Abstract
RNA-dependent RNA polymerase (RdRp) encoded by positive-strand RNA viruses is critical to the replication of viral RNA genome. Like other positive-strand RNA viruses, replication of hepatitis C virus (HCV) RNA is mediated through a negative-strand intermediate, which is generated through copying the positive-strand genomic RNA. Although it has been demonstrated that HCV NS5B alone can direct RNA replication through a copy-back primer at the 3′ end, de novo initiation of RNA synthesis is likely to be the mode of RNA replication in infected cells. In this study, we demonstrate that a recombinant HCV NS5B protein has the ability to initiate de novo RNA synthesis in vitro. The NS5B used HCV 3′ X-tail RNA (98 nucleotides) as the template to synthesize an RNA product of monomer size, which can be labeled by [γ-32P]nucleoside triphosphate. The de novo initiation activity was further confirmed by using small synthetic RNAs ending with dideoxynucleotides at the 3′ termini. In addition, HCV NS5B preferred GTP as the initiation nucleotide. The optimal conditions for the de novo initiation activity have been determined. Identification and characterization of the de novo priming or initiation activity by HCV NS5B provides an opportunity to screen for inhibitors that specifically target the initiation step.
Hepatitis C virus (HCV) is recognized as the causative agent for most cases of non-A and non-B hepatitis (5, 12), with an estimated prevalence of 170 million worldwide (21). Upon first exposure to HCV, about 10 to 20% of infected individuals develop acute clinical hepatitis, while others appear to resolve the infection spontaneously. In most cases (80 to 90%), however, the virus establishes a chronic infection that persists for decades, which may lead to more severe disease states such as cirrhosis and hepatocellular carcinoma (18, 19). Currently, there is no broadly effective treatment for the debilitating progression of chronic HCV.
HCV is a positive-strand RNA virus belonging to the Flaviviridae family (15, 16). The genome of HCV encodes a single open reading frame which is translated into a polyprotein of about 3,010 amino acids (for reviews, see references 3 and 17). This polyprotein is subsequently processed by host as well as virally encoded proteases into at least 10 separate proteins with the following order (from the amino to the carboxy terminus): NH2 - C - E1 - E2 - p7 - NS2 - NS3 - NS4A - NS4B - NS5A - NS5B - COOH. The nonstructural proteins are believed to provide catalytic machinery for viral replication. One key enzyme encoded by HCV is NS5B, which has been shown to possess an RNA-dependent RNA polymerase (RdRp) activity (1, 4, 6–8, 13, 14). NS5B is thus believed to be an essential component in the HCV replication complex.
By itself, HCV NS5B RdRp appears to lack specificity for HCV RNA and can copy-back heterologous nonviral RNA or elongate on an oligonucleotide primer annealed to a homopolymeric RNA template (4, 6, 7, 13, 14). This lack of specificity for HCV RNA may reflect the notion that additional viral or host factors are required for specific recognition of the replication signal(s). Although HCV NS5B is capable of initiating RNA synthesis in a primer-dependent or copy-back fashion, a de novo pathway is likely to be the mode of replication in HCV-infected cells. Recently, NS5B of bovine viral diarrhea virus (BVDV), a virus closely related to HCV, has been shown to be able to initiate RNA synthesis by both primer-dependent and primer-independent (de novo) mechanisms (9, 23). In this study, we demonstrate that a recombinant HCV NS5B protein expressed in Escherichia coli could initiate RNA synthesis by a de novo mechanism from both viral and nonviral templates. The optimal conditions and substrate requirement for de novo initiation were defined.
RNA synthesis using HCV 3′ X-RNA as a template.
