Abstract
Interferon (IFN) is an important immune system molecule capable of inducing an antiviral state within cells. Herpes simplex virus type 1 (HSV-1) replication is somewhat reduced in tissue culture in the presence of IFN, presumably due to decreased viral transcription. Here, we show mutations that inactivate immediate-early (IE) gene product ICP0 render HSV-1 exquisitely sensitive to IFN inhibition, resulting in greatly decreased levels of viral mRNA transcripts and the resulting polypeptides and a severe reduction in plaque formation ability. Mutations in other HSV-1 genes, including the genes coding for virion transactivator VP16 and the virion host shutoff protein vhs, IE gene ICP22, and the protein kinase UL13 gene, do not increase the IFN sensitivity of HSV-1. Interestingly, ICP0 mutants demonstrate the same level of sensitivity to IFN as wild-type virus on U2OS cells, an osteosarcoma cell line that is known to complement mutations in ICP0 and VP16. Thus, in some cell types, functional ICP0 is required for HSV-1 to efficiently bypass the inhibitory effects of IFN in order to ensure its replication. The significance of this link between ICP0 and IFN resistance is discussed.
Interferon (IFN) was initially characterized as a soluble factor mediating viral interference (21). Although it is now clear that both alpha/beta IFN (IFN-α/β) and gamma IFN (IFN-γ) IFNs are potent and pleiotropic immune modulators, antiviral defense is likely the major function of the alpha/beta IFNs (17). The IFN-induced cellular antiviral response is the primary defense mechanism against viral infection, and many viruses have therefore evolved strategies to bypass the inhibitory effects of IFN (reviewed in references 22 and 24). Members of the poxvirus family intercept IFN ligand molecules prior to cell-surface receptor engagement by producing IFN receptor decoys, thus preventing IFN signal transduction (3, 38). Human cytomegalovirus also perturbs IFN signal transduction, by decreasing the levels of two key components, JAK1 and p48 (35). Several IFN-induced pathways contribute to the intracellular antiviral response; however, the double-stranded RNA-dependent protein kinase PKR appears to be among the most effective (8). Accordingly, each step in the PKR pathway has been shown to be subject to negative regulation by a variety of RNA and DNA viruses (16).
While IFN severely inhibits the replication of several viruses, such as vesicular stomatitis virus (VSV) (31), replication of herpes simplex virus type 1 (HSV-1) is only marginally reduced in cultured cells (28, 29, 41). However, in vivo studies with IFN receptor-null mice and a variety of HSV-1 mutants illustrated the importance of IFN-α/βs in controlling early acute infection in intact animals and demonstrated a role for host factors in defining the phenotype of several HSV-1 mutants (27). HSV-1 is a large enveloped DNA virus that replicates in the nuclei of mammalian cells. HSV genes are transcribed by host RNA polymerase II in a regulatory cascade in which three distinct classes of genes (immediate-early [IE], early, and late) are sequentially activated in a defined temporal sequence (20, 47). Five IE genes are transcribed first, and four of these encode regulatory proteins (ICP0, -4, -22, and -27) that drive progression to the early and late phases of infection. Transcription of the IE genes is activated by the virion protein VP16, a 65-kDa phosphoprotein found within the HSV-1 tegument that contains a potent C-terminal acidic transcriptional activation domain (44).
Preliminary studies suggested that IFN or its inducers can specifically target HSV-1 translation (2, 29), while subsequent investigations showed that IFN treatment decreases IE gene transcription (36, 42, 43). IE transactivation is a major function of VP16; thus, it was initially believed that IFN blocks IE gene expression by interfering with the function of VP16 (10, 25). However, experiments with a VP16 activation mutant, in1814, demonstrated that inhibition of IE gene expression by IFN-α is VP16 independent (41). Relatively little is known concerning the mechanisms involved, although a recent study shows that the effects vary with both IFN and cell type (51).
