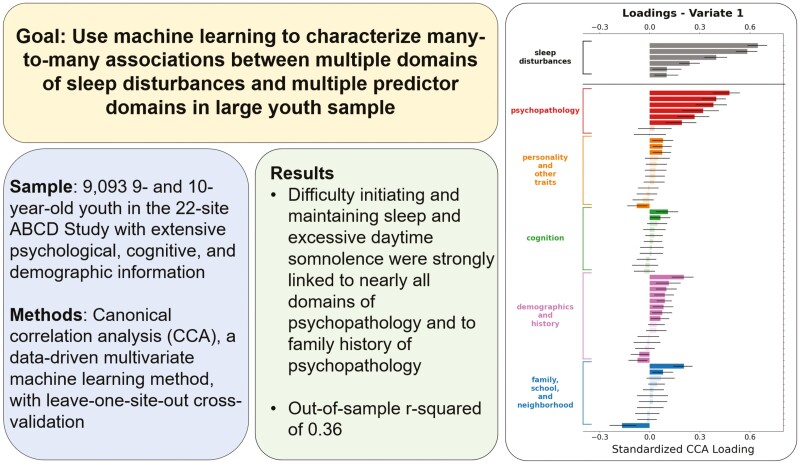
Abstract
Study Objectives
Sleep disturbances are common in adolescence and associated with a host of negative outcomes. Here, we assess associations between multifaceted sleep disturbances and a broad set of psychological, cognitive, and demographic variables using a data-driven approach, canonical correlation analysis (CCA).
Methods
Baseline data from 9093 participants from the Adolescent Brain Cognitive Development (ABCD) Study were examined using CCA, a multivariate statistical approach that identifies many-to-many associations between two sets of variables by finding combinations for each set of variables that maximize their correlation. We combined CCA with leave-one-site-out cross-validation across ABCD sites to examine the robustness of results and generalizability to new participants. The statistical significance of canonical correlations was determined by non-parametric permutation tests that accounted for twin, family, and site structure. To assess the stability of the associations identified at baseline, CCA was repeated using 2-year follow-up data from 4247 ABCD Study participants.
Results
Two significant sets of associations were identified: (1) difficulty initiating and maintaining sleep and excessive daytime somnolence were strongly linked to nearly all domains of psychopathology (r2 = 0.36, p < .0001); (2) sleep breathing disorders were linked to BMI and African American/black race (r2 = 0.08, p < .0001). These associations generalized to unseen participants at all 22 ABCD sites and were replicated using 2-year follow-up data.
Conclusions
These findings underscore interwoven links between sleep disturbances in early adolescence and psychological, social, and demographic factors.
Keywords: sleep, canonical correlation analysis, Adolescent Brain Cognitive Development Study, psychopathology, sleep-disordered breathing, adolescence, body mass index
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Adolescent sleep disturbances are associated with a host of negative outcomes. Previous research has identified individual predictors of sleep disturbances. Here, we break new ground by using machine learning methods to identify many-to-many mappings between multiple predictors across different domains and multifaceted sleep disturbances. Current levels of psychopathology among youth as well as a family history of psychopathology were strongly related to sleep disturbances, especially difficulties initiating and maintaining sleep and excessive daytime somnolence. Perceptions of family conflict and neighborhood safety were also associated with sleep disturbances, pointing to the influence of contextual factors. In addition, body mass index and African American/black race were linked to sleep-related breathing difficulties, highlighting the need for continued research to address sleep health disparities in emerging adolescents.
Introduction
Sleep plays a crucial role in healthy development during adolescence [1, 2]. Sleep disturbances—which may include inadequate duration, poor quality, problematic nighttime behaviors, and daytime sleepiness—are common in children and adolescents [3, 4], with an estimated 25% of youth experiencing significant sleep problems during childhood [5]. Adolescent sleep problems are associated with a wide range of psychiatric disorders [6–10] as well as other individual youth attributes [11, 12]. Characteristics of youth’s family [13–16], school [17, 18], and neighborhood [19, 20] environments have also been linked to sleep disturbances. Despite considerable evidence of individual associations of such constructs with sleep problems in adolescents, little is known about the complex, combined associations across these domains and their links to multifaceted sleep disturbances. This study aims to characterize the aggregated predictive associations among a broad set of psychological, cognitive, and demographic variables and multidomain sleep disturbances in emerging adolescents using data-driven multivariate machine learning methods.
Sleep disturbances [6] in adolescence have been linked with a host of individual factors [9, 16, 19, 21]. For example, sleep disturbances are associated with a wide range of psychiatric problems including attention deficit hyperactivity disorder [22], and mood and anxiety disorders [9, 23, 24]. Previous work suggests bidirectional links between adolescent sleep disturbances and both internalizing [25] and externalizing symptoms [26, 27]. Additionally, socioeconomic factors such as higher neighborhood disadvantage [19, 20] and lower parental education [13] have been linked to poorer sleep quality and shorter sleep duration, while family conflict [14, 15] and hostile school environments [18] have been found to contribute to disrupted sleeping habits. Moreover, many individual and health-related characteristics such as body mass index (BMI) and screen and media use have been shown to impact sleep hygiene and bedtime routines [12, 28]. Given the pervasive links between sleep disparities and adolescent health, these individual associations provide important insight into the broad range of risk factors associated with sleep.
From a socio-ecological perspective [29], there are likely rich interwoven links among multiple predictor domains [30] simultaneously with multiple aspects of sleep [31, 32], i.e. many-to-many associations; however, much of the current literature focuses on the examination of one-to-one associations between predictors and sleep problems [6, 12, 19, 26]. Furthermore, sleep disturbances are predominantly conceptualized using unidimensional approaches such as sum scores [5, 33], which may obscure important associations between subsets of predictors and specific domains of sleep problems. Multivariate machine learning methods can potentially address this issue by identifying shared and unique linkages between sets of variables from different domains [34]. However, these highly parameterized data-driven methods require large samples [34], and to date, few studies have implemented these methods to uncover shared and unique associations between distinct types of sleep disturbance and diverse multidomain predictors in youth.
This study seeks to identify the combined predictive associations among a diverse set of psychological, cognitive, and demographic variables in the Adolescent Brain Cognitive Development (ABCD) Study [35, 36], a population-based study of 11 875 9- and 10-year-olds. The ABCD Study is the largest longitudinal study of neurodevelopment in youth, with participants recruited from 22 research sites across the United States. To assess a broad array of sleep problems, we utilized the validated Sleep Disturbance Scale for Children (SDSC) [32], which indexes six dimensions of sleep disturbances. In addition, ABCD includes an extensive battery of assessments that capture wide-ranging potential predictors of sleep disturbances, spanning multiple participant-level domains (i.e. psychopathology, personality, cognition, and anthropomorphics) as well as additional levels of influence (i.e. family, school, and neighborhood).
Methods
Sample and data
Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA; https://nda.nih.gov). This is a multisite, longitudinal study designed to recruit more than 10 000 children age 9–10 and follow them over 10 years into early adulthood [35, 37, 38]. The ABCD data repository grows and changes over time. The ABCD data used in this report came from ABCD Release 4.0, NDA Study 1299, doi: 10.15154/1523041, which can be found at https://nda.nih.gov/study.html?id=1299. Study procedures were approved by a central institutional review board. Youth participants and their caregivers provided assent and written informed consent, respectively. Study methods and results are reported following the Strengthening the Reporting of Observational Studies in Epidemiology Statement for cohort studies [39].
