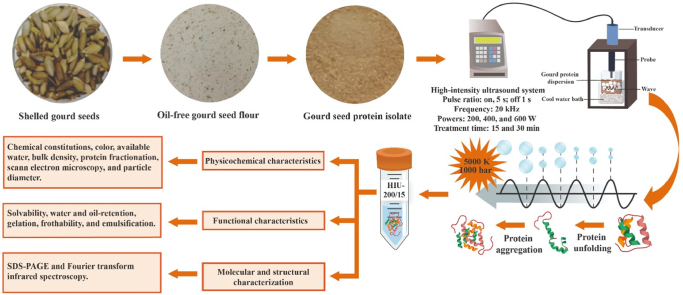
Abstract
The impact of high-intensity ultrasound (HIU, 20 kHz) on the physicochemical and functional characteristics of gourd seed protein isolate (GoSPI) was studied. GoSPI was prepared from oil-free gourd seed flour through alkaline extraction (pH 11) and subsequent isoelectric precipitation (pH 4). The crude protein concentration of GoSPI ranged from 91.56 ± 0.17 % to 95.43 ± 0.18 %. Aqueous suspensions of GoSPI (1:3.5 w/v) were ultrasonicated at powers of 200, 400, and 600 W for 15 and 30 min. Glutelins (76.18 ± 0.15 %) were the major protein fraction in GoSPI. HIU decreased the moisture, ash, ether extract, and nitrogen-free extract contents and the hue angle, available water and a* and b* color parameters of the GoSPI in some treatments. The L* color parameter increased (7.70 %) after ultrasonication. HIU reduced the bulk density (52.63 %) and particle diameter (39.45 %), as confirmed by scanning electron microscopy, indicating that ultrasonication dissociated macromolecular aggregates in GoSPI. These structural changes enhanced the oil retention capacity and foam stability by up to 62.60 and 6.84 %, respectively, while the increases in the solvability, water retention capacity, and emulsifying activity index of GoSPI were 90.10, 19.80, and 43.34 %, respectively. The gelation, foaming capacity, and stability index of the emulsion showed no improvement due to HIU. HIU altered the secondary structure of GoSPI by decreasing the content of α-helices (49.66 %) and increasing the content of β-sheets (52.00 %) and β-turns (65.00 %). The electrophoretic profile of the GoSPI was not changed by HIU. The ultrasonicated GoSPI had greater functional attributes than those of the control GoSPI and could therefore be used as a functional food component.
Keywords: Power ultrasound, Gourd, Physicochemical properties, Functional properties, Structural properties
Graphical abstract
Highlights
-
•
HIU altered the surface morphology and secondary structure of GoSPI.
-
•
The physicochemical and functional characteristics of GoSPI were modified by HIU.
-
•
HIU enhanced the protein concentration and color of GoSPI.
-
•
The oil retention capacity and foam stability of GoSPI increased due to HIU.
-
•
HIU decreased the bulk density and particle diameter of GoSPI.
1. Introduction
Human population growth, increasing protein demand, limited availability of protein-rich raw materials, and the safety of animal-based proteins pose challenges for protein supplies that can be overcome through the application of alternative sources of dietary protein [[1], [2], [3], [4]]. Thus, the production of proteins derived from agro-industrial wastes, including peels, seeds, and oilseed cakes, is an alternative that also minimizes food waste and environmental pollution [[5], [6], [7]]. Gourd is an important cucurbit vegetable crop worldwide [8], with a production of 22.8 million tons [9]. During the processing of gourd seed oil, a large quantity of pressed cake is generated, which is considered a byproduct that is principally used for feed or discarded [10,11]. However, this byproduct represents a potential protein source due to its crude protein concentration of approximately 45–65 % and could be used as a raw material for protein isolate (PI) or protein concentrate (PC) production [2,3].
Structural modifications improve the functionality of proteins, promoting and increasing the use of these polymers in the food industry [[12], [13], [14]]. These alterations in proteins have been achieved through physical, chemical and biological treatments [13,15]. The principal physical technologies for protein alteration include microwaves, ultrasonication, high isostatic pressure, cold plasma, and pulsed electric field exposure [16]. Among these methods, ultrasonication stands out as an emerging nonthermal, green, cost-effective, rapid, efficient, and reliable technology [[16], [17], [18], [19], [20], [21]]. The term “ultrasound” refers to acoustic waves with frequencies exceeding 20 kHz [22].
High-intensity ultrasound (HIU) or power ultrasound (20–100 kHz, 10–1000 W/cm2) [23] has been utilized for the physicochemical alteration of food proteins [24]. HIU wave application modifies the structural conformation and functionality of proteins in solution owing to acoustic cavitation [16]. This phenomenon refers to the cycle of the growth and collapse of microbubbles [25], which results in high temperatures (∼5000 K) and extreme pressure (∼1000 bar) [26], producing several physical and chemical effects [19]. Physical perturbations, namely, microjets, turbulence, shock waves, and shear forces [23], can cause protein folding and unfolding, with important effects on protein‒protein and protein‒water relationships that, in turn, modify the functional characteristics of polymers [16].
HIU has been used to change or improve the functional characteristics of different proteins [16,17]. For instance, HIU treatment was shown to modify the physicochemical and functional characteristics of PIs from orange [27], guamuchil [28], tamarind [29], bitter melon [30], album [31], and quinoa [32] seeds. Although there are studies on the modification of proteins from gourd seeds [11,17,33], there are no reports on the alteration of Cucurbita argyrosperma Huber seed protein using HIU. Consequently, the two main objectives of this research were to produce a protein isolate from gourd (C. argyrosperma Huber) seeds and to measure the impact of HIU on its physicochemical and functional characteristics.
2. Materials and methods
2.1. Materials
In a market in Tepic, Nayarit, Mexico, a 15 kg batch of shelled gourd seeds (C. argyrosperma Huber) was purchased; these seeds were subsequently transferred to the Centro de Tecnología de Alimentos of the Universidad Autónoma de Nayarit and stored for subsequent use. All the chemicals and reagents utilized were of analytical grade and were acquired from Sigma‒Aldrich and J. T. Baker (Mexico City, Mexico).
2.2. Preparation of oil-free gourd seed flour
Shelled gourd seeds were ground in a Nutribullet food processor to obtain gourd seed flour (GoSF). GoSF solutions were then prepared according to a method reported by Ma et al. [34], with minor modifications. Briefly, GoSF was added to ethyl ether (1:10 w/v) and mixed using magnetic agitation for 1 h. After sedimentation, the fat solvent was removed by decanting, and partially oil-free flour was obtained. Fresh solvent was then added to the partially oil-free flour, and the defatting process was continued for 1 h; this process was repeated five times. Oil-free gourd seed flour (OGoSF) was spread on a thin layer on Kraft paper under an extraction hood for 12 h. The OGoSF was stored in sealed containers until use. The concentrations of crude protein (N*6.25), moisture, ash, ether extract, and nitrogen-free extracts of GoSF and OGoSF were determined to be 40.33 ± 0.18 and 69.00 ± 0.06 %, 4.43 ± 0.07 and 7.25 ± 0.05 %, 4.15 ± 0.00 and 7.45 ± 0.01 %, 48.80 ± 0.07 and 1.41 ± 0.09 %, and 2.29 ± 0.23 and 14.90 ± 0.19 %, respectively, in accordance with the American Association of Official Analytical Chemists (AOAC) standard procedures [35].
2.3. Protein extraction from oil-free gourd seed flour
A study was carried out to determine the pH for the maximum and minimum extraction of OGoSF protein and to define the conditions for preparing the gourd seed protein isolate (GoSPI) by alkaline extraction and isoelectric precipitation. OGoSF samples were dispersed in distilled water (1:30 w/v). Then, the pH of the dispersions was adjusted to values of 1–12 using 1 N HCl or 1 N NaOH with continuous magnetic stirring at 525 rpm. The dispersions were then centrifuged at 2875×g for 30 min. The protein content of the supernatants was quantified by the macro-Kjeldahl method [35]. The yield of the protein extract was calculated with Eq. (1).
| (1) |
2.4. Preparation of gourd seed protein isolate
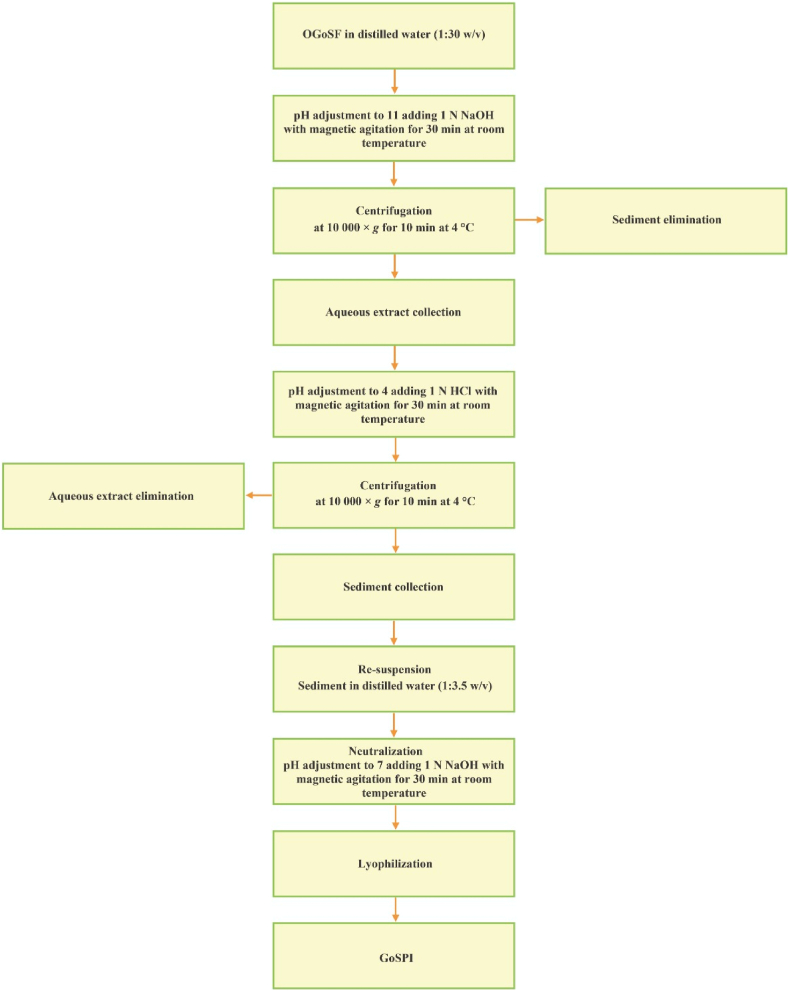
The GoSPI was obtained from OGoSF using the technique described by Flores-Jiménez et al. [28], with minor modifications (Fig. 1). The dispersion of OGoSF in distilled water (1:30 w/v) was adjusted to the pH for the maximum protein extraction (11) with 1 N NaOH with magnetic stirring for 30 min. Subsequently, the dispersion was centrifuged at 10 000×g for 10 min at 4 °C to obtain an aqueous extract, which was acidified with 1 N HCl to the pH of the minimum protein extraction (4) or isoelectric point (pI) using magnetic shaking for 30 min. The sediment was then separated from the aqueous extract by centrifugation under previously described conditions. Immediately, the sediment was resuspended in distilled water (1:3.5 w/v), and the pH of the dispersion was adjusted to 7 with 1 N NaOH using magnetic stirring for 30 min before being subjected to HIU treatment.
