Abstract
Background:
Weight reduction is a standard recommendation for obstructive sleep apnea (OSA) treatment in people with obesity or overweight; however, weight loss can be challenging to achieve and maintain without bariatric surgery. Currently, no approved anti-obesity medication has demonstrated effectiveness in OSA management. This study is evaluating the efficacy and safety of tirzepatide for treatment of moderate to severe OSA in people with obesity.
Methods:
SURMOUNT-OSA, a randomized, placebo -controlled, 52-week phase 3 trial, is investigating the efficacy and safety of tirzepatide for treatment of moderate to severe OSA (apnea hypopnea- index ≥15 events/hour) in participants with obesity (body mass index ≥30 kg/m2) and an established OSA diagnosis. SURMOUNT-OSA is made of 2 intervention-specific appendices (ISAs): ISA-1 includes participants with no current OSA treatment, and ISA-2 includes participants using positive airway pressure therapy. Overall, 469 participants have been randomized 1:1 to receive tirzepatide or placebo across the master protocol (ISA-1, n=234; ISA-2, n=235). All participants are also receiving lifestyle intervention for weight reduction.
Results:
The primary endpoint for the individual ISAs is the difference in apnea hypopnea- index response, as measured by polysomnography, between tirzepatide and placebo arms at week 52. Secondary endpoints include sleep apnea-specific hypoxic burden, functional outcomes, and cardiometabolic biomarkers. The trial employs digital wearables, including home sleep testing to capture time to improvement and accelerometry for daily physical activity assessment, to evaluate exploratory outcomes.
Conclusion:
SURMOUNT-OSA brings a novel design to investigate if tirzepatide provides clinically meaningful improvement in obesity-related OSA by targeting the underlying etiology.
Trial registration:
Keywords: sleep, obesity, apnea, lung, weight loss, daily functioning
1. Background
Obstructive sleep apnea (OSA) is a common disease with high prevalence across many countries [1]. Using the same diagnostic threshold as the SURMOUNT-OSA study to define hypopnea (4% decline in blood oxygen saturation), the Wisconsin Sleep Cohort study estimated that 17.4% of women and 33.9% of men in the United States aged 30 to 70 years had at least mild OSA, defined as an apnea-hypopnea index (AHI) of 5 to 14.9 events per hour of sleep, while 5.6% of women and 13.0% of men had moderate (AHI of 15–29.9 events/hour) or severe (AHI ≥30 events/hour) OSA [2,3]. OSA is increasing in prevalence due to the obesity pandemic and aging population [1]. Obesity is a major risk factor for OSA occurrence, although the mechanisms underlying obesity-related- OSA are not completely understood but may be related to increased tongue fat [4,5]. Weight reduction is an efficacious intervention for some patients with OSA as it is disease modifying [6,7]; however, it can be challenging to achieve and maintain weight loss in clinical practice with lifestyle interventions alone. Bariatric surgery has demonstrated a favorable impact on obesity-related OSA, although the risks and costs of surgical interventions are a deterrent for some patients. Pharmacotherapy for obesity is being used increasingly based on advances in this field. However, definitive data regarding the optimal management of people with obesity and OSA are lacking.
Weight reduction in patients with OSA is likely to be beneficial for many reasons [8,9]. Reduction of body weight, specifically excess adiposity surrounding the upper airways (particularly tongue and soft palate fat) and in the abdomen, is predicted to improve OSA severity based on the frequency of respiratory events and related to oxygen desaturation [10,11]. In addition, weight reduction in patients with OSA can improve cardiometabolic risk markers including high -sensitivity C-reactive protein (hsCRP), blood pressure, insulin resistance, and other factors [12]. Moreover, weight reduction often improves patient reported- outcomes (PROs) such as quality of life, fatigue, sleepiness, energy levels, depression, and overall sense of well-being [13]. Thus, strategies to improve weight reduction are clearly desirable for people living with obesity-related OSA.
Tirzepatide is a long-acting glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonist [13]. Treatment with tirzepatide improves control of carbohydrates, lipid metabolism, and body weight in patients with type 2 diabetes, beyond that observed with selective GLP-1 receptor agonists [14–17]. Results from the SURMOUNT-1 and SURMOUNT-2 studies in participants with obesity or overweight demonstrated mean weight reductions of up to 22.5% and 15.7% in those without and with type 2 diabetes, respectively, providing strong evidence that tirzepatide has the potential to be a highly effective anti-obesity medication, and these findings have been reinforced by data from other randomized controlled trials [13,18]. Tirzepatide received US Food and Drug Administration (FDA) approval for glycemic control in adults with type 2 diabetes and for chronic weight management. However, the role of tirzepatide in OSA management in people with obesity has not been explored.
Based on this conceptual framework, SURMOUNT-OSA will investigate if tirzepatide treatment in participants with OSA and obesity will yield clinically meaningful improvements in OSA severity as assessed by the AHI. In addition, this trial will assess PROs relevant to the disease burden and other clinically relevant outcome measures to determine a more comprehensive impact of tirzepatide treatment in participants with obesity-related OSA.
2. Methods
2.1. Study design and participants
SURMOUNT-OSA is a multicenter, randomized, parallel-arm, double-blind-, placebo-controlled phase 3 study to evaluate the efficacy and safety of tirzepatide at the maximum tolerated dose (MTD; 10 or 15 mg) once weekly versus placebo in participants with obesity (body mass index [BMI] ≥30 kg/m2) and moderate to severe OSA (AHI of ≥15 events/hour on polysomnography). The study design is a basket-type master protocol investigating 2 participant populations, described in 2 intervention-specific appendices (ISAs). ISA-1 includes 234 participants who are unwilling or unable to use positive airway pressure (PAP) therapy, and ISA-2 includes 235 participants who were using PAP therapy for at least 3 months at the time of screening and plan to continue PAP therapy during the study (Fig. 1). This trial is registered with ClinicalTrials.gov (NCT05412004), and the estimated study completion date is March 2024.
Fig. 1.
