Abstract
The latent structure of executive functions (EFs) remains controversial. Confirmatory factorial analysis (CFA) has provided support for both multidimensional (assumes EFs to be functionally separable but related components) and bifactor (proposes all components are nested within a common factor) models. However, these CFA models have never been compared in patient samples, nor regarding their neuroanatomical correlates. Here, we systematically contrast both approaches in neurotypicals and in a neurodegenerative lesion model (patients with the behavioral variant frontotemporal dementia, bvFTD), characterized by executive deficits associated with frontal neurodegeneration. First, CFA was used to test the models’ fit in a sample of 341 neurotypicals and 29 bvFTD patients based on performance in an executive frontal screening battery which assesses working memory, motor inhibition, verbal inhibition, and abstraction capacity. Second, we compared EFs factor and observed scores between patients and matched controls. Finally, we used voxel-based morphometry (VBM) to compare the grey matter correlates of factor and observed scores. CFA results showed that both models fit the data well. The multidimensional model, however, was more sensitive than the bifactor model and the observed scores to detect EFs impairments in bvFTD patients. VBM results for the multidimensional model revealed common and unique grey matter correlates for EFs components across prefrontal-insular, posterior, and temporal cortices. Regarding the bifactor model, only the common factor was associated with prefrontal-insular hubs. Observed scores presented scant, non-frontal grey matter associations. Converging behavioral and neuroanatomical evidence from healthy populations and a neurodegenerative model of EFs supports an underlying multidimensional structure.
Keywords: Executive functions, Confirmatory factorial analysis, Voxel-based morphometry, bvFTD, Lesion model
1. Introduction
Executive functions (EFs) are high-level cognitive processes that play crucial roles in the organization of mental resources to accomplish goals (Diamond, 2013). Despite the relevance of EFs in research and clinical settings, the construct itself remains poorly understood (Baggetta & Alexander, 2016; Jurado & Rosselli, 2007). While there is little controversy that EFs comprise multiple components (Baggetta & Alexander, 2016; Jurado & Rosselli, 2007), it is not clear whether their latent structure consists of distinct but related constructs, as proposed by multidimensional models, or whether they depend on a single underlying ability, as proposed by bifactor models (Friedman & Miyake, 2017; Karr et al., 2018). However, these competing latent approaches have never been systematically compared in patient samples, nor have they been compared regarding their neuroanatomical correlates. Here, we contrast a multidimensional and a bifactor model of EFs in neurotypicals and in a lesion model composed of behavioral variant frontotemporal dementia (bvFTD) patients, characterized by executive deficits mainly associated with frontal neurodegeneration. We performed confirmatory factorial analysis (CFA) and voxel-based morphometry (VBM) to evaluate which model is more sensitive to detect neurocognitive dysfunction in patients. We expect to bring novel integrated behavioral and neuroanatomical evidence to help elucidate the latent structure of EFs, which has been studied mainly based on performance measures.
Numerous components have been subsumed under the EFs umbrella term, including working memory (WM; the temporary storage and manipulation of information in mind (Baddeley, 2007; Wechsler, 1987)), inhibition (the ability to override a prepotent motor or verbal response (Aron, Robbins, & Poldrack, 2004), and abstraction capacity (Abs.C; conceptualization (Dubois, Slachevsky, Litvan, & Pillon, 2000)), among others. The organization of EFs components is still a matter of debate (Baggetta & Alexander, 2016; Jurado & Rosselli, 2007). Some lines of evidence suggest that EFs are a set of diverse cognitive capacities. EFs dissociate in some patient studies, in relation to the functional specialization of the frontal lobes (Godefroy, Cabaret, Petit-Chenal, Pruvo, & Rousseaux, 1999; Stuss, 2011; Stuss & Alexander, 2007; Tsuchida & Fellows, 2013). WM would be critically associated with the dorsolateral prefrontal cortex (D’Esposito et al., 1998; Smolker et al., 2015; Wager & Smith, 2003), inhibition with inferior, medial, and orbitofrontal regions (Aron et al., 2004; Collette et al., 2005; Stuss, 2011), and Abs.C with rostral-prefrontal areas (Dumontheil, 2014; Nee, Jahn, & Brown, 2014). On the other hand, the existence of a single ability underlying all EFs has also been proposed (Duncan, Johnson, Swales, & Freer, 1997; Friedman & Miyake, 2017; Obonsawin et al., 2002). Notably, different EFs share fronto-parietal engagement (Fedorenko, Duncan, & Kanwisher, 2013; Hedden & Gabrieli, 2010; Niendam et al., 2012).
A crucial shortcoming in EFs conceptualization lies in the interference of non-executive processes during task performance, or “task impurity” (Burgess, 1997). Factorial analysis, such as CFA, can reduce this confounding by extracting common variance across different tasks to represent a latent (i.e., pure) construct (Miyake et al., 2000). In healthy adult populations, both factorial multidimensional and bifactor models have received empirical support (Karr et al., 2018). The first one assumes that EFs components are functionally separable (although related) constructs (Miyake et al., 2000). Bifactor models2 propose that all components are nested within a common factor (CF) that predicts performance in all tasks (Friedman & Miyake, 2017). More specifically, bifactor models include a CF that comprises the commonality of all observed variables, and multiple domain-specific factors representing the unique influence of each specific component (Chen, West, & Sousa, 2006). Thus, the observed variables are directly influenced by the common and domain-specific factors, which are assumed to present orthogonal relationships (Chen et al., 2006). This aspect represents an advantage over unidimensional models, where the observed variables are uniquely affected by the CF (Brunner, Nagy, & Wilhelm, 2012). Consequently, unidimensional models cannot capture simultaneously domain-specific factors, which are frequently reported in CFA models of EFs (Karr et al., 2018). A re-analysis of 46 CFA in healthy populations (Karr et al., 2018) found that both multidimensional and bifactor models present similar fit indices and none could be unequivocally preferred. However, models have never been directly compared in clinical samples, nor regarding their brain correlates. Evidence from lesion and neuroanatomical approaches may help to characterize and compare both models.
Lesion models, including the neurodegenerative (García-Cordero et al., 2016; Melloni et al., 2016; Santamaría-García et al., 2017), can be useful to scrutinize the predictions of EFs. Patients with distinctive neurocognitive profiles allow for direct testing of the hypothesis regarding a model’s brain-behavior associations. In this context, patients with bvFTD are characterized by early and selective deficits in EFs associated with frontal neurodegeneration (Harciarek & Cosentino, 2013; Johnen & Bertoux, 2019; Piguet et al., 2011; Possin et al., 2013). The pattern of progressive degeneration among patients with bvFTD begins in the medial and orbitofrontal regions, followed by the anterior temporal pole, dorsolateral prefrontal cortices, and eventually the hippocampus and the basal ganglia (Kril & Halliday, 2004). Despite the fact that the initial site of atrophy does not lie in dorsolateral prefrontal cortices –which are typically engaged in EFs– current meta-analytic evidence (Beeldman et al., 2018; Kamath, Chaney, Deright, & Onyike, 2019) points to executive dysfunctions as core symptoms of bvFTD, with changes in mentalizing abilities being secondary to them (Schroeter et al., 2014). Moreover, executive impairment has been associated with the ventromedial compromise in bvFTD (Baez et al., 2019; Ducharme, Price, & Dickerson, 2018; Garcia-Cordero et al., 2019; Lu et al., 2013). Thus, the bvFTD constitutes a dysexecutive-frontal lesion model to test EFs models’ outcomes, especially in mild stages of the disease (such as the current sample).
With some exceptions (Ambrosini, Arbula, Rossato, Pacella, & Vallesi, 2019; Bettcher et al., 2016; Smolker et al., 2015, 2018), the majority of CFA studies in this field are based exclusively on behavioral measures claiming an integrated approach with brain measures (Ambrosini et al., 2019). In this line of research, VBM is a widely used method to study the grey matter correlates of EFs which greatly overlap with their functional bases (Ruscheweyh, Deppe, Keller, et al., 2013; Smolker et al., 2018, 2015; Weise et al., 2019). Also, structural neuroimaging has the advantage of being easy to implement in patient samples given its brevity and low demand relative to task-based functional methods.
Against this background, this work aims to compare two competing approaches to the organization of EFs in neurotypicals and a lesion model composed by a group of patients with bvFTD by combining CFA and VBM. First, we implemented CFA to assess the fit of robust multidimensional and bifactor models of EFs based on the performance of a large sample of participants (n = 370) on the INECO Frontal Screening (IFS) (Torralva et al., 2009), a validated battery that evaluates WM, motor inhibition (M.Inh), verbal inhibition (V.Inh), and Abs.C. We chose the IFS given its reliable psychometric properties (see details in Materials and methods, section 2.2). As age has strong effects on EFs structure (Bock, Haeger, & Voelcker-Rehage, 2019), we performed measurement invariance testing across that variable. We then compared IFS factor and observed scores between bvFTD patients (n = 29) and a sub-sample of controls (n = 24) matched in relevant demographic variables. Finally, we used VBM to assess the grey matter correlates of IFS factor and observed sores in the bvFTD group in tandem with its paired controls.
Based on previous evidence (Karr et al., 2018), we expect both models to fit the data well. Also, in light of evidence from frontal lesions (e.g., Tsuchida and Fellows, 2013) suggesting a fractionated structure of EFs, we hypothesize that the multidimensional model will provide a more sensitive discrimination of frontal-executive deficits in patients compared to the bifactor model and the observed IFS scores.
2. Materials and methods
We report how we determined our sample size, all data exclusions (if any), all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
2.1. Participants
A total of 370 participants were enrolled in this study −341 healthy controls and 29 bvFTD patients. For further statistical analyses, a subsample of 24 controls was sex, age, and education-matched with the bvFTD group (paired controls) –See demographics in Table 1. Sample size adequacy was determined using GPower 3.1 software. Our statistical design [two-group comparisons using Mann–Whitney U tests (predicting worse performance in bvFTD vs controls)] requires a minimum of 24 subjects per group to achieve an effect size d of 1, with α = .05 and β = .95. Participants’ inclusion and exclusion criteria (as detailed below) were established prior to assessment and data analysis.
Table 1 –
Participants’ demographic data.
| Healthy controls (full sample) n = 341 | bvFTD n = 29 | Paired controls n = 24 | bvFTD versus paired controls (stats) | |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Female | 164 | 15 | 13 | χ2 = .0 |
| Male | 172 | 14 | 11 | p = 1.0 |
| Age | 44.14 (16.7) [18–89] | 69.2 (7.45) [55–84] | 69.2 (7.47) [57–83] | t = .0p = 1.0 |
| Years of formal education | 11.9 (4.8) [0–25] | 14.0 (3.97) [4–19] | 15.2 (3.52) [6–20] | U = 1.0 p = .17 |
| ACE-III | – | 72.5 (15.9) [46–92] | 93.7 (4.6) [83–100] | U = 4.2 p < .001 |
Data are presented as mean (SD) [range], excepting sex. For categorical variables, we used χ2 test. For continuous variables, we used Student-t or Mann-Whitney U tests depending on data distribution. ACE-III: Addenbrooke’s Cognitive Examination-III; bvFTD: behavioral variant frontotemporal dementia.
Patients were diagnosed by an expert team composed of cognitive neurologists, psychiatrists, and neuropsychologists, following current revised criteria (Rascovsky et al., 2011). They were in mild stages of the disease according to expert criteria and atrophy pattern (Supplementary Table 1 and Supplementary Figure 1), compatible with a score of 1 in the Clinical Dementia Rating scale (Seeley et al., 2008) –index of mild impairment (Morris, 1993). On average, bvFTD patients presented declined cognitive state, as measured with the Addenbrooke’s Cognitive Examination test-III (ACE-III) (< 83, cut-off for mild dementia (Mathuranath, Nestor, Berrios, Rakowicz, & Hodges, 2000) (Table 1). Yet, patients’ ACE-III scores were highly variable, ranging from severely impaired to normal performance (Table 1), as usually reported (e.g., Chen et al., 2020; Dottori et al., 2017; Hornberger et al., 2011), even in advanced stages of the disease (Sheelakumari et al., 2020). Performance on general cognitive screening tests does not always accurately reflect bvFTD patients’ clinical manifestations or functionality (Hornberger, Piguet, Kipps, & Hodges, 2008; Kipps, Nestor, Fryer, & Hodges, 2007; Rahman et al., 1999; Schroeter et al., 2014), with executive and social cognition assessments being more sensitive to the hallmark features of this condition (Harciarek & Cosentino, 2013; Johnen & Bertoux, 2019; Piguet et al., 2011). No diagnosis nor signs of motor neuron disease/ALS nor motor impairments were registered in any patient. Controls declared no history of psychiatric or neurological conditions, substance abuse disorder, heart or vascular diseases, did not report symptoms of cognitive decline, and had normal executive functioning skills (see section 3.2). All subjects signed an informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the host institution.
