Abstract
Background
Berberine (BBR), an isoquinoline alkaloid from Coptidis rhizoma, has been found to have powerful activities against various human malignancies, including breast cancer. However, the underlying antitumor mechanisms of BBR in breast cancer remain poorly understood.
Methods
Breast cancer cells were cultured and treated with different doses (0, 20, 40, and 60 μM) of BBR for 48 h. Cell viability, proliferation, apoptosis, invasion, and migration were assessed using 3‐(4, 5‐dimethyl‐2‐thiazolyl)‐2, 5‐diphenyl‐2H‐tetrazolium bromide (MTT), 5‐ethynyl‐2′‐deoxyuridine (EdU), flow cytometry, transwell, and wound healing assays. Fibroblast growth factor 7 (FGF7), methyltransferase‐like 3 (METTL3), and insulin‐like growth factor‐2 mRNA‐binding protein 3 (IGF2BP3) mRNA levels and protein levels were measured using real‐time quantitative polymerase chain reaction (RT‐qPCR) and western blot. Interaction between METTL3 and FGF7 m6A was assessed using methylated RNA immunoprecipitation (MeRIP)‐qPCR and RNA immunoprecipitation (RIP) assay. Binding ability between IGF2BP3 and FGF7 mRNA was analyzed using RIP assay.
Results
BBR treatment hindered breast cancer cell proliferation, invasion, migration, and induced apoptosis. FGF7 expression was upregulated in breast cancer tissues, while its level was reduced in BBR‐treated tumor cells. FGF7 upregulation relieved the repression of BBR on breast cancer cell malignant behaviors. In mechanism, METTL3 stabilized FGF7 mRNA through the m6A‐IGF2BP3‐dependent mechanism and naturally improved FGF7 expression. BBR treatment inhibited breast cancer growth in vivo.
Conclusion
BBR treatment blocked breast cancer cell growth and metastasis partly by regulating METTL3‐mediated m6A modification of FGF7 mRNA, providing a promising therapeutic target for breast cancer treatment.
Keywords: BBR, breast cancer, FGF7, IGF2BP3, METTL3
BBR treatment suppressed breast cancer cell growth and metastasis partly by regulating METTL3‐mediated m6A modification of FGF7 mRNA in an m6A‐IGF2BP3‐dependent manner.
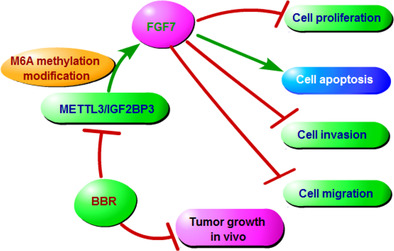
INTRODUCTION
As the most common occurring malignancy in women, breast cancer has been considered an increasing health and economic burden all over the world, with about 3 million new cases and 1 million deaths by 2040. 1 , 2 In recent years, breast cancer incidence rates have been slowly increasing, especially in younger women associated with poorer clinical outcomes. 3 Despite the fact that 70%–80% of individuals with early‐stage breast cancer are considered curable, thanks to the advancement of multimodal therapies, including mammography screening, surgical resection, adjuvant chemotherapy, and immunotherapy, outcomes for subjects with advanced/metastatic disease remain dismal. 4 , 5 Therefore, it is imperative to identify more effective therapies for breast cancer to achieve better treatment outcomes. In recent years, traditional Chinese medicine (TCM) and some extracted monomers have become more and more popular against different diseases due to their unique advantages such as multitargeting and low side effects. 6 , 7 As a nature‐driven phytochemical isoquinoline alkaloid from Chinese herbs and used in TCM, BBR has been reported to possess multiple protective properties, such as antibacterial, antiapoptosis, anti‐inflammatory, and anticancer. 8 , 9 Mounting evidence has indicated that BBR is a multitarget drug that prevents and treats many cancers by inhibiting proliferation and inducing apoptosis. 10 , 11 In fact, work in a number of laboratories has revealed that BBR diminished breast cancer cell growth and metastasis by altering several gene expressions. 12 , 13 Yet, the detailed underlying mechanism of the anticancer ability of BBR is far from being addressed in breast cancer.
