Abstract
Background
Risk factors for cardiovascular disease, including elevated blood pressure, are known to increase risk of Alzheimer’s disease. There has been increasing awareness of the relationship between long-term blood pressure (BP) patterns and their effects on the brain. We aimed to investigate the association of repeated BP measurements with Alzheimer’s and vascular disease markers.
Methods
We recruited 1,952 participants without dementia between August 2015 and February 2022. During serial clinic visits, we assessed both systolic BP (SBP) and diastolic BP (DBP), and visit-to-visit BP variability (BPV) was quantified from repeated measurements. In order to investigate the relationship of mean SBP (or DBP) with Alzheimer’s and vascular markers and cognition, we performed multiple linear and logistic regression analyses after controlling for potential confounders (Model 1). Next, we investigated the relationship of with variation of SBP (or DBP) with the aforementioned variables by adding it into Model 1 (Model 2). In addition, mediation analyses were conducted to determine mediation effects of Alzheimer’s and vascular makers on the relationship between BP parameters and cognitive impairment.
Results
High Aβ uptake was associated with greater mean SBP (β = 1.049, 95% confidence interval 1.016–1.083). High vascular burden was positively associated with mean SBP (odds ratio = 1.293, 95% CI 1.015–1.647) and mean DBP (1.390, 1.098–1.757). High tau uptake was related to greater systolic BPV (0.094, 0.001–0.187) and diastolic BPV (0.096, 0.007–0.184). High Aβ uptake partially mediated the relationship between mean SBP and the Mini-Mental State Examination (MMSE) scores. Hippocampal atrophy mediated the relationship between diastolic BPV and MMSE scores.
Conclusions
Each BP parameter affects Alzheimer’s and vascular disease markers differently, which in turn leads to cognitive impairment. Therefore, it is necessary to appropriately control specific BP parameters to prevent the development of dementia. Furthermore, a better understanding of pathways from specific BP parameters to cognitive impairments might enable us to select the managements targeting the specific BP parameters to prevent dementia effectively.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01483-y.
Keywords: Blood pressure, Blood pressure variability, Amyloid-beta, Tau, Vascular burden, Hippocampal volume, Cognition
Background
Alzheimer’s disease (AD) is characterized by amyloid-beta (Aβ) and tau deposition [1]. Neuronal injury, neuroinflammation and vascular disease also play a crucial role in the pathogenesis of AD [2–4]. Cerebral small vessel disease (CSVD) is characterized by extensive white matter hyperintensities (WMH) [5]. Pathological studies have demonstrated that dementia with AD-type is more frequently associated with concurrent CSVD loads compared to other neurodegenerative illnesses [5]. The advancement of Aβ and tau positron emission tomography (PET) also enabled us to detect AD imaging markers in patients with CSVD lesions [4]. In fact, AD combined with CSVD is reported to be the most prevalent mixed pathology [6–8]. Out of the total number of individuals with dementia, 38.0% (19 out of 50) have both AD and infarcts, 30.0% (15 out of 50) have only pure AD, and 12% (6 out of 50) have vascular dementia [6].
Epidemiological studies have shown that hypertension is associated with increased risk for dementia [4, 9–11]. Increased mean blood pressure (BP) promotes white matter alterations, resulting in the development of WMH [12]. It is also associated with an increased rate of brain atrophy with or without the mediation of WMH [13, 14]. Furthermore, several studies suggest that the presence of hypertension may enhance the deposition of Aβ and tau in the brain [15, 16]. More recently, BP variability (BPV) has been related to an increased risk of dementia [17], which highlights the importance of understanding the role of various BP parameters in the development of dementia. Notably, there has been a growing focus on BPV over months to years (e.g., visit-to-visit BPV), as a modifiable risk factor for cerebrovascular illness and cognitive decline, independent of average BP levels [18–21]. However, the associations between specific BP parameters and markers of AD and vascular disease have not been extensively established yet. Specific BP parameters might have various associations with markers of AD and vascular disease, eventually contributing to the development of dementia [22, 23]. In addition, recent study has shown that managing patients at increased risk for cardiovascular events, intensive treatment to reduce BP was linked to decreased rates of fatal and nonfatal cardiovascular events, as well as overall mortality, compared to standard treatment [24, 25]. Thus, in the treatment of hypertension, it is necessary to target specific BP parameters to prevent the development of dementia. Furthermore, a better understanding of pathways from specific BP parameters to cognitive impairment might enable us to select the specific medications targeting the specific BP parameters to prevent dementia effectively.
Thus, in the present study, we investigated the effects of specific BP parameters on AD and vascular disease markers in individuals without dementia. Furthermore, we determined whether these AD and vascular disease markers might mediate the relationship between specific BP parameters and cognitive impairment. We hypothesized that each BP parameter would affect Aβ, tau uptake, hippocampal atrophy and development of WMH differently, which in turn leads to cognitive impairment.