2.2. The INECO Frontal Screening
All participants completed the IFS, a 10-minute easy-to-administer, robust screening tool (Torralva et al., 2009) that includes eight subtests to tap four EFs components: WM, M.Inh, V.Inh, and Abs.C. This battery has shown good internal consistency, and high reliability and concurrent validity (Ihnen, Antivilo, Muñoz-Neira, & Chonchol, 2013; Torralva et al., 2009). Performance on the IFS is related with gold-standard EFs tests such as the Frontal Assessment Battery, the Trail Making Test part B, the Wisconsin Card Sorting Test, and verbal phonological fluency (Baez et al., 2014; Custodio et al., 2016; Gleichgerrcht, Roca, Manes, & Torralva, 2011; Ihnen et al., 2013; Torralva et al., 2009). The IFS was created on the basis of clinical experience, integrating the most sensitive and specific tasks to detect executive dysfunction in dementia (Custodio et al., 2016; Gleichgerrcht et al., 2011; Torralva et al., 2009), has been validated in other neuropsychiatric disorders (Baez et al., 2014, 2019; Bruno et al., 2015; Custodio et al., 2016; Fiorentino et al., 2013a, 2013b; Gleichgerrcht et al., 2011), and proved useful in both young and old healthy subjects (Fittipaldi et al., 2020; García-Cordero et al., 2017; Sierra Sanjurjo et al., 2019). The IFS cut-off is 18, with a sensitivity of .90 and a specificity of .86 to detect executive dysfunction (Ihnen et al., 2013). All IFS subtests exhibit high sensitivity by themselves (Moreira, Lima, & Vicente, 2014; Torralva et al., 2009).
WM is measured through the following subtests with a maximum 12 points:
Backwards digit span. This task captures the capacity to temporarily hold acoustic information in mind via phonological storage and an articulatory rehearsal mechanism (Baddeley, 2007; Hodges, 1994; Wechsler, 1987). Participants are required to repeat a progressively lengthening string of digits in reverse order (up to seven digits). Each length includes two trials and successful performance in at least one of them is necessary to move forward, with a maximum of six points.
Spatial working memory. It evaluates the ability to maintain in mind and manipulate visuo-spatial information to use in the task at hand (Baddeley, 2007; Wechsler, 1987). Participants are presented with a sequence of finger movements over four cubes, which are required to reproduce in reverse order. In total, four sequences are presented, and one point is given for each correctly performed. The maximum score is four points.
Verbal working memory. This subtest also tracks phonological WM (as backwards digit span), but with a less demanding cognitive load since the series is highly overlearned for most individuals (Hodges, 1994; Torralva et al., 2009). Participants are asked to list the months of the year in inverse order (starting with December). The maximum score for perfect performance is two (one error being penalized with one point, and two or more errors corresponding to zero points).
M.Inh (i.e., the capacity to cancel an intended movement) is measured through the following tasks with a maximum of nine points:
Motor programming. This subtest is sensible to inhibition deficits, which may be observed as perseveration (i.e., inappropriate repetition) of movements (Dubois et al., 2000). It consists of performing the Luria series “fist, edge, palm” six times after copying the examiner three times. The score is three points for a performance without errors, two points if at least three consecutive series are correct, one point if three series can be copied, and zero points otherwise.
Conflicting instructions. This subtest captures the ability to obey a verbal command while inhibiting automatic imitation of the examiner’s movements (Dubois et al., 2000). Participants are instructed to hit the table once or twice when the examiner hits it twice or once, respectively across a series. Three points are given for error-free performance, two points when one or two errors are committed, one point for more than two errors, and zero points otherwise.
Go-No Go. This subtest measures the capacity to inhibit a motor response that was previously given to a similar stimulus (Drewe, 1975; Dubois et al., 2000). The instruction is to hit the table once or do nothing when the examiner hits it once or twice, respectively, in a series. The task is applied immediately after conflict instructions, with identical scoring.
V.Inh (i.e., the capacity to inhibit a verbal response) is assessed through the following task, with a maximum of six points:
Modified version of the Hayling test. This subtest evaluates the capacity to override highly overlearned and expected verbal responses to behave in a contextually adequate manner (Burgess & Shallice, 1997). Participants are presented with three sentences whose last word is missing and are asked to complete them with a syntactically correct but semantically incorrect word as quickly as possible. Sentences are constructed to strongly constrain what the individual should say (e.g., “An eye for an eye and a tooth for a…”). The maximum score for each sentence is two points. If the participant completes the sentence with a semantically related word, only one point is given. Using the exactly expected word corresponds to zero points.
Abs.C is measured in the IFS through the following subtest, with a maximum of three points:
Proverb interpretation task. This task is usually employed to assess abstract thought (Dubois et al., 2000; Lezak, 1983). Three proverbs are given to the participant, who is required to explain their meaning. One point is given for each proverb correctly explained, .5 points are awarded if an example is given, and zero points are awarded in all other scenarios. For example, the English version of the IFS includes the following proverb: “A bird in the hand is worth two in the bush”. The participant would obtain one point if they were able to explain its abstract meaning; “it is better to be content with what you have than risk losing it by trying to get something better”. Alternatively, the participant would get one half of a point if they were to provide an example such as “you shouldn’t spend your savings in the lottery for the uncertain possibility of winning a high sum of money”, because it denotes some degree of abstraction. Finally, they would get zero points if they were to provide a literal response (e.g., “it is better to catch one bird than see two in the bush”). This scoring follows standard recommendations (Murphy et al., 2013).
2.3. MRI acquisition
Structural MRI recordings were obtained from bvFTD patients (n = 29) and their paired controls (n = 24) –See demographics in Table 1. Image acquisition and analysis are reported following the practical guide of the Organization for Human Brain Mapping (Nichols et al., 2017; Poldrack et al., 2017). Using a 1.5 T Phillips Intera scanner with a standard head coil (8 channels), we acquired T1-weighted anatomical 3D spin echo sequences parallel to the plane connecting the anterior and posterior commissures, covering the whole brain. The following parameters were used: 196 slices, TR = 7489 msec, TE = 3420 msec, flip angle = 8◦, matrix size = 256 × 240, voxel size = 1 × 1 × 1 mm3, total scan duration = 7 min.
2.4. Statistical analyses
2.4.1. Confirmatory factorial analysis
CFA was implemented to compare the fit of a multidimensional and a bifactor model from the observed IFS scores in the sample of 370 participants. The multidimensional model was composed of four components (WM, M.Inh, V.Inh, Abs.C), assuming functional differentiation with associations between them. The bifactor model included the same components as independent constructs nested in a CF. After removing the components’ shared variance in the CF, they are no longer related but explained as individual manifestations of a general domain ability.
Data analyses were performed in RStudio (R Core Team, 2020; RStudio Team, 2020), using various packages (Epskamp, 2019; Jorgensen et al., 2020; Rosseel, 2012; Wickham et al., 2019). CFA models were plotted using Ωnyx (Von Oertzen et al., 2015). Because the data were not normally distributed, we used maximum likelihood estimation with robust (Huber-White) standard errors. Full information maximum likelihood estimation method was implemented to handle missing data (.008% of the total measures). Goodness-of-fit of each model to the data was evaluated via global model fit indices that adjust for nonnormality: the Yuan-Bentler correction factor for the chi-square statistics (YB χ2), the robust comparative fit index (the robust CFI (Savalei, 2018)), and the robust root mean square error approximation (the robust RMSEA (Savalei, 2018)). For model selection, we used the Akaike’s Information Criterion (AIC). To discriminate between models, we used the AIC differences (ΔAIC) between the model with the smallest value and the other candidate models in the set. Differences between zero and two suggest little support to distinguish between models, from four to seven indicate less support for the model with the higher value, and a difference >10 suggest no support for the model with the higher value (Burnham & Anderson, 2002). The fixed variance method of identification was used in all models (Putnick & Bornstein, 2016).
The YB χ2 exams the exact-fit hypothesis that there is no difference between the model-implied covariance matrix and the population covariance matrix. A non-significant p-value (p ≥ .05) brings support to the exact-fit hypothesis. The robust RMSEA is an absolute fit index where a value of zero supports the exact-fit hypothesis (values > .08 considered as poor fit, values in the range of .05–.08 considered as adequate fit, and values ≤ .05 supporting the close-fit hypothesis (Browne & Cudeck, 1993)). The robust CFI assesses how the specified model improves fit over the null model (values > .95 considered as an acceptable fit, and values > .97 considered as excellent fit (Schermelleh-Engel et al., 2003). The AIC index has been formally proposed for the comparison of either nested or non-nested models of different complexity (those with the lowest values presenting a better fit (Burnham & Anderson, 2002)).
Due to the wide range of age of our participants (range = 18 – 89, median = 47) and expected age-related differences in the factors underlying the IFS, we dichotomized this variable into young and old adults (young ≤ 47, n = 187; older > 47, n = 183). This procedure is typical in this type of data modeling (Cheung & Rensvold, 2002; Little, 2013). To show that differences across age are due to differences in the factors underlying the IFS scores rather than differences related to unknown variables, we tested the IFS measurement invariance. This means that the factor structure and observed factor loadings and intercept values (i.e., the mean of the measured variables) are equal across age groups (i.e., scalar invariance model). Evidence for the latter model suggests that: (1) the latent variables have a common factorial structure across age groups; (2) age group differences in the factor means are unbiased; and (3) observed intercepts and factor loadings are directly related to the factor means (Kline, 2016). Details and criteria for testing measurement invariance are given in Supplementary Material 1.
We computed predicted factor scores of the IFS from the partial multidimensional and bifactor models to examine performance and correlations with grey matter volume in the group of bvFTD patients and its paired controls. Factor scores represent the prediction made by the model for each participant in each EFs component, as deviation units from the mean of the young group (0 ± 1). Lower scores represent lower predicted performance, and higher scores represent higher predicted performance.
2.4.2. Analysis of behavioral data
We compared the performance of bvFTD patients (n = 29) and their paired controls (n = 24) in the IFS scores predicted by the multidimensional and bifactor models (WM, M.Inh, V.Inh, Abs.C, CF) and those observed using Mann–Whitney U tests (since data were non-normally distributed). To consider results as significant, the α level was set at p < .05. Effect size for each comparison was estimated by the Cohen’s d, calculated with 5000 bootstrap resamples, using the package DABESTR for R (Ho, Tumkaya, Aryal, Choi, & Claridge-Chang, 2019).
2.4.3. MRI data
2.4.3.1. Images preprocessing.
T1-weighted images were processed using the Dartel Toolbox on SPM 12 running in MAT-LAB following validated VBM procedures (Ashburner & Friston, 2000). Preprocessing steps included segmentation into grey matter, white matter, and cerebrospinal fluid. Those images were used to estimate the total intracranial volume. Then, to improve between-subject alignment, a template based on grey and white matter segmentations was created for the complete data set (default parameters) (Ashburner, 2007). This template was used to affine transformation into MNI space to all individual grey matter images. Finally, images were modulated by Jacobian determinants and smoothed with a kernel of 12 mm.
2.4.3.2. Voxel-based morphometry analysis.
The atrophy pattern of bvFTD patients was calculated by comparing their grey matter maps with those of their paired controls, via two-sample t-tests (SPM module) (Supplementary Table 1 and Supplementary Figure 1). Then, we performed whole-brain multiple regression analyses (SPM module) to identify grey matter associations with scores predicted by CFA models and those observed. These analyses were made for the bvFTD group in tandem with its paired controls (n = 53) to increase behavioral variance, sample size, and statistical power (O’Callaghan et al., 2016; Sollberger et al., 2009). Total intracranial volume and a dummy variable codifying the group to which the participant belonged were included as nuisance covariates. The inclusion of these covariates reduces the inter-variability in head size (Pell et al., 2008) and the atrophy effect (Alkharusi, 2012).
To control for multiple comparisons, the statistical threshold was set at p < .05 at the cluster level with a voxel-level threshold of p < .001. The minimum cluster size (k) to consider results as significant was set using AlphaSim correction (Rest v1.8 software) (Song et al., 2011), which applies Monte Carlo simulations. The following parameters were used: individual voxel p = .005; rmm = 1; simulations = 1000. This approach is commonly used in VBM analysis (e.g., Peng et al., 2018; Tas et al., 2018; Zhang et al., 2013) to control for spurious findings while avoiding false negatives that could result when applying more conservative corrections such as FWE (Lieberman & Cunningham, 2009). Localization was derived from the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002).