Fig. 1.
Schematic diagram of the process of preparing gourd seed protein isolate (GoSPI) from oil-free gourd seed flour (OGoSF).
2.5. High-intensity ultrasound treatment
The HIU treatments were applied to the sediment obtained according to a method reported by Resendiz-Vazquez et al. [36], with minor modifications. The dissolved proteins were exposed to acoustic intensities of 41 W/cm2, 61 W/cm2, and 113 W/cm2 at various ultrasound powers (200, 400, and 600 W, respectively) at frequencies of 20 kHz for 15 and 30 min (pulse duration, 5 s; pulse interval, 1 s) using a Cole-Parmer ultrasonic homogenizer (model CPX-750; Vernon Hills, Illinois, USA) with a titanium probe, which was submerged 3 cm in the solutions in a 2 L glass beaker. A cool water bath was utilized to maintain the temperature of the ultrasonicated solution between 17 and 25 °C. Additionally, a control without HIU treatment was used. The treatments used were as follows: HIU-0, HIU-200/15, HIU-200/30, HIU-400/15, HIU-400/30, HIU-600/15 and HIU-600/30. All protein solutions were dried using a 12-Liter FreeZone console freeze dryer (Labconco, USA) and stored at −4.5 °C for later characterization.
2.6. Chemical constitution and physicochemical characteristics
2.6.1. Chemical constitution
The concentrations of crude protein (N*6.25), moisture, ether extract, and ash were determined following the standard procedures of the AOAC [35]. Moreover, the nitrogen-free extract concentration was calculated by subtracting the percentages of the other constituents from 100 %.
2.6.2. Color
The color characteristics of the samples were estimated with a CR-300 Chroma meter (Konica Minolta Co., Ltd., Tokyo, Japan). The values obtained are expressed in CIE-Lab coordinates, where L* indicates light/black (100/0), a* represents red/green (+a*/−a*), and b* designates yellow/blue (−b*/+b *); prior to measurement, the colorimeter was standardized (L*stand = 94.11, a* stand = 0.00, b* stand = 4.25). The hue angle (h°) was also determined, where 0, 90, 180 and 270° correspond to red, yellow, green and blue tones, respectively. The h° was calculated with Eq. (2) [37].
| (2) |
2.6.3. Available water
Available water (aw) was determined on an AquaLab 4 TE (Decagon Devices, Inc., Pullman, WA, USA).
2.6.4. Bulk density
The apparent density (ρb) was measured following the procedure of Abdollahi & Undeland [38]. A preweighed graduated cylinder was filled with sample to the 10 mL line. The cylinder weight was determined and used to calculate ρb (g/mL).
2.6.5. Protein fractionation
The protein fractions (albumins, globulins, prolamins and glutelins) of GoSF, OGoSF and GoSPI were obtained according to their solvability using the Osborne procedure [39] described by Kumar et al. [40], with minor modifications. Albumins were extracted by dispersing 0.5 g of sample (1:20 w/v) in distilled water and shaking for 1 h. Later, the protein suspension was centrifuged at 10 000×g for 20 min at 4 °C. The aqueous extract containing the albumin fraction was collected. The resulting sediment was used to obtain the subsequent fractions. The previous methodology was followed for the sequential recovery of globulins, prolamins and glutelins using 0.5 M NaCl, 70 % ethyl alcohol and 0.1 N NaOH as solvents, respectively. The macro-Kjeldahl method [35] was used for determination of the protein concentration in the recovered fractions.
2.6.6. Scanning electron microscopy
The surface morphology features of the GoSPI samples were observed with a Mini-SEM (SNE-3200 M, SEC Co., Ltd., Suwon, Korea) at an accelerating voltage of 20 kV and a magnification of 400 × . Prior to scanning electron microscopy (SEM) analysis, the samples were placed in a sputter coating device (MCM-100) and coated with a fine layer of gold.
2.6.7. Particle diameter
GoSPI samples were diluted in 0.01 M phosphate buffer (PB) at pH 9 to a protein concentration of 0.2 mg/mL. The particle diameter (Pd) was calculated on a Zetasizer Nano ZS (ZEN3600, Malvern Instruments Ltd., United Kingdom). The refractive index was set at 1.33.
2.7. Functional characteristics
2.7.1. Solvability
Solvability (So) was studied as reported by Resendiz-Vazquez et al. [36]. Sixty milligrams of GoSPI was dispersed in 40 mL of 0.01 M PB at pH 9. The solution was mixed for 1 h and subsequently centrifuged at 3430×g for 40 min at 17 °C. The protein concentration of the aqueous extract was estimated according to the Bradford technique [41], with bovine serum albumin (BSA) serving as the standard protein. Briefly, 0.1 mL of aqueous solution was gently mixed with 3 mL of Bradford reagent for 5 s. The samples were incubated for 10 min at room temperature, and the absorbance was read at 595 nm (A595). The net absorbance was plotted against the concentration of the standard protein. The So (mg/mL) was determined by comparing the net A595 values with the standard curve.
2.7.2. Water and oil retention
The water retention capacity (WRCa) and oil retention capacity (ORCa) of GoSPI were evaluated according to the procedures of Li et al. [42], with minor modifications. In a preweighed 50 mL conical tube, 0.5 g of GoSPI in 10 mL of distilled water or canola oil was shaken using a vortex shaker (IKA, Genius 3, Germany) for 30 s. The suspensions were allowed to rest for 30 min and subsequently centrifuged at 5000×g for 20 min. The aqueous or oil extract was subsequently decanted, and the sediment was weighed. WRCa and ORCa are expressed as grams of distilled water or oil retained per gram (g/g) of GoSPI.
2.7.3. Gelation
The minimum gelation concentration (MGCo) was measured according to the procedure defined by Mohan & Mellem [43]. GoSPI suspensions containing 0.20, 0.40, 0.60, 0.70, 0.80 and 1.00 g per 5 mL of distilled water were prepared in 15 mL graduated tubes. The tubes were placed in a water bath set at 100 °C for 1 h, after which the reaction was quenched with fresh water. Finally, the tubes were refrigerated at 4.5 °C for 2 h. The MGCo concentration was recorded as the lowest concentration at which the gel did not slide once the tube was inverted.
2.7.4. Foamability
The foaming characteristics were assessed by the technique of Zhao et al. [44] with minor modifications. Thirty milliliters of GoSPI dissolved in 0.01 M PB (1 % w/v, pH 9) was mixed using a high-speed homogenizer (Ultra-Turrax T25, IKA, Germany) at 10 000 rpm for 1 min. The content was subsequently added to a 100 mL graduated tube, after which the volume of the foam was recorded. The foaming capacity (FCa) and foam stability (FSt) were calculated with Eqs. (3) and (4), respectively, where V0 represents the foam volume at 0 min and Vt represents the foam volume 20 min after homogenization.
| (3) |
| (4) |
2.7.5. Emulsification
The emulsifying activity index (EAIn) and emulsion stability index (ESIn) of GoSPI were determined according to the methods of Lei et al. [45], with minor modifications. Soybean oil (4 mL) was added to 16 mL of GoSPI solution in 0.01 M PB (0.1 %, pH 9) and subsequently homogenized with a high-speed homogenizer at 12 000 rpm for 1 min. At 0 and 10 min after homogenization, 50 μL of the resulting emulsion was collected with a micropipette from the graduated cylinder and subsequently diluted in 5 mL of sodium dodecyl sulfate (SDS, 0.1 % w/v). The absorbance of the diluted emulsion was read at 500 nm against a white 0.1 % SDS solution with a UV–Vis spectrophotometer (FI-01620, Thermo Fisher Scientific, Vantaa, Finland). The EAIn and ESIn values were calculated using the following equations:
| (5) |
| (6) |
where A0 and A10 represent the absorbance of the diluted emulsion immediately after and 10 min after homogenization, respectively, C (g/mL) is the protein concentration before homogenization, and Φ (0.20) is the fraction of the oil volume (v/v) in the emulsion.
2.8. Molecular and structural characterization
2.8.1. Polyacrylamide gel electrophoresis with sodium dodecyl sulfate
The protein fractions of the GoSPIs were separated and identified by polyacrylamide gel electrophoresis with sodium dodecyl sulfate (SDS‒PAGE) according to the methods of Laemmli [46] using a Mini-Protean Tetra vertical electrophoresis cell (Bio-Rad Laboratories, Inc., USA). The samples were analyzed in gels prepared with 4 and 12 % w/v acrylamide (stacking and separation, respectively) under reducing conditions (with β-mercaptoethanol) and nonreducing conditions (without β-mercaptoethanol). Twenty micrograms of protein was loaded into each gel lane, including the lane for the 10–250 kDa molecular weight markers (Precision Plus Protein Dual Xtra, Bio-Rad Laboratories Inc., USA). Electrophoresis was performed first at 140 V for 10 min and then at 110 V for 40 min. Coomassie brilliant blue G-250 was used to stain the gels for 24 h. Finally, the gels were decolorized until the bands could be identified and scanned with GelAnalyzer Version 23.1 software.
2.8.2. Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectra of the GoSPI samples were obtained in the wavenumber range of 4000 cm−1 to 650 cm−1 using a spectrometer (Cary 630 FTIR Agilent Technologies, Inc., Santa Clara, CA, USA) at room temperature. Each sample was analyzed in triplicate and then averaged over a spectrum. Data transformation, deconvolution and peak separation analysis of the amide I band (1700 cm−1 to 1600 cm−1) were performed with OriginPro 9.0 software (OriginLab Corporation, Northampton, MA, USA). The secondary structures were determined from the sum of the relative areas of the peaks centered at 1610-1642 cm−1, 1643-1650 cm−1, 1650-1659 cm−1, and 1660-1699 cm−1, which are attributable to β-sheet, random coil, α-helix, and β-turn structures, respectively [47].
2.9. Statistical analysis
The results are presented as the mean ± standard deviation (SD). The data generated in triplicate were subjected to one-way analysis of variance (ANOVA) performed with InfoStat software (version 2020; FCA, Universidad Nacional de Córdoba, Argentina). The Tukey test (p < 0.05) was used to determine the statistical significance of differences among the analyzed groups. Graphs and figures were made using Microsoft Office programs, and an online converter was used to convert them to JPG format.
