ABSTRACT
Chikungunya virus (CHIKV) has emerged as a significant public health concern due to its rapid spread and potential for causing debilitating epidemics. In Argentina, the virus has garnered attention since its introduction to the Americas in 2013, due to its growing incidence and impact in neighbouring countries. Here we present a comprehensive analysis of the spatiotemporal dynamics of CHIKV in Argentina, focusing on the evolutionary trajectory of its genetic variants. Through a combination of active surveillance, screening of historical and recent samples, and whole-genome sequencing, we traced the evolutionary history of CHIKV lineages circulating within the country. Our results reveal that two distinct genotypes circulated in Argentina: The Asian lineage during the 2016 epidemic and the ECSA lineage in 2023. This distribution reflects the dominance of particular variants across Latin America. Since 2023, the ECSA lineage has led to a surge in cases throughout the Americas, marking a significant shift. The replacement of lineages in the American region constitutes a major epidemiological event, potentially affecting the dynamics of virus transmission and the clinical outcomes in impacted populations. The spatiotemporal analysis highlights CHIKV's distribution across Argentina and underscores the significant role of human mobility, especially when considering recent epidemics in neighbouring countries such as Paraguay and Uruguay, which have facilitated the spread and introduction of the viral strain into different districts. By integrating epidemiological data with genomic insights, we elucidate the patterns of virus dissemination, highlighting key areas of transmission and potential factors contributing to its spread.
KEYWORDS: Argentina, CHIKV, genomic surveillance, phylodynamic, molecular epidemiology, evolution
Introduction
Chikungunya virus (CHIKV), a member of the Togaviridae family, is predominantly transmitted through the bite of infected mosquitoes, especially those of the Aedes genus [1]. This arthropod-borne pathogen is notorious for causing chikungunya fever, characterized by fever, headache, muscle pain, and joint pain symptoms and severe joint pain. CHIKV exhibits genetic diversity and distinct spatiotemporal distributions, resulting in various genotypes and lineages [2]. Its rapid spread and potential for severe epidemics have elevated it to a global public health emergency [3]. Following the detection of a mutation in the ECSA CHIKV lineage in 2009, which conferred replication advantages in Aedes albopictus mosquitoes [4], Argentina initiated the development of diagnostic methods for CHIKV. The first confirmation of CHIKV transmission within the Americas occurred in 2012 on the island of Saint Martin, signalling the spread of the virus throughout the Caribbean and Latin American countries [5]. Argentina detected its first CHIKV cases in returning travellers in 2014, but autochthonous cases were reported for the first time in 2016, affecting only the Salta and Jujuy provinces in the northwest, with 322 confirmed cases reported between the 8th and 20th Epidemiological weeks (EW) [6–8]. Between 2017 and 2022, CHIKV cases were identified in returning travellers. However, a significant increase in CHIKV cases in early 2023 across various regions of Argentina reflected a broader trend in the Americas, which has reported over 214,000 cases since the virus's introduction. In Argentina, between the 1st and 43rd weeks, 2,314 confirmed cases were reported, mainly autochthonous, in 9 provinces of the country: Buenos Aires, CABA (Ciudad Autónoma de Buenos Aires), Córdoba, Chaco, Corrientes, Formosa, Misiones, Salta, and Santa Fe [8]. This study addresses the shifting epidemiological landscape of CHIKV in Argentina. Through a collaborative effort, we obtained 55 novel CHIKV genome sequences from February 2016 to April 2023, sourced from infected individuals in the most affected provinces. Our study has focused on providing valuable insights into the transmission dynamics and genetic characteristics of circulating CHIKV strains within Argentina, with a primary focus on understanding the genetic evolution and lineage diversity. Given the ongoing spread of this pathogen across the Americas, it has become crucial to comprehend not only its evolutionary dynamics within Argentina but also its interconnectedness with neighbouring countries. To achieve our objectives, we employed a comprehensive approach that includes active surveillance, the screening of historical and contemporary samples, and the utilization of cutting-edge whole-genome sequencing and phylodynamic techniques. Through these efforts, we have unravelled the intricate evolutionary trajectory of CHIKV lineages within the nation's borders, shedding light on its broader regional implications.
Results
To investigate the circulation of CHIKV genotypes in Argentina, we generated a total of 55 genome sequences. From the 2016 epidemic in Salta province, we obtained four sequences of the CHIKV-Asian genotype. During the 2023 epidemic, we sequenced 51 genomes of the CHIKV-ECSA genotype, of which 21 were identified as autochthonous cases, while 15 reported a travel history to Paraguay, which experienced a large epidemic by the end of 2022 (Table 1) [9]. The latter were collected from provinces most impacted by the 2023 epidemic: Buenos Aires, Chaco, Córdoba, Corrientes, Formosa, Misiones, and Santa Fe (Figure 1A and Table S1). Whole-genome sequences were obtained from RT-qPCR positive samples using a MiSeq (Illumina) sequencing instrument.
Table 1.
