Abstract
The main objective of this project was to compare in the field conditions two strategies of re‐nutrition of children with moderate acute malnutrition (MAM) aged from 6 to 24 months, targeting the microbiota in comparison with a standard regimen. A three‐arm, open‐label, pragmatic randomised trial was conducted in four countries (Niger, CAR, Senegal and Madagascar). Children received for 12 weeks either fortified blended flour (FBF control) = arm 1, or FBF + azithromycin (oral suspension of 20 mg/kg/day daily given with a syringe) for the first 3 days at inclusion = arm 2 or mix FBF with inulin/fructo‐oligosaccharides (6 g/day if age ≥12 months and 4 g if age <12 months) = arm 3. For each arm, children aged from 6 to 11 months received 100 g x 2 per day of flours and those aged from 12 to 24 months received 100 g × 3 per day of FBF. The primary endpoint was nutritional recovery, defined by reaching a weight‐for‐height z‐score (WHZ) ≥ −1.5 within 12 weeks. Overall, 881 children were randomised (297, 290 and 294 in arm 1, arm 2 and arm 3, respectively). Three hundred and forty‐four children were males (39%) and median/mean age were 14.6/14.4 months (SD = 4.9, IQR = 10.5–18.4). At inclusion, the three arms were comparable for all criteria, but differences were observed between countries. Overall, 44% (390/881) of the children recovered at week 12 from MAM, with no significant difference between the three arms (41.4%, 45.5% and 45.9%, in arm 1, arm 2 and arm 3, respectively, p = 0.47). This study did not support the true advantages of adding a prebiotic or antibiotic to flour. When using a threshold of WHZ ≥ −2 as an exploratory endpoint, significant differences were observed between the three arms, with higher success rates in arms with antibiotics or prebiotics compared to the control arm (66.9%, 66.0% and 55.2%, respectively, p = 0.005).
Keywords: Central African Republic, Madagascar, malnutrition, microbiota, Niger, prebiotic, Senegal
This open‐label randomised trial, conducted in the field conditions with locally produced supplements in 881 children with moderate acute malnutrition in four African countries, did not support the true advantages of adding a prebiotic or a treatment with antibiotic to flour in that it did not achieve, as it failed to meet the primary endpoint retained, that is, WHZ ≥ −1.5.
†No successive visits at Week‐12; Lost to follow‐up at week‐12; Missing data at Week‐12; Other (Two maternal deaths, 1 mother's inability to continue the study and one case of flour refusal of the child)
Arm 1: daily Fortified Blended Flour; Arm 2; daily Fortified Blended Flour + azithromycin; Arm 3: daily Fortified Blended Flour containing inulin/Fructo‐oligosaccharides
Key messages
Re‐nutrition of children with MAM is still a concern as the duration of recovery is always long and recurrences are frequent.
Literature suggests that prebiotics and antibiotics show potentially useful properties to speed this process of recovery.
However, in this open‐label study, prebiotics and antibiotics did not lead to advantages on the recovery rates defined by reaching the WHZ (−1.5) cut‐off.
1. INTRODUCTION
In 2020, based on prevalence surveys, UNICEF estimated that globally more than 40 million children under 5 years (UNICEF‐WHO, 2020) old had wasting defined by a weight‐for‐height Z‐score (WHZ) less than −2 (WHO, 2014). The number of children having an episode of wasting per year (yearly incidence) is much higher than this estimate (UNICEF‐WHO, 2020; de Clercq et al., 2016]. Childhood malnutrition is a major challenge facing countries today, particularly in low‐ and middle‐income countries. Undernutrition puts children at increased risk of dying from common infections, increases the frequency and severity of these infections and delays their recovery. Worldwide, nearly half of all deaths in children under five are attributable to undernutrition (UNICEF‐WHO, 2020).
Undernutrition is defined as a deficiency of energy or of more than one essential nutrient. It can develop from difficulties in obtaining, eating or absorbing food or a significantly increased need for energy (de Clercq et al., 2016).
Malnutrition may be related to intestinal dysfunction or social or psychological considerations, such as maternal depression, which disturbs child alimentation (Haithar et al., 2018). Providing supplemented foods does not always ensure recovery, and relapse occurs, especially for children with severe acute malnutrition (SAM), defined by WHZ < −3 or mid‐upper arm circumference (MUAC) < 115 mm or presence of nutritional oedema (UNICEF‐WHO, 2020).
Managing SAM requires more technical, logistical and financial means than moderate malnutrition.
Due to these difficulties and the cost of treatment, governments should take on the responsibility of managing moderate malnutrition to prevent children from developing severe malnutrition and the complications that this condition entails.
Malnutrition has several physiological, metabolic, immune or cognitive origins (Blanton et al., 2016; Poskitt 1992; Rytter et al., 2014). Diarrhoea may have an impact on a young child's weight gain for a short time but has no significant impact on height. The association between malnutrition and diarrhoea, for instance, is debated and the emphasis now is on the role of enteric dysfunction (Crane et al., 2015). One hypothesis is that an imbalance of the intestinal microbial flora (unrelated to a pathogenic agent causing diarrhoea) may have an effect on a child's long‐term growth. This imbalance seems to differ among children with SAM and those with moderate acute malnutrition (MAM). Children with SAM have lower microbial diversity and biomass and a significant enrichment of Enterobacteriaceae (Moya‐Alvarez and Sansonetti 2022). To date, no study has been conducted on microbiota in MAM. This project will therefore provide new insights on this topic.
Chronic intestinal inflammation, linked to permanent microbiological stimulation due to a diet heavily contaminated by non‐pathogenic bacteria, may induce villous atrophy and then anorexia and malnutrition (Moya‐Alvarez and Sansonetti 2022). These non‐pathogenic bacteria but also parasites may be introduced through contaminated water from wells or other unprotected water sources. Changes in microorganism colonisation of the gut for children suffering from MAM can be assessed by 16S and 18S mRNA amplification and sequencing. Species identification can be conducted using a specialised database like SYLVA (Singh et al., 2021). Adequate management of malnutrition should therefore include addressing food, micronutrient supplementation and regulation of intestinal flora with, for example, broad‐spectrum antibiotic therapy (Trehan et al., 2013).
Several research projects are studying the role of prebiotics in the prevention and treatment of malnutrition. Prebiotics are believed to stimulate the growth of normal resident bacteria in the microbiota, such as fusobacters and bacterioides. There are indigestible carbohydrates that are not suitable for other bacteria or human cells. It has also been shown that prebiotics have a role in regulating microbial flora and an effect on appetite (de Clercq et al., 2016; Piemontese et al., 2011). Prebiotics also have a role in preventing infections (van Stuijvenberg et al., 2011), but the effect of prebiotics on malnutrition requires clarification. Some recent well‐controlled studies support a small effect of prebiotic‐enriched flour in the treatment of MAM (Chen et al., 2021; Slattery et al., 2016; WHO, 2016). However, prebiotics produced in industrialised countries represent an additional cost for these flours, and national programmes against malnutrition have proposed local production of blended flours instead of distributing flours produced in northern countries. To reduce the cost of these flours, the WHO recommends that countries find local, less expensive and sustainable ingredients to better involve the population in the management of childhood malnutrition (WHO, 2016).
Before implementing new recommendations for the use of prebiotics and searching local sources of compounds usable for flours, scientific data are needed to confirm the effectiveness of these approaches under field conditions. This was the purpose of the MALINEA clinical trial, set up in Niger, Centre Africa Republic (CAR), Senegal and Madagascar, four African countries (Vray et al., 2018), which is part of a more global project aiming to analyse the impact of prebiotics on gut microbiota. MALINEA evaluated the effectiveness of two alternative strategies (prebiotics and antibiotics) for the management of MAM compared to a reference standard of care consistent with WHO recommendations (25 kcal/kg/day in addition to the standard nutrient of local flour) (WHO, 2012) on the nutritional recovery of children aged 6 to 24 months.
