Abstract
Background
New vaccine products were recently authorized for protection against invasive pneumococcal disease (IPD) in Canada. Our aim was to determine age- and serotype-specific trends in IPD incidence and severity in Canada's largest province, Ontario.
Methods
We included all confirmed IPD cases reported in Ontario and defined the pre–pneumococcal 13-valent conjugate vaccine (PCV13) era (01/2007 to 12/2010), post-PCV13 era (01/2011 to 12/2019), and coronavirus disease 2019 (COVID-19) pandemic era (01/2020 to 12/2022). We estimated incidence, hospitalization, and case fatality rate (CFR) by age. We grouped IPD cases by vaccine-specific serotypes (PCV13; PCV15-non-PCV13; PCV20-non-PCV13; PCV20-non-PCV15; polysaccharide 23-valent vaccine-non-PCV20; and non-vaccine-preventable [NVP]). We then compared incidence rates by age and serotype group in the pre- and post-PCV13 eras by calculating rate ratios (RRs) and their 95% CIs.
Results
Incidence and hospitalizations declined from the pre- to post-PCV13 era in children aged <5 years (RR, 0.7; 95% CI, 0.6–0.8; and RR, 0.8; 95% CI, 0.7–0.9, respectively), but the CFR increased (1.4% to 2.3%). Other age groups saw smaller declines or more stable incidence rates across the years; hospitalizations increased in adults aged 50–64 years (RR, 1.2; 95% CI, 1.1–1.4) and ≥65 years (RR, 1.1; 95% CI, 1.0–1.1). For all ages, IPD cases and hospitalizations attributable to PCV13 serotypes declined, and those attributable to PCV15-non-PCV13, PCV20-non-PCV13, and NVP serotypes increased. IPD incidence declined during the COVID-19 era.
Conclusions
IPD incidence and hospitalizations due to PCV13 serotypes decreased after PCV13 introduction but increased for other serotypes. Continued surveillance is required to evaluate changes to pneumococcal vaccination programs and ongoing changes to the distribution of IPD-causing serotypes.
Keywords: Invasive pneumococcal disease, epidemiology, vaccination program, serotype replacement
Invasive pneumococcal disease (IPD) is a serious acute illness caused by Streptococcus pneumoniae that is associated with severe morbidity and mortality worldwide [1, 2]. In 2016, S. pneumoniae led to almost 2 million episodes of illness worldwide and more deaths than all other lower respiratory infection etiologies combined [3]. In Ontario, Canada's largest province of nearly 16 million residents [4], publicly funded pneumococcal vaccine is routinely available for the age groups at highest risk of IPD, children aged <2 years and adults aged ≥65 years [5]. Individuals aged 2–64 years are also eligible for pneumococcal vaccination if considered at high risk of IPD due to an underlying medical condition [5].
The product and number of doses offered currently differ between the pediatric and adult pneumococcal vaccination programs in Ontario. For children <2 years, a 4-dose pneumococcal 7-valent conjugate vaccine (PCV7) program was first introduced in January 2005 [6]. PCV10, which protected against 3 additional serotypes of S. pneumoniae, replaced PCV7 in October 2009 [6]. In November 2010, PCV13, with coverage against an additional 3 serotypes, was introduced, and the number of recommended doses was reduced to 3 for healthy children [6]. Catch-up doses are recommended for all children up to age 5 years [7]. Since 1995–1996, polysaccharide 23-valent vaccine (PPV23) has been publicly funded for those aged ≥65 years or ≥2 years with an underlying medical condition [6]. Use of PCV13 in adults is currently only publicly funded for immunocompromised individuals aged ≥50 years [5]. Recently, between 2021 and 2023, 2 new vaccine products, PCV15 and PCV20, were authorized by Health Canada for use in individuals aged ≥6 weeks [8]. In consideration of these new products, the Canadian National Advisory Committee on Immunization (NACI), which makes recommendations for the use of currently or newly approved vaccines in Canada, updated their pneumococcal vaccination guidelines. NACI now recommends PCV20 for routine immunization for adults aged ≥65 years and adults aged 50–64 years at high risk of IPD [8]. NACI recommends either PCV15 or PCV20 for healthy children aged <5 years and PCV20 for individuals aged ≤18 years at high risk of IPD [9]. NACI guidance is used by provinces and territories in Canada to inform vaccination program decision-making. Ontario has not yet incorporated the new products into their publicly funded vaccination program.
Following the implementation of pediatric pneumococcal vaccination programs, there was a significant decrease in the incidence of IPD in children aged <5 years in Ontario [10, 11]. Comparatively, the overall incidence of IPD among adults remained relatively stable [10, 11]. This is thought to be mainly due to serotype replacement where non-vaccine-preventable serotypes have replaced the niche created by the reduction in vaccine-preventable serotypes [12]. IPD serotype replacement has been seen internationally, though the change in distribution of serotypes varies across jurisdictions [13–16]. Due to these increases in non-PCV13 serotypes, PCV15 and PCV20, which protect against additional serotypes, were developed and authorized in Canada, and others are currently in the pipeline.
Though serotype replacement post–PCV13 introduction has been reported in Ontario [10, 11], current trends are not yet fully understood. Moreover, little is known about the effect of PCV13 programs on IPD severity, and the COVID-19 pandemic may have led to further changes to the landscape of the disease. The objectives of our study were to (1) estimate age-specific IPD burden from 2007–2019 and the burden of IPD during the COVID-19 pandemic (2020–2022), (2) compare age-specific IPD incidence trends and severity pre– and post–PCV13 introduction (2007–2019), and (3) compare the distribution of disease (2007–2022) as well as IPD incidence and severity of disease by vaccine-specific and non-vaccine-preventable serotypes pre– and post–PCV13 introduction (2007–2019).
