Abstract
Skin biopsy samples from varicella-zoster virus (VZV)-infected patients examined by immunohistochemistry demonstrated VZV replication in nonepithelial cell types. ORF29p, a nonstructural nuclear protein, was found in nerves of two of six patients with chickenpox. In tissue culture, ORF29p was secreted by VZV-infected fibroblasts. Extracellular ORF29p can be taken up through endocytosis by human neurons, implying a novel role for this protein in pathogenesis.
Varicella-zoster virus (VZV) infects dorsal root ganglia (DRG), enters latency, and may later reactivate to cause zoster. Studies have detected VZV in specific sites at different stages of infection. VZV DNA is present in the oropharynx (27) and in peripheral blood mononuclear cells (PBMCs) of patients with chickenpox (3, 16, 20). Virus DNA, the glycoproteins gE and gB, and the immediate-early protein 63 (IE63p) are found in skin biopsy samples obtained from patients with chickenpox or zoster (1, 23–25). VZV is found in keratinocytes, antigen-presenting cells, and endothelial cells during acute zoster (23, 25) and in keratinocytes and inflammatory cells during chickenpox (1). VZV is present in neurons and satellite cells of DRG years following primary infection (6–8, 12, 17, 22) and has been observed by electron microscopy in sensory nerves during zoster (10). Other details of VZV pathogenesis remain speculative, including how the virus spreads from respiratory epithelial cells to PBMCs, keratinocytes, and DRG. Because PBMCs, sensory nerves, and epithelial cells are in close proximity in the dermis and epidermis, the skin is likely the site where virus enters the nervous system.
By analogy with herpes simplex virus (HSV), it is thought that VZV transcription is temporally regulated. Immediate-early (IE) genes are expressed first, followed by early (E) genes and lastly late (L) genes (5). Some VZV proteins encoded by IE and L genes are incorporated in the virion, including transactivators such as IE63p and structural proteins such as gC (14, 15). ORF29p (for open reading frame 29 protein), the major VZV DNA binding protein, is encoded by a putative E gene and is not detected in purified virions (13). During latency, VZV exhibits limited gene expression (6–9, 22), with the accumulation of specific IE and E gene-encoded proteins in neurons (18, 19). During reactivation, all kinetic classes of VZV genes are expressed in neurons (18). Whether VZV is in the lytic or latent state is reflected by the localization of expressed VZV gene products. VZV IE and E proteins that are present in both the nucleus and cytoplasm during productive infection are detected only in the cytoplasm of neurons during latency (18).
Early observations suggested that there were inclusion bodies in endothelial cells present in varicella lesions (29); however, there was no known association between histology and viral etiology at that time. To determine if, during primary infection, as in zoster, VZV infects endothelial cells and nerves in the dermis and to characterize the inflammatory cells in the epidermis and dermis infected by VZV, we performed comparative immunohistochemical analyses of skin biopsy samples obtained from patients with chickenpox and zoster.
Comparative immunohistochemical analysis of chickenpox and zoster lesions.