In our primary analysis, youth participants with complete baseline data for all variables of interest were included (N = 9093) while participants with missing data on one or more constructs of interest were excluded (N = 2783) from the analyses. The sample was roughly balanced by sex (47.5% female) and had a mean age of 9.92 years (SD = 0.63 years). Race and ethnicity of youth were determined based on parent report. Participants identified as Asian (2.1%), black (13.3%), Hispanic (19.1%), Other/multiracial (10.3%), and white (55.2%). More than two-thirds (69.8%) of youth participants lived in households in which the parent identified as married. Most parents (88.1%) reported at least some college, and most households (71.4%) reported an annual income of at least $50 000.
In our follow-up analysis, participants with complete data for the variables of interest at both baseline and 2-year follow-up visits were included (N = 4247). This subset of our baseline sample was 47.4% female, and the mean age of participants at the follow-up visit was 11.96 years (SD = 0.65 years). Participants identified as Asian (2.1%), black (9.2%), Hispanic (16.2%), Other/multiracial (9.5%), and white (62.9%). More than two-thirds (73.8%) of youth participants lived in households in which the parent identified as married. Most parents (74.3%) reported at least some college, and most households (80.3%) reported an annual income of at least $50 000.
In additional sensitivity analyses (detailed in the Supplementary Materials), we utilized multiple imputation for missing data [40–42]. We included all participants with available data on sleep disturbances and at least one other construct of interest (baseline N = 11 871, 2-year follow-up N = 10 340).
Measures
The rationale and selection of measures administered in the ABCD Study have previously been described in detail [37]. In brief, the selection of measures was guided by consideration of developmental appropriateness for the age range to be assessed during the longitudinal study, the feasibility and reliability of administration as part of a large multisite study, and evidence of scale validity and reliability [37]. For our analyses, a subset of ABCD Study measures was included that indexed multiple domains of sleep disturbance and key factors potentially associated with sleep disturbances across multiple levels including youth, family, school, and neighborhood characteristics.
Sleep disturbances
The SDSC [32] is a 26-item, parent-reported assessment of childhood sleep disturbance. The measure consists of six subscales corresponding to symptoms of six domains of sleep disorders, including disorders of initiating and maintaining sleep, sleep breathing disorders, disorders of arousal, sleep–wake transition disorders, disorders of excessive somnolence, and sleep hyperhydrosis. This well-established evidence-based assessment of multidimensional sleep problems [43] has been utilized in community and clinical settings, translated for use in multiple languages [44–46], and demonstrates adequate to good reliability [32, 47] and validity [32, 43, 45]. Additionally, the sleep breathing disorder subscale has shown significant discriminant validity with polysomnographic-confirmed sleep-related breathing disorders [46].
Psychopathology
The parent report form of the Achenbach Child Behavior Checklist [48] (CBCL) assesses internalizing and externalizing behaviors in children using eight subscales: Withdrawn, Somatic Complaints, Anxious/Depressed, Social Problems, Thought Problems, Attention Problems, Delinquent Behavior, and Aggressive Behavior. The CBCL has well-established psychometric properties, including test–retest reliability and internal consistency, and is one of the most utilized measures of emotional and behavioral problems in children.
To ensure our findings were not unduly influenced by sleep-related symptoms of psychopathology, we removed all items related to sleep [49] before calculating CBCL subscale scores.
Personality and other traits
The Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scale [50, 51] is a youth-reported 20-item measure designed to assess motivational systems. Specifically, the Behavioral Inhibition subscale indexes sensitivity to punishment while behavioral activation is captured by three subscales related to positive affect: Drive (intensity of goal-directed behavior), Fun seeking (spontaneity), and Reward Responsiveness (excitement over reinforcing outcomes). Preliminary analysis of a subset of ABCD participant data indicated adequate to good reliability of BIS/BAS subscales [37].
The Modified Urgency, Perseverance, Premeditation, and Sensation seeking (UPPS-P) Impulsive Behavior Scale for Children from PhenX Life Events is a 20-item youth-reported short version of the UPPS-P developed for use in the ABCD study [37] to index multidimensional trait impulsivity in youth [52]. The measure has five subscales: Negative Urgency, Positive Urgency, Lack of Perseverance, Lack of Planning, and Sensation Seeking. This abbreviated version of the UPPS-P which was modified to be consistent with the adult short form exhibits a factor structure consistent with the adult version and has good external validity [52].
The Prodromal Questionnaire—Brief Version (PQ-B) [53] is a 21-item youth-report measure designed to index subclinical prodromal psychosis risk phenotypes. The PQ-B has demonstrated good reliability [54] and convergent validity with clinician-administered assessments of psychosis risk [55].
The Prosocial Behavior subscale used in this study is an abbreviated version of the Prosocial Behavior subscale of the “Strengths and Difficulties Questionnaire” (SDQ) [56]. Specifically, the three items with the highest factor loadings were included. Our analyses separately include data from parent reporting on youth and youth reporting on self. Psychometric properties of the SDQ have been validated in large, representative samples of children and adolescents [56, 57].
Cognition
The NIH Toolbox Cognition Battery [58–60] contains seven tasks encompassing episodic memory, executive function, attention, working memory, processing speed, and language abilities. The subtests consist of Dimension Change Card Sort, Flanker Inhibitory Control and Attention, List Sorting Working Memory, Pattern Comparison Processing Speed, Picture Sequence Memory, Oral Reading Recognition, and Picture Vocabulary. It is favored for being conducive to longitudinal research, comprehensive, and brief [61]. The NIH Toolbox Cognition battery has been validated in pediatric samples and shows strong psychometric properties including excellent test–retest reliability and good construct validity [62].
The Pearson Rey Auditory Verbal Learning Test (RAVLT) [63–65] assesses auditory learning and memory. The RAVLT has been found to have adequate internal reliability as well as test–retest reliability [63]. Our analyses include subscale scores assessing short-term and long-term recall [63].
Wechsler Intelligence Scale for Children, 5th Edition (WISC-V) [66] Matrix Reasoning subtest is considered a measure of fluid reasoning. Specifically, this subtest requires youth to view an incomplete array of visual stimuli and select the best option among four pictures to complete the matrix [63, 66]. The Matrix Reasoning subtest is well validated and demonstrates good reliability [63].
The Little Man Task is intended to explore visuospatial processing, perspective-taking, and mental rotation [67]. Essentially, the test displays a man with a briefcase in different spatial orientations, and participants are asked to determine which hand is holding the briefcase.
Demographics and history
Family history of psychopathology
Based on previously published protocols, a family history composite score was constructed from parent responses on the Family History Assessment [37, 68, 69] which assesses suicidality and 15 psychiatric disorders in a youth’s parents and first-degree relatives.
Demographics
Parents reported on the demographics of their child as well as their child’s parents and grandparents in a comprehensive demographic survey [37] that employed items primarily from the PhenX toolkit [70]. Items of interest for this analysis include youth’s race, sex, age, parental education, and household income.
Anthropometrics
Participants’ weight and height were measured up to three times consecutively during the baseline visit. The average of the measurements was used to calculate BMI according to convention (weight [kg]/height [m2]).
Family, school, and neighborhood
The Family Conflict subscale of the Family Environment Scale [71, 72] is composed of nine items assessing the degree of outwardly expressed conflict among family members. The subscales generally show good face and content validity and adequate internal consistency validity [71, 73]. Parents and youth separately reported on this subscale [74].