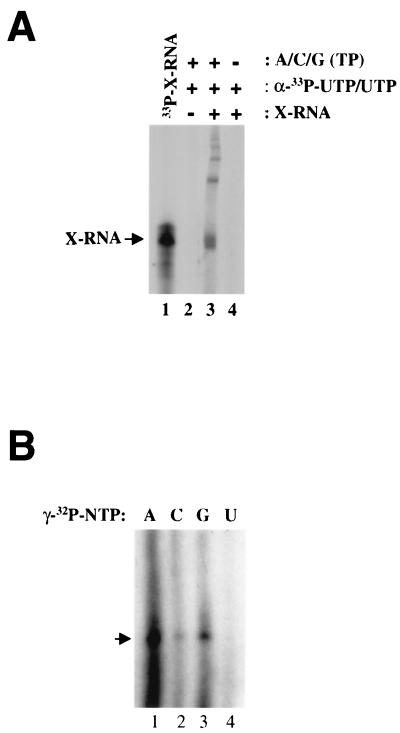
A recombinant HCV-1b (the BK isolate) NS5B was expressed in E. coli and purified to homogeneity as described previously (7). A 21-amino-acid hydrophobic region at the C terminus of the protein was removed, resulting in a highly soluble and enzymatically active NS5B protein (7). To determine whether the recombinant HCV NS5B could use a viral-specific sequence for primer-independent RNA synthesis in vitro, the 3′ X region, containing the last 98 nucleotides (nt) of HCV positive-strand RNA (11, 20, 22), was cloned into a transcription vector and the 3′ X-tail RNA (X-RNA) was transcribed in vitro using T7 RNA polymerase. We chose this sequence as the initial template because it is believed to contain a cis-acting promoter for directing the initiation of negative-strand RNA synthesis. As shown in Fig. 1A, no product was detected in the absence of the exogenous template RNA (lane 2), indicating a complete removal of host RNA during the purification process. When the 3′ X-RNA was added, HCV NS5B was able to direct synthesis of heterogeneous RNA products (Fig. 1A, lane 3). These products include template-length RNA as well as high-molecular-weight (HMW) species (Fig. 1A, lane 3). Synthesis of a monomer size product was surprising since it had been reported previously that HCV NS5B could use full-length HCV genomic RNA or the 3′-nontranslated region (341 nt) to produce near-dimer-size products via a copy-back mechanism. It was unlikely that the template-length product was the result of a contaminating host terminal transferase activity in the protein preparation, as it was produced only when all four nucleoside triphosphate (NTP) substrates were present (Fig. 1A, compare lanes 3 and 4). It is thus postulated that the template-length product was generated via a primer-independent (de novo) mechanism. To further support this notion, we established an alternative labeling method using various γ-32P-labeled NTPs to differentiate terminal labeling versus internal labeling. Only de novo-initiated products will retain the triphosphate (including the γ-phosphate label) at the 5′ terminus, while internally incorporated nucleotides (such as those incorporated via copy-back mechanism) will lose the γ-phosphate label. As shown in Fig. 1B, the template-length product could indeed be labeled with [γ-32P]ATP (lane 1), [γ-32P]GTP (lane 3), and weakly with [γ-32P]CTP (lane 2). [γ-32P]UTP failed to label the product (Fig. 1B, lane 4). This result indicated that the recombinant NS5B was capable of initiating de novo RNA synthesis. Furthermore, the labeling efficiency among the four [γ-32P]NTPs is in the order of ATP>GTP>>CTP>>>UTP and is consistent with the terminal sequence of the 3′ X-RNA (i.e., GU 3′) (note that GTP may form a base pair with the terminal uridylate in the template RNA). The weak labeling by [γ-32P]CTP might result from initiation at the penultimate position, forming a base pair with the guanylate.
FIG. 1.