3. Results and discussion
3.1. Protein extraction from oil-free gourd seed flour
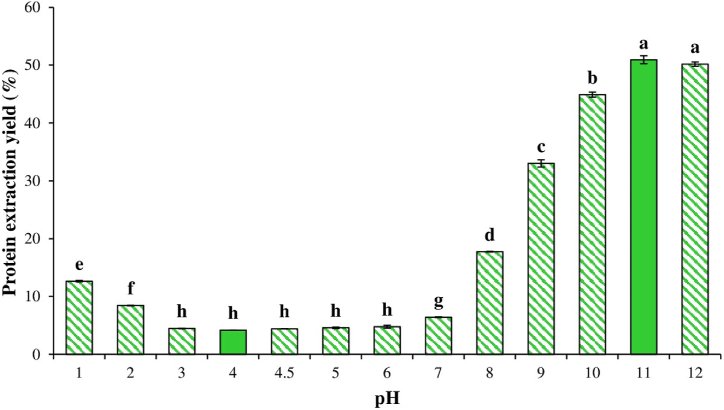
The influence of pH on the protein extraction of OGoSF is illustrated in Fig. 2. The maximal protein extraction (50.93 %) occurred at pH 11, while the minimal protein extraction (4.25 %) occurred at pH 4, corresponding to the pI. Therefore, these conditions were chosen for preparation of GoSPI by alkaline extraction with isoelectric precipitation. Generally, plant protein has a maximum extraction at an alkaline pH and a minimum extraction at pH 4–5, although values may vary depending on the source [28]. For example, the maximal protein extraction from orange [27], noni [48], guamuchil [28], and mango [49] oil-free seed flours was 90.5 % at pH 12, 90.8 % at pH 12, 66.3 % at pH 12, and 53.4 % at pH 11, respectively, while the minimal protein extraction was 30.7 % at pH 4.5, 13 % at pH 3, 30.7 % at pH 4.0, and 6.9 % at pH 5, respectively. Rezig et al. [50] reported that protein extraction from oil-free Cucurbita maxima seed flour was very low at pH < 5 but importantly increased at a pH above 6. The study also demonstrated that higher protein extraction yields can be attained at pH > 10 [50], which was similar to the results of our research.
Fig. 2.
Impact of pH on protein extraction yield from oil-free gourd seed flour. The different letters above the bars indicate significant differences (p < 0.05).
3.2. Chemical constitution and physicochemical characteristics
3.2.1. Chemical constitution
The impact of HIU on the chemical constitution of GoSPI is shown in Table 1. In contrast to what has been published in other works, ultrasonication significantly increased the crude protein concentration of GoSPI [27,28,51], except for in the HIU-400/15-treated GoSPI, in which the crude protein concentration was significantly equal (p < 0.05) to that in the HIU-0-treated GoSPI. The increase in crude protein concentration in HIU-treated GoSPI reached 4.10 % for the HIU-600/30 treatment group and at least 0.62 % for HIU-200/30 treatment group. The increase in crude protein content could be due to the structural change produced by HIU, which causes a reduction in moisture content with a consequent increase in the concentration of said polymers [52].
Table 1.
Impact of high-intensity ultrasound on the chemical constitution of gourd seed protein isolate.
| Constituents (%) | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| HIU-0 | HIU-200/15 | HIU-200/30 | HIU-400/15 | HIU-400/30 | HIU-600/15 | HIU-600/30 | |
| Crude protein | 91.67 ± 0.19e | 92.65 ± 0.16d | 92.24 ± 0.12d | 91.56 ± 0.17e | 93.21 ± 0.20c | 94.75 ± 0.17b | 95.43 ± 0.18a |
| Moisture | 2.99 ± 0.09bc | 2.61 ± 0.11d | 3.28 ± 0.04b | 4.03 ± 0.16a | 2.74 ± 0.08cd | 0.67 ± 0.08e | 0.57 ± 0.18e |
| Ash | 3.05 ± 0.05bc | 2.93 ± 0.10c | 2.66 ± 0.11d | 2.01 ± 0.02e | 3.24 ± 0.10b | 3.46 ± 0.01a | 3.04 ± 0.02c |
| Ether extract | 0.82 ± 0.11a | 0.89 ± 0.02a | 0.63 ± 0.09ab | 0.52 ± 0.07b | 0.71 ± 0.15ab | 0.83 ± 0.04a | 0.78 ± 0.14ab |
| Nitrogen-free extract* | 1.48 ± 0.04b | 0.92 ± 0.03d | 1.19 ± 0.13c | 1.88 ± 0.10a | 0.11 ± 0.03e | 0.29 ± 0.09e | 0.19 ± 0.12e |
* Calculated by the difference. The results are shown as the mean (n = 3) ± SD. Different letters within the same row indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
The crude protein concentration of GoSPI in this study (91.67 ± 0.19 %) was greater than that of the PIs from C. moschata Duch. seeds (88.96 %) [17], C. pepo seeds (86.07 %) [53] and gourd seeds (89.20 %) [11] but lower than that obtained for PIs from C. moschata seeds var. Kashi Harit (92.59 %) [3], C. pepo seeds (94.3 %) [10], and C. pepo L. seeds (94.04 %) [33].
With respect to moisture, ash, ether extract, and nitrogen-free extract concentrations, some HIU-treated GoSPIs exhibited decreases compared to those of HIU-0-treated GoSPI. The moisture concentration significantly decreased by 12.71, 77.60 and 80.94 % in the HIU-200/15, HIU-600/15 and HIU-600/30 treatment groups, respectively, compared to that in the HIU-0 treatment group (p < 0.05). The decrease in moisture concentration with the application of HIU may be associated with structural alterations in macromolecules, which promote greater water loss during freeze-drying [27]. The ash concentration was significantly lower (p < 0.05) in the HIU-200/30 and HIU-400/15 treated GoSPIs (12.79 and 34.10 %, respectively) than in the HIU-0-treated GoSPI. Compared to the HIU-0 treatment, the HIU-400/15 treatment significantly (p < 0.05) decreased the amount of ether extract by 36.59 %. Compared to that in the HIU-0-treated GoSPI, the nitrogen-free extract concentration was significantly (p < 0.05) reduced by 19.59, 37.84, 80.41, 87.16, and 92.57 % in the HIU-200/30-, HIU-200/15-, HIU-600/15-, HIU-600/30-, and HIU-400/30-treated GoSPIs, respectively. In research performed with PIs from guamuchil [28] and passion fruit [54] seeds, the application of HIU promoted modifications of the determined components, which may depend on the conditions of HIU exposure and the nature of the treated protein [27].
3.2.2. Color
Color influences the perception of food quality [37]. The impacts of HIU on the L*, a*, b*, and h° of GoSPI are presented in Table 2. The L* value increased significantly (p < 0.05) in the HIU-treated GoSPI. Among all the treatments, HIU-400/15 treatment induced the greatest increase in L* (7.70 % compared to that in the HIU-0 treatment). Previous studies reported an increase in L* values in PIs from album seeds [31] and gourd seeds [17] treated with HIU, which was similar to the results outlined in the present study.
Table 2.
Impact of high-intensity ultrasound on physicochemical characteristics of gourd seed protein isolate.
| Parameters | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| HIU-0 | HIU-200/15 | HIU-200/30 | HIU-400/15 | HIU-400/30 | HIU-600/15 | HIU-600/30 | |
| L* | 73.67 ± 0.04f | 77.66 ± 0.06b | 77.82 ± 0.13b | 79.34 ± 0.01a | 75.69 ± 0.05d | 74.90 ± 0.05e | 76.07 ± 0.06c |
| a* | 0.96 ± 0.08c | 0.18 ± 0.04f | 0.38 ± 0.01e | 0.95 ± 0.01c | 0.62 ± 0.03d | 1.21 ± 0.05b | 1.38 ± 0.01a |
| b* | 18.91 ± 0.04b | 16.43 ± 0.07e | 15.33 ± 0.15g | 15.54 ± 0.02f | 17.40 ± 0.01d | 19.29 ± 0.04a | 18.44 ± 0.02c |
| h° | 87.07 ± 0.22d | 89.39 ± 0.15a | 88.58 ± 0.03b | 86.50 ± 0.05e | 87.95 ± 0.09c | 86.40 ± 0.13e | 85.70 ± 0.03f |
| aw | 0.31 ± 0.00c | 0.33 ± 0.00b | 0.29 ± 0.00d | 0.35 ± 0.01a | 0.24 ± 0.01e | 0.12 ± 0.01f | 0.12 ± 0.01f |
| ρb (g/mL) | 0.38 ± 0.00a | 0.32 ± 0.00b | 0.30 ± 0.00c | 0.27 ± 0.01d | 0.18 ± 0.00e | 0.32 ± 0.00b | 0.29 ± 0.00c |
L* (light/black), a* (red/green), b* (yellow/blue), h° (hue angle), aw (available water), and ρb (bulk density). The results are shown as the mean (n = 3) ± SD. Different letters within the same row indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
For a*, the HIU-400/30, HIU-200/30, and HIU-200/15 treatments induced significant decreases (p < 0.05) of 35.42, 60.42, and 81.25 %, respectively, while the HIU-600/15 and HIU-600/30 treatments induced significant increases (p < 0.05) of 26.04 and 43.75 %, respectively, in comparison with the HIU-0 treatment. b* was significantly lower (p < 0.05) in all HIU-treated GoSPIs, except for in the HIU-600/15-treated GoSPIs, in which this value increased by 2 % with respect to that of HIU-0-treated GoSPIs. The greatest decrease in b* of 18.93 % occurred in the HIU-200/30-treated GoSPI. The GoSPI h° values in this study were less than 90, indicating that the apparent color was reddish yellow in all the treatments (Fig. 3).
Fig. 3.
Impact of high-intensity ultrasound on color of gourd seed protein isolate. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
The values of the GoSPI color parameters (L* = 73.67 ± 0.04, a* = 0.96 ± 0.08, b* = 18.91 ± 0.04, h° = 87.07 ± 0.22) were comparable to those published by Das et al. [53] for a PI from C. pepo seeds (L* = 72.47, a* = 0.40, b* = 20.20, h° = 88.86). Modifications in color coordinates due to the application of HIU have been observed for proteins from sunflower flour [55] and date palm pollen PCs [56], as well as for PIs from canola [51], gourd seeds [17], and guamuchil seeds [28]. Changes in color caused by HIU could be due to pigment degradation at high temperatures, which affects light absorption depending on the powder and exposure time [28].
In an alkaline environment, protein solutions acquire a dark color due to the oxidation of polyphenolic compounds, and when proteins sediment at their pI, the color cannot be removed from the PI [57]. Dark color is a factor that limits the use of a PI as a functional food component [58]. However, HIU can improve the color of products [59], as observed in this study.
3.2.3. Available water
The aw is a determining factor in food spoilage [60]. This factor is a measure of the amount of available water in a food item [61]. HIU treatment significantly (p < 0.05) decreased the aw, except for the HIU-200/15 and HIU-400/15 treatments. Compared to the value in the HIU-0 treatment group, the greatest reductions in aw (61.29 %) were observed for the HIU-600/15 and HIU-600/30 treatment groups. In general, HIU application reduces the aw value [62]. This difference is probably due to water loss during ultrasonic treatment through heat and mass transfer [63,64].
The aw GoSPI values were within the range of 0.12 ± 0.01 to 0.35 ± 0.01, and aw levels less than 0.60 were considered to indicate inhibited microbial growth [65]. Similar results were reported for PIs from safflower [66], canola [51] and guamuchil [28] seeds, which ranged from 0.286 to 0.341, 0.12–0.24, and 0.167–0.209, respectively.
3.2.4. Bulk density
The density of protein powder varies depending on the primary matter, the production process and the additional procedures applied [28]. Moreover, ρb is an important parameter in PI packaging [38] and depends on many factors, including the size of the particles, number of contact points, attractive forces between particles, concentration of moisture and shape of the particles [55]. Moreover, ρb decreased significantly (p < 0.05) with HIU exposure, from 0.38 ± 0.00 g/mL for HIU-0-treated GoSPIs to 0.18 ± 0.00–0.32 ± 0.00 g/mL for the HIU-treated GoSPIs. Compared to that in the HIU-0-treated GoSPI, the largest reduction in ρb (52.63 %) was observed in the HIU-400/30-treated GoSPI (Table 2). According to Zúñiga-Salcedo et al. [66], the decrease in ρb occurs because the HIU-treated and freeze-dried samples present larger and more heterogeneous structures than the untreated samples. The ρb of GoSPI was considerably lower than that of PIs from safflower (0.61 g/mL) [66], gourd seeds (0.61 g/mL) [53], and guamuchil seeds (0.408 g/mL) [28] but greater than that of the PI from canola flour (0.26 g/mL) [51].