Youth reported on School Risk and Protective Factors (SRPF) to assess their perceptions of their school environment [74]. This PhenX Toolkit measure, derived from the “Communities That Care Youth Survey” [75] yields three subscales: School Environment, School Involvement, and School Disengagement. Examination of the psychometric properties of this scale in an initial ABCD sample suggested all three scales showed discriminant validity when comparing higher-risk and lower-risk youth, with the School Environment and School Involvement subscales also demonstrating adequate internal reliability [74].
Area Deprivation Index (ADI) is a measurement from the American Community Survey to assess neighborhood deprivation (socioeconomic disadvantage based on income, education, employment, and housing quality) [76, 77]. Composite scores were available for each participant for up to three residences. A weighted average of ADI scores was computed based on months lived at each residence. Prior work has shown that the ADI is predictive of health outcomes [76, 78].
The Neighborhood Safety/Crime survey is a three-item parent-reported PhenX Toolkit [70] measure derived from “Safety from Crime” items [79, 80] assessing neighborhood characteristics. Our analysis included the three items separately to assess parent’s perspective of neighborhood safety related to walking, crime, and violence. In the ABCD sample, this scale has demonstrated good internal consistency and shown significant differences between higher-risk and lower-risk participants [81].
A brief Screen Time questionnaire was developed for ABCD [37] based on prior work by Sharif, Wills, and Sargent [82]. The two-question parent-report scale queries the overall amount of time the youth spends using visual media on a typical weekday and a typical weekend day.
Two-year follow-up measures
Two-year follow-up CCA analysis included all of the above-described measures except for a few which were not re-administered at that visit (i.e. Flanker Inhibitory Control and Attention tasks and the Wisconsin Card Sorting Test) or were not expected to change significantly from baseline (i.e. family history of psychopathology and ADI).
Additionally, to partially evaluate the extent to which common method variance may be biasing associations, we included an additional parent-rated questionnaire, first administered at the 2-year follow-up visit, that we did not hypothesize to be associated with multifaceted sleep disturbances. The PhenX Neighborhood Collective Efficacy—Community Cohesion and Informal Social Control measure [81, 83] comprised 10 items regarding feelings of trust and unity among neighbors. In the ABCD sample, this measure has shown good internal consistency [81].
Data analytic strategy
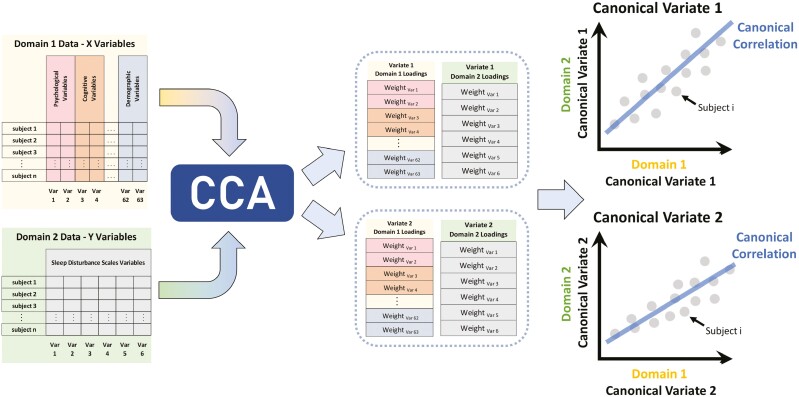
We used correlation analysis (CCA) [34] to identify associations between the six dimensions of sleep disturbances and a broad set of psychological, cognitive, and demographic variables (Figure 1). CCA is a multivariate statistical method that extracts many-to-many associations between two sets of variables. It identifies latent dimensions of largest common variation across two sets of measures by finding projections for each set of variables such that the correlations of these projections are maximized. The projections CCA discovers are called “canonical variates” (CVs), and the association between a pair of CVs from the variable sets is its “canonical correlation.” Each CV may also be expressed as a weighted linear combination of variables from the variable sets. These weights are called “variable loadings.” CCA has been previously utilized in high-dimensional multivariate fields such as genetics [84], fMRI imaging [85], and cognitive neuroscience [86].
Figure 1.
Depiction of canonical correlation analytic (CCA) approach. In this approach, multidomain data from the same set of participants were used to identify many-to-many associations between a broad set of psychological, cognitive, demographic, and other variables (Domain 1) and multifaceted sleep disturbances (Domain 2). CCA identifies weighted linear combinations of each set of variables (canonical variates, CVs) that maximize the correlation between the sets. The association between a pair of CVs is its canonical correlation.
To assess the generalizability of CCA models, we used leave-one-site-out cross-validation. For each fold of cross-validation, data from one of the ABCD sites were held out, and data from all other sites served as the training dataset. CCA models were trained on the training dataset for each fold and applied to the held-out test dataset. The canonical correlations of the leading two CVs in the test dataset were recorded as measurements of model performance per fold [87]. The statistical significance of canonical correlations was established by non-parametric permutation tests that accounted for twin, family, and site structure. Details on the preceding generalizability analyses are provided in Supplementary Material.
We assessed the statistical significance of the variable loadings for each CV using bootstrap analysis. In step 1, we resampled participants with replacement across the entire dataset. In step 2, a CCA model was fit to the data generated from the first step and the loadings for each variable for the leading two CVs were recorded. Since the signs of canonical variables and variable loadings are indeterminate [87], in step 3, we aligned the bootstrap loadings from step 2 to the observed loadings for each CV. That is, we multiplied each CV’s loadings by −1 if the sign of the sum of bootstrap loadings is different from the sign of the sum of observed loadings for the corresponding observed CV. These four steps were repeated 10 000 times yielding a distribution of loadings for each variable, for the first two CVs. An observed variable loading was calculated to be statistically significant if the 95% CI of its bootstrap distribution did not contain 0.
To assess the stability of the associations identified between multidomain sleep disturbances and a subset of our variables of interest, we repeated the CCA analysis in the subset of participants who had complete data for the included variables from their 2-year follow-up visit.
To evaluate the robustness of our findings to imputation of missing data, we conducted sensitivity analyses repeating the baseline and 2-year follow-up CCA analyses utilizing multiple imputation for missing data (Supplementary Methods).
Code availability:
Code for running analyses can be found at https://github.com/SripadaLab/ABCD_sleep_disturbances_CCA.
Results
Canonical correlation analysis of baseline sleep disturbances
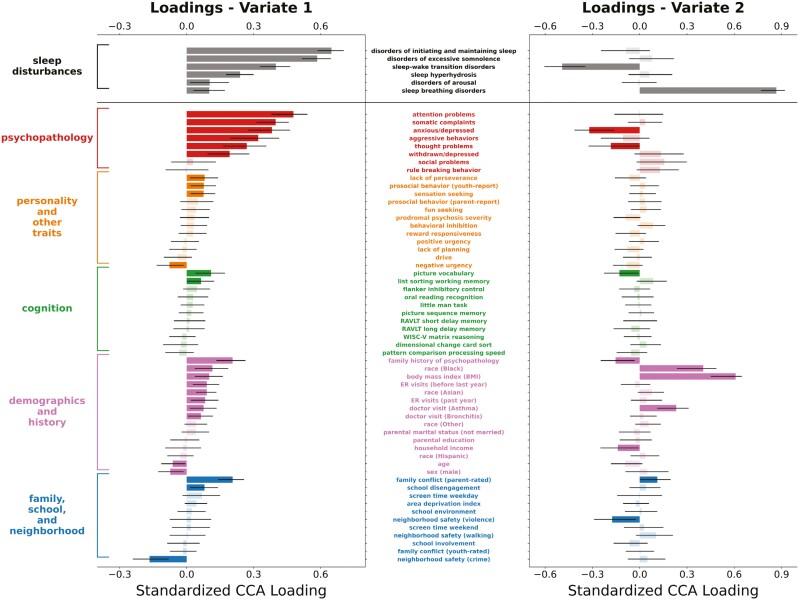
To examine the many-to-many associations between multidomain sleep disturbances and a broad set of psychological, cognitive, and demographic variables, we employed a multivariate statistical method, canonical correlation analysis (CCA), that identifies patterns of covariation between two sets of variables. Using a leave-one-site-out cross-validation framework and baseline data from 9093 participants, our analysis identified two statistically significant CVs (Figure 2). Averaging across the 22 cross-validation folds, the out-of-sample canonical correlations for the first two CVs were 0.58 and 0.25 (with permutation p-values < .001), respectively. Standardized loadings for CV1 and CV2 for all variables included in this analysis are detailed in Supplementary Table 1.
