Abstract
Bloodstream infections (BSIs) from oral organisms are a significant cause of morbidity and mortality in hematopoietic stem cell transplantation (HSCT) recipients. There are no proven strategies to decrease BSIs from oral organisms. The aim of this study was to evaluate the impact of daily xylitol wipes in improving oral health, decreasing BSI from oral organisms, and modulating the oral microbiome in pediatric HSCT recipients. This was a single-center 1:1 randomized controlled trial in pediatric HSCT recipients age >2 years. Age-matched healthy children were enrolled to compare the oral microbiome. The oral hygiene standard of care (SOC) group continued to receive the standard oral hygiene regimen. The xylitol group received daily oral xylitol wipes (with .7 g xylitol) in addition to the SOC. The intervention started from the beginning of the transplantation chemotherapy regimen and extended to 28 days following transplantation. The primary outcome was oral health at interval time points, and secondary outcomes included BSIs from oral organisms in the first 30 days following transplantation, oral microbiome abundance, and diversity and oral pathogenic organism abundance. The study was closed early due to efficacy after an interim analysis of the first 30 HSCT recipients was performed (SOC group, n = 16; xylitol group, n = 14). The xylitol group had a significantly lower rate of gingivitis at days 7, 14, and 28 following transplantation (P = .031, .0039, and .0005, respectively); oral plaque at days 7 and 14 (P = .045 and .0023, respectively); and oral ulcers >10 mm at day 14 (P = .049) compared with the SOC group. The xylitol group had no BSI from oral organisms compared with the SOC group, which had 4 (P = .04). The xylitol group had significantly lower abundance of potential BSI pathogens, such as Staphylococcus aureus (P = .036), Klebsiella pneumoniae (P = .033), and Streptococcus spp (P = .011) at the day after transplantation compared with the SOC group. Healthy children and young adults had significantly increased oral microbiome diversity compared with all HSCT recipients (P < .001). The addition of xylitol to standard oral care significantly improves oral health, decreases BSI from oral organisms, and decreases the abundance of pathogenic oral organisms in pediatric and young adult HSCT recipients.
Keywords: Hematopoietic stem cell transplantation, Bloodstream infection, Mucosal barrier injury laboratory-confirmed bloodstream infection, Microbiome, Xylitol
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is an effective treatment for many malignancies, marrow failure syndromes, and immune deficiencies in children, adolescents, and adults [1,2]. Transplantation strategies and supportive care have evolved over the past few decades, resulting in improved overall survival. However, despite these advances, HSCT recipients are at high risk for bloodstream infections (BSI), a significant cause of mortality [3–6]. HSCT recipients develop mucosal toxicity as a side effect of treatment [7]; that, along with a profound immunosuppressed state, predisposes to organism translocation through a nonintact oral mucosa into the bloodstream. Currently, there are no known strategies to reduce or prevent BSIs from oral organisms in HSCT recipients [7–12].
Gingivitis is an important feature of mucosal toxicity after HSCT and is closely associated with the amount of dental and gingival plaque; 1 mm3 of dental plaque contains ~100 million bacteria that serves as a persistent reservoir for potential BSI [13–15]. Oral plaque has been shown to be significantly associated with bacteremia in healthy subjects, as well as those undergoing HSCT [16,17]. Xylitol is a nonfermentable polysaccharide alcohol that has been shown to reduce dental caries, plaque accumulation, and oral disease progression and has been shown to decrease salivary counts of Streptococcus spp and Candida albicans in the oral cavity [18–24].
To date, studies of the microbiome have mainly used culture-based techniques and 16S rRNA, limiting the level of detail of the microbiome analysis. Recent advances using metagenomic shotgun sequencing analyze all genes present among organisms in a given sample to quantify and recognize pathogens at the strain level [25,26]. Metagenomic sequencing allows determination of the bacterial diversity and abundance of organisms specific to a patient and can accurately surveil the presence of and changes in pathogenic strains over time and correlate changes with clinical interventions [27]. The aim of this study was to evaluate the impact of daily xylitol application on oral health, BSI from oral organisms, microbiome abundance and diversity, and pathogenic oral organism abundance in pediatric and young adult HSCT recipients.
METHODS
Study Design
This single-center prospective randomized 1:1 controlled trial of HSCT recipients conducted at Cincinnati Children’s Hospital Medical Center’s Bone Marrow Transplant Unit. The protocol and all amendments were approved by the Center’s Institutional Review Board.
Study Intervention
Children and adolescents at least 2 years of age undergoing autologous or allogeneic HSCT were eligible. Study subjects were randomized into 2 groups: patients receiving the current oral hygiene standard of care (SOC group) and those randomized to receive the current standard of care as well as once-daily dental xylitol wipe application (xylitol group).
