Abstract
High levels of resistance to challenge with human immunodeficiency virus type 1 SF162 were observed in animals engrafted with peripheral blood mononuclear cells of four long-term nonprogressors (LTNPs). Resistance was abrogated by depletion of CD8+ T cells in vivo and was observed only in LTNPs with proliferative responses to p24. In a subgroup of nonprogressors, CD8+ T cells mediated restriction of challenge viruses, and this response was associated with strong proliferative responses to p24 antigen.
Although patients with normal CD4+ T-cell counts and low levels of virus in plasma are a heterogeneous group, a small subgroup of patients with truly nonprogressive human immunodeficiency virus (HIV) infection likely hold important clues to the basis of an effective immune response to HIV. It now appears clear that a large fraction of patients previously considered long-term nonprogressors (LTNPs) ultimately show a decline of CD4+-T-cell numbers. Members of a small subpopulation (<0.8% of HIV-infected individuals) show no signs of progression over a 10-year period (12, 22, 23, 36). Extensive studies have demonstrated strong cellular and humoral HIV-directed responses in LTNPs (2, 6, 7, 15, 18, 29, 31, 32). Regardless of the host or virus factors involved in nonprogression in these patients, a clear demonstration of immunity-mediated resistance to challenge virus and targets of such a response within HIV would enhance development of an effective HIV vaccine. Recently we established a human HIV-peripheral blood mononuclear cell (PBMC)-SCID mouse model, a modification of the method developed by Mosier et al. (13, 26, 28), to study the PBMC of infected patients (5). We determined whether PBMC of LTNPs support replication of patients' autologous viruses in this model and further whether these PBMC mediate restriction of challenge-virus replication.
Engraftment of CB-17 SCID mice and sample collection were performed as previously described (5). Animals were challenged intraperitoneally with HIVSF162 on day 7 and sacrificed on day 21. To deplete CD8+ T cells, on day 6 animals received 0.2 mg of 7ptF9 anti-CD8 monoclonal or 833ICG isotype control antibody (Coulter, Hialeah, Fla.). In preliminary experiments the 7ptF9 antibody was not blocked by the detecting antibody to CD8. Because there is no substantial lymphopoiesis, 7ptF9 treatment resulted in high-level (>98%) depletion of CD8+ T cells throughout the experimental period. Proviral DNA and plasma viral RNA assays were performed using the Perkin-Elmer (Foster City, Calif.) model 7700 sequence detector. Dunnett's test for multiple comparisons was used to compare the percentages of CD4+ T cells and the Wilcoxon two-sample test was used with the Bonferroni multiple-testing correction to compare levels of virus in plasma and provirus in spleen between groups of animals. In vitro cultures were performed as previously described (3). Standard enzyme-linked immunosorbent assays were used to quantify the CC chemokines MIP-1α, MIP-1β, and RANTES (R&D Systems, Minneapolis, Minn.) or p24 (Coulter). Standard 51Cr-release assays (37) and proliferation assays (33) were performed as previously described.
All patients have been infected for greater than 13 years (Table 1). Two patients typically classified as LTNPs (27, 35) were included as controls. These two patients (patients 1 and 2) had levels of HIV RNA in plasma of <500 to 14,650 copies/mm3 at three or four time points over the past 4 years of study. Patients 3 to 6 consistently had plasma HIV RNA levels of <50 copies/ml and no recovered virus in CD8+-T-cell-depleted cocultures or in UV-irradiated cultures (9).
TABLE 1.
Clinical data of study patientsa
| Patientb | Route of infection | Yr of diagnosis | No. of CD4+ T cells/mm3 | No. of CD8+ T cells/mm3 | HIV RNA in plasma (copies/ml) by:
|
|
|---|---|---|---|---|---|---|
| bDNA | RT-PCR | |||||
| 1 | Homosexual sex | 1985 | 902 | 714 | <500–14,650 | 26,400 |
| 2 | Homosexual sex | 1986 | 729 | 2,478 | <500–14,080 | 3,470 |
| 3 | Homosexual sex | 1985 | 941 | 1,015 | <500 | <10 |
| 4 | Homosexual sex | 1985 | 774 | 774 | <500 | <10 |
| 5 | Homosexual sex | 1985 | 914 | 656 | <500 | <10 |
| 6 | Received Factor VIII for hemophilia | 1986 | 734 | 672 | <500 | <10 |
Flow cytometry values indicated were determined at the time of donation. Levels of virus RNA in plasma were determined using a branched chain DNA (bDNA) assay with a sensitivity in plasma of 500 copies/ml and by real-time RT-PCR with a sensitivity of 10 copies/ml.
