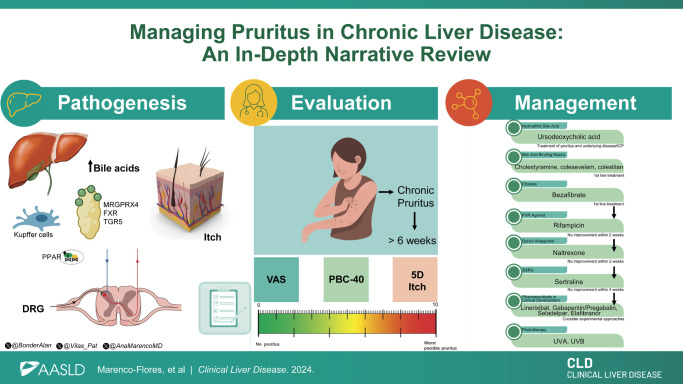
Abstract
INTRODUCTION
Pruritus is a prevalent symptom often linked to immune-mediated cholestatic liver diseases, such as primary biliary cholangitis (PBC). 1 Approximately 70% of patients diagnosed with PBC experience pruritus over their lifetime, and nearly 35% have refractory pruritus.2,3 In chronic liver disease, pruritus is intricately linked to both intrahepatic and extrahepatic cholestatic pathologies, with notable examples including PBC and primary sclerosing cholangitis. 4 Additionally, specific entities, such as intrahepatic cholestasis of pregnancy (ICP), contribute to pruritus etiology through mechanisms involving hepatocyte secretory failure and bile duct impairment. 4
Pruritus is a disabling symptom that can significantly impact one’s quality of life. Notably, intense nighttime itching, which is moderate to severe, results in sleep deprivation and worsens fatigue.5,6 These elements ultimately contribute to the emergence of depression and, in some cases, suicidal ideation. 7 Due to the subjective nature of pruritus, there is added difficulty in determining its etiology and severity. 8
Pharmacological treatments for pruritus, as per guideline recommendations, consist of bile acid–binding agents, rifampin, opioid antagonists, and sertraline. 2 Alternate therapies must be considered in patients who have pruritus and are refractory to these treatments. Many aspects of pruritus pathophysiology and the precise mechanisms of certain treatments are still under investigation. Addressing these often-unbearable symptoms demands close and ongoing monitoring, underpinned by a comprehensive, multidisciplinary approach that complements pharmaceutical interventions.
This review summarizes the clinical features, diagnosis, and current treatment options, along with a glimpse into potential future therapies for pruritus in patients with chronic liver disease.
PATHOGENESIS
The precise mechanisms of pruritus in cholestatic liver diseases remain to be definitively established; however, several potential mechanisms have been proposed.
First, it is known that bile acids are elevated in cholestasis and activate various receptors implicated in the transmission of itch signals, such as Takeda G protein–coupled receptor 5, MAS-related GPR Family Member X4, and farnesoid X receptor. 9 Nevertheless, studies examining the concentrations of bile acids in the skin of patients with chronic cholestasis and their correlation with the presence and severity of pruritus have yielded inconclusive results. 9 Similarly, studies exploring whether elevated bilirubin correlates to the itch associated with cholestasis have produced mixed findings. 10
Second, due to the antipruritic effects observed with opioid receptor antagonists and selective serotonin reuptake inhibitors, there is a hypothesis that pruritogens signal through endogenous opioidergic and serotonergic systems. 10
Third, an alternative avenue is the lysophosphatidic acid (LPA) and autotaxin (ATX) pathways. 10 LPA, generated by ATX activity, is found at elevated levels in the serum of patients with cholestatic diseases, and it has been associated with pruritus.10,11 The mechanism linking ATX-LPA activity and cholestatic liver disease remains unknown. 12 The association was identified when Kremer et al 11 observed intradermal LPA-induced scratch responses in mice. Subsequently, the same author 13 demonstrated that elevated ATX levels were specific to pruritus in cholestasis and not present in other forms of pruritus. Despite significant efforts in immunobiology, substantial gaps persist in comprehending the mechanisms of PBC-associated pruritus. 12
