Despite tremendous advances in the management of HCV, including the availability of a curative treatment, an estimated 57 million people worldwide are living with chronic HCV. 1 People who inject drugs (PWIDs) are a key at-risk population for both prevalent and incident HCV, and thus are a critical focus for HCV elimination efforts.2,3 Direct-acting antiviral (DAA) therapy is both safe and effective in PWID.4–6 Moreover, in the absence of an effective vaccine for HCV, modeling studies support scaling up DAA access in PWID in a “treatment-as-prevention” strategy. 7 However, HCV reinfection after successful DAA treatment remains a concern and could hinder elimination efforts. This review will summarize HCV reinfection incidence and outcomes among PWIDs and will describe a multifaceted approach to addressing HCV reinfection among PWIDs.
INCIDENCE OF HCV REINFECTION IN PWIDs
In the pre-DAA era, 2 systematic reviews evaluated the incidence of HCV reinfection among PWID who had achieved sustained virologic response (SVR) after HCV treatment. Although over 50 studies were included, they were relatively small with heterogenous populations.8,9 Since the development of DAAs, HCV treatment has become more widely available and effective, and over time, DAA uptake among PWID has increased. In this context, a recent large meta-analysis with over 3790 PWIDs found an overall HCV reinfection rate of 6.2/100 person years (PY) (95% CI: 4.3–8.9) among people reporting recent injection drug use (IDU) and 3.8/100 PY (95% CI: 2.5–5.8) among those receiving opioid agonist therapy (OAT). 10 Several additional studies have since been published, with reinfection rates ranging from 1.8/100 PY (95% CI: 0.6–5.6) in a UK cohort treated at an addiction center to 28.7/100 PY (95% CI: 16.3–50.6) among persons in an Australian prison who reported recent IDU and sharing of needles/syringes (Table 1).11–20 In addition to IDU, factors associated with higher rates of reinfection include younger age, experiencing unstable housing or homelessness, receipt of HCV treatment while in prison, and recent needle or syringe sharing.11,13,15,19 Notably, much of this research has been conducted outside of the United States. Given the highly regional nature of drug use epidemics, more data on reinfection rates and risk factors in the United States are needed.
TABLE 1.
Incidence of HCV reinfection after sustained virologic response among persons who use drugs
| References | Location | Population | Study design | No. subjects | No. reinfections | Reinfection rate, per 100 PY (95% CI) |
|---|---|---|---|---|---|---|
| Hajarizadeh et al 10 | International | Recent drug use or receiving OAT | Meta-analysis | 3790 (36 studies) | NR a | Recent drug use: 5.9 (4.1, 8.5) Recent IDU: 6.2 (4.3, 8.9) Receiving OAT: 3.8 (2.5, 5.8) |
| Cunningham et al 11 | International | People with recent IDU and receiving OAT | Clinical Trial | 177 | 8 | Overall: 3.1 (1.6–6.3) Sharing needles/syringes: 17.9 (5.8–55.6) |
| Lens et al 20 | Spain | Harm Reduction Center | Prospective | 168 | 42 | Overall: 31/100 |
| Hosseini-Hooshyar et al 12 | International | HIV/HCV | Meta-analysis | 9024 (41 studies) | 435 | Overall: 3.8 (2.8–5.1) MSM: 6.1 (4.5–8.0) IDU: 3.3 (2.0–5.4) |
| Carson et al 13 | Australia | Prison | Prospective | 161 | 18 | Overall: 12.5 (7.9–19.8) Sharing needle/syringe: 28.7 (16.3–50.6) |
| Beiser et al 19 | United States | Homeless | Retrospective | 535 | 74 | Overall: 12.0 (9.5–15.1) |
| Young et al 14 | Canada | HIV/HCV | Prospective | 814 | 62 | Interferon era overall: 2.6 (1.2–4.1) Interferon era IDU: 4.7 (1.4–.79) DAA era overall: 3.4 (2.5–4.4) DAA era IDU: 7.6 (5.3–10) |
| Lindqvist et al 15 | Sweden | Needle and Syringe Program | Prospective | 339 | 43 | Overall: 9.3 (7.0–12.3) |
| O’Sullivan et al 16 | UK | Addiction Center | Prospective | 146 | 19 | 2013–2017: 1.7 (0.9–3.4) 2017–2021: 1.8 (0.6–5.6) |
| Martinello et al 17 | International | Recently acquired HCV | Clinical Trial | 196 | 28 | Overall: 14.2 (9.8–20.5) |
| Sacks-Davis et al 18 | International | HIV/HCV | Pooled data from 6 contributing cohorts | 6144 | 643 b | Interferon era: 4.6 (4.1–5.1) Early DAA era: 3.4 (2.9–3.9) Broad DAA era: 3.1 (2.6–3.6) |
Not reported for all studies.
