Abstract
Introduction
Dermatofibrosarcoma protuberans (DFSP) is the most common sarcoma of the skin. Although distant metastases are infrequent, DFSP is highly aggressive locally with frequent local recurrences. It has been reported that the presence within the tumour of areas histopathologically mimicking fibrosarcoma may increase the risk of recurrence.
Objective
The objective of this study was to review the clinical features of our patients with DFSP and the factors associated with recurrence of the tumour, focussing on the presence of fibrosarcomatous areas.
Methods
Retrospective study of patients with DFSP diagnosed in 1990–2021 in a tertiary university hospital. The medical records were reviewed to obtain the following data: age, sex, tumour location, diameter, evolution time, presence of fibrosarcomatous areas, development of recurrence, and follow-up. Factors possibly associated with disease-free survival were analysed with Kaplan-Meier method and multivariate Cox regression.
Results
148 patients (74 women/74 men, mean age 46.28 years, SD 14.431) were included in the study. Tumours involved the head and neck in 15 cases, thorax in 31, abdomen in 16, upper back in 43, lower back in 10, upper extremities in 10, and lower extremities in 23. Fibrosarcoma-like areas were observed in 16 tumours (10.81%). In 17 patients (11.49%), recurrences were observed (13 local recurrences, 3 lung metastasis, and 1 local recurrence with lung metastasis). Fibrosarcomatous DFSP recurred more frequently than classic DFSP (50% vs. 6.82%, respectively), and its disease-free survival was significantly lower (p < 0.001). In multivariate Cox regression, the presence of fibrosarcomatous areas was the only factor influencing disease-free survival.
Conclusions
It is important to identify the fibrosarcomatous variant since it recurs more frequently and has lower recurrence-free survival. Distant metastases, mainly in the lung, are also more frequent in fibrosarcomatous DFSP.
Keywords: Dermatofibrosarcoma protuberans, Fibrosarcoma-like areas, Skin
Introduction
Dermatofibrosarcoma protuberans (DFSP) is the most common sarcoma of the skin [1]. Most DFSPs arise from the dermis; however, they may also develop in the subcutaneous tissue [2]. Histochemical and electron microscopy evidence points to a possible fibroblastic origin for DFSP [2–4]. However, CD34 positivity suggests that it originates in skin dendritic cells, probably derived from circulating haematopoietic progenitors [2, 5]. Regardless of the origin, the determining factor of cell proliferation is 17;22 translocation that is detected by FISH or PCR in more than 90% of DFSP [1, 2, 6]. This translocation determines the fusion gene PDGFB/COL1A1, leading to overproduction of PDGFB and autocrine stimulation of the PDGFB receptor and cell proliferation [1].
Histopathologically, DFSP shows a proliferation of homogeneous spindle cells with scant cytoplasm and elongated hyperchromatic nuclei involving the dermis and subcutaneous tissue. These cells are not pleomorphic and have low mitotic activity [1, 2, 7]. They are typically arranged in well-defined bands which interweave or radiate like the spokes of a wheel, forming an irregularly whorled or storiform pattern reminiscent of a straw mat [1, 2]. In early stages, a grenz zone can be observed between the tumour and the epidermis [8]. Tumour cells invade the subcutaneous tissue with irregular tentacle-like projections through septa and fat lobules [9]. Therefore, local recurrences after excision with apparently wide margins are common [2]. DFSP usually stains positively for CD34 and negatively for factor XIIIa [2]. Fibrosarcomatous DFSP is defined by the focal presence of areas with fascicles of spindle cells intersecting at acute angles in a typical “herringbone” pattern, almost identical to that seen in fibrosarcoma [1, 2]. In these areas, the mitotic activity is increased, and the cells show mild to moderate cellular and nuclear pleomorphism, and necrosis can be found [1, 2]. Fibrosarcomatous areas often show less intense or negative staining for CD34 [1]. In contrast, P53 expression is increased in these areas [1].
Although distant metastases are infrequent, DFSP is a tumour that is locally highly aggressive with frequent local recurrences [1]. It has been reported that the presence of fibrosarcomatous areas within the tumour increases the risk of recurrence [10]. However, the few studies on this topic have discordant results. Our objective was to review the clinical features of our patients with DFSP and the factors associated with recurrence of the tumour, focussing on the presence of fibrosarcomatous areas.
