Abstract
Acute malnutrition affects not only the growth and development but also the body composition of children. However, its specific effects have not yet been characterized. This study aims to compare the body composition of 5–7‐year‐old children with moderate acute malnutrition (MAM) to that of their well‐nourished (WN) peers and identify associated factors. A school‐based comparative cross‐sectional study was conducted from June to July 2022 in Jimma town, southwest Ethiopia. The study participants were selected from eight kindergartens and eight primary schools using a simple random sampling technique based on the proportional allocation of the sample to the size of the population in the respective school. Descriptive statistics and multivariable linear regression analyses were used to assess the mean differences and associations between variables and isolate independent predictors of body composition, respectively. The statistical significance was determined using ß‐coefficients with 95% confidence intervals and a p value of ≤ 0.05. Data were captured from 388 (194 MAM and 194 WN) children with a response rate of 97.9%. The mean fat‐free mass of WN children was significantly higher compared with those with MAM (p < 0.001). The mean (SD) of fat mass of MAM children was 4.23 ± 0.72 kg, 4.36 ± 0.88 kg and 4.08 ± 0.89 kg for 5, 6 and 7‐year‐olds, respectively. For WN children, the mean (SD) of fat mass was 4.92 ± 0.88 kg for 5 years old, 5.64 ± 1.01 kg for 6 years old and 5.75 ± 1.26 kg for 7 years old (p < 0.001). On the multivariable linear regression analysis after controlling for background variables, WN children exhibited 1.51 times higher fat‐free mass compared with MAM children (β = 1.51, p = 0.003). A unit increase in age of the study participants was associated with a 1.37 increment in fat‐free mass (β = 1.37, p < 0.001). WN children had 1.07 times higher fat mass compared with children with MAM (β = 1.07, p < 0.001). A unit increase in the age of the child resulted in 0.15 times increment in fat mass (β = 0.15, p = 0.020), and being female was associated with a 0.37 increase in fat mass (β = 0.37, p < 0.001). The results showed that the mean fat mass and fat‐free mass were significantly lower among moderately acute malnourished children than in WN children showing the loss of both body compartments due to malnutrition. The body mass index for age, age of the child and sex of the child were significantly linked to both fat‐free mass and fat mass.
Keywords: body composition, children, Ethiopia, fat‐free mass, fat mass, MAM, pre‐school
Our results showed that the mean fat mass (FM) and fat‐free mass (FFM) were significantly lower among moderate acute malnutrition (MAM) children than among NW children, showing the effect of moderate thinness on wasting of muscle mass, which can help to improve the continuum of care in the management of acute malnutrition.
Key messages
This study demonstrated that children aged 5–7 years suffering from moderate acute malnutrition (MAM) have lower mean body fat mass (FM) and fat‐free mass (FFM) as compared with well‐nourished (WN) children.
The substantial difference observed between MAM and WN children in body FM and FFM calls for the integration of MAM treatment into the routine health services to prevent their sliding down to SAM and associated mortality.
1. INTRODUCTION
According to the World Health Organization (WHO), a child is well‐nourished (WN) if their body mass index (BMI) for their age is between −2 and +1 and has moderate acute malnutrition (MAM) if their BMI is greater than −3 and less than −2 (Cashin & Oot, 2018). Malnutrition is broadly categorized as undernutrition (which includes wasting [low weight‐for‐height], underweight [low weight‐for‐age], stunting [low height‐for‐age], and micronutrient‐related malnutrition), and overnutrition (Dukhi, 2020; Kamruzzaman et al., 2021; World Health Organization, 2017).
Acute malnutrition (AM) is a significant global public health concern, with a more acute impact in low‐ and middle‐income countries (LMICs). Nearly 64% of people with AM are diagnosed with MAM, which is more common than severe acute malnutrition (SAM) (Karim et al., 2021).
Malnutrition has been linked to stunting, wasting and underweight conditions that are characterized by altered body composition and decreased physical growth (Wells, 2019). The muscle may be killed in favour of general organ protection, yet shrinkage of the thymus brought on by undernutrition has been linked to decreased immunological capability (Prentice, 1999). Fat mass (FM) supplies immune system function with substantial metabolic costs, in terms of metabolic precursors and energy (Wells, 2010). Leptin which acts as a ‘gateway’ for immunological activity is also secreted by FM (Lord, 2002). Malnutrition has a detrimental effect on the physical, intellectual, social and emotional development of children (Park et al., 2001). Evidence shows that malnutrition causes weak muscles and a decrease in motor skills in children (Badaru et al., 2023, Renault & Quesada, 1993).
Understanding the effect of wasting on fat‐free mass (FFM) and FM might substantiate public health and therapeutic interventions that are more successful in promoting survival, long‐term quality of life and decreasing functional consequences (Sinha et al., 2022). The basic body composition model divides body mass into two components, the FFM and FM, to better understand the physical impacts of malnutrition (Wells, 2019). Body composition is a potentially useful indicator as it is crucial for both long‐ and short‐term survival in both sick and healthy children (Radhakrishna et al., 2010). The body adiposity proportion is a useful predictor of children's health and nutritional status since they change with age, sex and environmental factors (Debnath et al., 2018). Adipocytes are found in numerous other cells and organs throughout the body (Gallagher, 2020).
Previous studies have shown the drawbacks of using body mass index for these objectives since it does not differentiate between increases in FFM and FM. Additionally, too much FM can conceal deficits in FFM (Kyle et al., 2015). Exclusive traditional weight measurement (one‐compartment model) does not provide as much information on nutritional storage and the type of tissue accumulation as a body composition assessment (McDonald et al., 2019).
Currently, anthropometric measurements based on middle upper arm circumference (MUAC), height, weight, weight for length z‐score or both, are used to assess and treat children with MAM, but neither FFM nor FM is used (Bartz et al., 2014). Evidence shows that body composition during childhood may predict the risk of developing a noncommunicable disease (NCD) during adulthood (Bander et al., 2023). Especially, rapid or catch‐up weight gain during early childhood has been linked to insulin resistance, overweight or obesity, adiposity and NCDs in later life (Kristjansson et al., 2015; Wells, 2019).
Children and adolescents aged 5–19 have heightened nutritional requirements due to their rapid growth, and undernutrition can lead to poor growth, delayed sexual maturation and impaired cognitive development. Furthermore, overweight and obesity during this age range are likely to persist into adulthood and increase the risk of chronic diseases. Therefore, assessing the nutritional status of this age group is crucial for further management and interventions (Agency for International Development [USAID], 2018). In Ethiopia, the treatment of MAM is not provided as a routine service except in some food‐insecure areas, like the Integrated Management of Acute Malnutrition (IMAM) woredas. It is believed that the health extension programme will take care of MAM cases (James et al., 2016). However, children suffering from MAM and with no access to supplementary feeding programmes experience high rates of deterioration and no improvement, as evidenced by a prospective cohort study conducted in rural Ethiopia (James et al., 2016).
Brain development continues for an extended period post‐natally. The brain increases in size fourfold during the pre‐school period, reaching approximately 90% of adult volume by age 6 (Lenroot & Giedd, 2006; Stiles & Jernigan, 2010). According to research by Luciana and Nelson, in typically developing children between the ages of 4 and 8, the prefrontal working memory system develops at about age 4 and gets better between the ages of 5 and 7 years (Luciana & Nelson, 1998), and there is a significant improvement in the ability to store visual short‐term memory. In diverse cultures, 5–7 years of age is the beginning of the ‘age of reason’ (Rogoff et al., 1975). The age range of 5–7 years old is a sensitive period/golden age, when the highest level of activity throughout life happens.