3. Results
3.1. Confirmatory factorial analysis
Measurement invariance revealed that the multidimensional model was not fully scalar invariant. Thus, we computed the partial scalar multidimensional model (from here on, multidimensional model). See Supplementary Material 1 and Supplementary Table 2 for details. In this model, the observed intercepts of verbal WM, the first trial of the modified Hayling test, and the third trial of the proverb interpretation task were freed across age. The goodness-of-fit indices indicated that the exact-fit hypothesis cannot be rejected (YB χ2(109) = 112.880, p = .38); the robust RMSEA indicated a close fit of the model to data (robust RMSEA = .014, 90% CI = .0–.042). Similarly, the robust CFI showed an excellent fit to data (robust CFI = .996).
Tests of measurement invariance for the bifactor model indicated that the scalar invariance hypothesis was not supported. Thus, in the partial scalar bifactor model (from here on, bifactor model), the intercepts of the conflicting instruction task and the third trial of the proverb interpretation task were freely estimated in both age groups (see Supplementary Material 1 and Supplementary Table 2). The bifactor model fitted the data well, as evidenced by the fit indices (YB χ2(108) = 115.522, p = .29; robust RMSEA = .02, 90% CI = .0–.044; robust CFI = .993). Although the multidimensional model appears to provide the best account of data (AIC = 8134.8), no strong goodness-of-fit measures distinguish it from the bifactor model (AIC = 8138.7; ΔAIC = 3.93).
Fig. 1A displays the standardized estimates of the multidimensional model with the symbols representing the types of variables, parameters, and relationships. All factor loadings were in the range of .42 and .81 and statistically significant, indicating that measured variables were directly influenced by each specific factor. In the multidimensional and bifactor models, all factor means were fixed to zero in the young group (“Y”) and freely estimated for the old group (“O”; see the parameters above the triangles pointing to each factor). Thus, factor means for WM (values provided above the triangles pointing to the WM factor) indicate that the old group’s performance was .55 standard deviations lower than the young group (p < .001). Similarly, relative to the young group, the old group performed worse in M.Inh (O = −.51, p < .01) and in V.Inh (O = −.25, p < .05). In Abs.C, age groups showed no differences in performance (O = −.17; p > .05). Correlations between latent variables were similar in both groups, in the range of .39 and .80. In the bifactor model (Fig. 1B), except for verbal WM task (p = .11), factor loadings were statistically significant (p < .05), evidencing that measured variables were directly influenced by the common and specific factors. The model reveals that age differences for the latent variables were statistically significant in M.Inh (O = −1.41, p = .04) and marginally significant in WM (O = −3.39, p = .06) and V.Inh (O = −1.33, p = .09).
Fig. 1 –
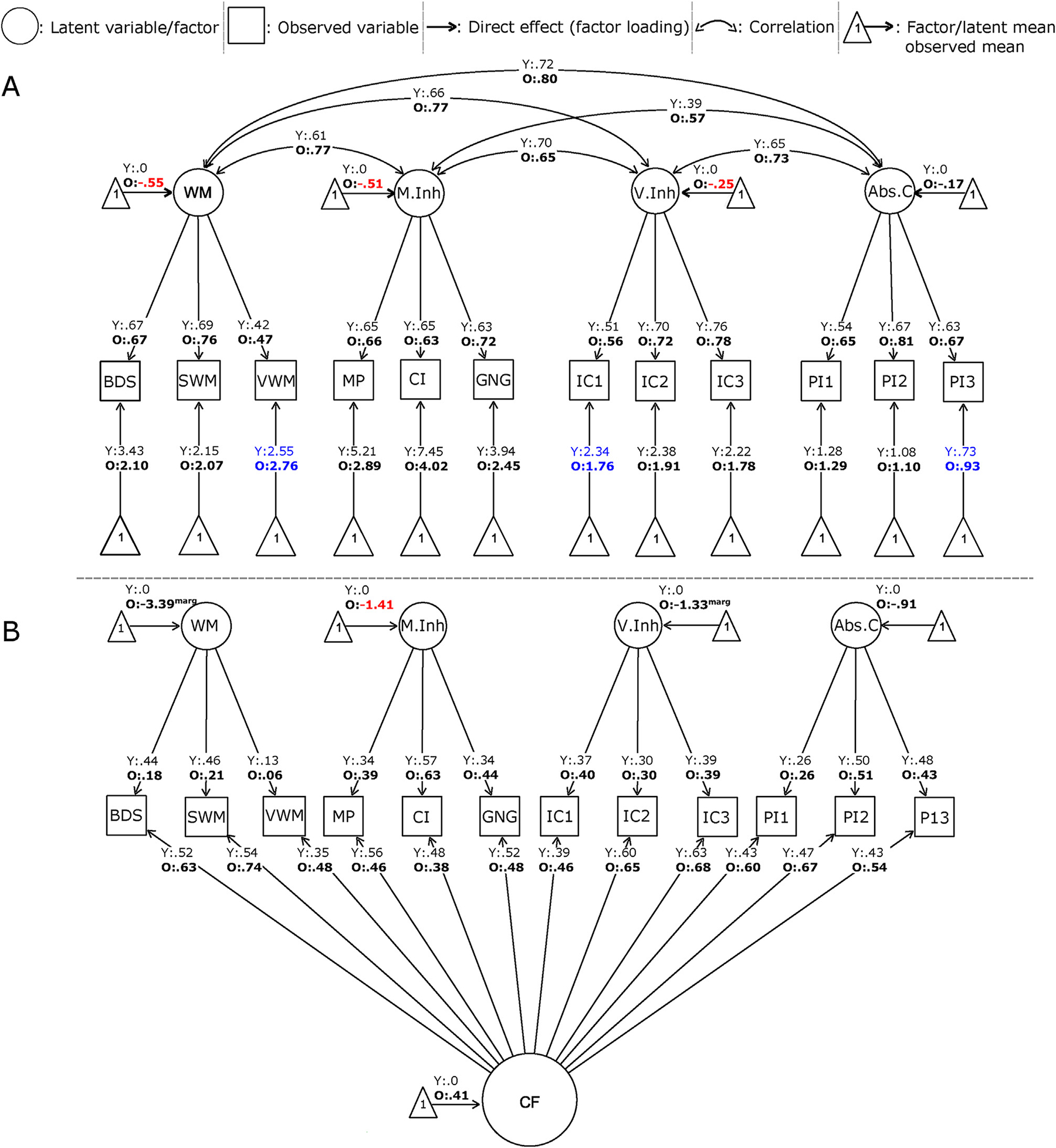
Standardized version of factorial models (partially scalar). A. Multidimensional model. B. Bifactor model. The legend displays the symbols representing the types of variables, parameters, and relationships. For identification of both models, factor means were fixed to zero in the young group (“Y”) and freely estimated for the old group (“O”). These estimated factor means are expressed as standard deviation units. For example, the factor mean for WM indicates that the old group’s performance was .55 SD lower than the young group (p < .001). Numbers in red denote significant age differences in the factor means (p < .05). In these models, factor variances/covariances, factor means, and residual variances were freely estimated. For simplicity of the figure, the model does not show residual variances and, in the case of bifactor model, neither observed intercepts. Abs.C: abstraction capacity; BDS: backwards digit span; CF: common factor; CI: conflicting instructions; GNG: Go-No Go; IC1: verbal inhibitory control, 1st trial; IC2: verbal inhibitory control, 2nd trial; IC3: verbal inhibitory control, 3rd trial, M.Inh: motor inhibition; MP: motor programming; SWM: spatial working memory; O: old, PI1: proverb interpretation, 1st trial; PI2: proverb interpretation, 2nd trial; PI3: proverb interpretation, 3rd trial; V.Inh: verbal inhibition; VWM: verbal working memory; WM: working memory; Y: young.
3.2. IFS performance
Descriptive statistics (mean, median, SD and range) of factor and observed IFS scores as well as between-group comparisons’ results (bvFTD patients vs paired controls) are summarized in Table 2.
Table 2 –
Participants performance on the IFS.
| Healthy controls (full sample) (n = 341) | bvFTD (n = 29) | Paired controls (n = 24) | bvFTD versus paired controls (Cohen’s d) | Stats | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Multidimensional model | WM | −.05 (−.09) [.81] | −1.22 (−1.04) [1.06] | .40 (.34) [.47] | −1.91 | U = 5.58 p < .001 |
| {−1.62–1.75} | {−3.39–.17} | {−.66–1.25} | ||||
| M.Inh | −.04 (.17) [.75] | −2.09 (−1.86) [1.94] | .26 (.39) [.64] | −1.47 | U = 4.90 p < .001 | |
| {−2.81–1.00} | {−6.37–.35} | {−1.96–.93} | ||||
| V.Inh | .13 (.19) [.76] | −1.11 (−.83) [1.24] | .76 (.83) [.34] | −1.91 | U = 5.69 p < .001 | |
| {−2.15–1.32} | {−3.04–.73} | {−.13–1.32} | ||||
| Abs.C | .15 (.09) [.91] | −.61 (−.62) [.91] | 1.16 (1.24) [.36] | −2.47 | U = 5.80 p < .001 | |
| {−1.36–1.58} | {−1.35–1.01} | {−.02–1.58} | ||||
| Bifactor model | WM | −.37 (−.11) [.92] | −1.23 (−1.25) [.09] | −1.23 (−1.23) [.10] | .05 | U = .13 p = .89 |
| {−1.50–1.43} | {−2.03–1.17} | {−1.40–1.03} | ||||
| M.Inh | −.37 (−.11) [.92] | −2.04 (−1.54) [1.75] | −1.39 (−1.18) [.86] | −.46 | U = 1.10 p = .27 | |
| {−5.29–1.04} | {−5.83–.40} | {−4.72–.48} | ||||
| V.Inh | −.33 (−.27) [.76] | −1.31 (−1.41) [.81] | −.91 (−.89) [.55] | −.56 | U = 2.29 p = .02 | |
| {−3.29–1.32} | {−2.85–.14} | {−1.92–.29} | ||||
| Abs.C | .37 (.26) [.88] | −.54 (−.42) [.78] | −.04 (.02) [.36] | −.81 | U = 2.77 p = .006 | |
| {−1.56–2.40} | {−1.94–.84} | {−1.30–.46} | ||||
| CF | .37 (.26) [.88] | −.53 (−.18) [1.32] | 1.53 (1.53) [.49] | −2.00 | U = 5.82 p < .001 | |
| {−1.56–2.40} | {−3.02–1.11} | {.56–2.39} | ||||
| Observed scores | WM | 7.36 (8.0) [2.24] | 5.83 (6.0) [2.45] | 8.58 (8.0) [1.59] | −1.31 | U = 4.09 p < .001 |
| {0–12} | {1–10} | {6–12} | ||||
| M.Inh | 8.09 (6.0) [1.47] | 5.86 (6.0) [2.67] | 8.42 (9.0) [1.21] | −1.19 | U = 3.88 p < .001 | |
| {0–9} | {0–9} | {4–9} | ||||
| V.Inh | 4.28 (1.48) [1.31] | 2.31 (2) [2.00] | 5.12 (5.0) [.74] | −1.80 | U = 4.81 p < .001 | |
| {0–6} | {0–6} | {3–6} | ||||
| Abs.C | 1.55 (1.0) [1.13] | .95 (1.0) [.97] | 2.77 (3.0) [.42] | −2.37 | U = 5.57 p < .001 | |
| {0–3} | {0–3} | {1.5–3} | ||||
| Total | 21.28 (22.0) [4.68] | 14.95 (16.5) [6.68] | 24.90 (25.0) [2.33] | −1.95 | U = 5.68 p < .001 | |
| {12–30} | {1–23} | {20–29} | ||||
Descriptive statistics are presented as mean (median) [SD] {range}. Factor scores represent the prediction made by the model for each participant in each EF component, as deviation units from the mean of the young group (0 ± 1). Lower scores represent lower predicted performance, and higher scores represent higher predicted performance. Statistical comparison was made through Mann-Witney U tests. In bold: results reaching the statistical threshold (p < .05). Abs.C: abstraction capacity; bvFTD: behavioral variant frontotemporal dementia; CF: common factor; M.Inh: motor inhibition; V.Inh: verbal inhibition; WM: working memory.