As a potent mitogen for different types of epithelial and cancer cells, fibroblast growth factor 7 (FGF7, also known as keratinocyte growth factor [KGF]) has been reported to modulate the migration and differentiation of these cells and defend them against various injuries under stress conditions. 14 Several studies have suggested that FGF7 is expressed at a high level in most human cancers, including breast cancer. 15 Further reports verified that the inhibition of FGF7 signaling might reduce breast cancer cell growth, migration, and invasion. 16 , 17 Beyond that, a recent study indicated that a TCM extract (aqueous extract of eucommia leaves) might significantly hinder the proliferation of osteoblasts by decreasing FGF7 expression. 18 However, whether FGF7 participates in BBR‐mediated antitumor effects in breast cancer remains unknown.
Numerous RNA modifications have been identified to possess the potential to change RNA function. As the most prevalent internal mRNA modification, N6‐methyadenosine (m6A) has received increasing attention as an important regulator of gene expression. 19 Indeed, some articles have presented that m6A methylation exerts a vital role in modulating gene expression in many human cancers, 20 including breast cancer. 21 As a dynamic and reversible process, it can be installed by methyltransferase complexes (“writers”) consisting of methyltransferase‐like 3 (METTL3), METTL14, and WTAP, and eliminated by demethylases (“erasers”), containing FTO and ALKBH5. 22 , 23 Moreover, the regulatory function of m6A is required for the recognition and binding of m6A sites via m6A binding proteins (“readers”), like insulin‐like growth factor‐2 mRNA‐binding protein 1/2/3 (IGF2BP1/2/3) and YTHDF1/2/3. 24 , 25 It has been reported that m6A modifications are involved in regulating mRNA stability, degradation, and translation efficiency. 24 Of note, the silencing of METTL3 might block breast cancer progression via decreasing PD‐L1 mRNA stability in an m6A‐IGF2BP3‐dependent way. 26 In addition, METTL3 has been reported to partake in BBR‐mediated neuroprotection in ischemic stroke. 27 In the present study, SRAMP software was used and it was discovered that FGF7 possessed the underlying m6A sites. Apart from that, FGF7 was identified as a possible target gene of METTL3. Accordingly, this study aimed to explore whether METTL3 might affect the antibreast cancer of BBR by modulating FGF7 expression.
METHODS
Cell culture and clinical samples
In this study, breast cancer cell lines (ER+/HER2− MCF‐7, CL‐0149; and triple‐negative MDA‐MB‐231, CL‐0150B; ER+/HER2+ BT‐474, CL‐0040; triple‐negative breast cancer BT‐549, CL‐549), and an immortalized, nontransformed human mammary epithelial cell line (MCF‐10A, CL‐0525, Procell) were respectively cultured in corresponding special medium (CM‐0149, CM‐0150B, CM‐0525, CM‐0040, CM‐549, Procell) at 37°C with 5% CO2. For the in vitro study, MCF‐7, MDA‐MB‐231, BT‐474, and BT‐549 cells were exposed to BBR (HPLC ≥99%, Meilum Biologics, dissolved in dimethylsulfoxide [DMSO]) in the dose range of 0, 20, 40, and 60 μM for 48 h at 37°C. For functional experiments, MCF‐7 and MDA‐MB‐231 cells were incubated with the media including 40 μM BBR for 48 h.
Breast cancer and matched adjacent normal tissues were provided by 29 patients at People's Hospital of Dongxihu District. No patient had received chemotherapy or radiation therapy prior to surgery. Subsequently, these excised specimens during the surgery were quickly frozen in liquid nitrogen and stored at −80°C. This research was approved by the Ethics Committee of People's Hospital of Dongxihu District with written informed consent from each participant.