Methods
Study participants
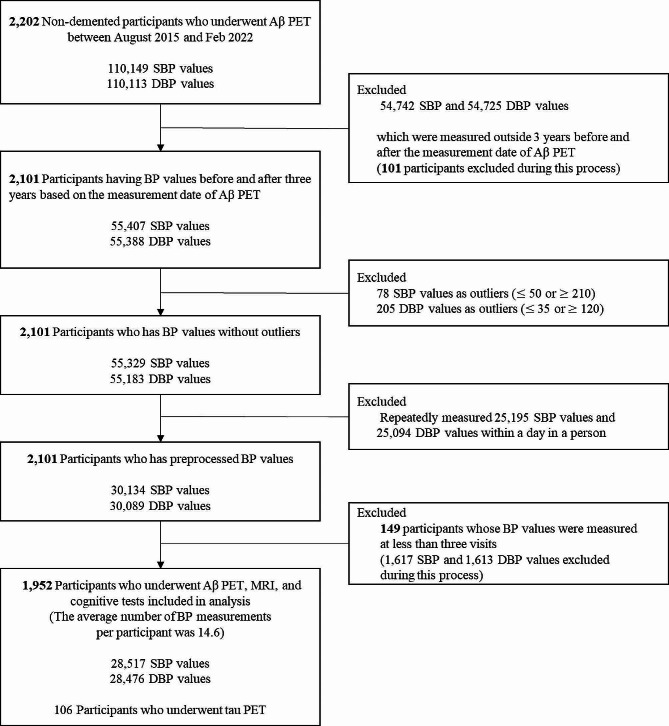
We enrolled 2,202 individuals without dementia who attended the memory clinic at Samsung Medical Center (SMC) in South Korea from August 2015 to February 2022 (Fig. 1). Health professionals conducted medical assessments utilizing standardized protocols. Every subject got a comprehensive evaluation that included a neurological examination, cognitive testing, standard blood tests, brain magnetic resonance imaging (MRI), and Aβ [18 F-florbetaben (FBB) or 18 F-flutemetamol (FMM)] PET scans. During the tests, we identified the vascular risk factors, including hypertension (defined as a self-reported medical history of hypertension or currently taking antihypertensive drugs), diabetes mellitus (defined as a self-reported history of diabetes mellitus or currently taking insulin or oral antidiabetic medications). The blood tests conducted on all individuals encompassed a complete blood count, blood chemistry analysis, vitamin B12/folate levels, syphilis serology, thyroid function panel, and apolipoprotein E (APOE) genotyping. Among them, 106 participants underwent tau [18 F-flortaucipir (FTP)] PET scans. We excluded participants with structural lesions such as brain tumor, large territorial infarct, and intracranial hemorrhage, as well as those with other causes of neurodegenerative disease including Parkinson’s disease, Lewy body dementia, progressive supranuclear palsy, cortico-basal syndrome, and frontotemporal dementia. Participants were further classified into cognitively unimpaired (CU) and mild cognitive impairment (MCI) groups. CU individuals in the study met the following criteria: (1) they had no medical history that would likely impact their cognitive function, as determined by Christensen’s health screening criteria [26], (2) they did not show any objective cognitive impairment in any cognitive domain, as measured by a comprehensive neuropsychological test battery, with scores at least − 1.0 standard deviation (SD) above age-adjusted norms, and (3) they were able to independently perform activities of daily living [27]. MCI participants met the following criteria [28]: (1) they or their caregiver reported subjective cognitive complaints; (2) they exhibited objective memory impairment below − 1·0 SD on verbal or visual memory tests; (3) they did not have significant impairment in their ability to do daily activities; and (4) they did not have dementia. When distinguishing between MCI and dementia, we used the Seoul-Instrumental Activities of Daily Living, and the cut-off score was 8 [29, 30].
Fig. 1.
Study flow chart
Abbreviations: Aβ, amyloid-beta; PET, positron emission tomography; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure
This study was approved by the Institutional Review Board of SMC (IRB No: 2019-11-177). Participants and their caregivers provided written informed consent for participating in the study and publication.
Cognitive assessment
The participants completed a standardized neuropsychological test battery known as the Seoul Neuropsychological Screening Battery (SNSB). This battery included tests that assessed attention, language, visuospatial abilities, memory, and frontal/executive functions [31]. The scored tests included the Digit Span Forward (DSF), Korean version of the Boston Naming Test (K-BNT), Rey-Osterrieth Complex Figure Test (RCFT: copying, immediate and 20-minute delayed recall, and recognition), Seoul Verbal Learning Test (SVLT: immediate, 20-minute delayed recall and recognition), phonemic and semantic Controlled Oral Word Association Test (COWAT), and the Stroop Test (color reading). The DSF was utilized to evaluate the level of attentiveness. Verbal and nonverbal learning and memory were evaluated using SVLT and RCFT. The K-BNT and RCFT assessments were conducted to assess language and visuospatial function, respectively. The phonemic and semantic COWAT and the Stroop Test were conducted to assess frontal/executive function. In addition, all participants underwent the Mini-Mental State Examination (MMSE) in order to evaluate their overall cognitive performance [32]. Abnormal scores were defined as those that fell below − 1.0 standard deviations from the age- and education-adjusted norms. The tests were conducted by experienced staff and overseen by clinical neuropsychologists who are certified by the board.
BP parameters
For each participant, an observation period of BP was defined as a period within three years before and after inspecting Aβ PET. We extracted systolic BP (SBP) and diastolic BP (DBP) records of all participants during their observation periods from the Clinical Data Warehouse of SMC. According to the guidelines in which the hospital follows [33, 34], the BP measurement was conducted with appropriate preparation, which included resting for 5 min in a quiet room and abstaining from smoking, alcohol, and caffeine for 30 min before to the measurement. Additionally, the cuff was placed at the level of the heart to ensure optimal posture. During each clinic visit, the patient’s seated BP and pulse were measured using an automated device. If needed, manual devices were used. The measurements were taken at regular intervals of 1–2 min [33]. We excluded patients with very severe hypertension or hypotension [24, 34–36]. Therefore, outliers of SBP (≤ 50 or ≥ 210) and DBP (≤ 35 or ≥ 120) values were removed. There were 1.84 SBP (range = 1–38) and 1.83 DBP (range = 1–36) records per participant on average in a single visit. When a participant had multiple records in a single day, the median of those records was selected as a BP value for the day. With this process, all subjects became have only one BP record per visit but could have multiple BPs over the entire follow-up time. For the next step, we excluded participants who had less than three BP values because BPV was not computable. Finally, 1,952 participants (729 CU and 1,223 MCI) were included in the analysis (Fig. 1). The average number of visits per participant during the entire follow-up time was 14.6 (range = 3-115).
Two different BP parameters, visit-to-visit mean BP and BPV for each subject, were considered in this study. Mean BPs and BPVs were defined as the averages and SDs of all BP values within a subject, respectively. SD was selected as the measure for BPV since the SD was a common measure for visit-to-visit variability [37].
First, the within-participant mean SBP and mean DBP were assessed with the averages of SBP and DBP values, respectively. Second, the within-participant systolic and diastolic BPVs were assessed with the SDs of SBP and DBP values since the SD is a common measure for visit-to-visit variability.
Brain MRI acquisition and measurement of hippocampal volumes
All participants received three-dimensional (3D) T1 turbo field echo images and 3D fluid-attenuated inversion recovery (FLAIR) at SMC utilizing a 3.0T MRI scanner (Philips 3.0T Achieva; Philips Healthcare, Andover, MA, USA), as previously described [38].
To measure hippocampal volumes (HV), we employed an automated method that involved a graph cut algorithm paired with an atlas-based segmentation and morphological opening as described in an previous study [39]. Intracranial volume (ICV) was determined by quantifying the combined the volumes of voxels contained within the the brain mask after the removal of the skull.
