Abstract
The highly specialized non-myelinating glial cells present at somatic peripheral nerve endings, known collectively as terminal Schwann cells (TSCs), play critical roles in the development, function and repair of their motor and sensory axon terminals and innervating tissue. Over the past decades, research efforts across various vertebrate species have revealed that while TSCs are a diverse group of cells, they share a number of features among them. In this review, we summarize the state-of-knowledge about each TSC type and explore the opportunities that TSCs provide to treat conditions that afflict peripheral axon terminals.
Keywords: glia, synaptic function, skin, muscle, ALS, SMA
Terminal Schwann cells are an integral part of end organ structures
Axon terminals of the peripheral nervous system (PNS) are present in every organ of the vertebrate body. These axonal terminals are points of contact at which bidirectional information is relayed between peripheral tissues and the central nervous system (CNS). Within the somatic division of the PNS, axon terminals transmit motor information to skeletal muscles at the neuromuscular junction (NMJ) or receive various types of sensory information from hair follicles, Meissner corpuscles (MCs), Pacinian corpuscles (PCs), muscle spindles, Golgi tendon organs (GTOs), Merkel cell-neurite complexes, and free nerve endings (FNEs). Each of these peripheral targets of innervation, to which we will refer collectively as “end organ structures”, is highly specialized yet shares a common feature of containing a unique population of peripheral glia. These glial cells were first reported in 1878[1] and later dubbed “teloglia”[2,3]. Nomenclature for these cells further evolved when they began to be referred to as “terminal Schwann cells”[3–5], based on observations that they appear to originate from the same progenitors as Schwann cells (SCs) along the nerve[5–9]. Although other common names for these cells persist, we will use terminal Schwann cells (TSCs) as a unifying designation of any non-myelinating Schwann cell that is associated with an axon terminal, regardless of end organ structure, in this review.
The last few decades have provided insights into how TSCs influence the development and physiology of adult end organ structures. We have learned that their association with axon terminals differs between each unique end organ structure. At the NMJ, TSCs cover the entire axon terminal[10]; at MCs and PCs, they elaborate lamellae around the axon terminal[11–13]; they wrap around the low-threshold mechanoreceptor (LTMR) axon terminals associated with hair follicles[14]; and, finally, they extend fine processes around nociceptive FNEs in skeletal muscle, connective tissue, and the epidermis [15–18]. Despite their different anatomical locations and cellular features, evidence continues to indicate that TSCs share many functional features with each other and with CNS glia, particularly astrocytes and microglia[19]. These include active participation in synaptic development[20], facilitating and maintaining neural transmission[21,22], and repairing damages following injury [23,24]. They perform these functions by sensing neurotransmission[21,25], releasing cytokines[26], influencing synaptic competition[27], and removing supernumerary axons[28]. Additionally, there is growing evidence that TSCs may undergo a myriad of changes that cause them to lose their homeostatic functions in diseases and aging[29–31]. Despite advances in understanding the molecular makeup of TSCs, including single cell and spatial transcriptomics (Table 1), the mechanisms that specify their identity and function remain largely unknown.
Table 1.
Genes highly expressed in TSCs as compared to myelinating Schwann cells.
| Gene | End organ structure | Method* | Reference |
|---|---|---|---|
| Adgrg6 | NMJ | RNA seq, IHC | [35,127] |
| Agrn | NMJ | RNA seq, WB | [35,128] |
| Ajap1 | NMJ | RNA seq, FISH | [35,38] |
| Apod | NMJ | RNA seq, FISH | [35,38,129] |
| Aqp1 | FNE | IHC | [16] |
| Asic2 | MC, PC | IHC | [79,80] |
| Bche | FNE, MC, NMJ, PC | RNA-seq, IHC, enzymatic activity | [35,75,130,131] |
| Calb1 | MC, PC | IHC | [132] |
| Calb2 | MC, PC | IHC | [132] |
| Chrm5 | NMJ | IHC | [64] |
| Cspg4 (aka NG2) | NMJ | RNA seq, FISH, IHC | [26,32,35,38] |
| Egfr | MC, PC | IHC | [132] |
| Gfap | PC | IHC | [133] |
| Grin2a (aka NMDA2A) | Hair Follicle | IHC | _[59]_ |
| Grm1 | PC | IHC | [83] |
| Kcnj10 (aka Kir4.1) | NMJ | RNA seq, IHC | [35,52] |
| L1cam | FNE | IHC | [134] |
| Mcam (aka CD146) | FNE | IHC | [134] |
| Ncam1 | FNE, MC, NMJ, PC | RNA seq, IHC | [35,54,134,135] |
| Nes | Hair Follicle | IHC | [59,136] |
| Ngfr | FNE, MC,PC | IHC | [53,132,134] |
| Ntrk1 | MC, PC | IHC | [132] |
| P2ry1 | NMJ | RNA seq, IHC | [35,64] |
| Pvalb | MC, PC | IHC | [132] |
| Sema3a | NMJ | RNA seq, IHC | [35,109] |
| Slc17a1 | PC | IHC | [83] |
| Trpv4 | MC | IHC | [78] |
| Ush2a | Hair Follicle, MC | IHC, functional analysis | [84] |
| Vim | FNE, MC, PC | IHC | [54,132,134,137] |
RNA seq = RNA sequencing, IHC = immunohistochemistry, WB = western blot, FISH = fluorescence in situ hybridization, NMJ = neuromuscular junction, MC = Meissner corpuscle, PC = Pacinian corpuscle, FNE = free nerve ending.
Recently discovered genetic markers that are specific for TSCs (Table 2) make it possible to interrogate TSCs to the exclusion of myelinating SCs. These advances have opened new possibilities in TSC research. This review aims to summarize progress made about the origin and function of TSCs with a particular focus on the better understood end organ structures, including NMJs, MCs, PCs, FNEs, and hair follicles. We will synthesize data generated across vertebrate species, including frogs, birds, rodents, cats, and humans, although differences between human TSCs and other vertebrates have been noted[32,33]. We will document similarities and differences among TSCs and point out unresolved questions about TSCs and their roles at end organ structures. We will also highlight how maladaptive changes in TSCs could have negative consequences for the function and health of the end organ structure and the neuron that innervates it. Additionally, we will discuss how current understanding of TSCs is providing clues to treat acute and chronic conditions that impair the function of neurons and peripheral tissues.
Table 2.
Transgenic mouse lines validated for TSC expression.
| SC population expression | Mouse line | Identifier | Reference |
|---|---|---|---|
| TSCs | NG2-DsRed | IMSR_JAX:008241 | [26,35] |
| Non-Myelinating SCs | Kir4.1-CreER | N/A | [52] |
| All SCs | Dhh-Cre | IMSR_JAX:012929 | [127] |
| All SCs | Plp1-CreER | MGI:2663093 | [49] |
| All SCs | Plp1-CreER | IMSR_JAX:005975 | [13,16,36,40,63] |
| All SCs | Plp1-GFP | IMSR_JAX:033357 | [22] |
| All SCs | S100b-GFP | IMSR_JAX:005621 | [95,100,101] |
| All SCs | S100b-rtTa | N/A | [138] |
| All SCs | Sox10-CreER | MGI:5910373 | [16,17,49] |
| All SCs | Wnt1-Cre | IMSR_JAX:009107 | [49,59,65] |
Morphological diversity of TSCs
TSCs are present at end organ structures in both the motor (Figure 1) and sensory divisions of the somatic PNS (Figure 2). Two end organ structure types are found in the motor PNS, the NMJ (Figure 1B) and the muscle spindle. The NMJ consists of the axon terminal of an α-motor neuron, an extrafusal muscle fiber and approximately 3 TSCs, also referred to as perisynaptic Schwann cells, in young adult mice[26,34–36]. The NMJ is a cholinergic synapse in mammals responsible for initiating voluntary movements by causing muscle contraction[34]. At the NMJ, TSCs completely cover the arborized axon terminal and separate the synaptic cleft from the surrounding cellular and molecular milieu of skeletal muscles[10,34]. Muscle spindles consist of axon terminals from γ-motor neurons and proprioceptive sensory neurons as well as intrafusal muscle fibers[37]. Muscle spindles modulate voluntary movements and their γ-motor axon terminals are associated with TSCs[37,38].
Figure 1. TSC morphology at skeletal muscle end organ structures.