Nevertheless, there is a paucity of evidence on body composition and associated factors among children in this age group in Ethiopia. Therefore, this study aimed to compare the body composition of WN and moderately wasted children aged 5–7 years in Jimma town, southwest Ethiopia.
2. METHODS AND MATERIALS
2.1. Study area and period
The study was carried out in kindergartens (KG) and elementary schools within Jimma Town, which is located 345 km from Addis Ababa, the capital of Ethiopia. The geographical coordinates of the town are approximately 7°41′N latitude and 36°50′E longitude. The town is bordered to the east by Kersa Woreda, to the west by Mana Woreda, to the north by Mana and Kersa Woreda, and the south by Seka Woreda. Data obtained from Jimma Town municipality show that the town has a total surface area of 4623 hectares and a population of 224,565 of which 39,352 children under the age of 5. Of the total of under 5 years old children, 19,645 were pre‐schoolers spread across 17 Kebeles. There were 25 public elementary schools in the Town. A total of 32,443 students were in public primary schools; of those, 10,123 were grade 1 students and 8770 were KG students in the age group of 5–7 years. Twenty‐five KG classes are primary schools called ‘0’ classes locally.
A school‐based comparative cross‐sectional study was conducted from June to July 2022 among 5–7‐year‐old children with MAM and WN children at eight KG and primary schools. Eligible participants were 5–7‐year‐old children attending KG and primary schools in Jimma Town, and whose parents or caregivers are residents of the town and willing to participate. Exclusions comprised mothers or caregivers who were seriously ill, children who were seriously ill, and physically disabled and children diagnosed with MAM and on an outpatient therapeutic programme (OTP). For children diagnosed with MAM, nutrition counselling was provided to their mothers or caregivers, and for those diagnosed with SAM, a linkage was established with a nearby health facility for further treatments.
2.2. Sample size and sampling techniques
The sample size was determined using G*power statistical software version 3.1.9.4 with the assumption of a difference between two independent samples (t test) for comparing the mean. Input parameters were: effect size of 0.4, a precision of 0.05, power (1‐β) of 80%, with a 95% level of confidence, and an allocation ratio of 1:1 which resulted in a sample size of 200. A design effect of 1.8 was applied for the cluster sampling, and 10% was added for nonresponse, resulting in a total sample size of 396. So, the study required the involvement of 198 WN and 198 MAM children.
2.3. Sampling procedure
In each of the 25 public primary schools in Jimma town, there are KG classes (locally called ‘0’ classes). The study's sampling process involved two steps. First, eight primary schools were randomly selected using a lottery method using the WHO recommendation as a rule of thumb to include at least 30% of the total schools (WHO & Unicef, 2009). Second, students were categorized into two groups based on age: KG (age group 5–6 years) and grade 1 (age group 7 years). For the age group of 5–7 years, the sample size was allocated based on the proportion of the age group that the school has, which is one‐third (n = 132) of the sample from grade 1 (7‐year‐old) children in the primary school and two‐third (n = 264) of the sample size from KG classes (5–6‐year‐old) children. The registration book of the school was used to select the study units from eligible children. Finally, a simple random sampling technique was applied to select the final sample after screening the children for their nutritional status as either MAM or WN.
2.4. Data collection and procedure
Data were collected using a semistructured questionnaire through face‐to‐face interviews of variables including socioeconomic and demographic factors, household food security (access) and child health characteristics. The wealth index of the household was assessed using ownership of durable assets. The wealth index was ranked into poor, medium and rich socioeconomic status tertiles. One physiotherapist, three nurses and two nutritionists who are fluent in the local language participated as data collectors and supervisors, respectively. Data collectors received 2‐day training on the questionnaire and data collection procedures. Throughout the data collection process, both the supervisors and the principal investigator followed the data collectors and performed quality checks.
2.5. Household food insecurity access scale measurement
Household food insecurity was measured using the Household Food Insecurity Access Scale (HFIAS), which was developed by the Food and Nutrition Technical Assistance (FANTA) project. For HFIAS measurement, each of the questions was asked with a recall period of 4 weeks. The respondent was first asked an occurrence question, that is, whether the condition in the question happened at all in the past 4 weeks (yes or no). If the respondent answered ‘yes’ to an occurrence question, a frequency‐of‐occurrence question was asked to determine whether the condition happened rarely (once or twice), sometimes (three to 10 times), or often (more than 10 times) in the past 4 weeks (Coates et al., 2007; Gebreyesus et al., 2015).
2.6. Anthropometric measurements
The measurements of height (cm) and weight (kg) were taken for each child after the standardization of measures and calibration of equipment. All measurements were taken three times, and an average value was recorded. Weight was measured with shoes taken off and in light clothing (underwear and t‐shirts only) using a Seca digital weighing scale (Seca, Model 770) and recorded to the nearest 0.1 kg. Height was measured using a stadiometer Seca 213 (Seca) recorded to the nearest 0.1 cm, after positioning the subject in a Frankfurt plane. In measuring height, the head was positioned in the Frankfort and the ankle, calf, buttocks and shoulders touched the vertical stand of the stadiometer.
2.7. Body composition measurement
Body composition (FFM and FM) was measured using bioelectrical impendence analysis on the right side of the body. The International Society for the Advancement of Kinanthropometry's recommendations for anatomical landmarks were followed (Kamruzzaman et al., 2021). Before beginning the body composition measurement, the study participants were asked to remove tight hand and wrist jewellery since it might interfere with the bioelectrical impedance analysis (BIA) body composition instrument. During BIA measurement, the study participants were asked to lie in a supine position on a flat table with the arms comfortably abducted from the body at 15° and the legs separated. The arms were separated from the trunk of the body and the legs were not touching each other (Health & Survey, 2000). Measurements were performed in duplicate at all points, and an average was taken. Bioelectrical impedance measurement was conducted by standard equipment, which is Bodystat QuadScan 4000.
2.8. Data quality management
Before actual data collection, a pretest was conducted on 20 subjects at a nearby school (Swiss‐Ginjo school) not included in the study. All the anthropometric measurements were performed by both the investigator and trained (data collector) three nurses, two nutritionists and supervisors to eliminate within‐examiner error. The weight scale was calibrated to zero level with no object on it and placed on a leveled surface before the measurement was performed. A continuous checkup of scales and bioelectrical impendence was carried out for their reliability. The data collection was supervised by the principal investigator and trained supervisors. The principal investigator supervised and reviewed every questionnaire for completeness and logical consistency and made corrections on the spot. A standardization exercise was conducted during training to capture technical errors in measurement (TEM). The TEM was performed to assess interobserver and interobserver errors using a published methodology (Perini et al., 2005) and the coefficient of variation was checked.
2.9. Data analysis
The data were checked for completeness, coded and entered EPI Data Version 3.1 and exported to SPSS Version 26 for cleaning and analysis. Principal component analysis (PCA) was done for the household wealth index. Household food security status was computed based on the HFIAS nine questions of occurrence and frequency of occurrence. The data were analysed based on food insecurity access classification criteria and categorized as a food‐secure or food‐insecure household. We performed a χ 2 test for the categorical data and an independent sample t test was used to compare differences in the mean value of body composition between MAM and WN children.