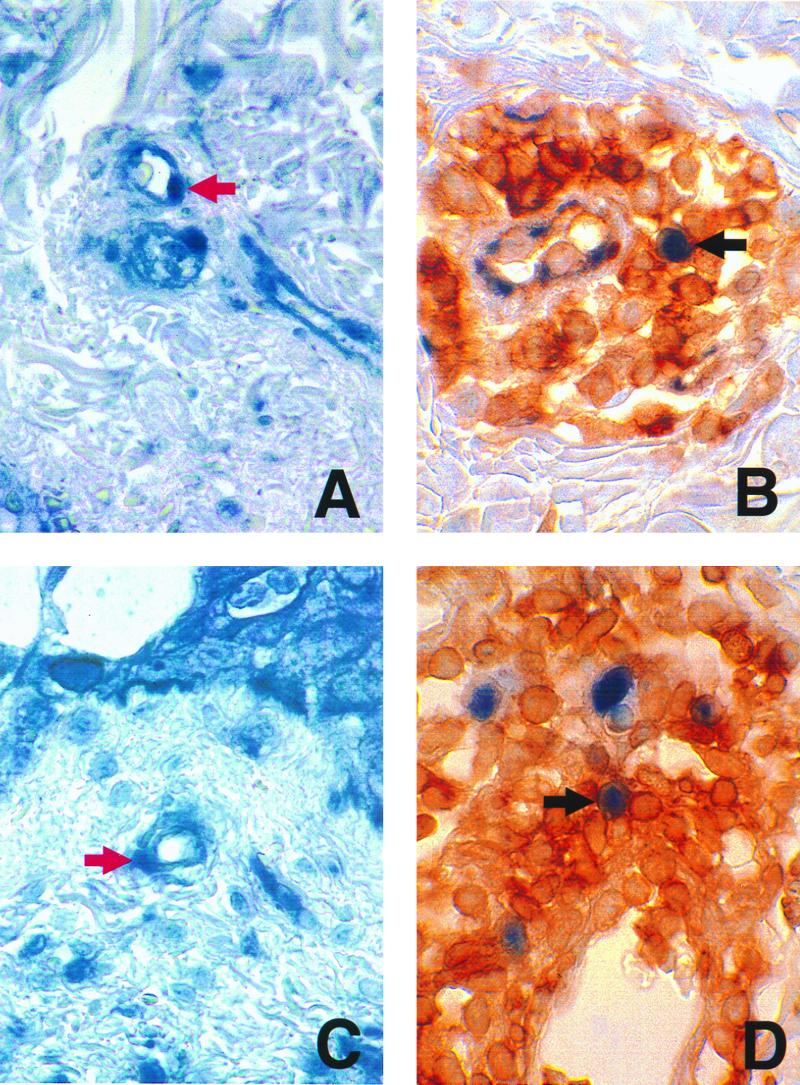
Six cases of chickenpox and eight cases of zoster were analyzed by immunohistochemistry using purified polyclonal antibodies generated against VZV proteins (18). Each specimen was analyzed for the presence of gC, a late gene product and component of the virus envelope (15); IE63p, a regulatory protein and component of the virus tegument (14); and ORF29p (13). In these specimens, antibodies to IE63p, ORF29p, and gC detected proteins in the expected intracellular compartments: ORF29p was found in cell nuclei (Fig. 1), gC was found in cell membranes and the cytoplasm, and IE63p was found in both the cell nucleus and cytoplasm (data not shown). VZV proteins were detected in epithelial cells, endothelial cells, nerves, and CD43+/CD68+ inflammatory cells in the epidermis and dermis in both chickenpox and zoster cases (Fig. 1 and 2). Among the six chickenpox specimens, all were positive for ORF29p, four were positive for IE63p, and five were positive for gC. Among the eight zoster specimens, all were positive for ORF29p, five were positive for IE63p, and five were positive for gC (Table 1). These results are consistent with our experience concerning the affinities of these antibodies for their target proteins. Five cases of Grover's disease, a noninfectious dermatosis, and three cases of HSV infection were included as controls and were negative (Table 1), confirming that each antibody signal was specific. Detection of ORF29p, a DNA binding protein not present in the virion (13), in the nucleus of infected cells demonstrates that VZV replication occurs in endothelial cells, epithelial cells, and cells of the monocyte/macrophage lineage expressing surface CD43 and CD68. Although VZV genomes were detected in circulating lymphocytes of patients with chickenpox and zoster (16, 20) we detected VZV proteins only in CD43+/CD68+ cells and not in cells expressing CD3 or CD20 in these specimens.
FIG. 1.