Furthermore, it has been established that changes in steroid and steroid metabolite levels, as well as lipophospholipids like lysophosphatidylcholine, may play a role in the development of pruritus in cholestatic liver diseases. 11
Clinical trials investigating the impact of farnesoid X receptor agonism have identified increased IL-31 expression in patients with cholestatic liver disease. This suggests a positive correlation between FXR agonism and elevated IL-31 levels, highlighting IL-31 as a trigger for pruritus in patients with PBC. 14 Importantly, medications with antipruritic effects, such as seladelpar, have demonstrated a dose-dependent reduction in IL-31 levels in patients with PBC, further supporting the multifactorial role of IL-31 in cholestatic pruritus. 15
Lastly, peroxisome proliferator-activated receptor (PPAR) α and δ are ligand-dependent transcription factors integral in regulating inflammatory processes and have emerged as crucial players in the pathogenesis of various skin diseases. 16 PPARs, as members of the nuclear receptor family, consist of 3 primary isoforms: PPARα, PPARβ/δ, and PPARγ. 17 PPAR-agonists have demonstrated beneficial effects on bile acid metabolism. For example, PPARγ agonists ease biliary inflammation in patients with PBC by curbing inflammatory factor expression. 17 In a mouse model simulating bile duct ligation, reduced PPARγ expression in KCs was associated with a decreased inhibitory effect on inflammatory cytokines. 18
PPARs possess notable potential as targets for the treatment of common skin diseases, including atopic dermatitis, psoriasis, and pruritus. It has been reported that PPARs are expressed in the itch sensory fibers and dorsal root ganglion. 16 Dorsal root ganglion neurons innervate the skin through C fibers and can be stimulated by exogenous or endogenous pruritogens released by immune cells, keratinocytes, or primary neuronal afferent terminals. However, the regions in the central nervous system that are involved in the itching process are poorly understood. 16
Moreover, a significant reduction in PPARγ expression was observed in the biliary epithelial cells of individuals with PBC. 18 Encouragingly, medications like seladelpar or elafibranor, targeting PPAR agonism, have shown promise in improving cholestatic pruritus, affirming a positive correlation between PPAR and this distressing symptom.15,19
EVALUATION OF PRURITUS
Individuals with cholestatic disorders frequently experience chronic pruritus, characterized by itching sensations that persist for longer than 6 weeks. 7 Pruritus is often a symptom of systemic illnesses, requiring an initial evaluation for other potential causes. 3 The initial evaluation of a patient experiencing pruritus should involve ruling out alternative dermatological and systemic factors. 7
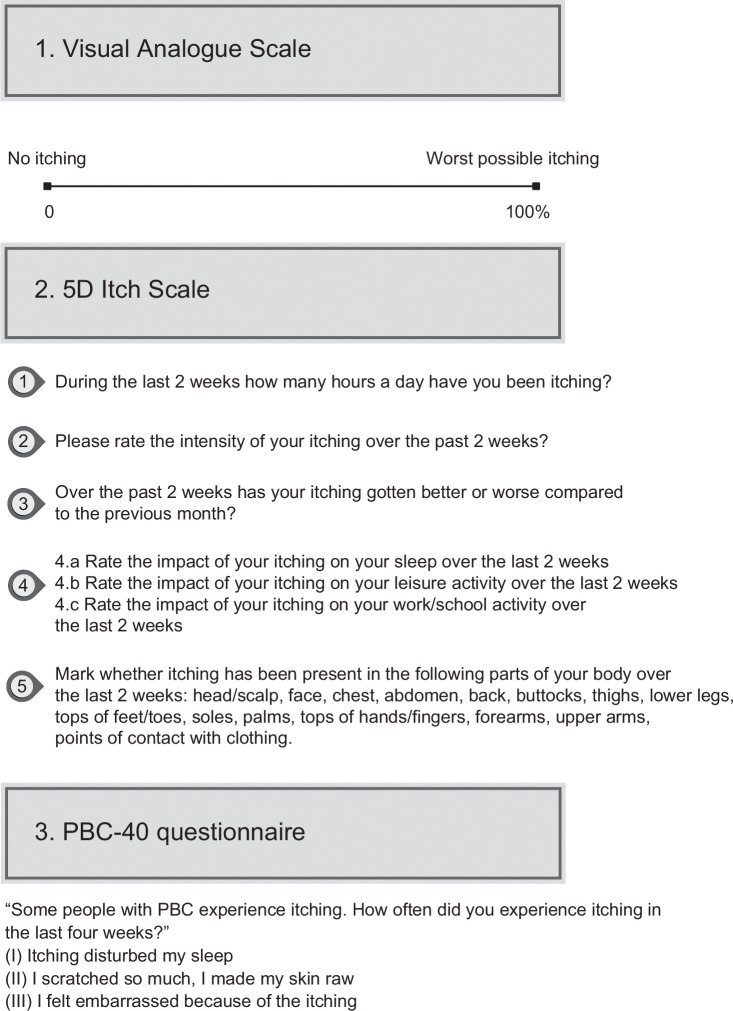
The inherent subjectivity of pruritus poses a considerable challenge in both evaluating its severity and devising effective treatment strategies. Therefore, simple and efficient questionnaires, like the Visual Analog Scale (VAS), PBC-40 questionnaire, and 5D itch scale, are preferred (Figure 1). 20
FIGURE 1.