Figure 2.
Plots of standardized loadings of CCA analysis relating sleep disturbances and multidomain predictors in the ABCD study baseline data. We used a multivariate machine learning method, CCA, to delineate associations between sleep disturbances on the one hand and a number of predictor domains on the other (i.e. psychopathology; personality and other traits; cognition; demographics and history; and family, school, and neighborhood). The y-axis lists the predictor variables (middle) and their respective domains (left), and the x-axis shows standardized beta weight values for these predictors. Error bars represent the 95% CI based on bootstrap analysis. Statistical significance is indicated by darkly shaded bars. For the first CV (left column), multidomain psychopathology was the strongest predictor of difficulty initiating and maintaining sleep and excessive daytime somnolence. For the second CV (right column), BMI and African American/black race were strongly predictive of sleep breathing disorders. Abbreviations: CCA, canonical correlation analysis; RAVLT, Rey Auditory Verbal Learning Test; WISC-V, Wechsler Intelligence Scale for Children-5th Edition; ER, emergency room.
First canonical variate loadings.
In CV1 (Figure 2, left column), which explained the largest amount of covariance, significant weights were seen for all six domains of sleep disturbances, with the highest loadings on problems initiating and maintaining sleep, disorders of excessive hypersomnolence, and sleep–wake transition disorders.
For the predictor variables of interest, the highest loadings on CV1 were seen for psychopathology-related measures (i.e. six of eight of the CBCL subscales), indicating that increasing severity of multidimensional psychopathology was associated with increasing severity of sleep disturbances, particularly for problems initiating and maintaining sleep and disorders of excessive hypersomnolence. Relatedly, family history of psychopathology also had a significant positive loading.
Other significant loadings included a positive weight for parent-rated family conflict and a negative weight for neighborhood safety related to crime, suggesting that increased levels of family conflict were linked to increased sleep disturbances while increasing neighborhood safety related to crime was associated with decreasing sleep disturbances.
Second canonical variate loadings.
In CV2 (Figure 2, right column), a significant positive loading was seen for sleep breathing disorders, suggesting unique associations between a subset of our variables of interest and this sleep disturbance specifically.
For the predictor variables of interest, the highest loadings were seen for BMI and African American/black race, suggesting that higher BMI and black race (compared to white race) were related to increased severity of sleep breathing disorders. A significant positive loading was also seen for parent report of youth having visited a doctor for asthma, indicating youth with a history of asthma-related doctor’s visits had increased severity of symptoms of sleep breathing disorders compared to youth with no history of visiting a doctor for asthma.
These findings were robust to sensitivity analyses using multiple imputation for missing data (detailed in Supplementary Results and Supplementary Table 3).
CCA of year 2 sleep disturbances
To examine the stability and generalizability of the associations identified in our baseline analysis, we conducted a similar analysis using data from the 2-year follow-up visit.
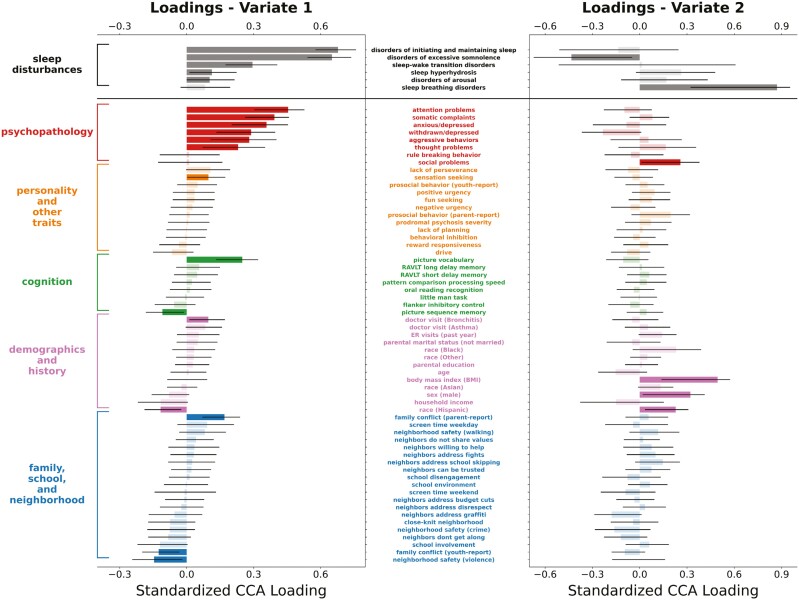
Using a leave-one-site-out cross-validation framework and 2-year follow-up data from 4247 participants, our analysis identified 2 statistically significant CVs (Figure 3). Averaging across the 21 cross-validation folds, the out-of-sample canonical correlations for the first 2 CVs were 0.54 and 0.20 (with permutation p-values < .001), respectively. Standardized loadings for CV1 and CV2 for all variables included in this analysis are detailed in Supplementary Table 2.
Figure 3.
Plots of standardized loadings of CCA analysis relating sleep disturbances and multidomain predictors in the ABCD study two-year follow-up data. We performed a similar CCA analysis on ABCD data from the two-year follow-up visit to assess the consistency of findings observed in baseline data. The y-axis lists the predictor variables (middle) and their respective categories (left), and the x-axis shows standardized beta weight values for these predictors. Error bars represent the 95% CI based on bootstrap analysis. Statistical significance is indicated by darkly shaded bars. On the first CV (left column), we once again saw multidomain psychopathology as the strongest predictor of difficulty initiating and maintaining sleep and excessive daytime somnolence. For the second CV (right column), we once again observed that BMI was strongly predictive of sleep breathing disorders. Abbreviations: CCA, canonical correlation analysis; RAVLT, Rey Auditory Verbal Learning Test; ER, emergency room.
In CV1 (Figure 3, left column), which explained the largest amount of covariance, significant weights were seen for five of the six domains of sleep disturbances, with the highest loadings on problems initiating and maintaining sleep and disorders of excessive hypersomnolence, similar to our findings using baseline data.
For the predictor variables of interest, the highest loadings on CV1 were again seen for psychopathology-related measures (i.e. the same six out of eight CBCL subscales identified in the baseline analysis), indicating that increasing severity of multidimensional psychopathology was associated with increasing severity of sleep disturbances, particularly for problems initiating and maintaining sleep and disorders of excessive hypersomnolence. In line with the baseline results, a significant positive loading was seen for parent-rated family conflict, and a significant negative weight was found for neighborhood safety due to violence.
In CV2 (Figure 3, right column), a significant positive loading was seen for sleep breathing disorders, in line with the baseline results. For the predictor variables of interest, the highest loading was for BMI, consistent with results from the baseline analysis showing increasing BMI associated with increasing severity of sleep breathing disorders.
