Abstract
Background
Adolescent idiopathic scoliosis (AIS) is a three‐dimensional deformity of the spine. While AIS can progress during growth and cause a surface deformity, it is usually not symptomatic. However, if the final spinal curvature surpasses a certain critical threshold, the risk of health problems and curve progression is increased. Interventions for the prevention of AIS progression include scoliosis‐specific exercises, bracing, and surgery. The main aims of all types of interventions are to correct the deformity and prevent further deterioration of the curve and to restore trunk asymmetry and balance, while minimising morbidity and pain, allowing return to full function. Surgery is normally recommended for curvatures exceeding 40 to 50 degrees to stop curvature progression with a view to achieving better truncal balance and cosmesis. Short‐term results of the surgical treatment of people with AIS demonstrate the ability of surgery to improve various outcome measures. However there is a clear paucity of information on long‐term follow‐up of surgical treatment of people with AIS.
Objectives
To examine the impact of surgical versus non‐surgical interventions in people with AIS who have severe curves of over 45 degrees, with a focus on trunk balance, progression of scoliosis, cosmetic issues, quality of life, disability, psychological issues, back pain, and adverse effects, at both the short term (a few months) and the long term (over 20 years).
Search methods
We searched the Cochrane Back Review Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, four other databases, and three trials registers up to August 2014 with no language limitations. We also checked the reference lists of relevant articles and conducted an extensive handsearch of the grey literature.
Selection criteria
We searched for randomised controlled trials (RCTs) and prospective controlled trials comparing spinal fusion surgery with non‐surgical interventions in people with AIS with a Cobb angle greater than 45 degrees. We were interested in all types of instrumented surgical interventions with fusion that aimed to provide curve correction and spine stabilisation.
Data collection and analysis
We found no RCTs or prospective controlled trials that met our inclusion criteria.
Main results
We did not identify any evidence comparing surgical to non‐surgical interventions for AIS with severe curves of over 45 degrees.
Authors' conclusions
We cannot draw any conclusions.
Keywords: Adolescent, Humans, Scoliosis, Scoliosis/therapy
Plain language summary
Surgical treatment compared to non‐surgical treatment (braces, exercise, or observation) for teens with idiopathic scoliosis
Background
Scoliosis is a condition where the spine is curved in three dimensions (from the back the spine appears to be shaped like a 'c' or an 's'). It is often idiopathic, or of unknown cause. The most common type of scoliosis, adolescent idiopathic scoliosis (AIS), is discovered around 10 years of age or older, and is defined as a curve that measures at least 10 degrees (known as a Cobb angle, which is measured on an x‐ray).
People with AIS usually have no symptoms, however the resulting surface deformity frequently negatively impacts adolescents. In addition, increased curvature of the spine can present health risks in adulthood. Different types of treatment, including physical therapy, bracing, and surgery, are advocated depending on the magnitude of the curvature and area affected, truncal balance, general health, level of function and satisfaction, and patient’s and parent’s treatment desire.
Surgery is normally recommended in curvatures exceeding 40 to 50 degrees to stop the progression of the curvature. Short‐term results of surgical treatment are improvements on outcome measures relating to self image, some functional aspects, and pain. However, the structured long‐term follow‐up needed to make meaningful conclusions is lacking. Recent papers highlight the long‐term complications of surgery, while other papers postulate that the medium‐ and long‐term complication rates following modern scoliosis surgery are low when compared to older techniques.
Study characteristics
We searched the literature for both randomised controlled trials and prospective non‐randomised studies with a control group examining the effects of surgical versus non‐surgical treatments for teens with idiopathic scoliosis. The evidence is current to August 2014.
Key results
We identified no evidence examining the effectiveness of surgical interventions compared to non‐surgical interventions for people with AIS. As a result, we cannot draw any conclusions regarding the benefits or harms of these treatments.
Summary of findings
for the main comparison.
| Surgical interventions compared with non‐surgical Interventions in people with adolescent idiopathic scoliosis | ||||||
|
Patient or population: Adolescent Idiopathic Scoliosis Settings: Anywhere Intervention: Surgical intervention Comparison: Non‐surgical intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐surgical interventions | Surgical Interventions | |||||
| Changes in trunk balance | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Progression of scoliosis | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Cosmetic issues | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Quality of life | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Back pain | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Disability | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| Psychological issues | See comment | See comment | Not estimable | 0 | See comment | We identified no randomised or non‐randomised prospective studies with a control group. |
| CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Scoliosis is a complex, three‐dimensional deformity of the spine that comprises a lateral curvature in the frontal plane (this is a vertical plane that divides the body into front and back halves), thoracic lordosis in the sagittal plane (this is a vertical plane that divides the body into right and left halves) and a posterior rib hump, which is produced by rotation of the vertebrae in the transverse plane (horizontal plane); this results in the posterior elevation of the rib cage on the convex side of the curve and a depression on the concave side (Bradford 1987). These underlying skeletal changes are usually reflected by a change in back shape, the unsightly shape of which is generally more of a concern to the patient than is the underlying skeletal deformity (White 1990). If left untreated, the condition results in altered spinal mechanics and degenerative changes that lead to pain, loss of spinal mobility, and possible loss of function or disability. Cardiac and respiratory dysfunction may accompany these symptoms, depending on the time of onset of the deformity (White 1990). There are also psychological consequences that result from the unsightly and deformed shape of the back: A restricted social life, lower marriage rate, higher divorce rate, fewer children per marriage, and increased psychiatric consultations, including eating disorders and increased suicide rate, have all been reported (Freidel 2002).
Scoliosis can also occur secondary to certain diseases and conditions that affect the nervous and muscular systems of the body. However, most cases of scoliosis (80% to 90%) are 'idiopathic', meaning the underlying cause is unknown. The most common form of scoliosis is adolescent idiopathic scoliosis (AIS), which appears in late childhood or adolescence ‐‐ a period of rapid growth (Kanayama 1996; Stokes 1996). According to the Scoliosis Research Society and the International Society on Scoliosis Orthopaedic Rehabilitation and Treatment (Negrini 2012), the prevalence of AIS in the general population is 2% to 3%. Nearly 10% of people with AIS will require some form of treatment, and up to 0.1% will eventually require surgery (Lonstein 2006).
AIS is more common in females. While it does not typically cause any health problems during growth, the resulting surface deformity frequently has a negative impact on adolescents that can give rise to quality‐of‐life issues and, in the worst cases, psychological disturbances (Reichel 2003). As the condition is most often painless, early diagnosis is difficult, especially in countries where school scoliosis screening is not implemented. External change to the body shape is minimal in the early stages, and most changes in back shape occur predominantly on the back of the trunk, which is difficult for patients to see, and can be concealed by clothing (Roaf 1980). Treatment of AIS is determined by the deformity itself. As most people with AIS progress during growth, the main aims of all interventions are to limit or stop the curvature progression, restore trunk balance (Goldberg 2002; Asher 2006), and prevent the long‐term consequences of the deformity.
Description of the intervention
Interventions for the prevention of AIS progression include scoliosis‐specific exercises, bracing, and surgery (Lenssinck 2005; Negrini 2005; Rowe 1997; Weiss 2006a). The goals of all interventions are to correct the deformity and prevent further deterioration of the curve (that is prevent progression) and to restore trunk symmetry and balance, while minimising morbidity and pain, allowing return to full function (Bridwell 1999; Lonstein 2006).
Non‐surgical interventions
Scoliosis‐specific exercises consist of individually adapted exercises that are taught to patients in a centre that is dedicated to scoliosis treatment. Patients learn an exercise protocol that is personalised according to their own medical and physiotherapeutic evaluation.
On the other hand, usual generalised physiotherapy is more generic, consisting of low‐impact stretching and strengthening activities like yoga, but can include many different exercise protocols.