Assessment of the severity of pruritus. Visual Analog Scale: 0% = no pruritus; 10%–30% = mild pruritus; 40%–60% = moderate pruritus; 70%–80% = severe pruritus; 90%–100% = very severe pruritus. 5D Itch Scale: The domain scores are added together, potentially ranging from 5 (no pruritus) to 25 (most severe pruritus). PBC-40: Items are scored from 1 to 5 (never, rarely, sometimes, most of the time, and always), and the individual item scores are summed to give a total domain. Abbreviation: PBC, primary biliary cholangitis.
VAS categorizes pruritus intensity, with 0% indicating the absence of pruritus, >0%–<40% percentage representing mild pruritus, ≥40 to <70 percentage denoting moderate pruritus, ≥70 to <90 points signifying severe pruritus, and ≥90 points indicating a state of very severe pruritus. 21
The 5-D itch questionnaire, a concise one-page tool, comprises 5 domains: duration, degree, direction, disability, and distribution, with each domain accounting for 5 points. 22 The domain scores are then added together for a total 5-D score, potentially ranging from 5 (no pruritus) to 25 (most severe pruritus). This survey assesses itch over the last 14 days.22,23
The PBC-40 includes 40 questions covering 6 domains related to PBC: general symptoms, itch, fatigue, cognition, social, and emotional. 23 Items are scored from zero or 1–5, contributing to total domain scores. This survey assesses symptoms over the past 4 weeks; clinically significant itch is defined as ≥7 points in the itch domain, while mild itch is ≥1 and <7 out of a maximum of 15. 23
MEDICAL THERAPIES
Various medical treatments, including bile acid–binding resins, rifampicin, and opioid receptor antagonists, have been studied for pruritus in chronic liver disease. Nonpharmacological approaches such as phototherapy are also highlighted. Here, we discuss some of these medical therapies for treating pruritus.
Bile acid–binding resins
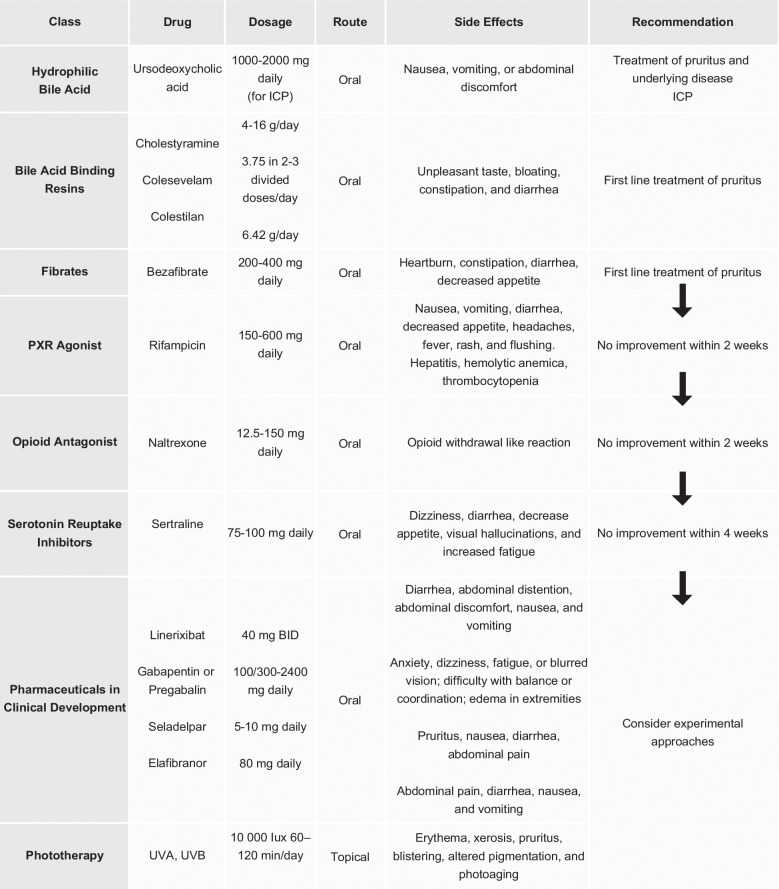
Cholestyramine, an anion-exchange resin, is the primary choice for cholestatic pruritus management (Figure 2). 6 This therapy eases symptoms by sequestering systemic bile salts.2,23 The proposed dose for cholestyramine in pruritus management is 4–16 g/d. 1 A double-blind study with microporous cholestyramine demonstrated its superiority over placebo in reducing itching intensity (p<0.01) and serum bile acid levels (p<0.01). 24 In a meta-analysis of 5 randomized controlled trials (RCTs), 3 showed significant pruritus reduction, while high-bias trials reported ns results. Zuin et al 25 RCT revealed cholestyramine significantly reduced pruritus by 45.5% versus 36.8% with the use of diethylaminoethyl-dextran (p<0.001). Diethylaminoethyl-dextran, a polycationic derivative of dextran, is used as an adjuvant in immunotherapeutics and has been reported to enhance interferon production induced by various mechanisms. 26
FIGURE 2.