On average, healthy controls reached the IFS cut-off for normal executive functioning (≥ 18 (Ihnen et al., 2013)). A 22% of them showed values below the cut-off, as expected and usually reported in Latin American samples, probably explained by educational level (controls with IFS < 18: Meducation = 7.5, SDeducation = 3.3, controls with IFS ≥ 18: Meducation = 13.4, SDeducation = 4.4; U = 9.4, p < .001) and potential differences in fluid intelligence. As occurs with many other EFs batteries from other regions (Diamond, 2013; Duncan, 2013; Julayanont & Ruthirago, 2018; Roca et al., 2010; Vigliecca & Baez, 2015; Wray et al., 2020) the IFS performance is not resistant to educational level and fluid intelligence (Roca et al., 2010) –See Discussion section. Indeed, there was a positive correlation (r = .7, p < .001) between IFS total score and years of education in the healthy controls’ sample. Low educational level could also explain poorer IFS performance in the full healthy controls’ sample (Mage = 44.1, SDage = 16.7) than in the sub-group of older paired controls (Mage = 69.2, SDage = 7.47), as revealed by a significant difference in years of education between non-paired (n = 317; Meducation = 11.7, SDeducation = 4.8) and paired (n = 24, Meducation = 15.2, SDeducation = 3.5) controls (U = 3.7, p < .001).
As expected (Harciarek & Cosentino, 2013; Johnen & Bertoux, 2019; Rascovsky et al., 2011), bvFTD patients showed executive decline (Table 2). Notably, patients’ performance was highly variable, suggesting different degree of executive impairment, as previously reported (Baez et al., 2019; Beeldman et al., 2018; Kamath et al., 2019).
3.2.1. Multidimensional model’s predicted scores
We found significant differences between groups in all EFs components as estimated by the multidimensional model, with bvFTD patients performing worse than controls (WM: U = 5.60 p < .001, Cohen’s d = 1.91; M.Inh: U = 4.90, p < .001, Cohen’s d = 1.47, V.Inh: U = 5.69, p < .001, Cohen’s d = 1.91, Abs.C: U = 5.87, p < .001, Cohen’s d = 2.47). Effect sizes were higher for these scores in comparison to those estimated by the bifactor model (Table 2 and Fig. 2, left panel).
Fig. 2 –
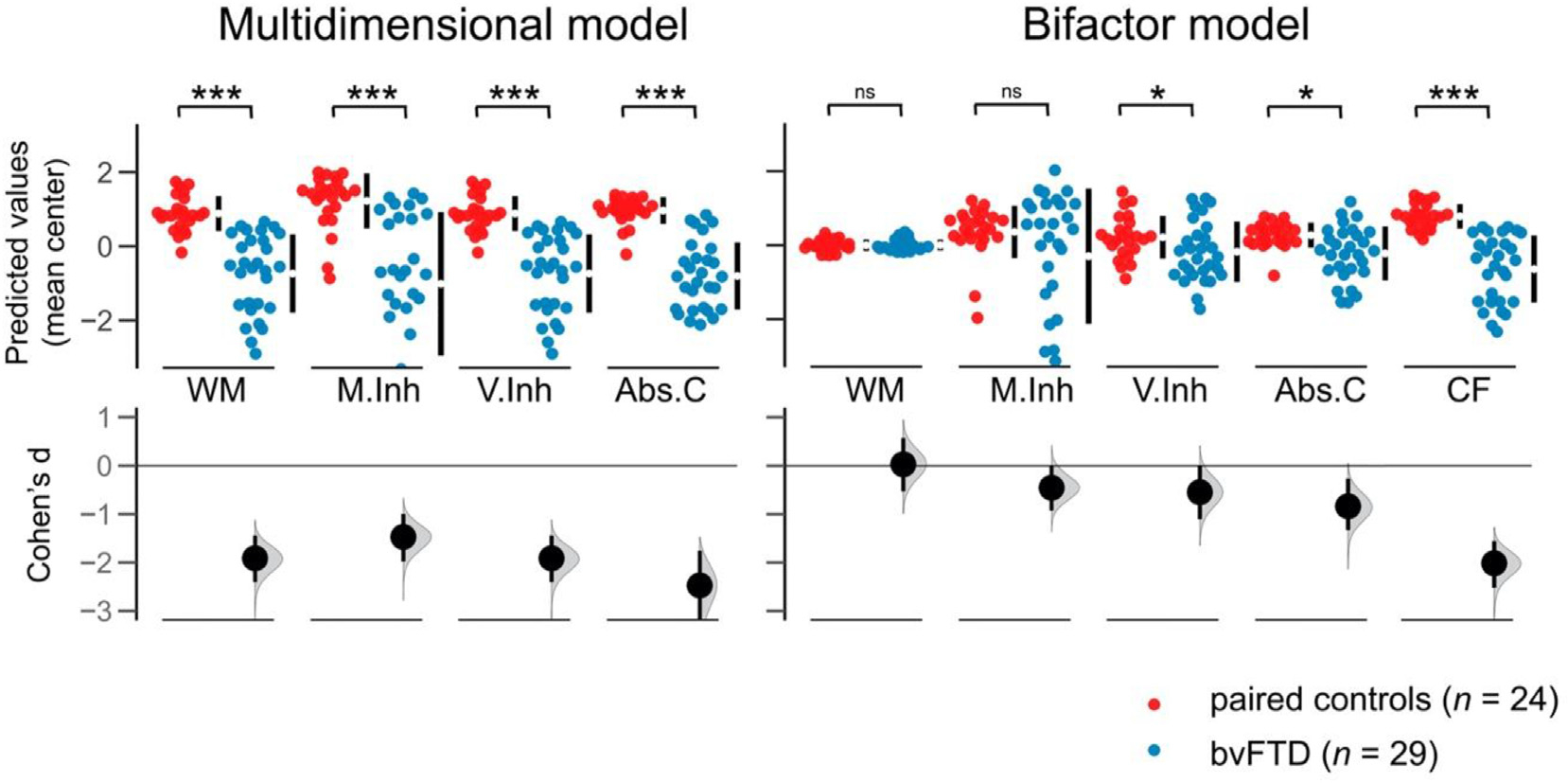
BvFTD patients and controls performance in predicted factor scores for each executive component tapped by the IFS. Estimation plots show Cohen’s d between groups calculated with 5000 bootstrap resamples. Statistical comparisons were performed through Mann-Witney U tests. Abs.C: abstraction capacity; bvFTD: behavioral variant frontotemporal dementia; CF: common factor; M.Inh: motor inhibition; V.Inh: verbal inhibition; WM: working memory. *: p < .05; ***: p < .001; ns: non-significant.
3.2.2. Bifactor model’s predicted scores
Significant differences between bvFTD patients and controls were found for V.Inh (U = 2.16, p < .05, Cohen’s d = .55), Abs.C (U = 2.90, p < .05, Cohen’s d = .84), and the CF (U = 5.82, p < .001, Cohen’s d = 2.37), with patients performing worse than controls. In contrast, no significant differences were found for WM and M.Inh. Effect sizes were lower for these scores in comparison to those estimated by the multidimensional model (Table 2 and Fig. 2, right panel).
3.2.3. Observed scores
Compared to controls, bvFTD patients presented lower IFS observed scores in all EFs components. Effect sizes were lower than those of the multidimensional model, but higher than those of the bifactor model (Table 2 and Supplementary Figure 2).
3.3. VBM results
3.3.1. Grey matter correlates of multidimensional model’s predicted scores
Higher WM scores were related with greater grey matter volume of the right dorsolateral, medial and orbitofrontal cortex (p < .05, AlphaSim cluster-corrected, k = 107 voxels). Superior M.Inh scores were related with higher volume in the right orbitofrontal cortex, in addition to the left parietal Rolandic operculum (p < .05, AlphaSim cluster-corrected, k = 114 voxels). Higher V.Inh scores were related with increased grey matter volume in the right medial and orbitofrontal cortex/gyrus rectus, and the bilateral anterior insula (p < .05, AlphaSim cluster-corrected, k = 116 voxels). Finally, higher scores in Abs.C were associated with more grey matter volume in the right medial and orbitofrontal cortex, the bilateral insula, the left Rolandic operculum, the bilateral mid/posterior cingulate gyri and precuneus, and the left (para)hippocampal/amygdala complex (p < .05, AlphaSim cluster-corrected, k = 123 voxels). See Fig. 3 and Supplementary Table 3 for further details.
Fig. 3 –
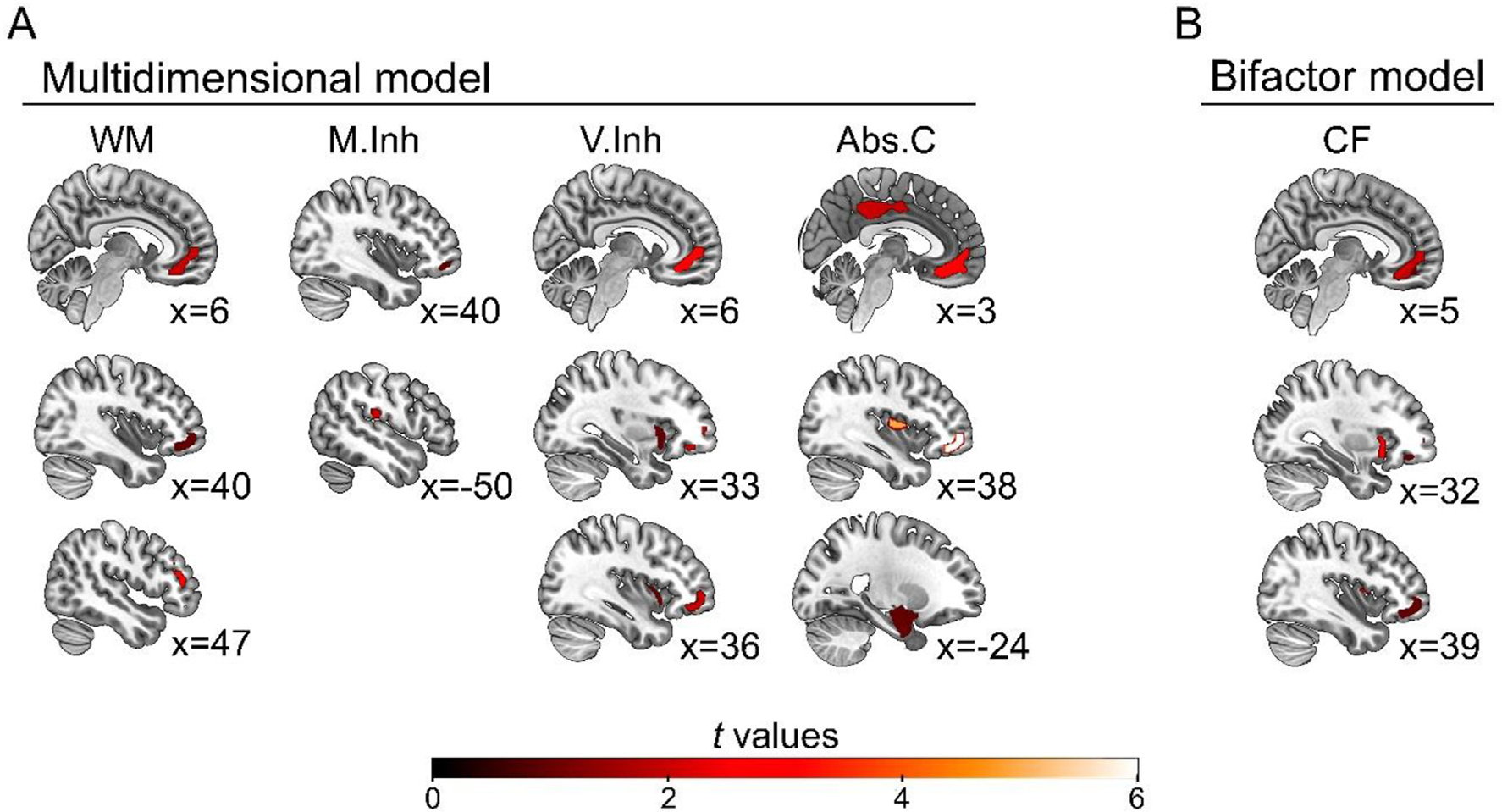
VBM results. A. Brain volume correlates of multidimensional model’s factor scores. Higher scores in EFs components were mainly associated with larger grey matter volume of prefrontal regions, as well as posterior and medial-temporal areas in the case of Abs.C. B. Grey matter correlates of bifactor model’s factor scores. Higher scores in the CF were associated with prefrontal and insular regions. No significant correlations were found for individual EFs components. See details in Supplementary Table 3. Images are displayed in neurological convention. The statistical threshold was set at p < .05, AlphaSim cluster-corrected. The numbers represent the slices coordinates in the sagittal plane. Abs.C: abstraction capacity; CF: common factor; M.Inh: motor inhibition; V.Inh: verbal inhibition; WM: working memory.