Epidemiological data the novel CHIKV samples sequenced as part of this study.
| Sample ID | Country | Colelction date | Age | Sex | CTs | Reads | Depth of Coverage | Coverage | Assignment | Lineages | Accession_Number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AR20230720_MS3113964_10557_CHIK|2023-03-03 | Argentina | 2023-03-03 | 37 | M | 21 | 182202 | 2018.9 | 94.5 | Chikungunya virus | East-Central-South-African | OR631864 |
| AR20230720_MS3113964_10558_CHIK|2023-03-01 | Argentina | 2023-03-01 | 63 | F | 21 | 182202 | 2018.9 | 94.5 | Chikungunya virus | East-Central-South-African | OR631866 |
| AR20230720_MS3113964_10559_CHIK|2023-02-27 | Argentina | 2023-02-27 | 41 | F | 19 | 249817 | 2739.9 | 95.5 | Chikungunya virus | East-Central-South-African | OR631865 |
| AR20230720_MS3113964_10560_CHIK|2023-02-23 | Argentina | 2023-02-23 | 58 | F | 25 | 190036 | 2103.5 | 94.4 | Chikungunya virus | East-Central-South-African | OR631863 |
| AR20230720_MS3113964_10561_CHIK|2023-02-20 | Argentina | 2023-02-20 | 26 | M | 32 | 293245 | 3218.4 | 95.3 | Chikungunya virus | East-Central-South-African | OR631867 |
| AR20230720_MS3113964_10562_CHIK|2023-02-10 | Argentina | 2023-02-10 | 29 | F | 24 | 220990 | 2444.9 | 94.6 | Chikungunya virus | East-Central-South-African | OR631868 |
| AR20230720_MS3113964_10563_CHIK|2023-02-08 | Argentina | 2023-02-08 | 24 | M | 26 | 232290 | 2588.8 | 93.4 | Chikungunya virus | East-Central-South-African | OR631872 |
| AR20230720_MS3113964_10564_CHIK|2023-02-06 | Argentina | 2023-02-06 | 83 | F | 29 | 141390 | 1664.2 | 87.7 | Chikungunya virus | East-Central-South-African | OR631870 |
| AR20230720_MS3113964_10565_CHIK|2023-02-08 | Argentina | 2023-02-08 | 34 | M | 22 | 311823 | 3428.1 | 94.6 | Chikungunya virus | East-Central-South-African | OR631869 |
| AR20230720_MS3113964_10566_CHIK|2023-02-05 | Argentina | 2023-02-05 | 50 | M | 20 | 188822 | 2087.9 | 94.5 | Chikungunya virus | East-Central-South-African | OR631871 |
| AR20230720_MS3113964_10568_CHIK|2023-02-02 | Argentina | 2023-02-02 | 40 | F | 31 | 139393 | 1718.5 | 83.8 | Chikungunya virus | East-Central-South-African | OR631873 |
| AR20230720_MS3113964_10569_CHIK|2023-01-25 | Argentina | 2023-01-25 | 35 | M | 25 | 200406 | 2221.9 | 94.1 | Chikungunya virus | East-Central-South-African | OR631875 |
| AR20230720_MS3113964_10570_CHIK|2023-01-25 | Argentina | 2023-01-25 | 35 | F | 20 | 235347 | 2615.9 | 94.6 | Chikungunya virus | East-Central-South-African | OR631874 |
| AR20230720_MS3113964_10571_CHIK|2023-01-19 | Argentina | 2023-01-19 | 16 | F | 24 | 217508 | 2398.3 | 94.5 | Chikungunya virus | East-Central-South-African | OR631876 |
| AR20230720_MS3113964_10572_CHIK|2023-01-13 | Argentina | 2023-01-13 | 51 | M | 19 | 330935 | 3654.5 | 94.5 | Chikungunya virus | East-Central-South-African | OR631877 |
| AR20230720_MS3113964_10573_CHIK|2023-01-19 | Argentina | 2023-01-19 | 26 | M | 26 | 261053 | 2862.3 | 94.5 | Chikungunya virus | East-Central-South-African | OR631878 |
| AR20230720_MS3113964_10574_CHIK|2023-03-03 | Argentina | 2023-03-03 | 48 | M | 25 | 296500 | 3269.7 | 94.5 | Chikungunya virus | East-Central-South-African | OR631879 |
| AR20230720_MS3113964_10575_CHIK|2023-02-27 | Argentina | 2023-02-27 | 11 | F | 24 | 234250 | 2593.2 | 94.5 | Chikungunya virus | East-Central-South-African | OR631881 |