RNA synthesis using HCV 3′ X-RNA as the template. (A) In vitro-transcribed HCV 3′ X-RNA consisting of the last 98 nt of HCV genomic RNA was tested for RNA synthesis by recombinant HCV NS5B. A standard reaction contained 20 mM Tris-Cl (pH 7.5), 0.6 mM MgCl2, 5 mM MnCl2, 5 mM dithiothreitol, 2% glycerol, 0.1% Tween 20, 50 μg of bovine serum albumin per ml, 200 to 300 ng of NS5B, 0.1 to 0.2 μg of 3′ X-RNA, 100 μM concentrations (each) of ATP, CTP, and GTP, 5 μM UTP, and 20 μCi of [α-33P]UTP label. The reaction mixture was incubated at 30°C for 1 h, and the labeled product was analyzed after separation on a 6% polyacrylamide–6 M urea–Tris-borate-EDTA gel (Norvex). Lane 1, end-labeled input template; lane 2, reaction with no RNA template added; lane 3, standard reaction in the presence of all four NTPs; lane 4, standard reaction in the presence of only UTP-[α-33P]UTP. (B) Labeling RNA product with [γ-32P]NTP. Standard RdRp reactions were performed with 100 μM (each) NTP plus 20 μCi of one of the four [γ-32P]NTP. Lane 1, [γ-32P]ATP; lane 2, [γ-32P]CTP; lane 3, [γ-32P]GTP; lane 4, [γ-32P]UTP.
De novo RNA synthesis using chemically synthesized small RNA as templates.
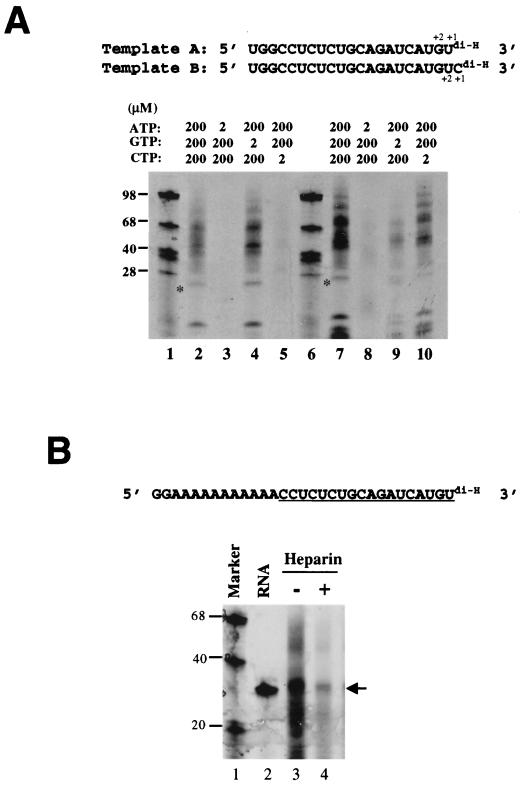
To further demonstrate that HCV NS5B can initiate de novo RNA synthesis, small RNAs (21 or 22 nt) representing the 3′-terminal sequence of HCV positive-strand RNA were chemically synthesized (templates A and B). Template B, with an extra nonviral cytidylate added to the 3′ terminus, was used to determine whether HCV NS5B uses a specific nucleotide for initiation. To prevent primer-dependent copy-back replication or nucleotidyl transfer reaction by any contaminating terminal transferase activity, the 3′-terminal uridylate (in template A) or cytidylate (in template B) was modified to have a dideoxyribose (Fig. 2A). This modification rendered the templates incapable of directing RNA synthesis via extension at the 3′ terminus. As shown in Fig. 2A, de novo-initiated products were observed with both RNA templates (lanes 2 and 7). The products include template-length (marked by asterisks in Fig. 2A), low-molecular-weight, as well as HMW, species. The template-length products represent complementary copies of the input RNAs, whereas the low-molecular-weight species probably resulted from abortive initiation and the HMW species probably resulted from template switching (2) or additional rounds of RNA synthesis due to the stuttering or slippage activities of the RNA polymerase (2, 9).
FIG. 2.