3.3.2. Grey matter correlates of bifactor model’s predicted scores
Higher CF scores were associated with increased grey matter volume in the right medial and orbitofrontal cortex/gyrus rectus, and the bilateral insula (p < .05, AlphaSim cluster-corrected, k = 111 voxels) –See Fig. 3 and Supplementary Table 3 for further details. In contrast, no significant grey matter correlates were found for the individual EFs’ components as estimated by the bifactor model (p < .05, AlphaSim cluster-corrected, k for WM = 119 voxels, k for M.Inh = 123 voxels, k for V.Inh = 126 voxels, and k for Abs.C = 148 voxels).
3.3.3. Grey matter correlates of observed scores
Observed IFS scores presented less specific brain volume correlates (Supplementary Table 3 and Supplementary Figure 3). No significant grey matter associations were obtained for WM (p < .05, AlphaSim cluster-corrected, k = 113 voxels). Higher M.Inh and V.Inh scores were related to higher volume in the left Rolandic operculum and the left posterior temporal gyrus, respectively (p < .05, AlphaSim cluster-corrected, k for M.Inh = 113 voxels, k for V.Inh = 115). Finally, higher Abs.C scores were associated with higher grey matter volume in the bilateral Rolandic operculum, the left hippocampus and amygdala, and the left mid/posterior cingulate gyrus (p < .05, AlphaSim cluster-corrected, k = 125 voxels).
4. Discussion
To our knowledge, this is the first work in examining the latent organization of EFs in both neurotypicals and in a neurodegeneration model of EFs. CFA was used to test the multidimensional and bifactor models of EFs. The multidimensional model featured four EFs components including WM, M.Inh, V.Inh and Abs.C as separate but correlated constructs. In the bifactor model, components were independent (i.e., unrelated) and their shared variance was captured by a CF, implying a general-domain ability underlying all EFs. Although both factorial models fit the data well, the multidimensional model was more sensitive to detect bvFTD’s EFs impairments and unique neuroanatomical correlates for EFs, in comparison with both the bifactor model and the observed scores. Thus, the converging behavioral and neuroanatomical evidence supports an undelaying multidimensional structure. Our framework offers novel insights to better understand the latent structure of EFs, which has been traditionally studied through performance measures.
The CFA in healthy participants evidenced that all fit indices (YB χ2, robust RMSEA, CI, and robust CFI) provided support for both models. While the multidimensional model appeared to provide a better account of behavioral data, goodness-of-fit measures did not provide a clear advantage for any model. This result confirms that, at a behavioral level, both models would work similarly (Friedman & Miyake, 2017; Karr et al., 2018; Miyake et al., 2000).
The bvFTD group showed impaired performance in all EFs components as predicted by the multidimensional model. This is consistent with the neuropsychological profile of this condition, characterized by impairments in an extended range of EFs (Harciarek & Cosentino, 2013; Johnen & Bertoux, 2019). A similar pattern of alterations was found for IFS observed scores, although with lower effect sizes (arguably due to the effect of task impurity). In contrast, the bifactor model only showed moderate impairments in patients in the CF, V.Inh, and Abs.C, with preserved performance in WM and M.Inh. Notably, effect sizes were lower than those obtained for the multidimensional model and observed scores, and, except for the CF, data from the bifactor model presented a very similar distribution across groups. Taken together, bvFTD behavioral results suggest more accurate predictions and sensitivity for the multidimensional model to detect executive dysfunction reflecting the selective involvement of the ventromedial PFC (Broe et al., 2003) in a very specific way.
VBM results for the multidimensional model revealed common grey matter correlates of EFs components. Higher scores in all components were associated with higher volume in the critical frontal EFs hubs (ventromedial/orbitofrontal cortex). Although these regions seem crucial for response inhibition (Aron et al., 2004; Collette et al., 2005; Stuss, 2011), they are also involved in other EFs (Fuster, 2019), and behavioral regulation (Stuss, 2011). Evidence from patients suggests that the integrity of the ventromedial/orbitofrontal cortex is critical for any complex executive task (Fuster, 2019), being these regions the earliest structures affected by the neuronal degeneration in bvFTD (Hodges & Piguet, 2018; Kril & Halliday, 2004).
Regarding specific EFs correlates of VBM’s multidimensional model, the better WM the larger the dorsolateral prefrontal cortex, confirming robust evidence (D’Esposito et al., 1998, 2000; Smolker et al., 2015; Wager & Smith, 2003). This result was right-lateralized. Previous evidence on brain lesions and healthy subjects has shown a lateralization effect on the dorsolateral prefrontal cortex in WM tasks, with the right and left hemispheres processing preferentially spatial and auditory-verbal material, respectively (D’Esposito et al., 1998; Jonides et al., 1993; Smith & Jonides, 1998; van Asselen et al., 2006). Since the WM component of the IFS involves both modalities, we would have expected a bilateral dorsolateral prefrontal pattern. However, there is evidence that the lateralization effects can change in the elderly (Jonides et al., 2000; Reuter-Lorenz et al., 2000), and in those with cerebral dysfunctions (Chiaravalloti et al., 2005; Walter et al., 2003). On the other hand, in contrast to brain lesion literature showing a critical involvement of posterior areas, such as the left angular gyrus (Warrington & Shallice, 1969), in WM, our results were circumscribed to frontal hubs. This may be explained by preserved posterior gray matter in our patients (Supplementary Table 1 and Supplementary Figure 1). Additionally, posterior regions are suggested to have a more generic role in EFs, specifically in processing low-level information that is shared across domains (Collette et al., 2005). Thus, CFA might have eliminated the variance associated with their function. Other VBM studies also failed to show associations between EFs and non-frontal areas (Smolker et al., 2015, 2018).
M.Inh presented a unique association with a cluster in the left parietal Rolandic operculum, putatively related to the sensorimotor aspects of this task (Eickhoff et al., 2010). Not surprisingly, V.Inh presented a positive correlation with the right ventromedial/orbitofrontal cortex, which has been previously referred to as the neural substrate of the Hayling test –the paradigm used in the IFS (Cipolotti et al., 2016; Robinson et al., 2015; Volle et al., 2012). Higher V.Inh was also associated with greater volume in the bilateral anterior insular cortex. Despite this region not being traditionally associated with EFs, several studies reveal its role in verbal response suppression tasks –similar to the Hayling test (De Zubicaray, Zelaya, Andrew, Williams, & Bullmore, 2000; Ruscheweyh, Deppe, Lohmann, et al., 2013)–, as well as in inhibitory failure (Ramautar et al., 2006), and in other higher-order cognitive aspects of language production (Oh, Duerden, & Pang, 2014). The anterior insula is the key node of the salience network for the facilitation of error monitoring and task control (Eckert et al., 2009; Menon & Uddin, 2010; Ruscheweyh, Deppe, Lohmann, et al., 2013). In this line, previous evidence has linked the insular damage with executive impairments in bvFTD (Baez et al., 2019). Taken together, these results suggest the insula might have a role in language-based inhibition tasks.
Finally, Abs.C was associated with a widespread grey matter pattern, comprising an extended prefrontal cluster as previously reported (Dumontheil, 2014; Murphy et al., 2013; Nee et al., 2014; Urbanski et al., 2016) but also including other temporo-posterior regions (Bohrn, Altmann, Lubrich, Menninghaus, & Jacobs, 2012). These last regions would be related to cognitive demands required by the proverb interpretation task used to assess Abs.C. The task relies on the medial temporal lobe’s long-term verbal memory involvement, which is putatively left-lateralized (Kaiser et al., 2013; McDonald, Delis, Kramer, Tecoma, & Iragui, 2008). Furthermore, proverb interpretation engages perspective-taking abilities (associated with medial frontal and posterior areas (Amodio & Frith, 2006; Saxe, 2006)) to decode meaning, while inhibiting literal responses (right ventromedial/orbitofrontal cortex). In brief, the multidimensional model presented common and unique neural signatures previously associated with the specific EFs components.
The bifactor model presented a positive association of the CF and ventromedial/orbitofrontal cortex volume (the main hubs of the multidimensional model) together with the bilateral insula. As discussed above, these regions encompass general purpose roles in EFs (Eckert et al., 2009; Fuster, 2019; Menon & Uddin, 2010; Stuss, 2011). Consistently, a previous work on latent variables also revealed a similar association (Smolker et al., 2015), however, no associations for individual EFs components were found using bifactor scores. These findings extend previous evidence of shared neuroanatomical bases among EFs and suggest a sensitivity loss of the individual components when their common variance is extracted.
Finally, the grey matter correlates of observed IFS scores were scant. Remarkably, no prefrontal involvement was detected in any component, possibly due to the effect of contaminating variables in performance (task impurity). Previous research has already pointed to difficulties and contradictions in brain results from observed (i.e., contaminated) EFs scores (Smolker et al., 2018; Weise et al., 2019; Yuan & Raz, 2014). Thus, our findings further reinforce the advantages of using CFA to characterize EFs constructs.
Taken together, while both the multidimensional and the bifactor models presented a good fit to observed IFS data, converging behavioral and neuroanatomical evidence from bvFTD supports the multidimensional model. This is especially relevant for the clinical field, as it has been previously suggested that EFs are fractionated in frontal patients (Godefroy et al., 1999; Stuss, 2011; Tsuchida & Fellows, 2013). The separability of EFs, however, should not be interpreted as complete independence. The multidimensional model presented moderate–high correlations among EFs components (ranging from .39 to .80), alongside common neuroanatomical hubs, suggesting a pattern of “unity and diversity” of EFs (Friedman & Miyake, 2017). The nature of such unity of EFs, namely whether it reflects general abilities (Obonsawin et al., 2002), goal neglect (Duncan et al., 1997), or reasoning (Salthouse, 2005) still remains to be determined.
The controversy regarding the unity and diversity structure of EFs has been largely addressed using behavioral measures in healthy participants (e.g., Huizinga, Dolan, & van der Molen, 2006; Miyake et al., 2000; Was, 2007), older adults (e.g., De Frias, Dixon, & Strauss, 2006; Hull, Martin, Beier, Lane, & Hamilton, 2008; Martin, Barker, Gibson, & Robinson, 2021), as well as in neurological (e.g., Robinson et al., 2012; Roca et al., 2010; Tsuchida & Fellows, 2013) and psychiatric (e.g., Martin, Mowry, Reutens, & Robinson, 2015) patients. Some works tackled this issue using structural and functional imaging (e.g., Collette et al., 2005; Fedorenko et al., 2013; Hedden & Gabrieli, 2010; Niendam et al., 2012). Overall, these studies support the differentiation of EFs across the lifespan. However, most of them rely on observed (i.e., contaminated) EFs measures, raising controversies regarding the latent organization of the different dimensions (i.e., whether they depend on a domain-general ability), and precluding specific brain-behavior associations (e.g., Weise et al., 2019; Yuan & Raz, 2014). On the other hand, studies using CFA to alleviate the problem of task impurity typically do not include brain measures. Moreover, the very few CFA studies that assess cortical correlates (Ambrosini et al., 2019; Bettcher et al., 2016; Smolker et al., 2015, 2018), do not test their hypotheses in patients. In sum, our study is the first in integrating robust convergent evidence from latent measures, structural imaging, and a neurodegenerative lesion model to investigate the multidimensional organization of EFs.
Results have relevant implications for neuropsychological assessment. EFs impairments are present in most neurological and psychiatric conditions (Huey et al., 2009; Snyder et al., 2015; Stopford et al., 2012). However, the use of tasks’ observed scores is sometimes problematic to accurately detect such deficits (e.g., to differentiate between types of dementia (Johns et al., 2009)). While decades of research and many resources have been invested to find specific tasks to solve this issue, the field still lacks a clear answer. Thus, the incorporation of factor scores as normative values in neuropsychological batteries represents a promising avenue towards the development of more sensitive EFs measures for diagnosis, detection of daily life impairments, and assessment of treatment outcomes.