First reinfections.
Abbreviations: DAA, direct-acting antiviral; IDU, injection drug use; MSM, men who have sex with men; NR, xxx; OAT, opioid agonist therapy; PY, person years.
OUTCOMES OF HCV REINFECTION IN PWID
Existing studies suggest that SVR rates are high among patients retreated with DAAs for reinfection, although multiple reinfections can occur.18,20,21 Importantly, high rates of HCV reinfection may be an indicator of high overall mortality. In one study of 94 individuals who underwent HCV treatment at a community needle and syringe program in the United Kingdom, HCV reinfection rate among those who achieved SVR was 21.5/100 PY (95% CI: 13.0–35.7). 21 The mortality rate was also high at 5.6/100 PY (95% CI: 2.8–11.1) and was predominantly due to drug overdose or other complications of drug use. In another UK study of 270 PWID who received HCV treatment at an addiction center observed 41 deaths (15%) during the 8-year study period; drug overdose was the most common cause of death among those whose cause of death could be ascertained. 16 In a third Canadian study of people living with HIV in clinical care, mortality was three times higher in individuals who experienced HCV reinfection after initial SVR. 14 These data underscore the importance of addressing non-liver outcomes and the syndemic of opioid use and viral infections in patients at high risk of HCV reinfection.
APPROACH TO HCV REINFECTION IN PWID
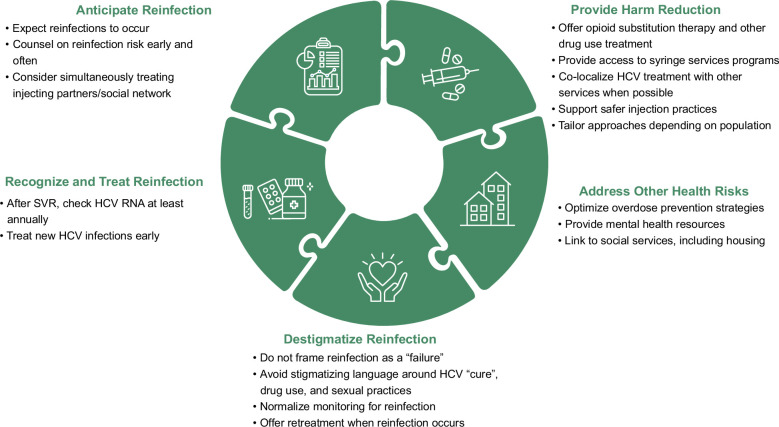
A framework for approaching reinfection in PWID can be found in Figure 1. First and foremost, treating clinicians should anticipate reinfection to occur in some patients. As more people with HCV are treated and cured, we can expect HCV infections to be concentrated in those at highest risk for exposure. Thus, rather than viewing reinfection as a failure, it should be seen as a positive sign that the population at greatest risk of HCV is being treated. 22 Indeed, a recent analysis of pooled data from 6 contributing cohorts of people living with HIV demonstrated that while the overall incidence of HCV declined in people living with HIV from 2015 to 2019, reinfection represented an increasing proportion of incident HCV cases. 18 In other words, new HCV infections are occurring in a shrinking population of people at risk, including those with prior successful HCV treatment. Reassuringly, the overall decline in incident cases in the study reinforced the concept of “treatment-as-prevention” and suggested that reinfections did not substantively impact HCV elimination efforts. Thus, providers should not withhold treatment out of fear of reinfection. Instead, providers should be mindful to counsel on reinfection risks early and often and monitor for reinfection with HCV RNA at least annually after SVR. Modeling studies also suggest that simultaneously treating HCV in injecting partners and social networks may reduce reinfection risk. 23
FIGURE 1.