3.2.5. Protein fractionation
The Osborne fractionation method categorizes proteins based on their So in different media [27]. GoSF and OGoSF demonstrated the following fractional compositions, respectively: glutelins, 67.44 ± 0.59 and 65.60 ± 0.29 %; albumins, 13.25 ± 0.35 and 13.40 ± 0.31 %; prolamins, 13.49 ± 0.56 and 12.50 ± 0.28 %; and globulins, 5.82 ± 0.45 and 8.51 ± 0.31 %. The protein fraction composition of OGoSF exhibited notable variation (p < 0.05) due to the GoSPI preparation process (Table 3). The majority of proteins in the GoSPI were glutelins (76.18 ± 0.15 %), followed by albumins (21.18 ± 0.11 %), prolamins (1.56 ± 0.11 %), and globulins (1.08 ± 0.01 %). Glutelins (in the alkaline fraction) also constitute the predominant fraction of C. maxima seed protein (42.1 %) [50], and that in the PI from C. moschata seeds is 45.82 % [3]. Moreover, glutelins and albumins were the main protein fractions produced from canola (glutelins 57.18 %, albumins 23.09 %) [51], guamuchil seeds (glutelins 61.10 %, albumins 26.92 %) [28], and noni seeds (glutelins 64.62 %, albumins 24.12 %) [48].
Table 3.
Fractionation of proteins from gourd seed flour (GoSF), oil-free gourd seed flour (OGoSF), and gourd seed protein isolate (GoSPI).
| Fraction (%) | GoSF | OGoSF | GoSPI |
|---|---|---|---|
| Albumins | 13.25 ± 0.35b | 13.40 ± 0.31b | 21.18 ± 0.11a |
| Globulins | 5.82 ± 0.45b | 8.51 ± 0.31a | 1.08 ± 0.01c |
| Prolamines | 13.49 ± 0.56a | 12.50 ± 0.28b | 1.56 ± 0.11c |
| Glutelins | 67.44 ± 0.59b | 65.60 ± 0.29c | 76.18 ± 0.15a |
The results are shown as the mean (n = 3) ± SD. Different letters within the same row indicate significant differences (p < 0.05) among the groups.
3.2.6. Scanning electron microscopy
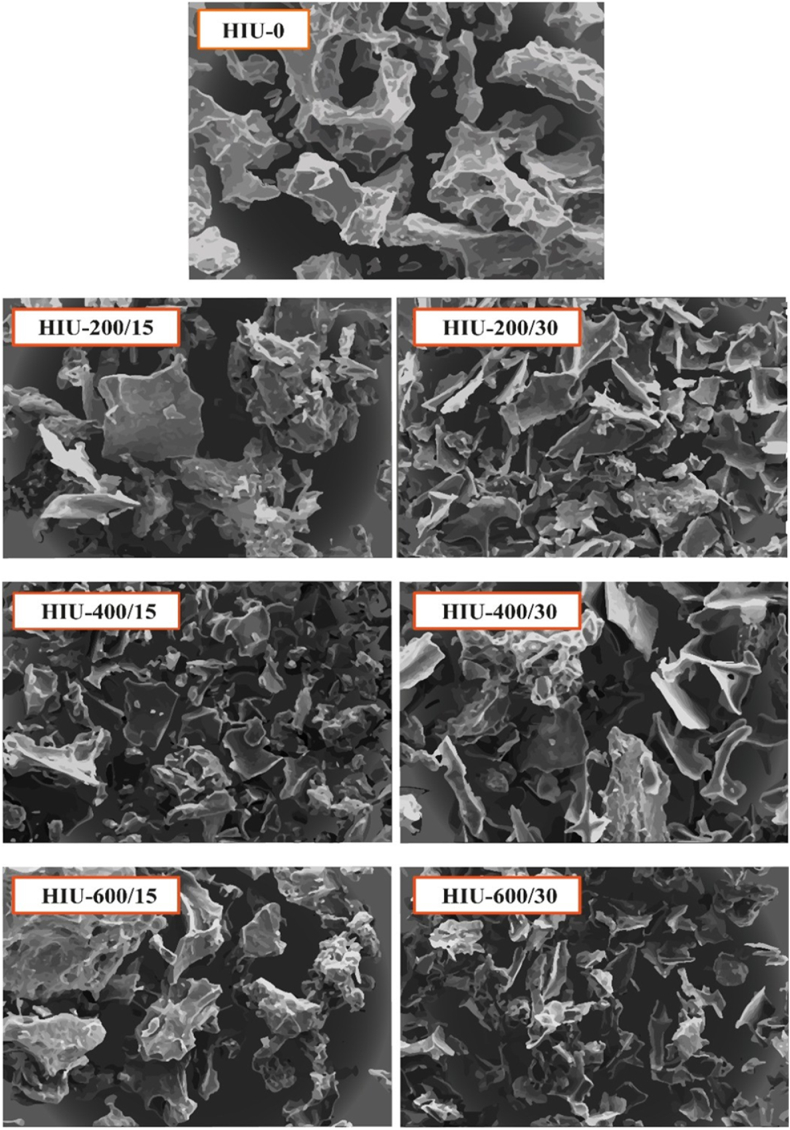
SEM images obtained at a magnification of 400 × showed the impact of HIU on the surface morphology of GoSPI (Fig. 4). The HIU-0-treated GoSPI had a larger and more compact structure with a rough surface than HIU-treated GoSPIs. After treatment with HIU, the protein aggregates fragmented into small and irregular particles with flat, smooth and sharp shapes. In HIU-400/15-treated GoSPI, the formation of smaller and more regular pieces with a more uniform distribution was observed. Such modifications in the molecular structure could be due to the shear, impact, and cavitation forces produced by HIU [67]. Other studies on PIs from pea [20], perilla seeds [4], tiger nut seeds [67] and gourd seeds [17] have shown the decomposition of protein aggregates into smaller aggregates.
Fig. 4.
Impact of high-intensity ultrasound on the surface morphology of gourd seed protein isolates at 400 × magnification. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
3.2.7. Particle diameter
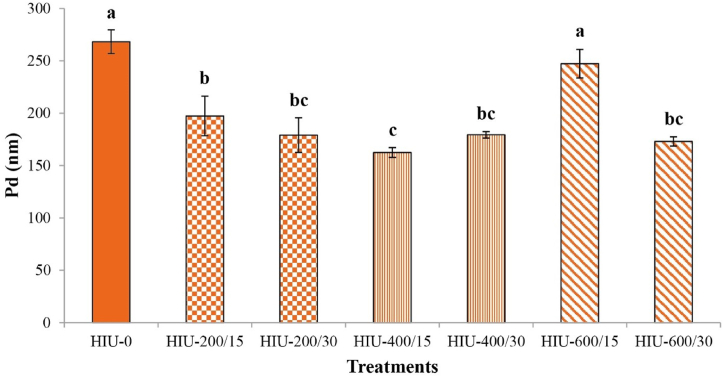
The Pd in a protein solution can affect several functional characteristics of proteins, such as solvability, foamability, emulsification and gelation [14,68,69]. The impact of HIU on the Pd of GoSPI is presented in Fig. 5. The HIU treatment reduced the Pd, except in HIU-600/15-treated GoSPI, in which the Pd was not significantly different (p < 0.05) from that in HIU-0-treated GoSPI. SEM confirmed these data (see Fig. 4). The greatest decrease in Pd (from 268.33 ± 11.31 to 162.47 ± 4.77 nm) was detected in the HIU-400/15-treated GoSPI. The decrease in Pd has been ascribed to the unfolding and deterioration of the aggregates by the strong impact of cavitation and high shear energy ultrasonic waves [69]. Studies of PIs from pea [20], walnut [70], perilla seeds [4], potato [69], guamuchil seeds [28], sunhemp seeds [71], and sesame seeds [72] demonstrated a decrease in Pd after HIU application.
Fig. 5.
Impact of high-intensity ultrasound on the particle diameter (Pd) of gourd seed protein isolates. Different letters above the bars indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time (mean ± SD, n = 3).
3.3. Functional characteristics
3.3.1. Solvability
The parameter So can be an index of other important functional characteristics of food proteins, such as emulsification, foamability, and gelation. In addition, flavor, aroma, color and consistency are important characteristics related to food products [73].
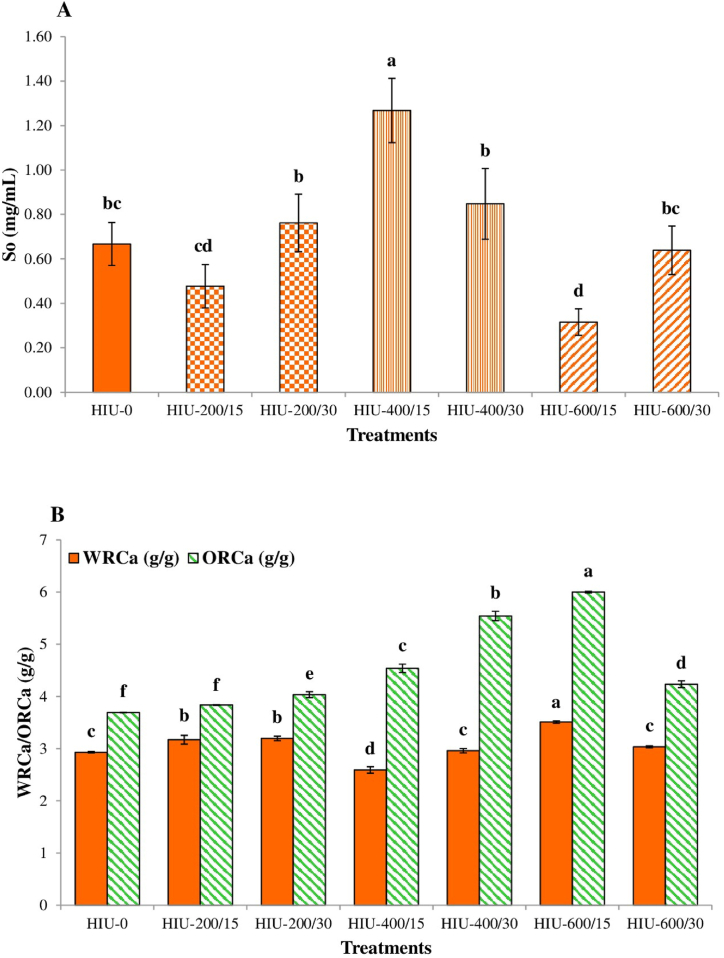
Fig. 6A shows the effect of HIU on the So of GoSPI. The behavior of So depended on the power and time of exposure to ultrasound. However, the So increased significantly (p < 0.05) in the HIU-400/15-treated GoSPI (90.10 %) compared with the HIU-0-treated GoSPI. A significant reduction (p < 0.05) in So was observed in HIU-600/15-treated GoSPI (52.62 %) compared to HIU-0-treated GoSPI, followed by HIU-200/15-treated GoSPI (28.48 %). There was no significant difference (p < 0.05) in the So among the HIU-0, HIU-200/30, HIU-400/30, and HIU-600/30 treatment groups. In this study, the So of GoSPI was inversely proportional to its Pd (see Fig. 5).