Some limitations must be acknowledged. First, despite its advantages, the IFS is not an exhaustive measure of EFs; it does not include all EFs components (e.g., cognitive flexibility, planning, organization) and does not account for processing speed (i.e., reaction times). Second, given its screening nature, a potential ceiling effect in healthy subjects cannot be ruled out. Yet, the IFS taps into complex behaviors (e.g., M.Inh domain is based on challenging hand movements that require interference control while having implicit the capacities of motor coordination and learning; Dubois et al., 2000) which allows for the tracking of inter-individual differences. Indeed, the IFS proved its utility in healthy young and old adults (Fittipaldi et al., 2020; García-Cordero et al., 2017; Sierra Sanjurjo et al., 2019). Relatedly, EFs are impacted by multiple factors, including education and fluid intelligence (Diamond, 2013; Duncan, 2013; Julayanont & Ruthirago, 2018; Roca et al., 2010; Vigliecca & Baez, 2015; Wray et al., 2020). Similarly, the IFS is sensitive to educational level (Ihnen et al., 2013; Moreira et al., 2014; Sierra Sanjurjo et al., 2019), and fluid intelligence (Roca et al., 2010), which can produce floor effects. Arguably, low-educated participants may present difficulties in attending, comprehending and following instructions, lack proper vocabulary (as required, for instance, in the proverb interpretation task), and struggle in creating adequate strategies to solve complex tasks (Hawkins & Bender, 2002; Le Carret et al., 2003; de Wachholz & Yassuda, 2011). This limitation is not exclusive of the IFS but typical of other gold-standard executive (Appollonio et al., 2005; Matioli et al., 2008; Rodrigues et al., 2009) and cognitive (e.g., Crum, Anthony, Bassett, & Folstein, 1993; Matías-Guiu et al., 2016; Zhou et al., 2015) screening tests widely used to track acquired deficits. In any case, future CFA works should more precisely address the issue of ceiling and floor effects on EFs tasks. Third, the Abs.C domain of the IFS is based on a single task (proverb interpretation), with a short range of possible scores (from 1 to 3). Low variability in this domain could impact VBM associations in addition to task impurity. Fourth, although we used a large sample for the construction of our models (n = 370), imaging analysis was performed only on a sub-sample (n = 53). Finally, the size of the bvFTD group was moderate (n = 29), but comparable or larger than that seen in similar studies using VBM in this population (e.g., Baez et al., 2019; Sheelakumari et al., 2020; Wilson et al., 2020). Nonetheless, our results should be replicated with a larger, more diverse pathological sample that includes other dysexecutive syndromes.
In conclusion, the multidimensional model seems to be more sensitive than the bifactor model and the observed scores to detect neurocognitive dysfunction. This suggests that EFs are better conceptualized as separate but related components. Also, our results strengthen the construct validity of the IFS across aging, highlighting its suitability for further applications with latent variables in other studies. Its robustness, alongside its brevity, low cost, easy application and interpretation (Roebuck-Spencer et al., 2017), confers to this battery several advantages over other alternatives. Thus, our findings provide a new agenda for further theoretical and clinical research regarding the conceptualization of EFs, the utility of factor scores in neuropsychological assessment, and the development of new evidence-based screenings for EFs examination.
Supplementary Material
Acknowledgements
The authors appreciate the kind disposition from all participants, especially patients and their caregivers.
Funding
This work is partially supported by grants from CONICET; ANID/FONDECYT Regular (1210195 and 1210176); FONCYT-PICT 2017–1820; ANID/FONDAP/15150012; Takeda CW2680521; Sistema General de Regalías (BPIN2018000100059), Universidad del Valle (CI 5316); Alzheimer’s Association GBHI ALZ UK-20–639295; and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer’s Association (SG-20–725707), Rainwater Charitable foundation - Tau Consortium, and Global Brain Health Institute)]. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these Institutions.
Abbreviations
- Abs.C
abstraction capacity
- bvFTD
behavioral variant frontotemporal dementia
- CF
common factor
- CFA
confirmatory factorial analysis
- M.Inh
motor inhibition
- V.Inh
verbal inhibition
- WM
working memory
- VBM
voxel-based morphometry
Footnotes
Declaration of competing interest
Authors have no competing interests to declare. There are not any financial and/or personal relationships with people or organizations that could inappropriately influence (bias) the present work.
Pre-registration statement
No part of the study procedures nor analyses was pre-registered prior to the research being conducted.
CRediT author statement
Raúl González-Gómez: Data Curation, Methodology, Formal analysis, Writing - Original Draft, Visualization.
Odir Antonio Rodríguez-Villagra: Data Curation, Methodology, Formal analysis, Writing - Original Draft, Visualization.
Michael Schulte: Writing - Review & Editing.
Teresa Torralva: Investigation.
Agustín Ibáñez: Conceptualization, Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
David Huepe: Conceptualization, Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
Sol Fittipaldi: Conceptualization, Data Curation, Methodology, Formal analysis, Writing - Review & Editing, Supervision, Project administration.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2021.08.015.
Recent research has also employed the term “nested” or “general-specific” model. Several aspects differentiate a bifactor model from a two-factor or two-dimensional model. Particularly, the first one can include more than two factors with a nested hierarchical structure.
Data availability statement
Given patients’ sensitive information and funding regulations, the databases, images, and code generated and/or used during the current study are not publicly archived. However, access to all materials will be granted from the corresponding author after the completion of a formal data sharing agreement and IRB approval.
references
- Alkharusi H (2012). Categorical variables in regression analysis: A comparison of dummy and effect coding. International Journal of Education, 4(2), 202–210. 10.5296/ije.v4i2.1962 [DOI] [Google Scholar]
- Ambrosini E, Arbula S, Rossato C, Pacella V, & Vallesi A (2019). Neuro-cognitive architecture of executive functions: A latent variable analysis. Cortex, 119, 441–456. 10.1016/j.cortex.2019.07.013 [DOI] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277. 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, et al. (2005). The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurological Sciences, 26(2), 108–116. 10.1007/s10072-005-0443-4 [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2000). Voxel-based morphometry — the methods. Neuroimage, 11, 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Baddeley A (2007). Oxfords psychology series (Vol. 45). Working memory, thought, and action. Oxford University Press. [Google Scholar]
- Baez S, Ibanez A, Gleichgerrcht E, Perez A, Roca M, Manes F, et al. (2014). The utility of IFS (INECO Frontal Screening) for the detection of executive dysfunction in adults with bipolar disorder and ADHD. Psychiatry Research, 216(2), 269–276. 10.1016/j.psychres.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Baez S, Pinasco C, Roca M, Ferrari J, Couto B, García-Cordero I, et al. (2019). Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia, 126, 159–169. 10.1016/j.neuropsychologia.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Baggetta P, & Alexander PA (2016). Conceptualization and operationalization of executive function. Mind, Brain, and Education, 10(1), 10–33. [Google Scholar]
- Beeldman E, Raaphorst J, Klein Twennaar M, Govaarts R, Pijnenburg YAL, De Haan RJ, et al. (2018). The cognitive profile of behavioural variant FTD and its similarities with ALS: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery, and Psychiatry, 89(9), 995–1002. 10.1136/jnnp-2017-317459 [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Mungas D, Patel N, Elofson J, Dutt S, Wynn M, et al. (2016). Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia, 85, 100–109. 10.1016/j.neuropsychologia.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Haeger M, & Voelcker-Rehage C (2019). Structure of executive functions in young and in older persons. Plos One, 14(5), 1–19. 10.1371/journal.pone.0216149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrn IC, Altmann U, Lubrich O, Menninghaus W, & Jacobs AM (2012). Old proverbs in new skins-an fMRI study on defamiliarization. Frontiers in Psychology, 3(JUL), 1–18. 10.3389/fpsyg.2012.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, & Halliday GM (2003). Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology, 60(6), 1005–1011. 10.1212/01.WNL.0000052685.09194.39 [DOI] [PubMed] [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit. In B KA, & Long JS (Eds.), Testing structural equation models (pp. 136e162). Beverly Hills, CA: Sage. [Google Scholar]
- Brunner M, Nagy G, & Wilhelm O (2012). A tutorial on hierarchically structured constructs. Journal of Personality, 80(4), 796–846. 10.1111/j.1467-6494.2011.00749.x [DOI] [PubMed] [Google Scholar]
- Bruno D, Torralva T, Marenco V, Ardilla JT, Baez S, Gleichgerrcht E, et al. (2015). Utility of the INECO frontal screening (IFS) in the detection of executive dysfunction in patients with relapsing-remitting multiple sclerosis (RRMS). Neurological Sciences, 36(11), 2035–2041. 10.1007/s10072-015-2299-6 [DOI] [PubMed] [Google Scholar]
- Burgess PW (1997). Theory and methodology in executive function research. In Rabbitt P(Ed.), Methodology of frontal and executive function (pp. 81–116). Hove, UK: Psychology Press. [Google Scholar]
- Burgess PW, & Shallice T (1997). The hayling and brixton test. Test manual. Thames Valley Test Company. [Google Scholar]
- Burnham KP, & Anderson DR (2002). Model selection and multimodel inference: A practical information-theoretic approach (2.a ed.). Springer-Verlag. [Google Scholar]
- Chen Y, Landin-Romero R, Kumfor F, Irish M, Hodges JR, & Piguet O (2020). Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex, 129, 57–67. 10.1016/j.cortex.2020.04.013 [DOI] [PubMed] [Google Scholar]
- Chen FF, West SG, & Sousa KH (2006). A comparison of bifactor and second-order models of quality of life. Multivariate Behavioral Research, 41(2), 189–225. 10.1207/s15327906mbr4102_5 [DOI] [PubMed] [Google Scholar]
- Cheung GW, & Rensvold RB (2002). Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling: A Multidisciplinary Journal, 9(2), 233–255. [Google Scholar]
- Chiaravalloti ND, Hillary FG, Ricker JH, Christodoulou C, Kalnin AJ, Liu WC, et al. (2005). Cerebral activation patterns during working memory performance in multiple sclerosis using fMRI. Journal of Clinical and Experimental Neuropsychology, 27(1), 33–54. 10.1080/138033990513609 [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Healy C, Spanò B, Lecce F, Biondo F, Robinson G, et al. (2016). Strategy and suppression impairments after right lateral prefrontal and orbito-frontal lesions. Brain, 139(2), e10. 10.1093/brain/awv269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van Der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, et al. (2005). Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping, 25(4), 409–423. 10.1002/hbm.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, & Folstein MF (1993). Population-based norms for the mini-mental state examination by age and educational level. JAMA: The Journal of the American Medical Association, 269(18), 2386–2391. 10.1001/jama.1993.03500180078038 [DOI] [PubMed] [Google Scholar]
- Custodio N, Herrera-Perez E, Lira D, Roca M, Manes F, Báez S, et al. (2016). Evaluation of the INECO frontal screening and the frontal assessment battery in Peruvian patients with alzheimer’s disease and behavioral variant frontotemporal dementia. ENeurologicalSci, 5, 25–29. 10.1016/j.ensci.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frias CM, Dixon RA, & Strauss E (2006). Structure of four executive functioning tests in healthy older adults. Neuropsychology, 20(2), 206–214. 10.1037/0894-4105.20.2.206 [DOI] [PubMed] [Google Scholar]
- de Wachholz TBO, & Yassuda MS (2011). The interpretation of proverbs by elderly with high, medium and low educational level: Abstract reasoning as an aspect of executive functions. Dementia & Neuropsychologia, 5(1), 31–37. 10.1590/s1980-57642011dn05010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zubicaray GI, Zelaya FO, Andrew C, Williams SCR, & Bullmore ET (2000). Cerebral regions associated with verbal response initiation, suppression and strategy use. Neuropsychologia, 38(9), 1292–1304. 10.1016/S0028-3932(00)00026-9 [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, & Lease J (1998). Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research, 7(1), 1–13. 10.1016/S0926-6410(98)00004-4 [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, & Rypma B (2000). Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research, 133(1), 3–11. 10.1007/s002210000395 [DOI] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Sedeño L, Martorell Caro M, Alifano F, Hesse E, Mikulan E, et al. (2017). Towards affordable biomarkers of frontotemporal dementia: A classification study via network’s information sharing. Scientific Reports, 7(1), 1–12. 10.1038/s41598-017-04204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe EA (1975). Go - No Go learning after frontal lobe lesions in humans. Cortex, 11(1), 8–16. 10.1016/S0010-9452(75)80015-3 [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B (2000). The FAB: A frontal assessment battery at bedside. Neurology, 55(11), 1621–1626. 10.1212/WNL.55.11.1621 [DOI] [PubMed] [Google Scholar]