Sensitivity analyses using multiple imputation for missing data (detailed in Supplementary Results and Supplementary Table 4) found a similar pattern of results as the primary analyses.
Discussion
Sleep disturbances are interconnected with adolescent health and behavior, but the complex, multidomain set of factors that contribute to their emergence is poorly understood. Using data from a population-based study of 9- and 10-year-olds [35, 36] and multivariate, data-driven machine learning methods, we mapped associations between multifaceted sleep disturbances and multidomain predictors spanning demographic, psychosocial, environmental, and health-related domains. We identified two prominent sets of associations: (1) difficulty initiating and maintaining sleep and excessive daytime somnolence were strongly linked to most domains of psychopathology and family history of psychopathology; (2) sleep breathing disorders were linked to BMI and black race. The primary canonical correlation was large (r2 of 0.36) and both sets of associations generalized very well out of sample. Moreover, the prominent associations were replicated using data from a second time point in the ABCD study. Our results add to our understanding of how sleep disturbances during emerging adolescence exhibit complex domain-spanning linkages to psychological, social, and demographic factors.
This study leveraged data from the baseline and 2-year follow-up of the ABCD longitudinal study of youth at 21 sites in the United States and includes substantial demographic and socioeconomic diversity. We coupled this dataset with novel machine learning methods. To our knowledge, this is the first study in youth to use data-driven multivariate methods to identify and quantify “many-many” associations between multiple types of sleep disturbances on the one hand and multidimensional psychopathology, cognition, personality, and socio-demographic factors, on the other. Our analytic approach was further strengthened by the use of leave-one-site-out cross-validation, allowing a clear demonstration that our findings are not spurious and that they generalize to new participants across the 21 sites of the ABCD study.
We found that psychopathology emerged as the strongest predictor of sleep disturbances even when considering other constructs thought to affect sleep, with nearly all psychopathology domains showing robust associations. This finding supports prior research identifying a general factor of psychopathology (“p factor”), reflecting a latent dimension that encompasses variance shared across psychiatric disorders [88–90]. It also points to the importance of considering psychopathology and sleep disorders as interrelated when assessing and treating adolescent patients. Relatedly, family history of psychopathology was linked to sleep disturbances. This finding expands on prior work indicating an increased risk of sleep problems in youth and adolescents with parental history of depression [91, 92] and suggests a potential risk factor for sleep disturbances that may be useful for early identification and intervention.
Our analysis also identified a significant association between parent-rated family conflict and sleep disturbances. This finding aligns with past research showing poor family functioning [16] and negative family relational factors [16, 93] are linked to poor sleep quality in adolescents. Conflict within the family unit may result in stress which, in turn, may contribute to other known correlates of sleep disturbances such as heightened physiological arousal and sensitivity to external threats [94]. This finding points to the importance of considering the family context in which youth sleep disturbances arise, as family conflict may be a modifiable target with appropriate interventions.
Sleep disturbances were also associated with neighborhood safety, suggesting an influence of the broader context in which youth reside. This finding parallels results from a longitudinal study which found that children living in neighborhoods characterized by safety concerns had a higher prevalence of serious sleep problems than those living in more favorable neighborhoods [20]. Similar to the experience of family conflict, concerns about neighborhood safety may contribute to psychological and physiological responses disruptive to sleep [95].
One strength of our analytic approach was the ability to examine additional associations that were present after accounting for the initial set of associations. In particular, we found BMI to be uniquely linked to sleep breathing disorders. Meta-analytic evidence supports a prospective association between short sleep duration and the development of obesity in childhood and adolescence [11]. Additionally, a recent study demonstrated support for causal roles of obesity and weight loss with sleep breathing disorder persistence and remission, respectively, in emerging adolescence [96]. Recent research on the ABCD Study sample demonstrated that children considered overweight or obese had significantly higher total scores on the sleep disturbances scale when compared to children with a normal BMI [97]. Our results add more specificity, suggesting that after accounting for other associations, BMI may be related to sleep breathing disorders specifically.
Our results also showed a significant association between sleep-disordered breathing and black race, which is consistent with prior work in smaller samples [98, 99]. Notably, even when controlling for obesity and increased rates of other respiratory problems such as asthma, research suggests that black youth experience both increased risk of developing sleep breathing disorders and greater severity of symptoms [98]. In interpreting these results, it is critical to recognize that race and ethnicity are socially defined constructs that exist within and intersect with a larger, intricate matrix of causally interrelated factors that span individuals, households, neighborhoods, schools, natural and built environments, and larger systems. Associations involving race and sleep disturbances, like other racial health disparities, should be interpreted thoughtfully in light of the complexity of this causal matrix in which both variables are embedded [100]. Given growing evidence of adverse neurocognitive consequences of untreated sleep breathing disorders in youth [101] and known disparities in access to treatment, our results highlight the need for continued research and targeted efforts aimed at reducing youth sleep health disparities [102].
Our findings should be considered with knowledge of the limitations of our study. First, our analysis is correlational, and thus, directionality or causality of the identified associations cannot be established [103]. Second, in our study, youth sleep disturbances and several constructs of interest including multidimensional psychopathology were measured exclusively via parent report at baseline. This protocol was adopted by the ABCD Study to reduce the time impacts of youth participation as well as due to known issues with reliability of youth reports in 9- and 10-year-old children. Thus, our findings (e.g. significant associations between multidimensional psychopathology and sleep disturbances) may be influenced by common method bias in which parent-report scales are used for the outcome (sleep disturbances) as well as many predictor domains [104, 105]. To partially address concerns about common method bias, in our 2-year follow-up analyses, we included an additional parent-reported questionnaire (i.e. The PhenX Neighborhood Collective Efficacy—Community Cohesion and Informal Social Control) that we did not hypothesize to be associated with multifaceted sleep disturbances (this questionnaire was not available at baseline). In this analysis, none of the parent-reported items from this questionnaire were found to be significantly associated with multifaceted sleep disturbances. This result provides some initial evidence that not all constructs based on parent-reported questionnaires were significantly associated with sleep disturbances. Future research should seek to corroborate our findings using data from multiple informants as well as other measures of sleep disturbances such as actigraphy and polysomnography.
Our analysis demonstrated the prominent associations were stable across time points (i.e. baseline and 2-year follow-up). As data from additional time points become available, this analytic approach may be useful for exploring how multidomain associations with sleep disturbances remain stable or change over time. While research suggests that parent-reported data on youth sleep disturbances are more reliable when children are young, as the participants grow older, youth self-reported data will be available and should be examined alongside and independently from parent-reported data to better account for rater effects. Additionally, a subset of participants from the ABCD Study provided actigraphic Fitbit data, which may provide an opportunity to examine multidomain associations with other sleep constructs such as sleep stages and sleep duration.
In sum, this study used novel, data-driven multivariate machine learning methods to identify robust and generalizable multidomain predictors of sleep disturbances, adding to our knowledge of the etiology of disturbed sleep in emerging adolescence. Our findings provide support for the prominent associations of multifaceted psychopathology with sleep disturbances while also identifying key contextual factors including family conflict and neighborhood safety, suggesting the importance of multidimensional assessment of adolescent sleep. Additionally, the association of BMI and African American/black race with sleep-related breathing difficulties underscores the need for continued research to address sleep health disparities in emerging adolescents.