Assessment of vascular burden
The WMH visual rating scale, developed by the Clinical Research Center for Dementia of South Korea, was utilized to examine the presence of WMH in the deep subcortical and periventricular areas on FLAIR images [38–40]. We defined vascular positivity (V+) as severe levels of WMH visual rating scales according to our classification system for ischemia [39]. This classification system differentiates the intensity of CSVD markers, such as the volume of WMH [39]. In summary, deep WMH were categorized as D1 (< 10 mm), D2 (10–25 mm), or D3 (≥ 25 mm) according to the lesions’ longest diameter. The classification of periventricular WMH was based on their maximum length measured perpendicular to the ventricle (cap) and horizontally (band). WMH were categorized as P1 if their length was less than 5 mm, P2 if it ranged from 5 to 10 mm, and P3 if it was equal to or greater than 10 mm. There was a total of 9 cells resulting from the combination of D and P ratings. The overall severity of WMH (minimal, moderate, and severe) was determined based on the following combinations of D and P ratings: minimum (D1P1, D1P2), moderate (D1P3, D2P1, D2P2, D2P3, D3P1, D3P2), and severe (D3P3) [40]. In order to evaluate the interrater reliability of our WMH visual rating, we randomly assigned 100 FLAIR images and had 3 experienced neurologists perform a visual rating of the WMH severity. The interrater agreement for the overall severity of WMH was excellent, with a Fleiss k value of 0.84.
Amyloid PET imaging acquisition and analysis
Each participant had either FBB or FMM PET at SMC using a Discovery STe PET/CT scanner (GE Medical Systems, Milwaukee, WI, USA) in 3D scanning mode. This mode examines 47 slices of 3.3 mm-thickness that cover the entire brain [41]. CT images were obtained using a 16-slice helical CT scanner with a 140 keV energy level and 80 mA current. This section width of each image was 3.75 mm, and these images were used for attenuation correction. As per the guidelines provided by the makers of the ligands, a 20-min emission PET scan was conducted using dynamic mode (consisting of 4 × 5 min frames). This scan was performed 90 min after injecting an average dose of 311.5 MBq of FBB or 185 MBq of FMM. The 3D PET images were created using the ordered-subsets expectation-maximization (OSEM) algorithm (FBB iterations = 4 and subset = 20; FMM iterations = 4 and subset = 20). The images were reconstructed in a 128 × 128 × 48 matrix with a voxel size of.
2.00 × 2.00 × 3.27 mm3. The PET data were aligned with individual 3D-T1 weighted MR images, which were then standardized to the T1-weighted MNI-152 template utilizing Statistical Parametric Mapping (SPM) 8.
In our previous study, to improve the prediction of prognosis and early detection, we developed an MRI-based regional modified Centiloid (rdcCL) method that harmonizes the overall and regional Aβ uptake among Aβ ligands [42]. The reference region used in the Centiloid pipeline was the whole cerebellum. More details of the analysis method followed are in the original Centiloid project paper and a previous paper [42, 43]. MRI and PET images underwent spatial normalization using the transformation parameters obtained from the SPM8 [44].
Tau PET imaging acquisition and analysis
18F-Flortaucipir PET images were obtained using a Discovery STE PET/CT (GE Healthcare) at SMC and a Biographic mCT PET/CT scanner (Siemens Medical Solutions) at Gangnam Severance Hospital. Following the injection of intravenous bolus doses of 280 MBq 18F-flortaucipir, PET images were obtained during a 20-minute acquisition period at 80 min post-injection. Prior to the PET scan, we affixed a head holder to reduce head movement and obtained brain CT images for the purpose of attenuation correction. PET images were reconstructed in a three-dimensional matrix with dimensions of 128 × 128 × 47 with 2.00 × 2.00 × 3.27 mm voxel size at SMC and in a 256 × 256 × 223 matrix with 1.591 × 1.591 × 1 mm voxel size at Gangnam Severance Hospital using the OSEM algorithm (iteration = 6 and subset = 16).
Flortaucipir PET images were realigned and co-registered to the structural MRIs of participants using SPM12. To perform regional standardized uptake value ratio (SUVR) analysis, FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/) was used to generate region of interest (ROI) masks in the native spaces. Cerebellar gray matter was used as the reference region. For partial volume correction (PVC) of ROI data on the flortaucipir PET images, we used the region-based voxel-wise correction (RBV) method according to the PETPVC toolbox [45]. Consequently, we computed the regional SUVR with the PVC in 41 cortical areas. Then, we created bilateral Braak stage ROIs that anatomically represented the Braak staging regions associated with tau pathology in AD [46–50]. By combining non-overlapping ROIs from FeeSurfer, we established Braak ROIs categorized as Braak I/II, Braak III/IV, and Braak V/VI [51]. Specifically, flortaucipir SUVR using PETPVC applied Braak III/IV ROI [parahippocampal, fusiform, lingual, amygdala, inferior and middle temporal, temporal pole, thalamus, caudal anterior and rostral anterior cingulate, isthmus cingulate, posterior cingulate, and insula] was used.
Statistical analysis
The baseline characteristics were summarized using the mean ± SD for continuous variables and frequency (percentage) for categorical variables. First, to investigate the relationships of each mean BP with AD markers such as Aβ uptake, tau uptake, and HV, or cognition scores from the MMSE, we used multivariable linear regression models with an adjustment for potential confounders (Model 1). However, multivariable logistic regression analyses were conducted to identify the association between mean BP and WMH because its type was binary. Variables having P-values ≤ 0.2 in univariable analyses were selected as potential confounders. While age, sex, duration of education, hypertension, diabetes mellitus, and ICV were selected for HV, the presence of the APOE4 allele was selected instead of ICV for other markers. Second, in Model 2, SDs of BP were included as independent variables to identify the relationships of BPV with AD, CSVD markers and cognitive scores respectively, further adjusting for mean SBP and mean DBP. Third, we added interaction term of BP parameters and the presence of hypertension in the multivariable regression models. It was performed to determine whether the presence of hypertension has moderated effect on the association of BP parameters with AD markers, CSVD marker, or MMSE scores.