METHODS
Surveillance Data
IPD is reportable under Ontario's Health Protection and Promotion Act [17]. Public health units (PHUs) are responsible for collecting and entering data for confirmed IPD cases into Ontario's integrated Public Health Information System (iPHIS) using the provincial surveillance case definition. A confirmed case of IPD is defined as an individual with clinical evidence of invasive disease with laboratory confirmation of infection, which includes the isolation of S. pneumoniae or detection of S. pneumoniae DNA by nucleic acid amplification test (NAAT) from a normally sterile site (eg, blood, cerebrospinal fluid, excluding middle ear) [18].
In Ontario, most isolates of S. pneumoniae from sterile sites are sent by the Public Health Ontario (PHO) laboratory to the National Microbiology Laboratory (NML) for serotyping [19]. Alternatively, for hospitals that are part of the Toronto Invasive Bacterial Diseases Network (TIBDN), isolates are sent to the TIBDN laboratory for identification and serotyping [19]. Prior to 2007, Ontario PHUs used their own version of a standalone provincial surveillance system called Reportable Disease Information System (RDIS). By 2007, all Ontario PHUs were using the integrated web-based surveillance system (iPHIS), which resulted in all provincial surveillance data being available in 1 centralized location. This change led to coordinated data-cleaning initiatives and active follow-up of cases and coincided with routine reporting of serotype information by Ontario labs to PHUs. Therefore, our analyses include all cases of IPD between January 1, 2007, and December 31, 2022.
Vaccine Eras
We defined 2 vaccine eras to align with the introduction of the PCV13 vaccine: pre-PCV13 era (January 2007 to December 2010) and post-PCV13 era (January 2011 to December 2019). We did not include 2020–2022 in the post-PCV13 era due to the potential effect of the COVID-19 pandemic on IPD. Instead, these 3 years were explored separately to assess whether any pandemic effects were seen on IPD rates.
Serotype Groupings
We grouped IPD cases according to the unique serotypes included in the different vaccines: PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), PCV15-non-PCV13 (22F and 33F), PCV20-non-PCV13 (22F, 33F, 8, 10A, 11A, 12F, 15B), PCV20-non-PCV15 (8, 10A, 11A, 12F, 15B), and PPV23-non-PCV20 (2, 9N, 17F, and 20) and non-vaccine-preventable (NVP; serotypes not protected against by any of the authorized vaccines). PPV23 does not contain the serotype 6A, whereas the conjugate vaccines do. Cases without serotype results were included in the overall IPD rate estimations but considered missing for serotype-specific analyses.
Statistical Analyses
We performed descriptive analyses using SAS Enterprise Guide 8.2 (SAS Institute Inc., Cary, NC, USA). Case details including hospitalizations and deaths were extracted from iPHIS and used in our overall and age-stratified analyses (<5, 5–49, 50–64, ≥65 years). We calculated incidence and hospitalization rates (per 100 000 population) by age group by dividing the number of cases or hospitalizations within population estimates (2007–2021) and projections (2022) obtained from Statistics Canada [20, 21]. We calculated rate ratios (RRs) and their 95% CIs, comparing rates in the post-PCV13 era with those in the pre-PCV13 era, by age group. We calculated case fatality rates (CFRs) by age group by dividing the number of deaths within each group by the number of IPD cases within the corresponding group. We also calculated the proportion of IPD cases attributable to the different serotype groupings for the entire study period (2007–2022) and estimated IPD incidence and hospitalizations caused by the serotype groupings pre- (2007–2010) and post-PCV13 (2011–2019) with corresponding RRs comparing the incidence and hospitalization rates between the 2 periods.
Ethics Approval
Our study contains data collected for the purpose of routine surveillance activities as outlined under provincial legislation (Ontario Agency for Health Protection and Promotion Act, SO 2007, c 10). Thus, ethics approval from Public Health Ontario's Ethics Review Board was not required.
RESULTS
Temporal Trends in IPD Incidence and Hospitalization Across the Entire Study Period (2007–2022)
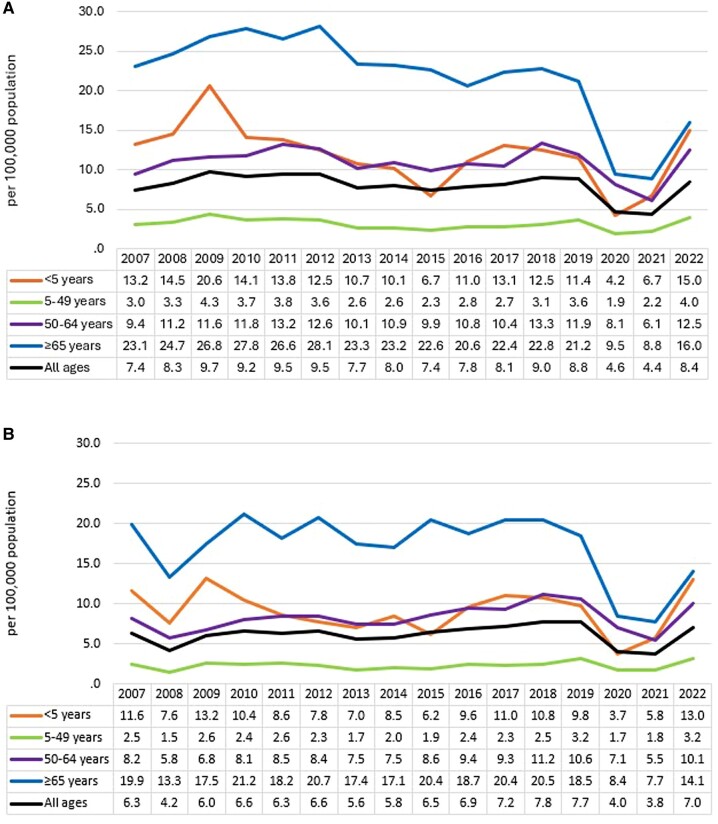
Overall in Ontario, the incidence of IPD remained stable until 2020. The highest incidence of IPD was in adults aged ≥65 years (Figure 1A, Table 1). Before 2020, IPD incidence rates remained relatively steady for those aged 5–64 years, whereas incidence seemed to decline, though with some fluctuations across the years, in those aged <5 years and ≥65 years. The rate of hospitalizations was also highest in adults aged ≥65 years and remained fairly stable across the different age groups until 2020, except for those aged ≤5 years, where more variability was seen across the years (Figure 1B, Table 1). In all age groups, a clear pandemic effect was seen in 2020 and 2021, where there was a significant decrease in both incidence and hospitalizations, with the most dramatic decrease observed in adults aged ≥65 years. In 2022, incidence and hospitalization rates increased to rates more similar to those seen in prepandemic years, but the level of increase varied across age groups. In children aged <5 years, the incidence and hospitalization rates in 2022 were the second highest in the entire study period. Conversely, the incidence and hospitalization rates in adults aged ≤65 years remained below most rates seen in prepandemic years.
