Abstract
Aims
Patients with structural heart disease (SHD) undergoing catheter ablation (CA) for ventricular tachycardia (VT) are at considerable risk of periprocedural complications, including acute haemodynamic decompensation (AHD). The PAINESD score was proposed to predict the risk of AHD. The goal of this study was to validate the PAINESD score using the retrospective analysis of data from a large-volume heart centre.
Methods and results
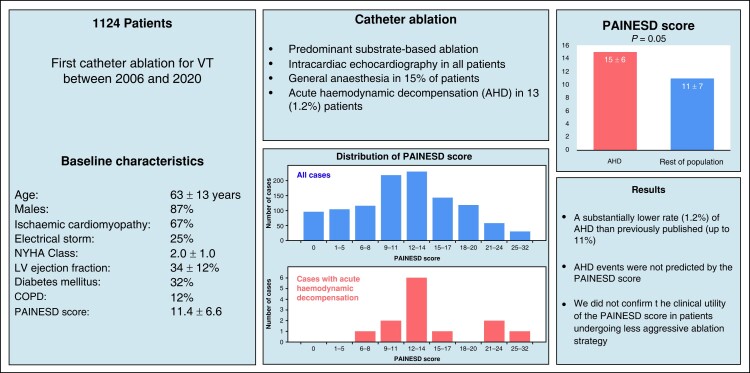
Patients who had their first radiofrequency CA for SHD-related VT between August 2006 and December 2020 were included in the study. Procedures were mainly performed under conscious sedation. Substrate mapping/ablation was performed primarily during spontaneous rhythm or right ventricular pacing. A purposely established institutional registry for complications of invasive procedures was used to collect all periprocedural complications that were subsequently adjudicated using the source medical records. Acute haemodynamic decompensation triggered by CA procedure was defined as intraprocedural or early post-procedural (<12 h) development of acute pulmonary oedema or refractory hypotension requiring urgent intervention. The study cohort consisted of 1124 patients (age, 63 ± 13 years; males, 87%; ischaemic cardiomyopathy, 67%; electrical storm, 25%; New York Heart Association Class, 2.0 ± 1.0; left ventricular ejection fraction, 34 ± 12%; diabetes mellitus, 31%; chronic obstructive pulmonary disease, 12%). Their PAINESD score was 11.4 ± 6.6 (median, 12; interquartile range, 6–17). Acute haemodynamic decompensation complicated the CA procedure in 13/1124 = 1.2% patients and was not predicted by PAINESD score with AHD rates of 0.3, 1.8, and 1.1% in subgroups by previously published PAINESD terciles (<9, 9–14, and >14). However, the PAINESD score strongly predicted mortality during the follow-up.
Conclusion
Primarily substrate-based CA of SHD-related VT performed under conscious sedation is associated with a substantially lower rate of AHD than previously reported. The PAINESD score did not predict these events. The application of the PAINESD score to the selection of patients for pre-emptive mechanical circulatory support should be reconsidered.
Keywords: Catheter ablation, Ventricular tachycardia, Acute haemodynamic decompensation, Mechanical circulatory support, Risk assessment, PAINESD score
Graphical Abstract
Graphical Abstract.
What’s new?
We observed a substantially lower rate (1.2%) of acute haemodynamic decompensation during catheter ablation (CA) of structural heart disease–related ventricular tachycardia (VT) than previously reported (up to 11%), and they were not predicted by the PAINESD score.
This observation may be explained by predominant substrate-based CA under conscious sedation that prevents prolonged low cardiac output state related to general anaesthesia–induced hypotension and repeated VT induction and mapping during VT.
The application of the PAINESD score to the selection of patients for pre-emptive mechanical circulatory support should be reconsidered.
Introduction
Radiofrequency catheter ablation (CA) is an established treatment method for the management of ventricular tachycardias (VTs) in patients with structural heart disease (SHD).1,2 Previous studies demonstrated the superiority of CA, compared with conventional treatment in managing the electrical storm,3–5 improving quality of life,6 and reducing VT recurrences and related hospitalizations.6,7 However, due to underlying SHD, concomitant heart failure, and a high burden of comorbidities, patients undergoing CA for VT are at considerable risk of periprocedural complications, including acute haemodynamic decompensation (AHD) with a reported rate of up to 11%.8 The PAINESD score was proposed to predict the risk of AHD and identify the patients who may benefit from pre-emptive use of mechanical circulatory support (MCS) devices.8–10 It is calculated as a sum of risk points for chronic obstructive pulmonary disease (5 points), age > 60 years (3 points), ischaemic cardiomyopathy (6 points), New York Heart Association (NYHA) Class > 2 (6 points), left ventricular (LV) ejection fraction < 25% (3 points), electrical storm (5 points), and diabetes mellitus (3 points). Other groups used the PAINESD score for this purpose with variable success.11,12 There are no data on the role of PAINESD in predicting haemodynamic deterioration during predominant substrate-based ablation without general anaesthesia (GA) and multiple inductions of VT. Therefore, the goal of this study was to investigate the incidence of AHD and the predictive power of the PAINESD score in a cohort of patients with ablation of SHD-related VT, based primarily on substrate mapping and pace mapping in sinus rhythm or right ventricular pacing.
Methods
Study population and study design
This single-centre study included consecutive patients who underwent their first CA for SHD-related VT in a large tertiary hospital between August 2006 and December 2020. We excluded those who had already LV assist device implanted. All patients signed informed consent with the procedure. The study was approved by the institutional ethics committee. Data were collected prospectively. We calculated the PAINESD score for each patient and assessed the distribution of AHD according to the terciles of PAINESD. The association of PAINESD and its components with all-cause mortality was also investigated.