- Ducharme S, Price BH, & Dickerson BC (2018). Apathy: A neurocircuitry model based on frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 89(4), 389–396. 10.1136/jnnp-2017-316277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I (2014). Development of abstract thinking during childhood and adolescence: The role of rostrolateral prefrontal cortex. Developmental Cognitive Neuroscience, 10, 57–76. 10.1016/j.dcn.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2013). The structure of cognition: Attentional episodes in mind and brain. In Neuron, 80(1), 35–50. 10.1016/j.neuron.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Johnson R, Swales M, & Freer C (1997). Frontal lobe deficits after head Injury: Unity and diversity of function. Cognitive Neuropsychology, 14(5), 713–741. [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, et al. (2009). At the heart of the ventral attention system: The right anterior insula. Human Brain Mapping, 30(8), 2530–2541. 10.1002/hbm.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. Journal of Neuroscience, 30(18), 6409–6421. 10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S (2019). semPlot: Path diagrams and visual analysis of various SEM packages’ output. [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences of the United States of America, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino N, Gleichgerrcht E, Roca M, Cetkovich M, Manes F, & Torralva T (2013a). INECO frontal screening: An instrument to assess executive dysfunction in schizophrenia. Dementia e Neuropsychologia, 7(1), 33–39. 10.1590/S1980-57642013DN70100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino N, Gleichgerrcht E, Roca M, Cetkovich M, Manes F, & Torralva T (2013b). Rastreio frontal ineco diferencia A variante comportamental da demência frontotemporal de depressão maior. Dementia e Neuropsychologia, 7(1), 33–39. 10.1590/S1980-57642013DN70100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi S, Abrevaya S, de la Fuente A, Pascariello GO, Hesse E, Birba A, et al. (2020). A multidimensional and multi-feature framework for cardiac interoception. Neuroimage, 212, 116677. 10.1016/j.neuroimage.2020.116677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (2019). The prefrontal cortex in the neurology clinic. In Handbook of clinical neurology (1st ed., Vol. 163). Elsevier B.V. 10.1016/B978-0-12-804281-6.00001-X. [DOI] [PubMed] [Google Scholar]
- García-Cordero I, Esteves S, Mikulan EP, Hesse E, Baglivo FH, Silva W, et al. (2017). Attention, in and out: Scalp-level and intracranial EEG correlates of interoception and exteroception. Frontiers in Neuroscience, 11, 411. 10.3389/fnins.2017.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cordero I, Sedeño L, Babino A, Dottori M, Melloni M, Martorell Caro M, et al. (2019). Explicit and implicit monitoring in neurodegeneration and stroke. Scientific Reports, 9(1), 1–10. 10.1038/s41598-019-50599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cordero I, Sedeño L, de la Fuente L, Slachevsky A, Forno G, Klein F, et al. (2016). Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708). 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Roca M, Manes F, & Torralva T (2011). Comparing the clinical usefulness of the institute of cognitive neurology (INECO) frontal screening (IFS) and the frontal assessment battery (FAB) in frontotemporal dementia. Journal of Clinical and Experimental Neuropsychology, 33(9), 997–1004. 10.1080/13803395.2011.589375 [DOI] [PubMed] [Google Scholar]
- Godefroy O, Cabaret M, Petit-Chenal V, Pruvo JP, & Rousseaux M (1999). Control functions of the frontal lobes. Modularity of the central-supervisory system? Cortex, 35(1), 1–20. 10.1016/S0010-9452(08)70782-2 [DOI] [PubMed] [Google Scholar]
- Harciarek M, & Cosentino S (2013). Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes. International Review of Psychiatry, 25(2), 178–196. 10.3109/09540261.2013.763340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KA, & Bender S (2002). Norms and the relationship of boston naming test performance to vocabulary and education: A review. Aphasiology, 16(12), 1143–1153. 10.1080/02687030244000031 [DOI] [Google Scholar]
- Hedden T, & Gabrieli JDE (2010). Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. Neuroimage, 51(1), 421–431. 10.1016/j.neuroimage.2010.01.089.Shared [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR (1994). Cognitive assessment for clinicians. Oxford: Oxford Univers. [Google Scholar]
- Hodges JR, & Piguet O (2018). Progress and challenges in frontotemporal dementia research: A 20-year review. Journal of Alzheimer’s Disease, 62(3), 1467–1480. 10.3233/JAD-171087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Kipps C, & Hodges JR (2008). Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology, 71(19), 1481–1488. 10.1212/01.wnl.0000334299.72023.c8 [DOI] [PubMed] [Google Scholar]
- Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, & Hodges JR (2011). Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 30(6), 547–552. 10.1159/000321670 [DOI] [PubMed] [Google Scholar]
- Ho J, Tumkaya T, Aryal S, Choi H, & Claridge-Chang A (2019). Moving beyond P values: Data analysis with estimation graphics. Nature Methods, 16(7), 565–566. 10.1038/s41592-019-0470-3 [DOI] [PubMed] [Google Scholar]
- Huey ED, Goveia EN, Paviol S, Pardini M, Krueger F, Zamboni G, et al. (2009). Executive dysfunction in frontotemporal dementia and corticobasal syndrome. Neurology, 72(5), 453–459. 10.1212/01.wnl.0000341781.39164.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, & van der Molen MW (2006). Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia, 44(11), 2017–2036. 10.1016/j.neuropsychologia.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Hull R, Martin RC, Beier ME, Lane D, & Hamilton AC (2008). Executive function in older adults: A structural equation modeling approach. Neuropsychology, 22(4), 508–522. 10.1037/0894-4105.22.4.508 [DOI] [PubMed] [Google Scholar]
- Ihnen J, Antivilo A, Muñoz-Neira C, & Chonchol AS (2013). Chilean version of the INECO Frontal Screening (IFS-Ch): Psychometric properties and diagnostic accuracy. Dementia e Neuropsychologia, 7(1), 40–47. 10.1590/s1980-57642013dn70100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnen A, & Bertoux M (2019). Psychological and cognitive markers of behavioral variant frontotemporal dementia - a clinical neuropsychologist’s view on diagnostic criteria and beyond. Frontiers in Neurology, 10(JUN), 1–24. 10.3389/fneur.2019.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns EK, Phillips NA, Belleville S, Goupil D, Babins L, Kelner N, et al. (2009). Executive functions in frontotemporal dementia and lewy body dementia. Neuropsychology, 23(6), 765–777. 10.1037/a0016792 [DOI] [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, & Hartley A (2000). Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. Journal of Cognitive Neuroscience, 12(1), 188–196. 10.1162/089892900561823 [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, & Mintun MA (1993). Spatial working memory in humans as revealed by PET. Nature, 363(6430), 623–625. 10.1038/363623a0 [DOI] [PubMed] [Google Scholar]
- Jorgensen TD, Pornprasertmanit S, Schoemann AM, Rosseel Y, Miller P, Quick C, et al. (2020). semTools: Useful tools for structural equation modeling (0.5–3). https://cran.r-project.org/package=semTools. [Google Scholar]
- Julayanont P, & Ruthirago D (2018). The illiterate brain and the neuropsychological assessment: From the past knowledge to the future new instruments. Applied Neuropsychology:Adult, 25(2), 174–187. 10.1080/23279095.2016.1250211 [DOI] [PubMed] [Google Scholar]
- Jurado MB, & Rosselli M (2007). The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review, 17(3), 213–233. 10.1007/s11065-007-9040-z [DOI] [PubMed] [Google Scholar]
- Kaiser NC, Lee GJ, Lu PH, Mather MJ, Shapira J, Jimenez E, et al. (2013). What dementia reveals about proverb interpretation and its neuroanatomical correlates. Neuropsychologia, 51(9), 1726–1733. 10.1016/j.neuropsychologia.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Kamath V, Chaney GAS, Deright J, & Onyike CU (2019). A meta-analysis of neuropsychological, social cognitive, and olfactory functioning in the behavioral and language variants of frontotemporal dementia. Psychological Medicine, 49(16), 2669–2680. 10.1017/S0033291718003604 [DOI] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, & Garcia-Barrera MA (2018). The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychological Bulletin, 144(11), 1147–1185. 10.1037/bul0000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Fryer TD, & Hodges JR (2007). Behavioural variant frontotemporal dementia: Not all it seems? Neurocase, 13(4), 237–247. 10.1080/13554790701594870 [DOI] [PubMed] [Google Scholar]
- Kline RB (2016). Principles and practice of structural equation modeling (4th ed.). [Google Scholar]
- Kril JJ, & Halliday GM (2004). Clinicopathological staging of frontotemporal dementia severity: Correlation with regional atrophy. Dementia and Geriatric Cognitive Disorders, 17(4), 311–315. 10.1159/000077161 [DOI] [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Letenneur L, Dartigues JF, Mayo W, & Fabrigoule C (2003). The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental Neuropsychology, 23(3), 317–337. 10.1207/S15326942DN2303_1 [DOI] [PubMed] [Google Scholar]
- Lezak MD (1983). Neuropsychological assessment (2nd ed.). Oxford University Press. [Google Scholar]
- Lieberman MD, & Cunningham WA (2009). Type I and type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience, 4(4), 423–428. 10.1093/scan/nsp052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T (2013). Longitudinal structural equation modeling. Guilford Press. [Google Scholar]
- Lu PH, Mendez MF, Lee GJ, Leow AD, Lee HW, Shapira J, et al. (2013). Patterns of brain atrophy in clinical variants of frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders, 35(1–2), 34–50. 10.1159/000345523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AK, Barker MS, Gibson EC, & Robinson GA (2021). Response initiation and inhibition and the relationship with fluid intelligence across the adult lifespan. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 36(2), 231–242. 10.1093/arclin/acz044 [DOI] [PubMed] [Google Scholar]
- Martin AK, Mowry B, Reutens D, & Robinson GA (2015). Executive functioning in schizophrenia: Unique and shared variance with measures of fluid intelligence. Brain and Cognition, 99, 57–67. 10.1016/j.bandc.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, & Hodges JR (2000). A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology, 55(11), 1613–1620. 10.1212/01.wnl.0000434309.85312.19 [DOI] [PubMed] [Google Scholar]
- Matías-Guiu JA, Fernández-Bobadilla R, Fernández-Oliveira A, Valles-Salgado M, Rognoni T, Cortés-Martínez A, et al. (2016). Normative data for the Spanish version of the addenbrooke’s cognitive examination III. Dementia and Geriatric Cognitive Disorders, 41(5–6), 243–250. 10.1159/000445799 [DOI] [PubMed] [Google Scholar]
- Matioli MNPS, Caramelli P, Marques BD, Rocha FD da, de Castro CC., Yamashita SR., et al. (2008). EXIT25 - executive interview applied to a cognitively healthy elderly population with heterogeneous educational background. Dementia & Neuropsychologia, 2(4), 305–309. 10.1590/s1980-57642009dn20400013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Kramer JH, Tecoma ES, & Iragui VJ (2008). A componential analysis of proverb interpretation in patients with frontal lobe epilepsy and temporal lobe epilepsy: Relationships with disease-related factors. Clinical Neuropsychologist, 22(3), 480–496. 10.1080/13854040701363828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni M, Billeke P, Baez S, Hesse E, De La Fuente L, Forno G, et al. (2016). Your perspective and my benefit: Multiple lesion models of self-other integration strategies during social bargaining. Brain, 139(11), 3022–3040. 10.1093/brain/aww231 [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5e6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex ‘“ frontal lobe ”‘ Tasks: A latent variable analysis and. Cognitive Psychology, 41, 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Moreira HS, Lima CF, & Vicente SG (2014). Examining executive dysfunction with the institute of cognitive neurology (INECO) frontal screening (IFS): Normative values from a healthy sample and clinical utility in alzheimer’s disease. Journal of Alzheimer’s Disease, 42(1), 261–273. 10.3233/JAD-132348 [DOI] [PubMed] [Google Scholar]
- Morris JC (1993). The clinical dementia rating (cdr): Current version and scoring rules. Neurology, 43(11), 2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Murphy P, Shallice T, Robinson G, MacPherson SE, Turner M, Woollett K, et al. (2013). Impairments in proverb interpretation following focal frontal lobe lesions. Neuropsychologia, 51(11), 2075–2086. 10.1016/j.neuropsychologia.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jahn A, & Brown JW (2014). Prefrontal cortex organization: Dissociating effects of temporal abstraction, relational abstraction, and integration with fMRI. Cerebral Cortex, 24(9), 2377–2387. 10.1093/cercor/bht091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, et al. (2017). Best practices in data analysis and sharing in neuroimaging using MRI. Nature Neuroscience, 20(3), 299–303. 10.1038/nn.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonsawin MC, Crawford JR, Page J, Chalmers P, Cochrane R, & Low G (2002). Performance on tests of frontal lobe function reflect general intellectual ability. Neuropsychologia, 40, 970–977. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, et al. (2016). Fair play: Social norm compliance failures in behavioural variant frontotemporal dementia. Brain, 139(1), 204–216. 10.1093/brain/awv315 [DOI] [PubMed] [Google Scholar]