Figure 1.
Incidence (A) and hospitalization rates (B) (per 100 000 population) of invasive pneumococcal disease cases, by age group and year, in Ontario, Canada, 2007–2022.
Table 1.
Comparison of Overall Incidence and Severity of IPD Cases in the Pre-PCV13 (2007–2010) and Post-PCV13 Vaccine Eras (2011–2019), by Age Group, in Ontario, Canada
| <5 Years | 5–49 Years | 50–64 Years | ≥65 Years | All Ages | |
|---|---|---|---|---|---|
| No. of cases | |||||
| Pre-PCV13 | 437 | 1144 | 1087 | 1806 | 4474 |
| Post-PCV13 | 731 | 2187 | 2957 | 4595 | 10 470 |
| IPD incidence rate (per 100 000) | |||||
| Pre-PCV13 | 15.6 | 3.6 | 11.0 | 25.6 | 8.6 |
| Post-PCV13 | 11.3 | 3.0 | 11.4 | 23.3 | 8.4 |
| IPD incidence, RR (95% CI) | 0.7 (0.6–0.8) | 0.8 (0.8–0.9) | 1.0 (1.0–1.1) | 0.9 (0.9–1.0) | 1.0 (0.9–1.0) |
| % of cases hospitalized (No.) | |||||
| Pre-PCV13 | 68.6 (300) | 62.8 (718) | 65.6 (718) | 70.2 (1267) | 67.0 (2998) |
| Post-PCV13 | 78.0 (570) | 76.9 (1681) | 78.8 (2330) | 82.3 (3780) | 79.9 (8361) |
| Hospitalization rate (per 100 000) | |||||
| Pre-PCV13 | 10.7 | 2.2 | 7.2 | 18.0 | 5.8 |
| Post-PCV13 | 8.8 | 2.3 | 9.0 | 19.1 | 6.7 |
| Hospitalizations, RR (95% CI) | 0.8 (0.7–0.9) | 1.0 (1.0–1.1) | 1.2 (1.1–1.4) | 1.1 (1.0–1.1) | 1.2 (1.1–1.2) |
| Case fatality rate, % (No.) | |||||
| Pre-PCV13 | 1.4 (6) | 5.9 (67) | 9.7 (105) | 18.8 (340) | 11.6 (518) |
| Post-PCV13 | 2.3 (17) | 5.8 (127) | 11.2 (330) | 16.4 (755) | 11.7 (1229) |
Abbreviations: IPD, invasive pneumococcal disease; PCV13, 13-valent pneumococcal conjugate vaccine; RR, rate ratio.
Age-Specific Trends in the Pre- and Post-PCV13 Eras (2007–2019)
The overall incidence of IPD in Ontario was similar pre- and post-PCV13 era (RR, 1.0; 95% CI, 0.9–1.0), but the rate of hospitalizations increased (RR, 1.2; 95% CI, 1.1–1.2) (Table 1). The largest decrease in IPD incidence pre– to post–PCV13 introduction was in children aged <5 years (RR, 0.7; 95% CI, 0.6–0.8). Slight declines in incidence were also seen in those aged 5–49 years (RR, 0.8; 95% CI, 0.8–0.9) and ≥65 years (RR, 0.9; 95% CI, 0.9–1.0), but incidence was stable in adults aged 50–64 years. For hospitalizations, a reduction in the age-specific hospitalization rate between the 2 eras was only observed in children aged <5 years (RR, 0.8; 95% CI, 0.7–0.9). Rates increased slightly in those aged 50–64 (RR, 1.2; 95% CI, 1.1–1.4) and ≥65 years (RR, 1.1; 95% CI, 1.0–1.1), and there was no change in those aged 5–49 years. The CFR increased in children aged <5 years and adults aged 50–64 years between the 2 eras. CFR between the 2 eras remained similar in those aged 5–49 years and decreased in adults aged ≥65 years.