| AR20230720_MS3113964_10576_CHIK|2023-02-10 | Argentina | 2023-02-10 | 14 | M | 28 | 196983 | 2269.1 | 89.7 | Chikungunya virus | East-Central-South-African | OR631880 |
| AR20230720_MS3113964_10577_CHIK|2023-03-02 | Argentina | 2023-03-02 | 66 | M | 29 | 129770 | 1495.2 | 90.2 | Chikungunya virus | East-Central-South-African | OR631882 |
| AR20230720_MS3113964_10578_CHIK|2023-03-01 | Argentina | 2023-03-01 | 41 | M | 23 | 208425 | 2293.3 | 94.5 | Chikungunya virus | East-Central-South-African | OR631883 |
| AR20230720_MS3113964_10579_CHIK|2023-03-01 | Argentina | 2023-03-01 | 45 | F | 24 | 202986 | 2218.0 | 95.4 | Chikungunya virus | East-Central-South-African | OR631886 |
| AR20230720_MS3113964_10580_CHIK|2023-02-16 | Argentina | 2023-02-16 | 55 | F | 23 | 203661 | 2241.7 | 94.5 | Chikungunya virus | East-Central-South-African | OR631885 |
| AR20230720_MS3148148_10811_CHIK|2023-03-03 | Argentina | 2023-03-03 | 42 | F | 36 | 62487 | 1137.6 | 54.2 | Chikungunya virus | East-Central-South-African | OR631884 |
| AR20230720_MS3148148_10812_CHIK|2023-02-26 | Argentina | 2023-02-26 | 13 | M | 23 | 226838 | 2483.4 | 94.5 | Chikungunya virus | East-Central-South-African | OR631887 |
| AR20230720_MS3148148_10813_CHIK|2023-02-10 | Argentina | 2023-02-10 | 38 | F | 33 | 219315 | 2414.3 | 93.3 | Chikungunya virus | East-Central-South-African | OR631888 |
| AR20230720_MS3148148_10814_CHIK|2023-03-04 | Argentina | 2023-03-04 | 60 | F | 32 | 226769 | 2512.3 | 93.3 | Chikungunya virus | East-Central-South-African | OR631889 |
| AR20230720_MS3148148_10815_CHIK|2023-03-29 | Argentina | 2023-03-29 | 40 | F | 20 | 243195 | 2647.9 | 95.9 | Chikungunya virus | East-Central-South-African | OR631891 |
| AR20230720_MS3148148_10816_CHIK|2023-02-16 | Argentina | 2023-02-16 | 11 | F | 25 | 233386 | 2562.4 | 94.3 | Chikungunya virus | East-Central-South-African | OR631890 |
| AR20230720_MS3148148_10817_CHIK|2023-02-13 | Argentina | 2023-02-13 | 44 | M | 17 | 252187 | 2724.5 | 96.9 | Chikungunya virus | East-Central-South-African | OR631892 |
| AR20230720_MS3148148_10818_CHIK|2023-02-16 | Argentina | 2023-02-16 | 22 | M | 19 | 272955 | 2991.6 | 95.2 | Chikungunya virus | East-Central-South-African | OR631893 |
| AR20230720_MS3148148_10819_CHIK|2023-04-12 | Argentina | 2023-04-12 | 32 | F | 20 | 237637 | 2538.2 | 96.9 | Chikungunya virus | East-Central-South-African | OR631895 |
| AR20230720_MS3148148_10820_CHIK|2023-03-29 | Argentina | 2023-03-29 | 32 | F | 18 | 189153 | 2057.8 | 95.2 | Chikungunya virus | East-Central-South-African | OR631896 |
| AR20230720_MS3148148_10821_CHIK|2023-03-30 | Argentina | 2023-03-30 | 58 | F | 23 | 223415 | 2438.4 | 94.5 | Chikungunya virus | East-Central-South-African | OR631894 |
| AR20230720_MS3148148_10822_CHIK|2023-04-24 | Argentina | 2023-04-24 | 53 | M | 18 | 259994 | 2805.2 | 96.7 | Chikungunya virus | East-Central-South-African | OR631897 |
| AR20230720_MS3148148_10823_CHIK|2023-04-25 | Argentina | 2023-04-25 | 67 | F | 25 | 223881 | 2456.4 | 94.5 | Chikungunya virus | East-Central-South-African | OR631898 |
| AR20230720_MS3148148_10824_CHIK|2023-04-28 | Argentina | 2023-04-28 | 15 | F | 20 | 256218 | 2760.2 | 97.1 | Chikungunya virus | East-Central-South-African | OR631899 |
| AR20230720_MS3148148_10825_CHIK|2023-03-29 | Argentina | 2023-03-29 | 32 | F | 21 | 233298 | 2586.7 | 94.5 | Chikungunya virus | East-Central-South-African | OR631901 |