Materials and Methods
With local Ethics Committee approval, all cases coded as DFSP between 1990 and 2021 in the database of the Pathology Department of Bellvitge University Hospital were included in the study. This is a tertiary university hospital with 800 beds that provides health care to approximately 1 million people. In cases oriented as DFSP, the sections examined were in relation to the size of the tumour, evaluating approximately one histological section per tumour centimetre. Histopathologically, fibrosarcomatous differentiation has been assessed by a pathologist specialised in soft tissue pathology or dermatopathology (Dr. Sanjuán and Dr. Penín, respectively), both qualitatively (presence or non-presence) and quantitatively (percentage of the entire tumour). Microscopically, the fibrosarcomatous transformation was defined by areas representing at least 10% of the tumour with herringbone or fascicular growth pattern usually in a background of classical storiform DFSP. If fibrosarcomatous areas were focal, the sample was re-embedded to verify the proportion of these areas. CD34 immunohistochemical staining has been performed on at least one section of the fibrosarcomatous areas. The clinical records of the patients were retrospectively reviewed to collect the following data: age at diagnosis of DFSP, sex, location of the lesion, tumour diameter, evolution time at diagnosis, presence of fibrosarcomatous areas within the tumour, development of recurrence (local or distant), and follow-up time.
Data obtained were analysed with SPSS 17.0 for Windows. Categorical variables were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using Student’s t test when normality of data distribution was confirmed. Otherwise, the Mann-Whitney U test was performed. Disease-free survival in patients with and without fibrosarcomatous areas was compared using the Kaplan-Meier method, and statistical significance was evaluated using the log-rank test. Univariate and multivariate Cox regression was performed to identify predictors of recurrence-free survival. Statistical significance was established for a p value <0.05.
Results
One hundred and forty-eight patients were diagnosed with DFSP in our hospital (74 women/74 men). Most patients were Caucasian (139), 6 were of African ethnicity, and 3 of South American descent. The age of the patients at diagnosis ranged between 16 and 89 years, with a mean age of 46.28 years (SD 14.431 years). The evolution time at diagnosis of DFSP ranged between 2 and 360 months (median 36 months, interquartile range [IQR] 72 months) and tumour diameter ranged between 0.4 and 30 cm (median 3 cm, IQR 3 cm).
Tumours were located in the head and neck region in 15 cases, anterior chest in 31, abdomen in 16, upper back (dorsal region) in 43, lower back (lumbar region) in 10, upper extremities in 10, and lower extremities in 23. Only 6 tumours (4.05%) involved distal regions of the extremities (1 on the wrist and 5 on the dorsum of the foot). Three cases corresponded to the pigmented variant of DFSP (Bednar’s tumour), all of them located on the back. Fibrosarcoma-like areas were histopathologically observed in 16 tumours (10.81%). The fibrosarcomatous areas showed increased cellularity, increased atypia (usually of low or intermediate grade and rarely high grade), higher mitotic activity (often >10 mitoses per 10 high-power fields), and, in some cases, focal necrosis. The FS component was sharply demarcated from the conventional areas and represented between 30 and 80% of the entire lesion in the tumours reviewed in this study. CD34 immunoreactivity was often diminished or lost compared to the surrounding conventional DFSP.
All patients were treated by wide surgical excision trying to achieve 2–4 cm tumour-free margins. Eight patients received adjuvant radiation therapy because adequate resection margins could not be achieved.
Seventeen patients developed tumour recurrence (11.49%). Thirteen patients had local recurrence, 3 pulmonary metastases, and 1 local recurrence and pulmonary metastases. Three of the 4 patients who developed pulmonary metastases had histopathologically fibrosarcoma-like areas. Therefore, the risk of distant metastasis was 18.75% for the fibrosarcomatous variant compared to 0.76% for the rest of DFSP. Follow-up ranged from 1 to 348 months (median 36 months, IQR 110). None of our 3 Bednar’s tumours had fibrosarcomatous areas, and none recurred.