Catheter ablation procedure
The procedures were performed mainly under conscious sedation with fentanyl and midazolam. Propofol was not used. General anaesthesia was used in patients requiring the epicardial access or in those who were on artificial ventilation because of haemodynamical or electrical instability before the CA. After obtaining vascular access, unfractionated heparin was administered as an initial bolus and continuous infusion to maintain the activated clotting time between 300 and 350 s. The LV was accessed either transseptally or retrogradely, depending on the substrate location, the actual INR level, the presence of peripheral arterial disease, and/or mechanical valve prosthesis. Procedures were navigated using a three-dimensional electroanatomic mapping system CARTO 3 (Biosense Webster) or Ensite (Abbott) and guided by intracardiac echocardiography (AcuNav, Siemens Medical Solutions). Radiofrequency energy was delivered using 30–40 W over a 3.5 mm irrigated-tip catheter (NaviStar ThermoCool, Biosense Webster, or Cool Flex, Abbott). One quadripolar catheter was inserted into the right ventricle for pacing. At baseline, programmed ventricular stimulation from the ventricular apex was performed at two drive trains (600 and 400 ms) and up to three extrastimuli except for incessant VT or focally triggered ventricular fibrillation (VF). Induced VTs that were not well-tolerated were immediately terminated by overdrive pacing or cardioverted. During the procedure, patients were carefully monitored, and mean arterial blood pressure was maintained at >65 mm Hg to reduce the risk of organ dysfunction. Activation and entrainment mapping were used only for well-tolerated VTs. Substrate mapping/ablation was performed primarily during spontaneous rhythm or right ventricular pacing using an integrated approach.13 Regions of abnormal wall morphology and motion as assessed by intracardiac echocardiography were explored first. Bipolar voltage maps (the lower threshold of 0.5 mV) were constructed and fragmented, or late potentials were tagged. Zones of slow conduction were identified by stimulus-to-QRS onset interval longer than 40 ms. The paced QRS morphology during sinus rhythm was used to match the exit sites of induced VT. The goal of subsequent CA was to abolish all abnormal signals or late potentials, often reaching isolation of the segment of the scar with no capture. The second goal was to abolish the inducibility of clinical VT and all inducible VTs. However, programmed ventricular stimulation at the end of the CA procedure was not performed systematically, mainly because of safety concerns in patients with severe LV dysfunction and/or fragile haemodynamics.
Clinical follow-up
Following the CA, most patients were routinely evaluated in our outpatient clinic at 3, 6, and every 6–12 months afterwards. A purposely established institutional registry for complications of invasive procedures was used to collect all periprocedural complications that were subsequently adjudicated using the source medical records. Acute haemodynamic decompensation triggered by CA was defined as intraprocedural or early post-procedural (<12 h) development of acute pulmonary oedema or refractory hypotension requiring urgent intervention including (but not limited to) inotropic/vasoactive agents and/or artificial ventilation and/or MCS. Data on mortality were obtained/verified for all subjects from the national registry of citizens.
Statistical analysis
All statistical analyses were conducted in R (http://www.R-project.org). Continuous variables are presented as means with standard deviations. Survival is displayed using Kaplan–Meier curves, and the differences between subgroups were assessed by the log-rank test. The Cox proportional hazard models were used to calculate the corresponding hazard ratios (HR) with 95% confidence intervals (CI) and to investigate the independent predictive value of individual risk factors for all-cause death. A P < 0.05 was considered significant.
Results
The baseline and procedural characteristics are shown in Tables 1 and 2. The study cohort consisted of 1124 patients (age, 63 ± 13 years; males, 87%; ischaemic cardiomyopathy, 67%; electrical storm, 25%; NYHA Class, 2.0 ± 1.0; LV ejection fraction, 34 ± 12%; diabetes mellitus, 32%; chronic obstructive pulmonary disease, 12%). The mean PAINESD score of the study cohort was 11.4 ± 6.6 [median, 12; interquartile range (IQR), 6–17].
Table 1.
Baseline characteristics of the study population
| Baseline characteristics | N = 1124 |
|---|---|
| Male (%) | 87 |
| Age (years) | 63 ± 13 |
| Age > 60 years (%) | 70 |
| Weight (kg) | 89 ± 17 |
| Height (cm) | 175 ± 9 |
| Body mass index (kg/m2) | 29 ± 5 |
| Body surface area (m2) | 2.0 ± 0.2 |
| Congestive heart failure (%) | 93 |
| CHA2DS2-VASc score | 3.6 ± 1.7 |
| Implantable cardioverter-defibrillator (%) | 78 |
| Cardiac resynchronisation therapy (%) | 35 |
| Arterial hypertension (%) | 66 |
| Diabetes mellitus (%) | 31 |
| Stroke/transient ischaemic attack (%) | 12 |
| Coronary (or peripheral) artery disease (%) | 68 |
| Chronic obstructive pulmonary disease (%) | 12 |
| NYHA Class | 2.0 ± 1.0 |
| NYHA Class ≥ III (%) | 31 |
| Left ventricular ejection fraction (%) | 34 ± 12 |
| Left ventricular ejection fraction < 25% (%) | 25 |
| Serum creatinine (μmol/L) | 112 ± 48 |
| Electrical storm (%) | 25 |
| PAINESD score | 11.4 ± 6.6 |
| Type of cardiomyopathy | |
| Ischaemic cardiomyopathy (%) | 67 |
| Dilated cardiomyopathy (%) | 18 |
| Arrhythmogenic cardiomyopathy (%) | 5 |
| Hypertrophic cardiomyopathy (%) | 1 |
| Valvular cardiomyopathy (%) | 11 |
| Other cardiomyopathy (%) | 13 |
Data are provided as means ± SD or percentages.