De novo initiation of RNA synthesis using small synthetic RNAs as templates. (A) Template A represents the last 21 nt of HCV genomic RNA, and template B contains an extra cytidylate at the 3′ terminus. Both RNAs contain a dideoxyribose at the 3′ termini (ddU and ddC), rendering them incapable of directing RNA synthesis via extension at the 3′ end. A standard RdRp assay was performed with 200 to 300 ng of NS5B protein, 0.1 μg of RNA template, 200 μM concentrations (each) of ATP, CTP, and GTP, and 20 μCi of [α-33P]UTP, unless indicated otherwise. Lanes 1 and 6, size markers; lanes 2 and 7, with 200 μM concentrations (each) of ATP, CTP, and GTP; lanes 3 and 8, with limiting concentration of ATP (2 μM); lanes 4 and 9, with limiting concentration of GTP (2 μM); lanes 5 and 10, with limiting concentration of CTP (2 μM). Lanes 2 to 5 and 7 to 10 were for templates A and B, respectively. (B) RdRp assay with an artificial template consisting of a nonviral sequence (GGAAAAAAAAAA) at the 5′ end and the HCV 3′-terminal sequence (underlined) at the 3′ end. Lane 1, size markers; lane 2, end-labeled input template; lane 3, labeled products from a standard reaction; lane 4, a 1 μM concentration of heparin was included in the reaction.
Interestingly, addition of the nonviral cytidylate to the 3′ terminus did not impair the efficiency of de novo initiation (Fig. 2A, lane 7). Rather, this addition reproducibly increased the synthesis by a factor of 3 to 5 (Fig. 2A, compare lanes 2 and 7). Products generated from template B were 1 nt larger than those from template A, suggesting that they both initiated at the 3′-terminal position. It has been shown with brome mosaic virus and BVDV RdRp that higher concentrations of the initiation nucleotide, which is GTP in both cases, are required for efficient initiation of de novo RNA synthesis (9, 10). We tested whether a similar feature also existed for HCV NS5B by examining the concentration effect of individual NTP substrate. As shown in Fig. 2A, de novo RNA synthesis from template A was completely abolished by low concentrations (2 μM) of ATP or CTP (lanes 3 and 5) but not of GTP (lane 4). Similarly, limiting ATP or GTP (Fig. 2A, lanes 8 and 9) significantly impaired synthesis from template B. These results demonstrated that HCV NS5B required higher concentrations of NTPs corresponding to the 3′-terminal (+1) and penultimate (+2) positions for efficient de novo initiation. This suggests that the initial priming steps at the +1 and +2 positions are rate limiting, confirming that HCV NS5B is capable of initiating de novo RNA synthesis. Based on the 3′-terminal sequence of the template RNA, HCV NS5B was able to use different NTPs as the initiation nucleotide (ATP for template A and GTP for template B), though GTP appeared to have a higher efficiency than ATP (compare RNA synthesis directed by template B versus template A in Fig. 2A, lane 7 versus lane 2).
The results in Fig. 2A also showed that a majority of the products generated from the two RNA templates were those of HMW species. The mechanism for synthesis of these HMW species is unclear. Several potential explanations have been proposed: (i) template switching (2) or (ii) continuous RNA synthesis from the nascent RNA without dissociation from the template (stuttering or slippage mechanism) (2, 9). To prevent the stuttering or template switching, a stretch of artificial sequence, GGAAAAAAAAAA, was designed and placed upstream of the HCV 3′-terminal sequence (the last 18 nt) (Fig. 2B). The GG pair reduces template switching by trapping the nascent RNA to the template (via stronger pairing of GC bases), and the stretch of A further reduces the stuttering or slippage of the polymerase since CC can't pair with A's. As expected, addition of this sequence significantly reduced production of the HMW species (Fig. 2B, lane 3), suggesting that generation of the HMW products was probably due to an intrinsic feature of the in vitro assay and may be prevented by modifying the template sequence. In addition, the total RNA synthesis was inhibited by heparin (Fig. 2B, lane 4). Since heparin can act as a polymerase trap, the reduction in RNA synthesis in the presence of heparin indicates that NS5B was capable of dissociating from its template and reinitiating on another template molecule for multiple rounds of RNA synthesis.