IPD by Vaccine and Non-Vaccine-Preventable Serotypes
Serotype information was reported for 14 887 (84.8%) cases. The proportion of IPD cases attributable to vaccine-specific and NVP serotypes remained stable up to 2010 (Supplementary Figure 1). Subsequently, a reduction in PCV13 serotypes and an increase in PCV15-non-PCV13, PCV20-non-PCV15, and NVP serotypes was seen for most years, as well as increases in PPV23-non-PCV20 serotypes in 2015–2017 for those aged ≥5 years (Supplementary Figure 1). As of 2013 onward in children aged <5 years, the proportion of cases attributable to the 7 additional serotypes in PCV20-non-PCV13 (proportion ranging between 32% and 53% of all cases) surpassed the proportion of cases attributable to PCV13 serotypes (Supplementary Appendix, Figure 2). The proportion attributable to 22F and 33F specifically ranged between 8% and 23% during those years. In 2022, the latest year of data available, the proportion of cases attributable to PCV20-non-PCV13 serotypes compared with PCV13 serotypes was 37.1% vs 27.0% in those aged <5 years and 26.8% vs 27.6% in those aged ≥65 years.
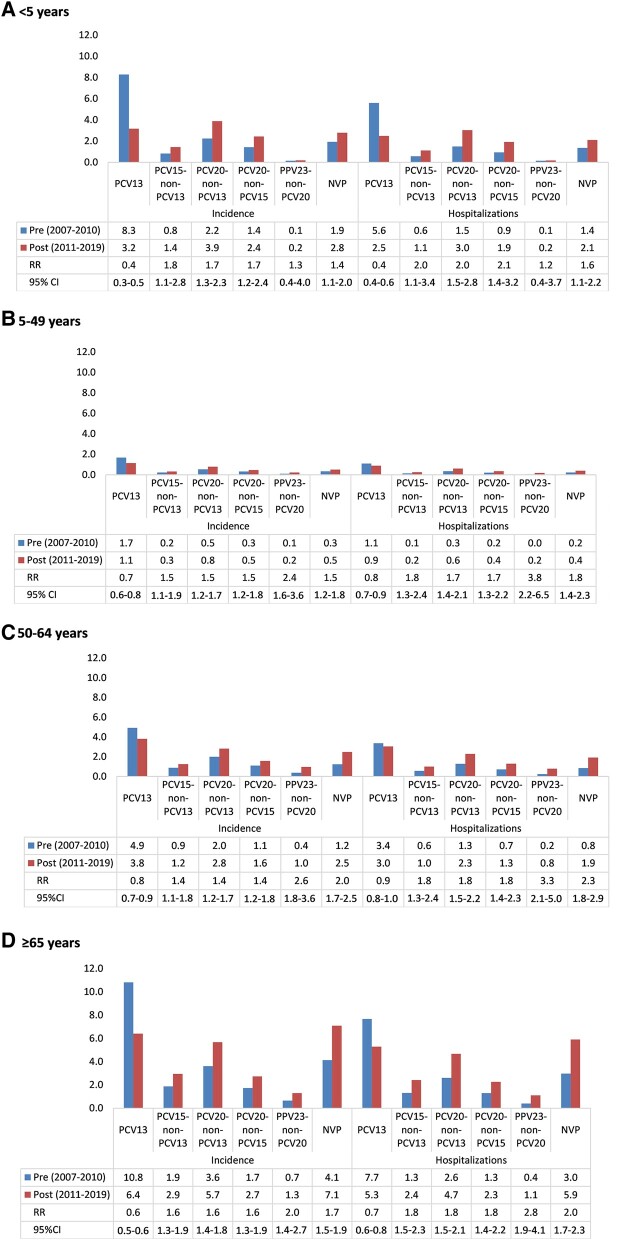
All age groups saw a reduction in IPD incidence and hospitalizations attributable to PCV13 serotypes pre- to post-PCV13 era (Figure 2). The largest decline was seen in children aged <5 years (incidence RR, 0.4; 95% CI, 0.3–0.5; and hospitalization RR, 0.4; 95% CI, 0.4–0.6). Conversely, all age groups saw an increase in IPD incidence and hospitalizations attributable to the PCV15-non-PCV13, PCV20-non-PCV13, and PCV20-non-PCV15 serotypes in the pre- to post-PCV13 era. In children aged <5 years and individuals aged 5–49 years, 50–64 years, and ≥65 years, the RR comparing the incidence of IPD attributable to PCV15-non-PCV13 serotypes pre- to post-PCV13 was 1.8 (95% CI, 1.1–2.8), 1.5 (95% CI, 1.1–1.9), 1.4 (95% CI, 1.1–1.8), and 1.6 (95% CI, 1.4–1.9), respectively. For PCV20-non-PCV15 serotypes, the RR was 1.7 (95% CI, 1.2–2.4), 1.5 (95% CI, 1.2–1.8), 1.4 (95% CI, 1.2–1.8), and 1.6 (95% CI, 1.3–1.9), for each age group, respectively. NVP serotypes saw the largest increase in cases for all age groups combined (pre-PCV13: 16.9% of cases; post-PCV13: 28.1% of cases) (Supplementary Appendix, Table 1). The next largest increase in cases from pre- to post-PCV13 was for serotype 22F (7.4% to 10.6% of cases), serotype 9N (2.5% to 5.4% of cases), and serotype 3 (9.2% to 11.9% of cases). The RR of hospitalizations attributable to PCV15-non-PCV13 and PCV20-non-PCV15 serotypes was highest in children aged <5 years at 2.0 (95% CI, 1.1–3.4) and 2.1 (95% CI, 1.4–3.2), respectively. The RR was similar for all other age groups. For all age groups, the rate of incidence and hospitalizations pre- and post-PCV13 for PCV20-non-PCV13 serotypes was double or greater than the rate for PCV15-non-PCV13 serotypes.
Figure 2.
