Abstract
Introduction
Alterations in microbiota composition have been implicated in a variety of human diseases. Patients with adenomyosis present immune dysregulation leading to a persistent chronic inflammatory response. In this context, the hypothesis that alterations in the microbiota may be involved in the pathogenesis of adenomyosis, by affecting the epigenetic, immunologic, and biochemical functions of the host, has recently been postulated. The aim of the present study was to compare the microbiota composition in the vagina, endometrium, and gut of individuals with and without adenomyosis.
Material and Methods
Cross‐sectional study including 38 adenomyosis patients and 46 controls, performed between September 2021 and October 2022 in a university hospital‐based research center. The diagnosis of adenomyosis was based on sonographic criteria. Fecal, vaginal, and endometrial samples were collected. Study of the microbiota using 16S rRNA gene sequencing.
Results
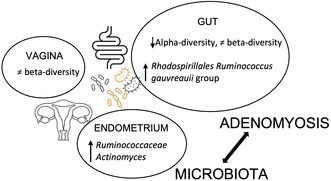
Patients with adenomyosis exhibited a significant reduction in the gut microbial alpha diversity compared with healthy controls (Chao1 p = 0.012, Fisher p = 0.005, Observed species p = 0.005). Beta‐diversity analysis showed significant differences in the compositions of both gut and vaginal microbiota between adenomyosis patients and the control group (Adonis p‐value = 0.001; R 2 = 0.03 and Adonis p‐value = 0.034; R 2 = 0.04 respectively). Specific bacterial taxa were found to be either overrepresented (Rhodospirillales, Ruminococcus gauvreauii group, Ruminococcaceae, and Actinomyces) or underrepresented in the gut and endometrial microbiota of adenomyosis patients compared with controls. Distinct microbiota profiles were identified among patients with internal and external adenomyosis phenotypes.
Conclusions
The study revealed reduced gut microbiota diversity in adenomyosis patients, accompanied by distinct compositions in gut and vaginal microbiota compared with controls. Overrepresented or underrepresented bacterial taxa were noted in the gut and endometrial microbiota of adenomyosis patients, with variations in microbiota profiles among those with internal and external adenomyosis phenotypes. These findings suggest a potential association between microbiota and adenomyosis, indicating the need for further research to comprehensively understand the implications of these differences.
Keywords: 16S rRNA sequencing, adenomyosis, endometrial microbiota, gut microbiota, vaginal microbiota
The study revealed reduced gut microbiota diversity in adenomyosis patients, accompanied by distinct compositions in gut and vaginal microbiota compared with controls. Overrepresented or underrepresented bacterial taxa were noted in the gut and endometrial microbiota of adenomyosis patients, with variations in microbiota profiles among those with internal and external adenomyosis phenotypes. These findings suggest a potential association between microbiota and adenomyosis, indicating the need for further research to comprehensively understand the implications of these differences.
Abbreviations
- LEfSe
linear discriminant analysis effective size
- SCFA
short‐chain fatty acid
Key message.
Our findings imply a possible link between gut, endometrial, and vaginal microbiota compositions and adenomyosis. Moreover, we observed variations in microbiota profiles among patients with internal and external adenomyosis phenotypes, suggesting potential avenues for novel diagnostic and therapeutic approaches.
1. INTRODUCTION
Adenomyosis is a benign gynecological condition classically defined by the presence of ectopic endometrial tissue within the myometrium. 1 Recent investigations have led to consider adenomyosis as a heterogeneous clinical condition with at least two different phenotypes based on whether the adenomyotic lesions are in the inner myometrium (internal adenomyosis) or more circumscribed in the outer myometrium (external adenomyosis). 2 , 3 , 4 , 5
Although the exact etiopathology of the disease remains controversial, it is known that patients with adenomyosis present immune dysregulation leading to a persistent chronic inflammatory response, sex steroidal hormone aberrations such as local hyperestrogenism and progesterone resistance, as well as dysregulation in neurogenesis and angiogenesis. 6 , 7 , 8 Moreover, adenomyosis often coexists with endometriosis. 3 , 9 , 10 , 11
The commensal microbial communities present along different sites of the human body contribute to the regulation of numerous immune and metabolic functions through a host–microbiota symbiotic interaction, such as the maintenance of mucosal structural integrity, protection against pathogens, synthesis and absorption of essential nutrients, development of the innate and adaptive immune system, modulation of host brain function, and regulation of estrogen metabolism by estrogen‐metabolizing enzyme production. Alterations in microbiota composition have been implicated in a variety of human diseases including intestinal alterations, autoimmune and metabolic conditions, cancer, neurological disorders, and gynecological pathologies. 12
In this context, the hypothesis that alterations in the microbiota may be involved in the pathogenesis of adenomyosis, by affecting the epigenetic, immunologic, and biochemical functions of the host, has recently been postulated. 13 , 14 , 15 , 16 , 17 Nonetheless, to date, there are still relatively few studies that have focused on characterizing the microbiota of the genital tract in women with adenomyosis, 13 , 14 , 15 , 16 and to the best of our knowledge, no study has investigated the intestinal microbiota of these patients.
The present study aimed to compare gut, vaginal, and endometrial microbiota composition, and its diversity between women with and without adenomyosis, and assess whether there are significant differences in microbiota profiles between women with external and internal adenomyosis.
2. MATERIAL AND METHODS
This observational cross‐sectional study was performed in a tertiary referral center between September 2021 and October 2022. Patient selection and sampling procedures were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the study participants.
All consecutive patients between the ages of 18 and 45 years old with a sonographic diagnosis of adenomyosis, with or without concomitant endometriosis, were included in the study. The control group included asymptomatic women visited for routine screening or for reproductive counseling with confirmation of normal uterus and adnexa based on sonographic examination. The exclusion criteria were as follows: (1) postmenopausal status, (2) previous diagnosis of autoimmune, inflammatory and/or neoplastic diseases, (3) use of gonadotropin‐releasing hormone agonist, vaginal contraceptive ring or intrauterine device during the 3 months before sample collection, (4) use of antibiotic or pre‐ or probiotics in the 3 months prior to sample collection, (5) presence of myomas or polyps in the ultrasound examination, and (6) current menstruation.