Optimal reaction conditions for de novo RNA synthesis.
Reaction conditions for de novo initiation of RNA synthesis by HCV NS5B were further optimized using a scintillation proximity assay (SPA). In this system, a 5′-biotinylated synthetic RNA (32 nt), Rcc (sequence in the legend to Fig. 3), which contains a dideoxynucleotide at the 3′ terminus, was used as the template. De novo-synthesized RNA products were then captured onto streptavidin-coated SPA beads through their complementarity to the biotinylated template RNA.
FIG. 3.
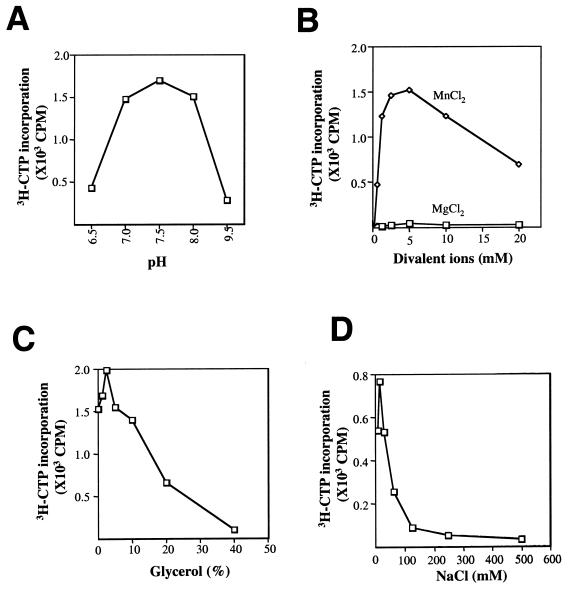
Effects of reaction conditions on de novo initiation by HCV NS5B. (A) Buffer pH; (B) Mg++ and Mn++ ions; (C) glycerol; and (D) NaCl. All other reagents were kept the same as described for the standard assay. The synthetic RNA, Rcc (32 nt), 5′ biotin-CGACUACUUACCGAUGGCUGAUUGCGACUACCdi-H 3′, was used as the template in the scintillation proximity assay containing 200 μM GTP, 10 μM CTP, ATP, and 2 μCi of 3H-UTP. The reactions were carried out at room temperature for 3 h in a 96-well plate, terminated by addition of 2 mM EDTA. The product was captured by adding strepavidin-coated SPA beads to the reaction mixture (note that the products were complementary to the biotinylated template strand and thus were captured along with the template RNA). After a 30-min binding period, the beads were washed thoroughly with standard washing buffer (7) and captured radioactivity was determined using a top counter (Packard, Meriden, Conn.).
As shown in Fig. 3, the optimal pH for de novo RNA synthesis was approximately 7.5 (Fig. 3A). Mn++ ions were essential for de novo initiation, with an optimal concentration of 5 mM. In contrast, Mg++ ion had minimal effect on the activity (Fig. 3B), suggesting that Mn++ is preferred for de novo initiation, whereas Mg++ has been shown to support primer-dependent RNA synthesis (4, 14). At lower concentrations (<25 mM), monovalent ion Na+ exhibited a modest stimulatory effect but was inhibitory at higher concentrations (Fig. 3D). In the case of glycerol, modest stimulation was observed at lower concentrations, which peaked at 2%, while higher concentrations were detrimental to the activity (Fig. 3C). Note that minimal amounts of salt and glycerol may be required to maintain the solubility of NS5B in the reaction mixture.
Substrate preference for de novo initiation of RNA synthesis by HCV NS5B.