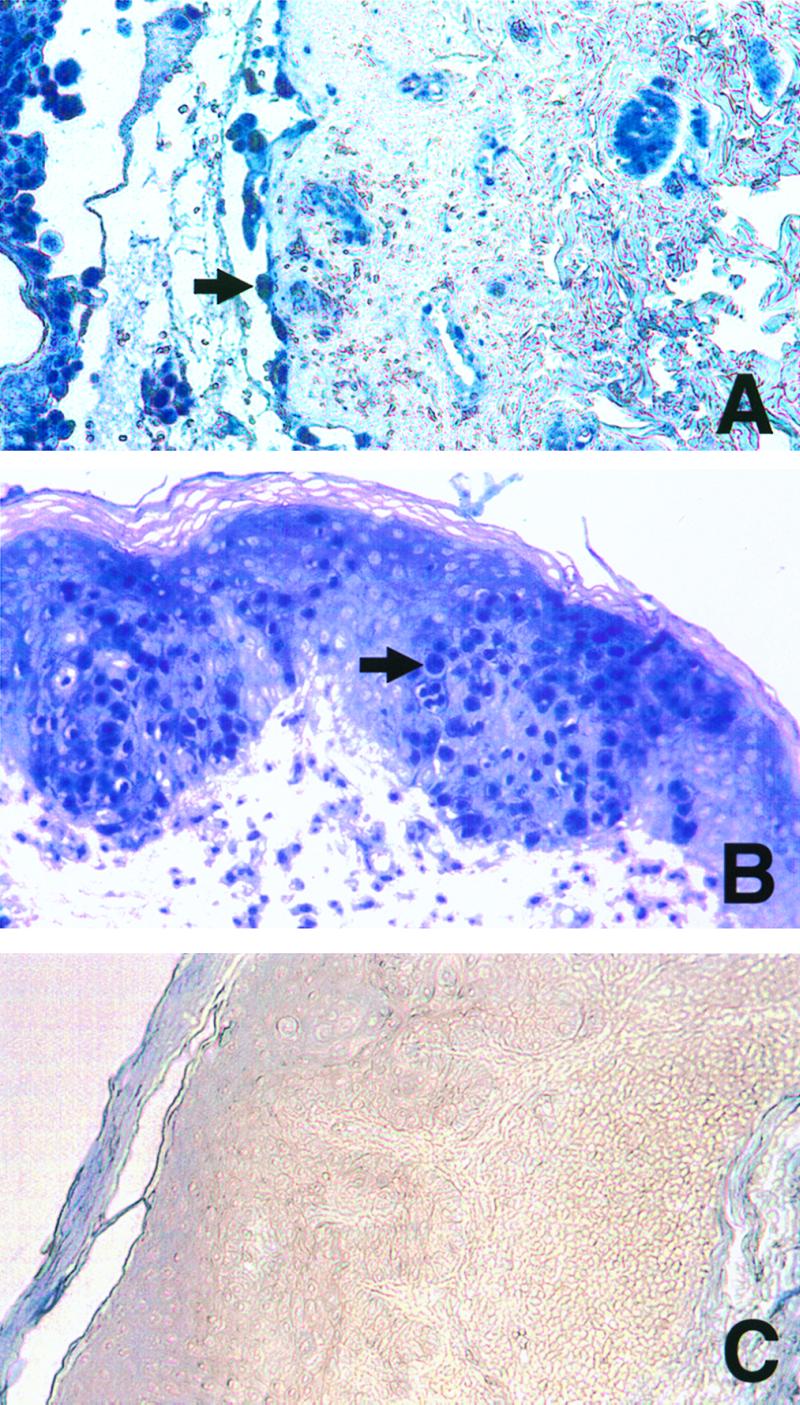
Immunohistochemical detection of ORF29p in skin biopsy samples. Chickenpox (A), zoster (B), and Grover's disease (C) skin lesions were analyzed for ORF29p as previously described (18), with the following exceptions. All washes were performed in Tris-buffered saline, and the signal was developed for 10 min in AP substrate (Vector Laboratories, Inc., Burlingame, Calif.), according to the manufacturer's recommendations, in the presence of levamisole to inhibit endogenous alkaline phosphatase activity. Arrows indicate positive epithelial cells. Magnification, ×100.
FIG. 2.