Fig. 6.
Impact of high-intensity ultrasound on the (A) solvability (So) and (B) water (WRCa) and oil (ORCa) retention capacities of gourd seed protein isolate. The different letters above the bars indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time (mean ± SD, n = 3).
The decrease in the So of GoSPI could be due to the reformation of insoluble protein aggregates through noncovalent interactions after treatment with HIU [74]. However, an improvement in So could be due to structural variations in the proteins generated by ultrasonic cavitation, a process that results in the exposure of hydrophilic groups on the surface and facilitates protein–water interactions. Furthermore, ultrasonic treatment can promote the breakdown of macromolecular aggregates, thereby releasing smaller protein aggregates and consequently increasing the number of interaction sites between water and protein molecules, leading to increased So [70]. According to certain studies, PIs from plum seeds [74], moringa seeds [75], perilla seeds [4], potato [69], and guamuchil seeds [28] improved upon the application of HIU.
3.3.2. Water and oil retention
The WRCa signifies the capacity of a food to retain its own weight, and water is added when subjected to force, pressing, centrifugation or heating. This parameter is crucial for the texture, mouth feel, and viscosity of many food products, such as sauces, meat products, and baked doughs [29,73,76]. HIU significantly (p < 0.05) enhanced the GoSPI WRCa in the HIU-200/15 (8.19 %), HIU-200/30 (9.22 %), and HIU-600/15 (19.80 %) treatment groups compared to the HIU-0 treatment group (Fig. 6B). In contrast, HIU significantly decreased the WRCa in the HIU-400/15-treated GoSPI (11.60 %). The WRCa values of the HIU-0, HIU-400/30 and HIU-600/30 treatment groups were not significantly different (p < 0.05).
The increase in WRCa may be due to the decrease in Pd and the increase in So caused by HIU treatment [32]. In addition, HIU can cause the dissociation and partial dissolution of protein molecules, which favors their interaction with water, thus enhancing the hydration of the polymers [4]. In contrast, the decrease in WRCa could be due to modifications in the three-dimensional molecular structure of GoSPI and an increase in the number of hydrophobic groups and sites on the surface of the protein, which reduces its interaction with the aqueous medium [32,36,72].
ORCa, the capacity of proteins to bind to oil, influences the flavor retention, shelf life, and emulsifying characteristics of food products [73]. Ingredients with high ORCa values are used in the cold meat industry, mainly in the production of sausages [76]. HIU significantly enhanced (p < 0.05) the ORCa of all GoSPIs treated with HIU, except for the HIU-200/15-treated GoSPI, for which the ORCa was not significantly different (p < 0.05) from that of the HIU-0-treated GoSPI (Fig. 6B). The HIU-600/15 treatment resulted in the greatest increase in ORCa (62.60 %).
The improvement in ORCa of GoSPI could be associated with the unfolding of the polypeptide chains, as well as with conformational modifications in the macromolecular structure, which are generated by the implosion of cavitation microbubbles surrounding the polypeptide due to treatment with HIU. These alterations expose the nonpolar hydrophobic side chains of the amino acids present in the polymer, which enhances the binding of oil molecules to protein molecules [29].
Flores-Jiménez et al. [28] demonstrated that HIU decreased the WRCa in guamuchil seed proteins and increased its ORCa, as was observed for GoSPI in this research. In contrast, Xue et al. [74] reported that HIU increased the WRCa while simultaneously decreasing the ORCa of plum seed protein. On the other hand, studies of PIs from quinoa seeds [32], tamarind seeds [29], perilla seeds [4] and sesame seeds [72] demonstrated that HIU treatment improved the WRCa and ORCa parameters.
3.3.3. Gelation
Proteins that have gelling characteristics are desirable ingredients for the manufacture of foods such as cheeses, yogurts, desserts, meat products, and eggs [21]. MGCo is defined as the minimum concentration of protein necessary to form a gel; thus, the lower the MGCo value is, the greater the gelling capacity [77,78].
Neither HIU-0-treated nor HIU-treated GoSPIs exhibited gelation characteristics. Similarly, Haque et al. [79] reported that the protein in jackfruit seed PI did not produce a gel in water. In this regard, surface hydrophobicity is considered one of the main forces linked to the formation of a protein gel [11]. Nonetheless, ultrasonic technology has been successfully used to improve the gelling characteristics of proteins from soy [80], guamuchil seeds [28], and bitter melon seeds [30].
3.3.4. Foamability
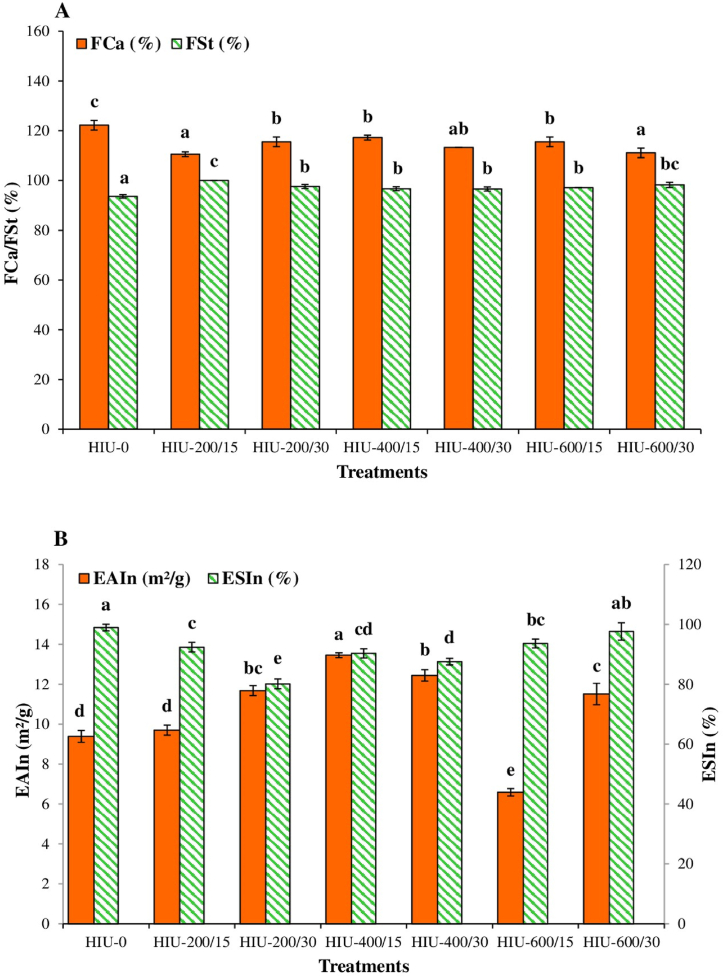
Protein foam is essential for baked products, foamy beverages, and ice cream mixes due to its influence on the glossiness, softness, and smoothness of foods [72,81]. The FCa of a protein is the amount of interfacial area that it can create [82]. Compared with HIU-0-treated GoSPI, all HIU-treated GoSPIs showed a significant reduction (p < 0.05) in FCa. The greatest reduction in FCa (9.49 %) was observed for HIU-200/15-treated GoSPI in comparison with HIU-0-treated GoSPI (Fig. 7A). This finding differs from the results reported for a PI from C. moschata Duch. seeds [17]. One reason for the decrease in FCa could be that HIU modified the protein structure in a process that prevents the molecule from unfolding at the interface, resulting in low surface activity [83]. Similarly, the application of HIU reduced the foaming of egg white protein [84] and soy PI [83].
Fig. 7.
Impact of high-intensity ultrasound on (A) foaming characteristics (FC = foaming capacity; FSt = foam stability) and (B) emulsifying (EAIn = emulsifying activity index; ESIn = emulsifying activity index) characteristics of gourd seed protein isolate. The different letters above the bars indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time (mean ± SD, n = 3).
The FSt refers to the period of time during which the initial characteristics of the foam can be maintained [85]. Unlike its effects on FCa, the HIU process significantly enhanced FSt (p < 0.05) with respect to that observed in the HIU-0-treated GoSPI. Compared with that for the HIU-0-treated GoSPI, the increase in FSt ranged from 3.20 % for the HIU-400/30-treated GoSPI to 6.84 % for the HIU-200/15-treated GoSPI (Fig. 7A). This could be mainly because fewer hydrophobic groups and sites were exposed on the surface during the ultrasonication process, causing a decrease in protein aggregation and resulting in an increase in FSt [17]. Studies of proteins from pea [20], tamarind seeds [29], guamuchil seeds [28], Qingke [86], and sesame seeds [72] have shown that HIU treatment enhances the foaming characteristics of these plants.
3.3.5. Emulsification
Emulsifiers are additives that prevent separation, improve texture, enhance flavors and extend the shelf life of processed foods such as beverages, baked goods, dressing, ice cream, mousse, margarine, mayonnaise and whipped dressing [87,88]. The emulsifying characteristics of proteins correspond to their ability to stabilize oil and water systems [29]. EAIn represents the capacity of proteins to quickly adsorb at an oil–water interface, while ESIn refers to the ability of polymers to maintain the stability of emulsions over a given time period [72].
EAIn was significantly greater (p < 0.05) in the HIU-treated GoSPIs than in the HIU-0-treated GoSPIs, except for in the HIU-200/15- and HIU-600/15-treated GoSPIs (Fig. 7B). Compared with that in the HIU-0-treated GoSPI, a 43.34 % increase in EAIn occurred in the HIU-400/15-treated GoSPI. The improvement in EAIn may be due to a higher concentration of small soluble proteins that tend to be adsorbed at the oil–water interface or due to modifications in the surface chemistry of the polymers caused by HIU that improve their surface activity [70].
Conversely, all HIU-treated GoSPIs experienced a significant (p < 0.05) decrease in ESIn, except for the HIU-600/30-treated GoSPI, for which the ESIn was not significantly different (p < 0.05) from that of the HIU-0-treated GoSPI (Fig. 7B). The decrease in ESIn ranged between 5.39 and 19.01 %, with the highest value being that of the HIU-200/30-treated GoSPI compared to the HIU-0-treated GoSPI. The decrease in ESIn could be due to the reorientation of polymers caused by the HIU process, which limits the stability of the oil–water interface film formed by the polymer [12].
Kamani et al. [12] reported that HIU decreased the ESIn while improving the EAIn of black-gram proteins, which is similar to the results of this work. Other studies have described the enhancement of the emulsifying characteristics of different proteins, including those obtained from walnut [70], tamarind seeds [29], tiger nuts [67], sesame seeds [72], and Qingke [86]. The improvement in the emulsifying characteristics was related to modifications in the structure and surface chemistry of the protein molecules after HIU. HIU induces the partial unfolding of globular proteins, thus exposing additional hydrophobic groups to the surrounding aqueous phase and increasing their surface activity [70].