5‐diphenyl‐2H‐tetrazolium bromide (MTT) assay
MCF‐7 and MDA‐MB‐231 cell viability were evaluated in this experiment. After being treated with or without BBR for 48 h at 37°C, 20 μL of 5 mg/mL MTT reaction solution (Sigma‐Aldrich) was added to 3 × 104 tumor cells in 96‐well plates. After 4 h, the precipitates generated were dissolved by the addition of 150 μL DMSO. Finally, the absorbance was measured based on a microplate reader.
5‐ethynyl‐2′‐deoxyuridine (EdU) assay
Briefly, 5 × 103 MCF‐7 and MDA‐MB‐231 cells were cultured in 96‐well plates with BBR or without for 48 h. Then, 50 μM EdU working solution (RiboBio) was added to each well for another 2 h, followed by fixture with 4% formaldehyde solution for 30 min. After reaction with Apollo dye solution for 30 min, the cells were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (identifying the nuclei) for 30 min. Finally, EdU‐positive cells (green cells) were visualized under a fluorescence microscope and counted using NIS‐Elements BR 3.0 software.
Flow cytometry for cell apoptosis
After treatment with or without BBR for 48 h, collected breast cancer cells were washed with phosphate buffered saline (PBS) and resuspended in binding buffer. Subsequently, 5 μL annexin V‐FITC and 10 μL PI (Solarbio) in the dark were used to stain the cells, which then were analyzed using the flow cytometer and FlowJo software version 10.5.0.
Matrigel invasion assay
In brief, 1 × 105 breast cancer cells in 200 μL serum‐free medium were introduced into the upper chamber (BD Biosciences) with coated Matrigel (BD Biosciences). Meanwhile, 600 μL medium with 10% FBS was added to the lower chambers. After incubation at 37°C for 24 h, cells invaded into the lower side were immobilized by 4% paraformaldehyde and stained with 0.1% crystal violet solution. Finally, the penetrated cells were counted using a microscope in five randomly selected fields.
Wound healing assay
The activity of tumor cell migration was measured in this experiment. Briefly, cells in 24‐well plates were cultured until they reached approximately 90% confluency. After that, 200 mL sterile pipette tips were used to scratch across the surface of the cell monolayer (record 0 h). After removing the nonadherent cells, the tumor cells were cultured in serum‐free medium for 24 h. Finally, wound closure was captured under a microscope and analyzed using Image J software.
Real‐time quantitative polymerase chain reaction (RT‐qPCR)
In general, total RNAs from clinical samples and cell lines were extracted using TRIzol reagent (Invitrogen). Then, 2 μg of total RNAs were utilized to synthesize cDNA based on HiScript 1st strand cDNA synthesis kit (Vazyme). On ABI7900HT fast real‐time PCR system (Applied Biosystems), amplification reaction was conducted with a real‐time fluorescent quantitative PCR kit (Vazyme). The PCR conditions were as follows: 30 s at 95°C, followed by 40 cycles at 95°C for 5 s, 45°C for 30 s, and 72°C for 30 s. After being normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), RNA expression fold changes were calculated by the 2−ΔΔCt method. The primers used are shown in Table 1.
TABLE 1.
Primer sequences used for PCR.
| Name | Primers for PCR (5′‐3′) | |
|---|---|---|
| METTL3 | Forward | CAGAGGCAGCATTGTCTCCA |
| Reverse | ATGGACACAGCATCAGTGGG | |
| IGF2BP3 | Forward | CCCCCTCGGACCTAGAAAGT |
| Reverse | TAAACTATCCAGCACCTCCCAC | |
| FGF7 | Forward | AGTTGCACCAGGCAGACAA |
| Reverse | TCAGTTGCTGTGACGCTGTT | |
| GAPDH | Forward | GGAGCGAGATCCCTCCAAAAT |
| Reverse | GGCTGTTGTCATACTTCTCATGG |
Abbreviations: FGF7, fibroblast growth factor 7; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IGFBP3, insulin‐like growth factor‐2 mRNA‐binding protein 3 METTL3, methyltransferase‐like 3; PCR, polymerase chain reaction.