Descriptive statistics (mean ± SD, frequencies and percentages) and bivariate analysis were utilized to select candidate variables for multivariable analysis. To determine the relationship between body composition and explanatory variables, a simple linear regression was conducted. Before the analysis, several assumptions were checked, including the linearity between the dependent and independent variables, normality, independence of observations (no multicollinearity) and homoscedasticity. The bivariate linear regression identified variables with p values less than 0.25 which were then considered for multivariable linear regression. This was done to determine the factors that independently affect body composition. During the analysis, all interactions were checked. The results were presented using ß‐coefficients and 95% confidence intervals. A p value less than 0.05 was considered statistically significant and the model's fitness was checked using adjusted R 2.
2.9.1. Operational definitions
-
1.
Food insecurity: ‘A situation that exists when people lack secure access to sufficient amounts of safe and nutritious food for normal growth and development and an active and healthy life’ (Coates et al., 2007).
-
2.
MAM: a child with BMI‐for‐age of ≥−3 to <−2 z scores (USAID, 2018).
-
3.
WN: a child with BMI‐for‐age ≥ −2 to < −1 z scores (USAID, 2018).
-
4.
FM: That portion of the human body that is composed strictly of fat (Health & Survey, 2000).
2.10. Ethics statement
The Institutional Review Board of Jimma University Institute of Health has ethically approved the proposal. Additionally, the administrative education office of Jimma Town received a letter of support from the Department of Human Nutrition and Dietetics, which this office then disseminated to each selected school. Each child's mother provided written informed consent. For children diagnosed with MAM mothers or caregivers received nutritional counselling, and those diagnosed with severe acute malnutrition were linked to local health facilities.
3. RESULTS
3.1. Sociodemographic characteristics of the mothers and caregivers
The response rate was 97.9%, with 388 of the 396 children and mothers/primary caregivers providing complete responses. These results were confirmed by the values of the technical error of measurement for weight and height in the acceptable range. For weight intra and interobserver variation was 0.11 kg which is acceptable (<0.21 kg), whereas for height it was 0.65 cm which is acceptable (<1.0 cm). The coefficient of variation was also 2.2% for height and 1.8% for weight which is within the acceptable range of less than 3%. The mean age of study the participants was 32.08 (±7.57) years. A total of 79.6% of the households were food insecure (Table 1).
Table 1.
Sociodemographic characteristics of parents/caregivers of children in Jimma town southwest Ethiopia, June to July 2022 (n = 388).
| Variables | Category | Nutritional status of the child (n = 388) | |||
|---|---|---|---|---|---|
| MAM (n = 194) | WN (n = 194) | Total | |||
| n = 194 (%) | n = 194 (%) | n = 388 (%) | p Value | ||
| Age (years) of mother/caregiver | ≤19 | 7 (3.6) | 0 | 7 (1.8) | <0.0001 |
| 20–29 | 54 (27.8) | 87 (44.8) | 141 (36.3) | ||
| 30–39 | 98 (50.5) | 88 (45.4) | 186 (47.9) | ||
| ≥40 | 35 (18.0) | 19 (9.8) | 54 (13.9) | ||
| Age (years) of mother/caregiver Mean (±SD) | 32.08 (±7.57) | ||||
| Marital status of the caregiver | Married & live together | 109 (56.2) | 168 (86.6) | 277 (71.4) | <0.0001 |
| Married & and live separately | 21 (10.8) | 5 (2.6) | 26 (6.7) | ||
| Divorced | 27 (13.9) | 14 (7.2) | 41 (10.6) | ||
| Widowed | 29 (14.9) | 6 (3.1) | 35 (9) | ||
| Single | 8 (4.1) | 1 (0.5) | 9 (2.3) | ||
| Educational status of the mother | Cannot read and write | 110 (56.7) | 39 (20.1) | 149 (38.4) | <0.0001 |
| Can read and write | 24 (12.4) | 32 (16.5) | 56 (14.4) | ||
| Primary school (0–8) | 47 (24.2) | 64 (33) | 111 (28.6) | ||
| Secondary school (9–12) | 11 (5.7) | 55 (28.4) | 66 (17) | ||
| Above secondary school ( >12) | 2 (1) | 4 (2.1) | 6 (1.5) | ||
| The educational status of the father | Cannot read and write | 41 (31.5) | 11 (6.4) | 52 (17.1) | <0.0001 |
| Can read and write | 13 (10) | 10 (5.8) | 23 (7.6) | ||
| Primary school (0–8) | 34 (26.2) | 50 (28.9) | 84 (27.7) | ||
| Secondary school (9–12) | 32 (24.6) | 64 (37) | 96 (31.7) | ||
| Above secondary school ( >12) | 10 (7.7) | 38 (22) | 48 (15.8) | ||
| Occupation of mother | Housewife | 80 (41.2) | 68 (35.1) | 148 (38.1) | <0.0001 |
| Merchant | 23 (11.9) | 32 (16.5) | 55 (14.2) | ||
| Government employee | 6 (3.1) | 41 (21.1) | 47 (12.1) | ||
| Private‐employee | 20 (10.3) | 27 (13.9) | 47 (12.1) | ||
| Daily labourer | 65 (33.5) | 26 (13.4) | 91 (23.5) | ||
| Occupation of father | Farmer | 3 (2.3) | 4 (2.3) | 7 (2.3) | <0.0001 |
| Merchant | 14 (10.8) | 52 (30) | 66 (21.8) | ||
| Government employee | 30 (23.1) | 73 (42.2) | 103 (34) | ||
| Private‐employee | 24 (18.5) | 12 (6.9) | 36 (11.9) | ||
| Daily labourer | 54 (41.5) | 29 (16.8) | 83 (27.4) | ||
| Othera | 5 (3.8) | 3 (1.7) | 8 (2.6) | ||
| Ethnicity | Oromo | 134 (69.1) | 129 (66.5) | 263 (67.8) | 0.218 |
| Amhara | 16 (8.2) | 23 (11.9) | 39 (10.1) | ||
| Kafa | 7 (3.6) | 13 (6.7) | 20 (5.2) | ||
| Yem | 20 (10.3) | 11 (5.7) | 31 (8) | ||
| Otherb | 17 (8.8) | 18 (9.3) | 35 (9) | ||
| Religion | Muslim | 103 (53.1) | 100 (51.5) | 203 (52.3) | 0.971 |
| Orthodox | 55 (28.4) | 58 (29.9) | 113 (29.1) | ||
| Protestant | 35 (18) | 35 (18) | 70 (18) | ||
| Otherc | 1 (0.5) | 1 (0.5) | 2 (0.5) | ||
| Family size | ≤5 members | 140 (72.2) | 162 (83.5) | 302 (77.8) | 0.007 |
| >5 members | 54 (27.8) | 32 (16.5) | 86 (22.2) | ||
| Head of household | Father | 133 (68.6) | 174 (89.7) | 307 (79.1) | <0.0001 |
| Mother | 61 (31.4) | 20 (10.3) | 81 (20.9) | ||
| Household wealth index | Poor | 136 (70.1) | 48 (24.7) | 184 (47.4) | <0.0001 |
| Medium | 34 (17.5) | 39 (20.1) | 73 (18.8) | ||
| Rich | 24 (12.4) | 107 (55.2) | 131 (33.8) | ||
| Food security status | Food secured | 9 (4.6) | 70 (36) | 79 (20.4) | <0.0001 |
| Food insecure | 185 (95.4) | 124 (64) | 309 (79.6) | ||
Note: Significant at p < 0.05.