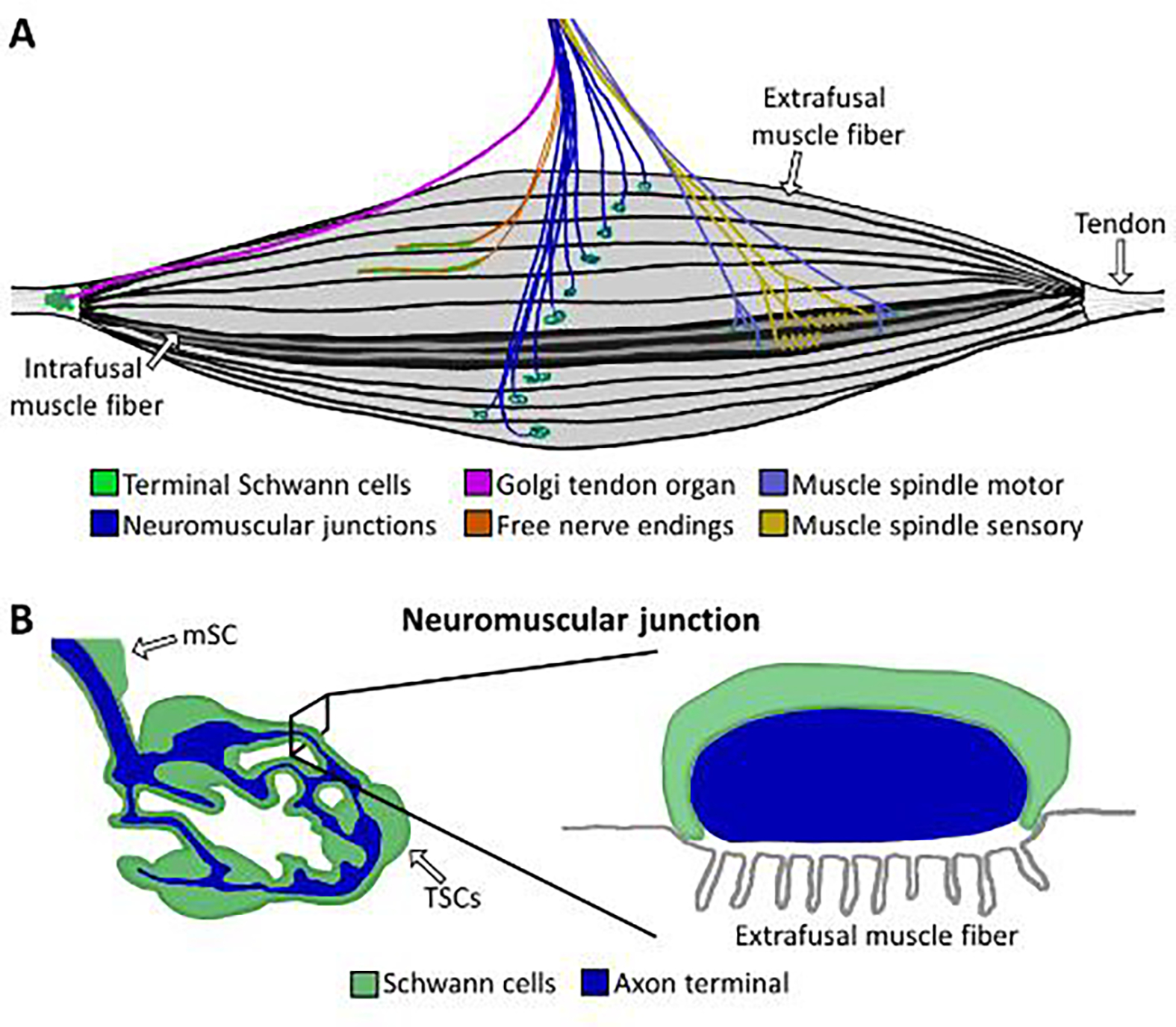
A) Innervation pattern of skeletal muscle, including neuromuscular junctions (dark blue), free nerve endings (orange), Golgi tendon organs (purple), and muscle spindles (yellow and light blue). B) The NMJ lies on an extrafusal muscle fiber and its axon terminal (blue) is entirely capped by TSC processes (green). In cross section, the axon terminal lies in the synaptic gutter (gray) and is capped by the TSC. mSCs = myelinating SCs.
Figure 2. TSC morphology at cutaneous end organ structures.
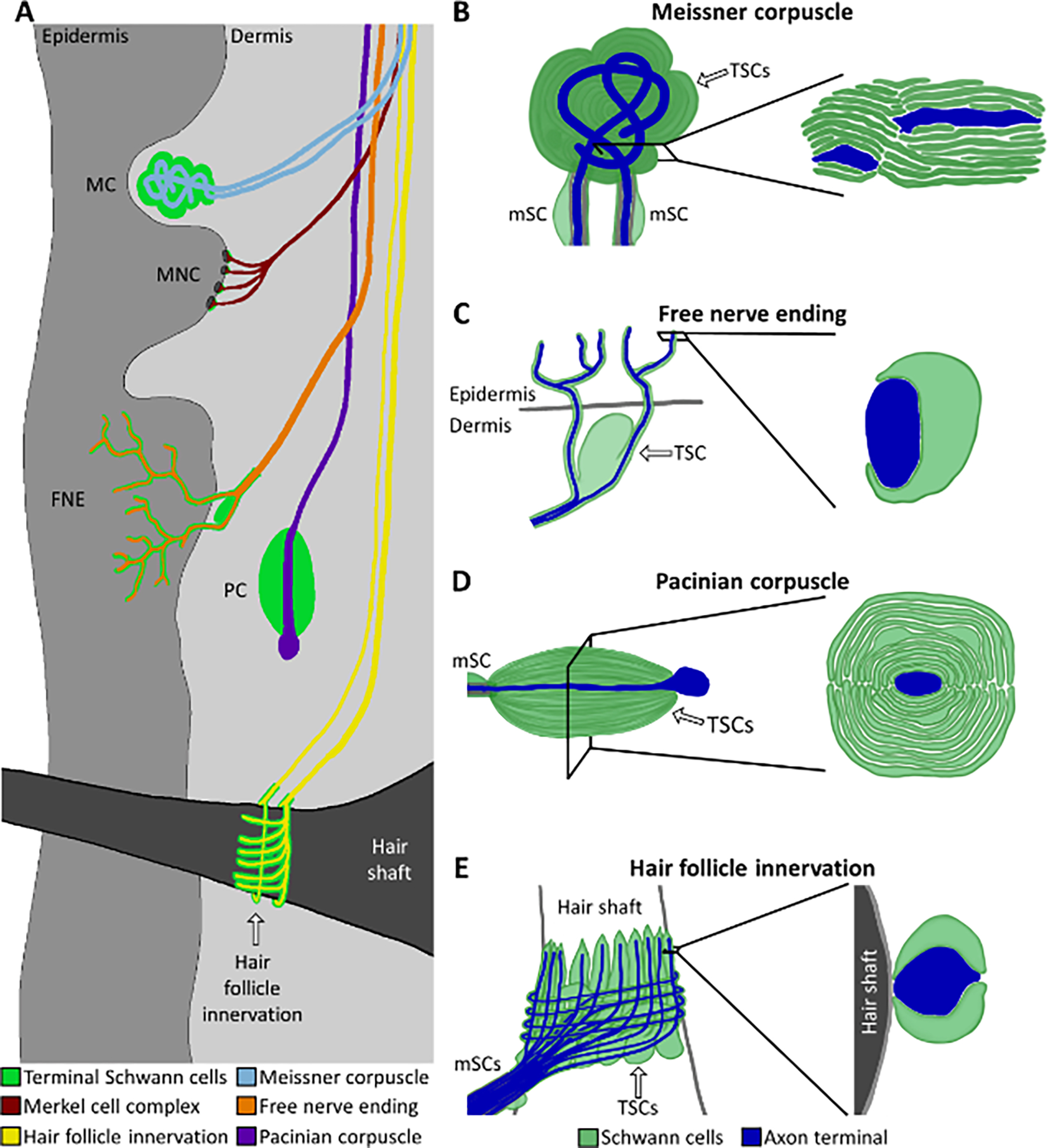
A) Peripheral nerve end organ structures in the skin, including Meissner corpuscles (MCs, blue), Merkel cell-neurite complexes (MNCs, maroon), free nerve endings (FNEs, orange), Pacinian corpuscles (PCs, purple), and hair follicles (yellow). B) MC innervated by two axon terminals. The TSCs produce lamellae in the form of a coin stack around the axon terminals. C) The complete coverage of FNE axon terminals in the skin by TSC processes transitions to partial coverage after crossing the dermal/epidermal border. D) In the PC, the axon terminal region is covered in TSC lamellae in the form of an onion bulb. E) Hair follicle innervated by lanceolate axon terminals running perpendicular to the follicle shaft and circumferential axon terminals wrapping around the follicle shaft (gray). In B-E, axon terminals are depicted in dark blue and TSC processes are depicted in green. mSCs = myelinating SCs.