Western blot assay
Using radioimmunoprecipitation (RIPA) lysis buffer (Beyotime), total proteins from clinical tissues and cell lines were prepared. After being mixed with loading buffer for 5 min at 100°C, samples were subjected to 12% separating gel and then shifted onto polyvinylidene fluoride (PVDF) membranes (Invitrogen). After incubation with appropriate diluent primary antibodies: FGF7 (ab131162, Abcam), METTL3 (ab195352, Abcam), IGF2BP3 (ab177477, Abcam), and GAPDH (ab9485, Abcam) at 4°C overnight, the membranes were hybridized with secondary antibody for 2 h at room temperature. Finally, signals were visualized using an ECL kit (Solarbio).
Cell transfection
For FGF7, IGF2BP3, and METTL3 overexpression, human FGF7 cDNA (NM_002009.4), IGF2BP3 cDNA (NM_006547.3), and METTL3 cDNA (XM_054376403.1) were respectively synthesized and cloned into pcDNA vector (GenePharma), which then were transfected into MCF‐7 and MDA‐MB‐231 cells, with empty vector as a negative control. For knockdown system, FGF7, IGF2BP3, and METTL3 small interfering RNAs (si‐FGF7, si‐IGF2BP3, or si‐METTL3) or their control (si‐NC) from RiboBio were introduced into MCF‐7 and MDA‐MB‐231 cell using lipofectamine 3000 (Invitrogen) for 48 h at 37°C.
Methylated RNA immunoprecipitation (MeRIP)
In order to examine m6A modification of FGF7 mRNA, total RNAs from MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC, si‐METTL3 or without were isolated using TRIzol (Invitrogen). After being fragmented, one‐ninth of the mRNA was used as an “input” control. Meanwhile, the A/G immunomagnetic beads and anti‐m6A (Abcam) or IgG (Abcam) were premixed and then incubated with the rest mRNA. After being digested, m6A enrichment was subjected to RT‐qPCR analysis and input normalization.
RNA immunoprecipitation (RIP)
To further prove the binding ability between FGF7 mRNA and METTL3 or IGF2BP3, this assay was performed in MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC or si‐METTL3. In summary, complete RIP lysis buffer (Millipore) was used to obtain sufficient cell lysates, which were incubated with magnetic beads coupled with anti‐IGF2BP3 (Abcam) or anti‐IgG (Millipore) at 4°C overnight. After being digested and purified, RNAs from the RNA‐protein complex were determined using RT‐qPCR assay.
Measurement of mRNA stability
In brief, MCF‐7 cells with si‐NC or si‐METTL3 were harvested at 0, 3, 6, and 9 h after treatment with actinomycin D (act D, a transcriptional inhibitor, Sigma‐Aldrich). After being extracted, the half‐life of FGF7 mRNA was assessed using RT‐qPCR.
Tumor xenograft assay
In this animal experiment, 100 μL MCF‐7 cells (1 × 106 cells) were subcutaneously injected into female nude mice (5‐week‐old, Slaike Jingda Laboratory, Hunan, China). When the tumors attained volumes of about 40 mm3, the mice were randomly assigned to two groups (n = 5 each group): (1) the control group, mice were treated with saline; (2) the BBR group, mice were treated with 10 mg/kg BBR by gavage administration. During tumor growth, size was examined and calculated every 3 days. Then, 22 days later, tumor weight was measured after mice were euthanized by cervical dislocation after deep anesthesia with 2% isoflurane (Baxter Healthcare Corporation). In addition, dissected tumors were subjected to western blot and immunohistochemical (IHC) staining analysis. All processes involving this experiment were authorized by the Animal Ethics Committee of People's Hospital of Dongxihu District.