| AR20230720_MS3148148_10826_CHIK|2023-04-21 | Argentina | 2023-04-21 | 70 | F | 22 | 285612 | 3129.0 | 94.6 | Chikungunya virus | East-Central-South-African | OR631900 |
| AR20230720_MS3148148_10833_CHIK|2023-03-09 | Argentina | 2023-03-09 | 39 | F | 21 | 183797 | 2032.9 | 94.5 | Chikungunya virus | East-Central-South-African | OR631906 |
| AR20230720_MS3148148_10834_CHIK|2023-03-14 | Argentina | 2023-03-14 | 54 | F | 27 | 160358 | 1730.3 | 96.5 | Chikungunya virus | East-Central-South-African | OR631907 |
| AR20230720_MS3148148_10835_CHIK|2023-03-22 | Argentina | 2023-03-22 | 51 | F | 21 | 247977 | 2659.1 | 97.8 | Chikungunya virus | East-Central-South-African | OR631908 |
| AR20230720_MS3148148_10836_CHIK|2023-03-24 | Argentina | 2023-03-24 | 3 | F | 28 | 193699 | 2115.0 | 94.5 | Chikungunya virus | East-Central-South-African | OR631910 |
| AR20230720_MS3148148_10837_CHIK|2023-03-09 | Argentina | 2023-03-09 | 17 | M | 25 | 264238 | 2891.5 | 94.5 | Chikungunya virus | East-Central-South-African | OR631909 |
| AR20230909_11016_CHIKV|2023-03-03 | Argentina | 2023-01-25 | 28 | F | 24 | 208425 | 2293.3 | 94.5 | Chikungunya virus | East-Central-South-African | OR631911 |
| AR20230909_11017_CHIKV|2023-03-01 | Argentina | 2023-01-15 | 35 | F | 24 | 202986 | 2218.0 | 95.4 | Chikungunya virus | East-Central-South-African | OR631912 |
| AR20230909_11018_CHIKV|2023-02-27 | Argentina | 2023-01-15 | 57 | M | 32 | 203661 | 2241.7 | 94.5 | Chikungunya virus | East-Central-South-African | OR631913 |
| AR20230909_11019_CHIKV|2023-02-23 | Argentina | 2023-01-22 | 49 | M | 22 | 62487 | 1137.6 | 54.2 | Chikungunya virus | East-Central-South-African | OR631914 |
| AR20230909_11020_CHIKV|2023-02-20 | Argentina | 2023-01-11 | 10 | F | 23 | 226838 | 2483.4 | 94.5 | Chikungunya virus | East-Central-South-African | OR631915 |
| AR20230909_11021_CHIKV|2023-02-10 | Argentina | 2023-05-04 | 42 | F | 20 | 219315 | 2414.3 | 93.3 | Chikungunya virus | East-Central-South-African | OR631916 |
| AR20230909_11022_CHIKV|2023-02-08 | Argentina | 2023-04-25 | 12 | M | 18 | 233298 | 2586.7 | 94.5 | Chikungunya virus | East-Central-South-African | OR631917 |
| AR20230720|MS3148148|10827|CHIK|BUENOSAIRES|2023-04-21 | Argentina | 2016-02-06 | 22 | F | 17 | 287566 | 3023.2 | 95.2 | Chikungunya virus | Asian and Caribbean | OR631902 |
| AR20230720|MS3148148|10828|CHIK|SALTA|2016-02-06 | Argentina | 2026-03-14 | 32 | M | 14 | 206969 | 2186.9 | 95.3 | Chikungunya virus | Asian and Caribbean | OR631903 |
| AR20230720|MS3148148|10829|CHIK|SALTA|2026-03-14 | Argentina | 2016-03-12 | 32 | F | 22 | 217779 | 2318.9 | 94.6 | Chikungunya virus | Asian and Caribbean | OR631904 |
| AR20230720|MS3148148|10830|CHIK|SALTA|2016-03-12 | Argentina | 2016-03-09 | 58 | F | 24 | 241838 | 2574.0 | 94.6 | Chikungunya virus | Asian and Caribbean | OR631905 |
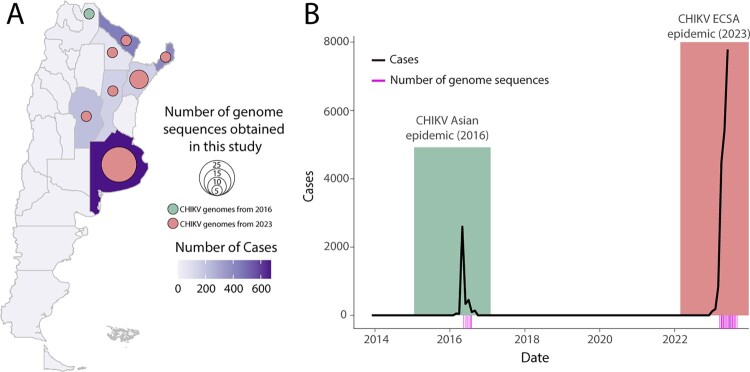
Figure 1.
Spatiotemporal Dynamics of CHIKV in Argentina. A) A map of Argentina shows districts coloured according to the number of reported CHIKV cases. Circles indicate the number of CHIKV genome sequences generated in this study, with the size of each circle representing the number of genomes isolated from that specific district. B) The number of autochthonous chikungunya cases reported weekly per 100,000 individuals from 2014 to 2023. The 2016 epidemic is highlighted in green, while the 2023 epidemic is highlighted in salmon. Pink bars represent the sample collection dates for the genomes generated in this study.