The end organ structures of the sensory PNS are far more diverse than those of the motor system and, for the most part, do not utilize chemical synapses to transmit sensory information from peripheral tissues to the CNS. The largest group of end organ structures in the sensory PNS are in the skin and fall under the category of mechanoreceptors and nociceptors that are innervated by axon terminals from Aβ, Aδ and C sensory neurons[22,33]. These end organ structures include PCs, MCs, hair follicles, and FNEs[15,16,22], covering the sensory modalities of touch, vibration, skin stretch, pain, temperature, and proprioception. PCs (Figure 2D) are the largest of these end organ structures with axon terminals at the center surrounded by multiple thin layers of TSCs that together form an onion bulb-like structure. Each PC contains upwards of 70 TSCs and functions to transmit forces associated with vibration in the skin to the CNS[11,13,39]. MCs (Figure 2B) are comprised of one or two axon terminals that intermingle with around 4 TSCs that form stacked layers and occupy the bulk of the end organ structure[13]. MCs detect fine touch against the skin. Because of their layered morphology, TSCs of both the PC and MC have been classically referred to as lamellar cells[22]. Hair follicles (Figure 2E) contain axon terminals that are either parallel with the hair shaft, known as lanceolate nerve endings, or encircle it, known as circumferential nerve endings[13,40]. The nerves innervating hair follicles sense hair movement. These axon terminals intermingle with one another and are flanked by multiple TSCs that maintain distinct territories within the hair follicle structure[13,14]. However, individual TSCs simultaneously associate with multiple axon terminals[13,40]. FNEs (Figure 2C), which sense pain among other modalities, are classically called “free” because they do not terminate in some larger structure such as a corpuscle. Nevertheless, morphological studies indicate that the axon terminals of FNEs are associated with TSC processes throughout their length[15,16].
Far less is known about the TSCs associated with proprioceptive sensory end organ structures in skeletal muscle, which include muscle spindles and Golgi tendon organs (GTOs). GTOs are present near the interface of myofibers and tendons, known as the myotendinous junction, and relay information about the amount of force that is being placed on this junction as muscles are stretched[41]. The branched axon terminals in this organ are surrounded by as many as 15 TSCs[41]. Sensory axons in muscle spindles wrap around intrafusal muscle fibers and detect muscle stretch[42]. To date, TSCs have not been found associated with these types of proprioceptive sensory axon terminals[37].
Origin, differentiation, maturation, and expansion of TSCs
Origin of TSCs:
It is generally agreed that TSCs and other SCs found along the length of peripheral nerves are derived from neural crest cells (NCCs) (Figure 3). Studies across vertebrate species, including frogs, birds, and rodents, indicate that during embryonic development, NCCs exit the neural tube, attach to nascent axons and differentiate into multipotent Schwann cell precursors (SCPs)[43]. SCPs migrate along growing axons as they approach their peripheral tissues. During this time, they continuously proliferate, leaving a trail of multipotent cells along the way, which give rise to nerve SCs. It has been presumed that TSCs come from those same SCPs because TSC precursors have consistently been described in ultrastructural studies as having similar morphologies to SCPs[5,6,8,9,44–47]. However, direct experimental evidence of the origin of TSCs has been sparse with the notable exception of a lineage tracing study that showed TSCs of the avian PC analogue do in fact have the same origin as nerve SCs[48]. Lineage tracing via single cell transcriptomics in mice has also provided evidence that NMJ TSCs likely arise from SCPs[49]. These experiments showed that SCP-derived cells induce expression of TSC-specific genes as they differentiate into NMJ TSCs. Moreover, mature NMJ TSCs retain expression of numerous SCP and immature SC markers[35,49]. It is also worth noting that TSCs in different end organ structures, or possibly within the same end organ structure, may not universally share the same progenitor. This was highlighted in a study showing that a subpopulation of TSCs in FNEs and hair follicles arise from Prss56+ boundary cap cells[50].
Figure 3. Developmental lineage of TSCs.
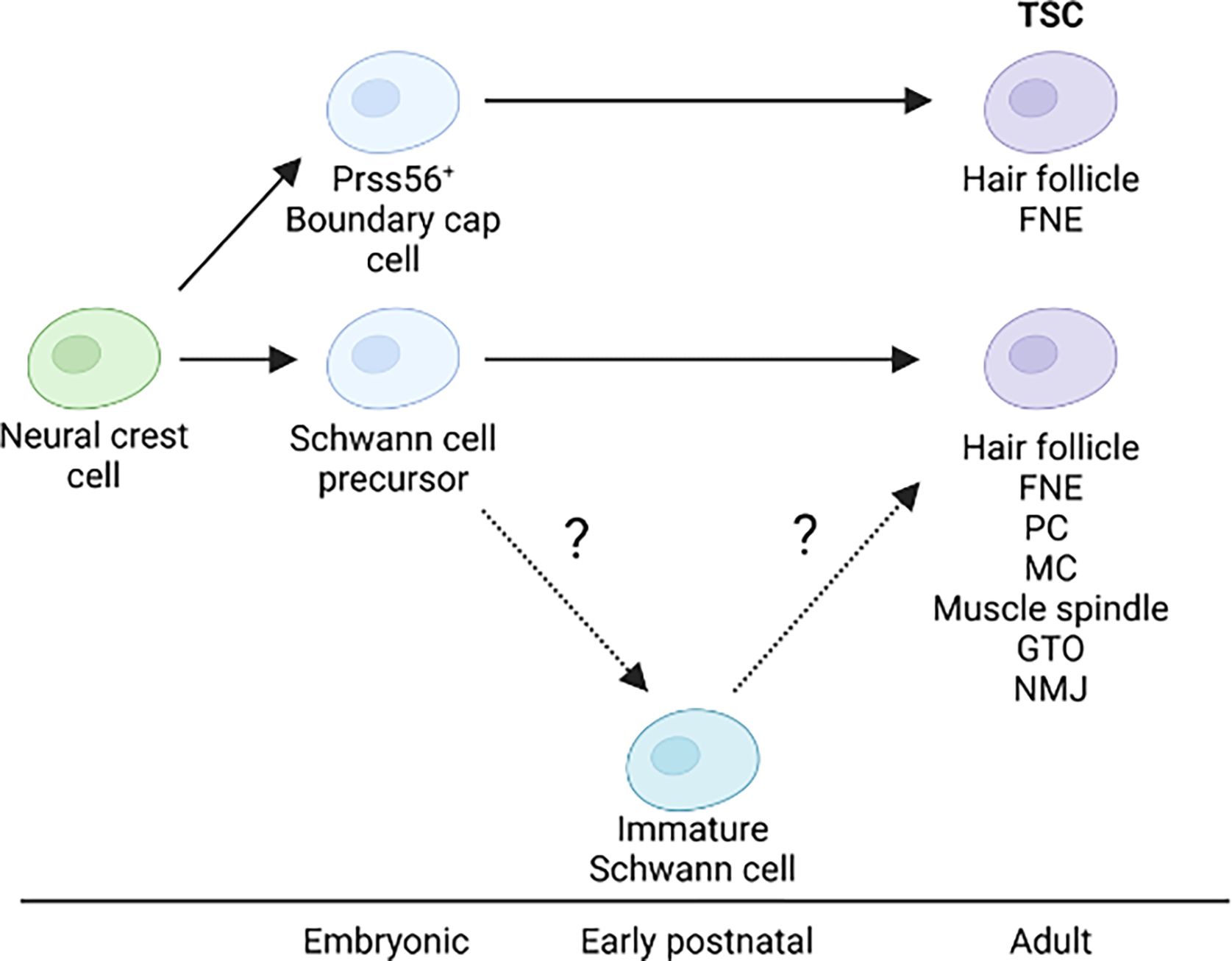
All TSCs originate from the neural crest. A subset of TSCs at hair follicles and FNEs are derived from a transient pool of boundary cap cells. TSCs at other end organ structures descend from the same Schwann cell precursors as SCs in the nerve, although it is unknown whether developing TSCs go through an immature Schwann cell state as do nerve SCs. Abbreviations: FNE: free nerve ending, PC: Pacinian corpuscle, MC: Meissner corpuscle, GTO: Golgi tendon organ, NMJ: neuromuscular junction.
TSC differentiation and maturation:
While the factors that drive precursor cells to differentiate into TSCs remain largely unknown, there is a close relationship between TSCs acquiring their distinctive cellular and molecular characteristics and the maturation of the end organ structure. For instance, as the mouse NMJ matures, TSCs form cellular processes that envelop the axon terminal arbor[28], organize into tiled territories[51], begin to express neuron-glial antigen 2 (NG2), a cell surface proteoglycan[35], and continue to express Kir4.1, an inward-rectifying K+ channel, unlike myelinating SCs[52]. A similar relationship has been found at PCs and MCs. In rodents and humans, as both of these end organ structures mature, TSCs express high levels of vimentin[53,54]. These relationships and the dependence of TSCs on axonal contact during development[41,55,56] suggest that end organ structure specific factors contribute to the differentiation and subsequent maturation of TSCs.
TSCs expansion:
The number of TSCs increases as end organ structures mature and grow in size during development. The number of TSCs increases from 1 to about 3 per NMJ in maturing rodents[57]. This increase in TSCs correlates with the transformation of the NMJ from a simple and small plaque-like structure into a large pretzel-like synapse[57]. There is a similar relationship in PCs and MCs. At maturing PCs in rodents and humans, TSCs greatly expand in number and alter their shape to form the thin lamellae of the onion bulb structure that surrounds the axon terminal ending[45,46,54]. At maturing murine MCs, TSCs increase from 1 to around 4 per end organ structure and elaborate lamellae in which the axon terminals are interspersed[13,47].