All patients had not received antiretroviral agents during or prior to the study period except as indicated. Patient 2 was receiving zidovudine (AZT) monotherapy during the study. Patient 3 received IFN-α–AZT (January 1990 to December 1995) or IFN-α–AZT–didanosine (DDI) (January 1996 to December 1996) as part of a National Institute of Allergy and Infectious Diseases protocol. He did not receive antiretroviral agents during the study period and has remained off antiretroviral agents and without viremia since that time.
In a previous study, engraftment of CB-17 SCID mice with PBMC from HIV-infected patients resulted in rapid replication of patient-derived (autologous) viruses (5). Plasma viremia peaked on day 10 and was associated with depletion of CD4+ T cells to 5% of human cells. This pattern was repeatedly observed in animals engrafted with PBMC of seven patients with progressive disease (not shown). However, in some experiments there was no replication of the patients' autologous viruses in animals engrafted with cells from two true LTNPs (patients 3 and 6). This observation permitted the study of restriction of challenge-virus replication in animals engrafted with PBMC of these patients. In preliminary experiments, upon challenge with 5 to 125 50% tissue culture infectious doses (TCID50) of HIVLAV, CD4+ T cells were maintained in animals engrafted with cells from patients 3 and 6 (not shown). We then determined if similar resistance would be observed upon challenge with the macrophage-tropic primary isolate HIVSF162. In animals engrafted with cells from an uninfected donor, significant depletion of CD4+ T cells (P = 0.05) and increases in levels of virus in plasma (P < 0.03) and of proviral DNA (P = 0.03) were observed at the 5- to 125-TCID50 doses when results were compared to results with unchallenged animals (Fig. 1). In both challenged and unchallenged animals engrafted with PBMC from patients 1 and 2, virus replication and CD4+-T-cell depletion were similar to those previously observed in animals engrafted with PBMC from progressors (5).
FIG. 1.
Changes in CD4+-T-cell numbers and levels of HIV-1 in animals engrafted with human PBMC and challenged with 1 to 125 TCID50 of HIVSF162. Values for similarly prepared animals that received a human CD8+-T-cell-depleting antibody on day 6 (1 day prior to challenge) are also shown. Values shown are those at the time of sacrifice (day 21). The percentages of human cells within the peritoneal wash (PW) which are CD4+ are shown in the top panels. Levels of virus in plasma and provirus in spleen determined by real-time PCR are shown in the two lower panels. The percentage of CD4+ T cells and levels of virus RNA in plasma and provirus DNA in spleen indicated by a given symbol within a column correspond to the same animal. The fraction of animals with detectable virus refers to the number of animals with virus RNA in the plasma or provirus in the spleen, or spleen coculture, divided by the number of animals in that group.
In contrast, animals engrafted with the cells from three of the four LTNPs (patients 4 to 6) did not replicate autologous viruses above levels of detection. Although unchallenged animals engrafted with cells from patient 3 had a lower percentage of CD4+ T cells than those of patients 4 to 6, no CD4+ T-cell depletion from this lower baseline was detected in challenged animals. No depletion of CD4+ T cells was observed in the majority of groups of animals engrafted with PBMC of patients 3 to 6 over a broad range of challenge doses when levels were compared to levels in unchallenged animals. Significant depletion of CD4+ T cells was found only in animals engrafted with the cells of patient 6 and challenged with 125 TCID50 (P = 0.05). No significant increase in virus in plasma was detected in challenged animals engrafted with PBMC of patients 3 to 6 when levels were compared with levels in unchallenged animals. Although several animals engrafted with the PBMC of patient 4 had plasma viremia, the distribution of these values was not significantly greater than that of unchallenged animals when values were corrected for multiple comparisons (P = 0.07). Significant increases in provirus in spleen were observed only in animals engrafted with PBMC of patient 4 and challenged with 125 TCID50 (P < 0.003). Sequence analysis of proviral DNA from the spleens of two of these animals confirmed that the virus detected was HIVSF162.