Current therapeutic recommendation for the management of pruritus in cholestatic liver diseases. Abbreviations: ICP, intrahepatic cholestasis of pregnancy; mg, milligrams; PXR, pregnane X receptor.
Less commonly used bile acid resins include colesevelam (3.75 g in 2–3 divided doses/day) and colestilan (6.42 g/d). 20 However, in an RCT, colesevelam showed a 40% reduction in VAS in 3 weeks, which was not significant when compared to placebo.20,27 More studies are needed to evaluate the benefits of this medication. Another RCT using colestilan demonstrated better results, showing a significant reduction in the Numeric Rating Scale (NRS) for pruritus compared to placebo (p<0.001). 28 Another bile acid resin use, colestipol, is less firmly established for PBC-pruritus usage. 28 Side effects include an unpleasant taste (cholestyramine) and bloating, constipation, and diarrhea (less with colesevelam). 20
Fibrates
Fibrates, along with bile acid resins, can also be considered the first choice for the management of pruritus. 23 Bezafibrate is the most used, starting with 200 mg up to a maximum of 400 mg daily. 7 Recent studies have investigated bezafibrate, a nonselective PPAR agonist, to assess its potential as a treatment for cholestasis, inflammation, and itching in people with cholestatic diseases. 3 In one study by de Vries et al, 29 involving 74 participants, bezafibrate treatment led to a significant reduction in VAS scores (a reduction of ≥50% in pruritus) in patients with PBC and primary sclerosing cholangitis, as compared to the placebo group (45% vs. 11%; p=0.003) after 3 weeks of treatment.
Similarly, a systematic literature review and meta-analysis, which encompassed all accessible double-blind, randomized, placebo-controlled clinical trials assessing the effectiveness of pharmacotherapy for symptomatically managing pruritus in PBC using the VAS, determined that bezafibrate had a significant positive impact on pruritus. 2 The analysis revealed that bezafibrate significantly improved pruritus compared to placebo, with a CI of −1.41 to −0.68. 2
Fenofibrate can be considered an alternative, though its antipruritic efficacy is not as firmly established. 7 Clinical trials investigating the use of fenofibrate in cholestatic pruritus are currently unavailable.30,31 However, a retrospective study by Lemoinne et al, 32 including 20 patients with primary sclerosing cholangitis who did not respond adequately to ursodeoxycholic acid (UDCA) monotherapy, was conducted to assess the impact of fibrates (fenofibrate micronized 200 mg/d or bezafibrate 400 mg/d) in combination with UDCA. About 88% of the patients experienced significantly decreased pruritus intensity, including 3 who reported complete remission (p=0.021). 32
URSODEOXYCHOLIC ACID
Although UDCA does not benefit cholestatic chronic pruritus, it is included in this review due to its use in ICP. A meta-analysis by Bacq et al 33 supports the efficacy of UDCA in reducing pruritus and improving liver markers in ICP. The recommended dose is 2 daily oral tablets (500 mg UDCA twice daily). Health care professionals may increase the dose by 1 tablet every 3–14 days, up to a maximum of 4 daily tablets (2000 mg UDCA daily). 33
Moreover, in the PITCHES trial conducted from December 2015 to August 2018, 605 women were enrolled and randomly assigned to receive either UDCA (n=305) or placebo (n=300). 34 In this RCT, the postrandomization maternal itch score was lower in the UDCA group compared to the placebo group (mean difference −5·7 [95% CI: −9·7 to −1·7], p=0·0054), which was less than the 30 points difference predetermined by clinicians and women as clinically meaningful.34,35 Therefore, the 5-point reduction in itch score reported in this RCT is unlikely to be clinically useful. 34
In a systematic review by Ovadia et al, 36 women with intrahepatic cholestasis of pregnancy who were treated with UDCA had lower rates of preterm birth and a composite outcome of stillbirth and preterm birth than did women who were not treated with UDCA. This study showed the benefit of UDCA treatment in reducing spontaneous preterm birth was statistically significant for women with higher bile acid concentrations (≥40 μmol/L). 36 Adverse events in pregnancy are rare, though some women may have gastrointestinal side effects like diarrhea; the largest trial to date showed no significant differences in reported side effects between UDCA and placebo groups. 37
Rifampicin
It is a second-line treatment for patients who are intolerant or refractory to bile acid resins. 9 It can be used starting with a dose of 150 mg up to 600 mg daily. 7 The antipruritic effect of rifampicin occurs through pregnane X receptor agonism, leading to the downregulation of ATX transcription in HepG2 cells. These cells, which are nontumorigenic and commonly used in metabolism studies, exhibit high proliferation.3,11 A comprehensive meta-analysis assessing the effectiveness of pharmacotherapy for symptomatically managing PBC through the use of the VAS revealed that rifampicin had a significant positive impact on pruritus (95% CI: −5.78 to −0.80). 2