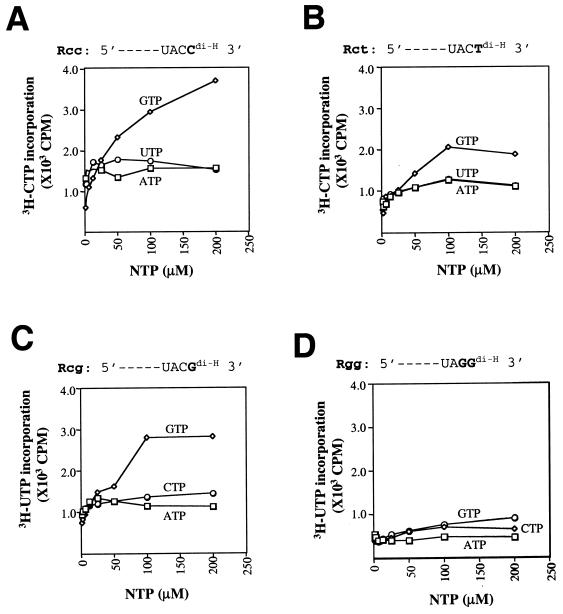
The results depicted in Fig. 2A suggest that, depending on the 3′-terminal nucleotide (uridylate in template A and cytidylate in template B), both ATP and GTP are capable of initiating RNA synthesis. Addition of the cytidylate to the 3′ terminus of template A, however, improved the activity by a factor of 3 to 5 (Fig. 2A, compare lanes 2 and 7). To further analyze more quantitatively whether a substrate preference or specificity exists at the initiation step, three small (32-nt) RNA templates, Rcc, Rct, and Rcg, were synthesized (Fig. 4A to C). Sequences of the RNAs were designed so that they each contained (i) similar numbers of each nucleotide, (ii) no stable secondary structure based on computer predictions, and (iii) a different dideoxynucleotide at the 3′ terminus (ddC, ddT, or ddG). The SPA was used to measure the activity of de novo RNA synthesis using the synthetic RNAs as templates and was subjected to various concentrations of a particular nucleotide (from 1.5 to 200 μM) (Fig. 4). It has been reported that higher concentrations of the initiation nucleotide are needed by brome mosaic virus and BVDV RdRp for the rate-limiting initiation step, whereas lower concentrations are sufficient for subsequent elongation steps (9, 10). These findings suggest that de novo initiation or priming of RNA synthesis is sensitive to the concentration of the initiation nucleotide, but not so to those of the noninitiating (elongating) nucleotides. As shown in Fig. 4A, de novo RNA synthesis directed by Rcc (5′ UACCdi-H 3′) was dependent on the GTP concentration; a dose response between GTP and RNA synthesis was observed. In comparison, varying concentrations of UTP or ATP had no significant impact. This result indicates that GTP is the initiation nucleotide for Rcc-directed RNA synthesis. In the cases of Rct (Fig. 4B) and Rcg (Fig. 4C), whose terminal nucleotides are ddT (UACTdi-H 3′) and ddG (UACGdi-H 3′), respectively, de novo RNA synthesis was not noticeably affected by varying ATP or CTP concentrations (Fig. 4B and C). This is surprising since they can form a base pair with the respective 3′-terminal nucleotide. Nevertheless, RNA synthesis from Rct or Rcg depends on higher concentrations of GTP, which reached the plateau at about 100 μM, suggesting that initiation at the penultimate cytidylate (+2) position may have occurred. To confirm this prediction, a fourth RNA template, Rgg, was made in which guanylate was present at both 3′-terminal (+1) and penultimate (+2) positions (UAGGdi-H 3′) (Fig. 4D). As expected, an increasing GTP concentration no longer stimulated RNA synthesis, confirming that cytidylate was required at the +1 or +2 position for efficient de novo initiation. In conclusion, despite the lack of a strict nucleotide specificity (as shown in Fig. 2A), HCV NS5B preferred GTP as the initiation nucleotide. Moreover, RdRp may be able to initiate at the less preferred penultimate cytidylate position if the terminal nucleotide mismatches with GTP (Fig. 4B and C). These data, taken collectively, confirmed the ability of recombinant HCV NS5B to initiate RNA synthesis by a de novo mechanism.