Implementation of the protocol in different countries was proposed to account for the effects of different livelihoods, different foods, and potentially different microbiota of children on the effectiveness of the regimens.
2. PATIENTS, MATERIALS AND METHODS
2.1. Study design
This three‐arm, open‐label, pragmatic randomised trial was conducted in four countries (Niger, CAR, Senegal and Madagascar). The protocol of this open‐label study was previously described in Trials (Vray et al., 2018). Randomisation of children was centralized and stratified by country, health centre and age (6–11 months vs. 12–24 months) using 1:1:1 ratio in computer‐generated blocks of 6. Centralized randomisation was carried out as follows: when a child met all inclusion/noninclusion criteria, the study data collector was asked to call a centre by telephone to enquire about treatment to be given to the child. After checking the inclusion criteria (including consent) and collecting identifying variables as the first letter of the name, the surname and the birthdate, the treatment allocated was provided.
2.2. Primary and secondary endpoints
In this study, the primary endpoint was nutritional recovery, defined by reaching a WHZ ≥ −1.5 within 12 weeks, confirmed by two consecutive visits (planned every week), and without hospitalisation or hospital transfer for SAM, death or lost to follow‐up during these 12 weeks. WHZ was estimated using the table from WHO (de Onis, et al., 2009). Secondary endpoints were weight and height gains, mid‐upper arm circumference (MUAC), other clinical outcomes such as intercurrent illness, hospitalisation and hospital transfer for SAM and adherence to and tolerance of the strategy.
2.3. Inclusion/noninclusion criteria
The enrolment of children at primary health centres selected for the study in the peri‐urban sites of the 4 countries is described in detail (Vray et al., 2018 ). In brief, to be included in this trial, children were first screened by community health workers on a MUAC value between 115 and 130 mm. Then, to be definitively included, children from 6 to 24 months should (1) have a WHZ between −3 and −2, without nutritional oedema, which defines MAM according to WHO (WHO, 2016), (2) be followed for at least 12 weeks, (3) be able to return for 3 (possibly 4) consecutive days and (4) have written consent to participate in the study signed by parents or legal guardians (Vray et al., 2018).
Among children initially screened with MUAC, the main noninclusion criteria were: severe malnutrition defined by WHZ < −3, nutritional oedema, complications requiring inpatient clinical care, any conditions requiring nasogastric tube feed and/or parenteral nutrition, bloody diarrhoea, treatments potentially interfering with the protocol (antacids, cetirizine, digoxin, ergotamine, zidovudine) or known hypersensitivity to macrolides, albendazole, flour or to the prebiotics used in the study. Only one child per household could be included.
2.4. Follow‐up
The project implemented close monitoring to ensure the smooth running of the planned interventions. Children were followed up weekly for 12 weeks at the primary health centre of registration. During each visit, flour was given to the parent to last for an entire week. Weight, height and MUAC were assessed and a clinical examination was performed, during which data on safety and food consumption (including flour) were gathered by a data collector. If a child missed a visit or experienced no weight gain, community health workers organised a home visit to collect additional information and to bring the research products to the families to avoid ruptures and protocol deviations, particularly in flour consumption. In case of non‐recovery at week 12, children benefited from new care and new monitoring according to national recommendations. Home visits were also carried out in cases of underconsumption of flour to ensure proper flour preparation and to improve consumption.
2.5. Interventions
The children were randomly given one of the following strategies for 12 weeks:
-
–
Arm 1 (control): daily fortified blended flour.
-
–
Arm 2 (antibiotic): daily fortified blended flour + azithromycin for 3 days at inclusion.
-
–
Arm 3 (prebiotic): daily fortified blended flour containing inulin/fructo‐oligosaccharides.
The composition of the flours and of the prebiotics was previously described (Vray et al., 2018 ). The quantity of flour provided to the mothers was greater than the dose recommended by WHO (25 kcal/kg/day) to anticipate the sharing of flour among the siblings. Fortified blended flours were packaged in 100 g sachets. The regular daily dose of flour was 200 g for children aged 6–11 months and 300 g for children aged 12–24 months.
The flour used in this study was produced in Abidjan (Ivory Coast) in a single batch from a factory already working for the World Food Programme. For the four countries, 25 tons of flour were produced and sent to the sites.
Azithromycin was given for the first three consecutive days of the study (or 4 days in case of a missed day) at a dose of 20 mg/Kg/day. A single dose of albendazole (200 mg) was given to all children older than 12 months according to the national protocol of malnutrition management in the four country sites of this trial (Kambale and Francisca 2022). Treatment and laboratory testing were free of charge for all participants.
2.6. Data collection and statistical analysis
2.6.1. Data collection
At inclusion, a standardised questionnaire was implemented for each parent or legal guardian to collect general characteristics and socioeconomic and clinical data. At each visit, the following data were collected: associated pathologies (diarrhoea, vomiting, fever, malaria episode or acute respiratory infection), event of transfer for hospital care safety and adherence to the strategy. Each week, mothers were asked to bring back the flour packets not consumed by their children. They would then receive the additional quantity of flour needed for the following week. As the quantity of flour given was greater than needed to compensate for possible sampling, the quantity of flour consumption reported by mothers was only qualitatively assessed by staff involved in the trial as complete or incomplete.
Anthropometric measures were also registered weekly, that is, weight (with SECA UNICEF Scales), height (using Fathom Infant/Child ShorrBoard), and mid‐upper arm circumference (UNICEF MUAC tape). Adverse events were recorded at each visit. All secondary endpoints were measured each week by staff dedicated to the study at each site.
2.6.2. Sample size
A 60% likelihood of nutritional recovery after 3 months of treatment with the control regimen was expected according to previously published data (varying from 50% to 88%). A difference of recovery between the arms of 15% was expected. Using a power of 90%, a two‐sided alpha level of 0.017 (Bonferroni correction for multiple comparisons), and a 10% proportion of participants lost to follow‐up, the number of children required for the study was set at a total of 840 children, with 280 children per arm and 210 per country.
2.6.3. Statistical analysis
Medians, interquartile ranges (IQR) and proportions with 95% confidence interval were used to describe continuous and categorical variables, respectively. Comparisons between arms and countries were done using the chi‐squared test or the exact Fischer test. Comparisons on continuous variables were done using variance analysis or the Kruskall–Wallis test, depending on the equality of variance.
The primary endpoint was analysed using 2 strategies: (1) an intention to treat (ITT) population involving all children randomised and documented for the primary outcome at 12 weeks and those lost to follow‐up, deaths or transferred to hospital, thus considered failures, (2) a per protocol (PP) population including only children documented for the WHZ at 12 weeks, whatever the flour amount consumed. A global comparison was first performed between the three arms, and in case of a statistically significant difference, comparisons between arms 2 by 2 were performed.
To be considered cured, children had to have a WHZ ≥ −1.5 at visit week 12 and at visit week 11 or at visit week 13. Additional exploratory analysis (not planned in the protocol) was also performed using a WHZ threshold of −2, a less severe criteria used in practice and recommended by the WHO to define MAM children (WHO, 2014). Children with a WHZ ≥ −1.5 (or −2) only at one visit (week 12) were considered failures both in intention‐to‐treat and per protocol analyses. The comparison of the time of recovery between the three arms was represented by Kaplan–Meier curves and tested by a Wilcoxon test by arm on the total population. A secondary analysis was performed only for children who recovered at week 12.
Relation between recovery and children's eating habits (defined as breast‐ or mixed‐feeding vs. no breastfeeding) was performed using the chi‐squared test.
2.6.4. Ethics statement
The study protocol and informed consent forms were reviewed and approved by the National Ethic Committees of the four countries and by the Pasteur IRB and the French Data Protection Authority (CNIL) in France. The trial was registered prior to before the enrolment of the first participant under the ClinicalTrials.gov ClinicalTrials.gov Identifier: NCT03474276. An independent Data and Safety Monitoring Board (DSMB) was set up prior to before the beginning of the trial.