Bracing is defined as the application of external supports to the trunk; these are usually rigid and are applied with the aim of achieving maximum correction of the pathological curve (Rigo 2006a). Treatment commences when the curve is diagnosed as progressive, or when it exceeds a threshold of 30 degrees Cobb angle (Lonstein 2006; Negrini 2005; Weiss 2006a). Braces generally need to be worn for a considerable period of time per day (at least 20 hours) over several years until the end of bone growth, which usually occurs at 16 years of age for girls and 18 years of age for boys (Katz 2001). This causes a significant negative impact on the quality of life of children and adolescents (Climent 1999; Fallstrom 1986; Noonan 1997).
Surgical interventions
Fusion is generally considered to be necessary when AIS exceeds a certain degree (approximately 45 to 50 degrees), when previous treatments have failed, or when AIS causes symptoms, but indications vary widely according to the preference of the treating physician/surgeon (Dolan 2007). The literature describes a large multitude and variety of interventions (Maruyama 2008), including different surgical approaches (posterior, anterior, or combined) and various types of metal implant systems.
Posterior fusion with instrumentation has long been a standard for the surgical treatment of AIS (Lonner 2007; Maruyama 2008). The Harrington technique and instrumentation was introduced in the 1960s, followed by the Cotrel‐Dubousset instrumentation technique in 1980s (Kadoury 2009). The last two decades have seen a gradual progression or shift of usage from hook‐based instrumentation systems to hybrid‐based systems (hooks and pedicle screws) to all‐pedicle screw systems with more anchors to the spine. This shift has enabled greater and better anchorage and correction manoeuvres and reduced the implant failure rate (Olgun 2013).
Anterior instrumentation surgery was previously favoured for thoracolumbar and lumbar scoliosis, as it enabled shorter fusion levels. However, since the recent introduction and usage of all‐pedicle screw construct posterior surgery, the use of anterior surgery has been limited (Maruyama 2008). Modern surgical techniques follow the principles of segmental spinal instrumentation, where each vertebra of the spine is attached to a metal rod by means of screws or hooks.
Modern surgical techniques are believed to reliably provide a better arrest of progression of the deformity and achieve greater correction of the curves in the coronal plane (30% to 90%) with minimal loss at follow‐up (Westrick 2011). Furthermore, these techniques also achieve a spontaneous partial correction of the compensatory curves and improve appearance. A recent prospective multicentre study on the outcomes of surgical treatment using participant‐based outcomes demonstrates the ability of surgery to improve some outcome measures at the two‐year postsurgical follow‐up (Merola 2002). However, there is a lack of high‐quality prospective studies with a control group on long‐term follow‐up.
All types of spinal fusion surgery are associated with significant risk, both in the short term and long term. The short‐term risk for spinal fusion surgery is estimated to be approximately 5%, while long‐term risks over a lifetime are estimated to be much higher (Weiss 2008a), with reoperation rates ranging from 6% to 29% (Asher 2006). A recently published meta‐analysis on 1565 participants with a mean follow‐up of 14.9 years demonstrated a reoperation rate between 0% to 8.3% (Lykissas 2013), the highest incidence being related to the use of Harrington rods and the lowest to all‐pedicle screws. The same study reported a mean infection rate of 3.6%, the lowest incidence being encountered with the use of all‐pedicle screws (1.18%) and the highest with Harrington rods (5.5%).
How the intervention might work
Scoliosis‐specific exercises can be used in three main clinical scenarios: (1) as the primary treatment of AIS for mild curves, (2) in conjunction with braces for moderate curves, and (3) during adulthood if the scoliosis curves exceed certain thresholds (Romano 2012). In the treatment of mild scoliosis, scoliosis‐specific exercises can be used on curves greater than 10 to 15 degrees but less than 25 or 30 degrees Cobb. These intense three‐dimensional spine‐and‐rib‐cage‐specific exercises are used to try to limit the progression of the curve and thereby avoid the need for a brace. This critical Cobb angle is generally regarded as the threshold for brace prescription (Lonstein 2006; Weiss 2006b). The key objectives of physical exercise in mild cases of AIS include stabilisation of the spine, three‐dimensional auto‐correction of the pelvis, rib cage, and shoulders together with isometric muscle contractions (Romano 2012; Weiss 2006b).
Braces use external corrective forces to correct the trunk; this is usually achieved with the use of rigid supports. However, some braces (known as soft braces) are made of material similar to elastic bands that is comparable with materials used in physical therapy treatments (Coillard 2003; Rigo 2006a). The mechanical forces of the brace are used to straighten the spine and de‐rotate the pelvis and shoulders to bring the whole body into normal alignment. Negrini 2010 states that the external and proprioceptive inputs due to bracing change the unnatural loading on the spine and rib cage, decrease asymmetrical movements, and improve neuromuscular control; this facilitates proper spinal growth, neuromotor reorganization, and changes in motor behaviours (Castro 2003; Coillard 2002; Grivas 2008; Lupparelli 2002; Negrini 2006c; Odermatt 2003; Smania 2008; Stokes 2006; Weiss 2004). Unfortunately, most braces have the disadvantage of not being very comfortable to wear, especially for long periods. Furthermore, weakening of the back muscles may occur if bracing is not combined with scoliosis‐specific exercises.
The aim of surgical treatment of the scoliotic spine is to correct the spinal curvature (reduction of the Cobb angle) and fuse the spine with the help of bone grafts that allow the spine to heal to a solid and stable bone fusion mass (spinal fusion), supported by the instrumentation (Haher 2003).
Why it is important to do this review
After conducting a comprehensive literature search, we identified only two key reviews that addressed surgical versus non‐surgical interventions for AIS (Hawes 2006a; Weiss 2008b). Hawes 2006a was a very comprehensive narrative review, and hence had all the biases and limitations generally associated with such reviews (Bettany‐Saltikov 2012). Weiss 2008b, although entitled a "systematic review", did not in actuality meet the criteria for a systematic review; the search strategy as well as the precise methods used to identify and select papers were not detailed. Furthermore, it was unclear which primary papers were included and/or excluded, and the processes for the methodological evaluation and data extraction of included primary papers, if undertaken, were not described.
The significant limitations of these two reviews strongly highlighted the need for a state‐of‐the‐art Cochrane review. Additionally, it was important to undertake this review so that people with AIS with severe curves and their parents understand both the short‐ and long‐term effects, as well as the side effects and consequences, of surgical and non‐surgical interventions, and can make evidence‐based decisions.
Objectives
To examine the impact of surgical versus non‐surgical interventions in people with AIS who have severe curves of over 45 degrees, with a focus on trunk balance, progression of scoliosis, cosmetic issues, quality of life, disability, psychological issues, back pain, and adverse effects, at both the short term (a few months) and the long term (over 20 years).
Methods
Criteria for considering studies for this review
Types of studies
For the primary analysis we intended to combine the results of randomised control trials (RCTs) and quasi‐randomised control trials (QRCTs). We also planned to include prospective non‐randomised studies (NRSs) with a control group because we anticipated finding very few RCTs. We planned to include primary studies that compared surgical interventions with non‐surgical interventions.
Types of participants
We intended to include people with AIS who were diagnosed and managed when they were between 10 and 18 years of age, with a Cobb angle greater than 45 degrees (Negrini 2012). We planned to exclude studies on participants with early‐onset scoliosis (infant or juvenile) and scoliosis secondary to other conditions.
Types of interventions
We intended to include all types of instrumented surgical interventions with fusion that aimed to provide curve correction and spine stabilisation. We planned to exclude studies describing non‐instrumented spinal correction and fusion, because the outcomes these interventions provide have been shown to be no better than those seen with untreated scoliosis (Bradford 1987).
We aimed to compare instrumented surgical interventions with different types of non‐surgical treatments, such as scoliosis‐specific exercises, bracing, physiotherapy, chiropractic treatment, electrical stimulation, and other non‐surgical interventions for people with AIS with severe curves of over 45 degrees.
Types of outcome measures
We planned to examine outcomes (primary and secondary) measured in the immediate term (perioperative to six weeks postoperative), the short term (results at the end of bone growth), within two years, and over the long term (results in adulthood and in old age).
Primary outcomes
-
Change in trunk balance, measured in centimetres:
Frontal (coronal) balance (refers to the plane that divides the body into front and back halves);
Lateral trunk shift; and
Apical vertebral translation.