Other: drivers, temporary employees, cajoler& retired.
Other: Silte, Dawro, and Tigre.
Other: Adventist and catholic.
3.2. Sociodemographic characteristics of the study participants' children
Out of 388 participants, 212 (54.6%) were females. The mean age of these participants was 6.08 (±0.76). A large (44.1%) proportion was in KG‐3. The majority (92.3%) of the participants travel to school by foot. The mean distance from the living place to the school amounts to 15.13 (±6.38) minutes (Table 2).
Table 2.
Sociodemographic characteristics of children aged between 5–7 years in Jimma town southwest Ethiopia, June to July 2022 (n = 388).
| Variables | Category | Nutritional status of the child (n = 388) | |||
|---|---|---|---|---|---|
| MAM (n = 194) | WN (n = 194) | Total | |||
| n = 194 (%) | n = 194 (%) | n = 388 (%) | p Value | ||
| Sex | Male | 78 (40.2) | 98 (50.5) | 176 (45.4) | 0.041 |
| Female | 116 (59.8) | 96 (49.5) | 212 (54.6) | ||
| Age of child (years) | 5 | 55 (28.4) | 43 (22.2) | 98 (25.3) | 0.308 |
| 6 | 75 (38.7) | 87 (44.8) | 162 (41.8) | ||
| 7 | 64 (33) | 64 (33) | 128 (32.9) | ||
| Grade Level | KG2 | 67 (34.5) | 53 (27.3) | 120 (30.9) | 0.307 |
| KG3 | 81 (41.8) | 90 (46.4) | 171 (44.1) | ||
| Grade 1 | 46 (23.7) | 51 (26.3) | 97 (25.0) | ||
| Distance from the school (minutes) | <10 | 24 (12.4) | 16 (8.2) | 40 (10.3) | 0.003 |
| 10‐14 | 53 (27.3) | 30 (15.5) | 83 (21.4) | ||
| ≥15 | 117 (60.3) | 148 (76.3) | 265 (68.3) | ||
| Distance from the school (minutes)a Mean ( ± SD) | 15.13 (±6.38) | ||||
| Means of transportation | Foot | 183 (94.3) | 175 (90.7) | 358 (92.3) | 0.177 |
| Taxi | 11 (5.7) | 19 (9.3) | 30 (7.7) | ||
|
Time to start CFb |
Before 6 months | 147 (75.8) | 53 (27.3) | 200 (51.5) | <0.001 |
| At 6 months | 36 (18.6) | 65 (33.5) | 101 (26.0) | ||
| After 6 months | 11 (5.7) | 76 (39.2) | 87 (22.4) | ||
Note: Significant at p < 0.05.
Indicate that this is based on the estimated parental report.
CF: complementary feeding time as reported by the mother.
3.3. Comparison of body composition
There were significant differences between MAM and WN children in body composition. In comparison to MAM children of 5, 6 and 7 years of age, WN children had a significantly larger FM (p < 0.001) (Table 3).
Table 3.
Comparison of FM and FFM per age category between WN and MAM children of age 5–7 years in Jimma town southwest Ethiopia, June to July 2022.
|
Outcomes variables |
Age (years) | BMI for age category | N | Mean | Std | Difference | p a | |
|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | |||||||
| Fat mass (kg) | 5 | MAM | 55 | 4.23 | 0.72 | −0.69 | −1.02, −0.36 | <0.001 |
| Well‐nourished | 43 | 4.92 | 0.88 | |||||
| 6 | MAM | 75 | 4.36 | 0.88 | −1.28 | −1.57, −0.98 | <0.001 | |
| Well‐nourished | 87 | 5.64 | 1.01 | |||||
| 7 | MAM | 64 | 4.08 | 0.89 | −1.67 | −2.05, −1.28 | <0.001 | |
| Well‐nourished | 64 | 5.75 | 1.26 | |||||
| Fat‐free mass (kg) | 5 | MAM | 55 | 11.26 | 1.24 | −0.68 | −1.26, −0.10 | 0.022 |
| Well‐nourished | 43 | 11.95 | 1.57 | |||||
| 6 | MAM | 75 | 12.38 | 1.73 | −0.88 | −2.20, 0.42 | 0.184 | |
| Well‐nourished | 87 | 13.27 | 5.89 | |||||
| 7 | MAM | 64 | 13.46 | 1.83 | −2.03 | −3.02, −1.04 | <0.001 | |
| Well‐nourished | 64 | 15.49 | 3.53 | |||||
p value deducted from the independent sample t test.
There were significant differences between MAM and WN children in body fat percentage, dry lean mass, basal metabolic rate, body FM index and FFM index (p < 0.001) (Table 4).
Table 4.
Independent sample t‐test of body composition children of age 5–7 years in Jimma town southwest Ethiopia, June to July 2022.
| Body composition | BMI for age category | N | Mean | Std | Difference | p * | |
|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | ||||||
| Body fat percentage | MAM | 194 | 25.28 | 4.798 | −4.590 | −5.61, −3.56 | <0.001 |
| Well‐nourished | 194 | 29.87 | 5.436 | ||||
| Fat mass (kg) | MAM | 194 | 4.23 | 0.849 | −1.288 | −1.48, −1.08 | <0.001 |
| Well‐nourished | 194 | 5.52 | 1.121 | ||||
| Lean mass (%) | MAM | 194 | 74.19 | 6.835 | 4.412 | 3.02, 5.79 | <0.001 |
| Well‐nourished | 194 | 69.78 | 7.032 | ||||
| Dry lean mass (kg) | MAM | 194 | 2.80 | 0.491 | −0.323 | −0.49, −0.15 | <0.001 |
| Well‐nourished | 194 | 3.13 | 1.082 | ||||
| Fat‐free mass (kg) | MAM | 194 | 12.42 | 1.852 | −1.291 | −2.00, −0.57 | <0.001 |
| Well‐nourished | 194 | 13.71 | 4.686 | ||||
| Total body water (%) | MAM | 194 | 56.20 | 5.036 | 1.793 | 0.44, 3.14 | 0.010 |
| Well‐nourished | 194 | 54.40 | 8.149 | ||||
| Total body water (lt) | MAM | 194 | 9.61 | 3.386 | −0.659 | −1.26, −0.05 | 0.032 |
| Well‐nourished | 194 | 10.27 | 2.605 | ||||
| Body cell mass | MAM | 194 | 9.78 | 2.818 | −1.890 | −2.61, −1.16 | <0.001 |
| Well‐nourished | 194 | 11.67 | 4.286 | ||||
| Intracellular water (%) | MAM | 194 | 41.58 | 8.350 | −1.110 | −2.80, 0.58 | 0.199 |
| Well‐nourished | 194 | 42.69 | 8.658 | ||||
| Intracellular water in volume | MAM | 194 | 7.17 | 3.329 | −0.592 | −1.13, −0.04 | 0.033 |
| Well‐nourished | 194 | 7.76 | 1.945 | ||||
| Extracellular water (%) | MAM | 194 | 44.00 | 5.732 | 2.429 | 1.27, 3.58 | <0.001 |
| Well‐nourished | 194 | 41.57 | 5.881 | ||||
| Extracellular water in volume | MAM | 194 | 7.65 | 4.998 | −0.164 | −1.02, 0.69 | 0.708 |
| Well‐nourished | 194 | 7.82 | 3.516 | ||||
| Basal metabolic rate (Kcal) | MAM | 194 | 877.13 | 39.40 | −37.00 | −45.5, −28.4 | <0.001 |
| Well‐nourished | 194 | 914.14 | 45.71 | ||||
| Basal metabolic rate/body weight (Kcal/kg) | MAM | 194 | 52.51 | 3.665 | 2.525 | 1.59, 3.45 | <0.001 |
| Well‐nourished | 194 | 49.99 | 5.494 | ||||
| Body fat mass index | MAM | 194 | 3.38 | 0.724 | −1.001 | −1.16, −0.83 | <0.001 |
| Well‐nourished | 194 | 4.38 | 0.931 | ||||
| Fat‐free mass index | MAM | 194 | 9.18 | 0.814 | −1.036 | −1.32, −0.74 | <0.001 |
| Well‐nourished | 194 | 10.22 | 1.871 | ||||
Abbreviations: BMI, body mass index; MAM, moderate acute malnutrition.
p value deducted from the independent sample t test.