3.4. Molecular and structural characterization
3.4.1. Polyacrylamide gel electrophoresis with sodium dodecyl sulfate
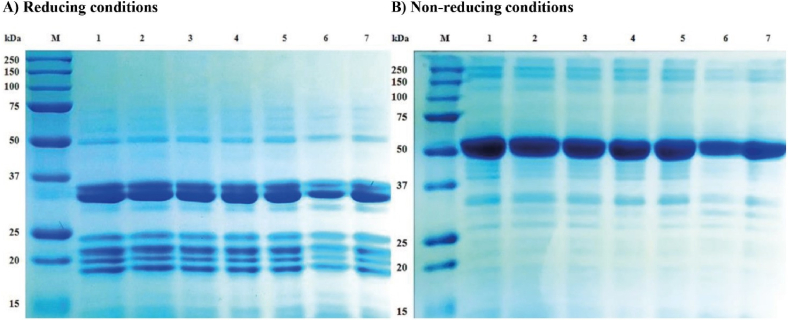
SDS‒PAGE analysis is used to determine the molecular weight of proteins [4]. The impact of HIU on the electrophoretic profile of GoSPI under reducing and nonreducing conditions is shown in Fig. 8 sections A and B, respectively. Under reducing conditions, eight major polypeptides with molecular weights of ∼48, 34, 32, 29, 24, 22, 20, and 19 kDa were detected in both HIU-0-treated and HIU-treated GoSPIs (Fig. 8A). These results were consistent with those obtained via SDS‒PAGE under reducing conditions from alkali-extracted C. maxima seed protein (44.5–17.9 kDa) [50]. Previous studies have shown that the majority fraction of gourd seed protein consists of an 11S globulin known as cucurbitin, a globular hexameric protein with a molecular weight of 54 kDa per subunit. Each subunit consists of a large acidic polypeptide of 33 kDa linked by disulfide bonds to a basic polypeptide of 22 kDa [50], in agreement with the results of this study (48-29 and 24-19 kDa).
Fig. 8.
Electrophoretic profile in A) reducing and B) nonreducing SDS‒PAGE of the gourd seed protein isolate treated with high-intensity ultrasound: Lane M, markers of known molecular weights; Lane 1, HIU-0; Lane 2, HIU-200/15; Lane 3, HIU-200/30; Lane 4, HIU-400/15; Lane 5, HIU-400/30; Lane 6, HIU-600/15; and Lane 7, HIU-600/30. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
SDS‒PAGE under nonreducing conditions revealed that HIU treatment did not induce major changes in the protein electrophoretic profiles of GoSPI. In all treatments, six main protein fractions with molecular weights of ∼245, 185, 48, 32, 29 and 27 kDa were identified (Fig. 8B). According to Li & Xiong [89], HIU primarily disrupts noncovalent interactions and has a minimal effect on disulfide bonds and other intermolecular covalent bonds. After HIU treatment at 600 W, the main protein bands became lighter in color, indicating that the protein content at this molecular weight decreased [17].
Similar findings were reported for seed proteins from tamarind [29], guamuchil [28], apple [90], and pumpkin [17] seed proteins, where no change was detected between the HIU-treated and untreated samples, showing that HIU did not disrupt the peptide bonds and therefore had no impact on the molecular weight of the proteins or the primary structure of the proteins [29,90]. In contrast, Resendiz-Vazquez et al. [36] reported that HIU caused fragmentation of the molecular structure of jackfruit seed protein, which could be attributed to the effects of turbulence and high shear forces generated by ultrasonic cavitation. The differences between the results suggest that ultrasonic protein hydrolysis could depend on the intrinsic properties of the polymers, solution conditions, and ultrasound treatment conditions [91].
3.4.2. Fourier transform infrared spectroscopy
FTIR measures the intensity and wavelength of the absorption of infrared radiation (IR) by a sample [92]. Characteristic absorption regions found in the infrared spectra of proteins and polypeptides include amides A, B, and I-VII [93]. The IR absorption associated with the amide I region leads to stretching vibrations of the C O bond (80 %), stretching vibrations of the N–H bond (10 %), and bending vibrations of the C–N bond (10 %) [93]. Amide I vibrations are sensitive to the content of the secondary structure of a protein because both the C O bond and the N–H bond are involved in the hydrogen bonding that occurs between the different elements of the secondary structure [94].
Table 4 shows content assignments of the secondary structures of the HIU-0-treated and HIU-treated GoSPIs. Compared with those subjected to HIU-0, the secondary structures of the GoSPI samples subjected to HIU were significantly different (p < 0.05). In HIU-treated GoSPIs, β-sheets were the predominant secondary structure, followed by α-helices and β-turns. HIU increased the relative contents of β-sheets and β-turns from initial values of 34.73 ± 0.45 and 14.66 ± 0.03 % (HIU-0) to 52.79 ± 1.56 and 24.19 ± 0.35 % (HIU-200/15 and HIU-600/15), respectively. In contrast, the relative content of α-helices decreased from an initial value of 50.62 ± 0.42 % (HIU-0) to 25.48 ± 0.74 % (HIU-200/15). A random coil structure was not detected in the analysis. The same result was reported by Yan et al. (2021) [95], who reported that HIU reduced the α-helix content and increased the β-sheet content of soy protein isolate. The shear force generated by cavitation breaks intramolecular hydrogen bonds, decreasing the α-helix structure, while the increase in the β-sheet structure is an indication of the exposure of hydrophobic regions that are generally found inside the folded protein [96].
Table 4.
Impact of high intensity ultrasound on secondary structure of gourd seed protein isolate.
| Treatment | Secondary structure (%) |
|||
|---|---|---|---|---|
| β-Sheet | Random coil | α-Helix | β-Turn | |
| HIU-0 | 34.73 ± 0.45d | ND | 50.62 ± 0.42a | 14.66 ± 0.03e |
| HIU-200/15 | 52.79 ± 1.56a | ND | 25.70 ± 0.06e | 21.53 ± 1.62ab |
| HIU-200/30 | 49.42 ± 0.02c | ND | 30.57 ± 0.12c | 20.01 ± 0.09bc |
| HIU-400/15 | 48.74 ± 0.53c | ND | 33.06 ± 0.57b | 18.21 ± 1.08cd |
| HIU-400/30 | 49.68 ± 0.05bc | ND | 27.62 ± 0.65d | 22.78 ± 0.70ab |
| HIU-600/15 | 50.42 ± 1.10 abc | ND | 25.48 ± 0.74e | 24.19 ± 0.35a |
| HIU-600/30 | 52.23 ± 0.63ab | ND | 30.62 ± 0.13c | 17.15 ± 050de |
ND = not detected. The results are shown as the mean (n = 3) ± SD. Different letters within the same row indicate significant differences (p < 0.05) among the groups. HIU-0 is the nonultrasound control treatment. The numbers in the treatment labels correspond to the ultrasonic power/treatment time.
Similarly, other researchers have reported that HIU causes alterations in the secondary structure of proteins in walnut [70], soy [95], guamuchil seeds [28] and mung beans [97]. According to Meng et al. [91], the changes in the secondary structure of proteins treated with HIU might be due to ruptures in the hydrogen bonds involved in maintaining the secondary structure of the polymers. However, Jin et al. [98] suggested that the free radicals, microjets, shear forces, shock waves and turbulence induced by ultrasound disrupt the interactions within local amino acid sequences and between different parts of the protein molecule, altering the content of the secondary structure.
4. Conclusions
HIU treatment modified the physicochemical, functional, and structural characteristics of GoSPI. The ORCa, FSt, and EAIn values of GoSPI increased when HIU was applied, while the So, FCa, ESIn, and ρb values decreased. The WRCa and MGCo values were not improved by HIU. HIU altered the secondary structure of GoSPI by decreasing the content of α-helices and increasing the contents of β-sheets and β-turns. An improvement in the functional characteristics of GoSPI was observed and was due to the impact of HIU cavitation, which caused a decrease in Pd. Therefore, HIU is an effective technology for modifying gourd seed proteins. Additional research on the structural and nutritional characteristics of GoSPI could extend their use in functional food production.
Data availability statement
The data supporting the findings of this research are available in the article. Original/derived data endorsing the findings of this report are obtainable upon request to the corresponding author.
Funding
The Patronato para Administrar el Impuesto Especial Destinado a la Universidad Autónoma de Nayarit provided the funds to carry out this study through grant PU2021/03.
Ethics declarations
Not applicable.
CRediT authorship contribution statement
Yessica Silva Carrillo: Writing – original draft, Methodology, Investigation. José Armando Ulloa: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Data curation, Conceptualization. Judith Esmeralda Urías Silvas: Validation, Supervision, Software, Resources. José Carmen Ramírez Ramírez: Visualization, Supervision, Conceptualization. Ranferi Gutiérrez Leyva: Visualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT) for financial support in the form of an Institutional Scholarship to the first author (CVU: 334476).