Patients in the SOC group continued to receive the prescribed 3 daily oral rinses of chlorhexidine gluconate and nystatin, as described previously [8,28–30]. The xylitol group used oral xylitol wipes (with .7 g xylitol) to wipe the patient’s teeth and gums once daily for a minimum of 30 seconds in addition to the standard of care. The study period for both groups started from the beginning of the HSCT recipient’s transplantation chemotherapy regimen to day 30 following transplantation.
Age-Matched Healthy Children
We enrolled 30 age-matched healthy children and young adults from the Cincinnati Children’s Hospital Medical Center’s Dental Clinic to compare differences in oral microbiome diversity between HSCT recipients and healthy children. Healthy children and young adults presenting to the dental clinic for routine follow up were eligible for the study.
Randomization
Consenting patients were randomized to the SOC or xylitol group (1:1 randomization) using a sealed envelope system. This was on open-label study, with clinicians and participants aware of the treatment allocation.
Primary Outcome Measures: Oral Heath
Oral assessment measures were performed in the SOC and xylitol group at baseline (before the start of transplantation chemotherapy), day 0 (day of stem cell infusion), and at days 7, 14, and 28 post-HSCT by a member of the study team (M.D. or D.D.S.). Oral assessments included evaluation of dental and lingual plaque, gingival inflammation, and oral ulcerations. Dental plaque was evaluated using the Simplified Oral Hygiene Index [31], lingual plaque was evaluated using the Miyazaki Tongue Coating Index [32], and gingival inflammation was assessed with the Gingival Index [33]. The presence of mucosal ulcerations along the buccal mucosa, labial mucosa, and tongue were evaluated clinically with an intraoral mirror and a flashlight. The number and size of lesions present were documented based on location. Mucosal ulcerations were categorized by size as <5 mm, >5 mm, or >10 mm.
Secondary Outcome Measure: BSIs from Oral Organisms
We compared the incidence of BSIs from oral organisms during the study period, starting at enrollment into the study and extending through 30 days post-transplantation. Oral organisms were identified as described in previous oral microbiota studies [34,35].
Sample Collection
Oral sample collection for microbiome analysis was the same for the SOC and xylitol groups. Teeth and gums were swabbed for approximately 30 seconds at baseline (before or at the start of transplantation chemotherapy) and at days 7, 14, and 28 post-transplantation. Gingival and oral mucosa specimens from healthy age-matched children and young adults were collected only once using the same mechanism.
Microbiome Analysis and Metagenomic Sequencing
DNA was extracted from oral samples and libraries were prepared with Nextera XT adapter tagmentation using dual indices with the Illumina PCR amplification protocol consisting of 12 cycles (Illumina, San Diego, CA). DNA distribution was analyzed on a fragment analyzer (Advanced Analytical Technologies, Orangeburg, NY). Samples were pooled to a uniform DNA concentration of 1 to 10 ng, and pooled fragments were eluted at 250 to 600 base pairs in length using Pippin Prep (Sage Science, Beverly, MA). The eluted pooled product was cleaned using the Qiagen DNA Mini Kit (Qiagen, Hilden, Germany). Denaturation of pools was performed by sequencing of 150 paired-end reads on the Illumina NextSeq 500 sequencing system. Amplified library generation was done with Illumina Nextera XT adapters, and sequencing was performed to obtain 150-bp DNA paired-end reads using the Illumina NextSeq 500 system. The depth of sequencing for metagenome samples was targeted to be between 16 and 20 million reads per metagenome sample. The Bracken microbial genome database was used to determine the relative abundance of oral organisms. The Kraken database was used to derive probabilities of how much sequence from each genome was identical to other genomes in the database and then to combine this information with the assignments for a particular sample to estimate abundance at the species level and genus level, as described previously [25].
Microbiome Analysis
Microbiome analysis included oral organism abundance and diversity and pathogenic oral organism abundance through metagenomic sequencing at baseline (before the start of transplantation chemotherapy) at days 7, 14, and 28 post-transplantation. Oral microbiome analysis of age-matched healthy children and young adults was compared with that of all HSCT recipients.
Adverse Events
Serious adverse events were defined as any local or systemic grade 4 toxicity that was unexpected and at least probably related to xylitol application.
Statistical Analysis
We initially anticipated the enrollment of a total of 60 HSCT recipients to have 52 evaluable HSCT recipients, including 26 in the SOC group and 26 in the xylitol group. This target enrollment was based on our findings of plaque accumulation and severe gingival inflammation in the first 28 days following transplantation in 85% of HSCT recipients in our previous study [8]. The target enrollment number was calculated to control the level of significance at .05, with a .80 power of success. The study was closed early owing to efficacy after an interim analysis of the first 30 HSCT recipients was performed. These HSCT recipients’ baseline values were compared with their values at days 0, 7, 14, and 28 post-transplantation using a paired t test, and scores were compared using a 2 × 2 between-participant factor and a within-participant factor mixed analysis of variance to determine whether there was any significant difference between treatments.