Studies have identified local factors important for the expansion of TSCs. Axon-derived NRG1 type-III has been shown to significantly impact the number of TSCs per NMJ during development and in adult mice[58]. In human PCs, evidence suggests that NCAM-1 may play a role in increasing the number of TSCs during development, as it is localized to axon-TSC junctions and upregulated as PCs mature[54]. In hair follicles of mice, glutamatergic signaling between TSCs and axon nerve endings has been shown to impact TSC proliferation[59]. TSCs express the glutamate receptor, NMDA. Here, disruption of glutamatergic signaling via conditional knockout of vesicular glutamate transporter 2 (VGLUT2) in sensory axons reduced the number of TSCs at the developing hair follicle. Along the same lines, administration of an NMDA antagonist stunted the formation of TSC processes that intermingle with axon terminals at hair follicles.
TSCs at maturing end organ structures
While TSCs do not appear to be required for axon terminals to reach and connect with end organ structures[60–63], there is evidence indicating important roles for TSCs in the maturation of the axon terminal and end organ structure. This has been best characterized at the rodent NMJ where TSC precursors have been shown to stabilize initial contacts between the axon and myofiber by inhibiting muscle derived thrombins[60,61]. TSCs are necessary for NMJ maturation to proceed past initial contact, as selective ablation of their precursors in-utero causes axons to degenerate shortly after they contact the myofiber[60–63]. Furthermore, those nascent NMJs are initially innervated by multiple motor axons[34], and TSCs participate in the elimination of all but one motor axon. In the context of NMJ development, this process is often referred to as synaptic elimination. TSCs participate in synaptic elimination by detecting differences in synaptic efficacy of competing axonal inputs using the P2RY1 receptor and calcium-mediated signaling[64,65]. Inhibition of P2YRY1 receptors renders TSCs unable to sense synaptic transmission and delays synaptic elimination[27]. TSCs also jockey with motor axons for contact with the muscle fiber surface and phagocytose axons that lose this competition[28]. Through these mechanisms, NMJ TSCs sense the relative strengths of competing synaptic inputs and influence the removal of weaker ones. Elsewhere in the PNS, it remains unknown whether and how TSCs participate in the maturation of their end organ structures.
TSCs at healthy adult end organ structures
TSCs insulate the site of contact between the axon terminal and end organ structure:
In each end organ structure, TSCs separate the axon terminal and its partner end organ structure region from the surrounding peripheral tissue. TSCs work together within a given end organ structure to create a physical barrier using finger-like cellular processes that tightly wrap around the perimeter of the axon terminal. This leaves the leading portion of the axon terminal exposed to communicate with the end organ structure. In the case of the NMJ, the exposed part of the axon terminal directly abuts the myofiber, and is the site of synaptic transmission[34]. In FNEs [15,16], MCs, PCs, and hair follicles [13], protrusions of the axon terminals beyond the TSC covering have been proposed to be involved in the conversion of external stimuli to afferent neuronal signal.
Research efforts in rodents and humans are revealing the identity of molecules with potential roles in the formation and function of TSCs processes. NMJ TSCs have been found to express high levels of transcripts for molecules involved in phagocytosis, cell adhesion, and extracellular matrix formation[35]. These include Ncam1, Ajap1, Megf10, members of the dystroglycan complex as well as connexin, nidogen, laminin and collagen subtypes. NCAM1 has also been found in TSCs of MCs and PCs[54]. In addition to anchoring TSC processes to the site of contact between the axon terminal and end organ structure, these molecules may also be involved in TSCs transducing signals among each other and to axon terminals. In support of this model, adherens junctions have been shown to connect TSCs and axon terminals and to possibly play roles in sensory signal transduction in hair follicles, MCs, and PCs[13].
There are a number of outstanding questions about the barrier TSC processes form. For instance, it is unknown which molecules are able to pass the TSC barrier. If the permeability of the TSC barrier is stringent, how are nutrients and waste carried in and out of the end organ structure? One possibility is that TSCs perform these functions by forming a barrier that is reminiscent in some ways of the blood brain barrier (BBB), and thereby connecting axon terminals and abutting end organ structure region with the vasculature. The BBB, which is the physical barrier that strictly regulates the influx of molecules from the blood stream into the CNS[66], is paralleled in the PNS by the blood-nerve barrier (BNB) that ensconces large peripheral nerves, such as the sciatic nerve[67]. Like astrocytes in the CNS, Schwann cells regulate the permeability of capillaries at the BNB[67]. Whether the BNB continues to extend along axons that branch out from these larger nerve structures as they fan out into peripheral tissues and whether TSCs influence its permeability to blood-derived molecules is largely unknown. Addressing these questions may be needed to fully understand the function of TSCs in the maturation and later in the maintenance of axon terminals and end organ structures.
TSCs detect and modulate neurotransmission:
TSCs across various end organ structures have been consistently shown to be involved in, and indeed critical for, proper neuronal signaling. NMJ TSCs in mice and frogs detect synaptic activity through muscarinic acetylcholine receptors[64,68], adenosine receptors[69], and purinergic receptors[70], which trigger a release of intracellular stores of Ca2+ and initiates G protein mediated signaling within the TSCs[70–74] (Figure 4A). NMJ TSCs also express butyrylcholinesterase (BChE), which fine tunes their modulation of synaptic plasticity in response to acetylcholine spillover [75]. Furthermore, differential nerve stimulation patterns result in different TSC Ca2+ wave patterns, and when that Ca2+ activity was optically mimicked in TSCs, the synaptic plasticity seen with nerve stimulation was replicated[76]. Despite decades of evidence that TSCs respond to synaptic activity in a way that can alter the function of the synapse itself, the precise mechanisms TSCs use to influence synaptic plasticity at NMJs remain to be fully elucidated.
Figure 4. TSCs actively participate in the physiology of their axon terminals.
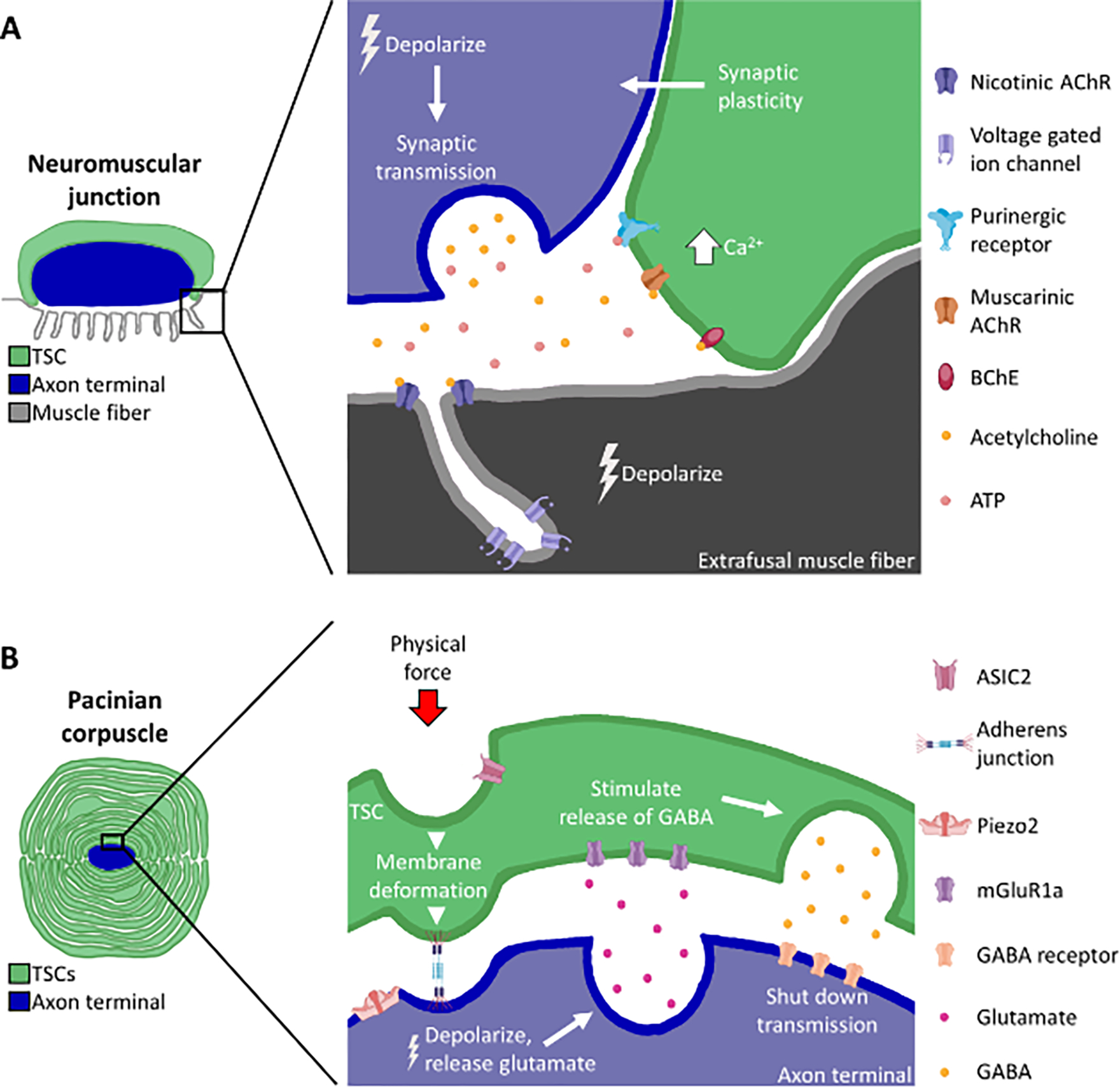
A) At the neuromuscular junction, axon terminals release vesicles containing transmitters such as acetylcholine and ATP. These interact with muscarinic acetylcholine receptors (AChRs) and purinergic receptors on TSCs, which cause an increase in intracellular Ca2+, leading to synaptic function modulation by the TSC. Butyrylcholinesterase (BChE), expressed by the TSC, also plays a role in modulating synaptic plasticity. B) At the Pacinian corpuscle, TSCs and the axon terminal express ion channels involved in mechanosensitivity (ASIC2 and Piezo2, respectively). Membrane deformation via physical force depolarizes the axon terminal. This causes the axon to release glutamate onto the TSC, inducing the TSC to release GABA onto the axon, which in turn shuts down neuronal transmission.