Statistical analysis
Data were analyzed using GraphPad Prism7 software and expressed in the form of mean ± standard deviation (SD). The statistical significance of the difference was assessed by student's t‐test or one‐way analysis of variance (ANOVA) with Tukey's tests. Statistical significance was accepted for p < 0.05.
RESULTS
BBR repressed breast cancer cell proliferation and metastasis
To investigate the effect of BBR in breast cancer cells, MCF‐7 and MDA‐MB‐231 cells were exposed to different concentrations of BBR (0, 20, 40, or 60 μM) for 48 h. After that, cell viability was assessed using MTT assay. Data exhibited that BBR treatment might decrease MCF‐7 and MDA‐MB‐231 cell viability in a dose‐dependent way (Figure 1a). Considering that no significant changes in suppression of tumor cell viability after 40 μM or 60 μM BBR treatment, we selected 40 μM for subsequent experiments. As shown in Figure 1b, BBR treatment might apparently block the number of EdU‐positive cells in MCF‐7 and MDA‐MB‐231 cells relative to the control group. On the contrary, an obvious enhancement in apoptosis rate was observed after BBR exposure in MCF‐7 and MDA‐MB‐231 cells (Figure 1c). Furthermore, transwell results showed that the number of MCF‐7 and MDA‐MB‐231 cell invasions was clearly hindered after BBR exposure (Figure 1d, e). Meanwhile, the wound healing assay also presented the inhibitory role of BBR treatment on MCF‐7 and MDA‐MB‐231 cell migratory ability (Figure 1f). Collectively, these results suggested that BBR treatment might constrain breast cancer cell growth and metastasis.
FIGURE 1.
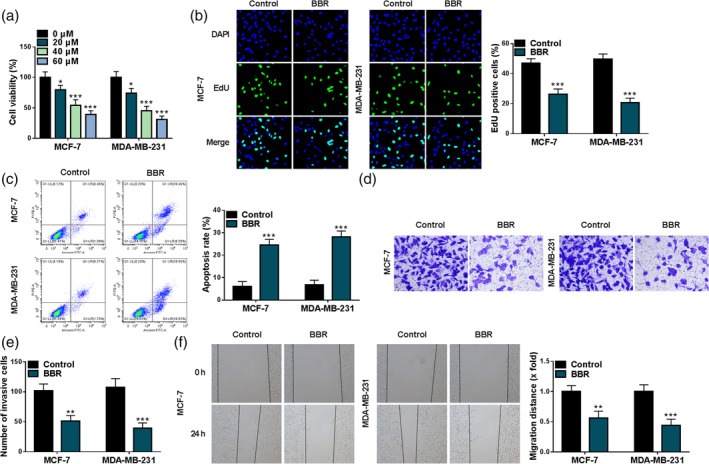
Effects of berberine (BBR) on breast cancer cell proliferation and metastasis. (a) A 5‐diphenyl‐2H‐tetrazolium bromide (MTT) assay was applied to measure cell viability in MCF‐7 and MDA‐MB‐231 cells exposed to different concentrations of BBR (0, 20, 40, and 60 μM). (b–f) MCF‐7 and MDA‐MB‐231 cells were treated with BBR (40 μM) or without (control). (b) A 5‐ethynyl‐2′‐deoxyuridine (EdU) assay was used to assess cell proliferation in treated MCF‐7 and MDA‐MB‐231 cells. (c) The cell apoptosis rate was examined using flow cytometry assay in treated MCF‐7 and MDA‐MB‐231 cells. (d and e) A transwell assay was performed to measure cell invasion in treated MCF‐7 and MDA‐MB‐231 cells. (f) Migration ability was detected using wound healing assay in treated MCF‐7 and MDA‐MB‐231 cells. *p < 0.05, **p < 0.01, ***p < 0.001.
