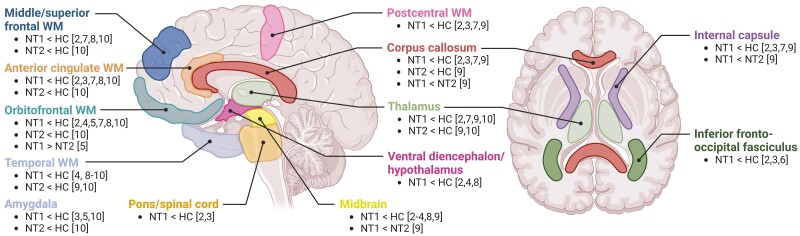
Narcolepsy is a chronic neurological sleep disorder characterized by excessive daytime sleepiness and is generally divided into two subtypes: narcolepsy type 1 and type 2. Besides the daytime sleepiness with sleep attacks, both narcolepsy subtypes show sleep-onset rapid eye movement (REM) sleep periods and are frequently accompanied by hypnagogic/hypnopompic hallucinations, sleep paralysis, disturbed nocturnal sleep, vigilance deficits, and in case of narcolepsy type 1, cataplexy. Narcolepsy type 1 is caused by hypocretin-1 deficiency, whereas the etiology of narcolepsy type 2 remains unknown [1]. The multitude of affected symptom domains in narcolepsy has interested many research groups to investigate underlying neural signatures. Using diffusion-weighted imaging (DWI), studies have suggested widespread structural white matter differences in narcolepsy type 1 compared to controls in practically all brain regions (Figure 1) [2–9].
Figure 1.
Summary figure of diffusion-weighted imaging (DWI) studies investigating structural connectivity in narcolepsy. Regions were generally included in case there were at least three independent studies indicating white matter changes within this region in people with narcolepsy compared to controls. Reported results were limited to reduced structural connectivity in narcolepsy as the vast majority of studies solely reported reduced structural connectivity. Reported differences could reflect any DWI outcome measure that represents white matter integrity, such as fractional anisotropy (FA) or mean diffusivity/apparent diffusion coefficient (MD/ADC). The numbers between brackets correspond to the reference numbers of the studies. The study by Chen et al. [10] did not divide narcolepsy into type 1 and type 2 when comparing structural connectivity to controls. Post hoc comparisons between narcolepsy type 1 and type 2 did not reveal differences between narcolepsy subtypes, and the study by Chen et al. [10] was thus reported for both the “NT1 < HC” and the “NT2 < HC” contrasts. HC, healthy controls; NT1, narcolepsy type 1; NT2, narcolepsy type 2; WM, white matter.
Chen and colleagues are the first to multimodally combine white matter assessment in narcolepsy with healthy individuals’ genetic expression and glutamatergic PET/SPECT profiles [10]. Limited drug-naïve samples of both narcolepsy subtypes were included in the DWI analyses, which showed generalized reduced structural connectivity, which also correlated with shorter objective sleep latency. Comparisons between narcolepsy type 1 and type 2 did not show significant differences in connectivity between subtypes, which contrasts with the few previous structural magnetic resonance imaging (MRI) studies comparing these populations (Figure 1) [5, 9, 11, 12]. This could be due to the small sample sizes included by Chen and colleagues, or indicate that the observed alterations are consequential to excessive daytime sleepiness instead of being specific for hypocretin deficiency. In the absence of hypocretin-1 measurements, it is also possible that hypocretin-1 deficiency without cataplexy could have led to misdiagnosis of narcolepsy type 2 in some individuals. Cautious interpretation of the genetic transcription and glutamatergic positron emission tomography-single photon emission computed tomography (PET-SPECT) findings is needed as these analyses relied exclusively on an external healthy population, lacking individuals with narcolepsy. The suggested pathophysiological relationship between glutamatergic signaling and narcolepsy mainly underscores the dire need for magnetic resonance spectroscopy studies, as neurometabolite concentrations in this context have been minimally explored [13, 14]. Future studies should combine larger groups of both narcolepsy types with idiopathic hypersomnia, and include control groups of sleep-deprived individuals to assess the specificity of the structural connectivity findings.