To date, no studies have explored the basis for the relatively IFN-resistant phenotype established by HSV-1 in cell culture. While the late gene products γ134.5 and US11 have been implicated in the inhibition of PKR activity (6, 18, 19, 37, 45), no direct link between these proteins and resistance to IFN has been established. To determine whether the relative resistance of HSV-1 to IFN depends on a specific regulatory protein, we examined the effects of mutations that alter known regulatory proteins on the sensitivity to IFN-α in a plaque reduction assay. For technical reasons, our initial screen was limited to viral regulatory gene products that are dispensable for virus replication in tissue culture.
Control and IFN-α-pretreated monolayers of Vero cells were infected with serial dilutions of wild-type HSV-1 strains KOS and 17syn+, and mutants of these strains bearing lesions in the genes encoding IE proteins ICP0 and IC22, the virion transactivator VP16, the predicted protein kinase encoded by the UL13 gene, and the virion host shutoff protein (vhs) encoded by the UL41 gene. Viral titers were then determined by counting plaques, and the results were expressed as the ratio of the titers observed in the absence and presence of IFN (Table 1). Consistent with previous data (23), plaque formation by wild-type strains KOS and 17syn+ was only marginally reduced by IFN-α (two- to fivefold reduction). In striking contrast, ICP0 mutants of both strains exhibited greatly increased sensitivity to IFN. Isolates 7134 (5), dl1403 (50), and n212 (5) were inhibited more than 200-fold, while dlX3.1 (48) displayed a greater than 1,000-fold reduction in titer. Isolates 7134, dl1403, and dlX3.1 bear large deletions of ICP0 coding sequences, while n212 is truncated by an amber codon following residue 212. The deletion in dlX3.1 also removes the majority of the transcriptional regulatory signals upstream of the ICP0 gene. ICP0 mutants display impaired expression of IE, early, and late genes during infection of cultured cells (15), and so it was possible that the hypersensitivity exhibited by these mutants was an indirect consequence of reduced expression of one or more viral proteins that are downstream in the lytic cascade. However, this seems unlikely, since the VP16 in1814 (1) and V422 (49) mutations, which eliminate VP16 transactivation, cause defects in viral gene expression similar to those exhibited by ICP0-null mutants (1, 39, 49), but are much less sensitive to IFN-α (Table 1). In agreement with previous results (23), in1814 was inhibited to a slightly greater extent than parental wild-type strain 17syn+, while V422 was approximately 5- to 10-fold more sensitive than parental wild-type strain KOS. This latter result is consistent with our previous data indicating that V422 displays a somewhat more severe phenotype than in1814 (39). Mutants defective in the protein kinase encoded by the UL13 gene (d13lacZ) (30), IE protein ICP22 (d22lacZ) (30), and the virion host shutoff protein vhs (ΔSma) (46) were as resistant to IFN-α as parental wild-type HSV-1 strain KOS (Table 1). Taken in combination, these data demonstrate that expression of functional ICP0 is required for the relatively IFN-resistant phenotype of wild-type HSV-1.
TABLE 1.
Effects of IFN-α and cell type on plaque formation efficiency of different virusesa
| Virus (strain) | Gene inactivated | −IFN-α/+IFN-α ratio
|
|
|---|---|---|---|
| Vero cells | U2OS cells | ||
| KOS | 5.5 | 3.9 | |
| 4 | 4.6 | ||
| 5.8 | 4.8 | ||
| 6.2 | |||
| 17syn+ | 7 | 2.2 | |
| 2.5 | 1.9 | ||
| 2.4 | |||
| 2.0 | |||
| n212 (KOS) | ICP0 | 230 | 10.9 |
| 207 | 10 | ||
| 285 | 8.5 | ||
| 260 | |||
| dlX3.1 (KOS) | ICP0 | 1,550 | 4.4 |
| >1,200 | 5.1 | ||
| 7134 (KOS) | ICP0 | 300 | 1.6 |
| >200 | |||
| dl1403 (17syn+) | ICP0 | 320 | 3 |
| 200 | |||
| in1814 (17syn+) | VP16 | 14 | 2.9 |
| 22 | 3.8 | ||
| 10.9 | |||
| V422 (KOS) | VP16 | 16 | 10 |
| 45 | 28 | ||
| d13lacZ (KOS) | UL13 | 4 | 5.4 |
| d22lacZ (KOS) | ICP22 | 5 | 10 |
| ΔSma (KOS) | vhs | 5.8 | 4.4 |
| VSV | >20,000 | 6.7 | |
Vero and U2OS cells were mock treated or pretreated for 16 h with 1,000 U of human lymphoblastoid IFN-α (Sigma) per ml prior to infection. Results from individual experiments are reported for each virus in each cell line, with the ratio determined by dividing the viral titer in the absence versus presence of IFN-α pretreatment. The symbol > indicates that no plaques were visible at the lowest dilution of virus tested in the presence of IFN-α, and thus the exact level of inhibition is unknown in that experiment.