Multifaceted approach to addressing HCV reinfection among people who inject drugs. Abbreviation: SVR, sustained virologic response.
Patients who experience reinfection report strong negative emotional responses, especially shame. 24 Therefore, it is critical that clinicians are mindful to destigmatize reinfection. Conversations about reinfection should be normalized, and HCV RNA screening after SVR should be standard of practice among individuals with active IDU. Stigmatizing language around HCV “cure,” drug use, and sexual practices should be avoided. Lastly, retreatment should be offered when reinfection occurs.
Studies demonstrate a lower risk of reinfection in people receiving OAT; therefore, drug use treatment in addition to DAAs should be offered to all patients with active drug use. 10 However, this should not be a requirement prior to DAA treatment. Studies show that reducing payor restrictions (eg, substance use abstinence) leads to increased DAA uptake, particularly among patients with a history of drug use. 25 Access to syringe services and safer injection practices is also critical, as sharing needles and syringes is associated with the highest risk of reinfection.11,26 Harm reduction is essential not only for preventing reinfection but also for providing comprehensive whole-person care. PWIDs who experience reinfection have a significantly higher risk of overdose-related death than liver-related death. Thus, optimizing overdose prevention strategies and providing mental health resources is necessary. Finally, homelessness is prevalent among PWIDs. 27 People experiencing homelessness have lower rates of SVR with initial treatment and higher rates of reinfection.6,15,19 This may be attributed to their unique challenges with medication retention, including frequent relocation and high rates of property theft, as well as the association of homelessness and injection risk behaviors.17,28 Efforts should be made to link people with unstable housing to social services and to design tailored interventions to improve their access to HCV and drug use treatment.
CONCLUSIONS
In summary, HCV reinfection should be anticipated, screened for, and treated, particularly among high-risk populations such as PWIDs. HCV retreatment should not be restricted by payers, and persons at higher risk of reinfection should be counseled on harm reduction strategies while also screened for reinfection. Normalization of this approach and destigmatization of reinfection are critical. Nationally, HCV elimination among PWID is a priority, and the Centers for Disease Control and Prevention has numerous approaches to achieve this. Specifically, the Centers for Disease Control and Prevention commits to increase the utilization of HCV prevention services among PWID, to establish comprehensive national viral hepatitis surveillance for public health action, including additional data collection on reinfection rates in the United States, and to reduce barriers to HCV treatment by eliminating eligibility restrictions and expand treatment sites to include substance use treatment centers and correctional facilities. 29 The Centers for Disease Control and Prevention’s 2025 Strategic Plan to address viral hepatitis emphasizes the need to support clean needle exchange programs and local policies like the Harm Reduction Community Linkage Projects in Illinois. 30 At the systems level, health care systems can support the use of telehealth to improve health care access throughout the HCV care continuum, including evidence-based practices like project Extension for Community Healthcare Outcomes and community-based facilitated telemedicine programs.31,32 Safe medication storage devices to improve medication adherence and achieve SVR with initial treatment and after reinfection should be considered, particularly among people experiencing homelessness. 33 HCV point of care testing, when available, can be used to shorten the time to HCV diagnosis and initiation of treatment and to monitor for reinfection. 34 Lastly, funding for HCV elimination efforts, innovative treatment approaches like shorter-duration treatment options and long-acting injectables, and research into effective HCV vaccines must be prioritized.
Acknowledgments
CONFLICTS OF INTEREST
Jennifer Price received grants from Gilead, AbbVie, Vir, Zydus, and Genentech. The remaining author has no conflicts to report.
Footnotes
Abbreviations: DAA, direct-acting antiviral; IDU, injection drug use; OAT, opioid agonist therapy; PWID, people who inject drugs; PY, person years; SVR, sustained virologic response.
Contributor Information
Rebecca G. Kim, Email: rebecca.g.kim@hsc.utah.edu.