In separate experiments, the effect of CD8+-T-cell depletion on restriction of virus replication was investigated (Fig. 1). In animals engrafted with cells from patient 3 or 5 and depleted of CD8+ T cells, challenge virus replicated to levels comparable to those of animals engrafted with PBMC from an uninfected control. This replication resulted in significant increases in virus in plasma (P = 0.05) and provirus in spleen (P = 0.03) when such levels were compared to levels in 25-TCID50-challenged, nondepleted animals. Because CD8+-T-cell depletion increases the percentage of CD4+ T cells in the peritoneal wash, comparisons were made with similarly depleted, unchallenged animals. Increased virus replication in CD8+-T-cell-depleted animals resulted in only partial CD4+-T-cell depletion in those animals engrafted with the cells of patient 3 but significant depletion in the mice engrafted with cells from patient 5 (P < 0.03).
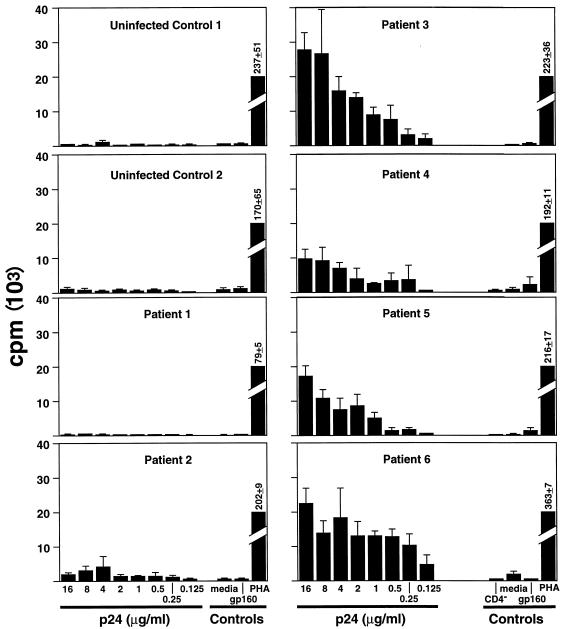
Because it is possible that restricted challenge-virus replication might not be entirely due to a CD8+-T-cell response but also to restriction of replication at the level of the CD4+ T cell, the ability to replicate virus in the absence of CD8+ T cells was investigated in vitro. In cultures depleted of CD8+ T cells (<1% residual CD8+ T cells), no residual restriction of the replication of HIVSF162 or HIVLAV was observed (not shown). The lack of a deletion within the CCR5 gene was confirmed by restriction fragment length polymorphism analysis. We also investigated whether patients 3 to 6 could be distinguished from patients 1 and 2 in standard assays of CD8+ T-cell-mediated HIV-specific immunity. PBMC of patients 3 to 6 did not show a greater ability to suppress replication of HIVSF162 or HIVLAV than those of patients 1 and 2. Similar results were obtained with 5, 50, and 125 TCID50 of HIVSF162 or HIVLAV or when CD8+ T cells were depleted and then repleted at various ratios (100:0 to 100:100 CD4/CD8 ratios) (not shown). No differences in the levels of the CC chemokines MIP-1α, MIP-1β, and RANTES were found in the supernatants of cultures. No difference in the magnitudes of the direct cytotoxic T lymphocyte (CTL) responses was detected in patients 1 and 2 when their responses were compared to those of patients 3 to 6. PBMC of uninfected patients and patients with detectable virus in plasma (patients 1 and 2) showed no proliferative response to p24 (Fig. 2). In contrast, patients 3 to 6 had strong responses to p24 (stimulation index, 10 to 140) that were abrogated by CD4+-T-cell depletion in vitro.
FIG. 2.
In vitro proliferation to HIV p24 antigen. PBMC (105) from two uninfected controls and patients 1 to 6 were incubated with the indicated antigens or media in a standard [H3]thymidine uptake assay. Results of triplicate cultures are shown. For patients 3 to 6, controls included CD4+-T-cell-depleted PBMC stimulated with 10 μg of p24 per ml. PHA, phytohemagglutinin.
The data from this study establish this resistance phenotype in a unique subgroup of nonprogressors that likely make up less than 0.8% of the HIV-infected population. These patients are characterized by nonprogressive disease, a level of virus in plasma below 50 copies/ml, a lack of readily detectable virus by culture, a lack of autologous virus replication in the SCID-Hu mouse, and strong proliferative responses to HIV antigens. Data from this study as well as previous epidemiologic data (12, 22, 23, 30, 33, 36) indicate that each of these characteristics is quite rare among patients with slowly progressive or nonprogressive disease, yet each of these was associated with the subgroup described here. Although the in vivo phenotype has been repeatedly observed over the past 4 years of study of these patients, no in vitro correlate which clearly distinguished patients 3 to 6 from progressors or other nonprogressors was found until the proliferative responses to HIV antigens in such patients and experimental animals was recently described (10, 11, 33).