TSCs of sensory end organ structures have been far less studied compared to those at NMJs. It is clear, however, that TSCs at sensory end organ structures facilitate the transduction of forces associated with stretch and vibration into neural signaling. Current evidence in mice, ducks, and humans suggests TSCs express mechanoreceptors, as do other PNS glia[77]. TSCs may modulate afferent signaling through mechanosensitivity, vesicular release of molecules onto the axon terminal, and/or by forming anchors with the axon terminal membrane. TSCs in the MC express receptors involved in mechanosensitivity, TRPV4[78] and ASIC2[79,80], which is also expressed by TSCs in PCs. Depolarization of TSCs via these mechanosensitive receptors may be important for sensory signaling[16,81], as evidenced by the depolarization of FNE TSCs being sufficient to induce a pain response in mice[16,82].
Evidence in mice and cats also suggests that TSCs influence the depolarization of their axon terminals by releasing select molecules and transferring mechanical force to axon terminals. TSCs have long been known to contain vesicles[11,39,40], and PC TSCs express components required for glutamatergic and GABAergic signaling[83]. Experiments with PCs utilizing glutamate and GABA antagonists suggest a model where mechanical excitation of the axon terminal causes it to release glutamate, which in turn causes TSCs to emit GABA onto the axon, inhibiting the release of glutamate and shutting down transmission shortly after response onset[25] (Figure 4B). TSCs may also influence neuronal excitation by transferring mechanical force from the surrounding tissue to the axon terminal. Mouse MC and hair follicle TSCs express the transmembrane protein Ush2A, which is necessary for proper sensation in these end organ structures, and may cause axon terminals to be mechanically affected when the TSC membrane is deformed by an external stimulus[84]. In the PC, MC and in hair follicles, adherens junctions between TSCs and the axon terminal are thought to be responsible for mechanical stretching of the terminal membrane in response to force. It has been proposed that this TSC-mediated deformation of the axonal membrane is necessary for activation of Piezo2 mechanoreceptors, which are exclusively located on axon terminals within these end organ structures[13]. These studies suggest that TSCs are conduits for transducing mechanical forces into action potentials by providing structural support or possibly through mechanoreceptor-mediated signaling. At the same time, TSCs may have a critically important role in fine-tuning the depolarization responses of sensory axons to mechanical stimuli through the release of GABA and glutamate, or possibly other signaling molecules.
TSCs maintain the function and structure of adult axon terminals:
Given that TSCs create a physical barrier to protect the end organ structure from the external environment and are important for proper neuronal signaling, it stands to reason that they may be necessary for the integrity and health of axon terminals. To examine whether that is the case, a number of studies have ablated TSCs at various end organ structures. TSC ablation at frog and mouse NMJs does not immediately impact electrophysiological properties, suggesting that TSCs are not required for synaptic transmission per se[62,85]. However, after 3–7 days without TSCs, neurotransmitter release and compound muscle action potential amplitude are decreased[62,63,85]. Likewise, when FNE TSCs are ablated, pain responses in mice are altered[17]. When TSCs are stripped away from PCs in cats, the axon terminal’s response to stimuli shifts from a rapidly adapting response to a slowly adapting response[86]. These effects may be, at least in part, due to full or partial degeneration of axon terminals in the absence of TSCs or off-target ablation of the SCs that myelinate these axons. While adult murine motor and circumferential LTMR axon terminals seem to be more resilient to TSC ablation[36,40], lanceolate LTMR and FNEs degenerate without their TSCs[17,40]. Altogether, published data support roles for TSCs in the function and stability of adult axon terminals and end organ structures.
TSCs in injury, disease, and aging
Injuries to peripheral nerves:
In adult rodents, TSCs repair end organ structures following axonal degeneration by clearing axonal debris, recruiting axons back to the end organ structure, and assisting with rebuilding the end organ structure upon reinnervation. With the exception of axon terminals at FNEs[17] and circumferential LTMR endings[40], where TSCs die shortly after axon retraction, TSCs remain at end organ structures following axonal degeneration in adults[24,40,87,88]. Upon denervation, TSC enter a reactive state characterized by a number of morphological and molecular changes. At the NMJ[24] and the PC[23], TSCs engulf components of the degenerating axon terminal. NMJ TSCs upregulate markers of phagocytosis (galectin-3[30]), migration (galectin-1[89]), and astrocytic reactivity (GFAP[90]), as well as other repair markers (p75-NTR[91], GAP-43[92], Nestin[93–95], and Zipro1[96]). The lamellae of MC TSCs shrink in size, with reduced expression of BChE after axotomy[88].
Once the debris from the former axon terminal has been cleared, NMJ TSCs have been posited to attract motor axons back to denervated NMJs by extending cellular processes far away from their original synaptic territory[91]. This includes extension of processes towards healthy neighboring NMJs, where they provide a path for the axon terminal of the innervated NMJ to sprout towards the denervated NMJ, in a process known as compensatory reinnervation[95,97–101]. TSC repair responses have not been as extensively studied in other end organ structures. While elimination of redundant inputs occurs in the PC after nerve injury, the role of TSCs in this process is not known[102]. TSCs do actively phagocytose debris from injured axons in the PC, suggesting that they may participate in the repair of this end organ structure following denervation. Re-innervation of the MC causes TSCs to regain healthy phenotypes, though the extent to which TSCs facilitate MC reinnervation is unknown[103].
Disease:
There is evidence that TSCs impact the health of motor and sensory end organ structures afflicted by disease. The NMJ is an early site of motor neuron degeneration in amyotrophic lateral sclerosis (ALS), and work in rodent ALS models suggests that TSCs acquire properties that contribute to deterioration of this synapse[31,104]. TSCs at the NMJ show altered sensitivity to synaptic transmission[105], migration away from the NMJ[106], aberrant insertion of processes into the synaptic cleft[107], impaired phagocytosis of degenerating axonal debris[108], and increased expression of harmful factors such as complement proteins[32] and Sema3A[109]. Many of these deleterious changes in TSCs have been observed before NMJ degeneration is evident. Thus, TSCs appear to acquire unique characteristics in ALS that may precipitate NMJ degeneration. Mouse models of spinal muscular atrophy (SMA) show that NMJ TSCs are impacted during the early stages of NMJ degeneration. This includes subtle changes in morphology, reduced S100B expression, increased presence of TSC sprouts associated with motor axons, and fewer TSCs in SMA mice[110–113]. In the mdx mouse model of Duchenne muscular dystrophy, NMJ TSCs form extensive sprouts that are seldomly associated with motor axons, exhibit reduced coverage of motor axon terminals, and show an increased number of TSCs per NMJ[114,115]. In the early stages of peripheral neuropathy associated with diabetes, the nerve endings at FNEs degenerate and TSCs are lost[116,117]. These findings indicate that TSCs at other end organ structures are at a minimum indirectly impacted by diseases.
Aging:
The ability of TSCs to repair end organ structures becomes particularly important as these structures begin to degrade during the course of aging. In the case of the NMJ, degeneration has been linked to sarcopenia and loss of motor function[104,118] and evidence suggests that TSCs may be involved in this process in both a beneficial and harmful manner. TSCs at aged NMJs in mice upregulate inflammatory and phagocytic genes, expand in numbers, and form processes, often associated with an axonal sprout, that contact neighboring NMJs[26]. They are thought to slow age-related muscle dysfunction by promoting compensatory reinnervation of denervated muscle fibers by nearby healthy motor axons[26,119,120]. However, there is also evidence that age-related changes in TSCs may contribute to NMJ damage. In aging humans and rodents, TSCs not only fail to tightly cap the synaptic cleft but aberrantly insert their processes into it[26,121–123]. Age-related deficits are not limited to the motor PNS. There is evidence that some sensory axon terminals at muscle spindles degenerate in aged mice[124] and humans[42], and given that proprioception is necessary for proper motor function, this degeneration may contribute to age-related loss of motor function[124]. Aged humans lose the ability to sense touch, impacting their ability to grasp objects[125], and this has been linked to age-related loss of PCs and MCs[126]. The roles of TSCs in preserving the health of aging end organ structures have not yet been fully established. Future research should address the fate of TSCs and their contribution to the health of end organ structures during aging.