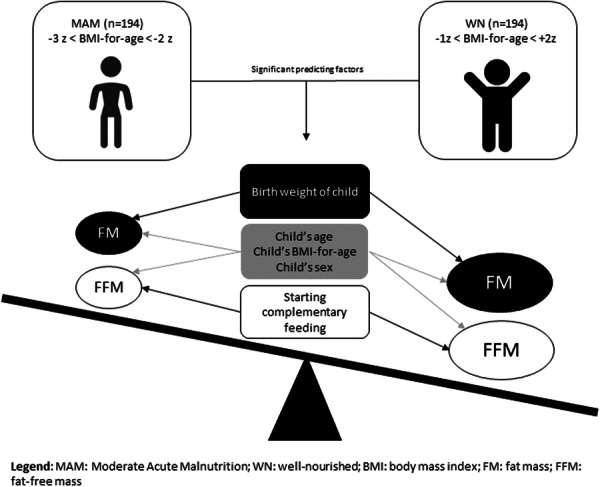
3.4. Predictors of body composition (FFM and FM)
The following variables had a p value less than 0.25 for the bivariate linear regression for the FFM BMI for age, age of mother/caregiver, sex of the child, age of the child, head of the house, complimentary food starts after 6 months, school absenteeism on days, the child's immunization status, distance from the school in minutes, history of current illness, household food insecurity, maternal education and wealth index were candidate variables for the multivariable linear regression analysis (Table A1).
The following variables in bivariate linear regression had a p value of less than 0.25 for FM: BMI for age, child's sex, age and birthweight, head of household, complementary food started before 6 months, complementary food started after 6 months, child's exclusive breastfeeding (EBF) for the first 6 months, child's immunization status, history of current illness, household food insecurity, maternal education, and wealth index. These variables were candidate for the multivariable linear regression analysis (Table A2).
3.5. Independent predictors of FFM
On multivariable linear regression analyses, after adjusting for other variables, BMI for age, sex of the child, age of the child and starting complementary feeding after 6 months were significantly associated with FFM. WN children have 1.51 times higher FFM than MAM children (β = 1.51, p = 0.003). An increase in the child's age by 1 year is associated with a 1.37‐fold increment in FFM (β = 1.37, p < 0.001). Being female was significantly associated with a decrease in FFM by 0.76 (β = −0.76, p = 0.03). Starting complementary feeding after 6 months was significantly negatively associated with FFM (β = −1.06, p = 0.023) (Table 5).
Table 5.
Multivariable linear regression model predicting fat‐free mass among children 5–7 years old in Jimma town southwest Ethiopia, June to July 2022.
| Model | Unstandardized Coefficients | p | 95% CI | ||
|---|---|---|---|---|---|
| B | Std. Err | Lower | Upper | ||
| BMI for age (without MAM) | 1.511 | 0.504 | 0.003 | 0.520 | 2.502 |
| Age of mother/caregiver | −0.041 | 0.023 | 0.074 | −0.087 | 0.004 |
| Maternal education (literate) | 0.454 | 0.612 | 0.459 | −0.750 | 1.657 |
| Age of the child (years) | 1.374 | 0.229 | <0.001 | 0.924 | 1.823 |
| Sex of the child (female) | −0.763 | 0.349 | 0.030 | −1.450 | −0.076 |
| After 6‐month starting complementary feeding | −1.067 | 0.467 | 0.023 | −1.986 | −0.147 |
| Head of the house (mother) | −0.468 | 0.662 | 0.480 | −1.770 | 0.835 |
| School absenteeism on days | −0.032 | 0.033 | 0.334 | −0.096 | 0.033 |
| Distance from the school (minutes) | −0.025 | 0.028 | 0.359 | −0.080 | 0.029 |
| Immunization status of the child | 0.010 | 0.494 | 0.985 | −0.963 | 0.982 |
| History of current illness | −0.349 | 0.461 | 0.449 | −1.255 | 0.556 |
| Food insecurity (secure) | −0.039 | 0.494 | 0.937 | −1.010 | 0.933 |
| Wealth index (rich) | 0.115 | 0.443 | 0.796 | −0.756 | 0.986 |
Note: Maximum VIF = 2.361.
Abbreviations: BMI, body mass index; MAM, moderate acute malnutrition.
3.6. Independent predictors of FM
On the multivariable linear regression model, after adjusting for other variables, BMI for age, sex of the child, age of the child and birthweight of the child were significant predictors of FM. WN children have 1.07 times higher FM than MAM children (β = 1.07, p < 0.001). A unit increase in the age of the child leads to a 0.15‐fold increment of the FM (β = 0.15, p = 0.020). Being female significantly increased the FM by 0.37 (β = 0.37, p < 0.001). The FM increases 0.37 times (β = 0.37, p = 0.012) if the birthweight of the children increases by 1 g (Table 6).
Table 6.
Multivariable linear regression model predicting fat mass among children 5–7 years old in Jimma town southwest Ethiopia, June to July 2022.
| Model | Unstandardized Coefficients | P | 95% CI | ||
|---|---|---|---|---|---|
| B | Std. Err | Lower | Upper | ||
| BMI for age (without MAM) | 1.072 | 0.145 | <0.001 | 0.787 | 1.357 |
| Maternal education (literate) | −0.084 | 0.122 | 0.492 | −0.324 | 0.156 |
| Age of the child (years) | 0.155 | 0.066 | 0.020 | 0.025 | 0.286 |
| Sex of the child (female) | 0.378 | 0.102 | <0.001 | 0.179 | 0.578 |
| Birthweight of the child (gram) | 0.376 | 0.148 | 0.012 | 0.085 | 0.667 |
| Before 6‐month starting complementary feeding | 0.137 | 0.239 | 0.568 | −0.334 | 0.607 |
| After 6‐month starting complementary feeding | 0.072 | 0.149 | 0.630 | −0.221 | 0.365 |
| Immunization status of the child | 0.263 | 0.149 | 0.077 | −0.029 | 0.555 |
| Child EBF for the first 6 months | −0.178 | 0.234 | 0.448 | −0.638 | 0.283 |
| History of current illness | 0.049 | 0.136 | 0.717 | −0.218 | 0.317 |
| Dewormed in last 6 months | 0.019 | 0.119 | 0.872 | −0.216 | 0.254 |
| Food insecurity (secure) | −0.253 | 0.144 | 0.081 | −0.537 | 0.031 |
| Wealth index (rich) | 0.000 | 0.130 | 0.998 | −0.256 | 0.256 |
Note: Maximum VIF = 5.896.