Microbiome metagenomic statistical analysis was performed using advanced machine learning algorithms, including random forest and generalized linear mixed model trees that consider microbiome distribution properties, longitudinal sampling with random effects, and feature selection or tree classification using R. Differences in oral microbiome alpha diversity, as defined by the Shannon Index, were assessed at each time point using analysis of variance and multivariate methods (generalized linear methods) to control for variables that have a clear impact on microbiome diversity. A generalized linear mixed model was used to determine whether the longitudinal trend of microbiome diversity was altered by xylitol wipe use. Changes in pathogenic oral bacteria over time were studied using a generalized linear mixed-effects model with random slope and intercept terms for each patient, using unstructured covariance.
RESULTS
A total of 35 HSCT recipients were enrolled and randomized between January 2017 and July 2018. In August 2018, the study was closed early owing to efficacy after an interim analysis was performed. All HSCT recipients had completed all oral evaluations, and all the oral samples had been obtained by this date.
The 35 HSCT recipients enrolled in the study included 16 in the SOC group and 14 in the xylitol group. Five HSCT recipients withdrew from the study, 2 from the SOC group and 3 from the xylitol group. The 2 HSCT recipients who withdrew from the SOC group were excluded due to acute illness not related to BSI. The 3 HSCT recipients from the xylitol group withdrew secondary to feeling overwhelmed and nonadherent with frequent oral exams and sample collection (Figure 1).
Figure 1.

Study participation and trial flow. Thirty-five study subjects were enrolled in the study: 18 in the SOC group, 17 in the xylitol group, and 30 healthy children and young adults. Two of the 18 subjects randomized to the SOC group and 3 of the 17 subjects randomized to the Xylitol group withdrew from the study; thus, the study analysis included 16 subjects in the SOC group and 14 subjects in the xylitol group.
Demographics
Characteristics of all eligible HSCT recipients are described in Table 1. The characteristics of the SOC group and xylitol group were similar except for a greater number of HSCT recipients undergoing allogeneic HSCT in the SOC group (P= .018).
Table 1.
Patient Demographics (N = 30)
| Characteristic | Xylitol Group (n = 14) |
SOC Group (n = 16) |
|---|---|---|
| Sex, n (%) | ||
| Female | 8(57) | 6(36) |
| Male | 6(43) | 10(64) |
| Age, yr, median (range) | 14.0 (2–26) | 11.5(3–29) |
| Diagnosis, n (%) | ||
| Malignancy | 8(57) | 8(50) |
| Immune deficiency | 1(7) | 3(18) |
| Bone marrow failure | 5(36) | 3(18) |
| Nonmalignant hematology | 0 | 1(6) |
| Genetic/metabolic | 0 | 1(6) |
| Conditioning regimen, n (%) | ||
| Myeloablative | 10(71) | 12(75) |
| Reduced intensity | 4(29) | 4(25) |
| Donor type,n(%) | ||
| Autologous | 8(57) | 2(12) |
| Allogeneic | 6(43) | 14(88) |
| Stem cell source, n(%) | ||
| Bone marrow | 2(14) | 6(38) |
| Peripheral blood stem cells | 12(86) | 9(56) |
| Cord blood | 0 | 1(6) |
Primary Outcomes
Compared with the SOC group, the xylitol group had a significantly lower rate of gingivitis at days 7, 14, and 28 post-transplantation (P= .031, .0039, and .0005, respectively); significantly lower rates of lingual plaque at days 7 and 14 post-transplantation (P= .045 and .0023, respectively); and significantly lower rates of oral ulcers larger than 10 mm at day 14 post-transplantation (P= .049). There was no significant between-group difference in dental plaque (Figure 2). Assessment of interrater agreement using the intraclass correlation coefficient showed 96% agreement, validating the evaluation by the 2 physicians.
Figure 2.