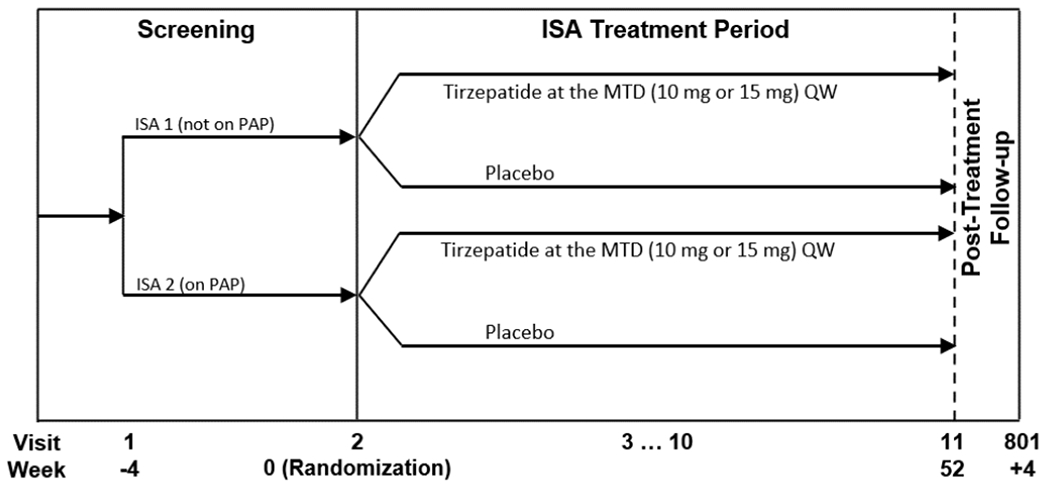
Study design.
Abbreviations: ISA, intervention-specific appendix: MTD, maximum tolerated dose; PAP, positive airway pressure; QW, once weekly.
Eligible participants are adults aged ≥18 years with moderate to severe OSA, a BMI ≥30 kg/m2, and a history of ≥1 self-reported unsuccessful dietary effort to lose weight. Exclusion criteria include a history of type 1 or 2 diabetes and a self-reported change in body weight >5 kg within 3 months before screening. Further trial eligibility criteria are shown in Table 1. As OSA is more common in males, the trial capped male enrollment at approximately 70% to ensure sufficient female participation.
Table 1.
Key eligibility criteria.
| Key inclusion criteria | |
| • Adult males or females aged ≥18 years • Body mass index ≥30 kg/m2 |
• Apnea-hypopnea index ≥15 events/hour • History of ≥1 self-reported unsuccessful dietary effort to lose body weight |
| Key exclusion criteria | |
| Diabetes related | |
| • HbA1c ≥6.5% at V1, and history of type 1 or 2 diabetes mellitus, ketoacidosis, or hyperosmolar state/coma | |
| OSA related | |
| • Any previous or planned surgery for sleep apnea or major ear, nose, or throat surgery • Significant craniofacial abnormalities that may affect breathing • Diagnosis of central or mixed sleep apnea, or diagnosis of Cheyne-Stokes respiration |
• Active device treatment of OSA other than positive airway pressure therapy • Respiratory and neuromuscular diseases that could interfere with the trial results |
| Obesity related | |
| • Have a self-reported change in body weight >5 kg within 3 months prior to screening |
• Have a prior or planned surgical or endoscopic treatment for obesity |
| Medical | |
| • History of disorder, other than OSA, associated with insomnia or excessive daytime sleepiness • Impaired renal function, defined as eGFR <30 mL/min/1.73 m2 • Known clinically significant gastric emptying abnormality or use of drugs affecting gastrointestinal motility • History of chronic or acute pancreatitis • Thyroid-stimulating hormone outside the range of 0.4–6.0 mIU/L • Obesity induced by other endocrinologic disorders, or monogenetic or syndromic forms of obesity • Deemed to be at significant risk for suicide per C-SSRS or investigator’s judgment • Patient Health Questionnaire-9 score of ≥15 at V1 or V2, prior to randomization • Uncontrolled hypertension (SBP ≥160 mmHg and/or DBP ≥100 mmHg) • Acute MI, cerebrovascular accident (stroke), unstable angina, or hospitalization due to CHF within 3 months prior to randomization • History of (<3 months prior to V1) or planned cardiovascular procedure • NYHA functional class IV CHF • Calcitonin level ≥20 ng/L at screening, if eGFR ≥60 mL/min/1.73 m2, or ≥35 ng/L, if eGFR <60 mL/min/1.73 m2 • Acute or chronic hepatitis or other liver disease (excluding nonalcoholic fatty liver disease) • ALT >3 × ULN for the reference range or ALP >1.5 × ULN or total bilirubin >1.2 × ULN (except for Gilbert syndrome) |
• History of or in remission from malignancy (other than basal or squamous cell skin cancer, in situ carcinomas of the cervix, or in situ prostate cancer) for <5 years • Family or personal history of MTC or MEN2 • History of marijuana use within <3 months of V1 and unwillingness to abstain from it during the trial • Have had a transplanted organ (corneal transplants [keratoplasty] allowed) or awaiting an organ transplant • Requires the use of supplemental oxygen • Concomitant therapy (current or within 3 months prior to screening) • Use of a GLP-1 RA <3 months prior to V1 • Use of metformin or any other glucose-lowering medication (whether prescribed for PCOS or diabetes prevention) • Use of systemic glucocorticoid therapy • Use of medications that may cause weight gain such as tricyclic antidepressants, atypical antipsychotics, and mood stabilizers • Use of medication or alternative therapies that promote weight loss • Use of stimulants <3 months prior to V1 • Use of hypnotics, mirtazapine, opioids, or trazodone <3 months prior to V1 • Use of any over-the-counter or prescription medications that could affect the evaluation of excessive sleepiness |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; DBF, diastolic blood pressures; CHF, congestive heart failure; C-SSRS, Columbia-Suicide Severity Rating Scale; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide 1 receptor agonist; MEN2 multiple endocrine neoplasia type 2; MI, myocardial infarction; MTC, medullary thyroid cancer; NYHA, New York Heart Association; OSA, obstructive sleep apnea; PCOS, polycystic ovary syndrome; SBP, systolic blood pressure; ULN, upper limit of normal; V1, Visit 1; V2, Visit 2.
The trial is being conducted in accordance with good clinical practice guidelines and the principles of the Declaration of Helsinki. Independent ethics committee or institutional review board approvals were received for each participating site. All participants provided written informed consent before trial participation.
2.2. Procedures
All participants are receiving lifestyle intervention throughout the study. This includes regular lifestyle counseling sessions delivered by experienced healthcare professionals, focused on healthy balanced meals with a 500-kcal/d deficit and at least 150 minutes per week of physical activity in accordance with guidelines [19].