FIG. 4.
Nucleotide preference for de novo initiation of RNA synthesis. The standard SPA assays were performed with one of the following synthetic RNA templates: Rcc (5′ biotin-CGACUACUUACCGAUGGCUGAUUGCGACUACCdi-H 3′) (A), Rct (5′ biotin-CGACUACUUACCGAUGGCUGAUUGCGACUACTdi-H 3′) (B), Rcg (5′ biotin-CGACUACUUACCGAUGGCUGAUUGCGACUACGdi-H 3′) (C), or Rgg (5′ biotin-CGACUACUUACCGAUGGCUGAUUGCGACUAGGdi-H 3′) (D). Two microcurie of 3H-UTP or 3H-CTP was used as the labeling nucleotide. Various concentrations (1.5 to 200 μM) of each individual nucleotide were tested, with the concentrations of the remaining nucleotides at 10 μM.
Positive-strand RNA viruses cause a variety of diseases in humans. Critical to the replication of these viruses is the virally encoded RdRp. As the only known class of template-dependent polymerases that can initiate RNA synthesis de novo from the 3′ terminus of the template and that was found only in virus-infected cells, viral RdRp provides a very attractive target for development of antiviral therapeutics. Understanding the mechanism of HCV RNA replication provides potential benefits in identifying compounds that specifically target the replication process. In this study, we demonstrated that recombinant HCV NS5B could initiate RNA synthesis by a de novo mechanism. It seems to prefer GTP as the initiation nucleotide, though other nucleotides (such as ATP) were capable of initiating lower-level RNA synthesis when cytidylate was not present at the 3′-terminal or penultimate position, as in the cases of 3′ X-RNA (Fig. 1B) and template A (Fig. 2A). The initiation nucleotide requirement for HCV NS5B seemed to be less strict than that for BVDV NS5B, which only uses GTP for de novo initiation of RNA synthesis (9). This could be attributed to the fact that cytidylate is the 3′-terminal nucleotide for both positive- and negative-strands of BVDV RNA. For HCV, however, uridylate and cytidylate are present at the 3′ termini of positive- and negative-strand RNAs, respectively. It is possible that the less strict GTP requirement by HCV NS5B observed in the de novo initiation assay reflects the ability of HCV NS5B to use ATP as the initiation nucleotide for negative-strand RNA synthesis and GTP for positive-strand synthesis in vivo. In addition, the preference for GTP over ATP is also consistent with the asymmetric replication of positive-strand RNA viruses in that positive-strand RNA is more abundant than negative-strand RNA in infected cells. Lastly, our results also showed that no template specificity was observed in the de novo initiation by HCV NS5B. Both viral and nonviral sequences can be used as the templates in this assay. This may be due to the fact that the recombinant NS5B protein represents only a part of the viral replication complex and the other components in this complex may be required to provide the specificity function.
Establishment of an in vitro system for analyzing de novo RNA replication directed by HCV NS5B represents the first step in elucidating the requirements for initiation of RNA synthesis. With this system, it may be feasible to test whether other virally encoded proteins play a role in the initiation process and what elements in these viral proteins (including NS5B) are critical for such function.
Acknowledgments
We thank Gregory Reyes for support and Michael Endres, Bahige M. Baroudy, Fred Lahser, and Nanhua Yao for their critical reading of the manuscript.