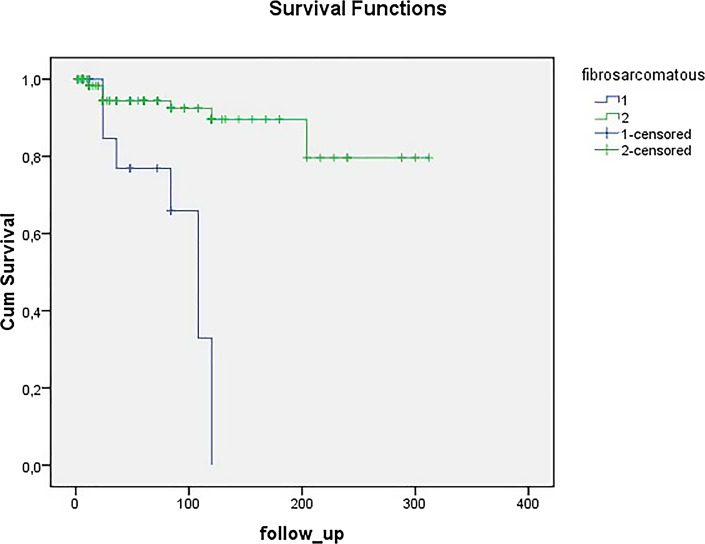
The comparison of the clinical features of the patients according to sex is shown in Table 1. The median tumour size was greater in males (3.3 cm, IQR 2.5 vs. 2.75 cm, IQR 3.43, p = 0.029). Table 2 shows comparison of the features of the patients with and without fibrosarcomatous areas. Tumours with fibrosarcomatous areas had a significantly greater median diameter (6.4 cm, IQR 7.5 vs. 3 cm, IQR 2.2, p = 0.003). They also had a higher mean age (54.44 years, DE 20.970 vs. 45.29 years, DE 13.199) and a greater median evolution time at diagnosis than the rest of the lesions (42 months, IQR 99 vs. 36 months, IQR 63), although these later differences were not significant. Regarding the behaviour of the patients, 50% of the tumours with fibrosarcomatous areas recurred versus only 6.82% of the rest of DFSP (p < 0.001). Figure 1 shows recurrence-free survival for 148 DFSP patients with and without fibrosarcomatous areas (Kaplan-Meier method, log-rank test, p < 0.001).
Table 1.
Clinical features of the 148 patients with DFSP according to sex
| Total | Female | Male | ||
|---|---|---|---|---|
| Patients, n (%) | 148 | 74 (50) | 74 (50) | |
| Ethnicity, n (%) | ||||
| Caucasian | 139 (93.92) | 69 (93.24) | 70 (94.59) | |
| African | 6 (4.05) | 2 (2.70) | 4 (5.41) | |
| South American | 3 (2.03) | 3 (4.05) | 0 (0) | |
| Age at diagnosis, mean (SD), years | 46.28 (14.431) | 45.88 (14.461) | 46.68 (14.489) | |
| Evolution at diagnosis, median (IQR), months | 36 (72) | 48 (63) | 36 (57) | |
| Diameter median (IQR), cm | 3 (3) | 2.75 (3.43) | 3.3 (2.5) | p = 0.029 |
| Site (148 tumours), n (%) | ||||
| Head and neck | 15 (10.14) | 5 (6.76) | 10 (13.51) | |
| Chest | 31 (20.95) | 18 (24.32) | 13 (17.57) | |
| Abdomen | 16 (10.81) | 8 (10.81) | 8 (10.81) | |
| Upper back | 43 (29.05) | 20 (27.03) | 23 (31.08) | |
| Lower back | 10 (6.76) | 6 (8.11) | 4 (5.41) | |
| Upper extremities | 10 (6.76) | 4 (5.41) | 6 (8.11) | |
| Lower extremities | 23 (15.54) | 13 (17.57) | 10 (13.51) | |
| Distal extremities (forearm 1, foot 5), n (%) | 6 (4.05) | 3 (4.05) | 3 (4.05) | |
| Previous scar, n (%) | 6 (4.05) | 1 (1.35) | 5 (6.76) | |
| Pigmented (Bednar tumour), n (%) | 3 (2.03) | 2 (2.70) | 1 (1.35) | |
| Fibrosarcomatous, n (%) | 16 (10.81) | 6 (8.11) | 10 (13.51) | |
| Recurrence, n (%) | 17 (11.49) | 8 (10.81) | 9 (12.16) | |
Table 2.