3. RESULTS
3.1. Recruitment
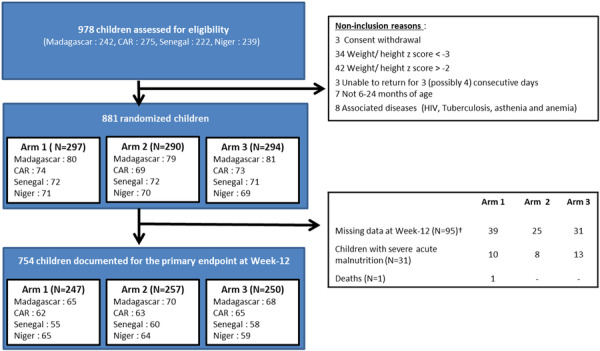
Between February 2018 and October 2018, 978 children were pre‐screened on MUAC (between 115 and 130 mm), that is, 242, 239, 275 and 222 in Madagascar, Niger, CAR and Senegal, respectively (Sampling profile for children in the trial summarised in Figure 1). Among these 978 children, 881 were definitively included according to the inclusion/noninclusion criteria and then randomised: 240, 210, 216 and 215 in Madagascar, Niger, CAR and Senegal, respectively, and 297, 290 and 294 in arm 1, arm 2 and arm 3, respectively.
Figure 1.
Sampling profile for children in the trial. †No successive visits at Week 12; Lost to follow‐up at Week 12; missing data at Week 12; others (two maternal deaths, one mother's inability to continue the study and one case of flour refusal of the child). Arm 1: daily fortified blended flour; Arm 2: daily fortified blended flour + azithromycin; Arm 3: daily fortified blended flour containing inulin/fructo‐oligosaccharides.
The main reasons for noninclusion were withdrawal of parental/guardian consent (n = 3), WHZ < −3 or > − 2 (n = 76), unable to return for 3 (or 4) consecutive visits (n = 3), age (n = 7) and associated diseases (n = 8). Two children in Niger were wrongly included (1 in arm 1 and 1 in arm 2) with a WHZ < −3. Both were followed until week 12 and maintained in the analyses.
At week 12, WHZ was documented for 754 children at two consecutive visits, 31 children were transferred to the hospital, one child died, and 95 children were lost to follow‐up (39 (13%), 25 (9%) and 31 (11%) in arm 1, arm 2 and arm 3, respectively, p = 0.21). In the ITT population, 881 children were analysed (297, 290 and 294 in arm 1, arm 2 and arm 3, respectively) with missing values, transfer to hospital or death, thus considered failures. Seven hundred fifty‐four children (247, 257 and 250 in arm 1, arm 2 and arm 3, respectively) were maintained in the per protocol analysis.
The two children wrongly included in Niger were maintained in the ITT analysis but excluded from the PP analysis.
3.2. Baseline characteristics of children by arm and by country
Table 1 shows the characteristics of the 881 children at inclusion. Baseline characteristics were similar in the three arms with no clinically relevant difference. Thirty‐nine per cent (344/881) were male, the median age was 14 months (IQR = [10‐18]), 97% (851/881) were born at term and 5.3% (47/881) had concomitant pathologies at inclusion, mainly acute respiratory infections (n = 12) and gastrointestinal diseases (n = 22).
Table 1.
Children characteristics at baseline by arm.
| Arm 1 (Control) | Arm 2 (Antibiotic) | Arm 3 (Prebiotic) | Total | p value | |
|---|---|---|---|---|---|
| n = 297 | n = 290 | n = 294 | n = 881 | ||
| Country n (%) | |||||
| Senegal | 72 (24.2) | 72 (24.8) | 71 (24.1) | 215 (24.4) | 0.99 |
| Madagascar | 80 (26.9) | 79 (27.2) | 81 (27.6) | 240 (27.2) | |
| Niger | 71 (23.9) | 70 (24.1) | 69 (23.5) | 210 (23.8) | |
| CAR | 74 (24.9) | 69 (23.8) | 73 (24.8) | 216 (24.5) | |
| General characteristics | |||||
| Sex | |||||
| Male n (%) | 120 (40.4) | 112 (38.6) | 112 (38.1) | 344 (39.0) | 0.83 |
| Age (months) a | 14.5 ± 5.0 | 14.9 ± 4.8 | 14.4 ± 4.7 | 14.6 ± 4.9 | |
| [6–18] months n (%) | 205 (69.0) | 190 (65.5) | 212 (72.1) | 607 (68.9) | 0.23 |
| [18–24] months n (%) | 90 (30.3) | 96 (33.1) | 81 (27.6) | 267 (30.3) | |
| Weight‐for‐height z‐score a | −2.23 ± 0.40 | −2.20 ± 0.39 | −2.17 ± 0.41 | −2.20 ± 0.40 | 0.17 |
| Weight‐for‐age z‐score a | −2.80 ± 0.76 | −2.85 ± 0.80 | −2.77 ± 0.82 | −2.81 ± 0.79 | 0.54 |
| Height‐for‐age z‐score a | −2.25 ± 1.27 | −2.39 ± 1.35 | −2.30 ± 1.40 | −2.31 ± 1.34 | 0.46 |
| MUAC a , b | 123.4 ± 4.7 | 123.3 ± 4.1 | 123.1 ± 4.6 | 123.3 ± 4.5 | 0.59 |
| Full‐term birth n (%) | 289 (97.3) | 279 (96.2) | 283 (96.3) | 851 (96.6) | 0.94 |
| Known diseases n (%) | 14 (4.7) | 13 (4.5) | 20 (6.8) | 47 (5.3) | 0.41 |
| Diet n (%) | |||||
| Exclusively breastfed | 2 (0.7) | 4 (1.4) | 2 (0.7) | 8 (0.9) | 0.85 |
| Breastfeeding with complementary foods | 246 (82.8) | 243 (83.8) | 244 (83.0) | 733 (83.2) | |
| Non‐breastfed | 49 (16.5) | 43 (14.8) | 48 (16.3) | 140 (15.9) | |
Mean ± SD.
Mid‐upper arm Circumference.
Table 2 shows the comparison at inclusion between countries. Significant differences were found in MUAC measurements with a lower mean in Niger compared to the three other countries (125, 124 and 122 cm in Senegal, Madagascar and CAR, respectively, vs. 122 cm in Niger, p < 0.001).
Table 2.
Children characteristics at baseline by country.
| MADAGASCAR | NIGER | CAR | SENEGAL | ||
|---|---|---|---|---|---|
| n = 240 | n = 210 | n = 216 | n = 215 | p value | |
| General characteristics | |||||
| Sex | |||||
| Male n (%) | 82 (34.2) | 93 (44.3) | 77 (35.6) | 92 (42.8) | 0.07 |
| Age (months) a | 15.1 ± 4.9 | 14.9 ± 4.4 | 14.5 ± 4.9 | 13.8 ± 5.1 | 0.03 |
| [6–18] months n (%) | 159 (66.3) | 143 (68.1) | 152 (70.4) | 153 (71.2) | 0.66 |
| [18–24] months n (%) | 81 (33.7) | 67 (31.9) | 64 (29.6) | 62 (28.8) | |
| Weight‐for‐height z‐score a | −2.10 ± 0.39 | −2.45 ± 0.37 | −2.14 ± 0.38 | −2.13 ± 0.37 | <0.001 |
| Weight‐for‐age z‐score a | −3.12 ± 0.76 | −3.11 ± 0.72 | −2.68 ± 0.73 | −2.30 ± 0.66 | <0.001 |
| Height‐for‐age z‐score a | −2.99 ± 1.22 | −2.59 ± 1.30 | −2.16 ± 1.24 | −1.43 ± 1.06 | <0.001 |
| MUAC a , b | 124.1 ± 4.6 | 121.7 ± 4.1 | 122.3 ± 3.56 | 124.9 ± 4.8 | <0.001 |
| Full‐term birth n (%) | 231 (96.2) | 208 (99.0) | 203 (94.0) | 209 (97.2) | 0.005 |
| Known diseases n (%) | 6 (2.5) | 32 (15.2) | 2 (0.9) | 7 (3.3) | <0.001 |
| Diet n (%) | <0.001 | ||||
| Exclusively breastfed | 1 (0.4) | 1 (0.5) | 2 (0.9) | 4 (1.9) | |
| Breastfed with complementary foods | 220 (91.7) | 170 (80.9) | 164 (75.9) | 178 (83.3) | |
| Nonbreastfed | 19 (7.9) | 39 (18.6) | 50 (23.1) | 32 (14.9) | |
Note: p, comparison between countries using χ2 test (or Fisher exact test) or ANOVA.