REFERENCES
- 1.Al R H, Xie Y, Wang Y, Hagedorn C H. Expression of recombinant hepatitis C virus non-structural protein 5B in Escherichia coli. Virus Res. 1998;53:141–149. doi: 10.1016/s0168-1702(97)00147-0. [DOI] [PubMed] [Google Scholar]
- 2.Arnold J J, Cameron C E. Poliovirus RNA-dependent RNA polymerase (3Dpol) is sufficient for template switching in vitro. J Biol Chem. 1999;274:2706–2716. doi: 10.1074/jbc.274.5.2706. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager R. Molecular targets in inhibition of hepatitis C virus replication. Antivir Chem Chemother. 1997;8:281–301. [Google Scholar]
- 4.Behrens S-E, Tomei L, De Francesco R. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 1996;15:12–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–364. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 6.De Francesco R, Behrens S E, Tomei L, Altamura S, Jiricny J. RNA-dependent RNA polymerase of hepatitis C virus. Methods Enzymol. 1996;275:58–67. doi: 10.1016/s0076-6879(96)75006-1. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari E, Wright-Minogue J, Fang J W S, Baroudy B M, Lau J Y N, Hong Z. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J Virol. 1999;73:1649–1654. doi: 10.1128/jvi.73.2.1649-1654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii K, Tanaka Y, Yap C C, Aizaki H, Matsuura Y, Miyamura T. Expression of hepatitis C virus NS5B protein: characterization of its RNA polymerase activity and binding. Hepatology. 1999;29:1227–1235. doi: 10.1002/hep.510290448. [DOI] [PubMed] [Google Scholar]
- 9.Kao C C, Del Vecchio A M, Zhong W. De novo initiation of RNA synthesis by a recombinant Flavivirus RNA-dependent RNA polymerase. Virology. 1999;253:1–7. doi: 10.1006/viro.1998.9517. [DOI] [PubMed] [Google Scholar]
- 10.Kao C C, Sun J H. Initiation of minus-strand RNA synthesis by the brome mosaic virus RNA-dependent RNA polymerase: use of oligonucleotide primers. J Virol. 1996;70:6826–6830. doi: 10.1128/jvi.70.10.6826-6830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo G, Choo Q-L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, Tegtmeier G E, Bonino F, Colombo M, Lee W-S, Kuo C, Berger K, Shuster J R, Overby L R, Bradley D W, Houghton M. An assay for circulating antibodies to a major etiologic virus of human non-A non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 13.Lohmann V, Korner F, Herian U, Bartenschlager R. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J Virol. 1997;71:8416–8428. doi: 10.1128/jvi.71.11.8416-8428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann V, Roos A, Korner F, Koch J O, Bartenschlager R. Biochemical and kinetic analyses of NS5B RNA-dependent RNA polymerase of the hepatitis C virus. Virology. 1998;249:108–118. doi: 10.1006/viro.1998.9311. [DOI] [PubMed] [Google Scholar]
- 15.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Javis A W, Martelli G P, Mayo M A, Summers M D. Classification and nomenclature of viruses: sixth report of the International Committee on Taxonomy of Viruses. New York, N.Y: Springer-Verlag; 1995. pp. 424–426. [Google Scholar]
- 17.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 931–960. [Google Scholar]
- 18.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe T Y, Koi S, Onji M, Ohta Y, Choo Q-L, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimotohno K. Hepatitis C virus as a causative agent of hepatocellular carcinoma. Intervirology. 1995;38:162–169. doi: 10.1159/000150427. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Kato N, Cho M-J, Sugiyama K, Shimotohno K. Structure of the 3′ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Lancet. 1998;351:1415. [Google Scholar]
- 22.Yamada N, Tanihara K, Takada A, Yorihuzi T, Tsutsumi M, Shimomura H, Tsuji T, Date T. Genetic organization and diversity of the 3′ noncoding region of the hepatitis C virus. Virology. 1996;223:255–261. doi: 10.1006/viro.1996.0476. [DOI] [PubMed] [Google Scholar]
- 23.Zhong W, Gutshall L, Del Vecchio A M. Identification and characterization of an RNA-dependent RNA polymerase activity within the nonstructural protein 5B of bovine viral diarrhea virus. J Virol. 1998;72:9365–9369. doi: 10.1128/jvi.72.11.9365-9369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]