Comparison of DFSP with and without fibrosarcomatous areas
| Total | Fibrosarcomatous | Rest of cases | ||
|---|---|---|---|---|
| Patients, n (%) | 148 | 16 (10.81) | 132 (89.19) | |
| Ethnicity, n (%) | ||||
| Caucasian | 139 (93.92) | 14 (87.5) | 125 (94.70) | |
| African | 6 (4.05) | 1 (6.25) | 5 (3.79) | |
| South American | 3 (2.03) | 1 (6.25) | 2 (1.51) | |
| Sex, n (%) | ||||
| Female | 74 (50) | 10 (62.5) | 64 (48.48) | |
| Male | 74 (50) | 6 (37.5) | 68 (51.52) | |
| Age at diagnosis, mean (SD), years | 46.28 (14.431) | 54.44 (20.970) | 45.29 (13.199) | |
| Evolution at diagnosis, median (IQR), months | 36 (72) | 42 (99) | 36 (63) | |
| Diameter, median (IQR), cm | 3 (3) | 6.4 (7.5) | 3 (2.2) | p = 0.003 |
| Site (148 tumours), n (%) | ||||
| Head and neck | 15 (10.14) | 2 (12.50) | 13 (9.85) | |
| Chest | 31 (20.95) | 4 (25.00) | 27 (20.45) | |
| Abdomen | 16 (10.81) | 1 (6.25) | 15 (11.36) | |
| Upper back | 43 (29.05) | 2 (12.5) | 41 (31.06) | |
| Lower back | 10 (6.76) | 1 (6.25) | 9 (6.82) | |
| Upper extremities | 10 (6.76) | 2 (12.50) | 8 (6.06) | |
| Lower extremities | 23 (15.54) | 4 (25.00) | 19 (14.39) | |
| Distal extremities (forearm 1, foot 5), n (%) | 6 (4.05) | 1 (6.25) | 5 (3.79) | |
| Previous scar, n (%) | 6 (4.05) | 0 (0) | 6 (4.55) | |
| Pigmented (Bednar tumour), n (%) | 3 (2.03) | 0 (0) | 3 (2.27) | |
| Recurrence, n (%) | 17 (11.49) | 8 (50) | 9 (6.82) | p < 0.001 |
Fig. 1.
Recurrence-free survival for the 148 DFSP patients with (1) and without (2) fibrosarcomatous areas (Kaplan-Meier method, log-rank test, p < 0.001).
Table 3 shows univariate and multivariate Cox regression analysis of factors possibly influencing recurrence-free survival. Only the presence of fibrosarcomatous areas was significantly associated with a shorter recurrence-free survival in the univariate analysis, and it maintained its statistical significance in the multivariate analysis. Other factors such as the age of the patients at diagnosis, gender, tumour diameter, and evolution time did not influence the risk of recurrence in either the univariate or the multivariate analysis.
Table 3.
Univariate and multivariate Cox regression analysis of predictors of recurrence-free survival
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Sex | ||||||
| Male | 1.316 | 0.506–3.424 | 0.573 | |||
| Female | Ref | |||||
| Age, years | ||||||
| <45 | Ref | |||||
| >45 | 1.750 | 0.664–4.609 | 0.258 | 1.621 | 0.512–5.130 | 0.411 |
| Diameter | ||||||
| <4.5 cm | Ref | Ref | ||||
| >4.5 cm | 2.864 | 0.764–10.736 | 0.119 | 1.418 | 0.334–6.016 | 0.636 |
| Site | ||||||
| Trunk | 1.011 | 0.354–2.886 | 0.983 | |||
| Other locations | Ref | |||||
| Fibrosarcomatous | ||||||
| Yes | 8.839 | 3.285–23.783 | <0.001 | 9.476 | 2.607–34.438 | <0.001 |
| No | Ref | Ref | ||||
Discussion
The reported incidence of DFSP ranges from 0.8 to 4.5 cases per million inhabitants per year [2]. However, there are important racial differences. In a study in the USA, the incidence in patients of African descent is double that of Caucasians (7.4 vs. 3.6 cases per million inhabitants and year) [11]. Unlike most skin neoplasms, it is more frequent in young people since it usually appears in the third or fourth decade of life [1, 2]. In our series, the mean age at diagnosis was 46.28 years (SD 14.431), with a peak around 35 and another around 55 years. In some studies, it is more common in males [10, 11], while in others, it has been more frequently reported in females [10, 12]. In the present study, the incidence was the same in both sexes (50% women and 50% men).