NYHA, New York Heart Association.
Table 2.
Procedural characteristics
| Procedural characteristics | N = 1124 |
|---|---|
| Radiofrequency time (min) | 23 ± 16 |
| Fluoroscopic dose (μGy m2) | 1114 ± 1803 |
| Fluoroscopic time (min) | 10.4 ± 8.1 |
| Procedure time (min) | 187 ± 79 |
| Major complications (%) | 7.7 |
| Major vascular access complications (%) | 4.4 |
| General anaesthesia (%) | 15.1 |
| Acute haemodynamic decompensation (%) | 1.2 |
Data are provided as means ± SD or percentages.
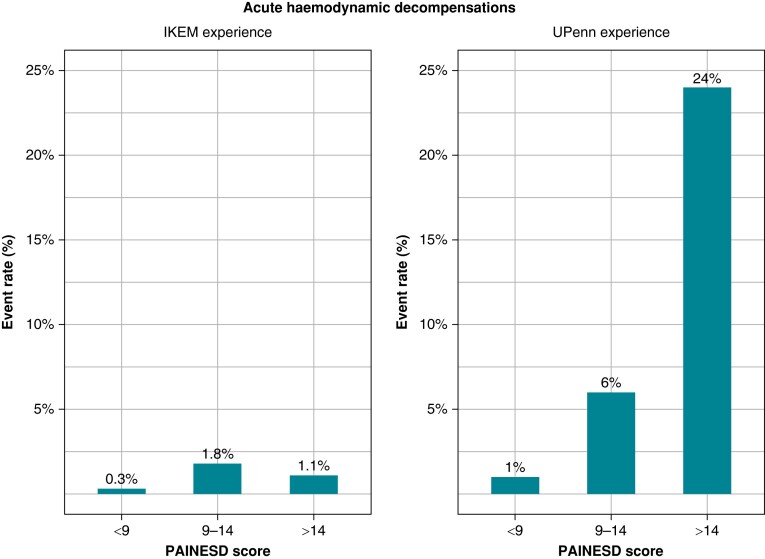
Catheter ablation was performed in GA in 170 (15%) patients; 115 (10%) patients had elective GA for procedures with epicardial access; 55 (5%) patients had GA as part of the management of arrhythmic storm. Catheter ablation (total duration, 187 ± 79 min; radiofrequency time, 23 ± 16 min) was complicated by AHD in 13 of 1124 (1.2%) patients, and these adverse events were not predicted by the PAINESD score. In subgroups by previously published PAINESD terciles [<9 (n = 318), 9–14 (n = 451), and >14 (n = 355)], a total of 1, 8, and 4 AHD events occurred with corresponding rates of 0.3, 1.8, and 1.1%, respectively (Figure 1). The clinical characteristics of the patients with AHD are summarized in Table 3. Four patients in the AHD group were intubated in the electrophysiology room due to incessant VT/VF; nine patients were treated with norepinephrine; three patients were treated with intravenous diuretics. Acute haemodynamic decompensation events did not accumulate in patients with PAINESD score in upper tercile in whom the highest AHD risk was reported up to 24% (Figure 1). Four of 1124 patients subsequently underwent MCS implantation early (>12 h, <5 days) after the procedure to treat pre-existing cardiogenic shock. None of them met the clinical criteria for acute cardiac decompensation during the procedure.
Figure 1.
Acute haemodynamic decompensation according to the PAINESD score. Comparison between IKEM and UPenn experience.8 IKEM, Institute for Clinical and Experimental Medicine; UPenn, University of Pennsylvania.
Table 3.
Acute heart decompensation during the ablation procedure
| Procedure date | Age (years) | Chronic obstructive pulmonary disease | Ischaemic cardiomyopathy | NYHA | Left ventricular ejection fraction (%) | Electrical storm | Diabetes mellitus | PAINESD score | Type of haemodynamic deterioration |
|---|---|---|---|---|---|---|---|---|---|
| 17/05/2007 | 54 | No | Yes | III | 25 | Yes | No | 17 | Periprocedural pulmonary oedema |
| 11/03/2009 | 40 | No | No | III | 15 | Yes | No | 14 | Periprocedural hypotension, CPR |
| 29/12/2011 | 69 | No | Yes | II | 40–45 | No | Yes | 12 | Incessant VT, CPR |
| 12/12/2013 | 73 | No | Yes | IV | 15–20 | Yes | Yes | 26 | Incessant VF, CPR |
| 14/09/2015 | 79 | No | Yes | II | 35–40 | Yes | No | 14 | Incessant VT, CPR |
| 04/07/2017 | 59 | Yes | Yes | III | 25–30 | Yes | No | 22 | Post-procedural pulmonary oedema |
| 14/09/2017 | 52 | No | No | II–III | 15–20 | Yes | No | 8 | Refractory VF, CPR |
| 06/10/2017 | 71 | No | Yes | II–III | 30–35 | Yes | No | 14 | Post-procedural pulmonary oedema |
| 18/12/2017 | 78 | No | Yes | II–III | 25–30 | No | No | 9 | Post-procedural pulmonary oedema |
| 24/01/2018 | 86 | Yes | No | III–IV | 35–40 | No | No | 14 | Post-procedural pulmonary oedema |
| 10/07/2019 | 67 | No | Yes | NK | NK | No | Yes | 12 | Pulseless electrical activity, CPR |
| 22/08/2019 | 69 | No | Yes | III | 20–25 | Yes | No | 23 | Post-procedural cardiogenic shock |
| 25/09/2019 | 68 | No | Yes | II–III | NK | No | No | 9 | Post-procedural pulmonary oedema |
Data are provided as means ± SD or counts (proportions).