Previous studies have shown that the growth defects of ICP0 and VP16 mutants are “complemented” in U2OS osteosarcoma cells (39, 49, 52). Interestingly, all of the mutants analyzed above demonstrated approximately the same sensitivity to IFN-α on U2OS cells as wild-type HSV-1 on Vero cells (Table 1). Thus, in Vero cells, HSV-1 mutants lacking functional ICP0 are exquisitely sensitive to IFN-α, while in U2OS cells, IFN-α inhibition is not affected by the functional status of ICP0. These data raise the possibility that the ability of U2OS cells to “complement” ICP0 mutants (52) may stem from a defect in one or more components of the IFN-inducible antiviral defense system. Consistent with this possibility, VSV is also highly resistant to IFN-α in U2OS cells (Table 1). Further experiments are required to test this hypothesis.
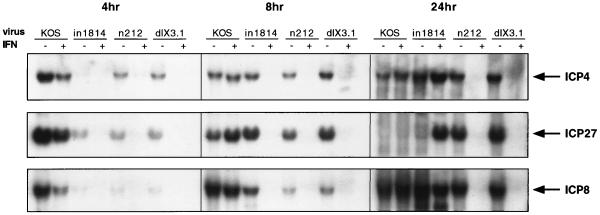
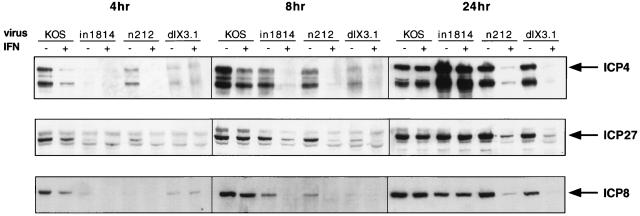
Given the published data indicating that IFN-α inhibits IE transcription during infection with wild-type HSV, it seemed possible that the hypersensitivity of ICP0 mutants revealed above stems at least in part from a failure to accumulate adequate quantities of IE mRNAs. We therefore examined the accumulation of viral transcripts in IFN-treated and control Vero cells at various times postinfection with 5 PFU of wild-type HSV-1, in1814, n212, and dlX3.1 per cell (Fig. 1). Viral titers were determined on U2OS cells in the presence of 3 mM hexamethylene-bis-acetamide (34, 39, 49) to control for the known increase in the particle/PFU ratio of VP16 and ICP0 mutants (1, 15). Untreated cells accumulated easily detectable amounts of the IE mRNAs encoding ICP4 and ICP27 by 8 h postinfection, regardless of the infecting virus, although in1814, n212, and dlX3.1 were significantly delayed relative to KOS. In contrast, only KOS displayed readily detectable IE RNA in IFN-α-treated cells at the 8-h time point. By 24 h postinfection, however, a clear distinction was observed between IFN-α-treated cells infected with in1814 and those infected with n212 or dlX3.1. High levels of IE RNA were observed in cells infected with in1814, while much less RNA was detected in cells infected with either ICP0 mutant. Identical results were obtained when the blots were stripped and reprobed for ICP0 mRNA, except that, as expected, dlX3.1 failed to accumulate the ICP0 transcript (data not shown). A similar trend was observed for the early transcript encoding ICP8, except for slightly delayed kinetics, as expected. These data indicate that ICP0 mutants display a severe defect in accumulation of IE RNAs in IFN-α-treated Vero cells. In marked contrast, all of the mutant viruses rapidly accumulated high levels of viral IE transcripts in both control and IFN-α-treated U2OS cells (data not shown). Concurrent Western blot analyses yielded a good correlation between mRNA levels and the levels of ICP4, ICP27, and ICP8 protein during infection of Vero cells (Fig. 2).