IPD incidence, hospitalization, RRs, and 95% CIs for RR comparing the pre- and post-PCV13 periods, by age and vaccine-specific and NPV serotype groupings (A: aged <5 y; B: aged 5–49 y; C: aged 50–64 y; D: aged 65+ y). PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), PCV15-non-PCV13 (22F and 33F), PCV20-non-PCV13 (includes serotypes in PCV15; 22F, 33F, 8, 10A, 11A, 12F, 15B), PCV20-non-PCV15 (8, 10A, 11A, 12F, 15B), and PPV23-non-PCV20 (2, 9N, 17F, and 20) and NVP (serotypes not protected against by any of the authorized vaccines). Abbreviations: IPD, invasive pneumococcal disease; NPV, non-vaccine-preventable; PCV, pneumococcal conjugate vaccine; PPV, polysaccharide vaccine; RR, rate ratio.
For the age groups 5–49 years, 50–64 years, and ≥65 years, the largest increase in incidence and hospitalization rates pre- to post-PCV13 era was seen for PPV23-non-PCV20 serotypes (incidence RR, 2.4; 95% CI, 1.6–3.6; incidence RR, 2.6; 95% CI, 1.8–3.6; incidence RR, 2.0; 95% CI, 1.4–2.7; and hospitalization RR, 3.8; 95% CI, 2.2–6.5; hospitalization RR, 3.3; 95% CI, 2.1–5.0; hospitalization RR, 2.8; 95% CI, 1.9–4.1, respectively). All age groups also saw an increase in incidence and hospitalizations attributable to NVP serotypes post-PCV13 introduction. Though there were very few fatalities in children aged <5 years, a higher proportion of fatalities was attributable to PCV20-non-PCV13 vs PCV13 serotypes in the post-PCV13 era (53.3% vs 33.3%) (Supplementary Table 2). Of the 8 PCV20-non-PCV13 fatalities, 3 were due to serotype 10A, 2 to 11A, 2 to 15BC, and 1 to 22F (Supplementary Table 3). In all ages combined, a 10.1% increase from pre- to post-PCV13 was seen for fatalities attributable to NVP serotypes (Supplementary Table 3). Among the vaccine-preventable serotypes, serotype 3 led to the most fatalities in the post-PCV13 era (16.0% of fatalities), and 22F had the largest increase in fatalities from the pre- to post-PCV13 era (6.2% to 10.5% of fatalities; 4.3% increase).
DISCUSSION
Despite the implementation of publicly funded pneumococcal conjugate vaccine programs, the overall incidence of IPD remained stable in Ontario from 2007 to 2019. The largest decline in incidence was seen in children aged <5 years, which includes children eligible for routine and catch-up pediatric pneumococcal vaccine in the province. IPD incidence and hospitalization rates declined in all age groups during the pandemic years of 2020 and 2021, particularly in adults aged ≥65 years. Rates returned to prepandemic levels in 2022 for those aged 5–64 years but were higher than average compared with prepandemic years for children aged <5 years and lower for adults aged ≥65 years. For all age groups post–PCV13 introduction, the incidence and severity of IPD attributable to all PCV13 serotypes combined decreased, whereas the incidence and severity attributable to serotypes in PCV15 and/or PCV20 but not in PCV13, as well as NVP serotypes, increased.
Similar to Ontario, many regions across the world saw a notable decline in IPD incidence during the pandemic years of 2020 and/or 2021 [22–24]. The reason for this decline was multifaceted, with one of the largest contributors likely being the public health measures that were employed to reduce the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including lockdowns, social distancing, and masking. These measures also reduced the spread of other transmissible infections, particularly viral infections (eg, influenza and respiratory syncytial virus [RSV]), which can lead to secondary bacterial infections, and thus led to reduced rates of IPD [25–27]. Further supporting this theory, in 2022, the rate of viral respiratory infections, particularly RSV, rose significantly in young children in many regions, including Ontario [28–30], leading to one of the worst respiratory seasons in years. This may also partly explain the substantial increase in IPD incidence and hospitalizations we saw in children aged <5 years in 2022. As IPD surveillance in Ontario is passive, declines in rates during the pandemic years may also have been impacted by factors such as fewer health care encounters resulting from reduced capacity, health care facilities accepting only urgent cases, and changes in health care seeking due to concern for contracting SARS-CoV-2 in such settings, meaning only the most severe cases were possibly being captured during this time [31, 32]. Competing priorities in public health during the pandemic may also have resulted in incomplete data entry for IPD cases.
In Ontario, pneumococcal vaccine coverage, which is voluntarily reported by parents to local public health units and thus is likely an underestimate, was 77% in the 2018–2019 school year (prepandemic) and 74% in the 2021–2022 school year among children aged 7 years [33]. The significant decline in IPD incidence in children aged <5 years post–PCV13 introduction reflects the direct effects and success of Ontario's pediatric pneumococcal vaccination program. However, the burden of serotype 3 remains an issue, one that has also been seen in other Canadian provinces [34]. Additionally, while overall IPD case counts decreased post-PCV13, there was a significant shift in the serotypes causing IPD in these children, with a decrease of PCV13 serotypes and an increase in PCV15-non-PCV13 and PCV20-non-PCV15 serotypes. From 2013 to 2022, the proportion of additional cases that could potentially be averted due to all 7 additional serotypes being protected against in PCV20 but not PCV13 ranged between 32% and 53%. Of these 7, the 2 also found in PCV15, 22F and 33F, could potentially avert 8%–23% of overall cases, which suggests that the 5 additional serotypes included in PCV20 but not PCV15 also contributed substantially to the burden of IPD. Of these 5 serotypes, serotype 8 saw the largest increase in cases from the pre- to post-PCV13 era. These findings suggest that implementation of PCV15 or PCV20 in pediatric pneumococcal vaccination programs may help further reduce the burden of IPD in eligible children, but the impact may be greater with PCV20. Nonetheless, the question of serotype replacement remains. Few individuals aged ≥2 years who are eligible for pneumococcal vaccination (ie, living with underlying risk factors) are eligible for PCV13 outside catch-up programs, yet there was also a considerable reduction in IPD cases attributable to PCV13 serotypes in those aged ≥5 years. These findings suggest that older children, adolescents, and adults are experiencing indirect effects from the pediatric vaccination program. Indirect effects of pneumococcal pediatric programs have also been seen in other regions internationally [35–37]. Similar to children aged <5 years, circulating PCV13 serotypes appear as if they are being replaced by non-PCV13 serotypes in other age groups since the introduction of the pediatric program.