Opioid antagonists
An increase in opioidergic tone has been observed in patients with pruritus. 3 This has culminated in the use of nonselective or κ-selective opioid receptor antagonists as a therapeutic approach to cholestatic pruritus. 38 Additionally, naltrexone, with its mechanism involving mu-opioid receptor antagonism, has also been used. It can be started with a dose of 12.5 mg up to 150 mg daily. 7
In a meta-analysis involving a 4-week intervention with 22 patients diagnosed with PBC, the assessment of pruritus scores demonstrated a significant reduction when oral medications (naltrexone and nalmefene) and i.v. naloxone was administered compared to the control placebo group. 2
Likewise, in a crossover, double-blind, placebo-controlled study, VAS exhibited statistically significant improvements using naltrexone when compared to the placebo group (p<0.0003). 39 Among patients receiving naltrexone, 45% experienced a reduction in pruritus of >50% when compared to their baseline, with 5 of them reporting a complete disappearance of pruritus. 39
In a placebo-controlled RCT involving 34 cholestatic patients experiencing pruritus, 47% exhibited side effects that were predominantly mild and transient, requiring no additional therapy in this study. 40 Two patients ceased treatment due to severe opioid withdrawal effects. Notably, naltrexone demonstrated a favorable safety profile compared to nalmefene, manifesting less severe complications in this study. 40
Sertraline
The serotonergic pathway might also contribute to the development of cholestatic pruritus. 3 Sertraline, suggested as a fourth-line treatment by both the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) guidelines, has shown effectiveness in pruritus treatment, although its antipruritic mechanisms are not yet fully understood. 20 A study sought to determine the effective dose of sertraline for cholestatic pruritus in 21 subjects with chronic pruritus related to liver diseases. It involved an open-label dose escalation phase to find the optimal dose, followed by a randomized, double-blind, placebo-controlled trial. The study showed that a sertraline dose of 75–100 mg/d was well tolerated and resulted in improved itch scores compared to a placebo (p=0.009). 41
Sertraline at 75 mg/d for 4 weeks showed the most improvement in itching, surpassing the effects of 25–50 mg/d doses. 41 Additionally, some nonresponsive patients responded positively to 100 mg/d for the same duration.
PHARMACEUTICALS IN CLINICAL DEVELOPMENT
Linerixibat
Recent insights into bile acid physiology and cholestatic pruritus pathophysiology hold promise for the development of novel therapies. Ileal bile acid transporter (IBAT) inhibitors, which decrease bile acid reabsorption, enhance excretion in stool, and disrupt enterohepatic circulation. 7 IBAT inhibitors, particularly linerixibat, have resulted in a significant reduction in pruritus compared to a placebo. 2 Collective analysis of IBAT inhibitors showed a positive trend in pruritus improvement; this was notably influenced by the GLIMMER trial. 2 This trial suggests that especially linerixibat at 40 mg twice a day (BID) significantly improved mean worst daily itch (MWDI) scores in patients with moderate to severe pruritus compared to placebo (mean change: −1.64, 95% CI: −3.19 to −0.10). 2 The monthly itch score also showed significant differences between placebo and linerixibat (180 mg every day, 40 mg BID, and 90 mg BID). 2 These findings suggest efficacy in relieving pruritus with linerixibat, particularly at a dose of 40 mg BID. 2 Side effects associated with IBAT inhibitors include abdominal pain and diarrhea. 42
Gabapentin or pregabalin
Anticonvulsants such as gabapentin and pregabalin, commonly used for neuropathic pain and chronic kidney disease–associated pruritus, have shown discouraging outcomes in hepatic pruritus treatment.24,29 The recommended starting dose is 100–300 mg daily, up to a maximum of 2400 mg daily. 7 Notably, a RCT conducted by Bergasa et al 43 found that pregabalin yielded negative results when compared to a placebo in a cohort of 16 patients over a 2-week period, as assessed by mean hourly scratching activity. Surprisingly, placebo demonstrated significantly superior efficacy than pregabalin at the end of the follow-up (mean hourly scratching activity=88.50 vs. 15.34). 43 It is essential to acknowledge the study’s limitations, including the small patient size and short duration, necessitating further investigations to understand the therapeutic potential of anticonvulsants for pruritus.