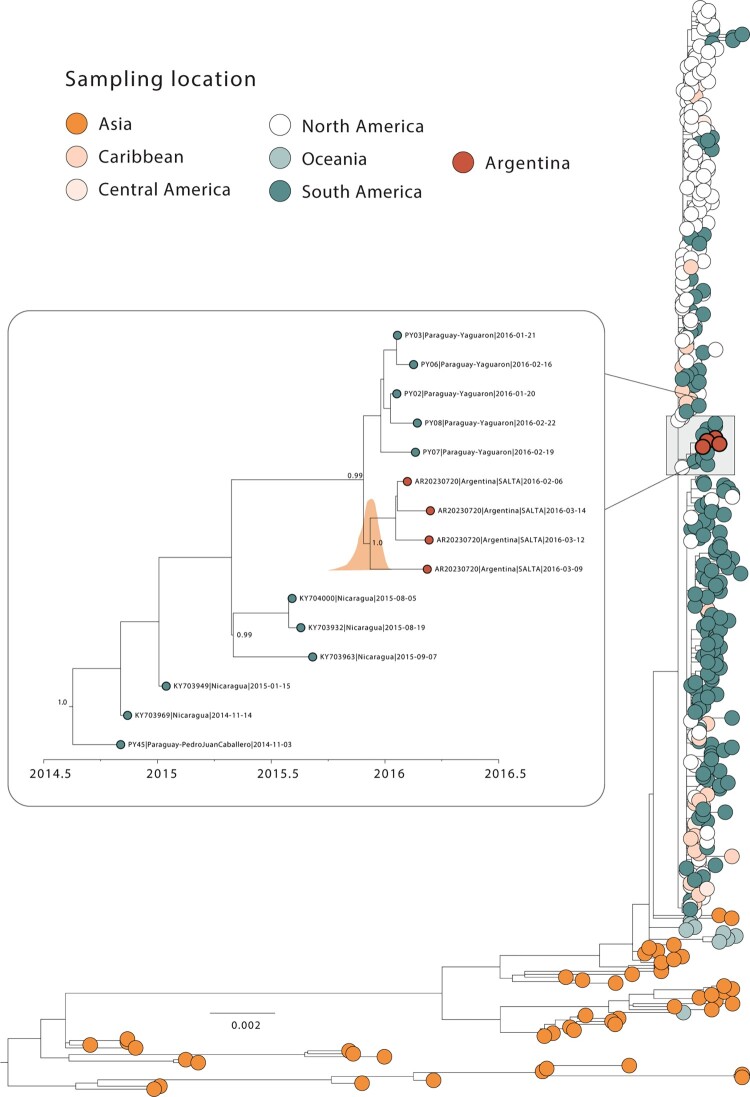
Screened samples presented a mean RT-qPCR cycle threshold value of 23.52, ranging from 14 to 36, and their associated average age was 40 for females and 36 for males (years), with 61% of patients identified as female (Table S1). All patients were classified as having chikungunya fever, and none were notified as severe. In total, the 55 new CHIKV genome sequences exhibited a mean genome coverage of 94.0% (coverage ranging from 74% to 98%), with a mean depth exceeding 1,000× for all samples. These sequences were obtained from samples collected between February 2016 and April 2023 and have been uploaded to the GenBank database under the accession numbers: OR631863-OR631917 (further details in Table S1). The CHIKV genotyping tool classified these new strains as either belonging to the Asian (n = 4) or ECSA (n = 51) genotypes (Table S1). Reported CHIKV cases identified two primary epidemic peaks in 2016 and 2023, as depicted in Figure 1B. The 2016 epidemic predominantly saw the circulation of the CHIKV-Asian genotype. By contrast, in 2023, there was a discernible change to the ECSA genotype, which has become prevalent in the Americas [9–11]. Accompanying this lineage replacement was a noticeable increase in case numbers, indicating a significant epidemiological shift. This pattern mirrors a larger trend seen across the Americas, where over 214,000 cases have been reported since the virus's introduction [10, 11]. To gain a better understanding of the phylodynamic history of the CHIKV Asian genotype in Argentina, we combined our 4 newly generated sequences with other CHIKV Asian strains from GenBank (n = 364). As shown in Figure 2A, our phylogenetic reconstruction revealed that these novel isolates form a cluster with strong support (posterior probability = 1.0). Delving further into the lineage's origins, our analysis suggests a significant connection with Paraguay, pointing to a potential cross-border transmission between these neighbouring countries. The likely source of this introduction appears to be associated with strains from South and Central America, as indicated by samples from Paraguay and Nicaragua (Figure 2). We estimated that this transmission event occurred around early December 2015, with a 95% highest posterior density (HPD) interval ranging from mid-June 2015 to early February 2016.
Figure 2.
Dispersion dynamics of CHIKV-Asian genotype in Argentina. Maximum likelihood (ML) phylogenetic analysis of 4 complete genome sequences from CHIKV-Asian genotype generated in this study alongside n = 364 CHIKV-Asian available complete genome sequences from GenBank. The scale bar is in units of nucleotide substitutions per site (s/s) and the tree is mid-pointed rooted. Colours represent different sampling locations. The highlight on the left showed the Maximum Clade Credibility (MCC) reconstruction of the Argentinian Clade. Values around key nodes represent posterior probability support.
In addition, to further investigate the phylodynamics of the CHIKV-ECSA genotype, we conducted a phylogenetic analysis of 51 newly sequenced genomes in addition with 924 complete CHIKV-ECSA genome sequences available on GenBank (Figure 3). Notably, our analysis revealed two independent introduction events. Given the genomic data available from neighbouring countries, our findings suggest a probable connection with the CHIKV epidemic observed in Uruguay and Paraguay, both of which have experienced an increase in CHIKV cases since early 2023. Additionally, our analysis uncovered a significant monophyletic cluster, indicating local transmission within Argentina, which we have designated as “AR-Clade 1’.