Symptoms of REM sleep behavior disorder (RBD) are common in narcolepsy. Chen and colleagues report high RBD prevalences of 71% in narcolepsy type 1 (30%–60% in previous studies) and 54% in narcolepsy type 2 (15%–40% in previous studies) [15]. In narcolepsy with RBD, a structurally enhanced subnetwork of the thalamus, striatum, and cingulate gyrus was identified [10]. These regions are usually activated during REM sleep but are not known for mediating REM sleep muscle atonia. In idiopathic RBD, REM sleep without atonia arises from the dysfunctioning of REM-regulating pontomedullary nuclei, primarily through glutamatergic neurons in the subcoeruleus [16]. Changes in neuron counts outside the hypothalamus have so far not been identified in narcolepsy type 1 [17, 18], and loss of hypocretinergic innervation of brainstem REM circuits and the basal ganglia seems a more reasonable hypothesis for RBD symptoms in narcolepsy type 1. Different underlying pathophysiology could account for the nuanced distinctions in RBD phenotypes, such as the typically younger onset in narcolepsy compared to idiopathic RBD [15]. This could also clarify why, until now, narcolepsy has not shown an association with elevated incidence rates of alpha-synucleinopathies.
The exact relationship between the structural and functional differences observed in narcolepsy type 1 remains a chicken or egg debate. Multiple resting-state and task-based functional MRI (fMRI) studies have identified extensive functional connectivity differences in narcolepsy type 1, including the default mode (DMN), limbic and attention networks [19–22]. Most consistent findings comprise altered limbic activity during emotional processing [23–25], and the compromised capacity of the DMN, the primary resting network, to disengage from active networks when cognitively challenged. This inability of the DMN to decouple from other networks suggests a dysregulation in narcolepsy type 1 in favor of staying awake over actual task performance [26]. Understanding whether structural distinctions directly contribute to altered brain activity or if they are an outcome of prolonged symptomatology remains ambiguous, as most studies focused on individuals with long disease durations. A plausible perspective is that the observed structural effects are intricately tied to the chronic symptoms associated with hypocretin deficiency. Further investigations should focus on individuals in early disease stages to gain a more comprehensive understanding.
Can we conclude that people with narcolepsy have less efficient structural brain wiring? DWI uses indirect measures of water diffusion to assess white matter integrity and presumes that diffusion is relatively unrestricted along axon bundles compared to directions perpendicular to the axonal bundles. From conventional DWI findings, it remains unclear whether myelin and/or axonal integrity are affected [27, 28], or whether other processes involving water diffusion play an important mediating role. Recent studies revealed that the brain undergoes clearance of waste metabolites through cerebrospinal fluid (CSF) flow during sleep [29]. The effects of disturbed sleep on brain clearance (and possibly water diffusion) remain unknown and require further investigations, especially in narcolepsy, where there are ample diurnal and nocturnal sleep–wake alterations [1]. Future research is essential before arriving at conclusive findings. Specifically, the incorporation of postmortem immunohistochemistry analyses or multishell DWI studies could offer resolutions for accurately interpreting the widespread DWI alterations in narcolepsy.
Compared to many other neurological and psychiatric disorders, neuroimaging in narcolepsy and idiopathic hypersomnia remains in its early stages with limited multimodal perspectives, few cross-disorder comparisons, and typically small sample sizes leading to inconsistent findings. Both technically advanced studies generating new hypotheses and large-scale international collaborations verifying these results are necessary to overcome these limitations. The multimodal methodology of Chen and colleagues guides future more extensive studies in which clearly distinct narcolepsy subtypes and controls will be included for all acquisition methods [10]. To confront the challenge of inconsistent outcomes, our recently established neuroimaging consortium on central disorders of hypersomnolence (NICHY) presents a possible solution [30]. This initiative involves the global integration of existing and future MRI datasets related to narcolepsy and idiopathic hypersomnia to conduct cross-disorder comparisons among hypersomnolence diagnoses with unprecedented statistical power. Where first analyses focus on T1-weighted structural measures, future projects will also target DWI-derived structural and fMRI connectivity measures.