Adenomyosis was diagnosed according to the following criteria established by the Morphological Uterus Sonographic Assessment (MUSA) group 18 , 19 : presence of myometrial cysts, myometrial hyperechoic islands, echogenic subendometrial lines, and buds (direct criteria), and asymmetrical thickening of uterine walls, fan‐shaped shadowing, translesional vascularity, irregular or interrupted junctional zone, and globular uterus (indirect criteria). Adenomyosis was diagnosed in the presence of two or more criteria, with at least one being direct. Internal adenomyosis was defined when the junctional zone (inner myometrium) was affected with or without involvement of the middle myometrium and without affecting the outer myometrium (subserosal layer). External adenomyosis, on the other hand, was considered when it involved the outer myometrium, with or without involvement of the middle myometrium, without altering the junctional zone. The presence of ovarian and deep endometriosis was also assessed according to the International Deep Endometriosis Analysis (IDEA) group consensus. 20 The following socio‐demographic and clinical data were collected from each patient during the medical visit performed following the ultrasound examination: age, ethnicity, body mass index, infertility, nulliparity, and hormonal treatment. Dysmenorrhea and non‐cyclic chronic pelvic pain were also assessed using a numerical rating scale, where 0 represented no pain and 10 indicated unbearable pain. Additionally, the presence of heavy menstrual bleeding was evaluated based on subjective patient assessments. 21
2.1. Sample collection and processing
Vaginal samples were collected using swabs (Cytobrush Plus GT; Medscan Medical AB) from the posterior vaginal fornix following the insertion of a sterile speculum and before performing any vaginal procedure including pelvic examination or transvaginal ultrasound. Each cytobrush was submerged in 1.0 mL of sodium chloride (NaCl) (9 mg/mL). Subsequently, the vagina and external cervix were swabbed with chlorhexidine. Endometrial samples were then collected using a double‐sheathed, sterile pipelle endometrial suction curette that was passed through the cervix to collect an endometrial biopsy, taking care to avoid contact with the vaginal wall and cervix. Biopsies were submerged in 1.0 mL of NaCl (9 mg/mL). Samples were transported to the laboratory on ice and preprocessed within 1 hour. Vaginal fluid and endometrial samples were centrifuged at 3000g at 4°C for 10 min and pellets were stored at −80°C until being processed.
Additionally, 0.5–1.0 g of fecal samples were collected at home on the same day of the visit by the participants using the DANASTOOL Sample Collection MICROBIOME Kit (Danagen‐Bioted, BCN, Spain) and provided to the clinic. After homogenization with phosphate‐buffered saline (PBS) buffer, the fecal samples were stored at −80°C.
2.2. DNA extraction, 16S ribosomal ribonucleic acid (16S rRNA) amplification, and sequencing
DNA from fecal, endometrial, and vaginal samples was extracted using the MagMAX Microbiome Ultra Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA) on the Thermo Scientific™ KingFisher™ Flex Purification System and following the Soil Flex protocol according to the manufacturer's instructions. The extracted DNA was quantified using the QuantiFluor ONE dsDNA Dye (Promega, Madison, WI, USA) on a Quantus Fluorometer (Promega, Madison, WI, USA).
The 16S rRNA V3‐V4 region was amplified using previously described primers with Illumina adapter sequences. 22 Libraries were prepared following the 16S Metagenomic Sequencing Library Preparation Illumina protocol (Part # 15044223 Rev. A, Illumina, CA, USA). Libraries were diluted to 1.2 nM concentration, pooled in equimolar proportions, and loaded at 5 pM concentrations with 20% 5 pM PhiX on a MiSeq platform (2 × 300 bp).
2.3. Sequence processing and statistical analyses
Sequences were processed using QIIME2‐2022.2 23 and DADA2 pipeline for quality control, applying the following parameters: −‐p‐trim‐left‐f 30, −‐p‐trim‐left‐r 0, −‐p‐trunc‐len‐f 280, and −‐p‐trunc‐len‐r 220. All samples were rarefied at 2000 sequencing depth based on α‐diversity rarefaction plots and taxonomy was assigned using the Silva v138.1 database trained on the V3‐V4 16S rRNA region with a Naive Bayes classifier. Alpha and beta diversity analyses were performed using phyloseq v.1.38.0, picante v.1.8.2, and vegan v.2.5–7 packages using R v.4.1.2 and Rstudio v.2022.2.3 environments.
For alpha diversity, Chao1, Faith's PD, Fisher, Observed species, Shannon, and Simpson indexes were calculated and compared using the nonparametric Wilcoxon test. Beta diversity was assessed with Non‐metric Multidimensional Scaling using Bray–Curtis dissimilarity index matrices. The statistical significance of sample groupings was calculated using the resulting distance matrices and the Adonis nonparametric analysis of variance. p < 0.05 was considered statistically significant in both alpha and beta diversity analyses.
Differentiation of microbial abundance at a genus level was assessed by the utilization of linear discriminant analysis effective size (LEfSe) 24 establishing a threshold of 2.0 linear discriminant analysis score and 0.05 α‐significance level to report significant taxa.
Other statistical analyses were performed with Stata software version 15.1. Continuous variables were analyzed by t‐tests while categorical variables were analyzed by Fisher's exact test; p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Samples and participant characteristics
A total of 84 women were included in the study, 38 (45.2%) in the adenomyosis group and 46 (54.8%) in the control group (Figure 1). The baseline socio‐demographic and clinical characteristics of the women included are shown in Table 1. No significant differences were found among groups regarding age, ethnicity, hormonal treatment, and body mass index, whereas women with adenomyosis were more frequently nulliparous. With respect to clinical symptoms, patients with adenomyosis presented significantly more dysmenorrhea, non‐cyclic chronic pelvic pain, heavy menstrual bleeding, and infertility than healthy controls. Thirty‐four (89.5%) of the patients with adenomyosis had associated endometriosis. Table 2 provides details of the sonographic findings on the patients with adenomyosis.
FIGURE 1.