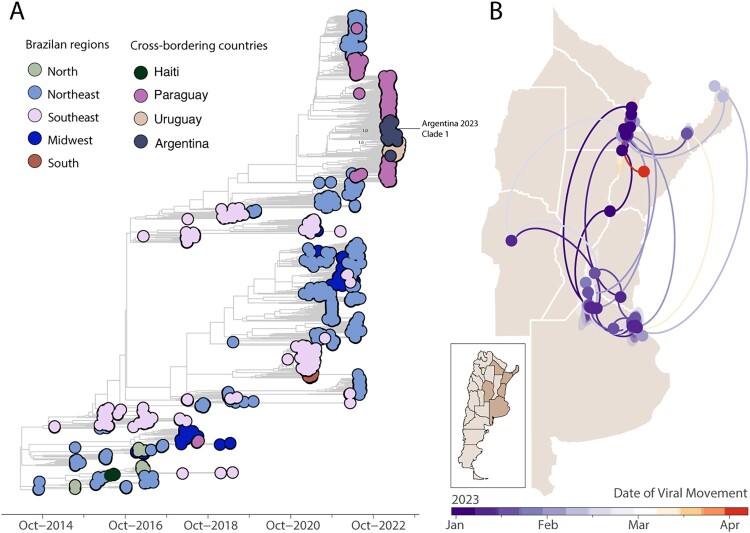
Figure 3.
Time-measured phylogeny of CHIKV-ECSA lineage in Argentina. A) MCC reconstruction of 51 complete genome sequences from CHIKV-ECSA genotype generated in this study plus n = 924 available sequences from GenBank. Numbers in black show clade posterior probabilities of main nodes. Colours represent different sampling locations. The highlight on the right showed the phylogeographic reconstruction of the large Clade 1 from Argentina (n = 43). Circles represent nodes of the MCC phylogeny and are coloured according to their inferred time of occurrence. Shaded areas represent the 80% highest posterior density interval and depict the uncertainty of the phylogeographic estimates for each node. Solid curved lines denote the links between nodes and the directionality of movement.
While relying solely on genetic data limits our ability to definitively determine transmission directionality, the evidence suggests a probable connection between the origin of this clade in Argentina and the recent explosive epidemic observed in Paraguay and Uruguay (Figure 3). We additionally reconstructed the viral movements within Argentina, particularly focusing on the AR-Clade 1 (n = 50). The analysis indicated that the mean time of origin for these samples was in late December 2022, with a 95% highest posterior density (HPD) ranging from mid-December 2022 to early January 2023. It's noteworthy that viruses encompassed within this clade appear to have spread multiple times from Argentina's northern regions (Formosa and Chaco provinces) toward the eastern provinces (Figure 3).
Discussion
CHIKV has emerged as a significant global health threat, characterized by its rapid transmission and potential to cause debilitating epidemics [9]. In 2023, Argentina experienced an increase in CHIKV infections as part of the virus's regional spread [9–11]. This increase prompted an in-depth investigation into the virus's evolutionary dynamics within Argentina. Our comprehensive approach included active surveillance, analysis of both historical and recent samples, and whole-genome sequencing to delineate the evolutionary trajectory of CHIKV lineages in the country. A critical outcome of this study was the discovery that the once-dominant Asian lineage has been replaced by the ECSA lineage, which is now the dominating genotype in the Americas. Further analysis of the CHIKV Asian genotype in Argentina revealed a substantial link with neighbouring countries, highlighting potential cross-border transmission. This finding is supported by samples from South and Central America, particularly Paraguay and Nicaragua. However, the lack of CHIKV sequences from Bolivia for 2015–2016 is noteworthy, especially considering the travel connections to Argentina's early 2016 outbreak, predominantly in the Salta and Jujuy provinces. This absence, coupled with Bolivia's significant case numbers during this period, complicates efforts to map the virus's transmission pathways and demonstrates its extensive regional impact. The replacement of the Asian lineage by the ECSA lineage represents more than just a shift in lineage prevalence; it represents a pivotal epidemiological event. Such a change can influence CHIKV's transmission dynamics and potentially shape clinical manifestations in affected individuals [12]. Notably, the ECSA lineage is known for its enhanced transmission efficiency and adaptability to Ae. albopictus mosquitoes, a primary vector for CHIKV in the Americas [13]. Notably, the mutation E1 A226 V, crucial for adaptation, was absent in the ECSA CHIKV sequences from Argentina analyzed in this study. Nonetheless, this lineage’s adaptability could significantly impact CHIKV’s epidemiological landscape in the country, potentially influencing the scope and intensity of future outbreaks. In addition, the interconnectedness of bordering countries amplifies these concerns [14]. Our analysis suggests a probable connection between the origin of the CHIKV-ECSA clade in Argentina and the recent explosive epidemic observed in Paraguay and the CHIKV circulation in Uruguay [10, 11]. The movement of populations and close ties between these countries could facilitate the spread and adaptation of the virus. In this respect, it's also crucial to acknowledge the limitations inherent in such analyses, primarily due to their dependency on sampling. Thus, we emphasize the imperative nature of increasing surveillance throughout the region. Enhanced monitoring would facilitate a more refined reconstruction of the virus's dissemination trajectory through epidemic seasons and across diverse sampling locations. The availability of technology to generate complete genomic sequences as epidemics occur will improve our ability to fully delineate and understand the molecular epidemiology of these viral strains at a granular regional level.