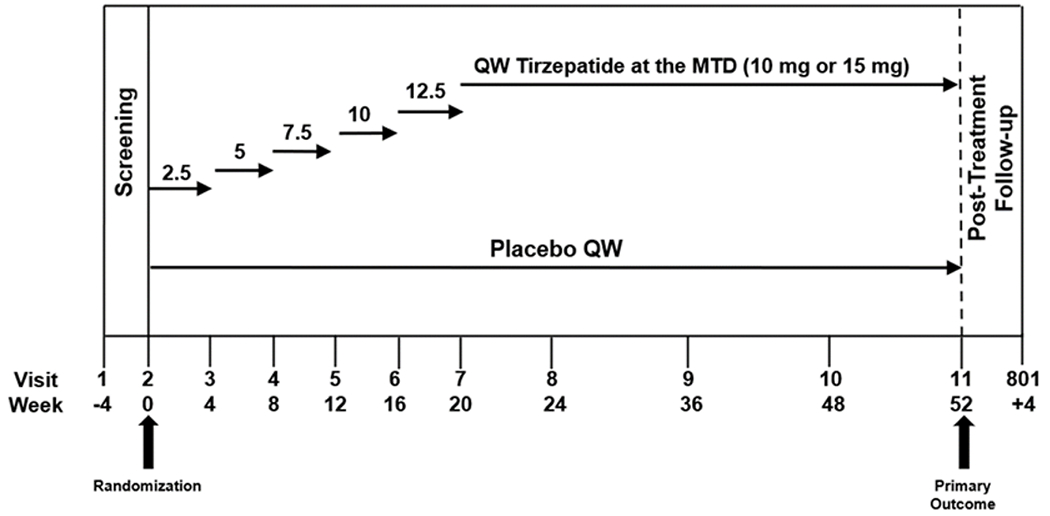
The SURMOUNT-OSA study is evaluating tirzepatide at the MTD (10 or 15 mg) with once-weekly subcutaneous administration. The starting tirzepatide dose of 2.5 mg once weekly is escalated in 2.5-mg increments at 4-week intervals until the participant reaches the MTD (10 or 15 mg); after this initial 20-week escalation period, participants remain on the MTD for an additional 32 weeks of the trial (Fig. 1). Dose de-escalation and re-escalation are allowed in the first 24 weeks after randomization to allow participants a greater opportunity to achieve the MTD. De-escalation and subsequent re-escalation are allowed only for management of intolerable gastrointestinal symptoms when other mitigations such as dietary counseling, symptomatic treatment, or temporary drug interruption for one dose have failed. The study drug will be discontinued for participants unable to tolerate the 10mg- dose; dose modifications beyond 24 weeks after randomization are not permitted. If a BMI ≤22 kg/m2 is reached during this trial, the recommended energy intake is recalculated with no caloric deficit for the remainder of the trial. If a BMI ≤18.5 kg/m2 is reached, the study drug is discontinued.
Treatment discontinuation is decided by the participant or the investigator. Clinical considerations for treatment discontinuation include initiation of prohibited medication, intolerable gastrointestinal symptoms, significantly elevated calcitonin levels, diagnosis of type 1 diabetes, pancreatitis, malignancy, major psychiatric disorder, or incident pregnancy. Participants who permanently stop the study drug during the double-blind treatment period are encouraged to continue attending all scheduled study visits for collection of all planned efficacy and safety measurements.
The expected total duration of study participation for each participant, including screening and the posttreatment follow-up periods, is 60 weeks, including 4 weeks of screening, 52 weeks of treatment, and 4 weeks of posttreatment follow-up (Fig. 1).
A steering committee comprised an academic/industry partnership including the lead author (Atul Malhotra) as chairperson, with participation from other members including coauthors. The committee had scheduled meetings throughout the design and execution of the study to make decisions regarding study conduct and for troubleshooting as issues arose. The steering committee remained blinded to all data, with an independent data monitoring committee (DMC) in place to meet to review unblinded data. The DMC is reviewing and evaluating unblinded trial data on a 6-month basis and ad hoc. The aim is to protect the study participants’ safety, evaluate the benefit-risk balance, and provide recommendations on trial continuation, modification, or termination.
2.3. Objectives and endpoints
The primary objective of the SURMOUNT-OSA trial is to demonstrate the superiority of tirzepatide at the MTD after 52 weeks of treatment, compared with placebo when given as an adjunct to diet and exercise, in the reduction of AHI for participants with moderate to severe OSA and obesity. AHI is measured by polysomnography and after 7 to 9 days of PAP therapy withdrawal for participants in ISA-2. The change in AHI is measured based on the Centers for Medicare & Medicaid Services criteria, i.e., hypopnea is considered any abnormal respiratory event lasting ≥10 seconds with a ≥30% reduction in thoracoabdominal movement or airflow, as compared to baseline, and with ≥4% oxygen desaturation.
Key secondary objectives (controlled for type 1 error) include percent change in AHI; change in the Functional Outcomes of Sleep Questionnaire (FOSQ) short version (FOSQ-10) score and in the FOSQ long version (FOSQ-30) vigilance and activity level subscales scores (assessed in hierarchical order using the win ratio*); responder analysis of participants achieving a meaningful change in AHI and OSA remission or mild nonsymptomatic OSA; change in sleep apnea specific hypoxic burden (SASHB); change in body weight, inflammatory status, and systolic blood pressure. Other secondary objectives include disease -relevant PROs, cardiometabolic biomarkers, and actigraphy measures (Table 2).
Table 2.
Objectives and endpoints.