Abbreviations: BMI, body mass index; EBF, early breastfeeding; MAM, moderate acute malnutrition.
On the multivariable linear regression model, after adjusting for other variables, BMI for age, sex, age and birthweight of the child were found to be significant predictors of FM. WN children exhibit 1.07 times higher FM than MAM children (β = 1.07, p < 0.001). A 1‐year increase in the age of the child leads to a 0.15‐fold increment of the FM (β = 0.15, p = 0.020). Being female was associated significantly with an increase in FM by 0.38 (β = 0.376, p < 0.001). For each gram increase in birthweight of the children, FM increases by 0.37 times (Table 6).
4. DISCUSSION
This study aimed to compare body FM and FFM between 5 and 7‐year‐old children with MAM and WN counterparts. The results showed that there is a substantial difference between MAM and WN children in both body FM and FFM. This means that MAM children exhibited less FM and FFM than WN children, which is consistent with the report of a study in India (Sinha et al., 2022). Evidence suggests that MAM or wasting is associated with notable deficiencies in FFM and FM and that these deficits could last for a long time (Wells, 2016) and undernutrition does, in fact, negatively affect muscle mass, which is linked to functional deficiencies, according to scientific records (Wells, 2019).
It was also observed that MAM children's mean FM is lower than that of WN children which is not consistent with the report of the study conducted in India (Savanur & Ghugre, 2016). The difference might be due to study setting, sociodemographic and socioeconomic factors, nutritional awareness, knowledge and culture affecting nutritional status among school‐age children.
Starting complementary feeding after 6 months was negatively associated with FFM (p = 0.023), which is inconsistent with a study conducted in Rotterdam, Netherlands (Van Beijsterveldt et al., 2021). The possible explanations might be that breastfeeding is sufficient in fulfilling all nutrients only up to six and hence not initiating complementary feeding at 6 months may lead to loss of the FFM. It was also observed that a monthly increase in the age of the study participants led to a 1.37‐fold increase in the FFM (p < 0.001) and a 0.15‐fold increase in the FM (p = 0.020). This finding is consistent with the reports of studies conducted in Rotterdam, the Netherlands, Sweden and Minnesota Masonic (Forsum et al., 2019; Scheurer et al., 2017; Van Beijsterveldt et al., 2021). Given the presence of a high prevalence of wasting among children in Ethiopia, the findings have wider practical implications for the identification and management of children with wasting and for correcting abnormalities of body composition across all age groups.
For the paediatric clinical and research field, bioimpedance analysis (BIA) is a relatively easy‐to‐use, fast and noninvasive technique (Wang & Hui, 2015). Unlike the BIA, the BMI has failed to appropriately represent changes in body composition evaluation such as changes in body FM (Zhao et al., 2023). Lack of muscle mass is linked to several issues, including weakened muscles, lowered immunity, and an increased chance of infection (Mastorci et al., 2017). Body composition assessment can be used to identify the body compartment affected by malnutrition because of its ease of use, affordability, safety and noninvasive nature, which will have an implication on the health of children. Children go through a lot of physical changes from the pre‐school years until puberty, including linear growth and gains in muscle and fat. The method of BIA is recognized for estimating low muscle mass (Gonzalez et al., 2018). To distinguish moderately acute malnourished children from nonmalnourished ones, it is recommended that future research focuses on developing body composition cut‐off points using larger sample sizes. A comprehensive understanding of the relationship between clinical outcomes, nutritional status and body composition in children with MAM and WN ones requires further research. In Ethiopia, the management of children with MAM is only carried out in a few IMAM woredas (James et al., 2016). This leaves children with MAM in other woredas untreated, assuming that the health extension programme will address their needs. However, there has been a recent effort to integrate the treatment of MAM into routine health care. The findings suggest that there is a need to strengthen the fast implementation of these efforts to reduce the negative consequences of MAM on children.
4.1. Limitations and strengths of the study
The fact that body composition was assessed by using Bioelectrical Bodystat QuadScan 4000 yields objective information on body composition characteristics, including FM and FFM, which is the strength of the study.
It is important to note that there is a chance that respondents may have answered questions in a way that they think is socially desirable, which could affect the accuracy of the results. Nevertheless, steps were taken to minimize the impact of this bias by informing respondents that their responses were only being used for comparison purposes and would not affect their privacy or service use.
5. CONCLUSION
The study found that children aged 5–7 with MAM have lower mean FM and FFM compared with WN children. These results highlight the negative impact of MAM and emphasize the need for improved efforts to manage AM. The child's BMI for age, age and sex were significantly associated with the FFM and FM of the children. Further research is required in Ethiopia to establish cut‐off points for normal and malnourished children's body composition, using larger sample sizes.
AUTHOR CONTRIBUTIONS
Melese Sinaga Teshome, Tefera Belachew Lema, and Eugene Rameckers conceptualized and designed the work. Data acquisition, analysis, interpretation, collection, drafting, and reviewing the paper were performed by Melese Sinaga Teshome, Tamirat Bekele, Evi Verbecque, Sarah Mingels, MaritaGranitzer, Teklu Gemechu Abessa, Tefera Belachew Lema and Eugene Rameckers. Melese Sinaga Teshome, who is the corresponding author, drafted the manuscript and submitted it for publication. All authors reviewed and approved the final version of the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The Department of Human Nutrition and Dietetics at Jimma University, as well as Hasselt University, deserve special appreciation for funding the execution of this study. Finally, we thank the study participants, mothers/caregivers, Jimma Town Educational Office staff, school directors, teachers, and data collectors. Jimma University's Institute of Health, Faculty of Public Health, and Department of Nutrition and Dietetics provided funding for this study.
APPENDIX A.
Table A1.
Bivariate analysis showing the candidate variables in the study fat‐free mass of children aged between 5 and 7 years in Jimma town southwest Ethiopia, June to July 2022.
| Predictors | Unstandardized coefficients | p | 95% CI for B | ||
|---|---|---|---|---|---|
| ß | Std. Err | Lower | Upper | ||
| BMI for age (without MAM) | 1.291 | 0.362 | <0.001 | 0.580 | 2.003 |
| Age of mother/caregiver | −0.043 | 0.024 | 0.076 | −0.091 | 0.005 |
| Family size of the caregiver | −0.079 | 0.099 | 0.430 | −0.274 | 0.117 |
| Maternal education (literate) | −0.604 | 0.406 | 0.138 | −1.401 | 0.194 |
| Paternal education (literate) | 0.077 | 0.378 | 0.839 | −0.666 | 0.820 |
| Age of the child (years) | 1.465 | 0.230 | <0.001 | 1.012 | 1.918 |
| Sex of the child (female) | −1.136 | 0.365 | 0.002 | −1.854 | −0.419 |
| Birthweight of the child (gram) | 0.446 | 0.428 | 0.298 | −0.395 | 1.287 |
| Complementary food starts before 6 months | 0.367 | 0.367 | 0.319 | −0.356 | 1.089 |
| Complementary food starts after 6 months | −0.532 | 0.440 | 0.228 | −1.397 | 0.333 |
| Head of the house (mother) | −0.897 | 0.450 | 0.047 | −1.782 | −0.012 |
| School absenteeism on days | −0.095 | 0.029 | 0.001 | −0.153 | −0.038 |
| Distance from the school in minutes | −0.041 | 0.029 | 0.153 | −0.098 | 0.015 |
| Immunization status of the child | −0.567 | 0.486 | 0.244 | −1.521 | 0.388 |
| Child EBF for the first 6 months | 0.411 | 0.367 | 0.263 | −0.311 | 1.133 |
| History of current illness | 0.575 | 0.374 | 0.125 | −0.160 | 1.310 |
| Dewormed in last 6 months | 0.144 | 0.388 | 0.710 | −0.618 | 0.907 |
| Food insecurity (secure) | −0.642 | 0.455 | 0.160 | −1.537 | 0.254 |
| Wealth index (medium) | −0.414 | 0.450 | 0.359 | −1.298 | 0.471 |
| Wealth index (rich) | −0.599 | 0.368 | 0.105 | −1.323 | 0.125 |
Abbreviations: BMI, body mass index; EBF, exclusive breastfeeding; MAM, moderate acute malnutrition.