-
Progression of scoliosis, measured by:
Cobb angle in degrees (absolute values);
Angle of trunk rotation in degrees (absolute values); and
Number of participants who progressed by more than 5 degrees Cobb (5 degrees Cobb is the standard clinical measure reported within various research papers and commonly used in clinical practice).
-
Cosmetic issues, as measured by:
Validated scales or questionnaires: Walter Reed Visual Assessment Scale (Pineda 2006), Spinal Appearance Questionnaire (Sanders 2007), Trunk Appearance Perception Scale (Bago 2010); and
Topographic measurements: Integrated Shape Imaging System or Integrated Shape Imaging System 2 (Berryman 2008), Quantec (Oxborrow 2000), Formetric (Knott 2010), measured in angles and millimetres.
-
Quality of life as measured by:
Generic questionnaires: 36‐Item Short Form Health Survey;
Scoliosis‐specific questionnaires: Scoliosis Research Society‐22 (Asher 2003);
Bad Sobernheim Stress Questionnaire (Weiss 2006b); and
Brace Questionnaire (Vasiliadis 2006).
-
Psychological issues as measured by:
Specific psychological questionnaires evaluating such concepts as self esteem, self image, etc., using specific questionnaires and subscales of Scoliosis Research Society‐22, Brace Questionnaire, 36‐Item Short Form Health Survey.
-
Back pain and disability as measured by:
Validated scales measuring pain intensity and pain duration, such as the visual analogue scale, McGill Pain Questionnaire, and other validated specific questionnaires, as well as use of medication.
Secondary outcomes
Secondary outcomes were any adverse effects reported in any of the included studies, such as blood loss, pseudarthrosis (a false joint where the bone has not healed adequately), deep wound infection, neurological complications, delayed infections, pedicle screw misplacement, delayed paraparesis (weakness or partial paralysis in the lower limbs) as well as the loss of normal spinal function and decompensation (spinal imbalance), increased spinal deformity and death. We planned to report any adverse events in our review, whether or not they were listed here.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases from inception to August 2014.
Cochrane Back Review Group Trials Register (Cochrane Register of Studies (CRS))
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library)
MEDLINE (OvidSP, 1946 to July Week 5, 2014) and MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP, 7 August 2014)
EMBASE (OvidSP, 1980 to 2014 Week 31)
Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO, 1981 to August 2014)
PsycINFO (OvidSP, 2002 to August Week 1, 2014)
Physiotherapy Evidence Database (PEDro, 1929 to August 2014)
PubMed (1946 to August 2014)
The search strategy combined the study design filter for observational studies adapted from the Scottish Intercollegiate Guidelines Network with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE, so that the search captured all study designs. We combined the study design terms with blocks of search terms for the disorder and the interventions. The strategy included subject headings (for example MeSH) and was adapted for the other databases (see Appendix 1 and Appendix 2).
Searching other resources
The following strategies were also used.
Screening the reference lists of all relevant papers.
Searching the main electronic sources of ongoing trials: UK Clinical Trials Gateway (UKCTG), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (see Appendix 2).
Searching the grey literature, including conference proceedings and PhD theses completed since 1980. For the latter, we searched the Electronic Theses Online Service (EThOS) provided by the British Library, which is an ‘open access single point digital repository of UK research theses‘. See Appendix 2 for the EThOS search strategy.
Contacting investigators and authors in the field for information on unpublished or incomplete trials.
All searches included non‐English language literature.
Data collection and analysis
Selection of studies
We first developed a study selection form on the basis of the inclusion criteria. This was piloted and tested for both intraobserver and interobserver reliability by two review authors, who then independently screened the search results by reading the titles and abstracts. Potentially relevant studies were obtained in full text, and once again they were independently assessed for inclusion by two review authors, who resolved any disagreement through discussion. A third review author was contacted if disagreements persisted. If a review author was also the author of a paper, another review author who had not authored any of the papers undertook the selection.
We did not select any papers before 1980 because research done on older instrumentation may not have been relevant. Although clear advances in the materials and the design of spinal instrumentation have been made since 1980, the surgical approach and training may still be the same.
We did not find any relevant RCTs, QRCTs, or NRSs with a control group. As such, we were unable to carry out most of the pre‐stated methodology. We have therefore described the planned methods in Appendix 3.
Data extraction and management
See Appendix 3 for planned methodology.
Assessment of risk of bias in included studies
See Appendix 3 for planned methodology.
Measures of treatment effect
See Appendix 3 for planned methodology.
Unit of analysis issues
See Appendix 3 for planned methodology.
Dealing with missing data
See Appendix 3 for planned methodology.
Assessment of heterogeneity
See Appendix 3 for planned methodology.
Assessment of reporting biases
See Appendix 3 for planned methodology.
Data synthesis
See Appendix 3 for planned methodology.
Subgroup analysis and investigation of heterogeneity
See Appendix 5 for planned methodology.
Sensitivity analysis
See Appendix 3 for planned methodology.
Clinical relevance of results
See Appendix 3 for planned methodology.
Results
Description of studies
Results of the search
Our search of databases identified 4798 records. After screening the records, we assessed eight full‐text articles, which we excluded because they were either retrospective cohort studies with a control group or retrospective case studies with a control group. There were no prospective studies with a control group (see study flow diagram, Figure 1).
1.
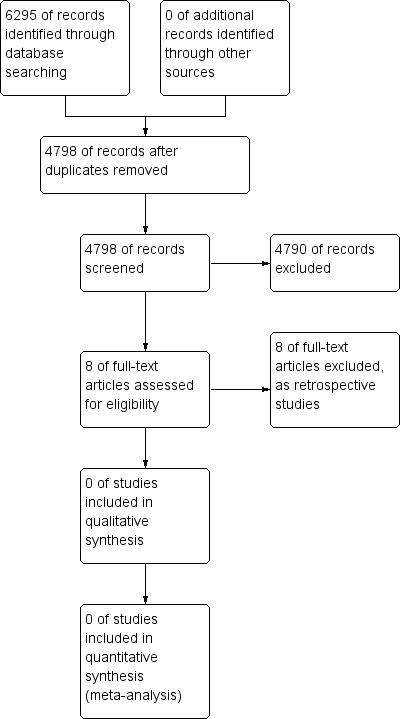
Study flow diagram.
Excluded studies
The eight studies we excluded were all retrospective studies with a control group (Anderson 2006; Bunge 2007; Danielsson 2001; Danielsson 2001a; Danielsson 2001b; Danielsson 2003; Danielsson 2006; Pehrsson 2001). See Characteristics of excluded studies tables.
Risk of bias in included studies
We evaluated no studies for methodological quality.
Effects of interventions
See: Table 1
We did not identify any evidence comparing surgical to non‐surgical interventions for people with AIS with severe curves of over 45 degrees (Table 1)
Discussion
Summary of main results
We did not identify any evidence of effectiveness of surgical compared to non‐surgical interventions for people with AIS with severe curves of over 45 degrees.
Overall completeness and applicability of evidence
This review is technically considered to be an 'empty review' as we did not find any studies that met all the inclusion criteria. Nonetheless, this review provides important information regarding research gaps in this field, which is consistent with previously published reviews on this topic (Weiss 2008c; Weiss 2013).
Quality of the evidence
There is currently no randomised or non‐randomised trial‐based evidence from prospective studies with a control group comparing the effectiveness of surgical to non‐surgical interventions for people with AIS with severe curves of over 45 degrees.
Potential biases in the review process
The most important potential bias is having missed any prospective controlled studies. However, given our extensive literature search, it is unlikely that we have missed any studies.
Agreements and disagreements with other studies or reviews
There is currently no randomised or non‐randomised trial‐based evidence from prospective studies with a control group that could be compared with other studies.
Authors' conclusions
Implications for practice.
In conclusion, we did not identify any evidence comparing surgical to non‐surgical interventions for people with AIS with severe curves of over 45 degrees. We were therefore unable to draw any conclusions in this review.
Implications for research.