Materials and methods
Sample collection, molecular screening and whole genome sequencing
Serum samples (n = 55) obtained from patients who tested positive for CHIKV and presented symptoms consistent with the infection, along with linked epidemiological metadata, were received at the Instituto Nacional de Enfermedades Virales Humanas “Dr. Julio I. Maiztegui” in Pergamino, Buenos Aires, Argentina. These samples were collected by the National Network for Arbovirus Diagnosis in Argentina. The samples underwent nucleic acid extraction using the QIAamp Viral RNA Mini Kit (QIAGEN), followed by real-time reverse transcription RT-qPCR as previously described [15]. Only samples with Ct values <36 were selected for sequencing (Table S1). Extracted RNA was first converted to cDNA, and libraries were prepared using the Illumina COVID-Seq Assay kit, replacing SARS-CoV-2 specific primers used for the generation of cDNA with specific primers for complete sequencing of CHIKV designed by Quick et al, 2017 [16]. The sequencing reaction was performed on a MiSeq (Illumina) sequencing instrument using the MiSeq v2 reagent kit on a 300-cycle programme.
Genome assembly and Phylodynamic analysis
The resulting sequence reads were screened for quality and assembled into complete consensus sequences using Genome Detective software [17]. To explore sequence genotypes, the chikungunya virus typing tool was used [18]. To investigate the origins and spatial dynamics of the different CHIKV genotypes identified in Argentina, we collected all genomes designated as CHIKV-Asian and CHIKV-ECSA from GenBank up to September 20, 2023. We excluded sequences that lacked sample date and location information, as well as those that represented less than fifty percent of the viral genome. All sequences were aligned with MAFFT [19] and manually edited with Aliview [20] to remove biological artifacts. The ModelFinder tool used in IQ-TREE2 [21] inferred the GTR nucleotide substitution model as the best-fit model, which was used to build maximum likelihood (ML) phylogenetic trees. The robustness of the tree topology was determined using 1,000 bootstrap replicates. The BEAST software [22] was used to infer time-scaled phylogenetic trees, and prior to that, TempEst [23] was used to assess the presence of a temporal signal. We employed a stringent model selection investigation that includes both path-sampling (PS) and steppingstone (SS) processes to determine the most suited molecular clock and coalescent model for the Bayesian phylogenetic analysis [24]. The uncorrelated relaxed molecular clock model was chosen for all datasets by evaluating marginal likelihoods with the codon-based SRD06 nucleotide substitution model and the nonparametric Bayesian Skyline coalescent model. We utilized a flexible relaxed random walk diffusion model [25] that allows branch-specific variation in dispersion rates with a Cauchy distribution and a jitter window site of 0.01 to predict the phylogenetic dissemination of the discovered CHIKV-ECSA community transmission clade. The MCMC analyses were run in duplicate for 10 million iterations and sampled every 10,000 steps in the chain using BEAST v1.10.4. Tracer was used to evaluate run convergence (effective sample size for all key model parameters >200). Maximum Clade Credibility (MCC) trees for each run were summarized using TreeAnnotator after eliminating the early 10% as burn-in. Finally, using the R package seraphim version 1.0 [26], we retrieved and mapped spatiotemporal information inherent in the posterior trees. The PAHO website was used to download epidemiological time series data for confirmed, suspected, and probable CHIKV illnesses [8].
Supplementary Material
Acknowledgments
M. Giovanetti's funding is provided by PON “Ricerca e Innovazione” 2014-2020. The authors would also like to acknowledge the Global Consortium to Identify and Control Epidemics – CLIMADE (https://climade.health/).
Funding Statement
This study was supported by the Ministry of Health of Argentina, the National Administration of Laboratories and Health Institutes (ANLIS) “Dr. Carlos Malbrán”, the Pan American Health Organization (PAHO) and the National Institutes of Health USA grant U01 AI151698 for the United World Arbovirus Research Network (UWARN) and the CRP-ICGEB RESEARCH GRANT 2020 Project CRP/BRA20-03, Contract CRP/20/03.
Author contributions
Conceptualization: C.F.; M.G., and M.A.M.; methodology: C.F., M.G., and V.F.; software: C.F., M.G., and V.F.; validation: C.F., and M.G.; formal analysis: C.F., M.G., V.L., V.F., J.G., C.B., M.F., S.P., D.C., S.G., M..A.L., M.D.F., M.F., K.S., S.L. N.R.D., G.C., G.B., N.J., C.S., T.P.C., L.F., C.G., J.M.R., L.C.J.A. and M.A.M.; Data collection: C.F., M.G., V.L., V.F., J.G., C.B., M.F., S.P., D.C., S.G., M..A.L., M.D.F., M.F., K.S., S.L. N.R.D., G.C., G.B., N.J., C.S., T.P.C., L.F., C.G., J.M.R., L.C.J.A. and M.A.M.; investigation: C.F., M.G., V.L., V.F., J.G., C.B., M.F., S.P., D.C., S.G., M..A.L., M.D.F., M.F., K.S., S.L. N.R.D., G.C., G.B., N.J., C.S., T.P.C., L.F., C.G., J.M.R., L.C.J.A. and M.A.M.; data curation: M.G., V.F.; writing – original draft preparation: M.G.; writing – review and editing: C.F., M.G., V.L., V.F., J.G., C.B., M.F., S.P., D.C., S.G., M..A.L., M.D.F., M.F., K.S., S.L. N.R.D., G.C., G.B., N.J., C.S., T.P.C., L.F., C.G., J.M.R., L.C.J.A. and M.A.M.; visualization: MG; supervision: M.A.M.; project administration: M.A.M; funding acquisition: M.A.M. All authors have read and agreed to the published version of the manuscript.