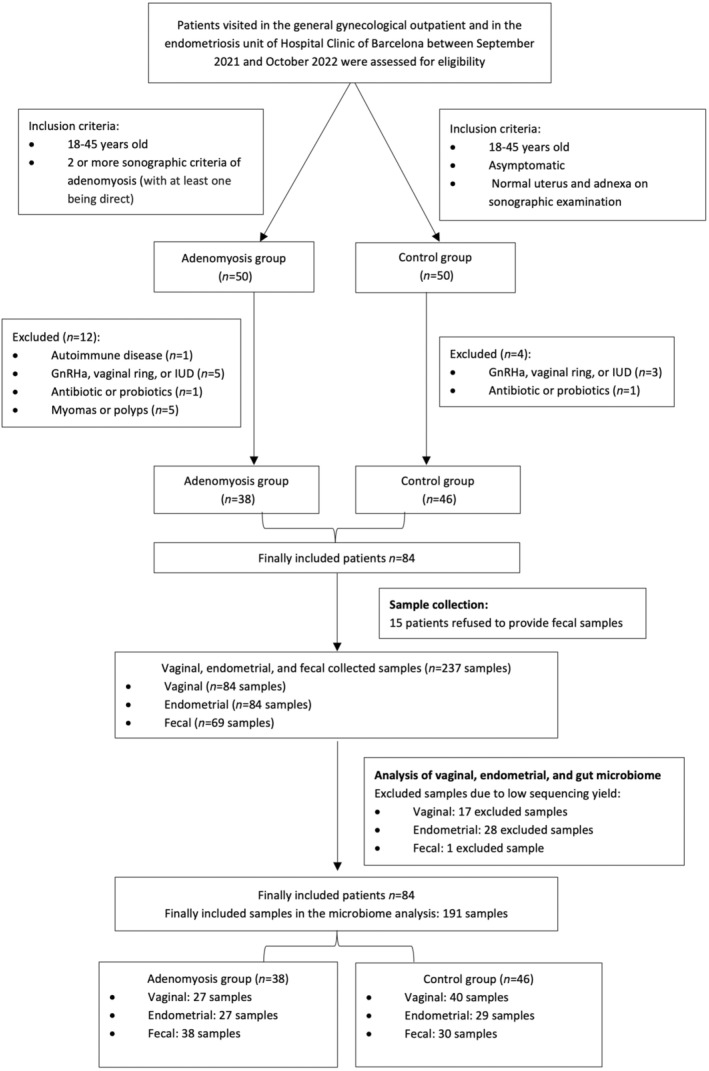
Flow chart of patient inclusion and drop‐out.
TABLE 1.
Baseline clinical characteristics of the women included.
| Total n = 84 | Adenomyosis n = 38 | Controls n = 46 | p‐value | |
|---|---|---|---|---|
| Age in years, mean (SD) | 37.6 (4.9) | 38.0 (4.9) | 37.1 (4.9) | 0.38 |
| BMI in kg/m2, mean (SD) | 24.4 (4.0) | 24.4 (4.2) | 24.4 (3.9) | 0.98 |
| Caucasian, n (%) | 72 (85.7) | 34 (89.5) | 42 (91.3) | 0.23 |
| Nulliparity, n (%) | 40 (47.6) | 27 (71.1) | 13 (28.2) | <0.001 |
| Hormonal treatment, n (%) | 68 (81.0) | 31 (81.6) | 37 (80.4) | 0.56 |
| Pain scores, NRS (0–10), mean (SD) | ||||
| Dysmenorrhea | 4.5 (3.6) | 5.7 (3.9) | 3.6 (3.1) | 0.007 |
| NCCPP | 2.1 (2.9) | 4.2 (3.1) | 0.4 (1.2) | <0.001 |
| HMB, n (%) | 20 (23.8) | 20 (52.6) | 0 (0.0) | <0.001 |
| Infertility | 12 (14.3) | 12 (31.6) | 0 (0.0) | <0.001 |
Abbreviations: BMI, body mass index; HMB, heavy menstrual bleeding; NCCPP, non‐cyclic chronic pelvic pain; NRS, numerical rating scale.
Note: Bold values indicate statistically significant results (p < 0.05).
TABLE 2.
Sonographic features in the adenomyosis group.
| Adenomyosis phenotype, n (%) | |
| Internal | 29 (76.3) |
| External | 9 (23.7) |
| Number of adenomyosis criteria, n (%) | |
| 2 | 1 (2.6) |
| 3 | 5 (13.2) |
| 4 | 18 (47.4) |
| 5 | 5 (13.2) |
| 6 | 6 (15.9) |
| 7 | 3 (7.9) |
| Adenomyosis criteria, n (%) | |
| Myometrial cysts | 17 (44.5) |
| Myometrial hyperechogenic islands | 37 (97.4) |
| Wall asymmetry | 19 (50.0) |
| Fan‐shaped shadowing | 31 (81.2) |
| Irregular or interrupted JZ | 29 (76.3) |
| Translesional vascularity | 18 (47.4) |
| Globular uterus | 22 (57.9) |
| Ovarian endometriosis, n (%) | 19 (50.0) |
| Deep endometriosis, n (%) | 34 (89.5) |
| Deep endometriosis location, n (%) | |
| Torus and/or uterosacral ligaments | 34 (89.5) |
| Rectosigmoid | 19 (50.0) |
| Bladder | 2 (5.3) |
Vaginal and endometrial samples were collected from each participant but only 69 (82.1%) women agreed to deliver fecal samples. Among the 237 samples collected and processed, 46 (19.4%) were excluded due to low sequencing yield (Figure 1; Supporting information Tables S1 and S2).
A total of 10 222 309 raw sequences, with 14 889 median sequences per sample (interquartile range 4321–25 511) were obtained. According to alpha rarefaction curve estimation, a sequencing depth of 2000 sequences per sample may represent the microbial diversity of each community. Considering all the collected samples, we were able to identify 312 different bacterial genera and 104 different bacterial families.
3.2. Diversity analysis
Alpha and beta diversity analyses were conducted to assess the differences in the composition of vaginal, endometrial, and gut microbiota in adenomyosis patients and healthy controls.
Alpha diversity comparison between groups showed that the Chao1 (p = 0.012), Fisher (p = 0.005), and the observed species (p = 0.005) indexes in the fecal samples of the control group were significantly higher than in the adenomyosis group (Figure 2). In contrast, no significant differences in alpha diversity were found between groups regarding vaginal and endometrial microbiota.
FIGURE 2.