Table A2.
Bivariate analysis showing the candidate variables in the study fat mass of children aged between 5 and 7 years in Jimma town southwest Ethiopia, June to July 2022.
| Predictors | Unstandardized coefficients | p | 95% CI for B | ||
|---|---|---|---|---|---|
| ß | Std. Err | Lower | Upper | ||
| BMI for age (without MAM) | 1.288 | 0.101 | <0.001 | 1.09 | 1.48 |
| Age of mother/caregiver | −0.002 | 0.008 | 0.763 | −0.01 | 0.01 |
| Family size of the caregiver | −0.013 | 0.033 | 0.685 | −0.07 | 0.05 |
| Maternal education (literate) | −0.606 | 0.130 | <0.001 | −0.86 | −0.35 |
| Age of the child (years) | 0.174 | 0.079 | 0.028 | 0.01 | 0.32 |
| Sex of the child (female) | 0.192 | 0.121 | 0.113 | −0.04 | 0.42 |
| Birthweight of the child (gram) | 0.922 | 0.132 | <0.001 | 0.66 | 1.18 |
| Complementary food starts before 6 months | −0.666 | 0.116 | <0.001 | −0.89 | −0.43 |
| Complementary food starts after 6 months | 0.631 | 0.141 | <0.001 | 0.35 | 0.90 |
| Immunization status of the child | −0.490 | 0.157 | 0.002 | −0.79 | −0.18 |
| Child EBF for the first 6 months | −0.707 | 0.115 | <0.001 | −0.93 | −0.48 |
| History of current illness | 0.887 | 0.114 | <0.001 | 0.66 | 1.11 |
| Dewormed in last 6 months | −0.448 | 0.125 | <0.001 | −0.69 | −0.20 |
| Food insecurity (secure) | −0.912 | 0.142 | <0.001 | −1.19 | −0.63 |
| Wealth index (medium) | −0.013 | 0.147 | 0.931 | −0.30 | 0.27 |
| Wealth index (rich) | −0.791 | 0.114 | <0.001 | −1.01 | −0.56 |
Abbreviations: BMI, body mass index; EBF, exclusive breastfeeding; MAM, moderate acute malnutrition.
Teshome, M. S. , Bekele, T. , Verbecque, E. , Mingels, S. , Granitzer, M. , Abessa, T. G. , Lema, T. B. , & Rameckers, E. (2024). Body composition and associated factors among 5–7‐year‐old children with moderate acute malnutrition in Jimma town in southwest Ethiopia: A comparative cross‐sectional study. Maternal & Child Nutrition, 20, e13655. 10.1111/mcn.13655
DATA AVAILABILITY STATEMENT
The corresponding author will provide the datasets used and analysed during the current work upon reasonable request.
REFERENCES
- Badaru, U. M. , Umar, A. L. , Abdullahi, A. , Usman, J. S. , & Ogwumike, O. O. (2023). Influence of malnutrition and body composition on the gross motor function of children with cerebral palsy in Kano, Nigeria: A cross‐sectional study. Bulletin of Faculty of Physical Therapy, 28(1), 1–8. [Google Scholar]
- Bander, A. , Murphy‐Alford, A. J. , Owino, V. O. , Loechl, C. U. , Wells, J. C. , Gluning, I. , & Kerac, M. (2023). Childhood BMI and other measures of body composition as a predictor of cardiometabolic non‐communicable diseases in adulthood: A systematic review. Public Health Nutrition, 26(2), 323–350. [DOI] [PubMed] [Google Scholar]
- Bartz, S. , Mody, A. , Hornik, C. , Bain, J. , Muehlbauer, M. , Kiyimba, T. , Kiboneka, E. , Stevens, R. , Bartlett, J. , St Peter, J. V. , Newgard, C. B. , & Freemark, M. (2014). Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. The Journal of Clinical Endocrinology and Metabolism, 99(6), 2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beijsterveldt, I. A. L. P. , de Fluiter, K. S. , Breij, L. M. , van der Steen, M. , & Hokken‐Koelega, A. C. S. (2021). Fat mass and fat‐free mass track from infancy to childhood: New insights in body composition programming in early life. Obesity, 29(11), 1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashin, K. , & Oot, L. (2018). Guide to anthropometry. A Practical tool for program planners, managers, and implementers. Food Nutr Tech Assist III Proj (FANTA)/FHI 360. pp. 1–231. https://www.fantaproject.org/sites/default/files/resources/FANTA-Anthropometry-Guide-May2018.pdf
- Coates, J. , Swindale, A. , & Bilinsky, P. , (2007). Household Food Insecurity Access Scale (HFIAS) for measurement of food access: Indicator guide: version 3. Available from: https://www.fantaproject.org/sites/default/files/resources/HFIAS_ENG_v3_Aug07.pdf
- Debnath, S. , Mondal, N. , & Sen, J. (2018). Percent of body fat, fat mass, fat‐free mass and assessment of body composition among rural school‐going children of eastern India. Anthropological Review, 81(2), 158–173. [Google Scholar]
- Dukhi, N. (2020). The global prevalence of malnutrition: Evidence from literature. Malnutrition, 1, 1–16. [Google Scholar]
- Forsum, E. , Eriksson, B. , Flinke, E. , Henriksson, H. , Henriksson, P. , & Löf, M. (2019). Fat and fat‐free mass of healthy Swedish children show tracking during early life, but there are differences. Acta Paediatrica, 108(9), 1704–1708. [DOI] [PubMed] [Google Scholar]
- Gallagher, D. A. (2020). A guide to methods for assessing childhood obesity. National Collaborative on Childhood Obesity Research. https://edward-elmhurst.cloud-cme.com/assets/edward-elmhurst/pdf/PedsGR_08.03.2021_NCCOR_MR_Guide_v7-web-small.pdf [Google Scholar]
- Gebreyesus, S. H. , Lunde, T. , Mariam, D. H. , Woldehanna, T. , & Lindtjørn, B. (2015). Is the adapted household food insecurity access scale (HFIAS) developed internationally to measure food insecurity valid in urban and rural households of Ethiopia? BMC Nutrition, 1, 2. [Google Scholar]
- Gonzalez, M. C. , Barbosa‐Silva, T. G. , & Heymsfield, S. B. (2018). Bioelectrical impedance analysis in the assessment of sarcopenia. Current Opinion in Clinical Nutrition and Metabolic Care, 21(5), 366–374. [DOI] [PubMed] [Google Scholar]
- Health, N. , & Survey, E. (2000). National Health and Nutrition Examination Survey body composition procedures. https://www.cdc.gov/nchs/data/nhanes/bc.pdf
- James, P. , Sadler, K. , Wondafrash, M. , Argaw, A. , Luo, H. , Geleta, B. , Kedir, K. , Getnet, Y. , Belachew, T. , & Bahwere, P. (2016). Children with moderate acute malnutrition with no access to supplementary feeding programmes experience high rates of deterioration and no improvement: Results from a prospective cohort study in rural Ethiopia. PLoS One, 11(4), e0153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamruzzaman, M. , Rahman, S. A. , Akter, S. , Shushmita, H. , Ali, M. Y. , Billah, M. A. , Kamal, M. S. , Elahi, M. T. , & Paul, D. K. (2021). The anthropometric assessment of body composition and nutritional status in children aged 2–15 years: A cross‐sectional study from three districts in Bangladesh. PLoS One, 16(9), e0257055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim, M. R. , Al Mamun, A. S. M. , Rana, M. M. , Mahumud, R. A. , Shoma, N. N. , Dutt, D. , Bharati, P. , & Hossain, M. G. (2021). Acute malnutrition and its determinants of preschool children in Bangladesh: Gender differentiation. BMC Pediatrics, 21, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson, E. , Francis, D. K. , Liberato, S. , Jandu, M. B. , Welch, V. , Batal, M. , Greenhalgh, T. , Rader, T. , Noonan, E. , Shea, B. , Janzen, L. , Wells, G. A. , & Petticrew, M. (2015). Food supplementation for improving the physical and psychosocial health of socio‐economically disadvantaged children aged three months to five years: A systematic review. Campbell Systematic Reviews, 11(1), 1–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle, U. G. , Earthman, C. P. , Pichard, C. , & Coss‐Bu, J. A. (2015). Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. European Journal of Clinical Nutrition, 69(12), 1298–1305. [DOI] [PubMed] [Google Scholar]