Seladelpar
Seladelpar, a PPAR-δ agonist, has shown promising efficacy in the management of pruritus in cholestatic liver disease.15,16 In a placebo-controlled, randomized trial, PBC patients with moderate to severe pruritus at baseline were administered seladelpar at a dose of 5 and 10 mg daily. 15 The results revealed a significant reduction in pruritus NRS in the seladelpar 10-mg group compared to the placebo group at month 3, with a noteworthy 36.8% of patients achieving a clinically relevant ≥4-point reduction in pruritus NRS. 15 Importantly, this medication exhibited improvements in the 5-domain itch scale and the PBC-40 itch domain scores. 14 Beyond its anticholestatic effects and reduction in liver injury signs, seladelpar’s efficacy in addressing pruritus suggests its potential role as a second-line therapy for managing both disease activity and symptoms in patients with PBC. 15 Ongoing research, including a 52-week phase 3 study (NCT04620733), aims to further confirm the efficacy and safety of seladelpar in PBC management.
Elafibranor
Elafibranor, a dual PPAR-α and δ agonist, demonstrated notable effectiveness in managing pruritus among patients with moderate to severe symptoms associated with PBC. 19 In a placebo-controlled RCT spanning 52 weeks using elafibranor 80 mg daily, secondary end points, including normalization of alkaline phosphatase levels and changes in pruritus intensity measured by the Worst Itch Numeric Rating Scale, showed a positive tendency supporting the medication. 19 However, the most substantial improvement was observed in patient-reported outcomes related to pruritus, assessed with the PBC-40 questionnaire, significantly favoring elafibranor 80 mg (mean difference, −2.3; 95% CI, −4.0 to −0.7). 19 These findings demonstrate the comprehensive therapeutic effects of this medication in addressing both biochemical and pruritus-related aspects of PBC.
LIVER TRANSPLANTATION
In cases where persistent pruritus remains unresponsive to therapy, liver transplantation can represent an appropriate course of action, even when liver failure is not apparent. 20
ULTRAVIOLET B PHOTOTHERAPY
The mechanism by which ultraviolet B phototherapy exerts antipruritic effects remains unknown. According to an observational case series, the median VAS showed a significant decrease from 8.0 to 2.0 after treatment with phototherapy (0<0.0001). 44
OTHER NONPHARMACOLOGIC THERAPIES
Patients who do not respond to or experience intolerable side effects from standard treatments should be directed to specialized centers for potential participation in clinical trials or experimental interventions, such as extracorporeal albumin dialysis (molecular adsorbent recirculating system or prometheus dyalisis), nasobiliary drainage, plasmapheresis, plasma separation, or anion absorption. 6 However, these therapies are beyond the scope of this review due to the limited data supporting their long-term efficacy and safety in PBC-related pruritus.
CONCLUSIONS
Managing pruritus in PBC remains challenging, as a significant number of patients still endure this unbearable symptom. The lack of effective, long-term treatments is a consequence of an incomplete understanding of the mechanisms behind cholestatic pruritus. Further research is needed to better enhance treatment options for those with chronic liver disease, ultimately improving their quality of life.
Acknowledgments
CONFLICTS OF INTEREST
Alan Bonder consults for and received grants from GSK, Intercept, Mirum, and Ipsen. He consults for Zydus, ChemomAb, CymbaBay, Gilead and CARA. He received royalties or holds licenses from UpToDate and DynaMed. He has given expert testimony for Expert Review, Inc. He has other interests with Pfizer. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ATX, autotaxin; BID, twice a day; EASL, European Association for the Study of the Liver; IBAT, ileal bile acid transporter; ICP, intrahepatic cholestasis of pregnancy; LPA, lysophosphatidic acid; NRS, Numeric Rating Scale; PBC, primary biliary cholangitis; PPAR, peroxisome proliferator-activated receptor; RCT, randomized controlled trials; UDCA, ursodeoxycholic acid; VAS, Visual Analog Scale.
Contributor Information
Ana Marenco-Flores, Email: amarenco@bidmc.harvard.edu.
Leandro Sierra, Email: lsierrac@bidmc.harvard.edu.
Daniela Goyes, Email: daniela.goyes@yale.edu.
Tamara Kahan, Email: tkahan@bidmc.harvard.edu.
Vilas R. Patwardhan, Email: vpatward@bidmc.harvard.edu.
Alan Bonder, Email: abonder@bidmc.harvard.edu.