| Primary objective | Endpoint |
|---|---|
| To demonstrate that tirzepatide QW at the MTD (10 or 15 mg) is superior to placebo for mean decrease in AHI | Change in AHI from baseline to week 52 (events/hour) |
| Key secondary objectives (controlled for type I error) | Endpoints |
| To demonstrate that tirzepatide QW at the MTD (10 or 15 mg) is superior to placebo for: • Change in AHI • A hierarchical assessment of PROs • Clinically meaningful change in AHI • Achieving OSA remission or mild non-symptomatic OSA • Change in body weight • Change in inflammatory status • Change in SBP |
From baseline to week 52: • Percent change in AHI • A hierarchical combination of change in: ○ FOSQ-10 score ○ FOSQ-30 vigilance subscale score ○ FOSQ-30 activity level subscale score • Percent of participants with ≥50% AHI reduction • Percent of participants with ○ AHI <5 events/hour or ○ AHI 5–14 events/hour with ESS score ≤10 • Percent change in body weight • Change in hsCRP concentration From baseline to week 48a: • Change in SBP |
| Other secondary objectives | Endpoints |
| To demonstrate that tirzepatide QW at the MTD (10 or 15 mg) is superior to placebo for: • Change in excessive daytime sleepiness • Change in patient-reported functional status as assessed by FOSQ (30-item) • Change in body weight • Change in lipid parameters • Change in PROs • Insulin • Hypoxic burden • Change in DBP |
From baseline to week 52: • Change in ESS score • Change in all other FOSQ domain scores • Percent of participants who achieve ○ ≥10% body weight reduction ○ ≥15% body weight reduction ○ ≥20% body weight reduction • Change in ○ HDL cholesterol ○ Non-HDL cholesterol ○ Triglycerides • Change in ○ PROMIS sleep-related impairment short form 8a score ○ PROMIS sleep disturbance short form 8b score ○ SF-36v2 acute form domain scores • Percent of participants with improved categorical shift in PGIS score for ○ OSA sleepiness ○ OSA fatigue ○ OSA snoring • Change in fasting insulin • Change in SASHB (% min/hour) From baseline to week 48a: • Change in DBP |
| Exploratory objectives | Endpoints |
| To demonstrate that tirzepatide QW at the MTD (10 or 15 mg) is superior to placebo for: • Change in PROs • To evaluate the effect of tirzepatide on sleep parameters as measured by actigraphy (Axivity AX6 accelerometer) |
From baseline to week 52: • Change in ○ EQ-5D-5L utility index ○ EQ-VAS scores • Percent of participants with improved categorical shift in PGIC score for: ○ OSA sleepiness ○ OSA fatigue ○ OSA sleep quality ○ OSA snoring • Mean change from baseline to endpoint assessment in: ○ Daytime sleep duration ○ Daily step counts ○ Average acceleration |
Abbreviations: AHI, apnea-hypopnea index; BP, blood pressure; DBP, diastolic blood pressure; EQ-5D-5L, EuroQol 5-Dimension 5-Level; EQ-VAS, EuroQol visual analogue scale; ESS, Epworth Sleepiness Scale; FOSQ-10, Functional Outcomes of Sleep Questionnaire short version; FOSQ-30, Functional Outcomes of Sleep Questionnaire long version; HDL, high -density lipoprotein; hsCRP, high -sensitivity C-reactive protein; MTD, maximum tolerated dose; OSA, obstructive sleep apnea; PGIC, Patient Global Impression of Change; PGIS, Patient Global Impression of Severity; PROs, patient -reported outcomes; PROMIS, Patient -Reported Outcomes Measurement Information System; QW, once weekly; SASHB, sleep apnea-specific hypoxic burden; SBP, systolic blood pressure; SF-36v2, Short-Form 36 Health Survey version 2.
BP will be assessed at week 48 because positive airway pressure suspension at week 52 may confound BP assessment.
All adverse events regardless of seriousness are reportable by the investigators, including events leading to treatment discontinuation, events for independent adjudication, and events requiring additional data collection. All adverse events and adjudication outcomes are being reviewed by an independent DMC, consisting of professionals with expertise in relevant specialties including statistics, sleep medicine, cardiology, endocrinology, etc.
2.3.1. Notable analysis and substudies
2.3.1.1. Neck anatomy and body composition.
Fat deposition in tissues surrounding the upper airway directly contributes to upper airway collapsibility [20]. Obesity also contributes indirectly to upper airway narrowing, especially in the hypotonic airway present during sleep, because lung volumes are markedly reduced by a combination of increased abdominal fat mass, including visceral fat, and being in a recumbent posture [21]. Tongue fat has been shown to be increased in patients with apnea and obesity when compared to those with obesity and no apnea, which helps explain the association between obesity and sleep apnea [22]. Moreover, reductions in tongue fat volume with weight reduction have been observed to be significantly correlated with AHI reductions among patients with OSA [11]. Upper airway imaging provides important insights into the biomechanical basis for OSA and the mechanisms underlying the efficacy of various therapeutic interventions including weight reduction.
The magnetic resonance imaging (MRI) addendum of SURMOUNT-OSA, in a sample of approximately 50 participants, is exploring changes in absolute tissue volume as well as fat volume and fat content (%) of the tongue, soft palate, pterygoid muscle, lateral pharyngeal walls, neck, and submandibular area. This addendum is also exploring changes in total, visceral, and subcutaneous abdominal adipose tissue volume. The MRI scan will be performed before and after the 52-week treatment intervention.
2.3.1.2. Polysomnography measures of interest.
Sleep apnea specific hypoxic burden is determined by measuring the respiratory event-associated area under the curve for oxygen desaturation from baseline before the event; as such, this represents the cumulative burden of intermittent hypoxia caused by OSA-related sleep -disordered breathing. Sleep apnea-specific hypoxic burden strongly predicts OSA-related cardiovascular disease mortality, overall mortality, and incident heart failure after accounting for important risk factors and comorbidities [23–25]. A recent post hoc analysis of the ISAACC randomized controlled trial demonstrated that treating those with a high hypoxic burden reduces risk of long-term cardiovascular disease [26].
Given its role as a predictor of OSA severity, sleep apnea-specific hypoxic burden will be assessed from polysomnography readings in SURMOUNT-OSA. The study will assess tirzepatide-related improvement in hypoxic burden and analyze the correlation of change in sleep apnea-specific hypoxic burden with changes detected in sleep, breathing, cardiometabolic indicators, and PROs.
Polysomnographic estimates of sleep apnea endotypic characteristics (pharyngeal collapsibility, muscle compensation, loop gain, and arousal threshold) will be calculated from polysomnography data [27–29] to explore the following:
Endotypic mechanisms of tirzepatide. Tirzepatide, and associated weight loss, may have physiological mechanisms of action beyond pharyngeal collapsibility. For example, obesity is associated with elevated loop gain (exaggerated chemoreflex control of breathing) [30]. This study will identify candidate physiological mechanisms by quantifying changes in each endotypic characteristic with treatment versus placebo (on treatment vs. baseline, vs. placebo; assessed with and without PAP).
Identifying characteristics of responders to tirzepatide. Using baseline data on endotypic characteristics, a multivariable analysis will be employed to assess the characteristics associated with greater responses to tirzepatide.
The primary analysis of endotypic characteristics will involve quantitative automated analysis of polysomnographic nasal pressure airflow signals. Briefly, each study is divided into 7-minute windows (2-minute overlap) from which an uncalibrated breath-by-breath ventilation signal (tidal volume × respiratory rate) is derived from linearized nasal pressure data. For each window, an estimated ventilatory drive signal is derived (least-squares fit using a model that converts reduced ventilation during events into increased drive during recovery periods); the model provides a numerical estimate of loop gain. Median values from available windows containing events during the non-rapid eye movement sleep stage are used to summarize each study. In addition, arousal threshold is taken as the median value of ventilatory drive preceding arousals from sleep. Collapsibility is taken as the median value of ventilation observed at normal ventilatory drive (lower values reflect greater collapsibility, i.e., zero ventilation is complete collapse). Compensation is taken as the increase in ventilation (from normal drive) observed when ventilatory drive rises to reach the level of the arousal threshold.