FGF7 expression was reduced in BBR‐treated breast cancer cells
Furthermore, the GSE85871 database microarray data set from the Gene Expression Omnibus (GEO) database was applied in an optimized analysis to identify differentially expressed genes in breast cancer treated with BBR. As a result, we found that FGF7 expression was significantly downregulated in the BBR group (Figure 2a), implying that FGF7 might partake in the BBR‐mediated breast cancer process. Moreover, we further validated that the mRNA level and protein level of FGF7 were remarkably hindered in BBR‐induced MCF‐7 and MDA‐MB‐231 cells compared with the control group (Figure 2b, c). In addition, FGF7 mRNA level and protein level were highly expressed in 29 breast cancer samples versus 29 normal tissues (Figure 2d, e). Simultaneously, the significant downregulation of FGF7 was found in breast cancer cell lines (MCF‐7 and MDA‐MB‐231) in comparison with MCF‐10A cells (Figure 2f). Together, these data highlighted that dysregulated FGF7 might be associated with BBR‐mediated breast cancer progression.
FIGURE 2.
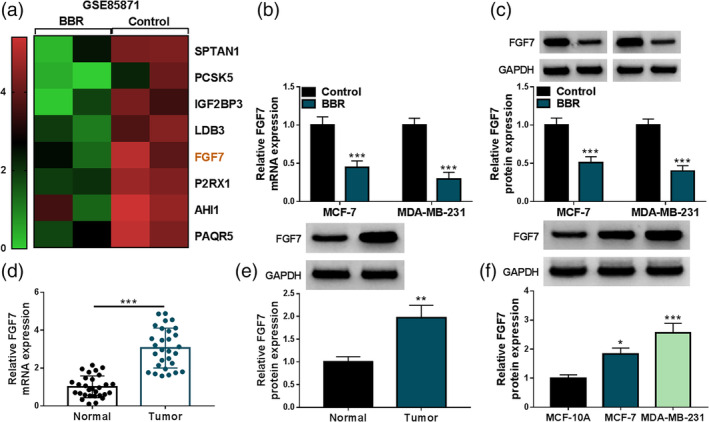
Berberine (BBR) might decrease the levels of fibroblast growth factor 7 (FGF7) in breast cancer cells. (a) The GSE85871 database was used to analyze the expression level of FGF7 in the control and BBR groups. (b and c) FGF7 mRNA level and protein level were determined in MCF‐7 and MDA‐MB‐231 cells treated with 40 μM BBR or control using real‐time quantitative polymerase chain reaction (RT‐qPCR) and western blot. (d and e) RT‐qPCR and western blot analysis of FGF7 mRNA level and protein level in 29 breast cancer tissues and 29 normal tissues. (f) FGF7 protein level was assessed in MCF‐10A cells and breast cancer cell lines (MCF‐7 and MDA‐MB‐231). *p < 0.05, ***p < 0.001.
BBR treatment suppressed breast cancer cell malignant phenotypes by modulating FGF7
Then, to check the impact of FGF7 on BBR‐mediated malignant phenotype inhibition of breast cancer cells, in vitro gain‐function analysis was conducted in MCF‐7 and MDA‐MB‐231 cells. At first, FGF7 protein level were markedly increased in pcDNA‐FGF7‐transfected cells compared with the pcDNA group (Figure 3a), suggesting that the overexpression efficiency is available. Under BBR treatment, MTT and EdU assays displayed that the overexpression of FGF7 might effectively abolish BBR‐mediated cell viability and proliferation inhibition in MCF‐7 and MDA‐MB‐231 cells (Figure 3b, c). Furthermore, BBR‐induced MCF‐7 and MDA‐MB‐231 cell apoptosis rates were distinctly relieved through FGF7 upregulation (Figure 3d, e). In addition, transwell and wound healing assays showed that the suppressive role of BBR exposure on MCF‐7 and MDA‐MB‐231 cell invasion and migration were evidently abrogated after pcDNA‐FGF7 introduction (Figure 3f, g). In addition, our data also confirmed that upregulation of FGF7 might partly relieve the repression of BBR exposure on the growth and metastasis of BT‐474 and BT‐549 cells (Figure S1). Collectively, these findings implied that FGF7 overexpression partially attenuated the inhibitory role of BBR treatment on breast cancer cell growth and metastasis in vitro.