Mean ± SD.
Mid‐upper arm circumference.
Similarly, the mean WHZ was significantly lower in Niger than in other countries (−2.13, −2.10 and −2.14 in Senegal, Madagascar and CAR, respectively, vs. −2.45 in Niger, p < 0.001).
Percentages of associated pathologies were also higher in Niger (15%) compared to the three other countries (p < 0.001). A significant difference was also seen in diet. While the majority of children (83%, 733/881) received a mixed diet that included complementary foods and milk feeding (breastfeeding or breast milk substitutes), this practice was more common in Madagascar, with fewer nonbreastfed children (7.9% in Madagascar vs. 14.9%, 18.6% and 23% in Senegal, Niger and CAR, respectively, p < 0.001).
3.3. Primary outcome
(Tables 3 and 4). Overall, 44% (390/881) of the children documented for the primary outcome (WHZ ≥ −1.5) recovered at week 12 from MAM, with no significant difference between the three arms (41.4%, 45.5% and 45.9% in arm 1, arm 2 and arm 3, respectively, [p = 0.47]). A similar result was observed for the per protocol analysis. Differences in recovery rates (95% CI) between arm 1 versus arm 2 and arm 3 were 4.1% (95% CI [−4.9–12.1], p = 0.32) and 4.5% (95% CI [−3.5 to 12.5], p = 0.27), respectively, in the ITT population, and 1.6% (95% CI [−7.2 to 10.3], p = 0.72) and 4.2% (95% CI [−4.6 to 13.0], p = 0.35), respectively, in the PP population.
Table 3.
Week‐12 recovery rates by arm.
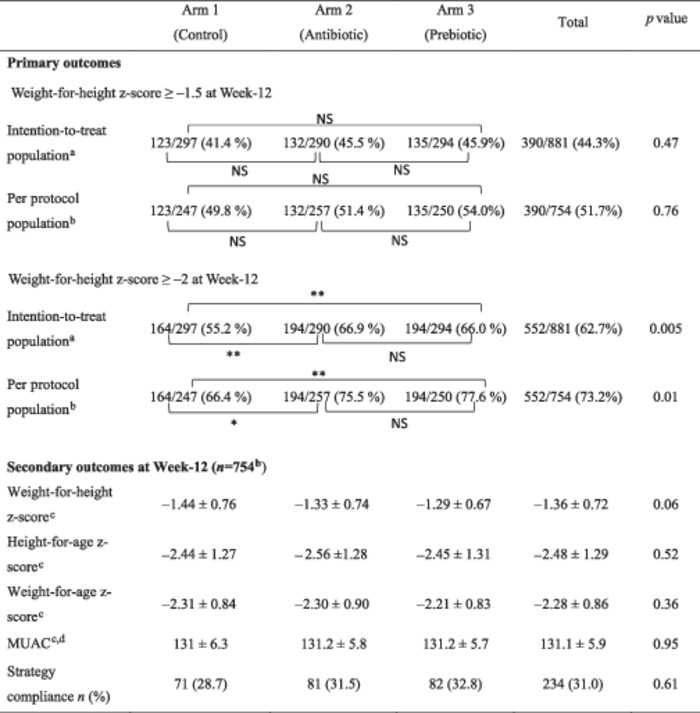
|
Note: p, comparison between countries using χ2 test (or Fisher exact test) or ANOVA.
Abbreviation: NS, nonsignificant.
†Transfer to hospital, death or missing data = failures.
‡Only documented children at 3 months.
§Mean ± SD.
¶Mid‐upper arm circumference.
*p < 0.05
**p < 0.01.
Table 4.
Week‐12 recovery rates by country.
| Madagascar | Niger | CAR | Senegal | p value | |
|---|---|---|---|---|---|
| Weight‐for‐height z‐score ≥ −1.5 at Week‐12 | |||||
| Intention‐to‐treat population a | 86/240 (35.8%) | 113/210 (53.8%) | 86/216 (39.8%) | 105/215 (48.8%) | <0.0001 |
| Per Protocol population b | 86/203 (42.4%) | 113/188 (60.1%) | 86/190 (45.3%) | 105/173 (60.7%) | <0.0001 |
| Weight‐for‐height z‐score ≥ −2 at Week‐12 | |||||
| Intention‐to‐treat population a | 138/240 (57.5%) | 135/210 (64.3%) | 128/216 (59.3%) | 151/215 (70.2%) | 0.02 |
| Per Protocol population b | 138/203 (68.0%) | 135/188 (71.8%) | 128/190 (67.4%) | 151/173 (87.3%) | <0.0001 |
Note: p, comparison between countries by χ2 test (or Fisher exact test).
Transfer to hospital, death or missing data = failures.
Only documented children at 3 months.
In contrast, significant differences were observed for recovery, defined as a WHZ ≥ −2. Higher recovery rates were observed in arms 2 and 3 compared to arm 1 (control) on the two analyses, with a difference in recovery of 11.7% (95% CI [3.8–19.5], p = 0.004) and 10.8% (95% CI [2.9–18.6], p = 0.007), respectively.
For an outcome at WHZ ≥ −1.5 or at ≥ −2, the proportion of recovery was higher in Senegal and Niger compared to Madagascar and CAR regardless of the type of analysis: 49% and 54% in Senegal and Niger versus 36% and 40% in Madagascar and CAR in the intention to treat analysis. When adjusting for age, WHZ at inclusion, and feeding status, the differences observed between countries are still statistically significant for both ITT and PP analyses. For the cure rate, defined as WHZ ≥ −1.5, the results showed a higher rate in Niger and Senegal. On the other hand, when using the threshold of −2, only Senegal showed a benefit compared to the three other countries. A statistical difference was observed in the recovery rate between breastfed children ± complementary feeding versus those not breastfed (42% vs. 56%, p = 0.003).
3.4. Time of recovery
The time to recovery with a WHZ ≥ −1.5 at 12 weeks was not significantly different between the three arms, with median time of 70 days (IQR = [35−91]), 63 days (IQR = [25–91]) and 63 days (IQR = [29–90]), in arm 1, arm 2 and arm 3, respectively, (p = 0.17) (Figure 2). The same results were observed among the 552 children who recovered at week 12 defined by a WHZ ≥ −2: median treatment durations of 28 days (IQR = [14–56]), 21 days (IQR = [14–43]) and 23 days (IQR = [14–45]), respectively, (p = 0.11). No difference between arms was observed in each country except in CAR, where statistical difference was observed with a shorter recovery time for arm 2 and arm 3 compared to arm 1:36 days (IQR = [21–84]), 43 days (IQR = [21–90]) and 70 days (IQR = [35–91]), respectively, (p = 0.05).
Figure 2.