Supplementary Material
Acknowledgments
We gratefully acknowledge discussions with Michael Dolan, Maria Dziubinski, Aaron Hayes, Ally King, and Zeinab Makled and thank them for their research support.
Contributor Information
Katherine L McCurry, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Katherine Toda-Thorne, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Aman Taxali, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Mike Angstadt, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Felicia A Hardi, Department of Psychology, University of Michigan, Ann Arbor, MI, USA.
Mary M Heitzeg, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Chandra Sripada, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA.
Funding
This work was supported by the following grants from the United States National Institutes of Health, the National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism: R01MH123458 (CS), U01DA041106 (CS, MH), and T32 AA007477 (KM). This research was supported in part through computational resources and services provided by Advanced Research Computing at the University of Michigan, Ann Arbor. Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the NDA. This is a multisite, longitudinal study designed to recruit more than 10 000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Disclosure Statement
Financial Disclosure: none.
Non-financial Disclosure: none.
Data Availability
The ABCD data repository grows and changes over time. The ABCD data used in this report came from ABCD Release 4.0, NDA Study 1299, doi: 10.15154/1523041, which can be found at https://nda.nih.gov/study.html?id=1299. Code for running analyses can be found at https://github.com/SripadaLab/ABCD_sleep_disturbances_CCA.
References
- 1. Galván A. The need for sleep in the adolescent brain. Trends Cogn Sci. 2020;24(1):79–89. doi: 10.1016/j.tics.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Anastasiades PG, de Vivo L, Bellesi M, Jones MW.. Adolescent sleep and the foundations of prefrontal cortical development and dysfunction. Prog Neurobiol. 2022;218:102338. doi: 10.1016/j.pneurobio.2022.102338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paiva T. Epidemiology of sleep disorders in children and adolescents. In: Nevšímalová S, Bruni O, eds. Sleep Disorders in Children. Cham: Springer International Publishing; 2017:53-67. doi: 10.1007/978-3-319-28640-2_3 [DOI] [Google Scholar]
- 4. Lewien C, Genuneit J, Meigen C, Kiess W, Poulain T.. Sleep-related difficulties in healthy children and adolescents. BMC Pediatr. 2021;21(1):82. doi: 10.1186/s12887-021-02529-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baddam SKR, Canapari CA, Van de Grift J, McGirr C, Nasser AY, Crowley MJ.. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30(1):65–84. doi: 10.1016/j.chc.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 6. Alvaro PK, Roberts RM, Harris JK.. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Ji X, Pitt S, et al. Childhood sleep: physical, cognitive, and behavioral consequences and implications. World J Pediatr. 2024;20(2):122–132. doi: 10.1007/s12519-022-00647-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker SP. ADHD and sleep: recent advances and future directions. Curr Opin Psychol. 2020;34:50–56. doi: 10.1016/j.copsyc.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott J, Kallestad H, Vedaa O, Sivertsen B, Etain B.. Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101429. doi: 10.1016/j.smrv.2021.101429 [DOI] [PubMed] [Google Scholar]
- 10. Hasler BP, Graves JL, Wallace ML, et al. Self-reported sleep and circadian characteristics predict alcohol and cannabis use: a longitudinal analysis of the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcohol Clin Exp Res. 2022;46(5):848–860. doi: 10.1111/acer.14808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP.. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41(4). doi: 10.1093/sleep/zsy018 [DOI] [PubMed] [Google Scholar]
- 12. Bartel KA, Gradisar M, Williamson P.. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. doi: 10.1016/j.smrv.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 13. Cameron EE, Watts D, Silang K, et al. Parental socioeconomic status and childhood sleep: a systematic review and meta-analysis. Sleep Epidemiol. 2022;2:100047. doi:1011016/j.sleepe.2022.100047 [Google Scholar]
- 14. Gregory AM, Caspi A, Moffitt TE, Poulton R.. Family conflict in childhood: a predictor of later insomnia. Sleep. 2006;29(8):1063–1067. doi: 10.1093/sleep/29.8.1063 [DOI] [PubMed] [Google Scholar]
- 15. Leonard H, Khurana A.. Parenting behaviors and family conflict as predictors of adolescent sleep and bedtime media use. J Youth Adolesc. 2022;51(8):1611–1621. doi: 10.1007/s10964-022-01614-4 [DOI] [PubMed] [Google Scholar]
- 16. Khor SPH, McClure A, Aldridge G, Bei B, Yap MBH.. Modifiable parental factors in adolescent sleep: a systematic review and meta-analysis. Sleep Med Rev. 2021;56:101408. doi: 10.1016/j.smrv.2020.101408 [DOI] [PubMed] [Google Scholar]
- 17. Bacaro V, Carpentier L, Crocetti E.. Sleep well, study well: a systematic review of longitudinal studies on the interplay between sleep and school experience in adolescence. Int J Environ Res Public Health. 2023;20(6):4829. doi: 10.3390/ijerph20064829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donoghue C, Meltzer LJ.. Sleep it off: bullying and sleep disturbances in adolescents. J Adolesc. 2018;68:87–93. doi: 10.1016/j.adolescence.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 19. Tomfohr-Madsen L, Cameron EE, Dhillon A, et al. Neighborhood socioeconomic status and child sleep duration: a systematic review and meta-analysis. Sleep Health. 2020;6(5):550–562. doi: 10.1016/j.sleh.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 20. Singh GK, Kenney MK.. Rising prevalence and neighborhood, social, and behavioral determinants of sleep problems in US children and adolescents, 2003–2012. Sleep Disord. 2013;2013:394320. doi: 10.1155/2013/394320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu JW, Tu YK, Lai YF, et al. Associations between sleep disturbances and suicidal ideation, plans, and attempts in adolescents: a systematic review and meta-analysis. Sleep. 2019;42(6). doi: 10.1093/sleep/zsz054 [DOI] [PubMed] [Google Scholar]
- 22. Corkum P, Tannock R, Moldofsky H.. Sleep disturbances in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1998;37(6):637–646. doi: 10.1097/00004583-199806000-00014 [DOI] [PubMed] [Google Scholar]
- 23. Goodwin RD, Marusic A.. Association between short sleep and suicidal ideation and suicide attempt among adults in the general population. Sleep. 2008;31(8):1097–1101. doi: 10.5665/sleep/31.8.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Mill JG, Hoogendijk WJG, Vogelzangs N, van Dyck R, Penninx BWJH.. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry. 2010;71(3):9161. doi: 10.4088/JCP.09m05218gry [DOI] [PubMed] [Google Scholar]
- 25. Vazsonyi AT, Liu D, Blatny M.. Longitudinal bidirectional effects between sleep quality and internalizing problems. J Adolesc. 2022;94(3):448–461. doi: 10.1002/jad.12039 [DOI] [PubMed] [Google Scholar]
- 26. Shimizu M, Zeringue MM, Erath SA, Hinnant JB, El-Sheikh M.. Trajectories of sleep problems in childhood: associations with mental health in adolescence. Sleep. 2021;44(3). doi: 10.1093/sleep/zsaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williamson AA, Zendarski N, Lange K, et al. Sleep problems, internalizing and externalizing symptoms, and domains of health-related quality of life: bidirectional associations from early childhood to early adolescence. Sleep. 2021;44(1). doi: 10.1093/sleep/zsaa139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller AL, Lumeng JC, LeBourgeois MK.. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):41–47. doi: 10.1097/MED.0000000000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bronfenbrenner U. Ecological systems theory. In: Vasta R, ed. Six Theories of Child Development: Revised Formulations and Current Issues. London, England: Jessica Kingsley Publishers; 1992:187–249. [Google Scholar]