REFERENCES
- 1.Smith HT, de Souza AR, Thompson AH, McLaughlin MM, Dever JJ, Myers JA, et al. Cholestatic pruritus treatments in primary biliary cholangitis and primary sclerosing cholangitis: A systematic literature review. Dig Dis Sci. 2023;68:2710–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina-Morales E, Barba Bernal R, Gerger H, Goyes D, Trivedi HD, Ferrigno B, et al. Pharmacological therapy of pruritus in primary biliary cholangitis: Systematic review and meta-analysis of randomized clinical trials. J Clin Gastroenterol. 2022;57:143–152. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi HD, Lizaola B, Tapper EB, Bonder A. Management of Pruritus in Primary Biliary Cholangitis: A Narrative Review. Am J Med. 2017;130:744.e1–744.e7. [DOI] [PubMed] [Google Scholar]
- 4.Düll MM, Kremer AE. Newer approaches to the management of pruritus in cholestatic liver disease. Curr Hepatol Rep. 2020;19:86–95. [Google Scholar]
- 5.Selim R, Ahn J. Pruritus in chronic liver disease. Clin Liver Dis. 2023;27:47–55. [DOI] [PubMed] [Google Scholar]
- 6.Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: Emerging mechanisms and therapeutics. J Am Acad Dermatol. 2019;81:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Düll MM, Kremer AE. Evaluation and management of pruritus in primary biliary cholangitis. Clin Liver Dis. 2022;26:727–45. [DOI] [PubMed] [Google Scholar]
- 8.Bhalerao A, Mannu GS. Management of pruritus in chronic liver disease. Dermatol Res Pract. 2015;2015:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langedijk JAGM, Beuers UH, Oude-Elferink RPJ. Cholestasis-associated pruritus and its pruritogens. Front Med. 2021;8:639674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuers U, Wolters F, Oude-Elferink R. Mechanisms of pruritus in cholestasis: Understanding and treating the itch. Nat Rev Gastroenterol Hepatol. 2023;20:26–36. [DOI] [PubMed] [Google Scholar]
- 11.Kremer AE, Martens JJWW, Kulik W, Ruëff F, Kuiper EMM, van Buuren HR, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–18. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Zhang W, Evans JF, Floreani A, Zou Z, Nishio Y, et al. Pruritus and primary biliary cholangitis (PBC). Autoimmun Rev. 2016;15:795–800. [DOI] [PubMed] [Google Scholar]
- 13.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EMM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–400. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Wang Y, Khoshdeli M, Peach M, Chuang JC, Lin J, et al. IL-31 levels correlate with pruritus in patients with cholestatic and metabolic liver diseases and are farnesoid X receptor responsive in NASH. Hepatology. 2023;77:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschfield GM, Shiffman ML, Gulamhusein A, Kowdley KV, Vierling JM, Levy C, et al. ENHANCE Study Group*. Seladelpar efficacy and safety at 3 months in patients with primary biliary cholangitis: ENHANCE, a phase 3, randomized, placebo-controlled study. Hepatology. 2023;78:397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostadhadi S, Nikoui V, Haj-Mirzaian A, Kordjazy N, Dehpour AR. The role of PPAR-gamma receptor in pruritus. Eur J Pharmacol. 2015;762:322–5. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Zhang L, Jiang Z. The role of peroxisome proliferator‐activated receptors in the regulation of bile acid metabolism. Basic Clin Pharmacol Toxicol. 2024;134:315–324. [DOI] [PubMed] [Google Scholar]
- 18.Lv X. Decreased hepatic peroxisome proliferator-activated receptor-γ contributes to increased sensitivity to endotoxin in obstructive jaundice. World J Gastroenterol. 2011;17:5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, et al. Efficacy and safety of elafibranor in primary biliary cholangitis. N Engl J Med. 2024;390:795–805. [DOI] [PubMed] [Google Scholar]
- 20.Hegade VS, Bolier R, Oude-Elferink RP, Beuers U, Kendrick S, Jones DE. A systematic approach to the management of cholestatic pruritus in primary biliary cirrhosis. Frontline Gastroenterol. 2015;7:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich A, Heisig M, Phan N, Taneda K, Takamori K, Takeuchi S, et al. Visual Analogue Scale: Evaluation of the instrument for the assessment of pruritus. Acta Dermato Venereologica. 2012;92:497–501. [DOI] [PubMed] [Google Scholar]
- 22.Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: A new measure of pruritus. Br J Dermatol. 2009;162:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayo MJ, Carey E, Smith HT, Mospan AR, McLaughlin M, Thompson A, et al. Impact of pruritus on quality of life and current treatment patterns in patients with primary biliary cholangitis. Dig Dis Sci. 2023;68:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Padova C, Tritapepe R, Rovagnati P, Rossetti S. Double-blind placebo-controlled clinical trial of microporous cholestyramine in the treatment of intra- and extra-hepatic cholestasis: Relationship between itching and serum bile acids. Methods Find Exp Clin Pharmacol. 1984;6:773–6. [PubMed] [Google Scholar]