All results were presented as the regression coefficients (βs) and odds ratios (ORs) with 95% confidence intervals (CIs) from multivariable linear and logistic regression analyses, respectively. Log transformation was used to revise the skewed distribution of Aβ uptake before analyses, and all results were reported in the original scale. Especially, the results of multivariable linear regression analyses were reported with risk ratios (RRs) that are defined as the inverse-log transformed values of regression coefficients.
Causal mediation analyses were performed to examine the mediation effects of AD and vascular disease markers on the relationships between BP parameters and MMSE scores. Among BP parameters, candidate exposures were selected as those showing potential association with MMSE scores (P-values < 0.1) in the multivariable linear regression analyses with adjustment (Model 1 or 2). Among AD and vascular disease markers, candidate mediators were chosen as those having potential association with selected exposure (P-values < 0.1) in the multivariable analyses with adjustment (Model 1 or 2). We estimated the natural direct (NDE) and indirect effects (NIE) of BP parameters on MMSE scores using the imputation strategies of Vansteelandt based on the counterfactual framework with the medflex (version 0.6-7) package in R software [52]. Bootstrap-based standard errors were applied to calculate 95% CIs and P-values of NDEs and NIEs. The total effect was defined as the summation of NIE and NDE. By dividing NIE by the total effect, we assessed the proportion mediated that indicates the portion of the indirect effect among the total effect of the BP parameters on MMSE scores. Freedman’s proportion explained was also calculated to identify the extent of surrogacy of AD and vascular disease markers when only NIE was significant [53]. Statistical significance was declared with a two-sided p-value < 0.05. All analyses were performed using R 4.1.0 (Vienna, Austria; http://www.R-project.org).
Results
Clinical characteristics of participants
The demographic and clinical characteristics of the participants are shown in Table 1. Among 1,952 participants, 729 (37.4%) were CU and 1,223 (62.6%) were MCI. The mean age of participants was 71.4 ± 8.7 years and 1126 (57.7%) were females. In relation to vascular risk factors, the subjects had a prevalence of 955 (49.5%) for hypertension and 409 (21.2%) for diabetes mellitus, respectively.
Table 1.
Baseline characteristics of study participants
| Variables | Total (N = 1,952) |
|---|---|
| Demographic and clinical characteristics | |
| Age (years) | 71.4 ± 8.7 |
| Female | 1,126 (57.7) |
| Duration of education (years) | 12.1 ± 4.7 |
| Diagnosis | |
| CU | 729 (37.4%) |
| MCI | 1,223 (62.6%) |
| Ligand type | |
| 18F-Florbetaben | 566 (29.0%) |
| 18F-Flutemetamol | 1386 (71.0%) |
| Global Centiloid | 42.6 ± 52.0 |
| APOE ε4 carrier (missing N = 37) | 677 (35.4%) |
| Diagnosis of hypertension (missing N = 22) | 955 (49.5%) |
| Diagnosis of diabetes mellitus (missing N = 22) | 409 (21.2%) |
| ICV (cm3) | 1,320 ± 130 |
| BP measurements per participant | |
| Observation period (months) | 37.2 ± 18.7 |
| The average of intervals between BP values (months) | 3.2 ± 1.8 |
| Number of BP values | 14.6 ± 12.6 |
| SBP parameters (mm Hg) | |
| Mean SBP | 130.0 ± 12.0 |
| SD of SBP | 12.2 ± 4.2 |
| DBP parameters (mm Hg) | |
| Mean SBP | 69.6 ± 8.0 |
| SD of SBP | 7.8 ± 2.7 |
Continuous or categorical variables were represented as mean ± standard deviation or frequency (%), respectively
Abbreviations: N, number of participants; CU, cognitively unimpaired; MCI, mild cognitive impairment; APOE ε4, apolipoprotein E ε4 allele; ICV, Intracranial volume; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation
Association of BP parameters with Alzheimer’s and vascular disease markers
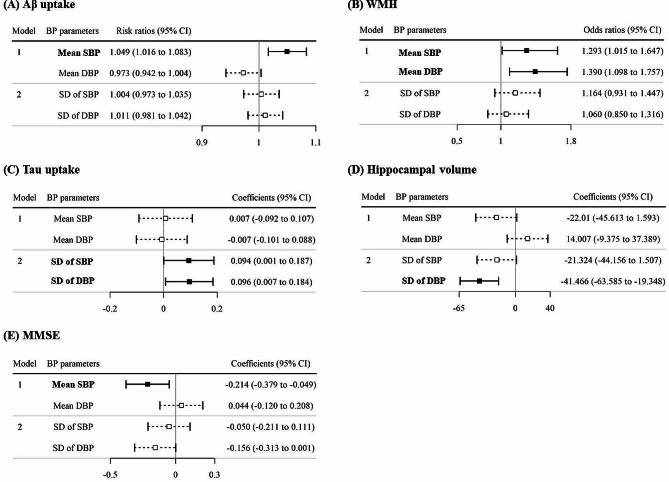
The forest plot in Fig. 2A shows the risk of Aβ uptake by BP parameters. The global Aβ Centiloid was positively associated with mean SBP (RR = 1.049, 95% CI, 1.016 to 1.083, P = 0.003). There was no association between the global Aβ Centiloid with the SDs of SBP (P = 0.805) and DBP (P = 0.476) or mean DBP (P = 0.085) (Supplementary Table 1).
Fig. 2.
Forest plots showing the association of BP parameters with Aβ uptake, WMH, tau uptake, hippocampal volume and MMSE expressed as risk ratios, odds ratios, or regression coefficients with the 95% confidence intervals. Bold lines in forest plots represent statistically significant associations
Abbreviations: Aβ, amyloid-beta; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; CI, confidence interval; Coefficients, regression coefficients; WMH, white matter hyperintensities; MMSE, Mini-Mental State Examination
In Fig. 2B, the forest plot shows the risk of developing WMH by BP parameters using the ORs. The risk elevation in WMH was associated with high mean SBP (OR = 1.293, 95% CI 1.015 to 1.647, P = 0.038) and mean DBP (OR = 1.390, 95% CI 1.098 to 1.757, P = 0.006). No association of WMH was found with SDs of SBP (P = 0.181) and DBP (P = 0.600) (Supplementary Table 2).
Figure 2C shows the expected tau uptake by BP parameters. Whereas the tau uptake did not show associations with mean BPs (P = 0.882 for SBP; P = 0.888 for DBP), it had positive associations with SDs of SBP (β = 0.094, 95% CI 0.001 to 0.187, P = 0.049) and DBP (β = 0.096, 95% CI 0.007 to 0.184, P = 0.034) (Supplementary Table 3).