- 30. Lupini F, Williamson AA.. Health disparities in pediatric sleep. Sleep Med Clin. 2023;18(2):225–234. doi: 10.1016/j.jsmc.2023.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meltzer LJ, Williamson AA, Mindell JA.. Pediatric sleep health: it matters, and so does how we define it. Sleep Med Rev. 2021;57:101425. doi: 10.1016/j.smrv.2021.101425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruni O, Ottaviano S, Guidetti V, et al. The sleep disturbance scale for children (SDSC) construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–261. doi: 10.1111/j.1365-2869.1996.00251.x [DOI] [PubMed] [Google Scholar]
- 33. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hotelling H. Relations between two sets of variates. Biometrika. 1936;28(3-4):321–377. doi: 10.2307/2333955 [DOI] [Google Scholar]
- 35. Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karcher NR, Barch DM.. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2021;46(1):131–142. doi: 10.1038/s41386-020-0736-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 40. Rubin DB. Multiple Imputation for Nonresponse in Surveys. 1st ed. New York, NY: John Wiley & Sons; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- 41. Rubin DB. Multiple imputation after 18+ Years. J Am Stat Assoc. 1996;91(434):473–489. doi: 10.2307/2291635 [DOI] [Google Scholar]
- 42. van Buuren S, Groothuis-Oudshoorn K.. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 43. Lewandowski AS, Toliver-Sokol M, Palermo TM.. Evidence-based review of subjective pediatric sleep measures. J Pediatr Psychol. 2011;36(7):780–793. doi: 10.1093/jpepsy/jsq119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spruyt K, Gozal D.. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011;15(1):19–32. doi: 10.1016/j.smrv.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ağca S, Görker I, Turan FN, Öztürk L.. Validity and reliability of the Turkish version of sleep disturbance scale for children. Sleep Med. 2021;84:56–62. doi: 10.1016/j.sleep.2021.05.016 [DOI] [PubMed] [Google Scholar]
- 46. Ferreira VR, Carvalho LBC, Ruotolo F, de Morais JF, Prado LBF, Prado GF.. Sleep disturbance scale for children: translation, cultural adaptation, and validation. Sleep Med. 2009;10(4):457–463. doi: 10.1016/j.sleep.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 47. Pagerols M, Bosch R, Prat R, et al. The sleep disturbance scale for children: psychometric properties and prevalence of sleep disorders in Spanish children aged 6–16 years. J Sleep Res. 2023;32(4):e13871. doi: 10.1111/jsr.13871 [DOI] [PubMed] [Google Scholar]
- 48. Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2009. [Google Scholar]
- 49. Becker SP, Ramsey RR, Byars KC.. Convergent validity of the Child Behavior Checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center. Sleep Med. 2015;16(1):79–86. doi: 10.1016/j.sleep.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 50. Pagliaccio D, Luking KR, Anokhin AP, et al. Revising the BIS/BAS scale to study development: measurement invariance and normative effects of age and sex from childhood through adulthood. Psychol Assess. 2016;28(4):429–442. doi: 10.1037/pas0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carver CS, White TL.. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1995;67(2):319. doi: 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- 52. Watts AL, Smith GT, Barch DM, Sher KJ.. Factor structure, measurement and structural invariance, and external validity of an abbreviated youth version of the UPPS-P Impulsive Behavior Scale. Psychol Assess. 2020;32(4):336–347. doi: 10.1037/pas0000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Loewy RL, Pearson R, Vinogradov S, Bearden CE, Cannon TD.. Psychosis risk screening with the Prodromal Questionnaire—Brief Version (PQ-B). Schizophr Res. 2011;129(1):42–46. doi: 10.1016/j.schres.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fonseca-Pedrero E, Gooding DC, Ortuño-Sierra J, Paino M.. Assessing self-reported clinical high risk symptoms in community-derived adolescents: a psychometric evaluation of the Prodromal Questionnaire-Brief. Compr Psychiatry. 2016;66:201–208. doi: 10.1016/j.comppsych.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 55. Kline E, Wilson C, Ereshefsky S, et al. Psychosis risk screening in youth: a validation study of three self-report measures of attenuated psychosis symptoms. Schizophr Res. 2012;141(1):72–77. doi: 10.1016/j.schres.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 56. Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015 [DOI] [PubMed] [Google Scholar]
- 57. Vugteveen J, de Bildt A, Theunissen M, Reijneveld M, Timmerman M.. Validity aspects of the strengths and difficulties questionnaire (SDQ) adolescent self-report and parent-report versions among dutch adolescents. Assessment. 2021;28(2):601–616. doi: 10.1177/1073191119858416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bleck TP, Nowinski CJ, Gershon R, Koroshetz WJ.. What is the NIH Toolbox, and what will it mean to neurology? Neurology. 2013;80(10):874–875. doi: 10.1212/WNL.0b013e3182872ea0 [DOI] [PubMed] [Google Scholar]
- 59. Gershon RC, Cook KF, Mungas D, et al. Language measures of the NIH toolbox cognition battery. J Int Neuropsychol Soc. 2014;20(6):642–651. doi: 10.1017/S1355617714000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hodes RJ, Insel TR, Landis SC; NIH Blueprint for Neuroscience Research. The NIH Toolbox: setting a standard for biomedical research. Neurology. 2013;80(11 Supplement 3):S1–S1. doi: 10.1212/WNL.0b013e3182872e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thompson WK, Barch DM, Bjork JM, et al. The structure of cognition in 9 and 10 year-old children and associations with problem behaviors: findings from the ABCD study’s baseline neurocognitive battery. Dev Cogn Neurosci. 2019;36:100606. doi: 10.1016/j.dcn.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bauer PJ, Zelazo PD.. IX. Nih toolbox cognition battery (cb): summary, conclusions, and implications for cognitive development. Monogr Soc Res Child Dev. 2013;78(4):133–146. doi: 10.1111/mono.12039 [DOI] [PubMed] [Google Scholar]
- 63. Luciana M, Bjork JM, Nagel BJ, et al. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67–79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor EM. Psychological appraisal of children with cerebral defects. In: Psychological Appraisal of Children with Cerebral Defects. Cambridge, MA: Harvard University Press; 1959. doi: 10.4159/harvard.9780674367494 [DOI] [Google Scholar]
- 65. Strauss E, Sherman EMS, Spreen O.. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2006:xvii, 1216. [Google Scholar]
- 66. Wechsler D. Wechsler Intelligence Scale for Children–Fifth Edition (WISC-V). Bloomington, MN: Pearson. 2014. [Google Scholar]
- 67. Acker W. A computerized approach to psychological screening—the Bexley-Maudsley Automated Psychological Screening and the Bexley-Maudsley Category sorting test. Int J Man Mach Stud. 1982;17(3):361–369. doi: 10.1016/s0020-7373(82)80037-0 [DOI] [Google Scholar]
- 68. Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x [DOI] [PubMed] [Google Scholar]
- 69. Brown SA, Brumback T, Tomlinson K, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 2015;76(6):895–908. doi: 10.15288/jsad.2015.76.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174(3):253–260. doi: 10.1093/aje/kwr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moos RH, Moos BS.. Family Environment Scale Manual: Development, Applications, Research. 4th. Palo Alto, CA: Center for Health Care Evaluation, Department of Veterans Affairs, Stanford University Medical Centers/Mind Garden. 2009. [Google Scholar]