FIG. 1.
Effect of IFN pretreatment on accumulation of viral mRNAs. Vero cells were mock treated (−) or pretreated (+) with 1,000 U of IFN-α per ml for 16 h prior to infection with 5 PFU of the indicated virus per cell. Total RNA was harvested either 4, 8, or 24 h postinfection by using Trizol (Gibco BRL). Aliquots (5 μg) were then analyzed for ICP4, ICP27, or ICP8 transcript levels by Northern blot analysis with 32P random-primer-labeled probe by using ExpressHyb (Clontech).
FIG. 2.
Effect of IFN pretreatment on accumulation of ICP4, ICP27, and ICP8. Vero cells were mock treated (−) or pretreated (+) with 1,000 U of IFN-α per ml for 16 h prior to infection with 5 PFU of the indicated virus per cell. Infected cells were harvested directly into sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer at 4, 8, and 24 h postinfection. Lysates were run on 9% polyacrylamide gels, transferred to nitrocellulose membranes, and blotted for ICP4, ICP27, or ICP8 protein by using a 1:2,000 dilution of GICR 1114, 1113, or 1115, respectively (Goodwin Institute for Cancer Research).
Taken together, these experiments demonstrate that ICP0 is required to overcome the antiviral effects of IFN-α. In the absence of VP16 activation function, accumulation of viral RNA and protein is significantly delayed, but eventually proceeds, giving rise to high levels of viral gene products. In contrast, ICP0 mutants are severely crippled in their ability to bypass the IFN-induced blockade. It is tempting to speculate that the slightly increased IFN sensitivity of VP16 mutants revealed in the plaque reduction assay stems from reduced and/or delayed production of ICP0.
The link between ICP0 and the resistance of HSV-1 to IFN revealed by our data is both intriguing and significant, given the results of recent studies of the role of ICP0 during HSV-1 infection. ICP0 has been shown to interact strongly and specifically with a ubiquitin-specific protease, HAUSP (13), as well as localize to and subsequently disrupt discrete nuclear domains called ND10 (33). ND10 are considered sites of DNA virus transcription and regulation, because many DNA viral genomes are deposited at the periphery of ND10 early in infection (32). ND10 are sites of accumulation of various proteins, most notably PML and Sp100, both of which are highly inducible by IFN, suggesting that ND10 may be involved in a nuclear defense mechanism (32). ICP0 appears to abrogate the SUMO-1 (ubiquitin-like) modification of ND10-specific proteins, thus causing the proteasome-dependent degradation of key cellular factors, whose normal protection by HAUSP is eliminated through its interaction with ICP0 (7, 11, 40). It has also been suggested that intact ND10 may inhibit viral DNA replication by masking important nuclear attachment sites, sites that are exposed upon disruption of ND10 by ICP0 (4).
Recent experiments have shown that the interaction with HAUSP contributes to the functional activities of ICP0 (12) and that inhibition of the ubiquitin-proteasome pathway inhibits the ability of ICP0 to stimulate lytic infection as well as reactivation of latent genomes (11). One interpretation of these data is that host repression proteins target incoming viral genomes, and ICP0 is required to induce the degradation of these inhibitory proteins (13, 14). Interpreted within the framework of this model, our data strongly suggest that some or many of the key inhibitory proteins targeted by ICP0 are IFN inducible. Identification of possible IFN-induced repressor proteins would solidify this hypothesis and warrants further investigation. If this model is correct, then it is possible that U2OS cells are defective in the repression mechanism, instead of expressing an ICP0-like activator as originally suggested (52). In this context, we are intrigued by the observation that VSV is highly resistant to IFN-α in these cells (Table 1). This result implies that U2OS cells are defective in one or more IFN-induced antiviral mechanisms. Although it is not yet clear if this defect accounts for the efficient replication of ICP0 mutants in this cell line in the absence of IFN (52), it is tempting to speculate that basal expression of one or more IFN-inducible inhibitors accounts for the requirement for ICP0 in other cell types. Consistent with this hypothesis, ICP0 mutants replicate poorly in wild-type mice, but replication is almost fully restored in IFN receptor-null mice, attesting to the importance of both ICP0 for HSV-1 and IFN for the host (9, 26, 27).