There was also a rise in IPD cases attributable to PPV23-non-PCV20 serotypes in those aged >5 years. The reduction of PCV13 serotypes could also be leading to an increase in PPV23-non-PCV20 serotypes due to replacement. This increase in PPV23-non-PCV20 serotypes could be as a result of eligible individuals not getting vaccinated or lack of effect from the PPV23 vaccine. Data were not available to estimate IPD rates specific to those aged 5–64 years and also eligible for the PPV23 vaccine, or to accurately estimate the proportion of individuals vaccinated against pneumococcal disease across Ontario. However, due to the low risk of severe disease among healthy individuals aged 5–64 years, it is likely that most IPD cases captured were among those at higher risk of disease, as has been seen by a network conducting enhanced IPD surveillance in 2 of the largest regions in Ontario [38, 39]. Self-reported adult pneumococcal vaccine uptake in Canada is collected through national coverage surveys and in 2023 was found to be quite low, at 42% among adults in Ontario aged ≥65 years or 18–64 years with at least 1 chronic health condition [40, 41]. Conjugate pneumococcal vaccines have superior effectiveness and a longer duration of protection compared with polysaccharide vaccines [42–44]. Introducing conjugate vaccines with more serotype coverage for adults aged ≥65 years and those at high risk of IPD and increasing vaccine coverage in these populations would likely further decrease the burden of disease. Older children and adults may also indirectly benefit from a pediatric program utilizing these vaccines, as was seen for PCV13.
Compared with the pre-PCV13 era, the CFR increased in children aged <5 years and adults aged 50–64 years in the post-PCV13 era, though the absolute number of fatalities was small. Though the CFR decreased in adults aged ≥65 years from pre– to post–PCV13 introduction, the rate of hospitalizations increased. These fluctuations in severity may partly be due to these observed changes in serotype distribution. For example, some serotypes that are protected against in PCV15 and PCV20 but not PCV13, including 8, 10A, 15BC, 22F, and 33F, may cause more severe clinical manifestations such as meningitis compared with PCV13 serotypes [45]. In our study, the proportion of deaths from pre- to post-PCV13 attributable to 22F increased by 4.9%, and those attributable to 11A increased by 2.2% among adults aged ≥65 years. Though there were few fatalities in children aged <5 years in the post-PCV13 era, more than half were attributable to the 7 additional serotypes protected against in PCV20, specifically 10A, 11A, 15BC, and 22F, but not PCV13.
Canada's NACI has a preferential recommendation for PCV20 for adults aged ≥65 years and ≥50 years living with underlying risk factors for IPD [8]. In cases where PCV20 is unavailable or inaccessible, PCV15 can be used but should be followed with a dose of PPV23 [8]. The preference for PCV20 over PCV15 was due to increased serotype coverage, the practical benefits of only requiring 1 vaccine product, and better cost-effectiveness [8]. PCV20 is also recommended for individuals aged ≤18 years who are at high risk of IPD [9]. Among healthy children aged <5 years, either PCV15 or PCV20 is recommended [9]. Unfortunately, it is unknown whether further serotype replacement will simply lead to other NVP serotypes, replacing the serotypes protected against in the higher-valency vaccines. All age groups also saw a significant rise in cases and fatalities attributable to NVP serotypes, suggesting that protection against additional serotypes is already needed. Many products are currently in development to help address this issue [46, 47], but additional strategies may also need to be considered. The significant reduction in IPD incidence rates seen during the pandemic years suggests that focusing efforts on reducing viral infections that may subsequently lead to infection by S. pneumoniae, such as increased efforts to vaccinate against influenza [48] and now RSV, could also be a strategy worth considering.
This study has some limitations. As Ontario uses a passive surveillance system to capture the burden of pneumococcal disease, it is likely that cases are underreported. This discrepancy can be caused by several factors, such as an individual's health care–seeking behaviors and differences in laboratory testing protocols. Our data may underestimate the overall IPD incidence and overestimate disease severity due to the nature of passive reporting, particularly during the pandemic years (eg, due to fewer health care visits and reduced capacity to enter cases into data systems, likely leading to the most severe cases being captured). Data regarding antimicrobial resistance are also absent and would have provided further insight for the increasing severity of vaccine-related serotypes [49]. Additionally, as previously mentioned, we lack data on accurate pneumococcal vaccine coverage for all ages in Ontario, making it difficult to make conclusions on the effect of vaccine uptake on incidence and hospitalization rates as well as changes in serotype distributions. We also lack data on risk factors and comorbidities, which limited our ability to gain a deeper understanding of IPD cases and the effect of these risk factors on severity of disease. Due to these data limitations, our analyses were conducted among all Ontarians aged 2–64 years without the ability to restrict to those who are eligible for high-risk pneumococcal vaccine programs in the province.
While the overall incidence of IPD in Ontario has generally remained unchanged, the PCV13 immunization program has led to a decline in IPD burden in children aged <5 years as well as strong effects on serotype distribution, as demonstrated by the shift in circulating serotypes. The incidence of IPD due to most PCV13 serotypes decreased after the introduction of PCV13 in 2010 across all ages. However, this decrease was largely offset by increasing incidence in other serotypes, and the burden of serotype 3 remains a problem. Ongoing surveillance of the circulating serotypes in Ontario is needed to continually monitor and evaluate pneumococcal vaccination programs as changes are made and as new vaccines are implemented. Moreover, data infrastructure such as a comprehensive immunization registry to allow for accurate vaccine coverage estimation in all ages is necessary to evaluate these programs effectively.