The risk of hippocampal atrophy by BP parameters is shown in Fig. 2D. While other BP parameters were not associated (P = 0.068 for mean SBP; P = 0.240 for mean DBP; P = 0.067 for SD of SBP), a low HV was associated with high SD of DBP (β = -41.466, 95% CI -63.585 to -19.348, P = 0.0002) (Supplementary Table 4).
As a result of multivariable regression model of MMSE scores in Fig. 2E, mean SBP showed the association with MMSE (β = -0.214, 95% CI -0.379 to -0.049, P = 0.011). SD of DBP tended to be associated with MMSE (β = -0.156, 95% CI -0.313 to 0.001 P = 0.051). (Supplementary Table 5).
There were no interactive effects of history of hypertension and BP parameters on AD and CSVD markers and MMSE scores (Supplementary Tables 6–10).
Association of BP parameters with MMSE scores through the mediation of Alzheimer’s and vascular disease markers
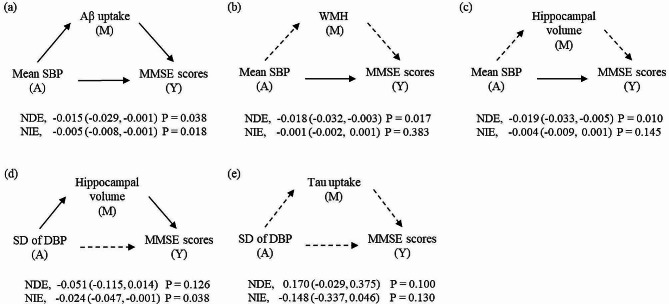
The relationship between mean SBP and MMSE scores was partially mediated by Aβ deposition (proportion mediated = 23.8%, 95% CI 22.7–44.2%), and was explained both directly and indirectly (NDE = -0.015, 95% CI -0.029 to -0.001, P = 0.038; NIE = -0.005, 95% CI -0.008 to -0.001, P = 0.018) (Fig. 3A). The direct effect between mean SBP and MMSE was observed (NDE = -0.018, 95% CI -0.032 to -0.003, P = 0.017), but there was no mediated effect of WMH (Fig. 3B). Likewise, only direct effect was identified between mean SBP and MMSE scores when the mediator was selected as HV (NDE = -0.019, 95% CI -0.033 to -0.005, P = 0.010; Fig. 3C). The relationship between SD of DBP and MMSE scores was mediated by HV (NIE = -0.024, 95% CI -0.047 to -0.001, P = 0.038) (Fig. 3D), and HV showed partial surrogacy (proportion explained = 0.32). Any significant effect was not observed when the exposure, mediator, and outcome were SD of DBP, tau uptake, and MMSE scores, respectively (Fig. 3E).
Fig. 3.
Causal relationship diagrams showing the natural direct and indirect effects of BP parameters on MMSE scores with mediation by Alzheimer’s and vascular markers. A, M, and Y in the diagram indicate the exposure, mediator, and outcome variables, respectively. Natural direct and indirect effects are represented as estimates (95% confidence intervals) with P-values. The solid or dashed lines indicate whether the effects are statistically significant or not
Abbreviations: Aβ, amyloid-beta; WMH, white matter hyperintensities; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; MMSE, Mini-Mental State Examination; NDE, natural direct effect; NIE, natural indirect effect
Discussion
In the present study, we investigated the effects of specific BP parameters on AD and vascular disease markers in carefully phenotyped and large-sized cohorts who underwent molecular and structural imaging. Our major findings are as follows. First, high Aβ uptake was associated with high mean SBP. Second, high vascular burden was associated with high mean SBP and DBP. Third, high tau uptake was associated with higher SBP and DBP variability. Fourth, higher DBP variability was predictive of hippocampal atrophy. Finally, high Aβ uptake partially mediated the relationships between high mean SBP and cognitive impairment. Hippocampal atrophy mediated the relationships between higher DBP variability and cognitive impairment. Taken together, our findings suggest that among the four BP parameters, each BP parameter affects AD and vascular disease markers differently, which in turn leads to cognitive impairment. Furthermore, our findings highlight the importance of targeting modifiable BP parameters to prevent the development of dementia effectively.
Our first major finding was that high Aβ uptake was associated with high mean SBP. Our finding is in line with previous studies suggesting that SBP increased Aβ burden in non-ε4 carriers [15]. Identifying the precise mechanism by which high SBP may contribute to the development of AD is challenging. However, our findings might be related to the fact that increased SBP is associated with microvascular damage and a compromised blood-brain barrier (BBB) [54]. The BBB plays a crucial function in the elimination of Aβ from the brain [55], therefore the impairment of the BBB could potentially lead to increased Aβ accumulation in the brain [56, 57].
We found that high vascular burden was associated with high mean SBP and mean DBP. According to a meta-analysis, SBP and DBP have a strong and largely consistent association with the severity of WMH [58]. It has been postulated that increased SBP and DBP might develop arterial stiffness, which in turn leads to alterations in cerebrovascular autoregulation, eventually resulting in decreased cerebral blood flow [59].
In the present study, higher SBP and DBP variabilities tend to be associated with high tau uptake. Our finding is consistent with a recent study showing that higher SBP and DBP variabilities were related to increased tau uptake in the temporal region [60]. Our finding might be explained by several hypotheses including ischemia-induced activation of cyclin-dependent kinase 5 (CDK-5) and glycogen synthase kinase 3β (GSK-3β), eventually resulting in the hyperphosphorylation of tau [61, 62].
Another notable finding is that a higher DBP variability was predictive of greater hippocampal atrophy. The results of our research align with a prior study that shown a correlation between a higher DBP variability over three years and a more pronounced decrease in brain atrophy [63]. This might be explained by several hypotheses including hemodynamic instability, inflammation and oxidative stress, arterial stiffness, small vessel disease, and autonomic dysfunction. Specifically, elevated BPV leads to fluctuations in cerebral blood flow, which in turn produce episodes of excessive and insufficient blood supply to the brain. This results in harm to the endothelial cells and smooth muscles in the brain, which in turn triggers damage to the neurovascular unit and initiates neuronal injury [22, 64]. The results further support the concept that BPV has a significant impact on brain structures. This is consistent with a previous study that proposed individuals with higher BPV undergo a faster neuronal shrinkage than expected during the normal aging process [65].