Concluding remarks and future perspectives
Much progress has been made to understand the developmental origins of TSCs as well as their roles in the maturation, function, health, and repair of associated axon terminals and end organ structures. TSCs are derived from NCCs and the majority appear to emanate directly from SCPs. They are also dependent on axonal contact to survive during development and are actively involved in the maturation of their end organ structures. Research over the last decades has revealed that TSCs play important roles in the proper function of adult end organ structures and their repair upon damage. Additionally, it is becoming apparent that TSCs lose their homeostatic functions in diseases and aging, and thus likely contribute to end organ structure degeneration. Despite these advances, many questions remain (see Outstanding Questions). Addressing these and other questions about the biology of TSCs could pave the path for developing therapies to treat a variety of PNS conditions. It may also provide leads for treating CNS conditions, given the many functional parallels between TSCs and CNS glia, such as their roles in synaptic pruning, axon guidance and modulation of neuronal activity.
Outstanding Questions.
The presence of TSCs at the axon terminals is conserved across various end organs, yet TSCs at different end organs display different morphologies and molecular functions. What are the TSC-intrinsic molecules that specify their identity and various functions?
Current evidence suggests that TSCs are derived from the same progenitors as SCs along the nerve. What factors, if any, do axon terminals and other end organ structures produce to promote TSC differentiation and maturation?
TSCs have been found to be altered in aging and various diseases. Which of the cellular and molecular changes to TSCs in these conditions are beneficial or detrimental to the sustained physiology of their associated axon terminal?
Do TSCs acquire different functional phenotypes and lose their homeostatic functions under chronic stress conditions, such as in disease and aging, as has been found for astrocytes and microglia in the CNS?
What is the consequence of TSCs’ losing the appropriate coverage of their axon terminal on the peripheral tissue and the innervating neuron residing either in the PNS or CNS?
Do TSCs influence the permeability of an end-organ level blood-nerve barrier? If so, is this structure damaged by disease and aging?
Highlights Box.
Terminal Schwann cells are highly specialized, non-myelinating glia, associated with axon terminals of the somatic peripheral nervous system.
Terminal Schwann cells mediate communication between the nervous system and peripheral tissue. They play key roles in the development, stability, and repair of axon terminals and peripheral tissues.
Accruing evidence points to maladaptive changes in terminal Schwann cells contributing to degeneration of peripheral tissues and innervating neurons in diseases and during aging.
Terminal Schwann cells contain a unique molecular fingerprint compared to myelinating Schwann cells.
Acknowledgments
This work was funded through grants from the National Institute on Aging (R01AG055545 and R56AG051501) and the National Institute of Neurological Disorders and Stroke (R21NS106313) awarded to GV. RLH was supported in part by NRSA Institutional Research Training Grant T32 AG041688-11.
Footnotes
Declaration of Interests
The authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ranvier LA (1878) Leçons sur l’histologie du système nerveux, F. Savy; [DOI] [PubMed] [Google Scholar]
- 2.Couteaux R (1947) Contribution à l’étude de la synapse myoneurale. Rev Canad Biol 6, 563–711 [Google Scholar]
- 3.Couteaux R (1960) Motor end-plate structure. Struct. Funct. muscle [Google Scholar]
- 4.Kaidoh T and Inoué T (2008) N‐cadherin expression in palisade nerve endings of rat vellus hairs. J. Comp. Neurol. 506, 525–534 [DOI] [PubMed] [Google Scholar]
- 5.Cauna N (1973) The free penicillate nerve endings of the human hairy skin. J. Anat. 115, 277. [PMC free article] [PubMed] [Google Scholar]
- 6.Boeke J (1949) THE SYMPATHETIC ENDFORMATION, ITS SYNAPTOLOGY, THE INTERSTITIAL CELLS, THE PERITERMINAL NETWORK, AND ITS BEARING ON THE NEURONE THEORY. DISCUSSION AND CRITIQUE. Cells Tissues Organs 8, 18–61 [DOI] [PubMed] [Google Scholar]
- 7.CAUNA N and MANNAN G (1959) Development and postnatal changes of digital Pacinian corpuscles (corpuscula lamellosa) in the human hand. J. Anat. 93, 271–27186 [PMC free article] [PubMed] [Google Scholar]
- 8.CAUNA N and ROSS LL (1960) The fine structure of Meissner’s touch corpuscles of human fingers. J. Biophys. Biochem. Cytol. 8, 467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly AM and Zacks SI (1969) The fine structure of motor endplate morphogenesis. J. Cell Biol. 42, 154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tello JF (1944) Sobre una vaina que envuelve toda la ramificacion del axon en las terminaciones motrices de los musculos estriados. Trab. Inst Cajal Invest Biol 36, 1–59 [Google Scholar]
- 11.Spencer PS and Schaumburg HH (1973) An ultrastructural study of the inner core of the Pacinian corpuscle. J. Neurocytol. 2, 217–235 [DOI] [PubMed] [Google Scholar]
- 12.García-Suárez O et al. (2009) Myelin basic protein-positive nerve fibres in human Meissner corpuscles. J. Anat. 214, 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handler A et al. (2023) Three-dimensional reconstructions of mechanosensory end organs suggest a unifying mechanism underlying dynamic, light touch. Neuron DOI: 10.1016/j.neuron.2023.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaidoh T and Inoué T (2000) Intercellular junctions between palisade nerve endings and outer root sheath cells of rat vellus hairs. J. Comp. Neurol. 420, 419–427 [DOI] [PubMed] [Google Scholar]
- 15.Heppelmann B et al. (1990) Ultrastructural three-dimensional reconstruction of group III and group IV sensory nerve endings (“free nerve endings”) in the knee joint capsule of the cat: Evidenence for multiple receptive sites. J. Comp. Neurol. 292, 103–116 [DOI] [PubMed] [Google Scholar]
- 16.Abdo H et al. (2019) Specialized cutaneous schwann cells initiate pain sensation. Science (80-. ). 365, 695–699 [DOI] [PubMed] [Google Scholar]
- 17.Rinwa P et al. (2021) Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain 162, 1816–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stacey MJ (1969) Free nerve endings in skeletal muscle of the cat. J. Anat. 105, 231–54 [PMC free article] [PubMed] [Google Scholar]
- 19.Vainchtein ID and Molofsky AV (2020) Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 43, 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darabid H et al. (2014) Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat. Rev. Neurosci. 15, 703–18 [PubMed] [Google Scholar]
- 21.Ko CP and Robitaille R (2015) Perisynaptic schwann cells at the neuromuscular synapse: Adaptable, multitasking glial cells. Cold Spring Harb. Perspect. Biol. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handler A and Ginty DD (2021) The mechanosensory neurons of touch and their mechanisms of activationNature Reviews Neuroscience, 22Nature Research, 521–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korthals JK et al. (1974) The fate of the axon and its terminal in the Pacinian corpuscle following sciatic nerve section. J. Neurocytol. 3, 385–403 [DOI] [PubMed] [Google Scholar]
- 24.Miledi R and Slater CR (1970) On the degeneration of rat neuromuscular junctions after nerve section. J. Physiol. 207, 507–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawson L et al. (2009) GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J. Neurosci. 29, 2695–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hastings RL et al. (2023) Cellular and molecular evidence that synaptic Schwann cells contribute to aging of mouse neuromuscular junctions. Aging Cell DOI: 10.1111/acel.13981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darabid H et al. (2018) Purinergic-Dependent Glial Regulation of Synaptic Plasticity of Competing Terminals and Synapse Elimination at the Neuromuscular Junction. Cell Rep. 25, 2070–2082.e6 [DOI] [PubMed] [Google Scholar]
- 28.Smith IW et al. (2013) Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J. Neurosci. 33, 17724–17736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuertes-Alvarez S and Izeta A (2021) Terminal schwann cell aging: Implications for age-associated neuromuscular dysfunctionAging and Disease, 12International Society on Aging and Disease, 494–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez‐Gonzalez AP et al. (2022) Functional adaptation of glial cells at neuromuscular junctions in response to injury. Glia 70, 1605–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbour D et al. (2017) New perspectives on amyotrophic lateral sclerosis: the role of glial cells at the neuromuscular junction. J. Physiol. 595, 647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhindi A et al. (2021) Terminal Schwann cells at the human neuromuscular junction. Brain Commun. 3, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suazo I et al. (2022) The Lamellar Cells of Vertebrate Meissner and Pacinian Corpuscles: Development, Characterization, and Functions. Front. Neurosci. 16, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanes JR and Lichtman JW (1999) DEVELOPMENT OF THE VERTEBRATE NEUROMUSCULAR JUNCTION, 22 [DOI] [PubMed] [Google Scholar]