Data availability statement
ewly generated sequences have been deposited in GenBank under accession numbers OR631863-OR631917.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Institutional review board statement
This project was reviewed and approved by the Pan American Health Organization Ethics Review Committee (PAHOERC) (Ref. No. PAHO-2016-08-0029).
References
- 1.Bartholomeeusen K, Daniel M, LaBeaud DA, et al. . Chikungunya fever. Nat Rev Dis Primers. 2023;9:17, doi: 10.1038/s41572-023-00429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suhrbier A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat Rev Rheumatol. 2019;15:597–611. doi: 10.1038/s41584-019-0276-9 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . (2023). https://www.who.int/health-topics/chikungunya#tab=tab_1.
- 4.Tsetsarkin KA, Vanlandingham DL, McGee CE, et al. . A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007 Dec;3(12):e201, doi: 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chastel C. Chikungunya virus: its recent spread to the southern Indian Ocean and Reunion Island (2005–2006). Bull Acad Natl Med. 2005;189:1827–1835. [PubMed] [Google Scholar]
- 6.Seijo, et al. 2014 but autochthonous cases were reported for the first time in 2016 (4, Boletín Integrado de Vigilancia | N° 327 – SE 37–2016 https://bancos.salud.gob.ar/recurso/boletin-integrado-de-vigilancia-n327-se37-20092016.
- 7.World Health Organization Chikungunya . (2016). Argentina. https://www.who.int/emergencies/disease-outbreak-news/item/14-march-2016-chikungunya-argentina-en (PODEMOS PONER BOLETIN EPIDEMIOLÓGICO DE ARGENTINA COMO REFERENCIA).
- 8.PAHO/WHO Data - Weekly Report . https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html.
- 9.Giovanetti M, Vazquez C, Lima M, et al. . Rapid epidemic expansion of chikungunya virus east/central/South African lineage, Paraguay. Emerg Infect Dis. 2023;29(9):1859–1863. doi: 10.3201/eid2909.230523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier J, Alcantara LCJ, Fonseca V, et al. . Increased interregional virus exchange and nucleotide diversity outline the expansion of chikungunya virus in Brazil. Nat Commun. 2023;14:4413, doi: 10.1038/s41467-023-40099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgueño A, Giovanetti M, Fonseca V, et al. Genomic and eco-epidemiological investigations in Uruguay reveal local Chikungunya virus transmission dynamics during its expansion across the Americas in 2023. medRxiv [Preprint]. 2023 Aug 20. doi: 10.1101/2023.08.17.23294156 [DOI] [PMC free article] [PubMed]
- 12.Tsetsarkin KA, Chen R, Leal G, et al. . Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7872–7877. doi: 10.1073/pnas.1018344108. Epub 2011 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vega-Rúa A, Marconcini M, Madec Y, et al. . Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Commun Biol. 2020 Jun 24;3(1):326, doi: 10.1038/s42003-020-1046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suk JE, Van Cangh T, Beauté J, et al. . The interconnected and cross-border nature of risks posed by infectious diseases. Glob Health Action. 2014 Oct 10;7:25287, doi: 10.3402/gha.v7.25287. Erratum in: Glob Health Action. 2015;8:27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson BW, Russell BJ, Goodman CH.. Laboratory diagnosis of chikungunya virus infections and commercial sources for diagnostic assays. J Infect Dis. 2016 Dec 15;214(suppl 5):S471–S474. doi: 10.1093/infdis/jiw274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quick J, Grubaugh ND, Pullan ST, et al. . Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–1276. doi: 10.1038/nprot.2017.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilsker M, Moosa Y, Nooij S, et al. . Genome Detective: an auto-mated system for virus identification from high-throughput sequencing data. Bioinformatics. 2019;35(5):871–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca V, Libin PJK, Theys K, et al. . A computational method for the identification of Dengue, Zika and Chikungunya virus species and genotypes. PLoS Negl Trop Dis. 2019 May 8;13(5):e0007231, doi: 10.1371/journal.pntd.0007231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh K, Rozewicki J, Yamada KD.. MAFFT online service: multiple sequence alignment, interac-tive sequence choice and visualization. Brief Bioinform. 2019;19;20(4):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bio-informatics. 2014;30:3276–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen L-T, Schmidt HA, von Haeseler A, et al. . IQ-TREE: a fast and effective stochastic al-gorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchard MA, Lemey P, Baele G, et al. . Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rambaut A, Lam TT, Carvalho M, et al. . Exploring the temporal structure of heter-ochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016;2:vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baele, G, Li WL, Drummond AJ, et al. . Accurate model selection of re-laxed molecular clocks in Bayesian phylogenetics. Mol. Biol. Evol. 2013;30:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemey P, Rambaut A, Welch JJ, et al. . Phylogeography takes a relaxed random walk in continuous space and time. Mol Biol Evol. 2010;27:1877–1885. doi: 10.1093/molbev/msq067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellicour S, Rose R, Faria NR, et al. . SERAPHIM:studying environ-mental rasters and phylogenetically informed movements. Bioinformatics. 2016;32:3204–3206. doi: 10.1093/bioinformatics/btw384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ewly generated sequences have been deposited in GenBank under accession numbers OR631863-OR631917.