- Lenroot, R. K. , & Giedd, J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews, 30(6), 718–729. [DOI] [PubMed] [Google Scholar]
- Lord, G. (2002). Role of leptin in immunology. Nutrition Reviews, 60(Suppl. 10), S35–S38. [DOI] [PubMed] [Google Scholar]
- Luciana, M. , & Nelson, C. A. (1998). The functional emergence of prefrontally‐guided working memory systems in four‐ to eight‐year‐old children. Neuropsychologia, 36(3), 273–293. [DOI] [PubMed] [Google Scholar]
- Mastorci, F. , Vassalle, C. , Chatzianagnostou, K. , Marabotti, C. , Siddiqui, K. , Eba, A. , Mhamed, S. , Bandopadhyay, A. , Nazzaro, M. , Passera, M. , & Pingitore, A. (2017). Undernutrition and overnutrition burden for diseases in developing countries: The role of oxidative stress biomarkers to assess disease risk and interventional strategies. Antioxidants, 6(2), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, C. M. , Ackatia‐Armah, R. S. , Doumbia, S. , Kupka, R. , Duggan, C. P. , & Brown, K. H. (2019). Percent fat mass increases with recovery but does not vary according to dietary therapy in young Malian children treated for moderate acute malnutrition. The Journal of Nutrition, 149(6), 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E. J. , Moon, H. K. , Lee, S. S. , & Park, W. H. (2001). A study on the food habits and nutritional status of developmentally disabled children. Korean J Nutr, 34(2), 188–197. [Google Scholar]
- Perini, T. A. , Oliveira, G. L. , Ornellas, J. S. , & Oliveira, F. P. (2005). Cálculo do erro técnico de medição em antropometria. Revista Brasileira de Medicina do Esporte, 11, 81–85. [Google Scholar]
- Prentice, A. M. (1999). The thymus: A barometer of malnutrition. British Journal of Nutrition, 81(5), 345–347. [PubMed] [Google Scholar]
- Radhakrishna, K. V. , Kulkarni, B. , Balakrishna, N. , Rajkumar, H. , Omkar, C. , & Shatrugna, V. (2010). Composition of weight gain during nutrition rehabilitation of severely undernourished children in a hospital‐based study from India. Asia Pacific Journal of Clinical Nutrition, 19(1), 8–13. [PubMed] [Google Scholar]
- Renault, F. , & Quesada, R. (1993). Muscle complications of malnutrition in children: A clinical and electromyographic study. Neurophysiologie Clinique/Clinical Neurophysiology, 23(4), 371–380. [DOI] [PubMed] [Google Scholar]
- Rogoff, B. , Sellers, M. J. , Pirrotta, S. , Fox, N. , & White, S. H. (1975). Age of assignment of roles and responsibilities to children. Human Development, 18(5), 353–369. [Google Scholar]
- Savanur, M. S. , & Ghugre, P. S. (2016). BMI, body fat and waist‐to‐height ratio of stuntedv. non‐stunted Indian children: A case–control study. Public Health Nutrition, 19(8), 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurer, J. M. , Zhang, L. , Gray, H. L. , Weir, K. , Demerath, E. W. , & Ramel, S. E. (2017). Body composition trajectories from infancy to preschool in children born premature versus full‐term. Journal of Pediatric Gastroenterology and Nutrition, 64(6), e147–e153. [DOI] [PubMed] [Google Scholar]
- Sinha, R. K. , Kumar, P. , Daniel, A. , Shah, H. , Sriswan, R. , Kokane, A. , Mohapatra, A. , Kashyap, V. , Goel, A. K. , Kumar, V. , Kiran, A. , Arlappa, N. , Joshi, A. , Nayak, R. R. , Sayal, S. , & de Wagt, A. (2022). Association between anthropometric criteria and body composition among children aged 6–59 months with severe acute malnutrition: A cross‐sectional assessment from India. BMC Nutrition, 8(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles, J. , & Jernigan, T. L. (2010). The basics of brain development. Neuropsychology Review, 20(4), 327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for International Development (USAID) . (2018). Children and adolescents 5–19 years of age. Food Nutr Tech Assist III Proj. pp. 64–93. https://www.fantaproject.org/node/1830
- Wang, L. , & Hui, S. S. (2015). Validity of four commercial bioelectrical impedance scales in measuring body fat among Chinese children and adolescents. BioMed Research International, 2015, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J. C. (2010). The evolutionary biology of human body fatness: Thrift and control (58). Cambridge University Press. [Google Scholar]
- Wells, J. C. (2016). The metabolic ghetto: An evolutionary perspective on nutrition, power relations, and chronic disease. Cambridge University Press.
- Wells, J. C. K. (2019). Body composition of children with moderate and severe undernutrition and after treatment: A narrative review. BMC Medicine, 17, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J. C. K. (2019). Using body composition assessment to evaluate the double burden of malnutrition. Annals of Nutrition and Metabolism, 75(2), 103–108. [DOI] [PubMed] [Google Scholar]
- WHO, U ., & Unicef (2009). AMDD. Monitoring emergency obstetric care: A handbook (p. 152). World Health Organization. [Google Scholar]
- World Health Organization . (2017). Guideline: Assessing and managing children at primary health‐care facilities to prevent overweight and obesity in the context of the double burden of malnutrition.Updates for the Integrated Management of Childhood Illness (IMCI). (pp. 1–73). [PubMed] [Google Scholar]
- Zhao, Y. , Gong, J. , Ji, Y. , Zhao, X. , He, L. , Cai, S. , & Yan, X. (2023). Cross‐sectional study of characteristics of body composition of 24,845 children and adolescents aged 3–17 years in Suzhou. BMC Pediatrics, 23(1), 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author will provide the datasets used and analysed during the current work upon reasonable request.