Jennifer C. Price, Email: Jennifer.Price@ucsf.edu.
REFERENCES
- 1.Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet. 2011;378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . National Progress Report 2025: Goal Reduce estimated* new hepatitis C virus infections by ≥20%. Accessed March 2, 2024. https://www.cdc.gov/hepatitis/policy/npr/2020/NationalProgressReport-HepC-ReduceInfections.htm#print
- 4.Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:754–67. [DOI] [PubMed] [Google Scholar]
- 5.Litwin AH, Lum PJ, Taylor LE, Mehta SH, Tsui JI, Feinberg J, et al. Patient-centred models of hepatitis C treatment for people who inject drugs: A multicentre, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2022;7:1112–7. [DOI] [PubMed] [Google Scholar]
- 6.Morris MD, McDonell C, Luetkemeyer AF, Thawley R, McKinney J, Price JC. Community-based point-of-diagnosis hepatitis C treatment for marginalized populations: A nonrandomized controlled trial. JAMA Netw Open. 2023;6:e2338792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajarizadeh B, Grebely J, Martinello M, Matthews GV, Lloyd AR, Dore GJ. Hepatitis C treatment as prevention: Evidence, feasibility, and challenges. Lancet Gastroenterol Hepatol. 2016;1:317–27. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(suppl 2):S80–89. [DOI] [PubMed] [Google Scholar]
- 9.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: A systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajarizadeh B, Cunningham EB, Valerio H, Martinello M, Law M, Janjua NZ, et al. Hepatitis C reinfection after successful antiviral treatment among people who inject drugs: A meta-analysis. J Hepatol. 2020;72:643–57. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham EB, Hajarizadeh B, Amin J, Hellard M, Bruneau J, Feld JJ, et al. Reinfection following successful direct-acting antiviral therapy for hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2021;72:1392–1400. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini-Hooshyar S, Hajarizadeh B, Bajis S, Law M, Janjua NZ, Fierer DS, et al. Risk of hepatitis C reinfection following successful therapy among people living with HIV: A global systematic review, meta-analysis, and meta-regression. Lancet HIV. 2022;9:e414–27. [DOI] [PubMed] [Google Scholar]
- 13.Carson JM, Dore GJ, Lloyd AR, Grebely J, Byrne M, Cunningham E, et al. Hepatitis C virus reinfection following direct-acting antiviral treatment in the prison setting: The SToP-C study. Clin Infect Dis. 2022;75:1809–19. [DOI] [PubMed] [Google Scholar]
- 14.Young J, Wang S, Lanièce Delaunay C, Cooper CL, Cox J, Gill MJ, et al. The rate of hepatitis C reinfection in Canadians coinfected with HIV and its implications for national elimination. Int J Drug Policy. 2023;114:103981. [DOI] [PubMed] [Google Scholar]
- 15.Lindqvist K, Thorin Z, Kåberg M. Real-world hepatitis C treatment outcomes and reinfections among people who inject drugs at a needle and syringe program in Stockholm, Sweden. Harm Reduct J. 2023;20:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Sullivan M, Jones AM, Mourad A, Haddadin Y, Verma S. Excellent hepatitis C virus cure rates despite increasing complexity of people who use drugs: Integrated-Test-stage Treat study final outcomes. J Viral Hepat. 2024;31:66–77. [DOI] [PubMed] [Google Scholar]
- 17.Martinello M, Carson JM, Van Der Valk M, Rockstroh JK, Ingiliz P, Hellard M, et al. Reinfection incidence and risk among people treated for recent hepatitis C virus infection. AIDS. 2023;37:1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacks-Davis R, van Santen DK, Boyd A, Young J, Stewart A, Doyle JS, et al. Changes in incidence of hepatitis C virus reinfection and access to direct-acting antiviral therapies in people with HIV from six countries, 2010-19: An analysis of data from a consortium of prospective cohort studies. Lancet HIV. 2024;11:e106–16. [DOI] [PubMed] [Google Scholar]