Immunohistochemical detection of ORF29p and CD43 in skin biopsy samples. Skin biopsy samples from a patient with chickenpox (A and B) or a patient with zoster (C and D) were probed for the presence of ORF29p (A and C) or ORF29p and CD43 (B and D) as described in the legend to Fig. 1. Red arrows indicate endothelial cells containing ORF29p. Black arrows indicate cells expressing CD43 that contain ORF29p. Magnification, ×600.
TABLE 1.
Detection of VZV proteins in skin biopsy samplesa
| Diagnosis | No. positive/no. examined | VZV proteinb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IE63p
|
ORF29p
|
gC
|
|||||||||
| EP | EN | NE | EP | EN | NE | WBCc | EP | EN | NE | ||
| CP | 6/6 | 4 | 3 | 0 | 6 | 4 | 2 | 4 | 4 | 5 | 3 |
| Z | 8/8 | 5 | 1 | 0 | 8 | 4 | 0 | 5 | 5 | 5 | 4 |
| G | 0/5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HSV-2 | 0/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Tissues from patients with clinical and histopathological diagnoses of chickenpox (CP), zoster (Z), Grover's disease (G) or HSV-2 were subjected to immunohistochemical analysis for immediate-early (IE63p), early (ORF29p), and late (gC) virus proteins in epithelial (EP), endothelial (EN), or inflammatory cells expressing CD43 (WBC) or in dermal nerves (NE).
Results are expressed as the absolute number of biopsy samples with detectable protein. Zero indicates the absence of detectable protein.
VZV-infected WBC from two chickenpox cases and two zoster cases were found to express CD68 and not CD3 or CD20.
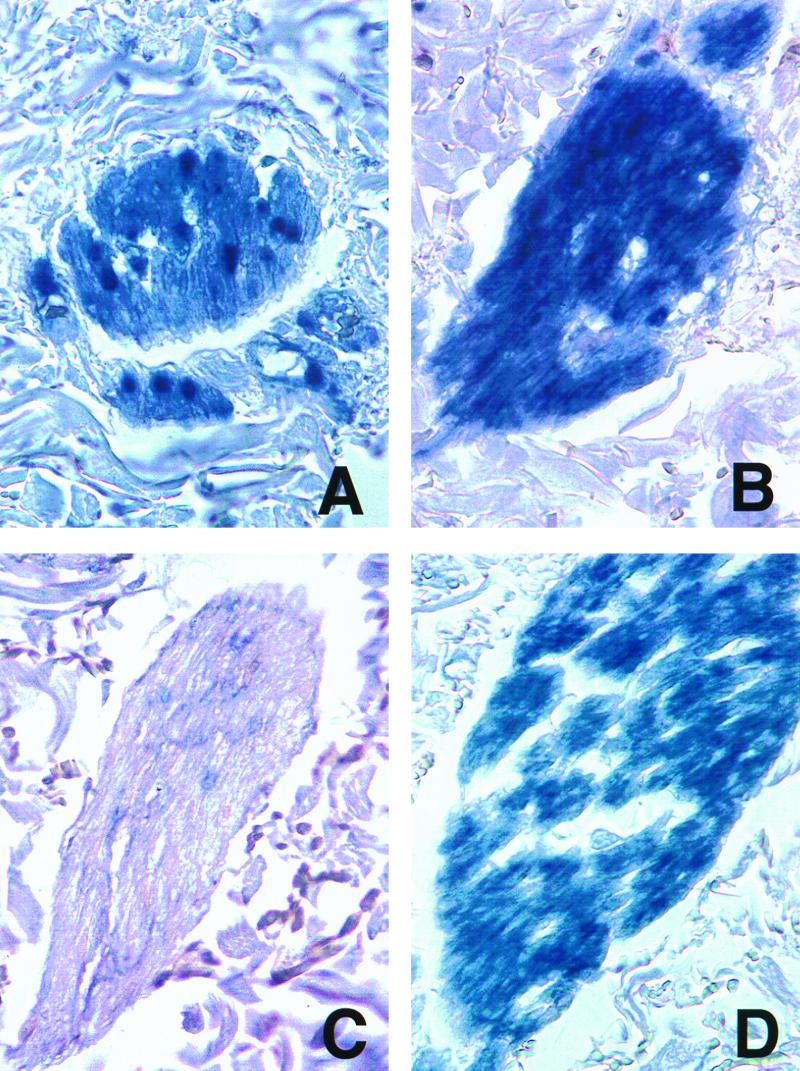
No differences in immunohistochemical staining were appreciated between specimens of chickenpox and zoster except for the presence of ORF29p in peripheral nerves in the dermis in two of the chickenpox specimens. ORF29p was detected in the Schwann cells and axons of nerves in these two cases (Fig. 3A), which included a biopsy sample that was obtained 2 days following the onset of the rash. Cytoplasmic localization of ORF29p is not consistent with the presence of replicating VZV or of formed virions. In contrast, ORF29p was not detected in axons or Schwann cells in the seven zoster biopsy samples with peripheral nerves apparent in the analyzed sections (Fig. 3C). The absence of ORF29p from axons during zoster was not surprising, as this protein localizes to the nucleus of productively infected cells, including neurons containing reactivating virus (18), and is not detected in virions (13). As expected, gC was found in both axons and Schwann cells of nerves in three chickenpox cases and four zoster cases (Fig. 3B and D). Detection of gC indicates the presence of virions or replicating VZV at these sites. The significance of infection of Schwann cells in chickenpox and zoster is unclear. Schwann cells cultivated in tissue culture are permissive for VZV infection (4), but the role of these cells in pathogenesis is not known.
FIG. 3.
Immunohistochemical detection of ORF29p and gC in skin biopsy samples. Sections of nerves in the dermis underlying chickenpox (A and B) or zoster (C and D) lesions underwent immunohistochemistry for ORF29p (A and C) or gC (B and D) as described in the legend to Fig. 1. Magnification, ×400.
Secretion of ORF29p by infected cells in tissue culture.
Clinical and laboratory evidence suggests that DRG are infected via the peripheral nerves during the exanthem of chickenpox (11, 21, 26). Although hematogenous and axonal spread of virus are not mutually exclusive, given the small numbers of VZV-infected circulating PBMCs (16, 20) it seems unlikely that a substantial number of neurons are infected without amplification in the epidermis and dermis. Nonetheless, the exact mechanism by which VZV reaches DRG remains unsettled.
Entry of virus particles into peripheral axons during chickenpox cannot account for the presence of ORF29p at this site because this protein is not a component of the virion (13). Assuming that the virus spreads from the skin to the peripheral nerves during the exanthem, the appearance of ORF29p in the nerve within 2 days of rash onset is surprising because of the distance between the peripheral axon and the sensory neuron in the DRG. By analogy with HSV, it is thought that VZV entering the axon in the epidermis travels by retrograde axonal transport (2) at a rate of 200 to 400 mm/day (28). Additional time would be required for VZV proteins to be produced in the neuron in the DRG and then to travel to the dermis and epidermis by anterograde axonal transport. If the DRG were infected during viremia prior to the onset of the rash, virus replication in the neuron and anterograde axonal transport of VZV proteins could occur. However, this would not explain the presence of ORF29p in peripheral axons, because ORF29p localizes to the nucleus rather than to the cytoplasm during productive infection (18). Moreover, ORF29p was not found in peripheral axons during zoster.