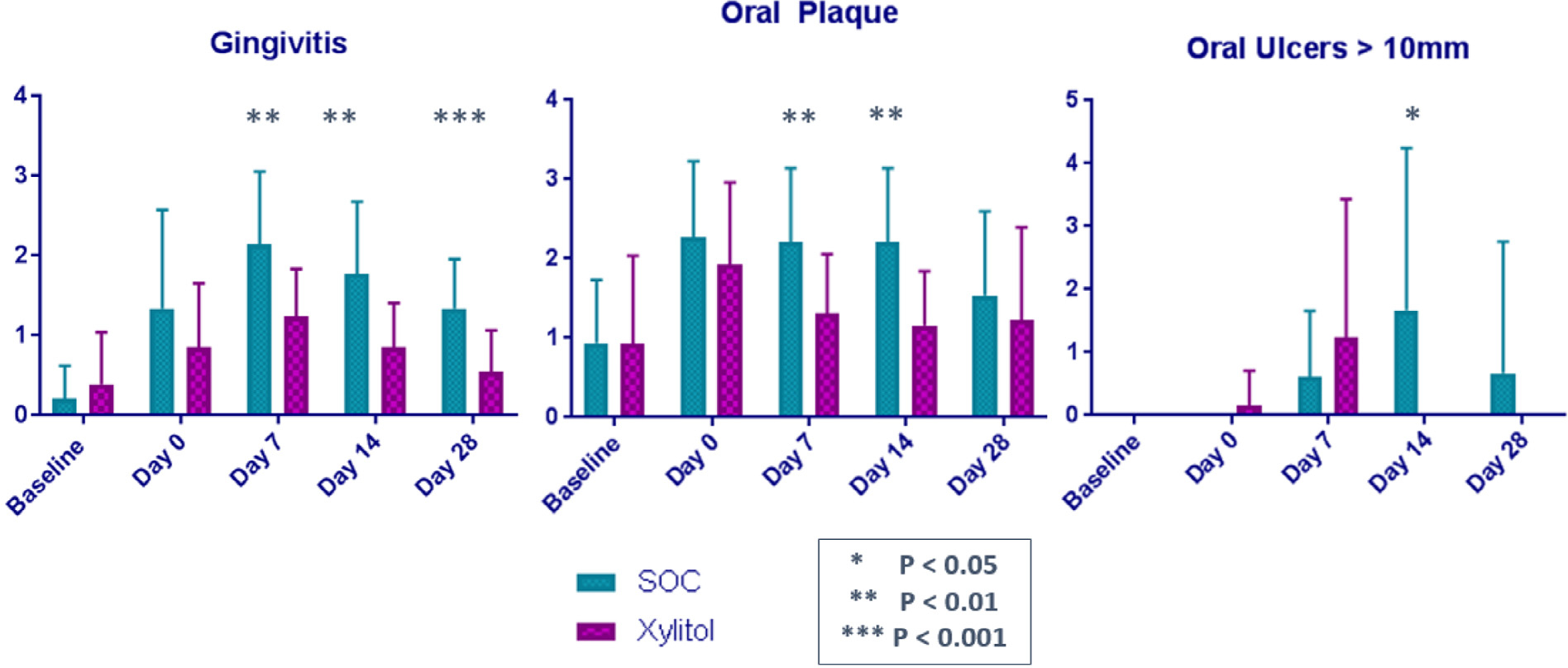
Comparison of oral hygiene in randomized to the xylitol group (n = 14) or the SOC group (n = 16). Compared with HSCT recipients in the SOC group, HSCT recipients in the xylitol group had decreased gingivitis at days 7, 14, and 28 post-transplantation; decreased oral plaque at days 7 and 14 post-transplantation; and fewer oral ulcers >10 mm at day 14 post-transplantation.
Secondary Outcomes
Four of the 16 (25%) HSCT recipients in the SOC group developed a BSI from oral organisms in the first 30 days post-HSCT, compared with none in the xylitol group (P = .04). The oral organisms identified in the 4 HSCT recipients in the SOC group were Streptococcus mitis/oralis in 3 patients, with a positive blood culture at days 2, 6, and 8 post-transplantation and Fusobacterium sp in 1 patient, with a positive blood culture at day 2 post-transplantation.
Microbiome Analysis
We compared the oral microbiome of age-matched healthy children and young adults with that of all HSCT recipients at baseline (before the start of transplantation chemotherapy), and then compared the oral microbiome of the SOC and xylitol groups at baseline and at days 7, 14, and 28 post-transplantation. The oral microbiome of healthy children and young adults showed a significantly different species abundance (P< .001) and increased oral microbiome diversity (P< .001) compared with that of all HSCT recipients at baseline (Figure 3A). Compared with all HSCT recipients, healthy children and young adults also had an increased abundance of commensal oral organisms, including S. mitis (P< .001), Gamella haemolysans (P< .001), Fusobacterium periodonticym (P< .001), and Streptococcus gordonii (P = .0068) (Figure 3B). Finally, all HSCT recipients at baseline had an increased abundance of potential pathogenic organisms in the oral microbiome, including E. coli (P< .001), K. pneumoniae (P< .001), Serratia marcescens (P< .001), Bacteroides vulgaris (P= .032), C. albicans (P= .12), and Malassezia restricta (P= .67) (Figure 3C). The microbiome analysis of HSCT recipients comparing the SOC group and xylitol group showed that both groups had similar species abundance at baseline (before the start of transplantation chemotherapy) (P= .39) (Figure 4A). HSCT recipients in the xylitol group had decreased species abundance of potential pathogenic organisms, such as S. aureus (P= .036) and K. pneumoniae (P= .022), compared with HCT recipients in the SOC group (Figure 4B). HSCT recipients in the xylitol group also had lower abundances of Streptococcus spp at day 7 (P= .11), C. albicans (P = .83), and M. restricta (P = .27) (Figure 4B).