References
- 1.Arrutia F., Binner E., Williams P., Waldron K.W. Oilseeds beyond oil: press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020;100:88–102. doi: 10.1016/j.tifs.2020.03.044. [DOI] [Google Scholar]
- 2.Sá A.G.A., Pacheco M.T.B., Moreno Y.M.F., Carciofi B.A.M. Processing effects on the protein quality and functional properties of cold-pressed pumpkin seed meal. Food Res. Int. 2023;169 doi: 10.1016/j.foodres.2023.112876. [DOI] [PubMed] [Google Scholar]
- 3.Vinayashree S., Vasu P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021;340 doi: 10.1016/j.foodchem.2020.128177. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q., Xie T., Hong X., Zhou Y., Fan L., Liu Y., Li J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130848. [DOI] [PubMed] [Google Scholar]
- 5.Görgüç A., Bircan C., Yılmaz F.M. Sesame bran as an unexploited by-product: effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019;283:637–645. doi: 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 6.Pojić M., Mišan A., Tiwari B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018;75:93–104. doi: 10.1016/j.tifs.2018.03.010. [DOI] [Google Scholar]
- 7.Sadh P.K., Duhan S., Duhan J.S. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess. 2018;5:1. doi: 10.1186/s40643-017-0187-z. [DOI] [Google Scholar]
- 8.Amin M.Z., Islam T., Uddin M.R., Uddin M.J., Rahman M.M., Satter M.A. Comparative study on nutrient contents in the different parts of indigenous and hybrid varieties of pumpkin (Cucurbita maxima Linn.) Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FAOSTAT . Food and agriculture organization of the United Nations (FAO); 2022. https://www.fao.org/faostat/es/#data/QCL [Google Scholar]
- 10.Bučko S., Katona J., Popović L., Vaštag Ž., Petrović L., Vučinić–Vasić M. Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. LWT-Food Sci. Technol. 2015;64:609–615. doi: 10.1016/j.lwt.2015.06.054. [DOI] [Google Scholar]
- 11.Zeng L., Wang Z., He Z., Zeng M., Qin F., Chen J. Physicochemical and gel properties of pumpkin seed protein: a comparative study. Int. J. Food Sci. Technol. 2022;58:1639–1651. doi: 10.1111/ijfs.16124. [DOI] [Google Scholar]
- 12.Kamani M.H., Semwal J., Meera M.S. Functional modification of protein extracted from black gram by-product: effect of ultrasonication and micronization techniques. LWT. 2021;144 doi: 10.1016/j.lwt.2021.111193. [DOI] [Google Scholar]
- 13.Nasrabadi M.N., Doost A.S., Mezzenga R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021;118 doi: 10.1016/j.foodhyd.2021.106789. [DOI] [Google Scholar]
- 14.Sengar A.S., Thirunavookarasu N., Choudhary P., Naik M., Surekha A., Sunil C.K., Rawson A. Application of power ultrasound for plant protein extraction, modification and allergen reduction – a review. Appl. Food Res. 2022;2 doi: 10.1016/j.afres.2022.100219. [DOI] [Google Scholar]
- 15.Zha F., Rao J., Chen B. Modification of pulse proteins for improved functionality and flavor profile: a comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021;20:3036–3060. doi: 10.1111/1541-4337.12736. [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Ma H., Wang Y.Y. Recent advances in modified food proteins by high intensity ultrasound for enhancing functionality: potential mechanisms, combination with other methods, equipment innovations and future directions. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H., Zhang J., Wang S., Manyande A., Wang J. Effect of high-intensity ultrasonic treatment on the physicochemical, structural, rheological, behavioral, and foaming properties of pumpkin (Cucurbita moschata Duch.)-seed protein isolates. LWT. 2022;155 doi: 10.1016/j.lwt.2021.112952. [DOI] [Google Scholar]
- 18.Guimarães J.T., Scudino H., Ramos G.L.P.A., Oliveira G.A.R., Margalho L.P., Costa L.E.O., Freitas M.Q., Duarte M.C.K.H., Sant'Ana A.S., Cruz A.G. Current applications of high-intensity ultrasound with microbial inactivation or stimulation purposes in dairy products. Curr. Opin. Food Sci. 2021;42:140–147. doi: 10.1016/j.cofs.2021.06.004. [DOI] [Google Scholar]
- 19.Rahman M.M., Lamsal B.P. Ultrasound-assisted extraction and modification of plant-based proteins: impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021;20:1457–1480. doi: 10.1111/1541-4337.12709. [DOI] [PubMed] [Google Scholar]
- 20.Xiong T., Xiong W., Ge M., Xia J., Li B., Chen Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018;109:260–267. doi: 10.1016/j.foodres.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Zheng T., Li X., Taha A., Wei Y., Hu T., Fatamorgana P.B., Zhang Z., Liu F., Xu X., Pan S., Hu H. Effect of high intensity ultrasound on the structure and physicochemical properties of soy protein isolates produced by different denaturation methods. Food Hydrocoll. 2019;97 doi: 10.1016/j.foodhyd.2019.105216. [DOI] [Google Scholar]
- 22.Carrillo-Lopez L.M., Garcia-Galicia I.A., Tirado-Gallegos J.M., Sanchez-Vega R., Huerta-Jimenez M., Ashokkumar M., Alarcon-Rojo A.D. Recent advances in the application of ultrasound in dairy products: effect on functional, physical, chemical, microbiological and sensory properties. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoo D.Y., Low Z.L., Low D.Y.S., Tang S.Y., Manickam S., Tan K.W., Ban Z.H. Ultrasonic cavitation: an effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan J.J., Park M., Beevers J., Greenwood R.W., Norton I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: a review. Food Hydrocoll. 2017;71:299–310. doi: 10.1016/j.foodhyd.2016.12.037. [DOI] [Google Scholar]
- 25.Ashokkumar M. The characterization of acoustic cavitation bubbles - an overview. Ultrason. Sonochem. 2011;18:864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Pandit A.V., Sarvothaman V.P., Ranade V.V. Estimation of chemical and physical effects of cavitation by analysis of cavitating single bubble dynamics. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas-Ulloa P., Ulloa J.A., Ulloa-Rangel B.E., López-Mártir K.U. Protein isolate from orange (Citrus sinensis L.) seeds: effect of high-intensity ultrasound on its physicochemical and functional properties. Food Bioprocess Technol. 2023;16:589–602. doi: 10.1007/s11947-022-02956-4. [DOI] [Google Scholar]
- 28.Flores-Jiménez N.T., Ulloa J.A., Urías-Silvas J.E., Ramírez-Ramírez J.C., Bautista-Rosales P.U., Gutiérrez-Leyva R. Influence of high-intensity ultrasound on physicochemical and functional properties of a guamuchil Pithecellobium dulce (Roxb.) seed protein isolate. Ultrason. Sonochem. 2022;84 doi: 10.1016/j.ultsonch.2022.105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas B., Sit N. Effect of ultrasonication on functional properties of tamarind seed protein isolates. J. Food Sci. Technol. 2020;57:2070–2078. doi: 10.1007/s13197-020-04241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik M., Natarajan V., Modupalli N., Thangaraj S., Rawson A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: kinetics and quality measurements. LWT. 2022;155 doi: 10.1016/j.lwt.2021.112997. [DOI] [Google Scholar]
- 31.Mir N.A., Riar C.S., Singh S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019;96:433–441. doi: 10.1016/j.foodhyd.2019.05.052. [DOI] [Google Scholar]
- 32.Mir N.A., Riar C.S., Singh S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104700. [DOI] [PubMed] [Google Scholar]
- 33.Sert D., Rohm H., Struck S. Ultrasound-assisted extraction of protein from pumpkin seed press cake: impact on protein yield and techno-functionality. Foods. 2022;11:4029. doi: 10.3390/foods11244029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma M., Ren Y., Xie W., Zhou D., Tang S., Kuang M., Wang Y., Du S.K. Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 2018;240:856–862. doi: 10.1016/j.foodchem.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 35.AOAC . Association of Official Analytical Chemists; Gaithersburg, MD, USA: 2019. Official Methods of Analysis. [Google Scholar]
- 36.Resendiz-Vazquez J.A., Ulloa J.A., Urías-Silvas J.E., Bautista-Rosales P.U., Ramírez-Ramírez J.C., Rosas-Ulloa P., González-Torres L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017;37:436–444. doi: 10.1016/j.ultsonch.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 37.Chao E., Tian J., Fan L., Zhang T. Drying methods influence the physicochemical and functional properties of seed-used pumpkin. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130937. [DOI] [PubMed] [Google Scholar]
- 38.Abdollahi M., Undeland I. Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food Bioprocess Technol. 2018;11:1733–1749. doi: 10.1007/s11947-018-2138-x. [DOI] [Google Scholar]
- 39.Osborne T.B., Voorhees C.L. Proteids of the wheat kernel. J. Am. Chem. Soc. 1894;16:524–535. doi: 10.1021/ja02106a003. [DOI] [Google Scholar]
- 40.Kumar A., Nayak R., Purohit S.R., Rao P.S. Impact of UV-C irradiation on solubility of Osborne protein fractions in wheat flour. Food Hydrocoll. 2021;110 doi: 10.1016/j.foodhyd.2020.105845. [DOI] [Google Scholar]
- 41.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 42.Li M., Wen X., Peng Y., Wang Y., Wang K., Ni Y. Functional properties of protein isolates from bell pepper (Capsicum annuum L. var. annuum) seeds. LWT. 2018;97:802–810. doi: 10.1016/j.lwt.2018.07.069. [DOI] [Google Scholar]
- 43.Mohan N., Mellem J.J. Functional properties of the protein isolates of hyacinth bean [Lablab purpureus (L.) Sweet]: an effect of the used procedures. LWT. 2020;129 doi: 10.1016/j.lwt.2020.109572. [DOI] [Google Scholar]
- 44.Zhao Q., Selomulya C., Xiong H., Chen X.D., Ruan X., Wang S., Xie J., Peng H., Sun W., Zhou Q. Comparison of functional and structural properties of native and industrial process-modified proteins from long-grain indica rice. J. Cereal. Sci. 2012;56:568–575. doi: 10.1016/j.jcs.2012.08.012. [DOI] [Google Scholar]
- 45.Lei L., Zhao Q., Selomulya C., Xiong H. The effect of deamidation on the structural, functional, and rheological properties of glutelin prepared from Akebia trifoliata var. australis seed. Food Chem. 2015;178:96–105. doi: 10.1016/j.foodchem.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 46.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. 1970;227:5259 680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 47.Sadat A., Joye I.J. Peak fitting applied to Fourier transform infrared and Raman spectroscopic analysis of proteins. Appl. Sci. 2020;10:17–5918. doi: 10.3390/app10175918. [DOI] [Google Scholar]
- 48.Hernández-Ramírez J.A., Ulloa J.A., Ulloa-Rangel B.E., Rosas-Ulloa P. Valorization of the Noni (Morinda citrifolia) seeds as source of a protein concentrate and its physicochemical, functional, and structural characterization. Waste Biomass Valorization. 2023 doi: 10.1007/s12649-023-02270-w. [DOI] [Google Scholar]
- 49.Pérez-Saucedo M.R., Ulloa J.A., Rosas Ulloa P., Ramírez-Ramírez-Ramírez J.C., Silva-Carrillo Y., Ulloa-Rangel B.E. Caracterización tecno-funcional de un concentrado proteínico obtenido de la semilla de mango (Mangifera indica L.) Biotecnia. 2021;23:120–126. doi: 10.18633/biotecnia.v23i1.1306. [DOI] [Google Scholar]
- 50.Rezig L., Chibani F., Chouaibi M., Dalgalarrondo M., Hessini K., Guéguen J., Hamdi S. Pumpkin (Cucurbita maxima) seed proteins: sequential extraction processing and fraction characterization. J. Agric. Food Chem. 2013;61:7715–7721. doi: 10.1021/jf402323u. [DOI] [PubMed] [Google Scholar]