DFSP initially presents as an asymptomatic, indurated plaque that slowly grows over months or years. The lesion may be covered by normal-appearing or yellowish-brown, erythematous, sclerodermiform, or atrophic skin, occasionally with telangiectasia [2]. As DFSP grows, it becomes raised, firm, and nodular. It is a slow-growing tumour that causes little discomfort, and its diagnosis is usually delayed [2]. In our patients, the median evolution time at diagnosis was 36 months, IQR 72. The pigmented variant or Bednar’s tumour shows a brownish colour due to colonisation of the tumour by melanin-producing dendritic melanocytes [2]. Bednar tumour accounts for less than 5% of DFSP cases and is more frequent in people of African descent [2]. In the present study, with 94% of patients being Caucasian, we only detected 3 cases of Bednar tumour (2.03%).
The most frequently reported location of DFSP is the trunk and the proximal region of the extremities [1, 2]. Involvement of hands and feet, particularly the fingers, is uncommon [1]. In a large database, tumours were located on the trunk in 42–49% of cases, upper extremities in 19–21%, lower extremities in 18–21%, and head and neck in 12–13% [11, 12]. In our patients, DFSP was located predominantly on the trunk (100 cases, 68%), especially the upper back (43 cases) and the thorax (31 cases). The location in the extremities was less frequent than in other series (upper extremities 7%, lower extremities 15%), and in the distal regions of the extremities, it was exceptional (only 6 cases, 4%).
DFSP is considered a low-grade sarcoma [2]. Local recurrence of DFSP has been reported to vary from 5.5% up to 60% of cases [1, 13]. Metastasis to lymph nodes and internal organs is extremely rare [1, 14, 15]. The lung is the organ most frequently affected by distant metastases, but brain, bone, and soft tissue metastases have also been reported [2]. In the present study, 17 of 148 DFSP recurred (11.49%), most of them locally in adjacent skin. Only four of our patients developed distant metastases, all of them in the lungs (2.70%).
Fibrosarcomatous DFSP is considered an intermediate-grade sarcoma and accounts for 5–20% of all DFSP [2, 16–20]. In our study, the presence of fibrosarcomatous areas was documented in 10.81% of patients. In agreement with most reported series [16, 20, 21], in our patients, fibrosarcomatous DFSP was more common in females (10 women/6 men, 1.7/1). Also as in previous studies [18], fibrosarcomatous DFSP developed at older ages than classic DFSP (mean age 54.44 vs. 45.29 years, respectively), although the differences were not significant. In the survey by Llombart et al. [16], fibrosarcomatous DFSP had larger diameter and longer evolution at diagnosis than the rest of DFSP. Our patients with fibrosarcomatous areas also had a median diameter significantly greater than that of the rest of the tumours (6.4 cm vs. 3 cm, p = 0.003) and greater median evolution time at diagnosis (42 months vs. 36 months), although this difference was not significant. In agreement with other studies, we did not observe differences in the locations of the DFSP and fibrosarcomatous DFSP lesions [14, 16, 20, 21].
It seems obvious that the presence of intermediate-grade sarcoma areas within a low-grade lesion may be associated with more aggressive behaviour. However, there are few studies on fibrosarcomatous DFSP, and their results are discordant [20]. While in some of them local recurrence rates were similar to those of classic DFSP [16], others detected local recurrence in around 20% of patients [14, 22], 34% [20], or even in more than 50% [19, 21]. In the present study, 50% of the DFSP with fibrosarcomatous areas relapsed compared to 6.82% of the rest of DFSP (p < 0.001). Regarding distant metastasis, discrepancies are also important, since they have been reported in 8.3–66.7% of fibrosarcomatous DFSP [10, 14, 16, 20, 21]. In our patients, 18.75% of fibrosarcomatous DFSP developed lung metastases versus 0.76% of the rest of DFSP.