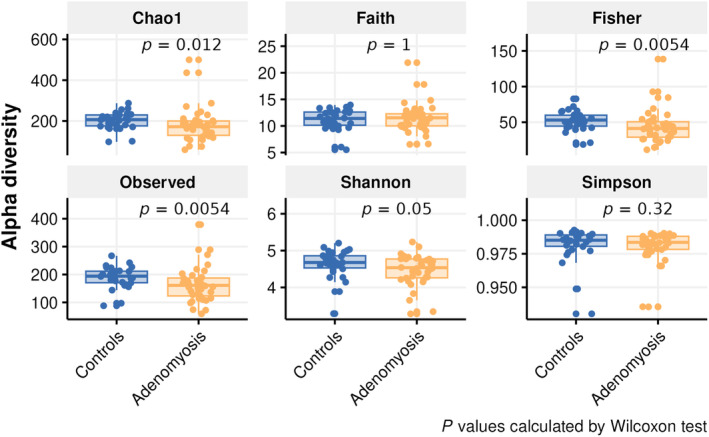
Boxplots representing alpha diversity analysis results for fecal samples including Chao1, Faith's PD, Fisher, observed species, Shannon, and Simpson indexes. The Chao1 (p = 0.012), Fisher (p = 0.005), and the observed species (p = 0.005) in the fecal samples of the control group were significantly higher than in the adenomyosis group (p < 0.05).
Regarding beta diversity, gut and vaginal microbiota of patients with adenomyosis and controls significantly differed (Adonis p‐value = 0.001; R 2 = 0.03 and Adonis p‐value = 0.034; R 2 = 0.04 respectively; Supporting information Figure S1), whereas there were no significant differences between groups regarding the composition of the endometrial microbiota (Adonis p‐value = 0.079; R 2 = 0.03).
3.3. Relative taxonomy abundance between adenomyosis and control groups
The distribution of the top 20 taxa of vaginal, endometrial, and gut microbiota at the family level is depicted in Figure 3. Bacteroidaceae was the most abundant family found in fecal samples in both groups, followed by Lachnospiraceae, Ruminococcaceae, Prevotellaceae, and Oscillospiraceae. In vaginal and endometrial samples, Lactobacillaceae was the most abundant family found in both groups, followed by Bifidobacteriaceae. The third most abundant family found in endometrial and vaginal samples of adenomyosis patients was Streptococcaceae, being Atopobiaceae in the control group.
FIGURE 3.
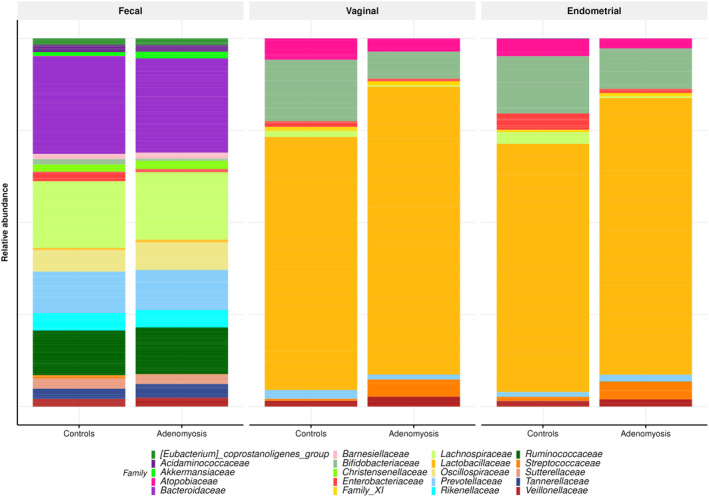
Distribution of the top 20 taxa of vaginal, endometrial, and gut microbiota at the family level.
3.4. Differential analysis of taxonomy profiles
LEfSe identified differentially abundant bacterial taxa in the gut and endometrial microbiota of patients with adenomyosis and controls (Figure 4). A significant increase in the abundance of the order Rhodospirillales and genus Ruminococcus gauvreauii group was identified in fecal samples in the adenomyosis group. On the other hand, we found that families Clostridiaceae, Rikenellaceae_RC9_gut_group, Peptostreptococcaceae and Atopobiaceae and genus Bifidobacterium, Streptococcus, Sutterella, Holdemanella, Coprococcus, Dorea, Butyricicoccus, Veillonella, Clostridium_sensu_stricto_1, UBA 1819, Acidaminococcus, Olsenella, Allisonella, and Howardella were significantly more abundant in the control group. In endometrial samples, the family Ruminococcaceae and genus Actinomyces were significantly enriched in the adenomyosis group. Moreover, the family Enterobacteriaceae and genus Enterobacter, Ligilactobacillus, Lacticaseibacillus, and Fastidiosipila were significantly more abundant in the control group. In vaginal samples, no differentially abundant bacterial taxa were found among groups.
FIGURE 4.
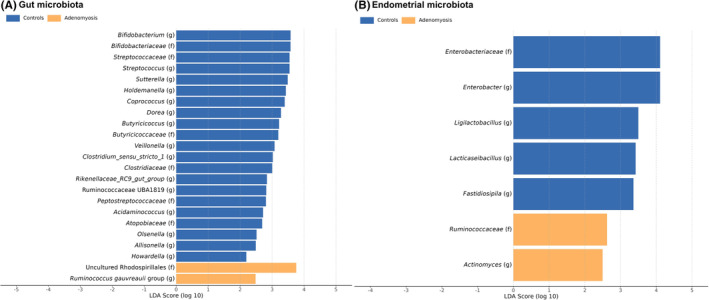
Differentially abundant bacterial taxa in the gut (A) and endometrial (B) microbiota of patients with adenomyosis and controls based on linear discriminant analysis (LDA) effect size technique (LEfSe) analysis.
3.5. Adenomyosis phenotypes and microbiota
No statistical significance in the alpha and beta‐diversity analysis was found between internal and external adenomyosis within the three studied sites (feces, vagina, and endometrium). Nonetheless, the beta‐diversity analysis showed that, albeit not statistically significant, there were more differences between the composition of vaginal and endometrial microbiota in patients with internal adenomyosis than in patients with external adenomyosis (Figure 5). Moreover, the LEfSe analysis identified differentially abundant bacterial taxa in the three studied sites of patients with internal and external adenomyosis as shown in Figure 6.
FIGURE 5.