Figure 3.
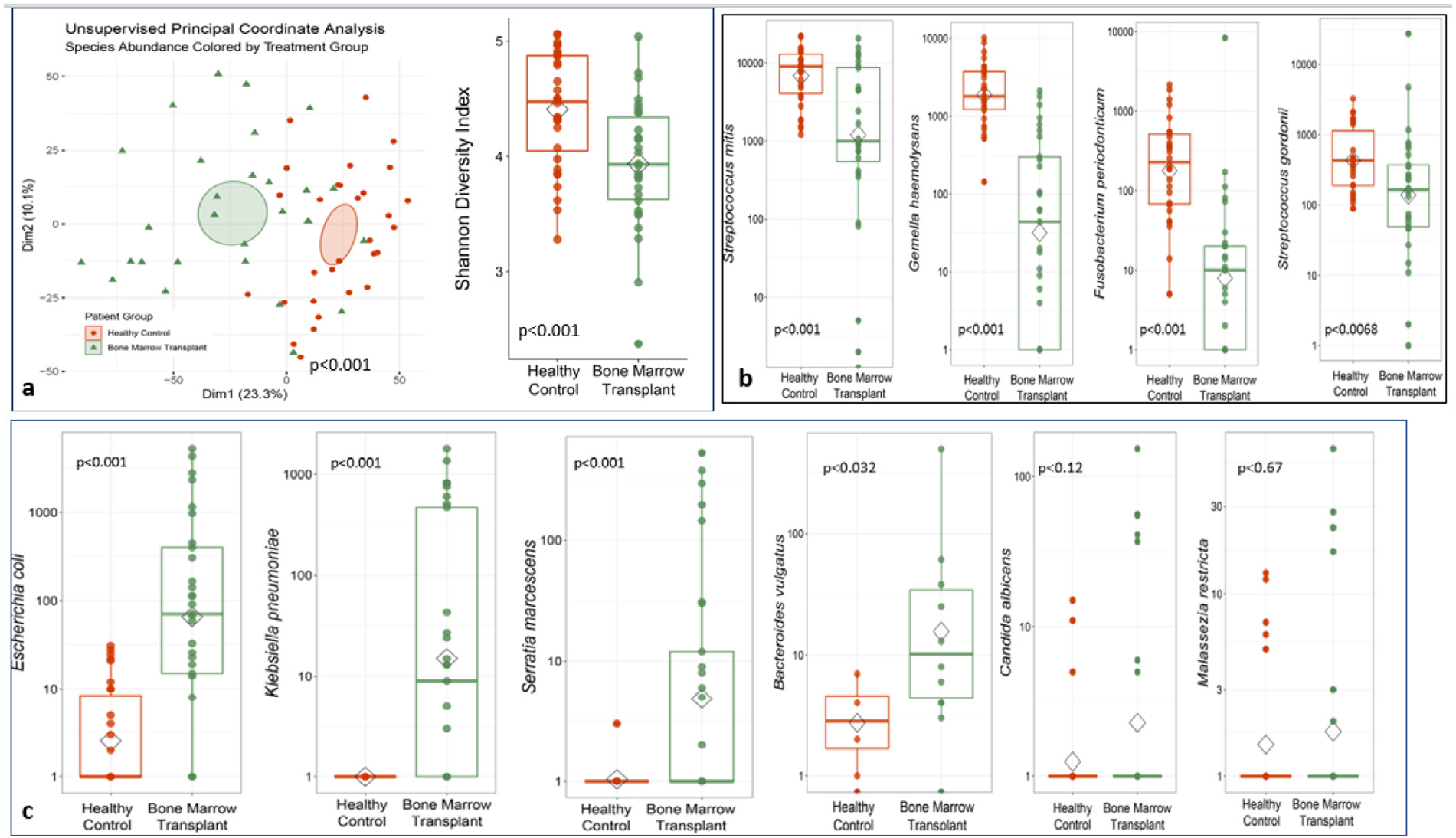
(A) Comparison of the oral microbiome species abundance and diversity in HSCT recipients at baseline (before transplantation chemotherapy) (n = 30) and healthy age-matched children and young adults (n = 30). (B) There was an increase abundance of commensal oral organisms in healthy children and young adults compared with all HSCT recipients. (C) There was an increase abundance of potential pathogenic oral organisms in all HSCT recipients compared with healthy children and young adults.
Figure 4.

(A) Species abundance at baseline (before the start of the transplantation chemotherapy regimen in the SOC and xylitol groups. (B) (1) Increased pathogenic species abundance at baseline and days 7, 14, 28 post-transplantation in the SOC group compared with the xylitol group. (2) Increased Streptococcus spp abundance (oral pathogenic bacteria) at day 7 post-transplantation in the SOC group compared with the xylitol group. (3) Increased fungal species abundance at days 7, 14, and 28 post-transplantation in the SOC group compared with the Xylitol group.