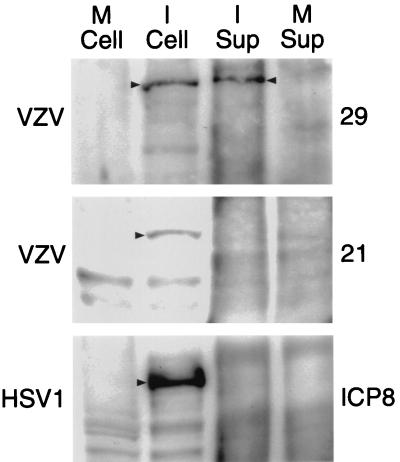
We therefore postulated that ORF29p may be secreted by VZV-infected cells in the dermis or epidermis and enter peripheral axons by endocytosis. In order to test whether ORF29p was secreted by VZV-infected cells, tissue culture media from uninfected human embryonic lung fibroblasts (HELF) or HELF infected with VZV or HSV-1 was clarified by centrifugation and filtration to remove detached cells. Immunoprecipitation with antibodies to ORF29p; ORF21p, a putative VZV accessory DNA binding protein; or ICP8, the HSV-1 homologue of ORF29p, was performed and the precipitated proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. ORF29p was detected in culture supernatants of infected cells but not in culture supernatants of uninfected cells (Fig. 4). ORF21p was not detected in supernatants of VZV-infected cells, and ICP8 was not detected in supernatants of HSV-1-infected cells. Thus, ORF29p is secreted by infected fibroblasts in tissue culture.
FIG. 4.
Western blot analyses of VZV and HSV-1 proteins. ORF29p, ORF21p, and ICP8 were detected in mock-infected cell extracts (M Cell) and supernatants (M Sup) or cell extracts (I Cell) and supernatants (I Sup) infected with the viruses denoted on the left. The proteins were immunoprecipitated and detected using the antibodies denoted on the right. Arrowheads denote the proteins of interest.
Endocytosis of secreted ORF29p by neurons in tissue culture.
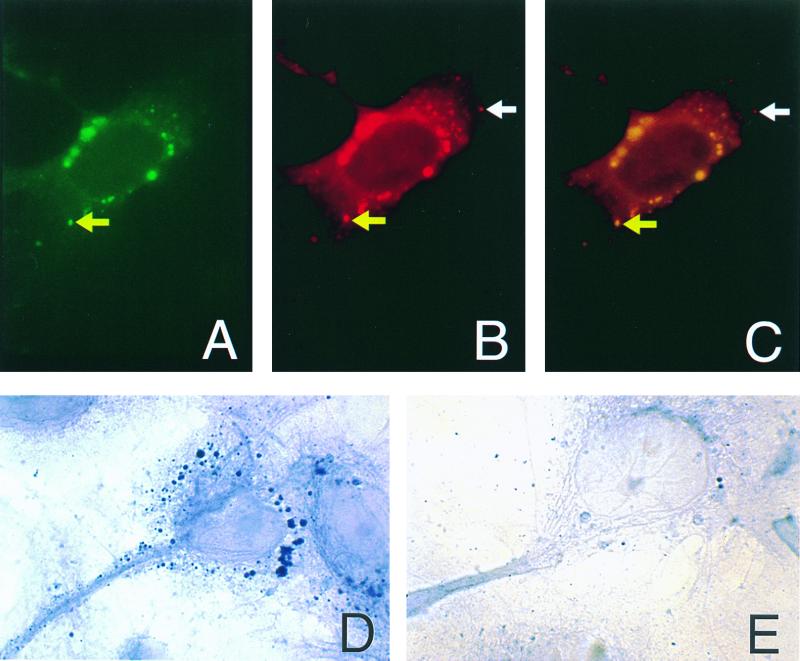
Filtered tissue culture medium from VZV-infected HELF and LysoTracker Red DND-99 (Molecular Probes, Eugene, Oreg.), a label for acidic endocytic vesicles, were applied to cultivated human neurons (hNTs) (Stratagene, La Jolla, Calif.) to determine if secreted ORF29p entered neurons by endocytosis. After incubating the hNTs with the filtered culture medium and LysoTracker for 2 h, the cells were examined by immunohistochemistry for the presence of ORF29p. ORF29p was detected in cytoplasmic vesicles (Fig. 5A and D) that colocalized with LysoTracker (Fig. 5C). ORF29p was not found in untreated hNTs (Fig. 5E). Therefore, extracellular ORF29p can enter hNTs by endocytosis, supporting our hypothesis that the presence of this protein in peripheral axons may result from its assimilation from surrounding cells that are infected with VZV.