2.3.1.3. Home sleep apnea test (HSAT) and actigraphy.
WatchPAT® 300 (ZOLL® Itamar®, Atlanta, GA, USA) is an HSAT monitor that works on the basis of peripheral arterial tonometry, a noninvasive measure for the arterial pulsatile volume changes at the fingertip. WatchPAT 300 has received FDA approval as a medical indication for sleep apnea diagnosis in adults and children aged 12 years and older. Despite numerous validation studies demonstrating a high degree of correlation in the respiratory disturbance index, AHI, and oxygen desaturation index during simultaneous recording of WatchPAT and polysomnography (respiratory disturbance index, r=0.879; AHI, r=0.893; oxygen desaturation index, r=0.942), no HSAT monitor has been approved for tracking OSA severity [31].
The ISA-1 (participants without PAP background therapy) in SURMOUNT-OSA is using WatchPAT 300 to explore early treatment-related changes in the respiratory event index after treatment initiation at weeks 4 and 12. The respiratory event index will be measured in parallel with polysomnography AHI measurements at baseline, week 20, and week 52. These data are expected to provide important evidence of the accuracy and reliability of WatchPAT 300 versus polysomnography in tracking OSA severity.
Actigraphy is a noninvasive method of estimating activity patterns through the monitoring of movement. The data on gross motor activity, collected with a small watch-sized device worn on the wrist, are subsequently translated to data points (e.g., epochs of wakefulness or sleep) using a device-specific algorithm. Actigraphy data have the potential to be a useful objective measure to complement traditional clinical outcome assessments (Ebony Dashiell-Aje, US FDA).
SURMOUNT-OSA is collecting actigraphy data for both ISAs through a 6-axis logging accelerometer device (AX6; Axivity, Newcastle upon Tyne, UK) at baseline, endpoint, and 3 other timepoints in the study to follow-up on mean changes in daytime sleep duration, daily step count, and average acceleration. These data are anticipated to complement traditional PRO data in assessing changes in participants’ functioning.
2.4. Statistical considerations
The master protocol guiding the evaluation of efficacy and safety of tirzepatide consists of 2 ISAs (see Section 2.1. Study design and participants). Within each ISA, participants are randomly assigned 1:1 to receive treatment with tirzepatide (MTD of 10 or 15 mg) or placebo once weekly. Randomization is stratified by country/geographic region, baseline AHI (moderate or severe), and gender.
The sample size in both ISAs provides at least 90% power to demonstrate superiority of tirzepatide to placebo for the mean percent change from baseline in AHI at a 2-sided alpha level of 0.05, assuming 50% improvement compared with placebo, with a common standard deviation of 50%, and up to 25% of participants discontinuing the study drug in each arm.
For each ISA, the superiority of tirzepatide versus placebo will be evaluated and aligned to 2 estimands: treatment regimen estimand and efficacy estimand. For both estimands, the population of interest is individuals who meet the eligibility criteria and receive at least 1 dose of study drug, the primary endpoint is the change from baseline in AHI at 52 weeks, and the population summary is the difference in mean changes in AHI from baseline to week 52 between tirzepatide and placebo. For the treatment regimen estimand, the treatment condition is the randomized treatment with allowance for potential dose interruptions and modifications regardless of adherence to study drug or initiation. For the efficacy estimand, the treatment condition is the randomized treatment with allowance for potential dose interruptions and modifications. The intercurrent event (ICE) of permanent discontinuation of study drug will be considered as part of the treatment condition in the analysis aligned to the treatment regimen estimand; hence, all available valid AHI values will be used in analysis. For the analysis aligned to the efficacy estimand, the ICE will be handled using a hypothetical strategy assuming the AHI after the ICE is as if participants remain on their randomly assigned treatment for 52 weeks.
An analysis of covariance model with the primary endpoint as a response variable, treatment and strata (pooled country/geographic region and gender) as fixed effects, and baseline AHI as a covariate will be used for analysis.
Key secondary endpoints will be controlled for type 1 error rate and include change from baseline in AHI; a hierarchical assessment of PROs based on change in FOSQ**,⸸ scores; clinically meaningful ≥50% change in AHI and responder analysis among those achieving OSA remission or mild non-symptomatic OSA (AHI<5 or AHI=5-14 with ESS≤10); and change from baseline in body weight, hsCRP, and SBP.
3. Demographics and baseline characteristics
As seen in Table 3, the enrolled participants have demographics and characteristics that reflect patients with OSA in usual clinical practice, comprising 30.3% females with a mean age of 49.7 years and mean BMI of 38.8 kg/m2 (roughly evenly distributed with class 1, class 2, and class 3 obesity) [2]. Participants are from diverse racial and ethnic backgrounds, including 17.5% Asian, 5.2% Black, and 8.1% American Indian or Alaska native, with 36.2% of Hispanic/Latino ethnicity. Regionally, 32.0% of patients are from North America, 31.8% from South America, 14.8% from Europe, and 21.4% from Asia/Pacific. The mean AHI of 50.1 events/hour is consistent with most participants (65.3%) having a diagnosis of severe OSA. Participants were mildly sleepy at baseline with a mean ESS score of 10.3. Baseline comorbidities were typical of this patient population (participants with diabetes were excluded) including prediabetes (61.7%), hypertension (76.3%), and dyslipidemia (83.4%) [32].
Table 3.
Demographics and baseline characteristics.
| Parameter | Total (N=459) |
|---|---|
| Age, years | 49.7 (11.4) |
| <50 | 221 (48.1%) |
| ≥50 | 238 (51.9%) |
| Female | 139 (30.3%) |
| Race | |
| American Indian or Alaska native | 37 (8.1%) |
| Asian | 80 (17.5%) |
| Black or African American | 24 (5.2%) |
| White | 316 (69.0%) |
| Multiple | 1 (0.2%) |
| Missing | 1 (0.2%) |
| Ethnicity | |
| Hispanic or Latino | 166 (36.2%) |
| Not Hispanic or Latino | 288 (62.7%) |
| Not reported | 5 (1.1%) |
| Region | |
| North America | 32.0% |
| South America | 31.8% |
| Europe | 14.8% |
| Asia-Pacific | 21.4% |
| Weight, kg | 115.0 (22.8) |
| BMI, kg/m2 | 38.8 (6.4) |
| <35 | 140 (30.5%) |
| ≥35 to <40 | 160 (34.9%) |
| ≥40 | 159 (34.6%) |
| HbA1c, % | 5.65 (0.4) |
| AHI, events/hour | 50.1 (28.6) |
| OSA severity | |
| Moderate | 152 (33.2%) |
| Severe | 299 (65.3%) |
| Waist circumference, cm | 121.0 (14.6) |
| Neck circumference, cm | 44.3 (4.7) |
| Prediabetes | 283 (61.7%) |
| Dyslipidemia | 383 (83.4%) |
| Hypertension | 350 (76.3%) |
| ESS score | 10.3 |
| Sleep efficiency | 75.8 (14.0) |
| Sleep onset | 15.9 (20.8) |
| WASO, min | 97.4 (59.8) |
| REM sleep, % | 13.1 (7.1) |
Note: Data are presented as mean (standard deviation) or n (%) unless otherwise noted.
