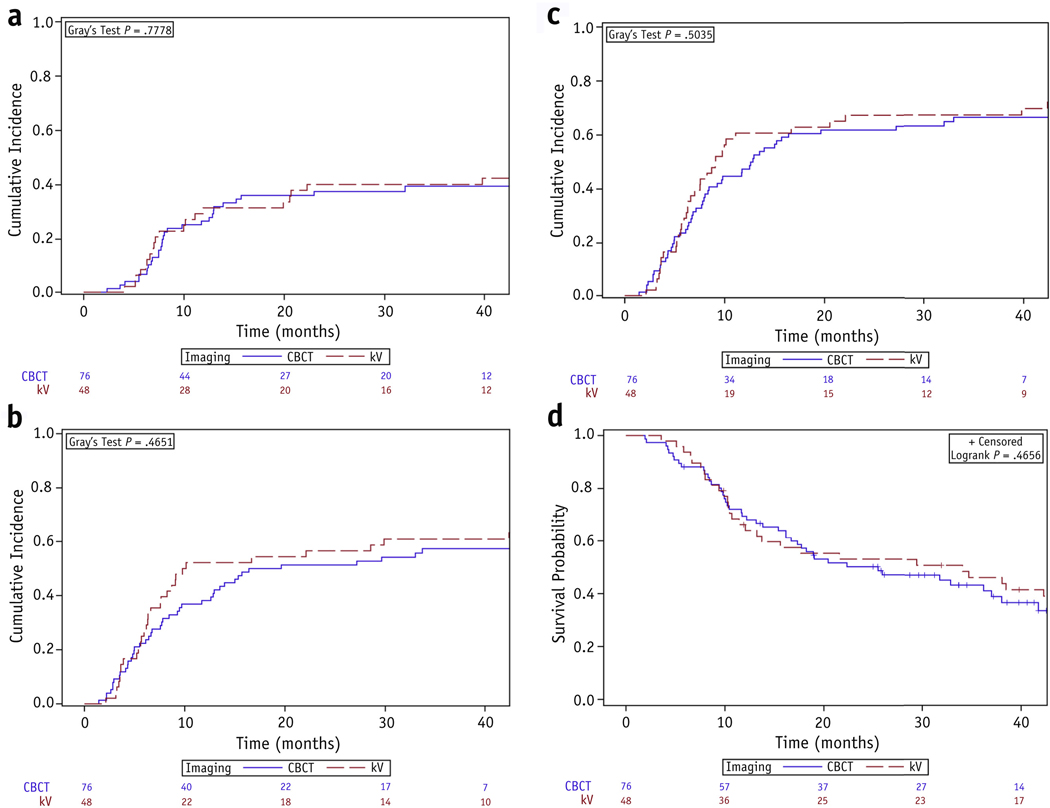
There was no difference in the 2-year cumulative incidence of locoregional progression (38% vs 40%, P = .78; 27% vs 30%, P = .68 when adjusting for the competing risk of distant metastasis), distant metastasis (51% vs 56%, P = .48), or any progression (62% vs 67%, P = .47) between the CBCT and orthogonal kV groups. Median OS for the cohort was 25.9 months (95% CI 16.7–38.0 months), with no difference between the respective groups (2-year OS, 50% vs 53%, P = .86). Figure 2 shows cumulative incidence functions and Kaplan-Meier curves for these endpoints.
Fig. 2.
Cumulative incidence functions for (a) locoregional progression, (b) distant metastasis, and (c) any progression, and (d) Kaplan-Meier curves for overall survival, stratified by choice of daily imaging. Abbreviations: CBCT = cone beam computed tomography; kV = orthogonal kilovoltage imaging.
Discussion
The transition to daily IGRT with CBCT after 4DCT simulation has theoretically improved tumor targeting, but improvements in clinical outcomes remain uncertain. Our retrospective study suggests that daily CBCT after 4DCT simulation is associated with a decrease in RP2 compared with daily orthogonal kV imaging. The findings are compelling given that the CBCT group had a higher median lung V20 and larger GTVs.