In conclusion, we did not identify any evidence comparing surgical to non‐surgical interventions for people with AIS with severe curves of over 45 degrees. We were therefore unable to draw any conclusions in this review.
Acknowledgements
We would like to thank Rachel Couban, Cochrane Back Review Group Trials Search Co‐ordinator, for her help with development of the search strategies. We would also like to thank Dr Teresa Marin for her help with all our queries in completing this review.
Appendices
Appendix 1. CBRG Trials Register, CENTRAL, MEDLINE, EMBASE and CINAHL search strategies
CBRG Trials Register
Last searched 11 August, 2014
#1 scoliosis
CENTRAL
Last searched 8 August, 2014
#1 MeSH descriptor: [Scoliosis] explode all trees
#2 scoliosis:ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Orthopedic Procedures] explode all trees
#5 MeSH descriptor: [Orthopedic Fixation Devices] explode all trees
#6 "spine fusion" or "spinal fusion" or "spinal instrumentation" or spondylodesis:ti,ab,kw (Word variations have been searched)
#7 surg* or operat* or realign* or screw* or hybrid or wire* or hook* or sublaminar:ti,ab,kw (Word variations have been searched)
#8 #4 or #5 or #6 or #7
#9 MeSH descriptor: [Orthotic Devices] explode all trees
#10 braces:ti,ab,kw (Word variations have been searched)
#11 bracing:ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Exercise] explode all trees
#13 MeSH descriptor: [Physical Therapy Modalities] explode all trees
#14 MeSH descriptor: [Rehabilitation] explode all trees
#15 MeSH descriptor: [Drug Therapy] explode all trees
#16 non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv* or taping or tape* or immobilis* or immobiliz* or therap* or electrotherap*:ti,ab,kw (Word variations have been searched)
#17 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #3 and #8 and #17 Publication Year from 2013 to 2014, in Trials
MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations
Last searched 8 August 2014
exp Spinal Diseases/
Scoliosis/
scoliosis.mp.
or/1‐3
Orthopedics/
exp Surgical Procedures, Operative/
surgery.fs.
surg$.tw.
operat$.tw.
realign$.tw.
spondylodesis.tw.
spine fusion.tw.
spinal fusion.tw.
spinal instrumentation.tw.
Bone Screws/
screw$.tw.
hybrid.tw.
Bone Wires/
sublaminar.tw.
wire$.tw.
hook$.tw.
or/5‐21
exp Rehabilitation/
rehabilit$.tw.
rehabilitation.fs.
exp Physical Therapy Modalities/
Physical Therapy Speciality.mp.
physiotherapy.tw.
physical therapy.tw.
exp Exercise/
exercise$.tw.
Exercise Movement Techniques/
exp Exercise Therapy/ (30988)
exp Musculoskeletal Manipulations/
Immobilization/
Braces/
brace$.mp.
bracing.mp.
exp Orthotic Devices/
Orthopedic Equipment/
limit 40 to yr="1902 ‐ 1975"
(non‐surg$ or nonsurg$ or non‐operat$ or nonoperat$ or conserv$).tw.
(immobilis$ or immobiliz$ or therap$ or taping or tape$ or electrotherapy$).tw.
or/23‐43
4 and 22 and 44
limit 45 to adolescent <13 to 18 years>
Adolescent/
adolescen$.mp.
47 or 48
45 and 49
46 or 50
Comparative Study/
exp Evaluation Studies/
exp Follow‐Up Studies/
exp Prospective Studies/
exp Cross‐Over Studies/
exp Epidemiologic Studies/
exp Case‐Control Studies/
exp Cohort Studies/
exp Cross‐Sectional Studies/
(cohort adj (study or studies)).mp.
cohort analy$.mp.
(follow up adj (study or studies)).mp.
(observational adj (study or studies)).mp.
longitudinal.mp.
retrospective.mp.
cross sectional.mp.
control$.mp.
prospective$.mp.
volunteer.mp.
or/52‐70
randomized controlled trial.pt.
controlled clinical trial.pt.
randomi#ed.ti,ab.
placebo.ti,ab.
drug therapy.fs.
randomly.ti,ab.
trial.ti,ab.
groups.ti,ab.
or/72‐79
(Animals not (Humans and Animals)).sh.
80 not 81
71 not 81
82 or 83
51 and 84
limit 85 to yr=2013‐2014
limit 85 to ed=20130705‐20140808
86 or 87
EMBASE
Last searched 8 August 2014
exp spine/
exp spine disease/
exp scoliosis/
exp idiopathic scoliosis/
scoliosis.mp.
or/1‐5
orthopedics/
exp surgery/
su.fs.
surg$.ti,ab.
operat$.ti,ab.
realign$.ti,ab.
spondylodesis.ti,ab.
spine fusion.ti,ab.
spinal fusion.ti,ab.
spinal instrumentation.ti,ab.
bone screw/
screw$.ti,ab.
hybrid.ti,ab.
Kirschner wire/
sublaminar.ti,ab.
wire$.ti,ab.
hook$.ti,ab.
or/7‐23
exp rehabilitation/
rehabilitat$.ti,ab.
rh.fs.
exp physiotherapy/
physiotherapist/
physiotherapy.ti,ab.
physical therapy.ti,ab.
exp exercise/
exercise$.ti,ab.
kinesiotherapy/
exp manipulative medicine/
immobilization/
brace/
brace$.mp.
bracing.mp.
exp orthotics/
exp orthopedic equipment/
(non‐surg$ or nonsurg$ or non‐operat$ or nonoperat$ or conserv$).ti,ab.
(immobilis$ or immobiliz$ or therap$ or taping or tape$ or electrotherap$).ti,ab.
or/25‐43
6 and 24 and 44
limit 45 to adolescent <13 to 17 years>
adolescent/
adolescen$.mp.
or/47‐48
45 and 49
46 or 50
exp Clinical Study/
exp Case Control Study/
exp Family Study/
exp Longitudinal Study/
exp Retrospective Study/
exp Prospective Study/
exp Cohort Analysis/
(cohort adj (study or studies)).mp.
(case control adj (study or studies)).mp.
(follow up adj (study or studies)).mp.
(observational adj (study or studies)).mp.
(epidemiologic$ adj (study or studies)).mp.
(cross sectional adj (study or studies)).mp.
exp Comparative Study/
evaluation study.mp.
follow‐up study.mp. or exp Follow Up/
Crossover Procedure/
prospective$.mp.