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; HbA1c, glycated hemoglobin; OSA, obstructive sleep apnea; REM, rapid eye movement; WASO, wakefulness after sleep onset.
4. Discussion
The SURMOUNT-OSA trial, by assessing gold-standard outcomes such as the AHI and targeting moderate to severe OSA, will be in a strong position to understand the role of tirzepatide treatment for managing moderate to severe OSA in people with obesity. In addition, the study is assessing cardiometabolic biomarkers to support a more comprehensive understanding of the impact of tirzepatide treatment in OSA.
Current management of OSA is limited, as existing therapies focus on treating symptoms rather than the underlying disease, and adherence to available therapies is quite variable. Nasal PAP is the OSA treatment of choice. Although many patients experience transformative benefits from PAP [33], others are unable to adhere to or refuse PAP treatment entirely because of the perceived burden [34]. Although OSA symptoms can improve with PAP therapy, current evidence does not strongly support the benefits of PAP in OSA for cardiometabolic outcomes (mortality, hypertension, myocardial infarction, stroke) [35,36]. Alternative therapies, such as mandibular advancement therapy or hypoglossal nerve stimulation, are supported so far with limited clinical evidence. Thus, alternative options for the treatment of OSA are needed to yield better long-term outcomes [37].
SURMOUNT-OSA is designed to answer questions about the efficacy and safety of tirzepatide treatment in participants with OSA and obesity. Efficacy will be measured by the effect of treatment on sleep-disordered breathing directly (change in AHI), responder rate analysis (percent of patients achieving a clinically meaningful change and percent of patient achieving either remission or mild asymptomatic OSA), hypoxic burden (change in sleep apnea-specific hypoxic burden), objective sleep patterns, and various PROs [23,38,39]. In addition to these outcome measures, SURMOUNT-OSA will generate further data about the effect of tirzepatide treatment on important cardiometabolic indicators (blood pressure, lipids, and fasting insulin) and inflammation (hsCRP) in people with obesity-related OSA. The study will uniquely explore the effect of tirzepatide treatment in 2 standalone ISAs of participants with and without use of PAP therapy. Thus, this study design is used to maximize generalizability in OSA management. For participants without use of PAP therapy in ISA-1, tirzepatide treatment is posited to provide weight reduction with improved body composition and associated improvements in cardiovascular risk markers [13], which would provide a compelling rationale to treat the underlying cause of obesity-related OSA rather than focus on symptomatic management. Similar benefits are expected for participants using PAP therapy in ISA-2. Indeed, a number of studies including meta-analyses have shown increases in body weight with PAP therapy, suggesting a major need to address body weight in the OSA population [40]. Given that these patients are at risk of weight gain, any observed improvements in body weight are likely to improve PROs and satisfaction [41–43]. Chirinos et al. [12] studied the impact of PAP therapy versus weight loss in a cohort of patients with OSA and obesity. They observed considerable benefits to weight reduction from the standpoint of hsCRP and other cardiovascular biomarkers in participants assigned to weight reduction alone and those assigned to a combination of weight reduction and PAP, but this was not observed in those receiving PAP alone [12]. Despite these compelling data, weight management is not prioritized in patients with OSA, leading to current efforts to improve this situation.
While objective outcomes are clearly important in OSA management, there is increasing appreciation for the role of PROs in providing a holistic understanding of the effect of therapeutic interventions. Thus, SURMOUNT-OSA will thoroughly assess a number of well-validated PROs to determine the impact of tirzepatide treatment on participants’ overall well-being. PROs are being assessed by employing the FOSQ-10, FOSQ-30 vigilance, and FOSQ-30 activity level subscales in a hierarchical design, along with the Patient-Reported Outcomes Measurement Information System sleep-related impairment and sleep disturbance forms, in addition to assessing excessive daytime sleepiness based on the Epworth Sleepiness Scale [44,45].
Despite the strengths of the SURMOUNT-OSA study, we acknowledge a number of potential limitations. First, the study is designed and powered to assess the efficacy of tirzepatide on sleep-disordered breathing and selected cardiometabolic risk factors; however, it is not designed or adequately powered for cardiovascular outcomes such as mortality, stroke, or myocardial infarction. Thus, the findings may provide a compelling rationale for subsequent large-scale studies. Second, we can conduct mediation analyses based on the study design, but ultimately, we may be unable to determine definitively which improvements are related to the resolution of obesity versus those related to the resolution of OSA per se. Moreover, changes in CPAP adherence, residual AHI and mask leak may have important confounding effects on AHI improvement or lack of improvement. Third, blinding can be quite challenging if major weight loss is obvious in the tirzepatide arm. This finding could influence the subjective reported outcomes, although we view PRO improvements in the context of major weight loss to be important. Indeed, we expect subjective improvements in the context of major weight loss to be real rather than a theoretical concern regarding potential misreporting. Despite these limitations, we view our study as rigorously designed and highly likely to provide clinically directive evidence for a multitude of patients with OSA.
5. Conclusion
The SURMOUNT-OSA study is rigorously designed to determine if tirzepatide provides clinically meaningful improvement in obesity-related OSA by targeting an underlying etiology. The study is evaluating participants without PAP use to assess the role of tirzepatide as a possible primary therapy for OSA as well as participants with PAP use to determine the role of tirzepatide as an adjunctive therapy. Carefully chosen objectives and subjective outcome measures should provide important guidance regarding the optimal management of OSA, role of weight management in treatment strategy, as well as a valuable information about the impact of tirzepatide treatment on relevant cardiometabolic indicators. The study may also provide indices whether tirzepatide influences sleep disordered breathing mostly by reduction in BMI, or by changes in other factors, including improvement in upper airway fat, loop gain, insulin resistance or inflammation. The study employs technology with HSAT/actigraphy to investigate changes in sleep and activity, and the MRI study addendum to visualize and measure the effect of treatment on upper airway caliber and tongue fat.