- 51.Flores-Jiménez N.T., Ulloa J.A., Urías-Silvas J.E., Ramírez-Ramírez J.C., Rosas-Ulloa P., Bautista-Rosales P.U., Silva-Carrillo Y., Gutiérrez-Leyva R. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019;121:947–956. doi: 10.1016/j.foodres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 52.López-Mártir K.U., Ulloa J.A., Urías-Silvas J.E., Rosas-Ulloa P., Ramírez-Ramírez J.C., Reséndiz-Vázquez J.A. Modification of the physicochemical, functional, biochemical and structural properties of a soursop seed (Annona muricata L.) protein isolate treated with high-intensity ultrasound. Ultrason. Sonochem. 2024;105 doi: 10.1016/j.ultsonch.2024.106870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das M., Devi L.M., Badwaik L.S. Ultrasound-assisted extraction of pumpkin seeds protein and its physicochemical and functional characterization. Appl. Food Res. 2022;2 doi: 10.1016/j.afres.2022.100121. [DOI] [Google Scholar]
- 54.Espinosa-Murillo N.D.C., Ulloa J.A., Urías-Silvas J.E., Rosas-Ulloa P., Ramírez-Ramírez J.C., Gutiérrez-Leyva R., Ulloa-Rangel B.E. Impact of high-intensity ultrasound on the physicochemical and functional properties of a protein isolate from passion fruit (Passiflora edulis) seeds. Int. J. Food Eng. 2021;17:609–618. doi: 10.1515/ijfe-2021-0050. [DOI] [Google Scholar]
- 55.Dabbour M., He R., Ma H., Musa A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process. Eng. 2018;41 doi: 10.1111/jfpe.12799. [DOI] [Google Scholar]
- 56.Sebii H., Karra S., Bchir B., Ghribi A.M., Danthine S., Blecker C., Attia H., Besbes S. Effect of sonication pretreatment on physico-chemical, surface and thermal properties of date palm pollen protein concentrate. LWT. 2019;106:128–136. doi: 10.1016/j.lwt.2019.02.041. [DOI] [Google Scholar]
- 57.Sosulski F. Organoleptic and nutritional effects of phenolic compounds on oilseed protein products: a review. J. Am. Oil Chem. Soc. 1979;56:711–715. doi: 10.1007/BF02663047. [DOI] [Google Scholar]
- 58.Hadidi M., Khaksar F.B., Pagan J., Ibarz A. Application of ultrasound-ultrafiltration-assisted alkaline isoelectric precipitation (UUAAIP) technique for producing alfalfa protein isolate for human consumption: optimization, comparison, physicochemical, and functional properties. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108907. [DOI] [PubMed] [Google Scholar]
- 59.Huang D., Men K., Li D., Wen T., Gong Z., Sunden B., Wu Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104950. [DOI] [PubMed] [Google Scholar]
- 60.Kumarakuru K., Reddy C.K., Haripriya S. Physicochemical, morphological and functional properties of protein isolates obtained from four fish species. J. Food Sci. Technol. 2018;55:4928–4936. doi: 10.1007/s13197-018-3427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pérez-Reyes M.E., Jie X., Zhu M.J., Tang J., Barbosa-Cánovas G.V. Influence of low water activity on the thermal resistance of Salmonella Enteritidis PT30 and Enterococcus faecium as its surrogate in egg powders. Food Sci. Technol. Int. 2021;27:184–193. doi: 10.1177/1082013220937872. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad F., Mohammad Z.H., Zaidi S., Ibrahim S.A. A comprehensive review on the application of ultrasound for the preservation of fruits and vegetables. J. Food Process. Eng. 2023;46 doi: 10.1111/jfpe.14291. [DOI] [Google Scholar]
- 63.Rawat R., Saini C.S. High-intensity ultrasound treatment: an effective way of modifying thermal and rheological properties of sunnhemp (Crotalaria juncea) protein isolate (SHPI) by varying ultrasound amplitude and time. J. Food Process. Eng. 2023;46 doi: 10.1111/jfpe.14290. [DOI] [Google Scholar]
- 64.Yao Y. Enhancement of mass transfer by ultrasound: application to adsorbent regeneration and food drying/dehydration. Ultrason. Sonochem. 2016;31:512–531. doi: 10.1016/j.ultsonch.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 65.Erkmen O., Bozoglu T.F. In: Food Microbiology: Principles into Practice. Erkmen O., Bozoglu T.F., editors. John Wiley & Sons; New York, NY: 2016. Food preservation by reducing water activity; pp. 44–58. [Google Scholar]
- 66.Zuñiga-Salcedo M.R., Ulloa J., Bautista-Rosales P., Ulloa P., Ramírez-Ramírez J., Carrillo Y., gutierrez L., Hernández C. Effect of ultrasound treatment on physicochemical, functional and nutritional properties of a safflower (Carthamus tinctorius L.) protein isolate. Ital. J. Food Sci. 2019;31:2019–2592. [Google Scholar]
- 67.Cui Q., Wang L., Wang G., Zhang A., Wang X., Jiang L. Ultrasonication effects on physicochemical and emulsifying properties of Cyperus esculentus seed (tiger nut) proteins. LWT. 2021;142 doi: 10.1016/j.lwt.2021.110979. [DOI] [Google Scholar]
- 68.Pezeshk S., Rezaei M., Hosseini H., Abdollahi M. Impact of pH-shift processing combined with ultrasonication on structural and functional properties of proteins isolated from rainbow trout by-products. Food Hydrocoll. 2021;118 doi: 10.1016/j.foodhyd.2021.106768. [DOI] [Google Scholar]
- 69.Zhao R., Liu X., Liu W., Liu Q., Zhang L., Hu H. Effect of high-intensity ultrasound on the structural, rheological, emulsifying and gelling properties of insoluble potato protein isolates. Ultrason. Sonochem. 2022;85 doi: 10.1016/j.ultsonch.2022.105969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Z., Zhu W., Yi J., Liu N., Cao Y., Lu J., Decker E.A., McClements D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018;106:853–861. doi: 10.1016/j.foodres.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 71.Rawat R., Saini C.S. High-intensity ultrasound (HIUS) treatment of sunnhemp protein isolate (Crotalaria juncea L.): modification of functional, structural, and microstructural properties. Food Bioprocess Technol. 2023;16:1464–1477. doi: 10.1007/s11947-023-03011-6. [DOI] [Google Scholar]
- 72.Gul O., Saricaoglu F.T., Atalar I., Gul L.B., Tornuk F., Simsek S. Structural characterization, technofunctional and rheological properties of sesame proteins treated by high-intensity ultrasound. Foods. 2023;12:1791. doi: 10.3390/foods12091791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngo N.T.T., Shahidi F. Functional properties of protein isolates from camelina (Camelina sativa (L.) Crantz) and flixweed (sophia, Descurainis sophia L.) seed meals. Food Prod. Process. Nutr. 2021;3:31. doi: 10.1186/s43014-021-00076-8. [DOI] [Google Scholar]
- 74.Xue F., Zhu C., Liu F., Wang S., Liu H., Li C. Effects of high-intensity ultrasound treatment on functional properties of plum (Pruni domesticae semen) seed protein isolate. J. Sci. Food Agric. 2018;98:5690–5699. doi: 10.1002/jsfa.9116. [DOI] [PubMed] [Google Scholar]
- 75.Tang S.Q., Du Q.H., Fu Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boukhari N., Doumandji A., Ait Chaouche F.S., Ferradji A. Effect of ultrasound treatment on protein content and functional properties of Spirulina powder grown in Algeria. Mediterr. J. Nutr. Metab. 2018;11:235–249. doi: 10.3233/MNM-180220. [DOI] [Google Scholar]
- 77.Hasmadi M., Noorfarahzilah M., Noraidah H., Zainol M.K., Jahurul M.H.A. Functional properties of composite flour: a review. Food Res. 2020;4:1820–1831. doi: 10.26656/fr.2017.4(6).419. [DOI] [Google Scholar]
- 78.Ma K.K., Grossmann L., Nolden A.A., McClements D.J., Kinchla A.J. Functional and physical properties of commercial pulse proteins compared to soy derived protein. Future Foods. 2022;6 doi: 10.1016/j.fufo.2022.100155. [DOI] [Google Scholar]
- 79.Haque M.A., Akter F., Rahman H., Baqui M. Jackfruit seeds protein isolate by spray drying method: the functional and physicochemical characteristics. Food Nutr. Sci. 2020;11:355–374. doi: 10.4236/fns.2020.115026. [DOI] [Google Scholar]
- 80.Wang J., Na X., Navicha W.B., Wen C., Ma W., Xu X., Wu C., Du M. Concentration-dependent improvement of gelling ability of soy proteins by preheating or ultrasound treatment. LWT. 2020;134 doi: 10.1016/j.lwt.2020.110170. [DOI] [Google Scholar]
- 81.Chen H., Guo Z., Wang Z., Yang B., Chen X., Wen L., Yang Q., Kan J. Structural and physicochemical properties of the different ultrasound frequency modified Qingke protein. Ultrason. Sonochem. 2023;94 doi: 10.1016/j.ultsonch.2023.106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diao Y., Zhang Y., Zhang W., Xu W., Hu Z., Yi Y., Wang Y. Acid-thermal-induced formation of rice bran protein nano-particles: foaming properties and physicochemical characteristics. Int. J. Food Sci. Technol. 2022;57:3624–3633. doi: 10.1111/ijfs.15686. [DOI] [Google Scholar]
- 83.Karki B., Lamsal B.P., Grewell D., Pometto A.L., van Leeuwen J., Khanal S.K., Jung S. Functional properties of soy protein isolates produced from ultrasonicated defatted soy flakes. J. Am. Oil Chem. Soc. 2009;86:1021–1028. doi: 10.1007/s11746-009-1433-0. [DOI] [Google Scholar]
- 84.Arzeni C., Pérez O.E., Pilosof A.M.R. Functionality of egg white proteins as affected by high intensity ultrasound. Food Hydrocoll. 2012;29:308–316. doi: 10.1016/j.foodhyd.2012.03.009. [DOI] [Google Scholar]
- 85.Falusi F., Berkó S., Kovács A., Budai-Szűcs M. Application of xanthan gum and hyaluronic acid as dermal foam stabilizers. Gels. 2022;8:413. doi: 10.3390/gels8070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H., Guo Z., Wang Z., Yang B., Chen X., Wen L., Yang Q., Kan J. Structural and physicochemical properties of the different ultrasound frequency modified Qingke protein. Ultrason. Sonochem. 2023;94 doi: 10.1016/j.ultsonch.2023.106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aryee A.N.A., Agyei D., Udenigwe C.C. In: Proteins in Food Processing. Yada R.Y., editor. Woodhead Publishing; Darya Ganj: 2018. Impact of processing on the chemistry and functionality of food proteins; pp. 27–45. [Google Scholar]
- 88.Pulido O.M., Rousseaux C.G., Cole P.I. In: Haschek and Rousseaux' S Handbook of Toxicologic Pathology. Haschek W.M., Rousseaux C.G., Wallig M.A., Bolon B., Heinz-taheny K.M., Rudmann D.G., Mahler B.W., editors. Academic Press; London, UK: 2023. Food and toxicologic pathology; pp. 33–103. [Google Scholar]
- 89.Li R., Xiong Y.L. Ultrasound-induced structural modification and thermal properties of oat protein. LWT. 2021;149 doi: 10.1016/j.lwt.2021.111861. [DOI] [Google Scholar]
- 90.Gani A., ul Ashraf Z., Noor N., Wani I.A. Ultrasonication as an innovative approach to tailor the apple seed proteins into nanosize: effect on protein structural and functional properties. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: implications for the structural and functional properties. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112272. [DOI] [Google Scholar]
- 92.Trache D., Hussin M.H., Chuin C.T.H., Sabar S., Fazita M.R.N., Taiwo O.F.A., Hassan T.M., Haafiz M.K.M. Microcrystalline cellulose: isolation, characterization and bio-composites application - a review. Int. J. Biol. Macromol. 2016;93:A 789–804. doi: 10.1016/j.ijbiomac.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 93.Rohmer M., Freudenberg J., Binder W.H. Secondary structures in synthetic poly(Amino Acids): homo- and copolymers of poly(Aib), poly(Glu), and poly(Asp) Macromol. Biosci. 2022;23:4. doi: 10.1002/mabi.202200344. 2200344. [DOI] [PubMed] [Google Scholar]
- 94.Gallagher W. FTIR analysis of protein structure, Course Man. Chem. 2009;455:1–8. [Google Scholar]
- 95.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: ultrasound-treated soybean protein isolate. LWT. 2021;142 doi: 10.1016/j.lwt.2021.110881. [DOI] [Google Scholar]
- 96.Mozafarpour R., Koocheki A., Nicolai T. Modification of grass pea protein isolate (Lathyrus sativus L.) using high intensity ultrasound treatment: structure and functional properties. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111520. [DOI] [PubMed] [Google Scholar]
- 97.Wang R.X., Li Y.Q., Sun G.J., Wang C.Y., Liang Y., Hua D.L., Chen L., Mo H.Z. The improvement and mechanism of gelation properties of mung bean protein treated by ultrasound. LWT. 2023;182 doi: 10.1016/j.lwt.2023.114811. [DOI] [Google Scholar]
- 98.Jin J., Ma H., Wang B., Yagoub A.E.G.A., Wang K., He R., Zhou C. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this research are available in the article. Original/derived data endorsing the findings of this report are obtainable upon request to the corresponding author.