CPR, cardiopulmonary resuscitation; NK, not known; NYHA, New York Heart Association; VT, ventricular tachycardia; VF, ventricular fibrillation.
During a mean follow-up of 4.2 (IQR: 2.1–7.3) years, a total of 318 patients (28%) underwent repeated CA. Forty (3.6%) patients underwent implantation of a LV assist device [median 65 (IQR: 26–378) days after the CA], and 51 (4.5%) patients underwent heart transplant. A total of 539 (48%) patients died during the follow-up. Patients with periprocedural AHD were more likely to have electrical storms, chronic obstructive pulmonary disease, worse NYHA Class, and adverse outcomes including the need for MCS implantation (Table 4).
Table 4.
Comparison between AHD and rest of population group
| AHD | Rest of population | P-value | |
|---|---|---|---|
| Male | 92.3% | 87.1% | 0.58 |
| Age (years) | 66.6 ± 12.4 | 63.4 ± 13 | 0.38 |
| Ischaemic cardiomyopathy | 76.9% | 66.7% | 0.44 |
| Dilated cardiomyopathy | 23.1% | 17.8% | 0.62 |
| Arterial hypertension | 69.2% | 66.4% | 0.83 |
| Congestive heart failure | 100% | 92.7% | 0.46 |
| NYHA Class | 2.8 ± 0.6 | 2.0 ± 1.0 | 0.01 |
| Diabetes mellitus | 23.1% | 31,4% | 0.52 |
| Stroke/transient ischaemic attack | 30.8% | 11,3% | 0.03 |
| Coronary (or peripheral) artery disease | 84.6% | 68.2% | 0.21 |
| CHA2DS2-VASc score | 4.3 ± 1.5 | 3.6 ± 1.7 | 0.28 |
| Chronic obstructive pulmonary disease | 15.4% | 11.6% | 0.67 |
| Serum creatinine (µmol/L) | 136.9 ± 56.3 | 112.2 ± 47.9 | 0.07 |
| Left ventricular ejection fraction (%) | 27.6 ± 9.3 | 34.2 ± 12.5 | 0.08 |
| Electrical storm | 61.5% | 24.3% | 0.002 |
| PAINESD score | 14.9 ± 5.6 | 11.4 ± 6.6 | 0.05 |
| Procedure time (min) | 171 ± 38 | 189 ± 60 | 0.34 |
| General anaesthesia (%) | 23.1% | 15.0% | 0.42 |
| Radiofrequency time (min) | 27.6 ± 15.8 | 22.7 ± 15.5 | 0.31 |
| Re-ablation during follow-up | 23.1% | 28.4% | 0.67 |
| MCS during follow-up | 23.1% | 3.3% | 0.0001 |
| Heart transplant during follow-up | 0.0% | 4.6% | 0.43 |
| Death | 76.9% | 47.6% | 0.04 |
Data are provided as means ± SD or counts (proportions).