Supplementary Material
Acknowledgments
The authors thank Ontario's public health units for their continued commitment to invasive pneumococcal disease case management, surveillance, and reporting. The authors also thank colleagues at the Public Health Ontario Laboratory, the National Microbiology Laboratory, and the Toronto Invasive Bacterial Diseases Network for their laboratory support in S. pneumoniae identification and serotyping. The data used in this analysis contain potentially identifying and sensitive patient information; therefore, Public Health Ontario (PHO) cannot disclose the underlying data. Doing so would compromise individual privacy, which is contrary to PHO's ethical and legal obligations. Restricted access to the data may be available under conditions prescribed by the Ontario Personal Health Information Protection Act, 2004, the Ontario Freedom of Information and Protection of Privacy Act, the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2 (2014)), and PHO privacy and ethical policies. Data are available for researchers who meet PHO's criteria for access to confidential data. Further information about PHO's data access request process is available online at https://www.publichealthontario.ca/en/data-and-analysis/using-data/data-requests.
Patient consent. This manuscript does not include factors necessitating patient consent.
Financial support. This work was supported by Public Health Ontario.
Contributor Information
Ramandip Grewal, Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Centre for Vaccine Preventable Diseases, University of Toronto, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Kelty Hillier, Health Protection, Public Health Ontario, Toronto, Ontario, Canada.
Shelley L Deeks, Department of Health and Wellness, Nova Scotia, Halifax, Nova Scotia, Canada.
Allison H Yeung, Centre for Immunization Surveillance, Public Health Agency of Canada, Ottawa, Ontario, Canada.
Sarah E Wilson, Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Centre for Vaccine Preventable Diseases, University of Toronto, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Shinthuja Wijayasri, Centre for Immunization Surveillance, Public Health Agency of Canada, Ottawa, Ontario, Canada.
Tara M Harris, Health Protection, Public Health Ontario, Toronto, Ontario, Canada.
Sarah A Buchan, Health Protection, Public Health Ontario, Toronto, Ontario, Canada; Centre for Vaccine Preventable Diseases, University of Toronto, Toronto, Ontario, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893–902. [DOI] [PubMed] [Google Scholar]
- 2. Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 2014; 20(S5):45–51. [DOI] [PubMed] [Google Scholar]
- 3. Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Government of Ontario . Ontario demographic quarterly: highlights of third quarter. 2024. Available at: http://www.ontario.ca/page/ontario-demographic-quarterly-highlights-third-quarter. Accessed March 27, 2024.
- 5. Ontario Ministry of Health and Long-Term Care . Publicly Funded Immunization Schedules for Ontario June 2022. Ontario Ministry of Health; 2022. [Google Scholar]
- 6. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Factors Affecting Reportable Diseases in Ontario Case Definition Changes and Associated Trends 1991–2016. Ontario Ministry of Health; 2018.
- 7. Ontario Ministry of Health and Long-Term Care . Guidance for Routine & Catch-Up Immunization Services. Ontario Ministry of Health; 2023. [Google Scholar]
- 8. National Advisory Committee on Immunization . Use of Pneumococcal Vaccines in Adults, Including the Use of 15-Valent and 20-Valent Conjugate Vaccines: NACI Public Health Level Recommendations. National Advisory Committee on Immunization; 2023. [Google Scholar]
- 9. National Advisory Committee on Immunization . Recommendations for Public Health Programs on the Use of Pneumococcal Vaccines in Children, Including the Use of 15-Valent and 20-Valent Conjugate Vaccines. National Advisory Committee on Immunization; 2024. [Google Scholar]
- 10. Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007–2017. PLoS One 2019; 14:e0226353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nasreen S, Wang J, Kwong JC, et al. Population-based incidence of invasive pneumococcal disease in children and adults in Ontario and British Columbia, 2002–2018: a Canadian Immunization Research Network (CIRN) study. Vaccine 2021; 39:7545–53. [DOI] [PubMed] [Google Scholar]
- 12. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease following pneumococcal vaccination: a discussion of the evidence. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 14. van der Linden M, Perniciaro S, Imöhl M. Increase of serotypes 15A and 23B in IPD in Germany in the PCV13 vaccination era. BMC Infect Dis 2015; 15:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tin Tin Htar M, Morato Martínez J, Theilacker C, Schmitt HJ, Swerdlow D. Serotype evolution in Western Europe: perspectives on invasive pneumococcal diseases (IPD). Expert Rev Vaccines 2019; 18:1145–55. [DOI] [PubMed] [Google Scholar]
- 16. Kandasamy R, Voysey M, Collins S, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis 2020; 221:1361–70. [DOI] [PubMed] [Google Scholar]
- 17. Ontario Agency for Health Protection and Promotion, Public Health Ontario . iPHIS User Guide: Vaccine Preventable Diseases. Queen's Printer for Ontario; 2019.