exp VOLUNTEER/
or/52‐70
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/72‐84
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/86‐100
85 or 101
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
103 and 104
103 not 105
102 not 106
71 not 106
107 or 108
51 and 109
limit 110 to yr=2013‐2014
limit 110 to em=201326‐201431
111 or 112
CINAHL
Last searched 8 August 2014
v S85 S83 or S84
S84 S82 and EM 20130705‐20140808
S83 S82 Limiters ‐ Published Date: 20130701‐20140831
S82 S77 OR S81
S81 S76 AND S80
S80 S78 OR S79
S79 adolescen*
S78 MH Adolescence+
S77 S34 AND S39 AND S75 Limiters ‐ Age Groups: Adolescent: 13‐18 years
S76 S34 AND S39 AND S75
S75 S56 OR S74
S74 S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73
S73 TI (immobilis* or immobiliz* or therap* or taping or tape* or electrotherap*) or AB (immobilis* or immobiliz* or therap* or taping or tape* or electrotherap*)
S72 TI (non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv*) or AB (non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv*)
S71 MH "Orthopedic Equipment and Supplies+"
S70 orthotic*
S69 (MH "Orthoses+") OR "orthoses"
S68 bracing
S67 brace*
S66 MH Immobilization
S65 MH Manipulation, Orthopedic
S64 MH Therapeutic Exercise+
S63 TI exercise* or AB exercise*
S62 MH Exercise+
S61 TI "physical therapy" or AB "physical therapy"
S60 TI physiotherapy or AB physiotherapy
S59 MH Physical Therapists
S58 MH Physical Therapy+
S57 MH Rehabilitation+
S56 (TI hook* or AB hook*) AND (S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55)
S55 TI hook* or AB hook*
S54 TI wire* or AB wire*
S53 TI sublaminar or AB sublaminar
S52 TI hybrid or AB hybrid
S51 TI screw* or AB screw*
S50 MH Orthopedic Fixation Devices
S49 TI "spinal instrumentation" or AB "spinal instrumentation"
S48 TI "spinal fusion" or AB "spinal fusion"
S47 TI "spine fusion" or AB "spine fusion"
S46 TI spondylodesis or AB spondylodesis
S45 TI realign* or AB realign*
S44 TI operat* or AB operat*
S43 TI surg* or AB surg*
S42 MW Surgery
S41 MH Surgery, Operative+
S40 MH Orthopedics
S39 S35 OR S36 OR S37 OR S38
S38 scoliosis
S37 MH Scoliosis+
S36 MH Spinal Diseases+
S35 MH Spine+
S34 S32 or S33
S33 S30 not S31
S32 S14 not S31
S31 MH Animals
S30 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29
S29 volunteer*
S28 prospective*
S27 control*
S26 retrospective
S25 longitudinal
S24 "observational studies" or "observational study"
S23 "follow‐up stud*" or "followup stud*"
S22 "cohort analys*"
S21 "cohort studies" or "cohort study"
S20 MH Epidemiological Research+
S19 MH Prospective Studies+
S18 MH Evaluation Research+
S17 MH Comparative Studies
S16 latin square
S15 MH Study Design+
S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 MH Random Sample
S12 random*
S11 MW Drug Therapy
S10 placebo*
S9 MH Placebos
S8 MH Placebo Effect
S7 TI groups or AB groups
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
Appendix 2. Other search strategies
PsycINFO
Last searched 8 August 2014
1 scoliosis.mp.
2 (surg* or operat* or realign* or spondylodesis or fusion or instrumentation or screw* or hook* or hybrid or wire* or sublaminar).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
3 (rehabilit* or therap* or physiotherapy or exercise* or braces or bracing or orthotic* or non‐surg* or nonsurg* or non‐operat* or nonoperat* or conserv* or immobilis* or immobiliz* or taping or tape* or electrotherapy).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
4 1 and 2 and 3
5 limit 4 to yr=2013‐2014
PEDro
Last searched 8 August 2014
Abstract & Title: scoliosis
AND
Method: clinical trial
New records added since 05/07/2013
PubMed
Searched 11 August 2014. This search was designed to capture citations not indexed in MEDLINE.
(((((surg* or fus* or orthopedic* or instrument* or screw* or wire* or hook*[Title/Abstract])) OR (nonsurg* or non‐surg or nonop* or non‐op* or immobiliz* or immobilis* or exercise* or therap* or braces or bracing or taping or tape* or electrotherap* or rehab* or conserv*[Title/Abstract])) AND (adolescen* AND scoliosis[Title/Abstract])) AND ("2013/07/05"[Date ‐ Publication] : "3000"[Date ‐ Publication])) NOT MEDLINE[sb]
UKCTG
Last searched August 2014
scoliosis
ClinicalTrials.gov
Last searched 8 August 2014
Condition: scoliosis
WHO ICTRP
Last searched 8 August 2014
Condition: scoliosis
EThOS
Last searched August 2014
scoliosis
Appendix 3. Planned Methodology
Data extraction and management
We planned to perform the review and meta‐analyses following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have performed the analyses using Review Manager (Review Manager (RevMan) 2014).
We had prepared a standardised data extraction form on the basis of all the inclusion criteria, which, had we identified any relevant trials, two review authors would have piloted and tested for intra‐ and interobserver reliability, and then used the form independently to extract data from the included papers. We would have extracted raw data, including information about the study design (RCT, QRCT, prospective controlled cohort study), study characteristics (country, recruitment modality, study funding, risk of bias), and participant characteristics (number of participants, age, sex, severity of scoliosis at baseline), as well as a description of experimental and comparison interventions, co‐interventions, adverse effects, duration of follow‐up, outcomes assessed and results, as well as any adverse effects. If a review author was the author of a paper, another review author would have undertaken the data extraction process. We would have discussed any disagreements and consulted a third review author if any disagreements persisted.
Assessment of risk of bias in included studies
We would have assessed the risk of bias for both randomised studies and NRSs using the criteria recommended by the Cochrane Back Review Group (Furlan 2009; Higgins 2011), together with items from the Downs and Black (Downs 1998) checklist, as outlined in Appendix 4. These criteria fall into five bias categories: selection bias, performance bias, attrition bias, detection bias, and selective outcome reporting. We piloted and tested the 'Risk of bias' assessment form for intraobserver and interobserver reliability. Two review authors would have independently assessed the internal validity of the included studies. The review authors would have resolved any disagreements by discussion, consulting a third independent review author if disagreements persisted. We planned to blind the 'Risk of bias' assessment to trial authors, institution, and journal. We planned to score the 'Risk of bias' criteria as high, low, or unclear and report these ratings in the 'Risk of bias' table. Next we would have rated the overall extent of risk of bias within each bias category (for example performance bias) as 'bias' or 'no bias'.
While it was difficult to provide an exhaustive list of all possible confounding variables at the start of the review, the review authors have experience in this field and are aware of most of the potential confounding variables that may occur when different treatment groups are compared. These may have included, for instance, demographic variables such as age, Risser sign (bone maturity), curve location, and curve magnitude.
In regard to grading the quality of the evidence, we would not have downgraded evidence from studies judged 'no bias' for all five categories. We would have downgraded evidence by ‐1 point when three or fewer categories for each study were judged to have bias. We would have downgraded evidence by ‐2 points when four or more categories for each study were judged to have bias. See the Data synthesis section that follows for additional details on how we would have assessed the quality assessment for each outcome.
Measures of treatment effect
We planned to analyse dichotomous outcomes by calculating the risk ratio for each trial, with uncertainty in each result expressed by 95% confidence intervals. We planned to analyse continuous outcomes by calculating the weighted mean difference or the standardised mean difference with 95% confidence intervals.
Unit of analysis issues
In cases where three or more interventions were evaluated in a single study, we pre‐stated in the protocol that we would include each pairwise comparison separately.
Dealing with missing data
For recent papers (within five years), we would have endeavoured to collect missing data by contacting the authors. When data were insufficient to be entered into the meta‐analysis (even after contacting the authors), we had planned to report the results qualitatively in the 'Characteristics of included studies' table and the 'Summary of findings' tables.
Assessment of heterogeneity
In the protocol we stated that we would combine the outcome measures from individual trials through meta‐analysis where possible (comparability of intervention and outcomes across trials) using a random‐effects model. We would have used the I2 statistic and the Chi2 test (P less than 0.1) to indicate whether significant statistical heterogeneity was present. If a meta‐analysis was not possible, we would have described the results from clinically comparable trials qualitatively in the text.
Assessment of reporting biases
To determine whether publication bias was present, we would have constructed funnel plots if at least 10 studies were available for the meta‐analysis (Sutton 2000).
Data synthesis
In the protocol we stated that we would analyse dichotomous outcomes by calculating the risk ratio. We also planned that continuous outcomes would be analysed by calculating the mean difference when the same instrument was used to measure outcomes or the standardised mean difference when different instruments were used to measure outcomes. We would express uncertainty with 95% confidence intervals. It was postulated to combine outcome measures from the individual trials through meta‐analysis where possible (clinical comparability of population, intervention/s and outcomes between trials) using a random‐effects model. A P value of the Chi2 test less than 0.1 would indicate significant statistical heterogeneity.
If meta‐analysis was not possible, we would have described the results from clinically comparable trials qualitatively in the text. Regardless of whether sufficient data were available for the use of quantitative analyses to summarise the data, we would have assessed the overall quality of the evidence for each outcome. To accomplish this, we would have used the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted in the updated Cochrane Back Review Group method guidelines (Furlan 2009). Factors that may have decreased the quality of the evidence included study design and risk of bias, inconsistency of results, indirectness (not generalisable), imprecision (sparse data), and other factors (for example reporting bias).