It should be stressed that these data do not directly indicate the mechanism by which patients 3 to 6 have avoided progressive disease. The model presented is not a model of nonprogression; rather, it is a model of immunity-mediated restriction of a challenge virus. These data do extend some recent data documenting the role of CD8+ T cells in restricting virus replication in macaques chronically infected with simian/human immunodeficiency virus or simian immunodeficiency virus (16, 24, 34). It should also be noted that the SCID-Hu mouse model does not directly model all of the aspects of resistance to challenge observed in virus infection of a natural host. In the SCID-Hu mouse model, the lack of lymphopoiesis and moderate lymphocyte activation leads to rapid virus replication and CD4+-T-cell depletion. The lack of virus replication and CD4+-T-cell depletion in many animals engrafted with cells from patients 3 to 6 suggests that the form of resistance mediated by the CD8+ T cells of these patients is quite potent.
We did not detect higher levels of HIV-specific CD8+-T-cell-mediated immunity by in vitro assays of PBMC from patients 3 to 6 compared to those of patients 1 and 2. In the PBMC of patients 1 and 2, who meet the widely used criteria of nonprogressors (27, 35), high levels of suppressive and direct CTL were observed, yet these responses were not associated with restriction of autologous virus replication in the mouse model. However, the CTL and CD8+-T-cell repletion assays used are crude and nonquantitative and thus might have underestimated greater HIV-specific CD8+-T-cell responses in patients 3 to 6. By a more quantitative assay, the numbers of antigen-specific CD8+ T cells detected by antigen-specific accumulation of intracellular gamma interferon (IFN-γ) of patients 3 to 6 were similar to those of patients 1 and 2 and other patients with progressive disease (J. Gea-Banacloche, unpublished data), consistent with one other recent report (8). It should also be noted that each of these assays measures responses to laboratory test strains and might not measure dominant responses to the in vivo virus strain. It is possible that the measured responses then underestimate the responses to the circulating viruses of patients 3 to 6. It is also possible that maintenance of CD4+-T-cell help in patients 3 to 6 results in the maintenance of high-avidity, HIV-specific CD8+ T cells, which is not adequately measured by these assays (1, 4, 14, 17, 19–21, 25).
Further definition of the mechanisms of CD8+-T-cell-mediated restriction of virus replication in the patients described in this study is likely to hold important clues to the parameters that should be measured or mechanisms that might be exploited as part of prophylactic or therapeutic vaccines for HIV. If such mechanisms are determined in vitro, the model described here may allow a further demonstration of their relationship with restriction of challenge-virus replication.
Acknowledgments
We thank Betsey Herpin, Stephanie Mizell, and Linda Ehler for arranging patient apheresis and handling of clinical samples. We especially thank the patients involved in this study for their time and dedication to its completion. The 7ptF9 antibody was kindly supplied by Gary Toedter of Coulter Corporation. HIV RNA standards (contributed by James Bremer and DAIDS, NIAID) and HIVSF162 challenge virus (contributed by Jay Levy) were provided by the NIH AIDS Research and Reference Reagent Program.
Juan C. Lopez Bernaldo de Quiros was partially supported by grant 97/5079 from the Fondo de Investigaciones Sanitarias, Madrid, Spain.