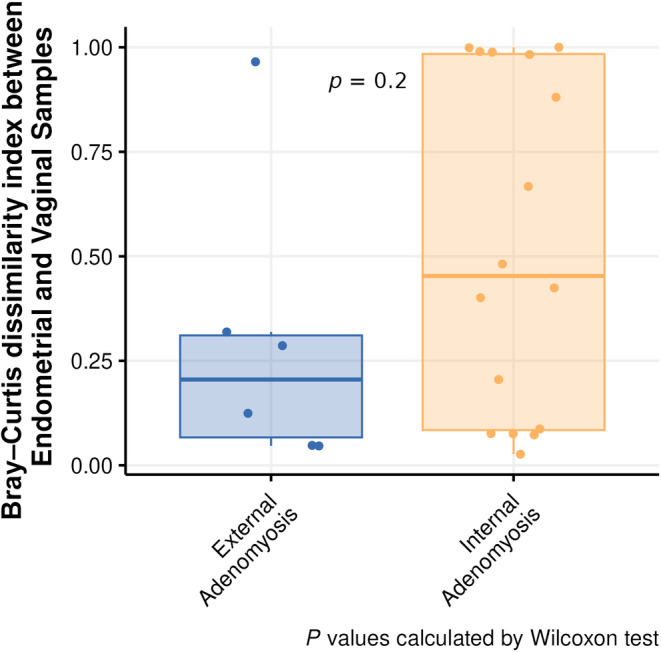
Boxplots representing differences in Bray–Curtis dissimilarity index between endometrial and vaginal samples of patients with internal and external adenomyosis.
FIGURE 6.
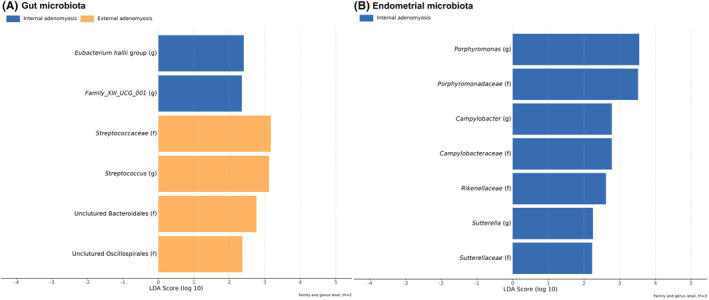
Differentially abundant bacterial taxa in the gut (A) and endometrial (B) microbiota of patients with internal and external adenomyosis based on linear discriminant analysis (LDA) effect size technique (LEfSe) analysis.
3.6. Concomitant endometriosis in patients with adenomyosis and microbiota
In the adenomyosis group, no statistical significance was found in the alpha and beta‐diversity analysis between with and without associated ovarian endometriosis, nor between patients with and without associated deep infiltrating endometriosis across the three studied sites (feces, vagina, and endometrium). However, the LEfSe analysis identified differentially abundant bacterial taxa in the fecal samples of patients with adenomyosis and associated rectosigmoid deep infiltrating endometriosis, as shown in Supporting information Figure S2.
4. DISCUSSION
In this study, we compared the vaginal, endometrial, and gut microbiota composition of patients with adenomyosis, with or without concomitant endometriosis, and healthy controls. The main findings of the present study are: the diversity of gut microbiota was significantly lower in the adenomyosis group (alpha‐diversity analysis); both gut and vaginal microbiota compositions significantly differed between adenomyosis patients and controls (beta‐diversity analysis); certain bacterial taxa were found to be overrepresented or underrepresented in the gut and endometrial microbiota of adenomyosis patients compared with controls, and finally, some differences in microbiota profiles were identified between patients with internal and external adenomyosis phenotypes.
It is known that the gut microbiota has the potential to impact the overall human physiology by influencing intestinal permeability, digestive and endocrine metabolism, and immune responses. 25 Eubiotic gut microbiota is characterized by being diverse and balanced and is primarily composed of bacteria dominated by the two phyla Firmicutes and Bacteroidetes which, when combined, represent 90% of gut microbials in healthy individuals. 26 In recent years, it has been acknowledged that gut dysbiosis, defined by an alteration in gut microbiota composition and reduction in its diversity, is associated with intestinal and extra‐intestinal conditions, such as irritable bowel syndrome, inflammatory bowel disease, celiac disease, colorectal cancer, metabolic disorders (obesity and type 2 diabetes), Alzheimer's and Parkinson's diseases and autism spectrum disorders. 27 Moreover, research has demonstrated that the gut microbiota can also have an impact on estrogen levels and estrogen‐related diseases. 17 , 28 To date, some previous studies have investigated the connection between gut microbiota and endometriosis in humans, 29 , 30 , 31 , 32 but only one study centered on adenomyosis, based on an animal model, has been published. 17 Therefore, to our knowledge this is the first study that analyzes changes in the intestinal microbiota in patients with adenomyosis, with or without associated endometriosis, compared with healthy controls.