The quality of the evidence for a specific outcome would have been reduced by a level, according to the performance of the studies against the following five factors.
High‐quality evidence: Consistent findings had been noted among at least 75% of RCTs with low risk of bias; consistent, direct, and precise data and no known or suspected publication biases. Further research is unlikely to change the estimate or our confidence in the results.
Moderate‐quality evidence: One of the domains was not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low‐quality evidence: Two of the domains were not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low‐quality evidence: Three of the domains were not met. We are very uncertain about the results.
No evidence: No studies were identified that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
If we noted significant statistical heterogeneity, we would have performed a subgroup analysis to consider the effects of the following variables: age, bone age, Cobb degrees, type of surgery and braces, and exercise.
Sensitivity analysis
To incorporate the 'Risk of bias' assessment into the review process, we would have started by stratifying the intervention effects estimates by risk. If we saw differences in results among studies at different risks of bias, we would have performed sensitivity analyses, excluding studies with high risk of bias from the analysis. Alternatively, we would have presented the results for RCTs and QRCTs separately from those of longitudinal studies.
Clinical relevance of results
The review authors would also have assessed each trial for its clinical relevance by using the five questions outlined by Shekelle 1994 and recommended by the Cochrane Back Review Group (see Appendix 5) (Furlan 2009; Van Tulder 2003). We would have discussed all important outcomes for each comparison. In the protocol the review authors had also planned for the main conclusion to be clinical, as our main aim was to give clinicians, researchers, patients, and service users state‐of‐the‐art information provided by relevant studies on this issue.
Appendix 4. Criteria for assessing risk of bias for internal validity for randomised and non‐randomised studies (Downs and Black 1998; Furlan 2009)
Selection bias
Random sequence generation
Risk of selection bias is low if the investigators described a random component in the sequence generation process, such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing lots, minimising (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
Risk of selection bias is high if the investigators described a non‐random component in the sequence generation process, such as sequence generated by odd or even date of birth, date (or day) of admission, hospital or clinic record number or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or a series of tests, or availability of the intervention.
If study is non‐randomised, this will be rated as high bias.
Allocation concealment
Risk of selection bias is low if participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, Web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; or sequentially numbered, opaque, sealed envelopes.
Risk of bias is high if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on using an open random allocation schedule (for example a list of random numbers); assignment envelopes were used without appropriate safeguards (for example if envelopes were unsealed or non‐opaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; or other explicitly unconcealed procedures.
If study is non‐randomised, this will be rated as high bias.
Selection bias (population)*
Risk of selection bias is low if participants in different intervention groups were recruited from the same population.
Selection bias (timing)*
Risk of selection bias is low if participants in different intervention groups were recruited over the same time. Surgical studies must be less than 10 years old for low risk of selection bias.
Adjustment for confounding*
Risk is low if no significant group differences were shown. Risk is high if the effect of the main confounders was not investigated or if no adjustment was made in the final analyses.
Performance bias
Blinding of participants
Risk of performance bias is low if blinding of participants was ensured and it was unlikely that the blinding could have been broken; or if no blinding or incomplete blinding was performed, but the review authors judge that the outcome was not likely to be influenced by lack of blinding.
Blinding of personnel/care providers
Risk of performance bias is low if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if no blinding or incomplete blinding was performed, but the review authors judge that the outcome was not likely to be influenced by lack of blinding.
Compliance (adherence)
Risk of bias is low if compliance with the interventions was acceptable on the basis of reported intensity/dosage, duration, number, and frequency for both index and control intervention(s). For single‐session interventions (for example surgery), this item is irrelevant.
Co‐interventions
Risk of bias is low if no co‐interventions were provided, or if they were similar between index and control groups.
Attrition bias
Incomplete outcome data
Risk of attrition bias is low if no outcome data were missing; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts were very large, imputation using even 'acceptable' methods may still suggest a high risk of bias). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but are arbitrary and are not supported by the literature).
Intention‐to‐treat analysis
Risk of bias is low if all randomly assigned participants were reported/analysed in the group to which they were allocated by randomisation.
Measurement/detection
Blinding of outcome assessment
Risk of detection bias is low if blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if no blinding or incomplete blinding was performed, but the review authors judge that the outcome was not likely to be influenced by lack of blinding, or:
for participant‐reported outcomes in which the participant was the outcome assessor (e.g. pain, disability): Risk of bias for outcome assessors is low if risk of bias for participant blinding is low;
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: Risk of bias for outcome assessors is low if risk of bias for care providers is low; and
for outcome criteria that are assessed from data from medical forms: Risk of bias is low if the treatment or adverse effects of the treatment could not be noticed in the extracted data.
Timing of outcome assessments
Risk of bias is low if all important outcome assessments for all intervention groups were measured at the same time, or if analyses adjust for different lengths of follow‐up.
Selective reporting
Data dredging
Risk of bias is low if all analyses were planned at the outset of the study.
Risk of bias is high if analyses were conducted retrospectively (for example retrospective unplanned subgroup analyses).
Outcome measures
Risk of reporting bias is low if the study protocol is available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way, or if the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
Risk of reporting bias is high if not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes were reported using measurements, analysis methods, or subsets of the data (for example subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely, so that they cannot be entered into a meta‐analysis; the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
*Items are relevant only to non‐randomised studies.
Appendix 5. Questions for assessing clinical relevance
1. Are the participants described in detail so that you can decide whether they are comparable with those that you see in your practice? Yes/No/Unsure 2. Are the interventions and treatment settings described well enough so that you can provide the same for your patients? Yes/No/Unsure 3. Were all clinically relevant outcomes measured and reported? Yes/No/Unsure 4. Is the size of the effect clinically important? Yes/No/Unsure 5. Are the likely treatment benefits worth the potential harms? Yes/No/Unsure
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2006 | Retrospective study with a control group |
| Bunge 2007 | Retrospective cross sectional study with a control group |
| Danielsson 2001 | Retrospective study with a control group |
| Danielsson 2001a | Retrospective study with a control group |
| Danielsson 2001b | Retrospective study with a control group |
| Danielsson 2003 | Retrospective study with a control group |
| Danielsson 2006 | Retrospective study with a control group |
| Pehrsson 2001 | Retrospective study with a control group |
Differences between protocol and review
The databases were searched from inception to August 2014.
Contributions of authors
Substantial contributions to conception and design: Josette Bettany‐Saltikov, Hans‐Rudolf Weiss, Razvan Taranu, Shreya Srinivas, Nachiappan Chockalingam, Vicky Whittaker, Raman V Kalyan
Study search and selection: Julie Hogg, Josette Bettany‐Saltikov, Hans‐Rudolf Weiss, Razvan Taranu, Shreya Srinivas, Tracey Arnell, Raman V Kalyan
Drafting of the article: Josette Bettany‐Saltikov, Hans‐Rudolf Weiss, Razvan Taranu, Shreya Srinivas, Nachiappan Chockalingam, Vicky Whittaker, Raman V Kalyan, Tracey Arnell
Critical revision for important intellectual content: Josette Bettany‐Saltikov, Hans‐Rudolf Weiss, Razvan Taranu, Shreya Srinivas, Nachiappan Chockalingam, Vicky Whittaker,Raman V Kalyan
Final approval of the version to be published: Josette Bettany‐Saltikov, Hans‐Rudolf Weiss, Nachiappan Chockalingam.
Sources of support
Internal sources
-
The University of Teesside, UK.
SAR Grant
External sources
No sources of support supplied
Declarations of interest
Nothing to declare.