- 35.Castro R et al. (2020) Specific labeling of synaptic schwann cells reveals unique cellular and molecular features. Elife 9, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hastings RL et al. (2020) Morphological remodeling during recovery of the neuromuscular junction from terminal Schwann cell ablation in adult mice. Sci. Rep. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patten RM and Ovalle WK (1991) Muscle spindle ultrastructure revealed by conventional and high‐resolution scanning electron microscopy. Anat. Rec. 230, 183–198 [DOI] [PubMed] [Google Scholar]
- 38.Seaberg BL et al. (2022) Validation of terminal Schwann cell gene marker expression by fluorescent in situ hybridization using RNAscope. Neurosci. Lett. 771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pease DC and Quilliam TA (1957) ELECTRON MICROSCOPY OF THE PACINIAN CORPUSCLE. J. Biophys. Biochem. Cytol. 3, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L and Ginty DD (2014) The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife 3, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopp DM et al. (1997) Glial Growth Factor Rescues Schwann Cells of Mechanoreceptors from Denervation-Induced Apoptosis. J. Neurosci. 17, 6697–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry M and Baudry S (2019) Age-related changes in leg proprioception: Implications for postural controlJournal of Neurophysiology, 122American Physiological Society, 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jessen KR and Mirsky R (2005) The origin and development of glial cells in peripheral nervesNature Reviews Neuroscience, 6Nat Rev Neurosci, 671–682 [DOI] [PubMed] [Google Scholar]
- 44.Cauna N (1959) The mode of termination of the sensory nerves and its significance. J. Comp. Neurol. 113, 169–209 [DOI] [PubMed] [Google Scholar]
- 45.Zelená J (1978) The development of Pacinian corpuscles. J. Neurocytol. 7, 71–91 [DOI] [PubMed] [Google Scholar]
- 46.Saxod R (1996) Ontogeny of the cutaneous sensory organs. Microsc. Res. Tech. 34, 313–333 [DOI] [PubMed] [Google Scholar]
- 47.Idé C (1977) Development of meissner corpuscle of mouse toe pad. Anat. Rec. 188, 49–67 [DOI] [PubMed] [Google Scholar]
- 48.Saxod R (1973) Developmental origin of the Herbst cutaneous sensory corpuscle. Experimental analysis using cellular markers. Dev. Biol. 32, 167–178 [DOI] [PubMed] [Google Scholar]
- 49.Kastriti ME et al. (2022) Schwann cell precursors represent a neural crest‐like state with biased multipotency. EMBO J. 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gresset A et al. (2015) Boundary Caps Give Rise to Neurogenic Stem Cells and Terminal Glia in the Skin. Stem Cell Reports 5, 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brill MS et al. (2011) Spatial constraints dictate glial territories at murine neuromuscular junctions. J. Cell Biol. 195, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Procacci NM et al. (2022) Kir4.1 is specifically expressed and active in non‐myelinating Schwann cells. Glia DOI: 10.1002/glia.24315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albuerne M et al. (2000) Development of Meissner-like and Pacinian sensory corpuscles in the mouse demonstrated with specific markers for corpuscular constituents. Anat. Rec. 258, 235–242 [DOI] [PubMed] [Google Scholar]
- 54.Feito J et al. (2018) The development of human digital Meissner’s and Pacinian corpuscles. Ann. Anat. 219, 8–24 [DOI] [PubMed] [Google Scholar]
- 55.Trachtenberg JT and Thompson WJ (1996) Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature 379, 174–177 [DOI] [PubMed] [Google Scholar]
- 56.Zelená J (1980) Rapid degeneration of developing rat Pacinian corpuscles after denervation. Brain Res. 187, 97–111 [DOI] [PubMed] [Google Scholar]
- 57.Love FM and Thompson WJ (1998) Schwann cells proliferate at rat neuromuscular junctions during development and regeneration. J. Neurosci. 18, 9376–9385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y il et al. (2016) Neuregulin1 displayed on motor axons regulates terminal Schwann cell-mediated synapse elimination at developing neuromuscular junctions. 113, E479–E487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo SH et al. (2012) Excitatory glutamate is essential for development and maintenance of the piloneural mechanoreceptor. Development 139, 740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gould TW et al. (2019) Glial cells maintain synapses by inhibiting an activity-dependent retrograde protease signal. PLOS Genet. 15, e1007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y et al. (2019) Blocking skeletal muscle DHPRS/RYR1 prevents neuromuscular synapse loss in mutant mice deficient in type III neuregulin 1 (CRD-NRG1). PLoS Genet. 15, 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy LV et al. (2003) Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron 40, 563–580 [DOI] [PubMed] [Google Scholar]
- 63.Barik A et al. (2016) Schwann cells in neuromuscular junction formation and maintenance. J. Neurosci. DOI: 10.1523/JNEUROSCI.0174-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darabid H et al. (2013) Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J. Neurosci. 33, 1297–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heredia DJ et al. (2018) Activity-induced Ca 2+ signaling in perisynaptic schwann cells of the early postnatal mouse is mediated by P2Y 1 receptors and regulates muscle fatigue. Elife 7, 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banks WA et al. (2021) Healthy aging and the blood–brain barrierNature Aging, 1Springer, 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malong L et al. (2023) Characterization of the structure and control of the blood-nerve barrier identifies avenues for therapeutic delivery. Dev. Cell 58, 174–191.e8 [DOI] [PubMed] [Google Scholar]
- 68.Wright MC et al. (2009) Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J. Neurosci. 29, 14942–14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rochon D et al. (2001) Synapse-glia interactions at the mammalian neuromuscular junction. J. Neurosci. 21, 3819–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robitaille R (1995) Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J. Neurosci. 15, 7121–7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jahromi BS et al. (1992) Transmitter release increases intracellular calcium in perisynaptic schwann cells in situ. Neuron 8, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 72.Reist NE and Smith SJ (1992) Neurally evoked calcium transients in terminal Schwann cells at the neuromuscular junction. Proc. Natl. Acad. Sci. U. S. A. 89, 7625–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robitaille R et al. (1997) Muscarinic Ca 2+ responses resistant to muscarinic antagonists at perisynaptic schwann cells of the frog neuromuscular junction. J. Physiol. 504, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robitaille R (1998) Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron 21, 847–55 [DOI] [PubMed] [Google Scholar]
- 75.Petrov KA et al. (2014) Schwann Cells Sense and Control Acetylcholine Spillover at the Neuromuscular Junction by α7 Nicotinic Receptors and Butyrylcholinesterase. J. Neurosci. 34, 11870–11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd KJ et al. (2010) Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J. Neurosci. 30, 11870–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brandt JP and Smith CJ (2023) Piezo1-mediated spontaneous calcium transients in satellite glia impact dorsal root ganglia development. PLOS Biol. 21, e3002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonso-González P et al. (2017) Human Digital Meissner Corpuscles Display Immunoreactivity for the Multifunctional Ion Channels Trpc6 and Trpv4. Anat. Rec. 300, 1022–1031 [DOI] [PubMed] [Google Scholar]
- 79.Cabo R et al. (2015) ASIC2 is present in human mechanosensory neurons of the dorsal root ganglia and in mechanoreceptors of the glabrous skin. Histochem. Cell Biol. 143, 267–276 [DOI] [PubMed] [Google Scholar]
- 80.Montaño JA et al. (2009) The expression of ENa+C and ASIC2 proteins in Pacinian corpuscles is differently regulated by TrkB and its ligands BDNF and NT-4. Neurosci. Lett. 463, 114–118 [DOI] [PubMed] [Google Scholar]
- 81.Nikolaev YA et al. (2020) Lamellar cells in Pacinian and Meissner corpuscles are touch sensors. Sci. Adv. 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ojeda-Alonso J et al. (2024) Sensory Schwann cells set perceptual thresholds for touch and selectively regulate mechanical nociception. Nat. Commun. 15, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pawson L et al. (2007) Possible glutaminergic interaction between the capsule and neurite of Pacinian corpuscles. Somatosens. Mot. Res. 24, 85–95 [DOI] [PubMed] [Google Scholar]
- 84.Schwaller F et al. (2021) USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans. Nat. Neurosci. 24, 74–81 [DOI] [PubMed] [Google Scholar]
- 85.Halstead SK et al. (2005) Anti-disialosyl antibodies mediate selective neuronal or Schwann cell injury at mouse neuromuscular junctions. Glia 52, 177–189 [DOI] [PubMed] [Google Scholar]
- 86.LOEWENSTEIN WR and MENDELSON M (1965) COMPONENTS OF RECEPTOR ADAPTATION IN A PACINIAN CORPUSCLE. J. Physiol. 177, 377–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zelená J (1982) Survival of Pacinian corpuscles after denervation in adult rats. Cell Tissue Res. 224, 673–683 [DOI] [PubMed] [Google Scholar]