- Oh A, Duerden EG, & Pang EW (2014). The role of the insula in speech and language processing. Brain and Language, 135(416), 96–103. 10.1016/j.bandl.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell GS, Briellmann RS, Chan C. H. (Patrick), Pardoe H., Abbott DF., et al. (2008). Selection of the control group for VBM analysis: Influence of covariates, matching and sample size. Neuroimage, 41(4), 1324–1335. 10.1016/j.neuroimage.2008.02.050 [DOI] [PubMed] [Google Scholar]
- Peng P, Li M, Liu H, Tian YR, Chu SL, van Halm-Lutterodt N, et al. (2018). Brain structure alterations in respect to tobacco consumption and nicotine dependence: A comparative voxel-based morphometry study. Frontiers in Neuroanatomy, 12(May), 1–11. 10.3389/fnana.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, & Hodges JR (2011). Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurology, 10(2), 162–172. 10.1016/S1474-4422(10)70299-4 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18(2), 115–126. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Feigenbaum D, Rankin KP, Smith GE, Boxer AL, Wood K, et al. (2013). Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology, 80(24), 2180–2185. 10.1212/WNL.0b013e318296e940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnick DL, & Bornstein MH (2016). Measurement invariance conventions and reporting: The state of the art and future directions for psychological research. Developmental Review, 41, 71–90. 10.1016/j.dr.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, & Robbins TW (1999). Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain, 122(8), 1469–1493. 10.1093/brain/122.8.1469 [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, & Ridderinkhof KR (2006). Probability effects in the stop-signal paradigm: The insula and the significance of failed inhibition. Brain Research, 1105(1), 143–154. 10.1016/j.brainres.2006.02.091 [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. (2000). Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience, 12(1), 174–187. 10.1162/089892900561814 [DOI] [PubMed] [Google Scholar]
- Robinson G, Cipolotti L, Walker DG, Biggs V, Bozzali M, & Shallice T (2015). Verbal suppression and strategy use: A role for the right lateral prefrontal cortex? Brain, 138(4), 1084–1096. 10.1093/brain/awv003 [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Bozzali M, & Cipolotti L (2012). The differing roles of the frontal cortex in fluency tests. Brain, 135(7), 2202–2214. 10.1093/brain/aws142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, et al. (2010). Executive function and fluid intelligence after frontal lobe lesions. Brain, 133(1), 234–247. 10.1093/brain/awp269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GR, Souza CP, Cetlin RS, De Oliveira DS, Pena-Pereira M, Ujikawa LT, et al. (2009). Use of the frontal assessment battery in evaluating executive dysfunction in patients with Huntington’s disease. Journal of Neurology, 256(11), 1809–1815. 10.1007/s00415-009-5197-0 [DOI] [PubMed] [Google Scholar]
- Roebuck-Spencer TM, Glen T, Puente AE, Denney RL, Ruff RM, Hostetter G, et al. (2017). Cognitive screening tests versus comprehensive neuropsychological test batteries: A national academy of neuropsychology education paper. Archives of Clinical Neuropsychology, 32(4), 491–498. 10.1093/arclin/acx021 [DOI] [PubMed] [Google Scholar]
- Rosseel Y (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48(1), 1–36. [Google Scholar]
- RStudio Team. (2020). RStudio: Integrated development Environment for R. RStudio. PBC. [Google Scholar]
- Ruscheweyh R, Deppe M, Keller SS, Knecht S, Ruscheweyh R, Deppe M, et al. (2013a). Executive performance is related to regional gray matter volume in healthy older individuals executive performance is related to regional gray matter volume in healthy older individuals. Human Brain Mapping, 34(12), 3333–3346. 10.1002/hbm.22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R, Deppe M, Lohmann H, Wersching H, Korsukewitz C, Duning T, et al. (2013b). Executive performance is related to regional gray matter volume in healthy older individuals. Human Brain Mapping, 34(12), 3333–3346. 10.1002/hbm.22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2005). Relations between cognitive abilities and measures of executive functioning. Neuropsychology, 19(4), 532–545. 10.1037/0894-4105.19.4.532 [DOI] [PubMed] [Google Scholar]
- Santamaría-García H, Baez S, Reyes P, Santamaría-García JA, Santacruz-Escudero JM, Matallana D, et al. (2017). A lesion model of envy and Schadenfreude: Legal, deservingness and moral dimensions as revealed by neurodegeneration. Brain, 140(12), 3357–3377. 10.1093/brain/awx269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalei V (2018). On the computation of the RMSEA and CFI from the mean-and-variance corrected test statistic with nonnormal data in SEM on the computation of the RMSEA and CFI from the mean-and-variance corrected test statistic with nonnormal data in SEM. Multivariate Behavioral Research, 53(3), 419–429. 10.1080/00273171.2018.1455142 [DOI] [PubMed] [Google Scholar]
- Saxe R (2006). Uniquely human social cognition. Current Opinion in Neurobiology, 16(2), 235–239. 10.1016/j.conb.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, & Muller H (2003). Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research, 8, 23–74. [Google Scholar]
- Schroeter ML, Laird AR, Chwiesko C, Deuschl C, Schneider E, Bzdok D, et al. (2014). Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses - the case of behavioral variant frontotemporal dementia. Cortex, 57, 22–37. 10.1016/j.cortex.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, & Gorno-Tempini ML (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology, 65(2), 249–255. 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheelakumari R, Bineesh C, Varghese T, Kesavadas C, & Verghese J (2020). Neuroanatomical correlates of apathy and disinhibition in behavioural variant frontotemporal dementia. Brain Imaging and Behavior, 14, 2004–2011. 10.1007/s11682-019-00150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra Sanjurjo N, Saraniti AB, Gleichgerrchtc E, Roca M, Manes F, & Torralva T (2019). The IFS (INECO Frontal Screening) and level of education: Normative data. Applied Neuropsychologye Adult, 26(4), 331–339. 10.1080/23279095.2018.1427096 [DOI] [PubMed] [Google Scholar]
- Smith EE, & Jonides J (1998). Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America, 95(20), 12061–12068. 10.1073/pnas.95.20.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker HR, Depue BE, Reineberg AE, Orr JM, & Banich MT (2015). Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Structure & Function, 220(3), 1291–1306. 10.1007/s00429-014-0723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolker HR, Friedman NP, Hewitt JK, & Banich MT (2018). Neuroanatomical correlates of the unity and diversity model of executive function in young adults. Frontiers in Human Neuroscience, 12(July), 1–23. 10.3389/fnhum.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6(MAR). 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, et al. (2009). Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia, 47(13), 2812–2827. 10.1016/j.neuropsychologia.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Dong Z, Long X, Li S, Zuo X, Zhu C, et al. (2011). REST: A toolkit for resting-state functional magnetic resonance imaging data processing. Plos One, 6(9), Article e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopford CL, Thompson JC, Neary D, Richardson AMT, & Snowden JS (2012). Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex, 48(4), 429–446. 10.1016/j.cortex.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Stuss DT (2011). Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society, 17(5), 759–765. 10.1017/S1355617711000695 [DOI] [PubMed] [Google Scholar]
- Stuss DT, & Alexander MP (2007). Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1481), 901–915. 10.1098/rstb.2007.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas C, Mogulkoc H, Eryilmaz G, Gogcegoz-Gul I, Erguzel TT, Metin B, et al. (2018). Discriminating schizophrenia and schizo-obsessive disorder: A structural MRI study combining VBM and machine learning methods. Neural Computing and Applications, 29(2), 377–387. 10.1007/s00521-016-2451-0 [DOI] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, López P, & Manes F (2009). INECO frontal screening (IFS): A brief, sensitive, and specific tool to assess executive functions in dementia. Journal of the International Neuropsychological Society, 15(5), 777–786. 10.1017/S1355617709990415 [DOI] [PubMed] [Google Scholar]
- Tsuchida A, & Fellows LK (2013). Arecorecomponentprocessesof executive function dissociable within the frontal lobes? Evidence from humans with focal prefrontal damage. Cortex, 49(7), 1790–1800. 10.1016/j.cortex.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Urbanski M, Bréchemier ML, Garcin B, Bendetowicz D, De Schotten MT, Foulon C, et al. (2016). Reasoning by analogy requires the left frontal pole: Lesion-deficit mapping and clinical implications. Brain, 139(6), 1783–1799. 10.1093/brain/aww072 [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RPC, Neggers SFW, Kappelle LJ, Frijns CJM, & Postma A (2006). Brain areas involved in spatial working memory. Neuropsychologia, 44(7), 1185–1194. 10.1016/j.neuropsychologia.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Vigliecca NS, & Baez S (2015). Screening executive function and global cognition with the Nine-Card Sorting Test: Healthy participant studies and ageing implications. Psychogeriatrics, 15(3), 163–170. 10.1111/psyg.12104 [DOI] [PubMed] [Google Scholar]
- Volle E, De Lacy Costello A, Coates LM, McGuire C, Towgood K, Gilbert S, et al. (2012). Dissociation between verbal response initiation and suppression after prefrontal lesions. Cerebral Cortex, 22(10), 2428–2440. 10.1093/cercor/bhr322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Oertzen T, Brandmaier AM, Tsang S, Von Oertzen T, Brandmaier AM, & Tsang S (2015). Structural equation modeling with U nyx. Structural Equation Modeling: A Multidisciplinary Journal, 22(1), 148–161. 10.1080/10705511.2014.935842 [DOI] [Google Scholar]
- Wager TD, & Smith EE (2003). Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective & Behavioral Neuroscience, 3(4), 255–274. 10.3758/CABN.3.4.255 [DOI] [PubMed] [Google Scholar]
- Walter H, Wunderlich AP, Blankenhorn M, Schäfer S, Tomczak R, Spitzer M, et al. (2003). No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophrenia Research, 61(2–3), 175–184. 10.1016/S0920-9964(02)00225-6 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & Shallice T (1969). The selective impairment of auditory verbal short-term memory. Brain, 92, 885–896. [DOI] [PubMed] [Google Scholar]
- Was C (2007). Further evidence that not all executive functions are equal. Advances in Cognitive Psychology, 3(3), 399–407. 10.2478/v10053-008-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler memory scale-revised. Psychological Corporation. [Google Scholar]
- Weise CM, Bachmann T, Schroeter ML, & Saur D (2019). When less is more: Structural correlates of core executive functions in young adults – a VBM and cortical thickness study. Neuroimage, 189(February), 896–903. 10.1016/j.neuroimage.2019.01.070 [DOI] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LD, Franç ois R., et al. (2019). Welcome to the tidyverse. Journal of Open Source Software, 4(43), 1686. 10.21105/joss.01686 [DOI] [Google Scholar]
- Wilson N-A, Ramanan S, Roquet D, Hodges JR, Piguet O, & Irish M (2020). Neuropsychologia Scene construction impairments in frontotemporal dementia : Evidence for a primary hippocampal contribution. Neuropsychologia, 137(107327). 10.1016/j.neuropsychologia.2019.107327 [DOI] [PubMed] [Google Scholar]
- Wray C, Kowalski A, Mpondo F, Ochaeta L, Belleza D, DiGirolamo A, et al. (2020). Executive functions form a single construct and are associated with schooling: Evidence from three low- and middle- income countries. Plos One, 15(11 November 2020), 1–14. 10.1371/journal.pone.0242936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, & Raz N (2014). Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neuroscience and Biobehavioral Reviews, 42, 180–192. 10.1016/j.neubiorev.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LJ, Wen J, Ni L, Zhong J, Lianq X, Zhenq G, et al. (2013). Predominant gray matter volume loss in patients with end-stage renal disease: A voxel-based morphometry study. Metabolic Brain Desease, 28(4), 647–654. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ortiz F, Nuñez C, Elashoff D, Woo E, Apostolova LG, et al. (2015). Use of the MoCA in detecting early alzheimer’s disease in a Spanish-speaking population with varied levels of education. Dementia and Geriatric Cognitive Disorders Extra, 5(1), 85–95. 10.1159/000365506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Given patients’ sensitive information and funding regulations, the databases, images, and code generated and/or used during the current study are not publicly archived. However, access to all materials will be granted from the corresponding author after the completion of a formal data sharing agreement and IRB approval.