Acknowledgements
The authors would like to thank Amelia Torcello Gomez PhD, and Gary Grant PhD, from Eli Lilly and Company, for writing and editorial contributions. Some data from this manuscript were presented at the SLEEP 2023 37th Annual Meeting of the Associated Professional Sleep Societies held on June 3–7, 2023 in Indianapolis, IN, USA.
Funding
This study was funded by Eli Lilly and Company.
Declaration of competing interests
AM is funded by the National Institutes of Health (NIH) and has received income related to medical education from Eli Lilly and Company, Jazz Pharmaceuticals, LivaNova, and Zoll Medical; ResMed provided a philanthropic donation to the employer of AM (University of California San Diego). TW has served as a consultant/advisory board member from the Alkermes Orexin Advisory Board, Bayer AG, Eli Lilly and Company, and the Idorsia Alliance for Sleep; and has received royalty fees for use of the FOSQ from Alkermes, Axsome Therapeutics, Bayer AG, Bioprojet Deutschland GmbH, Eli Lilly and Company, Harmony Biosciences, Ignis Therapeutics (Shanghai) Ltd., Inspire Medical Systems, IQVIA Technologies, Jazz Pharmaceuticals, LivaNova, Nyxoah, Philips Respironics, ResMed, Signant Health, Signifier Medical Technologies, Stratevi, Syneos Health, Vallis Bioscience, and Verily Life Sciences. RG has served as a consultant for Apnimed and Eli Lilly and Company. IF has served as a consultant for Eli Lilly and Company, Idorsia, ResMed, and Stada Arzneimittel AG; has served as a speaker for Hennig and Idorsia; and has received research grants from Löwenstein Medical and ResMed. SR has received grants from the NIH during the conduct of the study; has received grants and personal fees from Jazz Pharmaceuticals, personal fees from Eli Lilly and Company, and personal fees from Apnimed outside the submitted work; and is the first incumbent of an endowed professorship donated to the Harvard Medical School by Dr. Farrell (the founder and Board Chairman of ResMed) through a charitable remainder trust instrument, with annual support equivalent to the endowment payout provided to the Harvard Medical School during Dr. Farrell’s lifetime by ResMed through an irrevocable gift agreement. AA has received grant funding from the American Academy of Sleep Medicine Foundation and NIH; receives grant support from SomniFix; and serves as a consultant for Apnimed, Eli Lilly and Company, Respicardia, and SomniFix. Apnimed is developing pharmacological treatments for OSA. SAS has received grant funding from the NIH; has received grant support from Apnimed, DynaFlex, and ProSomnus; and serves as a consultant for Apnimed, Eli Lilly and Company, Forepont, Inspire Medical Systems, LinguaFlex, Nox Medical, and Respicardia. The interests of AA and SS were reviewed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their institutional policies. RJS reports grant and/or research support from ResMed, Inspire, and CryOSA, royalties from UpToDate and Merck Manual, research consulting for Eli Lilly and Company, and is on the Medical Advisory Board for eXciteOSA and Sleep Evolution. JB, SC, JPD, and MCB are employees and shareholders of Eli Lilly and Company.
Footnotes
Assessment of patient reported outcomes plan has not been finalized yet and final selection of endpoints controlled for type 1 error will be codified in the Statistical analysis plan before database lock and data unblinding.
The sample size was calculated for % change in AHI, which was the primary endpoint of the study. The primary endpoint was later changed to be absolute change in AHI, but the sample size was not recalculated because study enrollment was already complete at that time.
Assessment of patient reported outcomes plan has not been finalized yet and final selection of endpoints controlled for type 1 error will be codified in the Statistical analysis plan before database lock and data unblinding.
Data availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after a receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- [1].Young T, Peppard PE, Gottlieb DJ, Epidemiology of obstructive sleep apnea: a population health perspective, Am. J. Respir. Crit. Care Med 165 (9) (2002) 1217–1239. 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- [2].Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM, Increased prevalence of sleep-disordered breathing in adults, Am. J. Epidemiol 177 (9) (2013) 1006–1014. 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gottlieb DJ, Punjabi NM, Diagnosis and management of obstructive sleep apnea: a review, JAMA 323 (14) (2020) 1389–1400. 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- [4].Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, Wellman A, Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea, Am. J. Respir. Crit. Care Med 190 (8) (2014) 930–937. 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ, Tongue fat and its relationship to obstructive sleep apnea, Sleep 37 (10) (2014) 1639–48. 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST, Obstructive sleep apnea among obese patients with type 2 diabetes, Diabetes Care 32 (6) (2009) 1017–1019. 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST, A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study, Arch. Intern. Med 169 (17) (2009) 1619–1626. 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grunstein RR, Hedner J, Grote L, Treatment options for sleep apnoea, Drugs 61 (2) (2001) 237–251. 10.2165/00003495-200161020-00007. [DOI] [PubMed] [Google Scholar]
- [9].Grunstein RR, Wilcox I, Sleep-disordered breathing and obesity, Baillieres Clin. Endocrinol. Metab 8 (3) (1994) 601–628. 10.1016/s0950-351x(05)80288-5. [DOI] [PubMed] [Google Scholar]
- [10].Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER, Weight loss in mildly to moderately obese patients with obstructive sleep apnea, Ann. Intern. Med 103 (6 (Pt 1)) (1985) 850–855. 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- [11].Wang SH, Keenan BT, Wiemken A, Zang Y, Staley B, Sarwer DB, Torigian DA, Williams N, Pack AI, Schwab RJ, Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat, Am. J. Respir. Crit. Care Med 201 (6) (2020) 718–727. 10.1164/rccm.201903-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI, CPAP, weight loss, or both for obstructive sleep apnea, N. Engl. J. Med 370 (24) (2014) 2265–2275. 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A, SURMOUNT-1 Investigators, Tirzepatide once weekly for the treatment of obesity, N. Engl. J. Med 387 (3) (2022) 205–216. 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- [14].Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, Kuchibhotla U, Moyers JS, Benson CT, Gimeno RE, D’Alessio DA, Haupt A, LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept, Mol. Metab 18 (2018) 3–14. 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K, Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes, N. Engl. J. Med 385 (6) (2021) 503–515. 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- [16].Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, Bray R, Rodríguez Å, Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial, Lancet 398 (10300) (2021) 583–598. 10.1016/s0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- [17].Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ, Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial, Lancet 398 (10313) (2021) 1811–1824. 10.1016/s0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- [18].Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, Mao H, Zhang S, Ahmad NN, Bunck MC, Benabbad I, Zhang XM, SURMOUNT-2 Investigators, Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial, Lancet 402 (10402) (2023) 613–626. 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- [19].Human energy requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. https://www.fao.org/3/y5686e/y5686e00.htm, 2001. (accessed October 24, 2023).