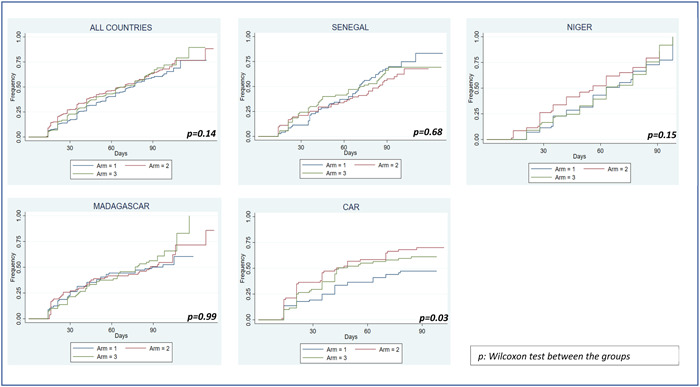
Time to recovery according to Z‐score ≥ −1.5 SD on total population and by country (Kaplan–Meier curves).
Among the 390 children who recovered at 12 weeks with a WHZ ≥ −1.5, the baseline WHZ was statistically higher in those who recovered early (at week 2) than in those who recovered later: −2.05 ± 0.31 (n = 63) versus −2.21 ± 0.41, (n = 327), p = 0.004.
3.5. Secondary outcomes
(Table 5) For the 754 children documented at week 12, no difference was observed at the end of the study between the three arms for MUAC measurements and height‐for‐age z‐score (HAZ). The same results were observed when considering the percentage of children with an HAZ < −2 or an HAZ < −3.
Table 5.
Secondary outcomes in children documented at Week 12 (N = 754).
| Arm 1 n = 247 | Arm 2 n = 257 | Arm 3 n = 250 | Total n = 754 | p value | |
|---|---|---|---|---|---|
| Secondary outcomes | |||||
| MUAC,a, b | 130 (127–135) | 130 (128–135) | 130 (128–135) | 130 (127–135) | 0.95 |
| Height‐for‐age z‐scoreb | −2.4 (−3.3; −1.6) | −2.5 (−3.4; −1.8) | −2.4 (−3.4; −1.6) | −2.5 (−3.4; −1.6) | 0.52 |
| HAZ < −2 n (%) | 155 (62.8) | 181 (70.4) | 152 (60.8) | 488 (64.7) | 0.06 |
| HAZ < −3 n (%) | 88 (35.6) | 91 (35.4) | 91 (36.4) | 270 (35.8) | 0.97 |
| Not completed consumption declared by mothers n (%) | 71 (28.7) | 81 (31.5) | 82 (32.8) | 234 (31.0) | 0.61 |
Note: p, comparison between countries using χ2 test (or Fisher exact test) or ANOVA.
Mid‐upper arm circumference.
Median (Q1–Q3).
The height gain from day 0 to 12 weeks was very similar between the three arms (2 cm [1.2, −2.9], 2 cm [1.3–2.7] and 2 cm [1.4–2.6] in arm 1, 2 and 3, respectively, p = 0.87), as well as the weight gain (0.90 kg [0.6–1.2], 0.85 kg [0.6–1.2] and 0.90 kg [0.6–1.2] in arm 1, 2 and 3, respectively, p = 0.74).
3.6. Flour consumption and reported compliance
Parents reported that 329/881 (37%) children did not fully consume flour. Compliance did not differ between the three arms: 35.0% (104/297), 37.2% (108/290) and 39.8% (117/294), in arm 1, 2 and 3, respectively, p = 0.49. On the other hand, a significant difference in the number of children who had not completely consumed flour between the countries was observed: 93% (201/215) in Senegal versus 20% (47/240) in Madagascar, 29% (61/210) in Niger and 10% (20/216) in CAR, (p < 0.001).
For azithromycin, all 290 children randomised in arm 2 received the three doses except two children (one in Senegal and one in CAR) who only received the first two doses. The first child could not receive the third dose because his mother was sick; the second child did not come to the primary health centre because of inclement weather. A mild side effect was reported in three children after taking one dose of the antibiotic, each with fever, diarrhoea and rhinorrhoea.
At inclusion, 98% (598/613) of children over 12 months of age received albendazole: 97.6% (200/205), 98.0% (199/203) and 97.1% (199/205) in arms 1, 2 and 3, respectively.
3.7. Adverse events
During the 12 weeks of follow‐up, 18 serious adverse events (SAEs) were reported from 18 children, including two deaths (one death related to tuberculosis in CAR [arm 1] and one death without diagnosis in Niger [arm 2], 13 hospitalisations due to complications following severe pathology (severe acute gastroenteritis, acute respiratory infection, severe pneumonia, severe malaria, measles) and three severe adverse events in arm 2 (a severe malaria attack, a fall resulting in a cervical contusion and a traffic accident resulting in multiple injuries). Out of the 18 children with reported SAEs, 6 were randomised to arm 1, 7 were randomised to arm 2 and 5 were randomised to arm 3. All SAEs were declared to be unrelated to the study. For 624 children (82.8%), at least one nonserious adverse event was reported and 7 (mainly diarrhoea) were considered by the mothers to be related to the flour (6 in Madagascar and 1 in Niger; 3 in arm 2 and 4 in arm 3). In all cases, the flour was not stopped, and children were followed until the end of the study.
The main reported unrelated events to the study were: fever (254/754, 33.7%), diarrhoea (384/754, 50.9%), acute respiratory infection (370/754, 49.1%) or malaria (100/754, 13.3. No differences were observed in the number of cases of diarrhoea reported between arms or between countries. Among the 384 cases of diarrhoea reported along the 3 months of follow‐up, 131/247 (53%), 128/257 (50%) and 125/250 (50%) were from arm 1, arm 2 and arm 3, respectively, p = 0.66, and 96/173 (56%), 98/203 (48%), 94/188 (50%) and 96/190 (51%) were from Senegal, Madagascar, Niger and CRA, respectively, p = 0.554.
4. DISCUSSION
The main objective of this pragmatic study was to compare in real‐life conditions the effectiveness of two strategies of re‐nutrition targeting the microbiota in comparison with a standard regimen in children with MAM. Enrolment for this trial followed an active strategy among community health workers to detect children with malnutrition in the suburbs of four African cities. This detection was based on MUAC measurements, and MAM was confirmed by WHZ at the primary health centre. Congruence of MUAC and WHZ was good during this study, which was previously reported for SAM but not for MAM (Bari et al., 2019; Lamsal et al., 2021), especially for older children (Briend et al., 2012; Grijalva‐Eternod et al., 2015; Roberfroid et al., 2015; Stephens et al., 2020; Wieringa et al., 2018). This community detection also confirmed the poor perception of MAM by mothers, because medical consultations had never been planned for any of the children.
During this study, 881 children were randomly assigned to an intervention arm and followed for 12 weeks in each of the four countries. At inclusion, the three arms were comparable for all anthropometric criteria and general characteristics. However, differences were observed between countries among children enroled. MUAC and weight were lower for children in Niger and Madagascar than in Senegal and CAR, which correlates well with the usual level of income in the countries.
Overall, the rate of recovery at 12 weeks (as defined by WHZ ≥ – 1.5 at 2 consecutive visits) both for the ITT and the PP populations did not differ between the three arms. The scientific committee of the trial decided to retain the threshold of −1.5 to consider the expected high recovery rates in this study, with supervision of flour every week and high relapse rates after 12 weeks. Significant differences, however, were observed in an exploratory analysis using recovery definition as a WHZ ≥ −2 between the standard arm and the two others (prebiotics mixed flour and antibiotics). This result must be considered cautiously, because it was not the primary endpoint of the trial and was not proposed in the protocol. However, this criterion is interesting because the threshold of −2, recommended by the WHO, is the dividing line defining MAM (WHO, 2014; Bergeron and Castleman [2012]).
Very few data are available in different countries to compare our data on MAM, especially since most other studies used probiotics, not prebiotics, in supplements. Kerac, in a double‐blind efficacy randomised controlled trial conducted in Malawi, failed to show the benefit of nutritional cure among severely malnourished children receiving Synbiotic2000 Forte (Kerac et al., 2009). Calder et al. did not show any differences in clinical outcomes in the MIMBLE study conducted in Uganda in children hospitalised with severe malnutrition using legume‐based feeds rich in fermentable carbohydrates (Calder et al., 2021).