AHD, acute haemodynamic decompensation; MCS, mechanical circulatory support; NYHA, New York Heart Association.
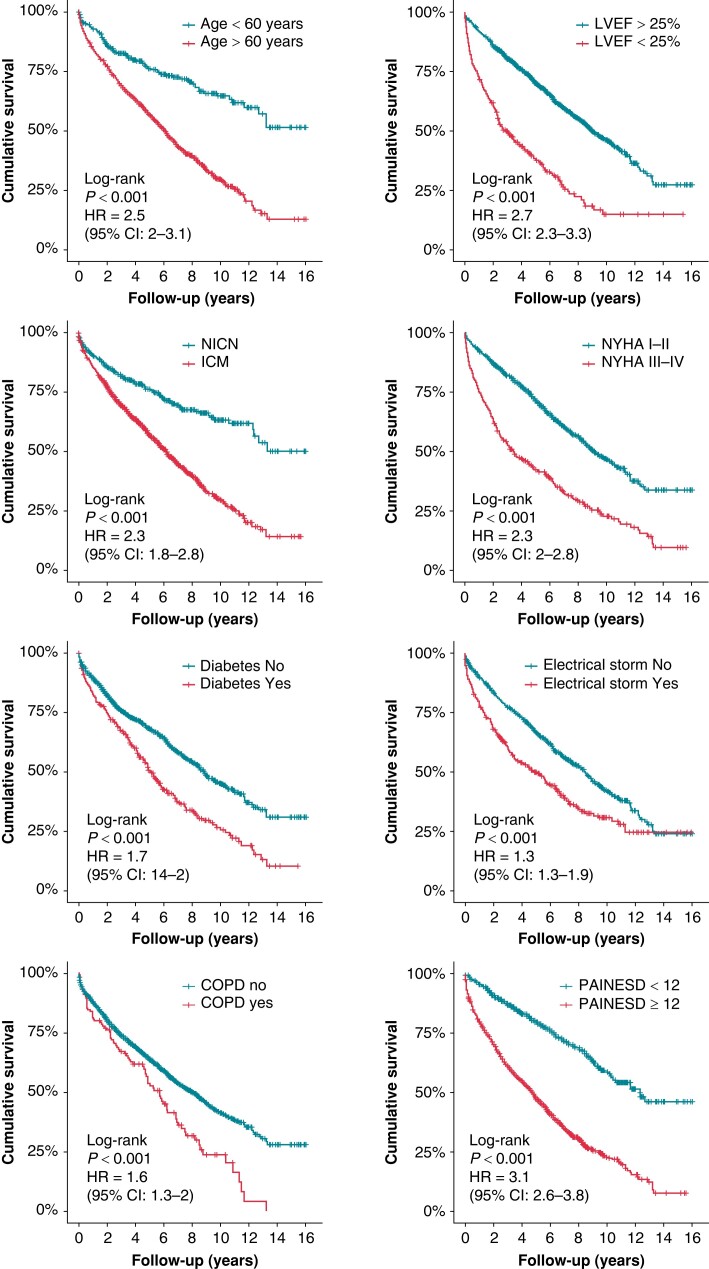
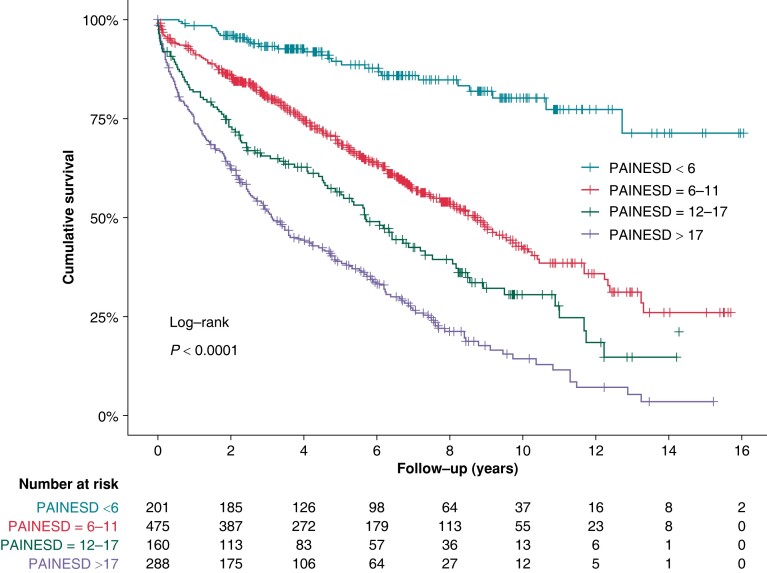
The impact of individual PAINESD risk factors on all-cause mortality in the univariate analysis is displayed in Figure 2. After multivariate adjustment, independent predictors of all-cause mortality were age > 60 years (HR 2.0, 95% CI 1.6–2.5, P < 0.0001), ischaemic cardiomyopathy (HR 1.6, 95% CI 1.2–2.0, P < 0.0001), NYHA Class ≥ III (HR 1.8, 95% CI 1.5–2.1, P < 0.0001), LV ejection fraction < 25% (HR 2.3, 95% CI 1.9–2.8, P < 0.00001), electrical storm (HR 1.4, 95% CI 1.2–1.7, P < 0.001), and diabetes mellitus (HR 1.4, 95% CI 1.1–1.6, P < 0.001). However, the presence of chronic pulmonary disease did not reach statistical significance after multivariate adjustment (HR 1.2, 95% CI 1.0–1.6, P = 0.08). The PAINESD score was a strong predictor of long-term mortality in this cohort (Figure 3).
Figure 2.
Dichotomized clinical factors associated with all-cause mortality (univariate Cox regression analysis). COPD, chronic obstructive pulmonary disease, LVEF, left ventricular ejection fraction; ICM, ischaemic cardiomyopathy; NICM, non-ischaemic cardiomyopathy; NYHA, New York Heart Association.
Figure 3.
All-cause mortality: the impact of the PAINESD score. Kaplan–Meier survival curves for the population categorized by quartiles of PAINESD score.
Discussion
To the best of our knowledge, this study investigated the largest single-centre cohort of patients undergoing CA for SHD-related VT. We retrospectively evaluated the predictive power of the PAINESD score for the incidence of AHD after the predominant substrate-based strategy of ablation. The results can be summarized as follows: (i) A substantially lower rate (1.2%) of AHD was observed than previously published (up to 11%),6 and (ii) AHD events did not accumulate in patients with upper-tercile PAINESD score in whom the highest AHD risk was reported (up to 24%). Therefore, we did not confirm the clinical utility of the PAINESD score in patients undergoing less aggressive ablation strategy.