There are few studies comparing disease-free survival of patients with fibrosarcomatous DFSP and those without fibrosarcomatous areas. A recent study of 158 patients with DFSP detected a lower recurrence-free time for the fibrosarcomatous variant [20]. Likewise, in our study, we also observed a shorter disease-free survival for DFSP with fibrosarcomatous areas (Kaplan-Meier method, log-rank, p < 0.001) (Fig. 1). In addition, in our multivariate Cox regression analysis, only the presence of fibrosarcomatous areas was associated with lower disease-free survival (p < 0.001) (Table 3).
The classical therapy of DFSP is wide surgical excision trying to achieve 2–4 cm tumour-free margins. However, several recent studies have demonstrated that Mohs micrographic surgery is associated with a much lower recurrence rate than wide local excision [9, 23, 24]. Mosh surgery would be especially indicated in cases with fibrosarcomatous areas, although in some cases, it may not be detected until the histopathological study of the completely removed tumour.
Limitations of the present study are that it is a retrospective observational survey with a limited number of patients. In addition, the data were obtained from a single reference hospital, and it is possible that the analysed population included patients who were more likely to have aggressive tumours.
In summary, in our population, DFSP is especially frequent in the upper part of the trunk, especially in the upper part of the back. The presence of fibrosarcomatous areas was observed in 10.81% of DFSP. Fibrosarcomatous DFSP had a significantly greater diameter at diagnosis than classic DFSP and a higher risk of local recurrence and distant metastasis. In multivariate analysis, the only factor associated with a higher risk of recurrence and a shorter recurrence-free survival time after the initial treatment was the presence of fibrosarcomatous areas. Therefore, our study confirms that fibrosarcomatous DFSP has a more aggressive course, with shorter recurrence-free survival and greater metastatic potential. For this reason, it is important to diagnose the fibrosarcomatous variant because it may require closer clinical follow-up, possibly with image tests to detect distant metastases, mainly in the lung.
Key Message
Fibrosarcomatous DFSP recurs more frequently and has lower recurrence-free survival.
Acknowledgment
We acknowledge CERCA Programme/Generalitat de Catalunya for institutional support.
Statement of Ethics
This work was approved by the Local Ethics Committee (Comitè d'Ètica de la Investigació de Medicaments Hospital de Bellvitge) – Ref PR284/23. Written informed consent was not required according to the Local Ethics Committee (Comitè d'Ètica de la Investigació de Medicaments Hospital de Bellvitge).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources for this work.
Author Contributions
Joaquim Marcoval contributed the central idea, analysed most of the data, and wrote the initial draft of the manuscript. Carlos Moreno-Vílchez, Clara Torrecilla-Vall-Llosera, and Clara Muntaner-Virgili collected the data. Diana Pérez Sidelnikova, Xavier Sanjuán, and Rosa María Penín revised the manuscript. All authors discussed the results.
Funding Statement
The authors have no funding sources for this work.