Adverse Events
There were no adverse events reported during the study period.
DISCUSSION
The data from this randomized controlled trial evaluating the addition of oral xylitol wipes to standard oral care and its impact on oral health and BSI arising from oral organisms demonstrate a significant decrease in gingivitis, oral plaque, and oral ulcerations >10 mm, as well as a lower rate of BSI from oral organisms, in HSCT recipients who received daily xylitol application in addition to the standard oral care. To our knowledge, this is the first randomized pediatric and young adult trial studying a clinical intervention to address oral health, BSI from oral organisms, and the oral microbiome in pediatric and young adult HSCT recipients. It is also important to emphasize that the extent of the innovation of the xylitol application lies in the simplicity and affordability of the intervention. Xylitol is commercially available, inexpensive ($.15 per application) and can be rapidly integrated into practice.
Gingivitis has been shown to be significantly associated with bacteremia in HSCT recipients [17], and it is known that a nonintact oral mucosa puts HSCT recipients at risk of bacteria translocating through the mucosa into the bloodstream, leading to BSI [7,36–38]. The reported incidence of BSI in the weeks following HSCT ranges from 21% to 58% [4,9]. Cappellano et al [39] found that nearly 21% of their HSCT recipients developed a BSI in the first 30 days post-HSCT, the majority (75%) from a gram-positive organism from the oral cavity. Wang et al [40] reported that nearly 24% of their HSCT recipients developed a BSI shortly after HSCT despite receiving levofloxacin prophylaxis, with nearly 70% of the infections related to organisms found in the oral cavity. In a subset analysis of HSCT recipients receiving fluoroquinolone prophylaxis who developed bacteremia from viridans streptococci (organisms generally confined to the oral cavity), Kimura et al [41] found viridans streptococci alone in 15% of patients receiving fluoroquinolone prophylaxis. Prospective studies have evaluated interventions that are effective in reducing mucositis, including keratinocyte growth factor [42] and cryotherapy [11], but these have not shown a beneficial effect in reducing BSI. Chlorhexidine has also been widely used as a bactericidal agent to reduce bacterial colony-forming units but has not been shown to reduce BSI from oral organisms [12,43,44].
In this randomized controlled trial, the HSCT recipients receiving daily oral xylitol wipes in addition to the standard oral care (chlorhexidine and nystatin mouthwash 3 times daily) had significantly lower rates of gingivitis, oral plaque and oral ulcers >10 mm post-transplantation compared with the HSCT recipients who received the standard oral care alone. BSI from oral organisms occurred in none of the HSCT recipients in the xylitol group, compared with 4 HSCT recipients in the SOC group. This finding indicates that HSCT recipients receiving xylitol had better oral health with decreased plaque and gingivitis, which led to a decreased abundance of oral pathogenic organisms and lowered the risk of pathogenic organisms translocating through the oral mucosa into the bloodstream and causing BSI. Of note, there were more allogeneic HSCT recipients in the SOC group compared with the xylitol group, and patients receiving an allogeneic transplant had an elevated rate of mucosal barrier injury-related BSIs. This observation will be investigated further in a subsequent study including only allogeneic HSCT recipients.
Our oral microbiome analysis comparing healthy children with HSCT recipients before transplantation revealed significant differences in the oral microbiome, including increased species abundance and diversity. This finding correlates with our finding that HSCT recipients had a significantly higher abundance of potential pathogenic organisms in the oral cavity, including E. coli, K. pneumoniae, and S. marcescens, most likely due to decreases in commensal organisms due to previous antibiotic exposure, changes in diet, chemotherapy, and radiation leading to dysbiosis of the oral microbiome and a predisposition for increased abundance of pathogenic organisms that can lead to infection.
The microbiome analysis showing the presence of S. aureus in the oral cavity is important. S. aureus has always been one of the major organisms causing central line- associated BSIs (CLABSIs), raising the question of whether BSI from S. aureus and other CLABSI-causing organisms arise not only from skin surfaces, but also from the oral cavity. Similar observations have been noted by others; for example, Tamburini et al [45] reported cases of nonenteric pathogens, such as Pseudomonas and Staphylococcus epidermidis, in the intestinal microbiome. These findings challenge the current thinking that CLABSIs originate from the environment, skin sources, or other mechanisms of central venous catheter contamination.