In principle, strategies of CA for VT in SHD can be divided into two groups. The first utilizes predominant activation and entrainment mapping during ongoing VT. The second relies mostly on substrate mapping, in which abnormal signals are identified during sinus rhythm or ventricular pacing.14,15 It may be complemented with imaging of the scar using magnetic resonance imaging, computed tomography, or intracardiac echocardiography.16–22 Unfortunately, a head-to-head comparison of the two strategies in large, randomized trials is not available. A meta-analysis of six studies (including two small randomized trials) comparing a strategy guided by activation and entrainment mapping with a substrate-based approach demonstrated comparable periprocedural efficacy, complications, VT recurrences, and mortality rates.23
The risk of acute haemodynamic decompensation during catheter ablation of ventricular tachycardia
Owing to the severity of underlying disease and comorbidities, patients undergoing CA for SHD-related VT are at a substantial risk of periprocedural complications including AHD, which is associated with increased short- and long-term mortality.9,24–26 The haemodynamic instability may have different causes. One could be the use of GA with cardio- and vasodepressive effects that often necessitate inotropic/vasoactive support. This can promote the spontaneous occurrence of less-tolerated VTs. The other reason could reflect repeated inductions of VT leading to coronary, cerebral, and renal hypoperfusion. Moreover, the low cardiac output state may even persist after the restitution of the normal sinus rhythm and precipitate further deterioration of cardiac systolic function.24
The PAINESD score was proposed by a group at the University of Pennsylvania to predict the risk of AHD and identify the patients who may benefit from the pre-emptive use of MCS devices. They had reported a high incidence of AHD events (in 22 of 193 patients, 11%), which, interestingly, occurred in the majority of patients (63%) during substrate ablation (not during ongoing VT).8,27 However, CA was performed under GA in a substantial proportion of cases (32%). The authors concluded that substrate-based ablation per Se cannot prevent AHD events. In contrast, the incidence of AHD in our cohort was substantially (10 times) lower and was not predicted by the PAINESD score (Table 5 and Figure 1). Our population had fewer patients with electric storm (25 vs. 47%) and fewer patients with chronic obstructive pulmonary disease (12 vs. 16%). On the other hand, it had more patients with ischaemic cardiomyopathy (67 vs. 56%), more patients with NYHA Class ≥ 3 (31 vs. 20%), and more patients with diabetes (31 vs. 20%). The mean PAINESD score in the development cohort6 was not presented, but its middle-tercile range of 9–14 indicates that it was close to our mean PAINESD of 11 ± 7. Two studies were published on patients undergoing CA of SHD-related VT with comparable results to ours. In the study by Martins et al.28 involving 102 patients, the PAINESD score did not predict early mortality or haemodynamic decompensations. In another study by Della et al.,29 intraprocedural AHD occurred in only 6 among 528 patients (1.1%).
Table 5.
Comparisons between our experience and previously published data
| IKEM experience | UPenn experience8 | International VT Ablation Center Collaborative group27 | |
|---|---|---|---|
| Number of patients | 1124 | 193 | 2061 |
| Age (years) | 63 ± 13 | 62 ± 15 | 62 ± 13 |
| Left ventricular EF (%) | 34 ± 12 | 37 ± 16 | 34 ± 13 |
| Follow-up duration | 4.2 years | 21 ± 7 months | 1 year |
| Electrical storm (%) | 25 | 47 | 35 |
| PAINESD score | 11 ± 7 | NA | 9.8a |
| Acute haemodynamic decompensation (%) | 1.2 | 11 | NA |
| Early (31 day) mortality (%) | 2.9 | NA | 5 |
| Late (21 month) mortality (%) | 18 | 16 | NA |
| Procedural time (min) | 187 ± 79 | 480 | 284 ± 117 |
Data are provided as means ± SD or counts (proportions).
EF, ejection fraction; IKEM, Institute for Clinical and Experimental Medicine; NA, not available; UPenn, University of Pennsylvania; VT, ventricular tachycardia.
aEstimated based on a weighted average of three study groups (early mortality group, late mortality group, and the rest of the population group)
Thus, it suggests that the risk of AHD highly varies between different centres and must be precipitated by other factors currently not included in the PAINESD score. Although we can only speculate, we believe that the crucial factors that can explain the lower risk of AHD observed in our patient population include careful intraprocedural haemodynamical monitoring (including monitoring of cardiac contractility with intracardiac echocardiography), preference for conscious sedation over GA, and use of substrate-based mapping as the dominant strategy.
The role of pre-emptive mechanical circulatory support in catheter ablation of ventricular tachycardia
The AHD is associated with a high risk of morbidity and mortality,8 and the use of the rescue extracorporeal membrane oxygenation in patients in whom AHD already occurred was associated with a high mortality rate (76% at a median follow-up of 10 days after the procedure).8 A propensity score–matched analysis by Muser et al.10 reported a benefit of pre-emptive use of MCS (Impella in all cases) in reducing the AHD events (7 vs. 23%, in MCS and control group, respectively, P < 0.01). However, this approach was not associated with improved mortality or arrhythmia-related survival. Moreover, there was a substantially increased risk of complication in the MCS group requiring surgical intervention. Increased risk of periprocedural complications when MCS was used was also reported by another group.30 In a study by Mathuria et al.,12 30-day mortality of patients undergoing VT ablation with pre-emptive MCS (n = 24, PAINESD 16.5) compared with those ablated without MCS (n = 57, PAINESD 13.4) was similar (4 vs. 3%). Of note, in our study, the 30-day mortality of patients with comparable PAINESD scores (15–18 vs. 12–15) who underwent CA without pre-emptive MCS was 7/218 (3%) vs. 15/297 (5%), respectively. In another study, Neuzner et al.11 used pre-emptive micro-axial MCS to prevent AHD in 26 patients undergoing VT ablation with a high PAAINESD (variant of PAINESD) score (21 ± 3), and they were successful in all but one case with bail-out use of MCS. However, such results are of limited relevance, since the control group is missing. Thus, the role of MCS and the selection of appropriate candidates during the CA of VT remain controversial.