REFERENCES
- 1.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker E, Mackewicz C E, Reyes-Teran G, Sato A, Stranford S A, Fujimura S H, Christopherson C, Chang S Y, Levy J A. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood. 1998;92:3105–3114. [PubMed] [Google Scholar]
- 3.Barker T D, Weissman D, Daucher J A, Roche K M, Fauci A S. Identification of multiple and distinct CD8+ T cell suppressor activities: dichotomy between infected and uninfected individuals, evolution with progression of disease, and sensitivity to gamma irradiation. J Immunol. 1996;156:4476–4483. [PubMed] [Google Scholar]
- 4.Bennett S R, Carbone F R, Karamalis F, Miller J F, Heath W R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle M J, Connors M, Flanigan M E, Geiger S P, Ford H, Jr, Baseler M, Adelsberger J, Davey R T, Jr, Lane H C. The human HIV/peripheral blood lymphocyte (PBL)-SCID mouse. A modified human PBL-SCID model for the study of HIV pathogenesis and therapy. J Immunol. 1995;154:6612–6623. [PubMed] [Google Scholar]
- 6.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 7.Cohen O J, Kinter A, Fauci A S. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalod M, Dupuis M, Deschemin J-C, Sicard D, Salmon D, Delfraissy J-F, Venet A, Sinet M, Guillet J-G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl-Hennig C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 11.Dittmer U, Spring M, Petry H, Nisslein T, Rieckmann P, Luke W, Stahl-Hennig C, Hunsmann G, Bodemer W. Cell-mediated immune response of macaques immunized with low doses of simian immunodeficiency virus (SIV) J Biotechnol. 1996;44:105–110. doi: 10.1016/0168-1656(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 12.Ferbas J, Kaplan A H, Hausner M A, Hultin L E, Matud J L, Liu Z, Panicali D L, Nerng-Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 13.Gulizia R J, Collman R G, Levy J A, Trono D, Mosier D E. Deletion of nef slows but does not prevent CD4-positive T-cell depletion in human immunodeficiency virus type 1-infected human-PBL-SCID mice. J Virol. 1997;71:4161–4164. doi: 10.1128/jvi.71.5.4161-4164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han W R, Mottram P L, Purcell L J, Plenter R J, McKenzie I F C. Infiltrating cells in mouse cardiac allografts after anti-CD4 monoclonal antibody treatment. Transplant Proc. 1995;27:2163. [PubMed] [Google Scholar]
- 15.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retrovirus. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karupiah G, Buller R M, Van Rooijen N, Duarte C J, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieger N R, Yin D P, Fathman C G. CD4+ but not CD8+ cells are essential for allorejection. J Exp Med. 1996;184:2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurts C, Carbone F R, Barnden M, Blanas E, Allison J, Heath W R, Miller J F. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 22.Lefrere J J, Mariotti M, Morand-Joubert L, Thauvin M, Roudot-Thoraval F. Plasma human immunodeficiency virus RNA below 40 copies/mL is rare in untreated persons even in the first years of infection. J Infect Dis. 1999;180:526–529. doi: 10.1086/314906. [DOI] [PubMed] [Google Scholar]
- 23.Lefrere J J, Morand-Joubert L, Mariotti M, Bludau H, Burghoffer B, Petit J C, Roudot-Thoraval F. Even individuals considered as long-term nonprogressors show biological signs of progression after 10 years of human immunodeficiency virus infection. Blood. 1997;90:1133–1140. [PubMed] [Google Scholar]
- 24.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 27.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L J, Margolick J B, Buchbinder S, Giorgi J V, Fauci A S. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 28.Picchio G R, Gulizia R J, Mosier D E. Chemokine receptor CCR5 genotype influences the kinetics of human immunodeficiency virus type 1 infection in human PBL-SCID mice. J Virol. 1997;71:7124–7127. doi: 10.1128/jvi.71.9.7124-7127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 30.Pontesilli O, Carotenuto P, Kerkhof-Garde S R, Roos M T, Keet I P, Coutinho R A, Goudsmit J, Miedema F. Lymphoproliferative response to HIV type 1 p24 in long-term survivors of HIV type 1 infection is predictive of persistent AIDS-free infection. AIDS Res Hum Retrovirus. 1999;15:973–981. doi: 10.1089/088922299310485. [DOI] [PubMed] [Google Scholar]
- 31.Rinaldo C, Piazza P, Wang Y Z, Armstrong J, Gupta P, Ho M, Petteway S, Reed D, Lyter D, Kingsley L. HIV-1-specific production of IFN-gamma and modulation by recombinant IL-2 during early HIV-1 infection. J Immunol. 1988;140:3389–3393. [PubMed] [Google Scholar]
- 32.Rinaldo C R, Jr, Beltz L A, Huang X L, Gupta P, Fan Z, Torpey D J., III Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retrovirus. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 35.Strathdee S A, Veugelers P J, Page-Shafer K A, McNulty A, Moss A R, Schechter M T, van Griensven G J, Coutinho R A. Lack of consistency between five definitions of nonprogression in cohorts of HIV-infected seroconverters. AIDS. 1996;10:959–965. doi: 10.1097/00002030-199610090-00005. [DOI] [PubMed] [Google Scholar]
- 36.Vesanen M, Stevens C E, Taylor P E, Rubinstein P, Saksela K. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. J Virol. 1996;70:9035–9040. doi: 10.1128/jvi.70.12.9035-9040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker B D. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. [Google Scholar]