While the antiviral mechanisms of IFN are exerted through both nuclear and cytoplasmic events, the majority of the viral proteins that counteract IFN act either outside the cell or within the cytoplasm. While our data do not identify the key targets of ICP0, it is possible that one or more targets are nuclear. Due to the design of our experiments, in which an IFN-induced antiviral state has been established prior to infection, it is fairly clear that ICP0 is not involved in disrupting the IFN-α signal transduction cascade, as is the case for human cytomegalovirus, but instead targets events which occur following production of IFN-stimulated antiviral proteins. Further studies are ongoing to elucidate more precisely where and how ICP0 functions relative to IFN, in order to further understand the mechanisms of action of both IFN and ICP0 in Vero and U2OS cells.
Acknowledgments
We thank Rob Maranchuk for technical assistance and P. Schaffer and R. Everett for generously providing mutant virus recombinants.
This work was supported by a grant from the Medical Research Council of Canada. K.L.M. is funded by fellowships from the MRC and the Alberta Heritage Foundation for Medical Research. J.R.S. was a Terry Fox Senior Scientist of the National Cancer Institute of Canada.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altinkilic B, Brandner G. Interferon inhibits herpes simplex virus-specific translation: a reinvestigation. J Gen Virol. 1988;69:3107–3112. doi: 10.1099/0022-1317-69-12-3107. [DOI] [PubMed] [Google Scholar]
- 3.Barry M, McFadden G. Virokines and viroceptors. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 251–261. [Google Scholar]
- 4.Burkham J, Coen D, Weller S K. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J Virol. 1998;72:10100–10107. doi: 10.1128/jvi.72.12.10100-10107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai W, Schaffer P A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassady K A, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72:8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelbi-Alix M K, de The H. Herpes virus induced proteosome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene. 1999;18:935–941. doi: 10.1038/sj.onc.1202366. [DOI] [PubMed] [Google Scholar]
- 8.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 9.Clements J B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 10.De Stasio P R, Taylor M W. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J Virol. 1990;64:2588–2593. doi: 10.1128/jvi.64.6.2588-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett R D, Meredith M, Orr A. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J Virol. 1999;73:417–426. doi: 10.1128/jvi.73.1.417-426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett R D, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett R D, Orr A, Preston C M. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 1998;17:7161–7169. doi: 10.1093/emboj/17.24.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett R D, Preston C M, Stow N D. Functional and genetic analysis of the role of Vmw110 in herpes simplex virus replication. In: Wagner E K, editor. The control of herpes virus gene expression. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 49–76. [Google Scholar]
- 16.Gale M, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 17.Gresser I. Wherefore interferon? J Leukoc Biol. 1997;61:567–574. doi: 10.1002/jlb.61.5.567. [DOI] [PubMed] [Google Scholar]
- 18.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Gross M, Roizman B. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1 α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc London Ser B. 1957;147:258–267. [PubMed] [Google Scholar]
- 22.Jacobs B L, Langland J O. Viral inhibitors of interferon action: inhibitors of the PKR and 2′5′ oligoadenylate synthetase/RNaseL pathways. In: Karupiah G, editor. Gamma interferon in antiviral defense. R. G. Austin, Tex: Landes Company; 1997. pp. 155–173. [Google Scholar]
- 23.Jamieson D R S, Robinson L H, Daksis J I, Nicholl M J, Preston C M. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus Vmw65 mutants. J Gen Virol. 1995;76:1417–1431. doi: 10.1099/0022-1317-76-6-1417. [DOI] [PubMed] [Google Scholar]
- 24.Kotwal G J. Microorganisms and their interaction with the immune system. J Leukoc Biol. 1997;62:415–429. doi: 10.1002/jlb.62.4.415. [DOI] [PubMed] [Google Scholar]
- 25.LaMarco K L, McKnight S L. Purification of a set of cellular polypeptides that bind to the purine-rich cis-regulatory element of herpes simplex virus immediate early genes. Genes Dev. 1989;3:1372–1383. doi: 10.1101/gad.3.9.1372. [DOI] [PubMed] [Google Scholar]