PAINESD score as the predictor of mortality
In a large multicentre registry of 2061 patients9 undergoing CA for SHD-related VT, the PAINESD score was a good predictor of early and long-term mortality. Our study confirmed these results. The key question remains whether the prognosis in such a high-risk group could be improved by more effective CA. Conversely, less aggressive CA aimed to prevent periprocedural AHD in high-risk patients may lead to a higher recurrence rate. In this regard, both early mortality, as an indicator of the safety and immediate risk associated with the procedure, and late mortality, which could reflect the efficacy of the procedure, were comparable in our cohort to those reported after more aggressive CA (Table 5). The outcome of the CA is always a balance between efficacy (i.e. the ability to abolish all inducible VTs resulting in lengthy procedures) and safety. In our hands, less aggressive CA aimed to avoid AHD was not associated with poorer outcomes. Overall, our results do not support the routine use of PAINESD score for the prediction of AHD and certainly not for the routine use of MCS devices based on a high PAINESD score.
Study limitations
This was a retrospective study. The enrolment period was very long so the ablation strategy could undergo some change. The study did not investigate all potential risk factors as we tried to be in line with the original definition of the PAINESD score. For example, creatinine level that is known as a strong risk predictor was not included although it was available. Similarly, VT inducibility was not considered as it was not tested consistently at the end of the procedure because of safety concerns.
Conclusions
In our large cohort of patients with CA of SHD-related VT, the incidence of AHD was substantially lower than previously reported. This observation may be explained by a strategy of predominant substrate-based CA under conscious sedation that prevents hypotension and prolonged low cardiac output state related to VT induction and activation mapping. In such a scenario, the PAINESD score may lead to unnecessary prophylactic use of MCS during the CA of VT.
Author’s contributions
Substantial contributions to the conception and design or the acquisition, analysis, or interpretation of the data: P.S., D.W., P.P., R.C., B.A., P.S., J.H., E.B., and J.K. Substantial contributions to the drafting of the articles or critical revision for important intellectual content: P.S., D.W., P.P., and J.K. Final approval of the version to be published: J.K. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved: P.S. and D.W.
Contributor Information
Predrag Stojadinović, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia; First Faculty of Medicine, Institute of Physiology, Charles University, Prague, Czechia.
Dan Wichterle, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Petr Peichl, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Robert Čihák, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Bashar Aldhoon, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Eva Borišincová, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Petr Štiavnický, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Jana Hašková, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Adam Ševčík, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Josef Kautzner, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9 Prague 140 21, Czechia.
Funding
This study was supported by the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, Project No. LX22NPO5104)—Funded by the European Union, NextGenerationEU. This work was also funded by the project (Ministry of Health, Czech Republic) for development of research organization 00023001 (IKEM, Prague, Czech Republic)—institutional support.
References
- 1. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. Esc guidelines for the management of V tachyarrhytmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 2. Natale A, Zeppenfeld K, Della BP, Liu X, Sabbag A, Santangeli P, et al. Twenty-five years of catheter ablation of ventricular tachycardia: a look back and a look forward. Europace 2023;25:euad225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conti S, Pala S, Biagioli V, Del Giorno G, Zucchetti M, Russo E, et al. Electrical storm: a clinical and electrophysiological overview. World J Cardiol 2015;7:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koželuhová M, Peichl P, Hlivák P, Čihák R, Wichterle D, Vančura V, et al. Spektrum idiopatických komorových tachykardií ve specializovaném centru. Interv a akutní Kardiol 2009;8:228–232. [Google Scholar]
- 5. Bennett RG, Turnbull S, Sood A, Aung M, Duncan E, Barman P, et al. Emergency out-of-hours catheter ablation for ventricular arrhythmia storm: a UK and Australian experience. Europace 2023;25:euad215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gula LJ, Doucette S, Leong-Sit P, Tang ASL, Parkash R, Sarrazin JF, et al. Quality of life with ablation or medical therapy for ventricular arrhythmias: a substudy of VANISH. J Cardiovasc Electrophysiol 2018;29:421–434. [DOI] [PubMed] [Google Scholar]
- 7. Zeppenfeld K, Wijnmaalen AP, Ebert M, Baldinger SH, Berruezo A, Catto V, et al. Clinical outcomes in patients with dilated cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol 2022;80:1045–1056. [DOI] [PubMed] [Google Scholar]
- 8. Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, et al. Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythmia Electrophysiol 2015;8:68–75. [DOI] [PubMed] [Google Scholar]