- 18. Ontario Ministry of Health and Long-Term Care . Ontario Public Health Standards: Requirements for Programs, Services and Accountability. Appendix 1: Case Definitions and Disease-Specific Information. Ontario Ministry of Health; 2022. Available at: https://files.ontario.ca/moh-ophs-pneumococcal-disease-en-2022.pdf. [Google Scholar]
- 19. Desai S, Policarpio ME, Wong K, Gubbay J, Fediurek J, Deeks S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: a population-based study. CMAJ Open 2016; 4:E545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Population Reporting . Population Estimates County/Municipality, 1986–2021. Statistics Canada; 2023. [Google Scholar]
- 21. Population Reporting . Population Projections Public Health Unit, 2022–2046. Ontario Ministry of Finance; 2023. [Google Scholar]
- 22. Ricketson LJ, Kellner JD. Changes in the incidence of invasive pneumococcal disease in Calgary, Canada, during the SARS-CoV-2 pandemic 2020–2022. Microorganisms 2023; 11:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izu A, Nunes MC, Solomon F, et al. All-cause and pathogen-specific lower respiratory tract infection hospital admissions in children younger than 5 years during the COVID-19 pandemic (2020–22) compared with the pre-pandemic period (2015–19) in South Africa: an observational study. Lancet Infect Dis 2023; 23:1031–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciruela P, Soldevila N, García-Garcia JJ, et al. Effect of COVID-19 pandemic on invasive pneumococcal disease in children, Catalonia, Spain. Emerg Infect Dis 2022; 28:2321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine 2009; 27:C9–14. [DOI] [PubMed] [Google Scholar]
- 26. Ouldali N, Deceuninck G, Lefebvre B, et al. Increase of invasive pneumococcal disease in children temporally associated with RSV outbreak in Quebec: a time-series analysis. Lancet Reg Health Am 2023; 19:100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med 2015; 12:e1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munkstrup C, Lomholt FK, Emborg HD, et al. Early and intense epidemic of respiratory syncytial virus (RSV) in Denmark, August to December 2022. Euro Surveill 2023; 28:2200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meng L, Bruce L, Buba M, et al. Paediatric health system impact of an early respiratory viral season in Eastern Ontario, Canada: a descriptive analysis. Paediatr Child Health. 2023:pxad082. [Google Scholar]
- 30. Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR 2023; 72:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glazier RH, Green ME, Wu FC, Frymire E, Kopp A, Kiran T. Shifts in office and virtual primary care during the early COVID-19 pandemic in Ontario, Canada. CMAJ 2021; 193:E200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeitouny S, Cheung DC, Bremner KE, et al. The impact of the early COVID-19 pandemic on healthcare system resource use and costs in two provinces in Canada: an interrupted time series analysis. PLoS One 2023; 18:e0290646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ontario Agency for Health Protection and Promotion, Public Health Ontario . Immunization Coverage Report for Routine Infant and Childhood Programs in Ontario: 2019–20, 2020–21 and 2021–22 School Years. Queen's Printer for Ontario; 2023. [Google Scholar]
- 34. Deceuninck G, Brousseau N, Lefebvre B, et al. Effectiveness of thirteen-valent pneumococcal conjugate vaccine to prevent serotype 3 invasive pneumococcal disease in Quebec in children, Canada. Vaccine 2023; 41:5486–9. [DOI] [PubMed] [Google Scholar]
- 35. Ciruela P, Broner S, Izquierdo C, et al. Indirect effects of paediatric conjugate vaccines on invasive pneumococcal disease in older adults. Int J Infect Dis 2019; 86:122–30. [DOI] [PubMed] [Google Scholar]
- 36. Hanquet G, Krizova P, Valentiner-Branth P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax 2019; 74:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kislaya I, Rodrigues AP, Sousa-Uva M, et al. Indirect effect of 7-valent and 13-valent pneumococcal conjugated vaccines on pneumococcal pneumonia hospitalizations in elderly. PLoS One 2019; 14:e0209428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ontario Agency for Health Protection and Promotion, Public Health Ontario . Recommendations: New Health Canada Authorized Pneumococcal Conjugate Vaccines for Adults Aged ≥18 Years. Queen's Printer for Ontario; 2023. [Google Scholar]
- 39. Shigayeva A, Rudnick W, Green K, et al. Invasive pneumococcal disease among immunocompromised persons: implications for vaccination programs. Clin Infect Dis 2016; 62:139–47. [DOI] [PubMed] [Google Scholar]
- 40. Public Health Agency of Canada . Vaccine uptake in Canadian adults: highlights from the 2020–2021 Seasonal Influenza Vaccination Coverage Survey. 2021. Available at: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/highlights-2020–2021-seasonal-influenza-survey.html. Accessed August 9, 2023.
- 41. Public Health Agency of Canada . Adult National Immunization Coverage Survey (aNICS): 2023 results. 2024. Available at: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/adult-national-immunization-coverage-survey-2023-results.html. Accessed March 27, 2024.
- 42. Berger A. Science commentary: why conjugate vaccines protect longer. BMJ 1998; 316:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 44. Latifi-Navid H, Latifi-Navid S, Mostafaiy B, Jamalkandi SA, Ahmadi A. Pneumococcal disease and the effectiveness of the PPV23 vaccine in adults: a two-stage Bayesian meta-analysis of observational and RCT reports. Sci Rep 2018; 8:11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grant LR, Slack MPE, Theilacker C, et al. Distribution of serotypes causing invasive pneumococcal disease in children from high-income countries and the impact of pediatric pneumococcal vaccination. Clin Infect Dis 2023; 76:e1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masomian M, Ahmad Z, Ti Gew L, Poh CL. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines (Basel) 2020; 8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chichili GR, Smulders R, Santos V, et al. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022; 40:4190–8. [DOI] [PubMed] [Google Scholar]
- 48. Smith AM, Huber VC. The unexpected impact of vaccines on secondary bacterial infections following influenza. Viral Immunol 2018; 31:159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mohanty S, Feemster K, Yu KC, Watts JA, Gupta V. Trends in Streptococcus pneumoniae antimicrobial resistance in US children: a multicenter evaluation. Open Forum Infect Dis 2023; 10:ofad098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.