This study has some limitations. The open-label design can lead to observational bias. Patients received different transplantation chemotherapy regimens, some of which are known to cause increased mucosal toxicity, which could have affected oral examination findings in HSCT recipients. The study included allogeneic and autologous HSCT recipients, with more allogeneic HSCT recipients randomized to the SOC group, and allogenic HSCT recipients are known to have a higher risk of mucosal barrier injury laboratory-confirmed bloodstream infections. We believe that these limitations will be addressed moving forward with a multicenter, randomized, double-blind, placebo-control trial (ClinicalTrials.gov identifier NCT04117477).
CONCLUSIONS
Among pediatric and young adult HSCT recipients, the addition of oral xylitol wipes to our current standard oral care resulted in a significant reduction in gingivitis, oral plaques, and oral ulceration, decreased BSIs from oral organisms, and decreased pathogenic oral bacteria abundance.
ACKNOWLEDGMENTS
Financial disclosure: The authors have nothing to disclose.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure: See Acknowledgments on page 1709.
REFERENCES
- 1.Barriga F, Ramírez P, Wietstruck A, Rojas N. Hematopoietic stem cell transplantation: clinical use and perspectives. Biol Res. 2012;45:307–316. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. [DOI] [PubMed] [Google Scholar]
- 3.Dandoy CE, Kim S, Chen M, et al. Incidence, risk factors, and outcomes of patients who develop mucosal barrier injury-laboratory confirmed bloodstream infections in the first 100 days after allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2020;3: e1918668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant. 2017;52:1091–1106. [DOI] [PubMed] [Google Scholar]
- 5.Dandoy CE, Kelley T, Gaur AH, et al. Outcomes after bloodstream infection in hospitalized pediatric hematology/oncology and stem cell transplant patients. Pediatr Blood Cancer. 2019;66:e27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dandoy CE, Haslam D, Lane A, et al. Healthcare burden, risk factors, and outcomes of mucosal barrier injury laboratory-confirmed bloodstream infections after stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhry HM, Bruce AJ, Wolf RC, et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant. 2016;22:605–616. [DOI] [PubMed] [Google Scholar]
- 8.Doss LM, Dandoy CE, Kramer K, et al. Oral health and hematopoietic stem cell transplantation: a longitudinal evaluation of the first 28 days. Pediatr Blood Cancer. 2018;65:e26773. [DOI] [PubMed] [Google Scholar]
- 9.Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40:63–70. [DOI] [PubMed] [Google Scholar]
- 10.Smith K, Robertson DP, Lappin DF, Ramage G. Commercial mouthwashes are ineffective against oral MRSA biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:624–629. [DOI] [PubMed] [Google Scholar]
- 11.Svanberg A, Ohrn K, Birgegard G. Oral cryotherapy reduces mucositis and improves nutrition—a randomised controlled trial. J Clin Nurs. 2010;19:2146–2151. [DOI] [PubMed] [Google Scholar]
- 12.Bortoluzzi MC, Santos FA. Amoxicillin and 0.12% chlorhexidine mouthwash may not be better than placebo for reducing bacteremia in third molar extractions. J Evid Based Dent Pract. 2014;14:34–35. [DOI] [PubMed] [Google Scholar]
- 13.Pilot T, Barmes DE, Leclercq MH, McCombie BJ, Sardo Infirri J. Periodontal conditions in adolescents, 15–19 years of age: an overview of CPITN data in the WHO Global Oral Data Bank. Community Dent Oral Epidemiol. 1987;15:336–338. [DOI] [PubMed] [Google Scholar]
- 14.Thoden van Velzen SK, Abraham-Inpijn L, Moorer WR. Plaque and systemic disease: a reappraisal of the focal infection concept. J Clin Periodontol. 1984;11:209–220. [DOI] [PubMed] [Google Scholar]
- 15.Mandel ID. Dental plaque: nature, formation and effects. J Periodontol. 1966;37:537–567. [PubMed] [Google Scholar]
- 16.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–407. [DOI] [PubMed] [Google Scholar]
- 17.Raber-Durlacher JE, Laheij AM, Epstein JB, et al. Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Support Care Cancer. 2013;21:1621–1627. [DOI] [PubMed] [Google Scholar]
- 18.Ur-Rehman S, Mushtaq Z, Zahoor T, Jamil A, Murtaza MA. Xylitol: a review on bioproduction, application, health benefits, and related safety issues. Crit Rev Food Sci Nutr. 2015;55:1514–1528. [DOI] [PubMed] [Google Scholar]
- 19.Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001;65:960–968. [PubMed] [Google Scholar]
- 20.Haghgoo R, Afshari E, Ghanaat T, Aghazadeh S. Comparing the efficacy of xylitol-containing and conventional chewing gums in reducing salivary counts of Streptococcus mutans: an in vivo study. J Int Soc Prev Community Dent. 2015;5(suppl 2):S112–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flagg A, Worley S, Foster CB. Characteristics of bacteremia in pediatric oncology patients based on pathogen classification as associated with the gastrointestinal mucosa or skin. Infect Control Hosp Epidemiol. 2015;36:730–733. [DOI] [PubMed] [Google Scholar]