The final major finding was that specific BP parameters affect cognitive impairment through specific pathways. That is, high mean SBP affected cognitive impairment with and without the mediation of high Aβ uptake. Higher DBP variability also affected cognitive impairment with the mediation of hippocampal atrophy. Therefore, our findings suggested that to prevent the progression to dementia, the clinicians should consider specific BP parameters, including mean SBP levels and DBP variability, for the treatment of BP. In addition, imaging markers related to specific BP parameters could also be taken into consideration when monitoring the effects of BP treatment. We also did not find there were interactive effects of history of hypertension and BP parameters on AD and CSVD markers. It suggested that the effect of each BP parameter on these markers would not depend on the presence of hypertension. Thus, it is necessary to appropriately control specific BP parameters to prevent the development of dementia regardless of the history of hypertension.
The strengths of the present study include its prospective setting and the use of standardized Aβ and tau PET and MRI protocols in carefully phenotyped cohorts with a large number of participants who do not have dementia. However, there are several limitations in this study. First, we used Aβ and tau PET uptakes, along with the presence of severe WMH and cortical thickness on MRI, to assess Aβ, tau, CSVD, and neurodegenerative pathologies due to the unavailability of pathological confirmation. Thus, it is not possible to take into account other neurodegenerative disorders that contribute to neurodegeneration, such as tau, transactive response DNA-binding protein (TDP-43), argyrophilic grain disease, and hippocampal sclerosis. Second, due to the inherent difficulties associated with conducting a retrospective cohort study, information regarding the neuroimaging biomarker status of individuals at the beginning of the study was not accessible. Consequently, we were unable to determine their causal relationships. Nevertheless, a retrospective cohort research may be a realistic alternative due to the gradual emergence of changes in neuroimaging biomarkers and the cost associated with their evaluation. Third, due to the retrospective acquisition of BP parameters from the clinical data warehouse, there were variations in the length of follow-up among participants, despite controlling for the duration of follow-up in the process of calculating BPV changes. However, these findings may accurately represent the practical circumstances in real-life environments, thus providing valuable real-world evidence for healthcare decision-making. Next, throughout the period of the MRI scanning, we were unable to account for changes in medication or subclasses of antihypertensive medications. Because different antihypertensive treatments have distinct methods of lowering BP or components of BP, they may also have distinctive impacts on brain [66]. In addition, the range of BPV in the current sample could be limited as participants with a SBP (≤ 50 or ≥ 210) and DBP (≤ 35 or ≥ 120) were excluded. Therefore, it could be difficult to generalize our findings with solely based on this study. Moreover, we did not find the mediation effects of vascular burden on the relationships between BP parameters and cognitive impairment. Previously, our WMH visual rating scale represented cerebral small vessel diseases such as such as the volume of WMH [39]. However, our WMH scale did not seem to fully represent microvascular damage, compromised BBB and alterations in autoregulation. Vascular burden on the association between BP and cognitive impairment could be better explained by applying quantification of WMH volume in the future study. Another limitation is that the number of participants with tau PET was small compared to those with Aβ PET. This might be related to our inability to demonstrate mediating effects of tau on the association between SD of DBP and HV. Finally, we used assumptions regarding confounders in causal mediation analysis. While it is not feasible to completely eliminate all unmeasured factors that could influence the results, we made an effort to incorporate all conceivable variables that could have a substantial impact on the aforementioned associations.
In conclusion, each BP parameter differently affects AD and vascular disease markers, which in turn leads to cognitive impairment. Furthermore, our findings highlight the importance of targeting modifiable BP parameters to prevent the development of dementia. In future studies, it is necessary to continue collecting long-term repeated measurement data on BP, cognitive, structural, and functional brain changes to develop a strong evidence-based understanding of the pathomechanisms of hypertension-induced cognitive impairment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Eli Lilly and Company, enabled use of the 18 F-flortaucipir tracer by providing precursor, but did not provide direct funding and was not involved in data analysis or interpretation.
Abbreviations
- AD
Alzheimer’s disease
- 3D
three-dimensional
- APOE
apolipoprotein E
- Aβ
amyloid-beta
- BP
blood pressure
- BPV
blood pressure variability
- CDK-5
cyclin-dependent kinase 5
- CIs
confidence intervals
- COWAT
Controlled Oral Word Association Test
- CSVD
cerebral small vessel disease
- CU
cognitively unimpaired
- DBP
diastolic blood pressure
- DM
diabetes mellitus
- DSF
Digit Span Forward
- FBB
18 F-florbetaben
- FLAIR
fluid-attenuated inversion recovery
- FBB
18 F-florbetaben
- FMM
18 F-flutemetamol
- FTP
18 F-flortaucipir
- GSK-3β
glycogen synthase kinase 3β
- HV
hippocampal volumes
- ICV
intracranial volume
- K-BNT
Korean version of the Boston Naming Test
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MRI
brain magnetic resonance imaging
- NDE
natural direct effects
- NIE
natural indirect effects
- ORs
odds ratios
- OSEM
ordered-subsets expectation-maximization
- PET
positron emission tomography
- PVC
partial volume correction
- RBV
region-based voxel-wise correction
- rdcCL
regional modified Centiloid
- ROI
region of interest
- RRs
risk ratios
- SBP
systolic blood pressure
- SD
standard deviation
- SMC
Samsung Medical Center
- SNSB
Seoul Neuropsychological Screening Battery
- SPM
Statistical Parametric Mapping
- SUVR
standardized uptake value ratio
- SVLT
Seoul Verbal Learning Test
- WMH
white matter hyperintensities
Author contributions
SL, SK, KK, SS devised the project and the main conceptual ideas. SL, SK made contribution to drafting the work and KK, SS reviewed and revised the manuscript. SL, KK, SS analyzed the data and investigated the findings of the work. HJ, JK led the data collection. GS, YP, HH, YG and CP critically reviewed and edited the manuscript. HK, DN supervised the work. All authors read and approved the final manuscript and had final responsibility for the decision to submit for publication.