Our study has several perceived limitations, including a small sample size and temporal and patient selection biases between the 2 groups. First, the small sample size limited the number of covariates that could be included in the multivariate models and decreased the power for detection of previously suggested risk factors for RP2, such as lung V20, tumor location in the lower lobe, and concurrent chemotherapy with carboplatin/paclitaxel (1, 16). Nevertheless, our findings are hypothesis-generating and could impact clinical practice with additional study. Second, CBCT was used in more recent years alongside other technological advances, including 4DCT and IMRT, which may be expected to synergize to reduce toxicity. However, the association between daily CBCT and RP2 persisted on a separate subgroup analysis of only patients who underwent 4DCT simulation. Additionally, even though prior studies associate IMRT with a decreased risk of radiation pneumonitis (15, 17), we did not find such an association likely because patients receiving IMRT did not benefit from lower lung V20s and MLDs (median values were 35.3% and 18.8 Gy for IMRT only, 34.4% and 19.4 Gy for IMRT/3D-CRT, and 32.4% and 19.0 Gy for 3D-CRT only). Our population is not ideal for ascertaining a benefit in IMRT, because IMRT was frequently used in combination with 3D-CRT and for tumors that could not meet normal tissue dose constraints with 3D-CRT. Less quantifiable advances include improvements in clinic workflow, treatment planning software, or RT field design, which could be associated with the transition from daily orthogonal kV imaging to daily CBCT. We analyzed the effect of treatment era to attempt to account for these advances, although we realize our methodology is imperfect. Third, although CBCT was introduced at our institution in late 2009, it was not routinely used for all patients until early 2013. From 2010 to 2012, in certain cases CBCT was preferentially used for tumors that were larger or exhibited greater motion. However, these biases might be unfavorable toward the CBCT group. The CBCT and orthogonal kV groups otherwise represent relatively homogeneous populations that received similar chemotherapy regimens, positron emission tomography scans for staging and RT planning, involved-field RT, and inhomogeneity corrections and multiple motion mitigation techniques during RT.
One potential explanation for the decrease in RP2 is that CBCT enhances tumor localization, providing volumetric alignment of the radiation beams to soft tissue anatomy instead of 2D alignment to osseous anatomy. For conventionally fractionated RT, Yeung et al (18) found that bony registration compared with soft tissue registration with CBCT led to additional residual errors of >5 mm in the superior—inferior dimension in 20% of patients. Similarly, in 28 patients undergoing daily CBCT for SBRT, Purdie et al (19) found a mean difference in the translational 3D vector of 6.9 mm ±4.9 mm between manual soft tissue registration and manual bony registration; the difference exceeded 13.9 mm in 10% of fractions. Thus, daily CBCT can improve the ability to respond to positional changes between fractions.
Moreover, aligning to soft tissue anatomy using CBCT can decrease day-to-day setup uncertainty and the required target volume margins (6, 18, 20). Although the CBCT group was treated with significantly smaller PTV margins, there was no association between PTV margins and RP2 among patients who received 4DCT. Nevertheless, considering that 21 patients in the orthogonal kV group did not have a planning 4DCT and were treated with 10-mm instead of 5-mm PTV margins, the decrease in setup margins associated with the use of CBCT and 4DCT may be one of the factors contributing to lower rates of RP2.
Apart from improved tumor localization and reduced setup uncertainty, CBCT has additional benefits over orthogonal kV imaging that could further improve outcomes for unresectable NSCLC. Reduction in tumor volume, as measured by CBCT, can allow for early assessment of the efficacy of CRT and appropriately tailored management to responders versus nonresponders (21). Additionally, CBCT guides adaptive RT, whereby periodic resimulations enable ongoing alterations to the target volume that may further reduce the risk of radiation pneumonitis (22, 23).
Drawbacks of daily CBCT versus orthogonal kV include the increased treatment time, need for additional resources, and higher imaging doses to the patient. For prostate cancer, CBCT may increase daily treatment time by up to 10 minutes (24) and may surprisingly result in decreased precision, albeit not necessarily decreased accuracy, in daily alignments compared with kV imaging with fiducial markers (25).
In our study there was no significant difference in locoregional progression or OS between groups. Although other retrospective studies suggest that the transition from weekly portal imaging to daily IGRT improves local tumor control (10, 11), the transition from daily orthogonal kV imaging to daily CBCT may represent a less pronounced change with a smaller benefit. Of our study population, 39% experienced distant metastasis as the sole first site of failure, a number that is unlikely to be affected by advances in radiation techniques alone. Distant metastasis thus probably contributed more to OS than did locoregional progression.
Incorporation of daily IGRT has varied across prospective trials for locally advanced NSCLC; its use is a requirement in Radiation Therapy Oncology Group (RTOG) 1106 and RTOG 1308, which was not the case in RTOG 0617 and RTOG 1306. However, RTOG 1106 and RTOG 1308 do not specify which IGRT technique should be used, allowing for either 2D or 3D imaging. Given our finding that CBCT may decrease RP2, we believe there is value in prospectively verifying the potential benefit of CBCT over orthogonal kV imaging when all patients are treated with the same target volume margins. Furthermore, RTOG 0617 required PTV margins of at least 5 mm, even when IGRT and motion management strategies were used. If daily CBCT after 4DCT simulation allows for a safe reduction in PTV margins below 5 mm, such a paradigm may represent a step toward safer dose escalation that could be explored in trials to come.