- 19.Beiser ME, Shaw LC, Shores SK, Carson JM, Hajarizadeh B. Hepatitis C virus reinfection in a real-world cohort of homeless-experienced individuals in Boston. Clin Infect Dis. 2023;77:46–55. [DOI] [PubMed] [Google Scholar]
- 20.Lens S, Miralpeix A, Gálvez M, Martró E, González N, Rodríguez-Tajes S, et al. HCV microelimination in harm reduction centres has benefits beyond HCV cure but is hampered by high reinfection rates. JHEP Rep. 2022;4:100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulkind J, Stephens B, Ahmad F F, Johnston L, Hutchinson S, Thain D, et al. High response and re-infection rates among people who inject drugs treated for hepatitis C in a community needle and syringe programme. J Viral Hepat. 2019;26:519–28. [DOI] [PubMed] [Google Scholar]
- 22.Dore GJ. HCV reinfection as a positive indication of high-risk population treatment access. J Viral Hepat. 2019;26:516–8. [DOI] [PubMed] [Google Scholar]
- 23.Hellard M, Rolls DA, Sacks‐Davis R, Robins G, Pattison P, Higgs P, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60:1861–70. [DOI] [PubMed] [Google Scholar]
- 24.Karasz A, Merchant K, Singh R, Thomas A, Borsuk C, McKee D, et al. The experience of re-infection among people who inject drugs successfully treated for hepatitis C. J Subst Use Addict Treat. 2023;146:208937. [DOI] [PubMed] [Google Scholar]
- 25.Herink MC, Geddes J, Vo K, Zaman A, Hartung DM. Effect of relaxing hepatitis C treatment restrictions on direct-acting antiviral use in a Medicaid program: An interrupted time series analysis. J Manag Care Spec Pharm. 2021;27:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson JM, Hajarizadeh B, Hanson J, O'Beirne J, Iser D, Read P, et al. Retreatment for hepatitis C virus direct acting antiviral therapy virological failure in primary and tertiary settings: The REACH-C cohort. J Viral Hepat. 2022;29:661–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arum C, Fraser H, Artenie AA, Bivegete S, Trickey A, Alary M, et al. Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: A systematic review and meta-analysis. Lancet Public Health. 2021;6:e309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudyal V, MacLure K, Buchanan C, Wilson L, Macleod J, Stewart D. ‘When you are homeless, you are not thinking about your medication, but your food, shelter or heat for the night’: Behavioural determinants of homeless patients’ adherence to prescribed medicines. Public Health. 2017;148:1–8. [DOI] [PubMed] [Google Scholar]
- 29.CDC 24/7: Saving Livers, Protecting People. Too Few People Treated for Hepatitis C. Updated 9/21/22. Accessed March 2, 2024. https://www.cdc.gov/vitalsigns/hepc-treatment/index.html#bedone
- 30.Division of Viral Hepatitis: 2025 Strategic Plan. CDC. Accessed March 2, 2024. https://www.cdc.gov/hepatitis/pdfs/DVH-StrategicPlan2020-2025.pdf
- 31.Arora S, Kalishman S, Thornton K, Dion D, Murata G, Deming P, et al. Expanding access to hepatitis C virus treatment—Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52:1124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talal AH, Markatou M, Liu A, Perumalswami PV, Dinani AM, Tobin JN, et al. Healthcare access through facilitated telemedicine for underserved populations: A stepped wedge cluster randomized controlled trial of hepatitis C virus treatment among persons with opioid use disorder [abstract #53]. The Liver Meeting 2023 American Association for the Study of Liver Disease. Boston, MA: 2023. November 10-14 PMID: 38568601. [Google Scholar]
- 33.Morris MD, McDonell C, Kim RG, Laguardia Y, Kanner R, Price JC. A pilot study to understand and respond to loss, theft, and misplacement of hepatitis C treatment medication for people who inject drugs. Clin Liver Dis (Hoboken). 2023;22:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trickey A, Fajardo E, Alemu D, Artenie AA, Easterbrook P. Impact of hepatitis C virus point-of-care RNA viral load testing compared with laboratory-based testing on uptake of RNA testing and treatment, and turnaround times: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:253–70. [DOI] [PubMed] [Google Scholar]