- [20].Jang MS, Kim HY, Dhong HJ, Chung SK, Hong SD, Cho HJ, Jung TY, Effect of parapharyngeal fat on dynamic obstruction of the upper airway in patients with obstructive sleep apnea, Am. J. Respir. Crit. Care Med 190 (11) (2014) 1318–1321. 10.1164/rccm.201408-1498LE. [DOI] [PubMed] [Google Scholar]
- [21].Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP, Pathophysiology of sleep apnea, Physiol. Rev 90 (1) (2010) 47–112. 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ, Tongue fat and its relationship to obstructive sleep apnea, Sleep 37 (10) (2014) 1639–1648. 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, Ancoli-Israel S, Ensrud K, Purcell S, White DP, Redline S, Wellman A, The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study, Eur. Heart J 40 (14) (2019) 1149–1157. 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Azarbarzin A, Sands SA, Taranto-Montemurro L, Vena D, Sofer T, Kim SW, Stone KL, White DP, Wellman A, Redline S, The sleep apnea-specific hypoxic burden predicts incident heart failure, Chest 158 (2) (2020) 739–750. 10.1016/j.chest.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Labarca G, Vena D, Hu WH, Esmaeili N, Gell L, Yang HC, Wang TY, Messineo L, Taranto-Montemurro L, Sofer T, Barr RG, Stone KL, White DP, Wellman A, Sands S, Redline S, Azarbarzin A, Sleep apnea physiological burdens and cardiovascular morbidity and mortality, Am. J. Respir. Crit. Care Med 208 (7) (2023) 802–813. 10.1164/rccm.202209-1808OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pinilla L, Esmaeili N, Labarca G, Martinez-Garcia M, Torres G, Gracia-Lavedan E, Mínguez O, Martínez D, Abad J, José Masdeu M, Mediano O, Muñoz C, Cabriada V, Duran-Cantolla J, Mayos M, Coloma R, Montserrat JM, de la Peña M, Hu WH, Messineo L, MohammadReza S, Wellman A, Redline S, Sands S, Barbé F, Sánchez-de-la-Torre M, Azarbarzin A, Hypoxic burden to guide CPAP treatment allocation in patients with obstructive sleep apnoea: a post-hoc study of the ISAACC trial, Eur. Respir. J Online ahead of print (2023). 10.1183/13993003.00828-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, Sands SA, Quantifying the ventilatory control contribution to sleep apnoea using polysomnography, Eur. Respir. J 45 (2) (2015) 408–418. 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, de Melo CM, Loring SH, Butler JP, White DP, Wellman A, Quantifying the arousal threshold using polysomnography in obstructive sleep apnea, Sleep 41 (1) (2018) zsx183. 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, Hess LB, White DP, Wellman A, Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea, Am. J. Respir. Crit. Care Med 197 (9) (2018) 1187–1197. 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sands SA, Alex RM, Mann D, Vena D, Terrill PI, Gell LK, Zinchuk A, Sofer T, Patel SR, Taranto-Montemurro L, Azarbarzin A, Rueschman M, White DP, Wellman A, Redline S, Pathophysiology underlying demographic and obesity determinants of sleep apnea severity, Ann. Am. Thorac. Soc 20 (3) (2023) 440–449. 10.1513/AnnalsATS.202203-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M, Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis, JAMA Otolaryngol. Head Neck Surg 139 (12) (2013) 1343–1350. 10.1001/jamaoto.2013.5338. [DOI] [PubMed] [Google Scholar]
- [32].Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S, CPAP versus oxygen in obstructive sleep apnea, N. Engl. J. Med 370 (24) (2014) 2276–2285. 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV, Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis, Chest 153 (4) (2018) 843–850. 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin HS, Zuliani G, Amjad EH, Prasad AS, Badr MS, Pan CJ, Rowley JA, Treatment compliance in patients lost to follow-up after polysomnography, Otolaryngol. Head Neck Surg. 136 (2) (2007) 236–240. 10.1016/j.otohns.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [35].McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, SAVE Investigators and Coordinators, CPAP for prevention of cardiovascular events in obstructive sleep apnea, N. Engl. J. Med 375 (10) (2016) 919–931. 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- [36].Patel S, White DP, Malhotra A, Stanchina M, Ayas NT, The effect of CPAP therapy on subjective and objective sleepiness in obstructive sleep apnea: a meta-analysis of randomized controlled trials, Arch. Intern. Med (2002) in press. [Google Scholar]
- [37].Jen R, Grandner MA, Malhotra A, Future of sleep-disordered breathing therapy using a mechanistic approach, Can. J. Cardiol 31 (7) (2015) 880–888. 10.1016/j.cjca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF, An instrument to measure functional status outcomes for disorders of excessive sleepiness, Sleep 20 (10) (1997) 835–843. [PubMed] [Google Scholar]
- [39].Sunwoo BY, Kaufmann CN, Murez A, Lee E, Gilbertson D, Bosompra NO, DeYoung P, Malhotra A, The language of sleepiness in obstructive sleep apnea beyond the Epworth, Sleep Breath. 27 (3) (2022) 1057–1065. 10.1007/s11325-022-02703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen B, Drager LF, Peker Y, Vgontzas AN, Phillips CL, Hoyos CM, Salles GF, Guo M, Li Y, Effect of continuous positive airway pressure on weight and local adiposity in adults with obstructive sleep apnea: a meta-analysis, Ann. Am. Thorac. Soc 18 (10) (2021) 1717–1727. 10.1513/AnnalsATS.202101-060OC. [DOI] [PubMed] [Google Scholar]
- [41].Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G, Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome, J. Am. Coll. Cardiol 62 (7) (2013) 569–576. 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJ, Fraga RF, Jun JC, Negrao CE, Krieger EM, Polotsky VY, Lorenzi-Filho G, The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome, PLoS One 5 (8) (2010) e12065. 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA, Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials, Thorax 70 (3) (2015) 258–264. 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- [44].Johns MW, A new method for measuring daytime sleepiness: the Epworth sleepiness scale, Sleep 14 (6) (1991) 540–545. 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- [45].Patel S, Kon SSC, Nolan CM, Barker RE, Simonds AK, Morrell MJ, Man WD, The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea, Am. J. Respir. Crit. Care Med 197 (7) (2018) 961–963. 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after a receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