Funding
This research was supported by a grant of the Korea Dementia Research Project through the Korea Dementia Research Center (KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (grant number: RS-2020-KH106434); the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare and Ministry of science and ICT, Republic of Korea (grant number: RS-2022-KH127756); the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (NRF-2019R1A5A2027340); the Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government(MSIT) (No.RS-2021-II212068, Artificial Intelligence Innovation Hub); the Future Medicine 20*30 Project of the Samsung Medical Center [#SMX1240561]; the “Korea National Institute of Health” research project (2024-ER1003-00); the Medical data-driven hospital support project through the Korea Health Information Service (KHIS), funded by the Ministry of Health & Welfare, Republic of Korea.
Data availability
Anonymized and statistical information of all the participants was made available to and shared only among qualified investigators.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No: 2019-11-177). Written informed consent for participating in the study and publication was obtained from participants and their caregivers.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sungjoo Lee and Si Eun Kim contributed equally to this work.
Kyunga Kim and Sang Won Seo contributed equally to this work.
Contributor Information
Kyunga Kim, Email: kyunga.j.kim@gmail.com.
Sang Won Seo, Email: sw72.seo@samsung.com.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12 2:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer L, Schnabel J, Kazmierczak P, Ewers M, Schönecker S, Prix C, et al. Neuronal injury biomarkers for assessment of the individual cognitive reserve in clinically suspected Alzheimer’s disease. Neuroimage Clin. 2019;24:101949. doi: 10.1016/j.nicl.2019.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14 4:388–405. doi: 10.1016/s1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SE, Kim HJ, Jang H, Weiner MW, DeCarli C, Na DL, et al. Interaction between Alzheimer’s Disease and Cerebral Small Vessel Disease: a review focused on neuroimaging markers. Int J Mol Sci. 2022;23:18. doi: 10.3390/ijms231810490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s coordinating centre. Brain. 2013 doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69 24:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 7.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease - lessons from pathology. BMC Med. 2014;12 1:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85 22:1930–6. doi: 10.1212/wnl.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21 1:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim SE, Lee JS, Woo S, Kim S, Kim HJ, Park S, et al. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology. 2019;93(11):e1045–57. doi: 10.1212/WNL.0000000000008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decarli C. Vascular factors in dementia: an overview. J Neurol Sci. 2004;226(1–2):19–23. doi: 10.1016/j.jns.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Dijk EJv, Breteler MMB, Schmidt R, Berger K, Nilsson L-G, Oudkerk M, et al. The Association between blood pressure, hypertension, and cerebral White Matter lesions. Hypertension. 2004;44 5:625–30. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Wang W, Sang F, Zhang Z, Li X. White matter changes underlie hypertension-related cognitive decline in older adults. NeuroImage: Clin. 2023;38:103389. doi: 10.1016/j.nicl.2023.103389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, et al. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J Hypertens. 2013;31 8:1502–16. doi: 10.1097/HJH.0b013e32836184b5. [DOI] [PubMed] [Google Scholar]
- 15.Fungwe TV, Ngwa JS, Johnson SP, Turner JV, Ramirez Ruiz MI, Ogunlana OO, et al. Systolic blood pressure is associated with increased brain amyloid load in mild cognitively impaired participants: Alzheimer’s disease neuroimaging initiatives study. Dement Geriatr Cogn Disord. 2023;52(1). 10.1159/000528117 [DOI] [PMC free article] [PubMed]
- 16.Rizvi B, Lao PJ, Brickman AM. Blood pressure is associated with tau pathology independent of beta-amyloid. Alzheimer’s Dement. 2020;16 S5:e047483. doi: 10.1002/alz.047483. [DOI] [Google Scholar]
- 17.de Heus RAA, Tzourio C, Lee EJL, Opozda M, Vincent AD, Anstey KJ, et al. Association between blood pressure variability with dementia and cognitive impairment: a systematic review and Meta-analysis. Hypertension. 2021;78 5:1478–89. doi: 10.1161/hypertensionaha.121.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sible IJ, Nation DA. Visit-to-visit blood pressure variability and longitudinal tau Accumulation in older adults. Hypertension. 2021;79:629–37. doi: 10.1161/HYPERTENSIONAHA.121.18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst ME, Ryan J, Chowdhury EK, Margolis KL, Beilin LJ, Reid CM, et al. Long-term blood pressure variability and risk of Cognitive decline and Dementia among older adults. J Am Heart Assoc. 2021;10 13:e019613. doi: 10.1161/jaha.120.019613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HX, Fan QX, Xue SZ, Zhang M, Zhao JX. Twenty-four-hour blood pressure variability plays a detrimental role in the neurological outcome of hemorrhagic stroke. J Int Med Res. 2018;46 7:2558–68. doi: 10.1177/0300060518760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Havenon A, Fino NF, Johnson B, Wong KH, Majersik JJ, Tirschwell D, et al. Blood pressure variability and Cardiovascular outcomes in patients with prior stroke: a secondary analysis of PRoFESS. Stroke. 2019;50 11:3170–6. doi: 10.1161/strokeaha.119.026293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lattanzi S, Brigo F, Vernieri F, Silvestrini M. Visit-to-visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens (Greenwich) 2018;20 5:918–24. doi: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17 10:639–54. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A Randomized Trial of Intensive versus Standard Blood-Pressure Control N Engl J Med. 2015;373 22:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Final Report of a Trial of Intensive versus Standard Blood-Pressure Control N Engl J Med. 2021;384 20:1921–30. doi: 10.1056/NEJMoa1901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. Psychol Assessment: J Consulting Clin Psychol. 1991;3 2:168–74. doi: 10.1037/1040-3590.3.2.168. [DOI] [Google Scholar]
- 27.Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25 7:1071–6. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7 3:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung YH, Lee S, Kim WJ, Lee JH, Kim MJ, Han HJ. Effect of Integrated Cognitive Intervention Therapy in patients with mild to moderate Alzheimer’s Disease. Dement Neurocogn Disord. 2020;19 3:86–95. doi: 10.12779/dnd.2020.19.3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku HM, Kim JH, Lee HS, Ko HJ, Kwon EJ, Jo S, et al. A study on the reliability and validity of Seoul-activities of Daily Living(S-ADL) Ann Geriatr Med Res. 2004;8(4):206–14. [Google Scholar]