- 9. Muser D, Castro SA, Liang JJ, Santangeli P. Identifying risk and management of acute haemodynamic decompensation during catheter ablation of ventricular tachycardia. Arrhythmia Electrophysiol Rev 2018;7:282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muser D, Liang JJ, Castro SA, Hayashi T, Enriquez A, Troutman GS, et al. Outcomes with prophylactic use of percutaneous left ventricular assist devices in high-risk patients undergoing catheter ablation of scar-related ventricular tachycardia: a propensity-score matched analysis. Hear Rhythm 2018;15:1500–1506. [DOI] [PubMed] [Google Scholar]
- 11. Neuzner J, Dietze T, Paliege R, Gradaus R. Effectiveness of a percutaneous left ventricular assist device in preventing acute hemodynamic decompensation during catheter ablation of ventricular tachycardia in advanced heart failure patients: a retrospective single-center analysis. J Cardiovasc Electrophysiol 2019;30:2864–2868. [DOI] [PubMed] [Google Scholar]
- 12. Mathuria N, Wu G, Rojas-Delgado F, Shuraih M, Razavi M, Civitello A, et al. Outcomes of pre-emptive and rescue use of percutaneous left ventricular assist device in patients with structural heart disease undergoing catheter ablation of ventricular tachycardia. J Interv Card Electrophysiol 2017;48:27–34. [DOI] [PubMed] [Google Scholar]
- 13. Kautzner J, Čihák R, Peichl P, Vančura V, Bytešník J. Catheter ablation of ventricular tachycardia following myocardial infarction using three-dimensional electroanatomical mapping. Pacing Clin Electrophysiol 2003;26:342–347. [DOI] [PubMed] [Google Scholar]
- 14. Kahle AK, Jungen C, Alken FA, Scherschel K, Willems S, Pürerfellner H, et al. Management of ventricular tachycardia in patients with ischaemic cardiomyopathy: contemporary armamentarium. Europace 2022;24:538–551. [DOI] [PubMed] [Google Scholar]
- 15. Hanaki Y, Komatsu Y, Nogami A, Kowase S, Kurosaki K, Sekiguchi Y, et al. Combined endo- and epicardial pace-mapping to localize ventricular tachycardia isthmus in ischaemic and non-ischaemic cardiomyopathy. Europace 2022;24:587–597. [DOI] [PubMed] [Google Scholar]
- 16. Yamashita S, Sacher F, Mahida S, Berte B, Lim HS, Komatsu Y, et al. Image integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol 2016;27:699–708. [DOI] [PubMed] [Google Scholar]
- 17. Yamashita S, Sacher F, Hooks DA, Berte B, Sellal JM, Frontera A, et al. Myocardial wall thinning predicts transmural substrate in patients with scar-related ventricular tachycardia. Hear Rhythm 2017;14:155–163. [DOI] [PubMed] [Google Scholar]
- 18. Bunch TJ, Weiss JP, Crandall BG, Day JD, Dimarco JP, Ferguson JD, et al. Image integration using intracardiac ultrasound and 3D reconstruction for scar mapping and ablation of ventricular tachycardia. J Cardiovasc Electrophysiol 2010;21:678–684. [DOI] [PubMed] [Google Scholar]
- 19. Engert F, Bahlke F, Erhard N, Krafft H, Popa M-A, Risse E, et al. VT ablation based on CT imaging substrate visualization: results from a large cohort of ischemic and non-ischemic cardiomyopathy patients. Clin Res Cardiol 2023. doi: 10.1007/s00392-023-02321-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esposito A, Palmisano A, Antunes S, Maccabelli G, Colantoni C, Rancoita PMV, et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging 2016;9:822–832. [DOI] [PubMed] [Google Scholar]
- 21. Siontis KC, Kim HM, Dabbagh GS, Latchamsetty R, Stojanovska J, Jongnarangsin K, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Hear Rhythm 2017;14:1487–1493. [DOI] [PubMed] [Google Scholar]
- 22. Komatsu Y, Cochet H, Jadidi A, Sacher F, Shah A, Derval N, et al. Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: assessment of structural and electrical substrate. Circ Arrhythmia Electrophysiol 2013;6:342–350. [DOI] [PubMed] [Google Scholar]
- 23. Kumar S, Baldinger SH, Romero J, Fujii A, Mahida SN, Tedrow UB, et al. Substrate-based ablation versus ablation guided by activation and entrainment mapping for ventricular tachycardia: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2016;27:1437–1447. [DOI] [PubMed] [Google Scholar]
- 24. Skhirtladze K, Mora B, Moritz A, Birkenberg B, Ankersmit HJ, Dworschak M. Impaired recovery of cardiac output and mean arterial pressure after successful defibrillation in patients with low left ventricular ejection fraction. Resuscitation 2010;81:1123–1127. [DOI] [PubMed] [Google Scholar]
- 25. Eckardt L, Doldi F, Anwar O, Gessler N, Scherschel K, Kahle A-K, et al. Major in-hospital complications after catheter ablation of cardiac arrhythmias: individual case analysis of 43 031 procedures CLINICAL RESEARCH. Europace 2023;26:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peichl P, Wichterle D, Pavlů L, ČihÁk R, Aldhoon B, Kautzner J. Complications of catheter ablation of ventricular tachycardia a single-center experience. Circ Arrhythmia Electrophysiol 2014;7:684–690. [DOI] [PubMed] [Google Scholar]
- 27. Santangeli P, Frankel DS, Tung R, Vaseghi M, Sauer WH, Tzou WS, et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J Am Coll Cardiol 2017;69:2105–2115. [DOI] [PubMed] [Google Scholar]
- 28. Martins A, Antonio PS, Pereira SC, Brito J, Silva BV, Da Silva PA, et al. Is it possible to predict mortality and recurrence of VT after ablation? PAINESD risk score applicability vs new predictors. EP Eur 2022;24:40695. [Google Scholar]
- 29. Della BP, Baratto F, Tsiachris D, Trevisi N, Vergara P, Bisceglia C, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation 2013;127:1359–1368. [DOI] [PubMed] [Google Scholar]
- 30. Kusa S, Miller MA, Whang W, Enomoto Y, Panizo JG, Iwasawa J, et al. Outcomes of ventricular tachycardia ablation using percutaneous left ventricular assist devices. Circ Arrhythmia Electrophysiol 2017;10:1–7. [DOI] [PubMed] [Google Scholar]