- 22.Zhan L, Cheng J, Chang P, et al. Effects of xylitol wipes on cariogenic bacteria and caries in young children. J Dent Res. 2012;91(7 suppl):85S–90S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan L, Featherstone JD, Lo J, et al. Clinical efficacy and effects of xylitol wipes on bacterial virulence. Adv Dent Res. 2012;24:117–122. [DOI] [PubMed] [Google Scholar]
- 24.Jain A, Bhaskar DJ, Gupta D, et al. Clinical efficacy and effects of xylitol wipes on bacterial virulence. Adv Dent Res. 2015;6:53–57. [DOI] [PubMed] [Google Scholar]
- 25.Andersen H, Connolly N, Bangar H, et al. Use of shotgun metagenome sequencing to detect fecal colonization with multidrug-resistant bacteria in children. J Clin Microbiol. 2016;54:1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood DE, Kraken Salzberg SL. ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Breitwieser FP, Thielen P, Salzberg SL. Bracken: estimating species abundance in metagenomics data. Peer J Comput Sci. 2017;3:e104. [Google Scholar]
- 28.Best D, Osterkamp E, Demmel K, et al. Increasing activities of daily living is as easy as 1–2-3. J Pediatr Oncol Nurs. 2016;33:345–352. [DOI] [PubMed] [Google Scholar]
- 29.Dandoy CE, Hausfeld J, Flesch L, et al. Rapid cycle development of a multifactorial intervention achieved sustained reductions in central line-associated bloodstream infections in haematology oncology units at a children’s hospital: a time series analysis. BMJ Qual Saf. 2016;25:633–643. [DOI] [PubMed] [Google Scholar]
- 30.Hickey V, Flesch L, Lane A, et al. Token economy to improve adherence to activities of daily living. Pediatr Blood Cancer. 2018;65:e27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki H, Sakao S, Katoh Y, Takehara T. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J Periodontol. 1995;66:679–684. [DOI] [PubMed] [Google Scholar]
- 33.Lo€e H. The Gingival Index, the Plaque Index and the Retention Index systems. J Periodontol. 1967;38(suppl 6):610–616. [DOI] [PubMed] [Google Scholar]
- 34.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasan NA, Young BA, Minard-Smith AT, et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One. 2014;9:e97699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.See I, Iwamoto M, Allen-Bridson K, Horan T, Magill SS, Thompson ND. Mucosal barrier injury laboratory-confirmed bloodstream infection: results from a field test of a new National Healthcare Safety Network definition. Infect Control Hosp Epidemiol. 2013;34:769–776. [DOI] [PubMed] [Google Scholar]
- 37.Metzger KE, Rucker Y, Callaghan M, et al. The burden of mucosal barrier injury laboratory-confirmed bloodstream infection among hematology, oncology, and stem cell transplant patients. Infect Control Hosp Epidemiol. 2015;36:119–124. [DOI] [PubMed] [Google Scholar]
- 38.Epstein L, See I, Edwards JR, Magill SS, Thompson ND. Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections (MBI-LCBI): descriptive analysis of data reported to National Healthcare Safety Network (NHSN), 2013. Infect Control Hosp Epidemiol.. 2016;37:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappellano P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A. Epidemiology and risk factors for bloodstream infections after allogeneic hematopoietic stem cell transplantion. New Microbiol. 2007;30:89–99. [PubMed] [Google Scholar]
- 40.Wang CH, Chang FY, Chao TY, et al. Characteristics comparisons of bacteremia in allogeneic and autologous hematopoietic stem cell-transplant recipients with levofloxacin prophylaxis and influence on resistant bacteria emergence. J Microbiol Immunol Infect. 2018;51:123–131. [DOI] [PubMed] [Google Scholar]
- 41.Kimura M, Araoka H, Yoshida A, et al. Breakthrough viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients receiving levofloxacin prophylaxis in a Japanese hospital. BMC Infect Dis. 2016;16:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vadhan-Raj S, Trent J, Patel S, et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Ann Intern Med. 2010;153:358–367. [DOI] [PubMed] [Google Scholar]
- 43.Antunes HS, de Sa Ferreira EM, de Faria LM, et al. Streptococcal bacteraemia in patients submitted to hematopoietic stem cell transplantation: the role of tooth brushing and use of chlorhexidine. Med Oral Patol Oral Cir Bucal. 2010;15:e303–e309. [DOI] [PubMed] [Google Scholar]
- 44.Erden Y, Ipekcoban G. Comparison of efficacy of cryotherapy and chlorhexidine to oral nutrition transition time in chemotherapy-induced oral mucositis. Eur J Cancer Care (Engl). 2017;26:e12495. [DOI] [PubMed] [Google Scholar]
- 45.Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med. 2018;24:1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]