Last, daily CBCT can particularly benefit NSCLC patients who receive immunotherapy in addition to definitive CRT, given that immunotherapy regimens themselves may increase the risk of pneumonitis (26, 27).
Conclusions
In this study, inoperable NSCLC patients treated with definitive CRT experienced a decreased risk of RP2 when receiving daily IGRT with CBCT after an initial 4DCT simulation compared with daily IGRT with 2D orthogonal kV imaging. Given the study’s retrospective design and sample size, our findings are hypothesis-generating and warrant additional study. Clinicians could consider the benefits of CBCT over other IGRT techniques, including its potential to improve tumor localization and reduce setup uncertainty.
Supplementary Material
Footnotes
Conflict of interest: S.K.J. has research funding from Merck and Nestle.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013; 85:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu HH, Balter P, Tutt T, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2007;68:531–540. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P, Yorke E, Hu YC, et al. Predictive treatment management: Incorporating a predictive tumor response model into robust prospective treatment planning for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;88:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelsman M, Damen EM, De Jaeger K, et al. The effect of breathing and set-up errors on the cumulative dose to a lung tumor. Radiother Oncol 2001;60:95–105. [DOI] [PubMed] [Google Scholar]
- 5.Dawson LA, Jaffray DA. Advances in image-guided radiation therapy. J Clin Oncol 2007;25:938–946. [DOI] [PubMed] [Google Scholar]
- 6.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol 2008;3:177–186. [DOI] [PubMed] [Google Scholar]
- 7.Korreman SS. Image-guided radiotherapy and motion management in lung cancer. Br J Radiol 2015;88:20150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bujold A, Craig T, Jaffray D, et al. Image-guided radiotherapy: Has it influenced patient outcomes? Semin Radiat Oncol 2012;22:50–61. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J, Bezjak A, Hope A, et al. Effect of image-guidance frequency on geometric accuracy and setup margins in radiotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys 2011;80: 1330–1337. [DOI] [PubMed] [Google Scholar]
- 10.Kilburn JM, Soike MH, Lucas JT, et al. Image guided radiation therapy may result in improved local control in locally advanced lung cancer patients. Pract Radiat Oncol 2016;6:e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deek MP, Kim S, Yue N, et al. Modern radiotherapy using image guidance for unresectable non-small cell lung cancer can improve outcomes in patients treated with chemoradiation therapy. J Thorac Dis 2016;8:2602–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan AJ, Mehta PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 2006;81:163–167. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour SK, Daroui P, Moore D, et al. A novel paradigm in the treatment of oligometastatic non-small cell lung cancer. J Thorac Dis 2011;3:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706–714. [DOI] [PubMed] [Google Scholar]
- 15.Liao ZX, Komaki RR, Thames HD Jr., et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;76:775–781. [DOI] [PubMed] [Google Scholar]
- 16.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol 2015;25:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung AR, Li JG, Shi W, et al. Tumor localization using cone-beam CT reduces setup margins in conventionally fractionated radiotherapy for lung tumors. Int J Radiat Oncol Biol Phys 2009;74: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 19.Purdie TG, Bissonnette JP, Franks K, et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: Localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007;68:243–252. [DOI] [PubMed] [Google Scholar]
- 20.Grills IS, Hugo G, Kestin LL, et al. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70:1045–1056. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour SK, Kim S, Haider SA, et al. Reduction in tumor volume by cone beam computed tomography predicts overall survival in non-small cell lung cancer treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys 2015;92:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil AA, Knap MM, Petersen MT, et al. OC-0143: Adaptive radiotherapy reduces pneumonitis without increasing the risk of failure in lung cancer. Radiother Oncol 2017;123(Suppl. 1):S70. [Google Scholar]
- 23.Ramsey CR, Langen KM, Kupelian PA, et al. A technique for adaptive image-guided helical tomotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2006;64:1237–1244. [DOI] [PubMed] [Google Scholar]
- 24.Barney BM, Lee RJ, Handrahan D, et al. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int J Radiat Oncol Biol Phys 2011;80:301–305. [DOI] [PubMed] [Google Scholar]
- 25.Goff PH, Harrison LB, Furhang E, et al. 2D kV orthogonal imaging with fiducial markers is more precise for daily image guided alignments than soft-tissue cone beam computed tomography for prostate radiation therapy. Adv Radiat Oncol 2017;2:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.