In conclusion, the question of whether disturbed white matter integrity in narcolepsy serves as a blueprint for functional complaints sparks an interesting debate. The findings of Chen and colleagues suggest a potential link between structural alterations and narcolepsy symptomatology that requires further investigation. Despite important methodological limitations to consider when interpreting findings by Chen and colleagues, the study illustrates the bright future of neuroimaging research in narcolepsy, where first efforts are being made to link brain structure with multimodal functioning [10]. Important questions remain: Are changes in DWI metrics in narcolepsy attributed to myelin, axonal density, or other poorly understood diffusion processes? To what degree are the extensive variations specific to hypocretin deficiency, or do these findings result from chronic exposure to excessive daytime sleepiness? What is the relationship between brain structure and functioning in narcolepsy? More multimodal efforts and upcoming multi-center collaborations like NICHY will be essential to build a comprehensive understanding of the neural signatures of central disorders of hypersomnolence.
Contributor Information
Jari K Gool, Anatomy and Neurosciences, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Stichting Epilepsie Instellingen Nederland (SEIN), Sleep-Wake Centre, Heemstede, Netherlands; Department of Neurology, Leiden University Medical Centre, Leiden, The Netherlands; Compulsivity, Impulsivity and Attention, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Thien Thanh Dang-Vu, Centre de recherche de l’Institut universitaire de gériatrie de Montréal, Centre intégré universitaire de santé et de services sociaux du Centre-Sud-de-l’Ile-de-Montréal, Montreal, QC, Canada; Center for Studies in Behavioral Neurobiology, Department of Health, Kinesiology and Applied Physiology, Concordia University, Montreal, QC, Canada.
Ysbrand D van der Werf, Anatomy and Neurosciences, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Compulsivity, Impulsivity and Attention, Amsterdam Neuroscience, Amsterdam, The Netherlands.
Disclosure Statement
Financial disclosure: none. Nonfinancial disclosure: none.
References
- 1. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9 [DOI] [PubMed] [Google Scholar]
- 2. Gool JK, Fronczek R, Leemans A, Kies DA, Lammers GJ, Van der Werf YD.. Widespread white matter connectivity abnormalities in narcolepsy type 1: a diffusion tensor imaging study. Neuroimage Clin. 2019;24:101963. doi: 10.1016/j.nicl.2019.101963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juvodden HT, Alnaes D, Lund MJ, et al. Widespread white matter changes in post-H1N1 patients with narcolepsy type 1 and first-degree relatives. Sleep. 2018;41(10). doi: 10.1093/sleep/zsy145 [DOI] [PubMed] [Google Scholar]
- 4. Menzler K, Belke M, Unger MM, et al. DTI reveals hypothalamic and brainstem white matter lesions in patients with idiopathic narcolepsy. Sleep Med. 2012;13(6):736–742. doi: 10.1016/j.sleep.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 5. Nakamura M, Nishida S, Hayashida K, Ueki Y, Dauvilliers Y, Inoue Y.. Differences in brain morphological findings between narcolepsy with and without cataplexy. PLoS One. 2013;8(11):e81059. doi: 10.1371/journal.pone.0081059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park HR, Kim HR, Seong JK, Joo EY.. Localising deficits in white matter tracts of patients with narcolepsy with cataplexy: tract-specific statistical analysis. Brain Imaging Behav. 2020;14(5):1674–1681. doi: 10.1007/s11682-019-00100-z [DOI] [PubMed] [Google Scholar]
- 7. Park YK, Kwon OH, Joo EY, et al. White matter alterations in narcolepsy patients with cataplexy: tract-based spatial statistics. J Sleep Res. 2016;25(2):181–189. doi: 10.1111/jsr.12366 [DOI] [PubMed] [Google Scholar]
- 8. Scherfler C, Frauscher B, Schocke M, et al. White and gray matter abnormalities in narcolepsy with cataplexy. Sleep. 2012;35(3):345–351. doi: 10.5665/sleep.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tezer FI, Erdal A, Gumusyayla S, Has AC, Gocmen R, Oguz KK.. Differences in diffusion tensor imaging changes between narcolepsy with and without cataplexy. Sleep Med. 2018;52:128–133. doi: 10.1016/j.sleep.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 10. Chen G, Wang W, Wu H, et al. Disrupted topological properties of structural brain networks present a glutamatergic neuropathophysiology in people with narcolepsy. Sleep. 2024;47(6):zsae002. doi: 10.1093/sleep/zsae002. [DOI] [PubMed] [Google Scholar]