- 31.Ryu HJ, Yang DW. The Seoul Neuropsychological Screening Battery (SNSB) for Comprehensive Neuropsychological Assessment. Dement Neurocogn Disord. 2023;22 1:1–15. doi: 10.12779/dnd.2023.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s Continuum. Dement Neurocogn Disord. 2019;18 3:77–95. doi: 10.12779/dnd.2019.18.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H-L, Lee EM, Ahn SY, Kim K-i, Kim HC, Kim JH, et al. The 2022 focused update of the 2018 Korean hypertension society guidelines for the management of hypertension. Clin Hypertens. 2023;29(1):11. 10.1186/s40885-023-00234-9 [DOI] [PMC free article] [PubMed]
- 34.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: A Scientific Statement from the American Heart Association. Hypertension. 2019;73 5:e35–66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75 6:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 36.Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension the Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA) J Hypertens. 2023;41 12:1874–2071. doi: 10.1097/hjh.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 37.Yano Y. Visit-to-visit blood pressure variability—what is the current challenge? Am J Hypertens. 2016;30 2:112–4. doi: 10.1093/ajh/hpw124. [DOI] [PubMed] [Google Scholar]
- 38.Kang SH, Kim ME, Jang H, Kwon H, Lee H, Kim HJ, et al. Amyloid positivity in the Alzheimer/Subcortical-Vascular Spectrum. Neurology. 2021;96 17:e2201–11. doi: 10.1212/wnl.0000000000011833. [DOI] [PubMed] [Google Scholar]
- 39.Noh Y, Lee Y, Seo SW, Jeong JH, Choi SH, Back JH, et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J Stroke Cerebrovasc Dis. 2014;23(4):636–42. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Choi SH, Lee YM, Kim MJ, Kim YD, Kim JY, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr. 2015;27 12:2069–77. doi: 10.1017/s1041610215001076. [DOI] [PubMed] [Google Scholar]
- 41.Jang H, Jang YK, Kim HJ, Werring DJ, Lee JS, Choe YS, et al. Clinical significance of amyloid β positivity in patients with probable cerebral amyloid angiopathy markers. Eur J Nucl Med Mol Imaging. 2019;46 6:1287–98. doi: 10.1007/s00259-019-04314-7. [DOI] [PubMed] [Google Scholar]
- 42.Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD, Sr., Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11 1:1–15. doi: 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S-J, Ham H, Park YH, Choe YS, Kim YJ, Jang H, et al. Development and clinical validation of CT-based regional modified centiloid method for amyloid PET. Alzheimers Res Ther. 2022;14(1):157. 10.1186/s13195-022-01099-0 [DOI] [PMC free article] [PubMed]
- 44.Jang H, Kim JS, Lee HJ, Kim C-H, Na DL, Kim HJ, et al. Performance of the plasma Aβ42/Aβ40 ratio, measured with a novel HPLC-MS/MS method, as a biomarker of amyloid PET status in a DPUK-KOREAN cohort. Alzheimers Res Ther. 2021;13(1):179. 10.1186/s13195-021-00911-7 [DOI] [PMC free article] [PubMed]
- 45.Thomas BA, Cuplov V, Bousse A, Mendes A, Thielemans K, Hutton BF, et al. PETPVC: a toolbox for performing partial volume correction techniques in positron emission tomography. Phys Med Biol. 2016;61 22:7975. doi: 10.1088/0031-9155/61/22/7975. [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112 4:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82 4:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, Aβ-amyloid and the onset of Alzheimer’s disease. Neurosci Lett. 1996;210 2:87–90. doi: 10.1016/0304-3940(96)12668-9. [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18 4:351–7. doi: 10.1016/S0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathology Experimental Neurol. 2011;70 11:960–9. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 51.Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89 5:971–82. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vansteelandt S, Bekaert M, Lange T. Imputation strategies for the estimation of Natural Direct and Indirect effects. Epidemiol Methods. 2012;1(1):131–58. doi: 10.1515/2161-962X.1014. [DOI] [Google Scholar]
- 53.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11 2:167–78. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 54.Montgolfier Od, Pinçon A, Pouliot P, Gillis M-A, Bishop J, Sled JG, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and Cognitive decline in mice. Hypertension. 2019;73(1):217–28. doi: 10.1161/HYPERTENSIONAHA.118.12048. [DOI] [PubMed] [Google Scholar]
- 55.Ma Q, Zhao Z, Sagare AP, Wu Y, Wang M, Owens NC, et al. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener. 2018;13(1):57. 10.1186/s13024-018-0286-0 [DOI] [PMC free article] [PubMed]
- 56.Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, et al. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009;30(2):222–8. doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020;140 6:793–810. doi: 10.1007/s00401-020-02215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson I, Webb AJS. Consistency of associations of systolic and diastolic blood pressure with white matter hyperintensities: a meta-analysis. Int J Stroke. 2022;17 3:291–8. doi: 10.1177/17474930211043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ihara M, Yamamoto Y. Emerging evidence for Pathogenesis of sporadic cerebral small Vessel Disease. Stroke. 2016;47 2:554–60. doi: 10.1161/strokeaha.115.009627. [DOI] [PubMed] [Google Scholar]
- 60.Sible IJ, Nation DA, Weiner M, Aisen P, Petersen R, Jack CR, et al. Visit-to-visit blood pressure variability and longitudinal tau Accumulation in older adults. Hypertension. 2022;79 3:629–37. doi: 10.1161/HYPERTENSIONAHA.121.18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J Exp Med. 2017;214 11:3151–69. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, et al. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278 33:31277–85. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 63.Gutteridge DS, Segal A, McNeil JJ, Beilin L, Brodtmann A, Chowdhury EK, et al. The relationship between long-term blood pressure variability and cortical thickness in older adults. Neurobiol Aging. 2023;129:157–67. doi: 10.1016/j.neurobiolaging.2023.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state-of-the-art review. Am J Hypertens. 2020;33 12:1059–66. doi: 10.1093/ajh/hpaa119. [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, Yilmaz P, Bos D, Blacker D, Viswanathan A, Ikram MA, et al. Blood pressure variation and subclinical brain disease. J Am Coll Cardiol. 2020;75 19:2387–99. doi: 10.1016/j.jacc.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Luo H, Ma Y, Si S, Zhao H. Effects of Antihypertensive drugs on cognitive function in Elderly patients with hypertension: a review. Aging Dis. 2021;12 3:841–51. doi: 10.14336/ad.2020.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized and statistical information of all the participants was made available to and shared only among qualified investigators.