New
References
References to studies excluded from this review
Anderson 2006 {published data only}
- Anderson MO, Christenesen SB, Thomsen K. Outcome at 10 years after treatment for adolescent idiopathic scoliosis. Spine 2006;31(3):350‐4. [DOI] [PubMed] [Google Scholar]
Bunge 2007 {published data only}
- Bunge EM, Juttmann RE, Kleuver M, Biezen FC, Koning HJ. Health‐related quality of life in patients with adolescent idiopathic scoliosis after treatment: short‐term effects after brace or surgical treatment. European Spine Journal 2007;16(1):83‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danielsson 2001 {published data only}
- Danielsson AJ, Nachemson AL. Radiologic findings and curve progression 22 years after treatment for adolescent idiopathic scoliosis. Spine 2001;26(5):516‐25. [DOI] [PubMed] [Google Scholar]
Danielsson 2001a {published data only}
- Danielsson AJ, Nachemson AL. Childbearing, curve progression, and sexual function in women 22 years after treatment for adolescent idiopathic scoliosis. Spine 2001;26(13):1449‐56. [DOI] [PubMed] [Google Scholar]
Danielsson 2001b {published data only}
- Danielsson AJ, Wiklund I, Pehrsson K, Nachemson AL. Health‐related quality of life in patients with adolescent idiopathic scoliosis: a matched follow‐up at least 20 years after treatment with brace or surgery. European Spine Journal 2001;10(4):278‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danielsson 2003 {published data only}
- Danielsson AJ, Nachemson AL. Back pain and function 22 years after brace treatment for adolescent idiopathic scoliosis: a case‐control study ‐ part 1. Spine 2003;28(18):2078‐86. [DOI] [PubMed] [Google Scholar]
Danielsson 2006 {published data only}
- Danielsson AJ, Romberg K, Nachemson AL. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis. Spine 2006;31(3):275‐83. [DOI] [PubMed] [Google Scholar]
Pehrsson 2001 {published data only}
- Pehrsson K, Danielson A, Nachemson A. Pulmonary function in adolescent idiopathic scoliosis: a 25 year follow up after surgery or start of brace treatment. Thorax 2001;56(5):388‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Asher 2003
- Asher M, Min Lai S, Burton D, Manna B. The reliability and concurrent validity of the Scoliosis Research Society-22 patient questionnaire for idiopathic scoliosis. Spine 2003;28(1):63‐9. [DOI] [PubMed] [Google Scholar]
Asher 2006
- Asher M, Burton D. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis 2006;1(1):2. [DOI: 10.1186/1748-7161-1-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bago 2010
- Bago J, Sanchez‐Raya J, Sanchez Perez‐Grueso F, Climent J. The Trunk Appearance Perception Scale (TAPS): a new tool to evaluate subjective impression of trunk deformity in patients with idiopathic scoliosis. Scoliosis 2010;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berryman 2008
- Berryman F, Pynsent P, Fairbank J, Disney S. A new system for measuring three‐dimensional back shape in scoliosis. European Spine Journal 2008;17(5):663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bettany‐Saltikov 2012
- Bettany‐Saltikov. How to Do a Systematic Review: A Step by Step Guide for Nursing Practice. 1st Edition. London: Open University Press, 2012. [Google Scholar]
Bradford 1987
- Bradford D, Lonstein J, Moe J, Ogilvie J, Winter R. Moe's Textbook of Scoliosis and Other Spinal Deformities. 2. Philadelphia: Saunders, 1987. [Google Scholar]
Bridwell 1999
- Bridwell K. Surgical treatment of idiopathic adolescent scoliosis. Spine (Focus Issue) 1999;24(24):2607‐16. [DOI] [PubMed] [Google Scholar]
Castro 2003
- Castro F Jr. Adolescent idiopathic scoliosis, bracing and the Hueter‐Volkmann principle. Spine 2003;3(3):180‐5. [DOI] [PubMed] [Google Scholar]
Climent 1999
- Climent J, Sanchez J. Impact of the type of brace on the quality of life of adolescents with spine deformities. Spine 1999;24(18):1903‐8. [DOI] [PubMed] [Google Scholar]
Coillard 2002
- Coillard C, Leroux M, Badeaux J, Rivard C. SPINECOR: a new therapeutic approach for idiopathic scoliosis. Studies in Health Technology and Informatics 2002;88(1):215‐7. [PubMed] [Google Scholar]
Coillard 2003
- Coillard C, Leroux M, Zabjek K, Rivard C. SpineCor-A non‐rigid brace for the treatment of idiopathic scoliosis: post‐treatment results. European Spine Journal 2003;12(2):141‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2007
- Dolan L, Weinstein S. Surgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence‐based review. Spine 2007;32(19):S91‐100. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology & Community Health 1998;52(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallstrom 1986
- Fallstrom K, Cochran T, Nachemson A. Long‐term effects on personality development in patients with adolescent idiopathic scoliosis: influence of type of treatment. Spine 1986;11(7):756‐8. [DOI] [PubMed] [Google Scholar]
Freidel 2002
- Freidel K, Petermann F, Reichel D, Steiner A, Warschburger P, Weiss Hans R. Quality of life in women with idiopathic scoliosis. Spine 2002;27(4):E87‐91. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Goldberg 2002
- Goldberg C, Moore D, Fogerty E, Dowling F. The natural history of early onset scoliosis. Studies in Health Technology and Informatics 2002;91(SUPP 3):68‐70. [PubMed] [Google Scholar]
Grivas 2008
- Grivas T, Vasiliadis E, Olga D, Triantafyllopoulos S, Triantafyllopoulos G. What a school screening program could contribute in clinical research of idiopathic scoliosis aetiology. Disability and Rehabilitation 2008;30(10):752‐62. [DOI] [PubMed] [Google Scholar]
Haher 2003
- Haher T, Merola A. Atlas of Spine Surgical Techniques. 1. Vol. 1, New York, NY: Thieme, 2003. [Google Scholar]
Hawes 2006a
- Hawes M. Impact of spine surgery on signs and symptoms of spinal deformity. Pediatric Rehabilitation 2006;9(4):318‐39. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley and Sons.
Kadoury 2009
- Kadoury S, Cheriet F, Beausejour M, Stokes I, Parent S, Labelle H. A three‐dimensional retrospective analysis of the evolution of spinal instrumentation for the correction of adolescent idiopathic scoliosis. European Spine Journal 2009;18(1):23‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanayama 1996
- Kanayama M, Tadano S, Kaneda K, Ukai T, Abumi K. A. mathematical expression of three dimensional configuration of the scoliotic spine. ASME Journal of Biomechanical Engineering 1996;2(118):247‐52. [DOI] [PubMed] [Google Scholar]
Katz 2001
- Katz D, Durrani A. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine 2001;26(21):2354‐61. [DOI] [PubMed] [Google Scholar]
Knott 2010
- Knott P, Mardjetko S, Rollet M, Baute S, Riemenschneider M, Muncie L, et al. Evaluation of the reproducibility of the formetric 4D measurements for scoliosis. Scoliosis 2010;5(Suppl 1):O10. [Google Scholar]
Lenssinck 2005
- Lenssinck ML, Frijlink AC, Berger MY, Bierman‐Zeinstra SM, Verkerk K, Verhagen AP, et al. Effect of bracing and other conservative interventions in the treatment of idiopathic scoliosis in adolescents: a systematic review of clinical trials. Physical Therapy 2005;85(12):1329–39. [PubMed] [Google Scholar]
Lonner 2007
- Lonner BS, Kondrachov D, Siddigi F, Hayes V, Scharf C. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. Surgical technique.. American Journal of Bone Joint Surgery 2007;Suppl 2(1):142‐56. [DOI] [PubMed] [Google Scholar]
Lonstein 2006
- Lonstein JE. Scoliosis: surgical versus non‐surgical treatment. Clinical Orthopaedics and Related Research 2006;443(1):248–59. [DOI] [PubMed] [Google Scholar]
Lupparelli 2002
- Lupparelli S, Pola E, Pitta L, Mazza O, Santis V, Aulisa L, et al. Biomechanical factors affecting progression of structural scoliotic curves of the spine. Studies in Health Technology and Informatics 2002;91(1):81‐5. [PubMed] [Google Scholar]