- 26.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotypes of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipp M, Brandner G. Herpes simplex virus gene expression in interferon-treated cells. In: Kirchner H, Schellekens H, editors. The biology of the interferon system. Amsterdam, The Netherlands: Elsevier; 1985. pp. 355–360. [Google Scholar]
- 29.Lipp M, Brandner G. Inhibition of herpes simplex virus type 1 specific translation in cells treated with poly(rI) · poly(rC) J Gen Virol. 1980;47:97–111. doi: 10.1099/0022-1317-47-1-97. [DOI] [PubMed] [Google Scholar]
- 30.Long M C, Leong V, Schaffer P A, Spencer C A, Rice S A. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J Virol. 1999;73:5593–5604. doi: 10.1128/jvi.73.7.5593-5604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus P I, Engelhardt D L, Hunt J M, Sekellick M J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971;174:593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- 32.Maul G G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Maul G G, Guldner H H, Spivack J G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0) J Gen Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 34.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes simplex virus immediate-early gene expression in the absence of trans-induction by Vmw65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 35.Miller D M, Zhang Y, Rahill B M, Waldman W J, Sedmak D D. Human cytomegalovirus inhibits IFN-α stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-α signal transduction. J Immunol. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 36.Mittnacht S, Straub P, Kirchner H, Jacobsen H. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology. 1988;164:201–210. doi: 10.1016/0042-6822(88)90637-x. [DOI] [PubMed] [Google Scholar]
- 37.Mohr I, Gluzman Y. A herpes virus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 38.Mossman K, Barry M, McFadden G. Regulation of gamma interferon gene expression and extracellular ligand function by immunomodulatory viral proteins. In: Karupiah G, editor. Gamma interferon in antiviral defense. R. G. Austin, Tex: Landes Company; 1997. pp. 175–188. [Google Scholar]
- 39.Mossman K L, Smiley J R. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 renders expression of the immediate-early genes almost entirely dependent on ICP0. J Virol. 1999;73:9726–9733. doi: 10.1128/jvi.73.12.9726-9733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller S, Dejean A. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J Virol. 1999;73:5137–5143. doi: 10.1128/jvi.73.6.5137-5143.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholl M J, Preston C M. Inhibition of herpes simplex virus type 1 immediate-early gene expression by alpha interferon is not VP16 specific. J Virol. 1996;70:6336–6339. doi: 10.1128/jvi.70.9.6336-6339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberman F, Panet A. Characterization of the early steps of herpes simplex virus replication in interferon-treated human cells. J Interferon Res. 1989;9:563–571. doi: 10.1089/jir.1989.9.563. [DOI] [PubMed] [Google Scholar]
- 43.Oberman F, Panet A. Inhibition of transcription of herpes simplex virus immediate early genes in interferon-treated human cells. J Gen Virol. 1988;69:1167–1177. doi: 10.1099/0022-1317-69-6-1167. [DOI] [PubMed] [Google Scholar]
- 44.O'Hare P. The virion transactivator of herpes simplex virus. Semin Virol. 1993;4:145–155. [Google Scholar]
- 45.Poon A P W, Roizman B. Differentiation of the shutoff of protein synthesis by virion host shutoff and mutant γ134.5 genes of herpes simplex virus. Virology. 1997;229:98–105. doi: 10.1006/viro.1996.8425. [DOI] [PubMed] [Google Scholar]
- 46.Read G S, Karr B M, Knight K. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J Virol. 1993;67:7149–7160. doi: 10.1128/jvi.67.12.7149-7160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fundamental virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1043–1107. [Google Scholar]
- 48.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smiley J R, Duncan J. 1814 linker insertion mutation. J. Virol. 71:6191–6193. 1997. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 produces a phenotype similar to that of the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stow N D, Stow E C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate-early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 51.Taylor J L, Little S D, O'Brien W J. The comparative anti-herpes simplex virus effects of human interferons. J Interferon Cytokine Res. 1998;18:159–165. doi: 10.1089/jir.1998.18.159. [DOI] [PubMed] [Google Scholar]
- 52.Yao F, Schaffer P A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]