In our study, the diversity of gut microbiota was significantly lower in adenomyosis patients. Moreover, beta‐diversity analysis showed that gut microbiota composition differs between adenomyosis patients and controls. The only previous study focused on gut microbiota and adenomyosis was performed in mice and did not find differences regarding alpha and beta diversity between groups. Nonetheless, loss of microbiota diversity is of note the most constant finding of intestinal dysbiosis and has previously been linked to different chronic conditions 33 including endometriosis. 34
On the other hand, most of the taxonomic groups identified as significantly differing in abundance in fecal samples between the adenomyosis and control groups by LEfSe analysis are known for either their favorable or detrimental effects on intestinal equilibrium. Adenomyosis patients presented a reduction in the genus Bifidobacterium in gut microbiota compared with the control group. Bifidobacterium is known to have beneficial effects such as short‐chain fatty acids (SCFA) production, biological barrier formation, and anti‐microbial compound secretion. 35 Interestingly, most of the other depleted bacterial taxa in the adenomyosis group (genus Copropcoccus, Dorea, Veillonella, Butyricicoccus, and Allisonella) are important butyrate producers 36 , 37 , 38 and some have also been found to be reduced in inflammatory bowel disease, 39 Crohn's disease, 40 and endometriosis. 30 Butyrate is a SCFA found in the gut metabolome that serves as a crucial energy source for enterocytes and stimulates the growth of the intestinal epithelium, repairing and fortifying the gut barrier. 41 A decrease in the abundance of taxa responsible for butyrate production may lead to compromised epithelial cell integrity, increased colonic permeability, bacterial infiltration, and local inflammation. 42 On the other hand, patients with adenomyosis exhibited an enrichment of the Ruminococcus gauverii group and the Rhodospirillales order compared with the control group. R. gauverii is a mucolytic bacterium from the Ruminococcaceae family which can induce chronic intestinal inflammation and disruption of the gut barrier. 43 , 44 , 45 , 46 Moreover, previous studies showed that genus from the Ruminococcaceae family are beta‐galactosidase and beta‐glucuronidase producers. 47 , 48 The estrobolome has been considered a significant contributor to adenomyosis and endometriosis by dysregulating circulating estrogen levels in women, through gut microbial enzymes involved in their metabolism. Particularly, beta‐glucuronidase, beta‐galactosidase, and beta‐glucosidase, are bacterial hydrolytic enzymes that play a key role in the deconjugation of estrogens from glucuronic acid, leading to their reabsorption in active form into the circulatory system. 28 , 49 Taxa from the order Rhodospirillales are Gram‐negative bacteria and, therefore, are characterized by having lipopolysaccharide (LPS) as the main component of their outer membrane. LPS functions as a powerful endotoxin, and its activation of pattern recognition receptors, such as toll‐like receptor 4, plays a crucial role in provoking pro‐inflammatory and immune responses, as well as promoting neo‐angiogenesis and the secretion of growth factors. All these mechanisms are known to be involved in the pathogenesis of adenomyosis. 8 , 25 , 50 , 51 , 52
Furthermore, it has been demonstrated that the complex interplay among bacteria and the host epithelial and immune cells within the female reproductive tract is crucial for preserving the homeostasis of the reproductive system, and that a shift in their equilibrium can potentially contribute to a wide range of conditions. 28 , 53 , 54 , 55 Even if healthy women may exhibit diverse vaginal microbial compositions, 56 eubiotic vaginal microbiota is normally associated with low bacterial diversity and the dominance of Lactobacillus spp. 57 Despite having been less studied to date, the uterine microbiota in healthy women is characterized by an abundance of genus Lactobacillus, genus Gardnerella and phyla Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria. 54 , 58 , 59 Compared with the vaginal microbiota, the endometrial microbiota presents a significantly lower density—with approximately 10 000 times fewer bacteria—but it displays a higher degree of bacterial diversity. 54 , 60 Some studies have previously examined the microbiota in the female reproductive tract of women with adenomyosis 13 , 14 , 15 , 16 revealing differences compared with control groups. These findings suggest a potential link between female reproductive tract dysbiosis and the pathogenesis of adenomyosis.
In terms of vaginal microbiota, we found no significant differences in alpha diversity, but beta diversity analysis revealed that vaginal microbiota composition significantly differed between women with adenomyosis and healthy controls. These findings align with data previously published by Chen et al. in 2020, 16 and Chao et al. in 2021. 15 In their recently published study, Kunaseth et al. 14 found that the richness of vaginal microbiota was significantly higher in patients with adenomyosis compared with healthy controls despite the absence of differences between groups in the beta diversity analysis.
Regarding endometrial microbiota, we found no significant differences in alpha‐diversity and beta‐diversity analysis among groups. Our findings are not in line with a prior study conducted by Lin et al., 13 which reported a significant reduction in endometrial microbial richness of patients with adenomyosis compared with healthy controls, along with significant variations in endometrial microbiota composition (beta diversity) between the adenomyosis and control groups. However, our LEfSe analysis showed significantly differential abundances of endometrial bacteria between groups: We found that the Ruminococcaceae family and the genus Actinomyces were significantly enriched in the adenomyosis group, whereas the genus Ligilactobacillus, Lacticaseibacillus, and Fastidiosipila were significantly more abundant in the control group. In their recent work, Lin et al. also detected an enrichment of Actinobacteria (class) in endometrial samples from patients with adenomyosis. 13 Taxa from the Ruminococcaceae family are SCFA‐producers. As mentioned before, SCFAs, such as acetate and butyrate, are known to play an anti‐inflammatory and protective role in the gut. Interestingly, in the female reproductive tract, SCFAs have been observed to promote dysbiosis and inflammation, especially when there is an associated deficiency in lactate‐producer lactobacilli. The increase in SCFAs in the female reproductive tract has been associated with enhanced pro‐inflammatory cytokine production through Toll‐like receptor activation and reduced neutrophil chemotaxis. Moreover, the reduction in the abundance of the genus Ligilactobacillus and Lacticaseibacillus among individuals with adenomyosis aligns with prior research findings that have reported lower levels of these lactate‐producer bacteria in cases of other pathologic gynecological conditions. 61 , 62 , 63
We identified differentially abundant bacterial taxa in the gut and endometrial microbiota of patients with internal and external adenomyosis. Moreover, although not statistically significant, it seems that there are more differences between the composition of vaginal and endometrial microbiota in patients with internal adenomyosis than in patients with external adenomyosis. These findings could be associated with the previously suggested theory indicating distinct pathways in the development of internal and external adenomyosis, with internal adenomyosis possibly originating from the endometrium within the uterus, and external adenomyosis arising from ectopic endometrial cells invading from a nearby endometriotic lesion. 3 , 4 , 64
Most of the patients with adenomyosis included in our study had associated endometriosis, which is not unexpected and is consistent with previously published literature. 3 , 9 , 10 , 11 Although it was not a primary outcome of our study, we evaluated whether there could be differences in the microbiota profiles of patients with adenomyosis due to the presence of concomitant endometriosis. We found no statistical significance in diversity analysis between patients with and without associated ovarian endometriosis, nor between patients with and without associated deep infiltrating endometriosis across the three studied sites. However, the LEfSe analysis identified differentially abundant bacterial taxa in the fecal samples of patients with adenomyosis and associated rectosigmoid deep infiltrating endometriosis. Further studies are warranted to comprehensively investigate the potential implications of these findings.