- 25.Zuin M, Grandinetti G, Camisasca M, et al. A comparison of cholestyramine and diethylaminoethyl-dextran for the treatment of hyperlipidemia and pruritus of primary biliary cirrhosis. Curr Ther Res. 1991;49:659–665. [Google Scholar]
- 26.Bakrania AK, Variya BC, Madan P, Patel SS. Repeated dose 28-day oral toxicity study of Deae-dextran in mice: An advancement in Safety Chemotherapeutics. Regul Toxicol Pharmacol. 2017;88:262–72. [DOI] [PubMed] [Google Scholar]
- 27.Yokomori H, Oda M, Ishii H. Effects of ursodeoxycholic acid and Colestilan versus ursodeoxycholic acid alone on serum bile acids and pruritus: A randomized, open-label study. Curr Ther Res. 2020;62:221–9. [Google Scholar]
- 28.Kuiper EMM, van Erpecum KJ, Beuers U, Hansen BE, Thio HB, de Man RA, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: Results of a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:1334–40. [DOI] [PubMed] [Google Scholar]
- 29.de Vries E, Bolier R, Goet J, Parés A, Verbeek J, de Vree M, et al. Fibrates for itch (fitch) in fibrosing Cholangiopathies: A double-blind, randomized, placebo-controlled trial. Gastroenterology. 2021;160:734–43. [DOI] [PubMed] [Google Scholar]
- 30.Ebhohon E, Chung RT. Systematic review: Efficacy of therapies for cholestatic pruritus. Therap Adv Gastroenterol. 2023;16:17562848231172829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dervout C, Boulais N, Barnetche T, Nousbaum J-B, Brenaut E, Misery L. Efficacy of treatments for cholestatic pruritus: A systemic review and meta-analysis. Acta Derm Venereol. 2022;102:adv00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemoinne S, Pares A, Reig A, Ben Belkacem K, Kemgang Fankem AD, Gaouar F, et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French–Spanish experience. Clin Res Hepatol Gastroenterol. 2018;42:521–8. [DOI] [PubMed] [Google Scholar]
- 33.Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: A meta-analysis. Gastroenterology. 2012;143:1492–501. [DOI] [PubMed] [Google Scholar]
- 34.Chappell LC, Bell JL, Smith A, Linsell L, Juszczak E, Dixon PH, et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (pitches): A randomised controlled trial. The Lancet. 2019;394:849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chappell LC, Gurung V, Seed PT, Chambers J, Williamson C, Thornton JG. Ursodeoxycholic acid versus placebo, and early term delivery versus expectant management, in women with intrahepatic cholestasis of pregnancy: Semifactorial randomised clinical trial. BMJ. 2012;344:e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovadia C, Sajous J, Seed PT, Patel K, Williamson NJ, Attilakos G, et al. Ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: A systematic review and individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chappell LC, Chambers J, Dixon PH, Dorling J, Hunter R, Bell JL, et al. Ursodeoxycholic acid versus placebo in the treatment of women with intrahepatic cholestasis of pregnancy (ICP) to improve perinatal outcomes: Protocol for a randomised controlled trial (pitches). Trials. 2018;19:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergasa NV. Effects of naloxone infusions in patients with the pruritus of cholestasis: A double-blind, randomized, controlled trial. Ann Int Med. 1995;123:161–7. [DOI] [PubMed] [Google Scholar]
- 39.Terg R, Coronel E, Sordá J, Muñoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37:717–22. [DOI] [PubMed] [Google Scholar]
- 40.Mansour-Ghanaei F. Effect of oral naltrexone on pruritus in cholestatic patients. World J Gastroenterol. 2006;12:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45:666–74. [DOI] [PubMed] [Google Scholar]
- 42.Al-Dury S, Marschall HU. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and Nash. Front Pharmacol. 2018;9:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergasa NV, McGee M, Ginsburg IH, Engler D. Gabapentin in patients with the pruritus of cholestasis: A double-blind, randomized, placebo-controlled trial. Hepatology. 2006;44:1317–23. [DOI] [PubMed] [Google Scholar]
- 44.Decock S, Roelandts R, Steenbergen WV, Laleman W, Cassiman D, Verslype C, et al. Cholestasis-induced pruritus treated with ultraviolet B phototherapy: An observational case series study. Hepatology. 2012;57:637–41. [DOI] [PubMed] [Google Scholar]