The recovery time did not differ between the three arms for the −2 WHZ and −1.5 criteria.
We did not observe a gain in height over the study period. Our study was only 12 weeks long, which was too short to measure any effect on height. Although young children at this age grow on average 1 cm per month, there exist such variability under real‐life conditions that it remains challenging to demonstrate the impact of the intervention.
Overall, adherence to flour consumption was good except in Senegal, where the amount of flour was not fully consumed. This could be explained by additional foods given to children there.
Tolerance of the treatments was good, with very few side effects reported at the same severity for the three arms and unrelated to the regimens.
At 12 weeks, global recovery rates are higher in Niger and Senegal than in Madagascar and CAR, regardless of the population analysed (ITT or PP population) and the recovery cut‐off (−1.5 or −2).
The main pitfall in the re‐nutrition strategy is the duration of the process, and thus compliance of mothers in charge of feeding the child is a major factor of re‐nutrition success.
Compliance was evaluated in the field conditions of MAM management in this trial, that is, a weekly visit to the primary health centre where the child is weighed and measured and where flours are delivered for the following week. A weekly interview was also conducted with each mother to investigate the amount of flour eaten by the child as well as any complementary foods consumed. An extra amount of flour was weekly given to each child to anticipate sharing with siblings. The amount of flour returned by the mother was used as an indicator of consumption and sharing.
Despite receiving greater quantity than those needed by their children, Malagasy mothers and guardians returned very little flour. In this country, for families with children who did not recover, sharing of flour to all children and even selling it was documented. Senegalese mothers, in contrast, returned large quantities of flour corresponding to the excess of flour given every week to the mothers, probably because of the high consumption of complementary foods provided as reported by mothers or caregivers. In Niger, children coming from rural areas with poor access to complementary food seemed to receive correct quantities of flour. They also recovered better than children included in other countries.
For this study, prebiotics were tested instead of probiotics, to avoid the problem of the large number of probiotic strains available in the market, as well as to support future development of local ingredient‐based production of flour.
Along the same lines, previous studies have underscored the role of bacteria in malnutrition, thus supporting the use of an antibiotic in the treatment of MAM children. Azithromycin was chosen as the antibiotic in this trial because it is not yet widely used in primary health centres and, moreover, the use of azithromycin in mass treatment during trachoma control campaigns underlined the good tolerance of the product, with very few side effects. More recently, azithromycin was also used during the MORDOR study (Keenan et al., 2019; Oron et al., 2020) as a yearly treatment of all the 1–59‐month children. During this study, azithromycin, in comparison to a placebo, reduced mortality by nearly 14% in this group (12‐59 months) (Keenan et al., 2018) and by 23% in children 1–11 months old. Since then, WHO proposed guidelines (Oldenburg et al., 2020; WHO, 2020) for the evaluation of this strategy in a larger community of children between 1 and 11 months old.
4.1. Limitations
The first limitation of our clinical trial was the number of subjects randomised and analysed for the primary endpoint. Due to longer than expected recruitment and children lost to follow‐up, the initially planned number of 280 children per arm could not be achieved: 247, 257 and 250 children in arm 1, arm 2 and arm 3, respectively, were analysed for the primary endpoint, which decreased the power of the study. Similarly, measuring the height of children between 6 and 24 months is very difficult, even with adequate devices (Bilukha et al., 2020). Standardised documentation of clinical data (especially the documentation of diseases and side events) was also challenging because the capacities of the medical teams were not the same in the four countries.
Socio‐cultural and environmental contexts can influence the effectiveness of a strategy. If multisite trials are important to validate strategies in different contexts, children included in our trial from 4 African countries resulted in many logistic constraints.
Finally, although free delivery of flour to the mother corresponds to real‐life conditions, the exact quantity of flour absorbed by each child could not be controlled.
5. CONCLUSION
Overall, this study conducted in the field conditions with locally produced supplements did not support the true advantages of adding a prebiotic or a treatment with antibiotic to flour in that it did not achieve, as it failed to meet the primary endpoint retained, that is, WHZ ≥ −1.5. Simultaneous enrolment of children in four different countries raised problem with comparisons across countries but not between the three arms. Differences between countries should be addressed in a subsequent study. How mothers engage with the trial and provide accurate quantities of flour and complementary foods is determinant. Moreover, the criteria chosen for the study (WHZ ≥ −1.5) can clearly sustain the recovery of the child and prevent relapse. The criteria, however, may be too stringent to estimate the effectiveness of the regimen, whereas (WHZ ≥ −2) might be a more operational milestone to assume that the child is no longer in the danger zone of moderate acute malnutrition (MAM) on the way to severe acute malnutrition (SAM). Nevertheless, this study confirmed the effectiveness of managing children with MAM at home as through a community‐based control strategy (Kimani‐Murage et al., 2019).
Other analyses are in process related to the composition of the gut microbiota of these children, highlighting many differences between countries and children (Dauga in process).
AUTHOR CONTRIBUTIONS
Muriel Vray, Ronan Jambou, André Briend and Antonio Vargas contributed to the design and development of the MALINEA trial. Muriel Vray is the principal investigator of the MALINEA trial. Cassandre Von Platen, Laura Tondeur and Boris Gildas Hedible supervised the operational management of the trial. Rindra Vatosoa Randremanana, Alexandre Manirakiza, Ramatoulaye Hamidou Lazoumar and Boris Gildas Hedible supervised data collection in the four countries. Laura Tondeur oversaw data collection activities and Laura Tondeur, Muriel Vray and Boris Gildas Hedible analysed the data. Muriel Vray, Ronan Jambou and Laura Tondeur wrote the first draught of the manuscript. André Briend provided support in interpreting results. All authors contributed to the revision of the manuscript and read and approved the final version.
MALINEA CLINICAL TRIAL GROUP
Rado Ernestau Marice Andrianantenaina (Institut Pasteur, Madagascar), Nirina Nadia Andrianomenjanahary (Institut Pasteur, Madagascar), Oumar Barry (Université Cheikh Anta Diop, Senegal), Yvette Batoumbou (Institut Pasteur, Senegal), Emilie Buttarelli (ACF, Senegal), Fabrice Carbonne (ACF, Senegal), Ousmane Moussa Djibo (Centre de Santé Intégré Tchaké, Niger), Abdias Ogobara Dougnon (ACF, Senegal), Abdal‐Aziz Ousma Gado (ACF, Niger), Mareme Gaye Diop (Institut Pasteur, Senegal), Viola Gnocchi (ACF, Senegal), Jean‐Chrysostome Gody (Centre Hospitalier Universitaire Pédiatrique de Bangui, Central African Republic), Dieynaba Kane (ACF, Senegal), Jean‐Pierre Lombart (Institut Pasteur, Central African Republic), Sani Mamane (Centre de Santé Intégré Issawane, Niger), Dior Maronne (Poste de Santé Hamo 5, Guediawaye, Senegal), Youmou Ndongo (ACF, Senegal), Deborah Nguiamba (Centre Hospitalier Universitaire Pédiatrique de Bangui, Central African Republic), Garda Ide Oumarou (CERMES, Niger), Hobitiana Marietta Rabemiafara (Institut Pasteur, Madagascar), Antsanirina Sitrakantenaina Rafanomezantsoa (Institut Pasteur, Madagascar), Voahanginirina Rakotoarimivo Andrianaly (Institut Pasteur, Madagascar), Rojovola Rakotondramanana (Institut Pasteur, Madagascar), Ravaka Randriamparany (Institut Pasteur, Madagascar), Christelle Mioranirina Randrianarisoa (Institut Pasteur, Madagascar), Rasetamalala (Centre de Santé Mitia Andavamamba, Madagascar), Hanta Viviane Ratrimonirina (Institut Pasteur, Madagascar), Marie‐Florence Razanarimanana (Centre de Santé Mitia Andavamamba, Madagascar), Olga Sakanga (Institut Pasteur, Central African Republic), Auguste Sarr (Institut Pasteur, Senegal), Abdoulaye Seck (Institut Pasteur, Senegal), Aminata Sy (Poste de Santé Hamo 5, Guediawaye, Senegal), Tokiravakiniaina Voarinirina (Institut Pasteur, Madagascar)
CONFLICT OF INTEREST STATEMENT
No conflicts of interest are declared by the authors.