Our study has several strengths that should be considered: it is the first study describing the composition of the gut, vaginal, and endometrial microbiota of patients with adenomyosis, with or without associated endometriosis; moreover, it is the first study comparing microbiota profiles between internal and external adenomyosis phenotypes. Additionally, this study was performed in a tertiary referral center where the transvaginal ultrasound evaluations were performed by two expert sonographers with more than 10 years of experience and who have previously demonstrated a high diagnostic accuracy with transvaginal ultrasound for determining the presence of adenomyosis. 65 , 66 Finally, all vaginal and endometrial samples were collected by the same investigator following a strict protocol to avoid contamination and were later processed and analyzed using a blinded approach to ensure the accuracy of the statistical analyses. On the other hand, we must acknowledge certain limitations. Our study population was selected from the Endometriosis Unit of a tertiary center and, therefore, patient selection bias may be present as the high rate of patients with associated endometriosis has decreased the external validity of our study making our results not applicable to the general population. Moreover, most of the patients included in our study were undergoing hormonal therapy, which is our first‐line treatment for adenomyosis and endometriosis. While this could be considered a potential source of bias, there were no significant differences in hormonal treatment intake among the groups (p = 0.56). Furthermore, a recent study has shown that the use of hormonal contraceptives is not associated with differences in microbiota composition or diversity. 67 Nevertheless, it would be valuable to investigate the potential impact of hormonal treatment on the microbiota of adenomyosis patients through prospective studies analyzing microbiota composition before and after the initiation of hormonal therapy for each individual patient. Such investigations could shed light on whether hormonal treatments influence the microbiota and whether any alterations in microbiota composition correlate with treatment outcomes or symptom management. Understanding these potential relationships could potentially lead to more tailored and effective treatment approaches. Another limitation of our study is that we used 16S rRNA gene sequencing, which limited the taxonomic resolution. Using alternative methods, such as shotgun metagenomic sequencing, would enable the identification and profiling of a broader range of microorganisms, including fungi, viruses, and microbial genes, thereby providing additional insights into the functional potential of the microbiota, as well as a better resolution for the bacterial taxonomic classification.
5. CONCLUSION
Our study investigated the composition of the microbiota in the vagina, endometrium, and gut of patients with and without adenomyosis. The results revealed that adenomyosis patients displayed reduced diversity in the gut microbiota. Moreover, significant variations were observed in both gut and vaginal microbiota compositions between adenomyosis patients and the control group, and specific bacterial taxa were either overrepresented or underrepresented in the gut and endometrial microbiota of adenomyosis patients compared with controls. Notably, differences in microbiota profiles were identified between patients with internal and external adenomyosis phenotypes. These findings shed light on the potential role of microbiota in the context of adenomyosis and warrant further research to better understand the implications of these differences.
AUTHOR CONTRIBUTIONS
Marta Valdés‐Bango, Meritxell Gracia, Andrea Vergara, Climent Casals‐Pascual, and Francisco Carmona planned and designed the study. Marta Valdés‐Bango, Meritxell Gracia, Mariona Rius, Maria Ángeles Martínez‐Zamora, Cristina Ros, and Lara Quintas included participants, collected samples, and provided clinical data. Marta Valdés‐Bango and Elisa Rubio processed samples. Marta Valdés‐Bango, Meritxell Gracia, Elisa Rubio, and Eduard Mension analyzed data. All authors contributed to data interpretation. Marta Valdés‐Bango, Meritxell Gracia, Elisa Rubio, and Francisco Carmona contributed to writing the manuscript. All authors contributed to the manuscript revision. All the authors approved the final version of the manuscript.
FUNDING INFORMATION
The study was funded by the scholarship “Emily Letang”—Hospital Clinic of Barcelona granted in May 2021.
CONFLICT OF INTEREST STATEMENT
No conflict of interest to declare.
ETHICS STATEMENT
Patient selection and sampling procedures were carried out in accordance with the Declaration of Helsinki and applicable local regulatory requirements after approval from the Ethics Committee of the Hospital Clinic of Barcelona (HCB/2021/0242; approved on March 11, 2021). Written informed consent was obtained from all the study participants.
Supporting information
Figure S1:
Figure S2:
Table S1.
Table S2.
Valdés‐Bango M, Gracia M, Rubio E, et al. Comparative analysis of endometrial, vaginal, and gut microbiota in patients with and without adenomyosis. Acta Obstet Gynecol Scand. 2024;103:1271‐1282. doi: 10.1111/aogs.14847
Marta Valdés‐Bango and Meritxell Gracia are joint first authors.
REFERENCES
- 1. Wc H, Ll S, Wc R. Uterine adenomyosis; incidence, symptoms, and pathology in 1,856 hysterectomies. Am J Obstet Gynecol. 1947;53:663‐668. [PubMed] [Google Scholar]
- 2. Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, Taniguchi F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207:114.e1‐114.e7. [DOI] [PubMed] [Google Scholar]
- 3. Bourdon M, Oliveira J, Marcellin L, et al. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod. 2021;36:349‐357. [DOI] [PubMed] [Google Scholar]
- 4. Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32:1393‐1401. [DOI] [PubMed] [Google Scholar]
- 5. Marcellin L, Santulli P, Bortolato S, et al. Anterior focal adenomyosis and bladder deep infiltrating endometriosis: is there a link? J Minim Invasive Gynecol. 2018;25:896‐901. [DOI] [PubMed] [Google Scholar]
- 6. Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592‐601. [DOI] [PubMed] [Google Scholar]
- 7. Bourdon M, Santulli P, Jeljeli M, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. 2021;4(27):108‐129. [DOI] [PubMed] [Google Scholar]
- 8. Zhai J, Vannuccini S, Petraglia F, Giudice LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;8(38):129‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;15(26):392‐411. [DOI] [PubMed] [Google Scholar]
- 10. Eisenberg VH, Arbib N, Schiff E, Goldenberg M, Seidman DS, Soriano D. Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. Biomed Res Int. 2017;2017:8967803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kishi Y, Shimada K, Fujii T, et al. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS One. 2017;12:e0189522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin Q, Duan H, Wang S, et al. Endometrial microbiota in women with and without adenomyosis: a pilot study. Front Microbiol. 2023;14:1075900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kunaseth J, Waiyaput W, Chanchaem P, et al. Vaginal microbiome of women with adenomyosis: a case‐control study. PLoS One. 2022;17:e0263283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao X, Liu Y, Fan Q, Shi H, Wang S, Lang J. The role of the vaginal microbiome in distinguishing female chronic pelvic pain caused by endometriosis/adenomyosis. Ann Transl Med. 2021;9:771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen S, Gu Z, Zhang W, et al. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s‐rRNA sequencing technique: a pilot study. Ann Transl Med. 2020;8:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen P, Wang K, Zhuang M, et al. An insight into gut microbiota and metabolites in the mice with adenomyosis. Front Cell Infect Microbiol. 2023;13:1075387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the morphological uterus sonographic assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284‐298. [DOI] [PubMed] [Google Scholar]