FIG. 5.
Immunohistochemical detection of ORF29p in hNTs. hNTs treated with VZV-infected cell supernatants and LysoTracker were analyzed by immunohistochemistry for the presence of ORF29p. Yellow arrows indicate ORF29p (A), LysoTracker (B), and colocalization of ORF29p and LysoTracker in the merged image (C). White arrows indicate an endocytic vesicle that does not contain ORF29p (B and C). ORF 29p is restricted to cyptoplasmic vesicles in the treated hNTs (D). Untreated hNTs do not contain ORF29p (E).
Our results illustrate key steps of VZV pathogenesis. During chickenpox, VZV infects epithelial cells, endothelial cells, cells of the monocyte/macrophage lineage, and nerves of the skin. After infecting the neuron, the virus enters latency. In some individuals, the virus reactivates in one or more neurons, travels via the axon to the skin, and infects the epithelial cells. In addition, endothelial cells are infected in zoster, which could potentially spread virus to other areas. That VZV does not typically spread outside of the dermatome during zoster implies that host immunity effectively halts cell-to-cell spread. This study suggests that entry of VZV into the nervous system during primary infection may not rely solely on axonal transport of mature virions from the skin during chickenpox, because ORF29p was present in axons early in the course of the rash.
Acknowledgments
This work was supported by NIH grants AI-01409 (to P.W.A.) and AI-124021 (to A.A.G. and S.J.S.) and by a Pilot Award from the Columbia Presbyterian Medical Center Office of Clinical Trials (to P.W.A.).
We thank Michael Gershon, Giorgio Catoretti, and Sharon Steinberg for assistance with this work.
REFERENCES
- 1.Annunziato P, Lungu O, Gershon A, Silvers D, LaRussa P, Silverstein S. In situ hybridization detection of varicella zoster virus in paraffin-embedded skin biopsy samples. Clin Diagn Virol. 1996;7:69–76. doi: 10.1016/s0928-0197(96)00252-8. [DOI] [PubMed] [Google Scholar]
- 2.Arvin A. Varicella-zoster virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2547–2585. [Google Scholar]
- 3.Asano Y, Itakura N, Hiroishi Y, Hirose S, Nagai T, Ozaki T, Yazaki T, Yamanishi Y, Takahashi M. Viremia is present in incubation period in nonimmunocompromised children with varicella. J Pediatr. 1985;106:69–71. doi: 10.1016/s0022-3476(85)80468-6. [DOI] [PubMed] [Google Scholar]
- 4.Assouline J G, Levin M J, Major E O, Forghani B, Straus S, Ostrove J M. Varicella-zoster virus infection of human astrocytes, Schwann cells, and neurons. Virology. 1990;179:834–843. doi: 10.1016/0042-6822(90)90152-h. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J, Straus S. Varicella-zoster virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2525–2546. [Google Scholar]
- 6.Cohrs R J, Barbour M, Gilden D. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J Virol. 1996;70:2789–2796. doi: 10.1128/jvi.70.5.2789-2796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohrs R J, Barbour M B, Mahlingham R, Wellish M, Gilden D. Varicella-zoster virus (VZV) transcription during latency in human ganglia: prevalence of VZV gene 21 transcripts in latently infected human ganglia. J Virol. 1995;69:2674–2678. doi: 10.1128/jvi.69.4.2674-2678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohrs R J, Srock K, Barbour M B, Owens G, Mahlingham R, Devlin M, Wellish M, Gilden D. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J Virol. 1994;68:7900–7908. doi: 10.1128/jvi.68.12.7900-7908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croen K D, Ostrove J M, Dragovic L Y, Straus S E. Patterns of gene expression and sites of latency in human ganglia are different for varicella-zoster and herpes simplex viruses. Proc Natl Acad Sci USA. 1988;85:9773–9777. doi: 10.1073/pnas.85.24.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esiri M, Tomlinson A. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 11.Hope-Simpson R E. The nature of herpes zoster: a long term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy P, Grinfeld E, Gow J. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc Natl Acad Sci USA. 1998;95:4658–4662. doi: 10.1073/pnas.95.8.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinchington P, Hougland J, Arvin A, Ruyechan W, Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992;66:359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinchington P R, Bookey D, Turse S E. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J Virol. 1995;69:4274–4282. doi: 10.1128/jvi.69.7.4274-4282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinchington P R, Ling P, Pensiero M, Ruyechan W T, Hay J. The glycoprotein products of varicella-zoster virus gene 14 and their defective accumulation in a vaccine strain (Oka) J Virol. 1990;64:4540–4548. doi: 10.1128/jvi.64.9.4540-4548.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koropchak C, Graham G, Palmer J, Winsberg M, Ting S, Wallace M, Prober C, Arvin A. Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis. 1991;163:1016–1022. doi: 10.1093/infdis/163.5.1016. [DOI] [PubMed] [Google Scholar]
- 17.Lungu O, Annunziato P, Gershon A, Stegatis S, Josefson D, LaRussa P, Silverstein S. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc Natl Acad Sci USA. 1995;92:10980–10984. doi: 10.1073/pnas.92.24.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lungu O, Panagiotidis C, Annunziato P, Gershon A, Silverstein S. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden D. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci USA. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mainka C, Fuss B, Geiger H, Hofelmayr H, Wolff M. Characterization of viremia at different stages of varicella-zoster virus infection. J Med Virol. 1998;56:91–98. doi: 10.1002/(sici)1096-9071(199809)56:1<91::aid-jmv15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Mazur H, Dolin R. Herpes zoster at the NIH: a 20 year experience. Am J Med. 1978;65:738–744. doi: 10.1016/0002-9343(78)90791-x. [DOI] [PubMed] [Google Scholar]
- 22.Meier J L, Holman R P, Croen K D, Smialek J E, Straus S E. Varicella-zoster virus transcription in human trigeminal ganglia. Virology. 1993;193:193–200. doi: 10.1006/viro.1993.1115. [DOI] [PubMed] [Google Scholar]
- 23.Nikkels A, Delvenne P, Debrus S, Sadzot-Delvaux C, Piette J, Rentier B, Pierard G. Distribution of varicella-zoster virus gpI and gpII and corresponding genome sequences in the skin. J Med Virol. 1995;46:91–6. doi: 10.1002/jmv.1890460202. [DOI] [PubMed] [Google Scholar]
- 24.Nikkels A F, Rentier B, Pierard G E. Chronic varicella-zoster virus skin lesions in patients with human immunodeficiency virus are related to decreased expression of gE and gB. J Infect Dis. 1997;176:261–264. doi: 10.1086/517262. [DOI] [PubMed] [Google Scholar]
- 25.Nikkels A F, Debrus S, Sadzot-Delvaux C, Piette J, Delvenne P, Rentier B, Pierard G E. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A. 1993;422:121–126. doi: 10.1007/BF01607163. [DOI] [PubMed] [Google Scholar]
- 26.Sadzot-Delvaux C, Merville-Louis M-P, Delree P, Marc P, Moonen G, Rentier B. An in vivo model of varicella-zoster virus latent infection of dorsal root ganglia. J Neurosci Res. 1990;26:83–89. doi: 10.1002/jnr.490260110. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer M H, Wu Y N, Chamberlin C J, Burgos C, Brodine S K, Bowler W A, LaRocco A, Oldfield E C, Wallace M R. Detection of varicella-zoster virus DNA in the oropharynx and blood of patients with varicella. J Infect Dis. 1992;166:885–888. doi: 10.1093/infdis/166.4.885. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz J. Biochemical control mechanisms in synaptic transmissions. In: Kandel E, Schwartz J, editors. Principles of neural science. New York, N.Y: Elsevier Science Publishing; 1984. pp. 121–131. [Google Scholar]
- 29.Tyzzer E E. The histology of skin lesions in varicella. J Med Res. 1906;14:361–392. [PMC free article] [PubMed] [Google Scholar]