ACKNOWLEDGEMENTS
The authors thank all mothers who accepted the follow‐up of their children, as well as staff of dispensaries, where the project occurred (Centres de Santé Mitia Andavamamba Antananarivo and Andohatapenaka in Madagascar; Centres de Santé Intégrés Issawane and Tchaké in Niger; Poste de Santé Hamo 5 Guédiawaye in Senegal; Centre Hospital‐Universitaire Pediatrique de Bangui in CAR). The authors are grateful to the Beneyd society for providing the prebiotic for the entire study. They also warmly thank the entire team of the International Direction of Institute Pasteur in Paris, who supported the administrative management of the project, and the CRT team, who assisted them with ethical regulation management. The trial was funded by two grants: one from the French Foreign Office (MEAE) and one from the international foundation, Action against Hunger.
Vray, M. , Tondeur, L. , Hedible, B. G. , Randremanana, R. V. , Manirakiza, A. , Lazoumar, R. H. , Platen, C. V. , Vargas, A. , Briend, A. , Jambou, R. (2024). Three‐arm clinical trial of improved flour targeting intestinal microbiota (MALINEA). Maternal & Child Nutrition, 20, e13649. 10.1111/mcn.13649
Contributor Information
Muriel Vray, Email: muriel.vray@pasteur.fr.
Malinea Clinical Trial Group:
Rado Ernestau Marice Andrianantenaina, Nirina Nadia Andrianomenjanahary, Oumar Barry, Yvette Batoumbou, Emilie Buttarelli, Fabrice Carbonne, Ousmane Moussa Djibo, Abdias Ogobara Dougnon, Abdal‐Aziz Ousma Gado, Mareme Gaye Diop, Viola Gnocchi, Jean‐Chrysostome Gody, Dieynaba Kane, Jean‐Pierre Lombart, Sani Mamane, Dior Maronne, Youmou Ndongo, Deborah Nguiamba, Garda Ide Oumarou, Hobitiana Marietta Rabemiafara, Antsanirina Sitrakantenaina Rafanomezantsoa, Voahanginirina Rakotoarimivo Andrianaly, Rojovola Rakotondramanana, Ravaka Randriamparany, Christelle Mioranirina Randrianarisoa, Rasetamalala, Hanta Viviane Ratrimonirina, Marie‐Florence Razanarimanana, Olga Sakanga, Auguste Sarr, Abdoulaye Seck, Aminata Sy, and Tokiravakiniaina Voarinirina
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Bari, A. , Nazar, M. , Iftikhar, A. , & Mehreen, S. (2019). Comparison of weight‐for‐height z‐score and mid‐upper arm circumference to diagnose moderate and severe acute malnutrition in children aged 6‐59 months. Pakistan Journal of Medical Sciences, 35, 337–341. 10.12669/pjms.35.2.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron, G. , & Castleman, T. (2012). Program responses to acute and chronic malnutrition: Divergences and convergences. Advances in Nutrition, 3(2), 242–249. 10.3945/an.111.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilukha, O. , Couture, A. , McCain, K. , & Leidman, E. (2020). Comparison of anthropometric data quality in children aged 6‐23 and 24‐59 months: Lessons from population‐representative surveys from humanitarian settings. BMC Nutrition, 6, 60. 10.1186/s40795-020-00385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton, L. V. , Barratt, M. J. , Charbonneau, M. R. , Ahmed, T. , & Gordon, J. I. (2016). Childhood undernutrition, the gut microbiota, and microbiota‐directed therapeutics. Science, 352, 1533. [DOI] [PubMed] [Google Scholar]
- Briend, A. , Maire, B. , Fontaine, O. , & Garenne, M. (2012). Mid‐upper arm circumference and weight‐for‐height to identify high‐risk malnourished under‐five children. Maternal & Child Nutrition, 8, 130–133. 10.1111/j.1740-8709.2011.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, N. , Walsh, K. , Olupot‐Olupot, P. , Ssenyondo, T. , Muhindo, R. , Mpoya, A. , Brignardello, J. , Wang, X. , McKay, E. , Morrison, D. , Holmes, E. , Frost, G. , & Maitland, K. (2021). Modifying gut integrity and microbiome in children with severe acute malnutrition using legume‐based feeds (MIMBLE): A pilot trial. Cell Reports Medicine, 2(5), 100280. 10.1016/j.xcrm.2021.100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. Y. , Mostafa, I. , Hibberd, M. C. , Das, S. , Mahfuz, M. , Naila, N. N. , Islam, M. M. , Huq, S. , Alam, M. A. , Zaman, M. U. , Raman, A. S. , Webber, D. , Zhou, C. , Sundaresan, V. , Ahsan, K. , Meier, M. F. , Barratt, M. J. , Ahmed, T. , & Gordon, J. I. (2021). A microbiota‐directed food intervention for undernourished children. New England Journal of Medicine, 384, 1517–1528. 10.1056/NEJMoa2023294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clercq, N. C. , Groen, A. K. , Romijn, J. A. , & Nieuwdorp, M. (2016). Gut microbiota in obesity and undernutrition. Advances in Nutrition, 7, 1080–1089. 10.3945/an.116.012914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, R. J. , Jones, K. D. J. , & Berkley, J. A. (2015). Environmental enteric dysfunction: an overview. Food and Nutrition Bulletin, 36(Suppl)., S76–S87. 10.1177/15648265150361S113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalva‐Eternod, C. S. , Wells, J. C. , Girma, T. , Kæstel, P. , Admassu, B. , Friis, H. , & Andersen, G. S. (2015). Midupper arm circumference and weight‐for‐length z scores have different associations with body composition: Evidence from a cohort of Ethiopian infants. The American Journal of Clinical Nutrition, 102, 593–599. 10.3945/ajcn.114.106419 [DOI] [PubMed] [Google Scholar]
- Haithar, S. , Kuria, M. W. , Sheikh, A. , Kumar, M. , & Vander Stoep, A. (2018). Maternal depression and child severe acute malnutrition: A case‐control study from Kenya. BMC Pediatrics, 18, 289. 10.1186/s12887-018-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambale, R. M. , & Francisca, I. N. (2022). Optimising the management of acute malnutrition. The Lancet Global Health, 10, e453–e454. 10.1016/S2214-109X(22)00087-0 [DOI] [PubMed] [Google Scholar]
- Keenan, J. D. , Bailey, R. L. , West, S. K. , Arzika, A. M. , Hart, J. , Weaver, J. , Kalua, K. , Mrango, Z. , Ray, K. J. , Cook, C. , Lebas, E. , O'Brien, K. S. , Emerson, P. M. , Porco, T. C. , & Lietman, T. M. (2018). Azithromycin to reduce childhood mortality in Sub‐Saharan Africa. New England Journal of Medicine, 378, 1583–1592. 10.1056/NEJMoa1715474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, J. D. , Arzika, A. M. , Maliki, R. , Boubacar, N. , Elh Adamou, S. , Moussa Ali, M. , Cook, C. , Lebas, E. , Lin, Y. , Ray, K. J. , O'Brien, K. S. , Doan, T. , Oldenburg, C. E. , Callahan, E. K. , Emerson, P. M. , Porco, T. C. , & Lietman, T. M. (2019). Longer‐term assessment of azithromycin for reducing childhood mortality in Africa. New England Journal of Medicine, 380, 2207–2214. 10.1056/NEJMoa1817213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerac, M. , Bunn, J. , Seal, A. , Thindwa, M. , Tomkins, A. , Sadler, K. , Bahwere, P. , & Collins, S. (2009). Probiotics and prebiotics for severe acute malnutrition (PRONUT study): A double‐blind efficacy randomised controlled trial in Malawi. The Lancet, 374(9684), 136–144. 10.1016/S0140-6736(09)60884-9 [DOI] [PubMed] [Google Scholar]