- 11. Gool JK, Cross N, Fronczek R, Lammers GJ, Van der Werf YD, Dang-Vu TT.. Neuroimaging in narcolepsy and idiopathic hypersomnia: from neural correlates to clinical practice. Curr Sleep Med Rep. 2020;6:251–266. doi: 10.1007/s40675-020-00185-9 [DOI] [Google Scholar]
- 12. Nemcova V, Krasensky J, Kemlink D, et al. Hippocampal but not amygdalar volume loss in narcolepsy with cataplexy. Neuro Endocrinol Lett. 2015;36(7):682–688. [PubMed] [Google Scholar]
- 13. Kim SJ, Lyoo IK, Lee YS, et al. Increased GABA levels in medial prefrontal cortex of young adults with narcolepsy. Sleep. 2008;31(3):342–347. doi: 10.1093/sleep/31.3.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Witt ST, Drissi NM, Tapper S, et al. Evidence for cognitive resource imbalance in adolescents with narcolepsy. Brain Imaging Behav. 2018;12(2):411–424. doi: 10.1007/s11682-017-9706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antelmi E, Pizza F, Franceschini C, Ferri R, Plazzi G.. REM sleep behavior disorder in narcolepsy: a secondary form or an intrinsic feature? Sleep Med Rev. 2020;50:101254. doi: 10.1016/j.smrv.2019.101254 [DOI] [PubMed] [Google Scholar]
- 16. Fraigne JJ, Torontali ZA, Snow MB, Peever JH.. REM sleep at its core - circuits, neurotransmitters, and pathophysiology. Front Neurol. 2015;6:123. doi: 10.3389/fneur.2015.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shan L, Balesar R, Swaab DF, Lammers GJ, Fronczek R.. Reduced numbers of corticotropin-releasing hormone neurons in narcolepsy type 1. Ann Neurol. 2022;91(2):282–288. doi: 10.1002/ana.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shan L, Linssen S, Harteman Z, et al. Activated wake systems in narcolepsy Type 1. Ann Neurol. 2023;94(4):762–771. doi: 10.1002/ana.26736 [DOI] [PubMed] [Google Scholar]
- 19. Huang YS, Liu FY, Lin CY, Hsiao IT, Guilleminault C.. Brain imaging and cognition in young narcoleptic patients. Sleep Med. 2016;24:137–144. doi: 10.1016/j.sleep.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 20. Drissi NM, Szakacs A, Witt ST, et al. Altered brain microstate dynamics in adolescents with narcolepsy. Front Hum Neurosci. 2016;10:369. doi: 10.3389/fnhum.2016.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvela M, Raatikainen V, Kotila A, et al. Lag analysis of fast fMRI reveals delayed information flow between the default mode and other networks in narcolepsy. Cereb Cortex Commun. 2020;1(1):tgaa073. doi: 10.1093/texcom/tgaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gool JK, van der Werf YD, Lammers GJ, Fronczek R.. The sustained attention to response task shows lower cingulo-opercular and frontoparietal activity in people with narcolepsy Type 1: An fMRI study on the neural regulation of attention. Brain Sci. 2020;10(7):419. doi: 10.3390/brainsci10070419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meletti S, Vaudano AE, Pizza F, et al. The brain correlates of laugh and cataplexy in childhood narcolepsy. J Neurosci. 2015;35(33):11583–11594. doi: 10.1523/JNEUROSCI.0840-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reiss AL, Hoeft F, Tenforde AS, Chen W, Mobbs D, Mignot EJ.. Anomalous hypothalamic responses to humor in cataplexy. PLoS One. 2008;3(5):e2225. doi: 10.1371/journal.pone.0002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwartz S, Ponz A, Poryazova R, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131(Pt 2):514–522. doi: 10.1093/brain/awm292 [DOI] [PubMed] [Google Scholar]
- 26. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 27. Basser PJ, Mattiello J, LeBihan D.. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A.. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20(2):209–225. doi: 10.1007/s11065-010-9129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reddy OC, van der Werf YD.. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020;10(11):868. doi: 10.3390/brainsci10110868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gool JK, Van Heese EM, Lammers GJ, Fronczek R, Joo EY, Schwartz S, et al. The NeuroImaging Consortium on Central Disorders of Hypersomnolence (NICHY): A Global Meta- and Mega-analysis Framework to Unravel the Neural Correlates of Central Disorders of Hypersomnolence. Bologna, Italy: Paper presented at: European Narcolepsy Days; 2022. [Google Scholar]