- 88.Idé C (1982) Degeneration of mouse digital corpuscles. Am. J. Anat. 163, 59–72 [DOI] [PubMed] [Google Scholar]
- 89.Plachta N et al. (2007) Identification of a lectin causing the degeneration of neuronal processes using engineered embryonic stem cells. Nat. Neurosci. 10, 712–719 [DOI] [PubMed] [Google Scholar]
- 90.Georgiou J et al. (1994) Synaptic regulation of glial protein expression in vivo. Neuron 12, 443–455 [DOI] [PubMed] [Google Scholar]
- 91.Reynolds ML and Woolf CJ (1992) Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J. Neurocytol. 21, 50–66 [DOI] [PubMed] [Google Scholar]
- 92.Woolf CJ et al. (1992) Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J. Neurosci. 12, 3999–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Astrow SH et al. (1994) Differential neural regulation of a neuromuscular junction-associated antigen in muscle fibers and Schwann cells. J. Neurobiol. 25, 937–952 [DOI] [PubMed] [Google Scholar]
- 94.Kang H et al. (2007) Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J. Neurosci. 27, 5948–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang H et al. (2014) Terminal schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. J. Neurosci. 34, 6323–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ellerton EL et al. (2008) Induction of zinc-finger proliferation 1 expression in non-myelinating Schwann cells after denervation. Neuroscience 153, 975–985 [DOI] [PubMed] [Google Scholar]
- 97.Son Y-J and Thompson WJ (1995) Schwann cell processes guide regeneration of peripheral axons. Neuron 14, 125–132 [DOI] [PubMed] [Google Scholar]
- 98.Son YJ and Thompson WJ (1995) Nerve sprouting in muscle is induced and guided by processes extended by schwann cells. Neuron 14, 133–141 [DOI] [PubMed] [Google Scholar]
- 99.Love FM et al. (2003) Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J. Neurobiol. 54, 566–576 [DOI] [PubMed] [Google Scholar]
- 100.Kang H et al. (2019) Schwann cell guidance of nerve growth between synaptic sites explains changes in the pattern of muscle innervation and remodeling of synaptic sites following peripheral nerve injuries. J. Comp. Neurol. 527, 1388–1400 [DOI] [PubMed] [Google Scholar]
- 101.Kang H et al. (2003) Terminal Schwann cells guide the reinnervation of muscle after nerve injury. J. Neurocytol. 32, 975–985 [DOI] [PubMed] [Google Scholar]
- 102.Zelená J (1984) Multiple axon terminals in reinnervated Pacinian corpuscles of adult rat. J. Neurocytol. 13, 665–684 [DOI] [PubMed] [Google Scholar]
- 103.Idé C (1982) Regeneration of mouse digital corpuscles. Am. J. Anat. 163, 73–85 [DOI] [PubMed] [Google Scholar]
- 104.Moloney EB et al. (2014) ALS as a distal axonopathy: Molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci. 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arbour D et al. (2015) Early and Persistent Abnormal Decoding by Glial Cells at the Neuromuscular Junction in an ALS Model. J. Neurosci. 35, 688–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carrasco DI et al. (2016) Altered terminal Schwann cell morphology precedes denervation in SOD1 mice. Exp. Neurol. 275, 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruneteau G et al. (2015) Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann. Clin. Transl. Neurol. 2, 362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martineau É et al. (2020) Properties of Glial Cell at the Neuromuscular Junction Are Incompatible with Synaptic Repair in the SOD1 G37R ALS Mouse Model. J. Neurosci. 40, 7759–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winter F De et al. (2006) The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol. Cell. Neurosci. 32, 102–117 [DOI] [PubMed] [Google Scholar]
- 110.Murray LM et al. (2013) Defects in neuromuscular junction remodelling in the Smn2B/− mouse model of spinal muscular atrophy. Neurobiol. Dis. 49, 57–67 [DOI] [PubMed] [Google Scholar]
- 111.Lee Y il et al. (2011) Muscles in a mouse model of spinal muscular atrophy show profound defects in neuromuscular development even in the absence of failure in neuromuscular transmission or loss of motor neurons. Dev. Biol. 356, 432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Voigt T et al. (2010) Ultrastructural changes in diaphragm neuromuscular junctions in a severe mouse model for Spinal Muscular Atrophy and their prevention by bifunctional U7 snRNA correcting SMN2 splicing. Neuromuscul. Disord. 20, 744–752 [DOI] [PubMed] [Google Scholar]
- 113.Neve A et al. (2016) Central and peripheral defects in motor units of the diaphragm of spinal muscular atrophy mice. Mol. Cell. Neurosci. 70, 30–41 [DOI] [PubMed] [Google Scholar]
- 114.Personius KE and Sawyer RP (2005) Terminal Schwann cell structure is altered in diaphragm of mdx mice. Muscle Nerve 32, 656–663 [DOI] [PubMed] [Google Scholar]
- 115.Haddix SG et al. (2018) Cycles of myofiber degeneration and regeneration lead to remodeling of the neuromuscular junction in two mammalian models of Duchenne muscular dystrophy. PLoS One 13, e0205926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reinisch CM et al. (2008) Rarefaction of the peripheral nerve network in diabetic patients is associated with a pronounced reduction of terminal schwann cells. Diabetes Care 31, 1219–1221 [DOI] [PubMed] [Google Scholar]
- 117.Umapathi T et al. (2007) Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle and Nerve 35, 591–598 [DOI] [PubMed] [Google Scholar]
- 118.Taetzsch T and Valdez G (2018) NMJ maintenance and repair in aging. Curr. Opin. Physiol. 4, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gutmann E and Hanzlíková V (1966) Motor unit in old age [26]. Nature 209, 921–922 [DOI] [PubMed] [Google Scholar]
- 120.Edström L and Larsson L (1987) Effects of age on contractile and enzyme-histochemical properties of fast- and slow-twitch single motor units in the rat. J. Physiol. 392, 129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wokke JHJ et al. (1990) Morphological changes in the human end plate with age. J. Neurol. Sci. 95, 291–310 [DOI] [PubMed] [Google Scholar]
- 122.Boaro SN et al. (1998) Comparative structural analysis of neuromuscular junctions in mice at different ages. Ann. Anat. - Anat. Anzeiger 180, 173–179 [DOI] [PubMed] [Google Scholar]
- 123.Kawabuchi M et al. (2001) The spatiotemporal relationship among Schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. Anat. Rec. Integr. Anat. Evol. Biol. 264, 183–202 [DOI] [PubMed] [Google Scholar]
- 124.Cao R et al. (2023) Intrafusal-fiber LRP4 for muscle spindle formation and maintenance in adult and aged animals. Nat. Commun. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campoi EG et al. (2023) The effects of age and postural constraints on prehension. Exp. Brain Res. 241, 1847–1859 [DOI] [PubMed] [Google Scholar]
- 126.García-Piqueras J et al. (2019) Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors. J. Anat. 234, 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jablonka‐Shariff A et al. (2020) Gpr126/Adgrg6 contributes to the terminal Schwann cell response at the neuromuscular junction following peripheral nerve injury. Glia 68, 1182–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang J-F et al. (2001) Schwann Cells Express Active Agrin and Enhance Aggregation of Acetylcholine Receptors on Muscle Fibers. J. Neurosci. 21, 9572–9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolbert J et al. (2020) Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 117, 9466–9476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.CAUNA N (1960) THE DISTRIBUTION OF CHOLINESTERASE IN THE CUTANEOUS RECEPTOR ORGANS, ESPECIALLY TOUCH CORPUSCLES OF THE HUMAN FINGER. J. Histochem. Cytochem. 8, 367–375 [DOI] [PubMed] [Google Scholar]
- 131.Dubový P (1986) Electron microscopical localization of non-specific cholinesterase activity in three principal parts of cat Pacinian corpuscles. Acta Histochem. 80, 1–12 [PubMed] [Google Scholar]
- 132.Vega JA et al. (1996) Immunohistochemistry of human cutaneous Meissner and Pacinian corpuscles. Microsc. Res. Tech. 34, 351–361 [DOI] [PubMed] [Google Scholar]
- 133.Pawson L et al. (2000) Immunocytochemical identification of proteins within the Pacinian corpuscle. Somatosens. Mot. Res. 17, 159–170 [DOI] [PubMed] [Google Scholar]
- 134.Reinisch CM and Tschachler E (2012) The dimensions and characteristics of the subepidermal nerve plexus in human skin – Terminal Schwann cells constitute a substantial cell population within the superficial dermis. J. Dermatol. Sci. 65, 162–169 [DOI] [PubMed] [Google Scholar]
- 135.Covault J and Sanes JR (1986) Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J. Cell Biol. 102, 716–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li L et al. (2003) Nestin expression in hair follicle sheath progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 100, 9958–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vega JA et al. (1994) The inner-core, outer-core and capsule cells of the human Pacinian corpuscles: an immunohistochemical study. Eur. J. Morphol. 32, 11–8 [PubMed] [Google Scholar]
- 138.Hayworth CR et al. (2006) Induction of Neuregulin Signaling in Mouse Schwann Cells In Vivo Mimics Responses to Denervation. J. Neurosci. 26, 6873–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]