- 72. Sanford K, Bingham CR, Zucker RA.. Validity issues with the Family Environment Scale: psychometric resolution and research application with alcoholic families. Psychol Assess. 1999;11(3):315–325. doi: 10.1037//1040-3590.11.3.315 [DOI] [Google Scholar]
- 73. Moos RH. Conceptual and empirical approaches to developing family-based assessment procedures: resolving the case of the family environment scale. Fam Process. 1990;29(2):199–208; discussion 209. doi: 10.1111/j.1545-5300.1990.00199.x [DOI] [PubMed] [Google Scholar]
- 74. Zucker RA, Gonzalez R, Feldstein Ewing SW, et al. Assessment of culture and environment in the Adolescent Brain and Cognitive Development Study: rationale, description of measures, and early data. Dev Cogn Neurosci. 2018;32:107–120. doi: 10.1016/j.dcn.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arthur MW, Briney JS, Hawkins JD, Abbott RD, Brooke-Weiss BL, Catalano RF.. Measuring risk and protection in communities using the Communities That Care Youth Survey. Eval Program Plann. 2007;30(2):197–211. doi: 10.1016/j.evalprogplan.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 76. Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. doi: 10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fan CC, Marshall A, Smolker H, et al. Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci. 2021;52:101030. doi: 10.1016/j.dcn.2021.101030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hu J, Kind AJH, Nerenz D.. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493–501. doi: 10.1177/1062860617753063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Echeverria SE, Diez-Roux AV, Link BG.. Reliability of self-reported neighborhood characteristics. J Urban Health. 2004;81(4):682–701. doi: 10.1093/jurban/jth151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T.. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040 [DOI] [PubMed] [Google Scholar]
- 81. Gonzalez R, Thompson EL, Sanchez M, et al. An update on the assessment of culture and environment in the ABCD Study®: emerging literature and protocol updates over three measurement waves. Dev Cogn Neurosci. 2021;52:101021. doi: 10.1016/j.dcn.2021.101021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sharif I, Wills TA, Sargent JD.. Effect of visual media use on school performance: a prospective study. J Adolesc Health. 2010;46(1):52–61. doi: 10.1016/j.jadohealth.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sampson RJ, Raudenbush SW, Earls F.. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918 [DOI] [PubMed] [Google Scholar]
- 84. Witten DM, Tibshirani RJ.. Extensions of sparse canonical correlation analysis with applications to genomic data. Stat Appl Genet Mol Biol. 2009;8(1):Article28. doi: 10.2202/1544-6115.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xia CH, Ma Z, Ciric R, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9(1):3003. doi: 10.1038/s41467-018-05317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang HT, Smallwood J, Mourao-Miranda J, et al. Finding the needle in a high-dimensional haystack: canonical correlation analysis for neuroscientists. Neuroimage. 2020;216:116745. doi: 10.1016/j.neuroimage.2020.116745 [DOI] [PubMed] [Google Scholar]
- 87. Winkler AM, Renaud O, Smith SM, Nichols TE.. Permutation inference for canonical correlation analysis. Neuroimage. 2020;220:117065. doi: 10.1016/j.neuroimage.2020.117065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cervin M, Norris LA, Ginsburg G, et al. The p factor consistently predicts long-term psychiatric and functional outcomes in anxiety-disordered youth. J Am Acad Child Adolesc Psychiatry. 2021;60(7):902–912.e5. doi: 10.1016/j.jaac.2020.08.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clark DA, Hicks BM, Angstadt M, et al. The general factor of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study: a comparison of alternative modeling approaches. Clin Psychol Sci. 2021;9(2):169–182. doi: 10.1177/2167702620959317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Michelini G, Barch DM, Tian Y, Watson D, Klein DN, Kotov R.. Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Transl Psychiatry. 2019;9(1):1–15. doi: 10.1038/s41398-019-0593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hamilton JL, Ladouceur CD, Silk JS, Franzen PL, Bylsma LM.. Higher rates of sleep disturbance among offspring of parents with recurrent depression compared to offspring of nondepressed parents. J Pediatr Psychol. 2020;45(1):1–11. doi: 10.1093/jpepsy/jsz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Taylor AK, Netsi E, O’Mahen H, Stein A, Evans J, Pearson RM.. The association between maternal postnatal depressive symptoms and offspring sleep problems in adolescence. Psychol Med. 2017;47(3):451–459. doi: 10.1017/S0033291716002427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Maratia F, Bacaro V, Crocetti E.. Sleep is a family affair: a systematic review and meta-analysis of longitudinal studies on the interplay between adolescents’ sleep and family factors. Int J Environ Res Public Health. 2023;20(5):4572. doi: 10.3390/ijerph20054572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dahl RE, El-Sheikh M.. Considering sleep in a family context: introduction to the special issue. J Fam Psychol. 2007;21(1):1–3. doi: 10.1037/0893-3200.21.1.1 [DOI] [PubMed] [Google Scholar]
- 95. Hill TD, Trinh HN, Wen M, Hale L.. Perceived neighborhood safety and sleep quality: a global analysis of six countries. Sleep Med. 2016;18:56–60. doi: 10.1016/j.sleep.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 96. Frye SS, Fernandez-Mendoza J, Calhoun SL, et al. Childhood obesity, weight loss and developmental trajectories predict the persistence and remission of childhood sleep-disordered breathing. Pediatr Obes. 2019;14(1):e12461. doi: 10.1111/ijpo.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mattey-Mora PP, Nelson EJ.. Sleep disturbances, obesity, and cognitive function in childhood: a mediation analysis. Curr Dev Nutr. 2021;5(10):nzab119. doi: 10.1093/cdn/nzab119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Williamson AA, Johnson TJ, Tapia IE.. Health disparities in pediatric sleep-disordered breathing. Paediatr Respir Rev. 2023;45:2–7. doi: 10.1016/j.prrv.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G.. Risk factors for sleep-disordered breathing in children. Am J Respir Crit Care Med. 1999;159(5):1527–1532. doi: 10.1164/ajrccm.159.5.9809079 [DOI] [PubMed] [Google Scholar]
- 100. Perez MF, Coutinho MT.. An overview of health disparities in asthma. Yale J Biol Med. 2021;94(3):497–507. [PMC free article] [PubMed] [Google Scholar]
- 101. O’Brien LM. Neurocognitive and Behavioral Consequences of Sleep-Disordered Breathing in Children. Sleep and psychiatric disorders in children and adolescents. 2008:165–178. doi: 10.3109/9781420048087-15 [DOI]
- 102. Jackson CL, Walker JR, Brown MK, Das R, Jones NL.. A workshop report on the causes and consequences of sleep health disparities. Sleep. 2020;43(8). doi: 10.1093/sleep/zsaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. MacKinnon DP, Cheong J, Pirlott AG.. Statistical mediation analysis. APA handbook of research methods in psychology, Vol 2: Research designs: Quantitative, qualitative, neuropsychological, and biological. 2012;313–331. doi: 10.1037/13620-018 [DOI]
- 104. Campbell DT, Fiske DW.. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56(2):81–105. doi: 10.1037/h0046016 [DOI] [PubMed] [Google Scholar]
- 105. Podsakoff PM, MacKenzie SB, Lee JY, Podsakoff NP.. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003;88(5):879–903. doi: 10.1037/0021-9010.88.5.879 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD data repository grows and changes over time. The ABCD data used in this report came from ABCD Release 4.0, NDA Study 1299, doi: 10.15154/1523041, which can be found at https://nda.nih.gov/study.html?id=1299. Code for running analyses can be found at https://github.com/SripadaLab/ABCD_sleep_disturbances_CCA.