Data Availability Statement
All data analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Calonje E, Tsai KY. Soft-tissue tumours and tumour-like conditions. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s textbook of dermatology. ed 9. London: Wiley; 2016. [Chapter 137]. [Google Scholar]
- 2. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. In: Post TW, editor. UpToDate. Waltham, MA. [Accessed February 4, 2022]. [Google Scholar]
- 3. Allan AE, Tsou HC, Harrington A, Stasko T, Lee X, Si SP, et al. Clonal origin of dermatofibrosarcoma protuberans. J Invest Dermatol. 1993;100(2):99–102. [DOI] [PubMed] [Google Scholar]
- 4. Dominguez-Malagón HR, Ordóñez NG, Mackay B. Dermatofibrosarcoma protuberans: ultrastructural and immunocytochemical observations. Ultrastruct Pathol. 1995 Jul-Aug;19(4):281–9. [DOI] [PubMed] [Google Scholar]
- 5. Nickoloff BJ. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi's sarcoma. Arch Dermatol. 1991;127(4):523–9. [PubMed] [Google Scholar]
- 6. Patel KU, Szabo SS, Hernandez VS, Prieto VG, Abruzzo LV, Lazar AJ, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39(2):184–93. [DOI] [PubMed] [Google Scholar]
- 7. Weedon D. Weedon’s skin pathology. 3rd ed.London: Churchill Livingstone Elsevier; 2010; p. 833–5. [Chapter 34]. [Google Scholar]
- 8. Lindner NJ, Scarborough MT, Powell GJ, Spanier S, Enneking WF. Revision surgery in dermatofibrosarcoma protuberans of the trunk and extremities. Eur J Surg Oncol. 1999;25(4):392–7. [DOI] [PubMed] [Google Scholar]
- 9. Llombart B, Serra-Guillén C, Monteagudo C, López Guerrero JA, Sanmartín O. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Semin Diagn Pathol. 2013;30(1):13–28. [DOI] [PubMed] [Google Scholar]
- 10. Jing C, Zhang H, Zhang X, Yu S. Dermatofibrosarcoma protuberans: a clinicopathologic and therapeutic analysis of 254 cases at a single institution. Dermatol Surg. 2021;47(2):e26–e30. [DOI] [PubMed] [Google Scholar]
- 11. Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42(Suppl 1):S24–31. [DOI] [PubMed] [Google Scholar]
- 12. Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: national Cancer Database analysis. J Am Acad Dermatol. 2018;78(6):1125–34. [DOI] [PubMed] [Google Scholar]
- 13. Kim BJ, Kim H, Jin US, Minn KW, Chang H. Wide local excision for dermatofibrosarcoma protuberans: a single-center series of 90 patients. BioMed Res Int. 2015;2015:642549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abbott JJ, Oliveira AM, Nascimento AG. The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans. Am J Surg Pathol. 2006;30(4):436–43. [DOI] [PubMed] [Google Scholar]
- 15. Brenner W, Schaefler K, Chhabra H, Postel A. Dermatofibrosarcoma protuberans metastatic to a regional lymph node. Report of a case and review. Cancer. 1975;36(5):1897–902. [DOI] [PubMed] [Google Scholar]
- 16. Llombart B, Monteagudo C, Sanmartín O, López-Guerrero JA, Serra-Guillén C, Poveda A, et al. Dermatofibrosarcoma protuberans: a clinicopathological, immunohistochemical, genetic (COL1A1-PDGFB), and therapeutic study of low-grade versus high-grade (fibrosarcomatous) tumors. J Am Acad Dermatol. 2011;65(3):564–75. [DOI] [PubMed] [Google Scholar]
- 17. Wrotnowski U, Cooper PH, Shmookler BM. Fibrosarcomatous change in dermatofibrosarcoma protuberans. Am J Surg Pathol. 1988;12(4):287–93. [DOI] [PubMed] [Google Scholar]
- 18. Connelly JH, Evans HL. Dermatofibrosarcoma protuberans. A clinicopathologic review with emphasis on fibrosarcomatous areas. Am J Surg Pathol. 1992;16(10):921–5. [PubMed] [Google Scholar]
- 19. Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88(12):2711–20. [PubMed] [Google Scholar]
- 20. Hoesly PM, Lowe GC, Lohse CM, Brewer JD, Lehman JS. Prognostic impact of fibrosarcomatous transformation in dermatofibrosarcoma protuberans: a cohort study. J Am Acad Dermatol. 2015;72(3):419–25. [DOI] [PubMed] [Google Scholar]
- 21. Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22(5):576–87. [DOI] [PubMed] [Google Scholar]
- 22. Goldblum JR, Reith JD, Weiss SW. Sarcomas arising in dermatofibrosarcoma protuberans: a reappraisal of biologic behavior in eighteen cases treated by wide local excision with extended clinical follow up. Am J Surg Pathol. 2000;24(8):1125–30. [DOI] [PubMed] [Google Scholar]
- 23. Lowe GC, Onajin O, Baum CL, Otley CC, Arpey CJ, Roenigk RK, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43(1):98–106. [DOI] [PubMed] [Google Scholar]
- 24. Veronese F, Boggio P, Tiberio R, Gattoni M, Fava P, Caliendo V, et al. Wide local excision vs Mohs tubingen technique in the treatment of dermatofibrosarcoma protuberans: a two-centre retrospective study and literature review. J Eur Acad Dermatol Venereol. 2017;31(12):2069–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.