Lykissas 2013
- Lykissas M, Jain V, Nathan S, Pawar V, Eismann E, Sturm P, et al. Mid‐ to long‐term outcome in adolescent idiopathic scoliosis after instrumented posterior spinal fusion: a meta‐analysis. Spine 2013;38(2):E113‐9. [DOI] [PubMed] [Google Scholar]
Maruyama 2008
- Maruyama T, Takeshita K. Surgical treatment of scoliosis: a review of techniques currently applied. Scoliosis 2008;3(6):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merola 2002
- Merola A, Haher TR, Brkaric M, Panagopoulos G, Mathur S, Kohani O, Lowe TG. A multicenter study of the outcomes of the surgical treatment of adolescent idiopathic scoliosis using the Scoliosis Research Society (SRS) outcome instrument. Spine 2002;27(18):2046‐51. [DOI] [PubMed] [Google Scholar]
Negrini 2005
- Negrini S, Aulisa L, Ferraro C, Fraschini P, Masiero S, Simonazzi P, et al. Italian guidelines on rehabilitation treatment of adolescents with scoliosis or other spinal deformities. Europa Medicophysica 2005;41(2):183‐201. [PubMed] [Google Scholar]
Negrini 2006c
- Negrini S, Marchini G. Efficacy of the symmetric, patient‐oriented, rigid, three‐dimensional, active (SPoRT) concept of bracing for scoliosis: a prospective study of the Sforzesco versus Lyon brace. Europa Medicophysica 2006;43(2):171‐81. [PubMed] [Google Scholar]
Negrini 2010
- Negrini S, Minozzi S, Romano M, Bettany‐Saltikov JA, Chokalingham N, Grivas T, et al. Braces for idiopathic scoliosis in adolescents. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006850.pub2] [DOI] [PubMed] [Google Scholar]
Negrini 2012
- Negrini S, Aulisa A, Aulisa L, Circo A, Mauroy J, Durmala J, et al. 2011 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis 2012;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noonan 1997
- Noonan K, Dolan L, Jacobson W, Weinstein S. Long‐term psychosocial characteristics of patients treated for idiopathic scoliosis. Journal of Pediatric Orthopaedics 1997;17(6):712‐7. [PubMed] [Google Scholar]
Odermatt 2003
- Odermatt D, Mathieu P, Beausejour M, Labelle H, Aubin C. Electromyography of scoliotic patients treated with a brace. Journal of Orthopaedic Resources 2003;21(5):931‐6. [DOI] [PubMed] [Google Scholar]
Olgun 2013
- Olgun ZD, Yazic M. Posterior instrumentation and fusion. Journal of Children's Orthopaedics 2013;7(1):69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxborrow 2000
- Oxborrow N. Methodology. Current topic: assessing the child with scoliosis: the role of surface topography. Archives of Disease in Childhood 2000;83(5):453‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pineda 2006
- Pineda S, Bago J, Gilperez C, Climent J. Validity of the Walter Reed Visual Assessment Scale to measure subjective perception of spine deformity in patients with idiopathic scoliosis. Scoliosis 2006;1(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reichel 2003
- Reichel D, Schanz J. Developmental psychological aspects of scoliosis treatment. Pediatric Rehabilitation 2003;6(34):221‐5. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) 2014
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) (Computer program). 2014.
Rigo 2006a
- Rigo M, Negrini S, Weiss H, Grivas T, Maruyama T, Kotwicki T, et al. SOSORT consensus paper on brace action: TLSO biomechanics of correction (investigating the rationale for force vector selection). Scoliosis 2006;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roaf 1980
- Roaf R. Spinal Deformities. 2nd Edition. Tunbridge Wells: Pitman Medical, 1980. [Google Scholar]
Romano 2012
- Romano M, Minozzi S, Bettany‐Saltikov J, Zaina F, Chockalingam N, Kotwicki T, et al. Exercises for adolescent idiopathic scoliosis. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007837.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 1997
- Rowe D, Bernstein S, Riddick M, Adler F, Emans J, Gardner‐Bonneau D, et al. A meta‐analysis of the efficacy of non‐operative treatments for idiopathic scoliosis. The Journal of Bone & Joint Surgery 1997;79(5):664‐74. [DOI] [PubMed] [Google Scholar]
Sanders 2007
- Sanders J, Harrast J, Kuklo T, Polly D, Bridwell K, Diab M, et al. The Spinal Appearance Questionnaire: results of reliability, validity, and responsiveness testing in patients with idiopathic scoliosis. Spine 2007;32(24):2719‐22. [DOI] [PubMed] [Google Scholar]
Shekelle 1994
- Shekelle P, Andersson G, Bombardier C, Cherkin D, Deyo R, Keller R, et al. A brief introduction to the critical reading of the clinical literature. Spine 1994;19(19):2028S‐31S. [DOI] [PubMed] [Google Scholar]
Smania 2008
- Smania N, Picelli A, Romano M, Negrini S. Neurophysiological basis of rehabilitation of adolescent idiopathic scoliosis. Disability and Rehabilitation 2008;30(10):763‐71. [DOI] [PubMed] [Google Scholar]
Stokes 1996
- Stokes I, Spence H, Aronsson D, Kilmer N. Mechanical modulation of vertebral body growth: implications for scoliosis progression. Spine 1996;21(10):1162‐7. [DOI] [PubMed] [Google Scholar]
Stokes 2006
- Stokes I, Burwell R, Dangerfield P. Biomechanical spinal growth modulation and progressive adolescent scoliosis-a test of the ’vicious cycle’ pathogenetic hypothesis: summary of an electronic focus group debate of the IBSE. Scoliosis 2006;1(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutton 2000
- Sutton A, Duval S, Tweedie R, Abrams K, Jones D. Empirical assessment of effect of publication bias on metaanalyses. BMJ 2000;320(1):1574‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board Cochrane Back Review Group. Updated method guidelines for systemic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Vasiliadis 2006
- Vasiliadis E, Grivas T, Gkoltsiou K. Development and preliminary validation of Brace Questionnaire (BrQ): a new instrument for measuring quality of life of brace treated scoliotics. Scoliosis 2006;1(7):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2004
- Weiss H, Hawes M. Adolescent idiopathic scoliosis, bracing and the Hueter‐Volkmann principle. Spine Journal 2004;4(4):485‐6. [DOI] [PubMed] [Google Scholar]
Weiss 2006a
- Weiss H, Negrini S, Hawes M, Rigo M, Kotwicki T, Grivas T, et al. Physical exercises in the treatment of idiopathic scoliosis at risk of brace treatment‐SOSORT Consensus Paper 2005. Scoliosis 2006;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2006b
- Weiss H, Reichel D, Schanz J, Zimmermann‐Gudd S. Deformity related stress in adolescents with AIS. Studies of Health and Technology Informatics 2006;123(1):347–51. [PubMed] [Google Scholar]
Weiss 2008a
- Weiss H, Goodall D. Rate of complications in scoliosis surgery: a systematic review of the PubMed literature. Scoliosis 2008;3(9):1‐18. [DOI: 10.1186/1748-7161-3-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2008b
- Weiss H. Adolescent idiopathic scoliosis (AIS)-an indication for surgery? A systematic review of the literature. Disability and Rehabilitation 2008;30(10):799‐807. [DOI] [PubMed] [Google Scholar]
Weiss 2008c
- Weiss H, Goodall D. The treatment of adolescent idiopathic scoliosis (AIS) according to present evidence. A systematic review. European Journal of Physical Rehabilitation Medicine 2008;44(2):177‐93. [PubMed] [Google Scholar]
Weiss 2013
- Weiss H, Moramarco M, Moramarco K. Risks and long‐term complications of adolescent idiopathic scoliosis surgery vs. non‐surgical and natural history outcomes. Hard Tissue 2013;2(3):27. [Google Scholar]
Westrick 2011
- Westrick E, Ward W. Adolescent idiopathic scoliosis: 5‐year to 20‐year evidence‐based surgical results. Journal of Pediatric Orthopaedics 2011;31(1 suppl):S61‐8. [DOI] [PubMed] [Google Scholar]
White 1990
- White A, Panjabi M. Chapter 7. Functional analysis and clinical applications. Clinical Biomechanics of the Spine. 2nd Edition. Baltimore: Lippincott, 1990. [Google Scholar]