- 19. Harmsen MJ, Van den Bosch T, de Leeuw RA, et al. Consensus on revised definitions of morphological uterus sonographic assessment (MUSA) features of adenomyosis: results of modified Delphi procedure. Ultrasound Obstet Gynecol. 2022;60:118‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the international deep endometriosis analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318‐332. [DOI] [PubMed] [Google Scholar]
- 21. RCOG . National Guideline Alliance, hosted by the Royal College of obstetrician and Gynaecologists. Evidence reviews for management of heavy menstrual bleeding. National Institute for Health and Clinical Excellence; 2018. [PubMed] [Google Scholar]
- 22. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next‐generation sequencing‐based diversity studies. Nucleic Acids Res. 2013;41(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laschke MW, Menger MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. 2016;215:68.e1‐68.e4. [DOI] [PubMed] [Google Scholar]
- 26. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salliss ME, Farland LV, Mahnert ND, Herbst‐Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021;28:92‐131. [DOI] [PubMed] [Google Scholar]
- 29. Ata B, Yildiz S, Turkgeldi E, et al. The Endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. 2019;9(1):2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang L, Liu B, Liu Z, et al. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front Cell Infect Microbiol. 2021;11:788836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Svensson A, Brunkwall L, Roth B, Orho‐Melander M, Ohlsson B. Associations between endometriosis and gut microbiota. Reprod Sci. 2021;28:2367‐2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shan J, Ni Z, Cheng W, et al. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet. 2021;304:1363‐1373. [DOI] [PubMed] [Google Scholar]
- 33. Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kobayashi H. Gut and reproductive tract microbiota: insights into the pathogenesis of endometriosis (review). Biomed Rep. 2023;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bottacini F, Ventura M, van Sinderen D, O'Connell MM. Diversity, ecology and intestinal function of bifidobacteria. Microb Cell Fact. 2014;13:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Notting F, Pirovano W, Sybesma W, Kort R. The butyrate‐producing and spore‐forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut Microbiome. 2023;4:e16. [Google Scholar]
- 37. Yang C, Deng Q, Xu J, et al. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high‐fat diet‐fed rats. Food Res Int. 2019;116:1202‐1211. [DOI] [PubMed] [Google Scholar]
- 38. Kolenbrander P. The Genus Veillonella. The Prokaryotes. Springer US; 2006:1022‐1040. [Google Scholar]
- 39. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gevers D, Kugathasan S, Denson LA, et al. The treatment‐naive microbiome in new‐onset Crohn's disease. Cell Host Microbe. 2014;15:382‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Fj T, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104‐119. [DOI] [PubMed] [Google Scholar]
- 42. Kinoshita M, Suzuki Y, Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARγ activation. Biochem Biophys Res Commun. 2002;293(2):827‐831. [DOI] [PubMed] [Google Scholar]
- 43. Domingo MC, Huletsky A, Boissinot M, Bernard KA, Picard FJ, Bergeron MG. Ruminococcus gauvreauii sp. nov., a glycopeptide‐resistant species isolated from a human faecal specimen. Int J Syst Evol Microbiol. 2008;58:1393‐1397. [DOI] [PubMed] [Google Scholar]
- 44. Png CW, Lindén SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420‐2428. [DOI] [PubMed] [Google Scholar]
- 45. Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8(4):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fang S, Chen X, Ye X, Zhou L, Xue S, Gan Q. Effects of gut microbiome and short‐chain fatty acids (SCFAs) on finishing weight of meat rabbits. Front Microbiol. 2020;11:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu S, Ding Q, Zhang W, Kang M, Ma J, Zhao L. Gut microbial beta‐glucuronidase: a vital regulator in female estrogen metabolism. Gut Microbes. 2023;15:2236749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu Z, Pfeiffer RM, Byrd DA, et al. Associations of circulating estrogens and estrogen metabolites with fecal and Oral microbiome in postmenopausal women in the Ghana breast health study. Microbiol Spectr. 2023;11:e0157223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10:324‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guo J, Chen L, Luo N, et al. LPS/TLR4‐mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci Rep. 2016;6:21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uzuner C, Mak J, El‐Assaad F, Condous G. The bidirectional relationship between endometriosis and microbiome. Front Endocrinol (Lausanne). 2023;14:1110824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan KN, Fujishita A, Hiraki K, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65(3):190‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine‐related diseases. Nat Commun. 2017;8:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS. The microbiome and endometriosis. The Mmicrobiome and Eendometriosis Reprod Fertil. 2022;3:R163‐R175. [Google Scholar]
- 56. Moosa Y, Kwon D, de Oliveira T, Wong EB. Determinants of vaginal microbiota composition. Front Cell Infect Microbiol. 2020;10:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020;19(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker JM, Chase DM, Herbst‐Kralovetz MM. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018. Mar;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moreno I, Codoñer FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684‐703. [DOI] [PubMed] [Google Scholar]
- 60. Mitchell CM, Haick A, Nkwopara E, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212:611.e1‐611.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Medina‐Bastidas D, Camacho‐Arroyo I, García‐Gómez E. Current findings in endometrial microbiome: impact on uterine diseases. Reproduction. 2022;163:R81‐R96. [DOI] [PubMed] [Google Scholar]
- 62. Gholiof M, Adamson‐De Luca E, Wessels JM. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front Reprod Health. 2022;4:963752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wei W, Zhang X, Tang H, Zeng L, Wu R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob. 2020;19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. García‐Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109:371‐379. [DOI] [PubMed] [Google Scholar]
- 65. Ros C, Martínez‐Serrano MJ, Rius M, et al. Bowel preparation improves the accuracy of transvaginal ultrasound in the diagnosis of rectosigmoid deep infiltrating endometriosis: a prospective study. J Minim Invasive Gynecol. 2017;24:1145‐1151. [DOI] [PubMed] [Google Scholar]
- 66. Gracia M, de Guirior C, Valdés‐Bango M, et al. Adenomyosis is an independent risk factor for complications in deep endometriosis laparoscopic surgery. Sci Rep. 2022;12:7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krog MC, Hugerth LW, Fransson E, et al. The healthy female microbiome across body sites: effect of hormonal contraceptives and the menstrual cycle. Hum Reprod. 2022;37:1525‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Figure S2:
Table S1.
Table S2.