- Kimani‐Murage, E. W. , Pythagore, H. , Mwaniki, E. , Daniel, T. , Samburu, B. , Cuellar, P. C. , Mbochi, R. , Njiru, J. , Wangare, L. , Karimurio, L. , Agutu, O. , Maina, L. G. , Okoth, P. , Raburu, J. , Wanjohi, M. , Macharia, T. , & Zerfu, T. A. (2019). Integrated and simplified approaches to community management of acute malnutrition in rural Kenya: A cluster randomized trial protocol. BMC Public Health, 19, 1253. 10.1186/s12889-019-7497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsal, K. P. , Parajuli, K. R. , Pun, B. K. , Adhikari, R. P. , Bashyal, M. , Dangol, B. , & Cunningham, K. (2021). Accuracy of using mid‐upper arm circumference to detect wasting among children aged 6‐59 months in Nepal. Global Health: Science and Practice, 9, 881–889. 10.9745/GHSP-D-20-00450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya‐Alvarez, V. , & Sansonetti, P. J. (2022). Understanding the pathways leading to gut dysbiosis and enteric environmental dysfunction in infants: The influence of maternal dysbiosis and other microbiota determinants during early life. FEMS Microbiology Reviews, 46(3), fuac004. 10.1093/femsre/fuac004 [DOI] [PubMed] [Google Scholar]
- Oldenburg, C. E. , Arzika, A. M. , Maliki, R. , Lin, Y. , O'Brien, K. S. , Keenan, J. D. , & Lietman, T. M. (2020). Optimizing the number of child deaths averted with mass azithromycin distribution. The American Journal of Tropical Medicine and Hygiene, 103, 1308–1310. 10.4269/ajtmh.19-0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis, M. , Garza, C. , Onyango, A. W. , & Rolland‐Cachera, M. F. (2009). Les standards de croissance de l'organisation mondiale de la santé pour les nourrissons et les jeunes enfants. Archives de Pédiatrie, 16, 47–53. 10.1016/j.arcped.2008.10.010 [DOI] [PubMed] [Google Scholar]
- Oron, A. P. , Burstein, R. , Mercer, L. D. , Arzika, A. M. , Kalua, K. , Mrango, Z. , West, S. K. , Bailey, R. L. , Porco, T. C. , & Lietman, T. M. (2020). Effect modification by baseline mortality in the MORDOR azithromycin trial. The American Journal of Tropical Medicine and Hygiene, 103, 1295–1300. 10.4269/ajtmh.18-1004020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piemontese, P. , Giannì, M. L. , Braegger, C. P. , Chirico, G. , Grüber, C. , Riedler, J. , Arslanoglu, S. , van Stuijvenberg, M. , Boehm, G. , Jelinek, J. , & Roggero, P. (2011). Tolerance and safety evaluation in a large cohort of healthy infants fed an innovative prebiotic formula: A randomized controlled trial. PLoS One, 6, e28010. 10.1371/journal.pone.0028010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt, E. M. E. (1992). Protein energy malnutrition archives diseases. Children, 67, 1416. [Google Scholar]
- Roberfroid, D. , Huybregts, L. , Lachat, C. , Vrijens, F. , Kolsteren, P. , & Guesdon, B. (2015). Inconsistent diagnosis of acute malnutrition by weight‐for‐height and mid‐upper arm circumference: contributors in 16 cross‐sectional surveys from South Sudan, the Philippines, Chad, and Bangladesh. Nutrition Journal, 14, 86. 10.1186/s12937-015-0074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytter, M. J. H. , Kolte, L. , Briend, A. , Friis, H. , & Christensen, V. B. (2014). The immune system in children with malnutrition ‐ a systematic review. PLoS One, 9, e105017. 10.1371/journal.pone.0105017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. , Potani, I. , Griswold, S. P. , Suri, D. , Langlois, B. , Shen, Y. , Walton, S. M. , Kwan Ho Chui, K. , Manary, M. J. , Webb, P. , Rogers, B. L. , & Rosenberg, I. H. (2021). Host fecal mRNAs predicted environmental enteric dysfunction among children with moderate acute malnutrition in Sierra Leone. The American Journal of Tropical Medicine and Hygiene, 105, 1376–1382. 10.4269/ajtmh.21-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery, J. , MacFabe, D. F. , & Frye, R. E. (2016). The significance of the enteric microbiome on the development of childhood disease: A review of prebiotic and probiotic therapies in disorders of childhood. Clinical Medicine Insights: Pediatrics, 10, CMPed.S38338. 10.4137/CMPed.s38338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, K. , Orlick, M. , Beattie, S. , Snell, A. , Munsterman, K. , Oladitan, L. , & Abdel‐Rahman, S. (2020). Examining mid‐upper arm circumference malnutrition z‐score thresholds. Nutrition in Clinical Practice, 35, 344–352. 10.1002/ncp.10324 [DOI] [PubMed] [Google Scholar]
- van Stuijvenberg, M. , Eisses, A. M. , Grüber, C. , Mosca, F. , Arslanoglu, S. , Chirico, G. , Braegger, C. P. , Riedler, J. , Boehm, G. , & Sauer, P. J. For the Multicenter Infection Prevention Study‐1 (MIPS‐1) Study Group . (2011). Do prebiotics reduce the number of fever episodes in healthy children in their first year of life: A randomised controlled trial. British Journal of Nutrition, 106, 1740–1748. 10.1017/S0007114511004053 [DOI] [PubMed] [Google Scholar]
- Trehan, I. , Goldbach, H. S. , LaGrone, L. N. , Meuli, G. J. , Wang, R. J. , Maleta, K. M. , & Manary, M. J. (2013). Antibiotics as part of the management of severe acute malnutrition. New England Journal of Medicine, 368, 425–435. 10.1056/NEJMoa1202851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF‐WHO . (2020). The World Bank group joint child malnutrition estimates: levels and trends in child malnutrition: Key findings of the 2020 edition. https://www.who.int/publications/i/item/jme-2020-edition
- Vray, M. , Hedible, B. G. , Adam, P. , Tondeur, L. , Manirazika, A. , Randremanana, R. , Mainassara, H. , Briend, A. , Artaud, C. , von Platen, C. , Altmann, M. , & Jambou, R. (2018). A multicenter, randomized controlled comparison of three renutrition strategies for the management of moderate acute malnutrition among children aged from 6 to 24 months (the MALINEA project). Trials, 19, 666. 10.1186/s13063-018-3027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO , 2014. Integrated management of childhood illness: Distance learning course [Internet]. Geneva: World Health Organization; [cité 25 oct 2023]. 15 p. https://iris.who.int/handle/10665/104772
- WHO . (2012). Technical note: Supplementary foods for the management of moderate acute malnutrition in infants and children 6–59 months of age. https://apps.who.int/iris/handle/10665/75836
- WHO . (2016). Management of severe and moderate acute malnutrition in children. https://www.ncbi.nlm.nih.gov/books/NBK361900/ [PubMed]
- WHO . (2020). Guideline on mass drug administration of azithromycin to children under five years of age to promote child survival. https://www.who.int/publications/i/item/9789240009585 [PubMed]
- Wieringa, F. , Gauthier, L. , Greffeuille, V. , Som, S. , Dijkhuizen, M. , Laillou, A. , Chamnan, C. , Berger, J. , & Poirot, E. (2018). Identification of acute malnutrition in children in Cambodia requires both mid upper arm circumference and weight‐for‐height to offset gender bias of each indicator. Nutrients, 10, 786. 10.3390/nu10060786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