FIGURE 3.
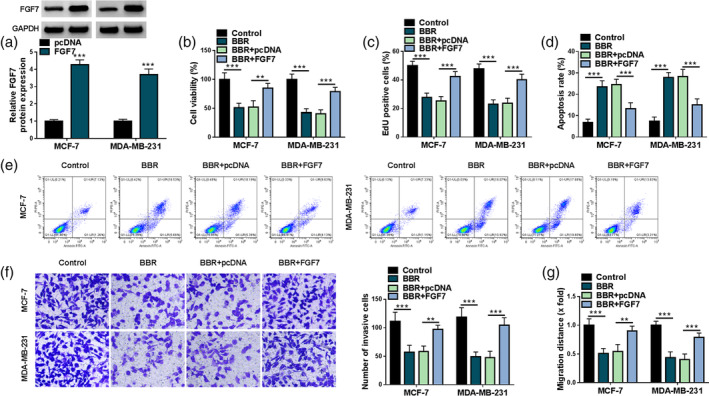
Overexpression of fibroblast growth factor 7 (FGF7) might overturn the effects of berberine (BBR) on breast cancer cell malignant phenotypes. (a) FGF7 protein level was determined in MCF‐7 and MDA‐MB‐231 cells transfected with pcDNA or FGF7 using western blot. (b–g) MCF‐7 and MDA‐MB‐231 cells were treated with control, BBR, BBR + pcDNA, and BBR + FGF7. (b) Cell viability was assessed using a 5‐diphenyl‐2H‐tetrazolium bromide (MTT) assay in treated MCF‐7 and MDA‐MB‐231 cells. (c) A 5‐ethynyl‐2′‐deoxyuridine (EdU) assay was used to measure cell proliferation in treated MCF‐7 and MDA‐MB‐231 cells. (d and e) Flow cytometry assay was applied to analyze cell apoptosis rate in treated MCF‐7 and MDA‐MB‐231 cells. (f and g) Transwell and wound healing assays were employed to examine cell invasion and migration in treated MCF‐7 and MDA‐MB‐231 cells. **p < 0.01, ***p < 0.001.
METTL3 might improve the stability and expression of FGF7 mRNA by regulating m6A methylation
Based on MeRIP analysis, we found that FGF7 contained abundant m6A methylation (Figure 4a). Subsequently, SRAMP (an online tool to predict m6A sites) database discovered that FGF7 mRNA possessed the potential m6A sites (Figure 4b). In addition, the knockdown efficiency of METTL3 in MCF‐7 and MDA‐MB‐231 cells was detected and shown in Figure 4c. After that, RT‐qPCR results showed that the FGF7 mRNA level was apparently blocked by METTL3 knockdown in MCF‐7 and MDA‐MB‐231 cells (Figure 4d). Moreover, MeRIP‐qPCR analysis presented that the downregulation of METTL3 might apparently decrease the m6A level of FGF7 mRNA in MCF‐7 and MDA‐MB‐231 cells (Figure 4e). To further validate their interaction, RIP assay was carried out. Data showed that FGF7 RNA enrichment was significantly reduced in the si‐METTL3 group relative to the si‐NC group (Figure 4f). In addition, the actinomycin D experiment showed that METTL3 knockdown might enhance the degradation rate of mRNA in MCF‐7 cells (Figure 4g). Beyond that, western blot assay presented that METTL3 content remarkably declined in BBR‐treated MCF‐7 and MDA‐MB‐231 cells compared with the control group (Figure 4h). Collectively, these results indicated that METTL3 might sustain FGF7 mRNA stability through m6A modification.
FIGURE 4.
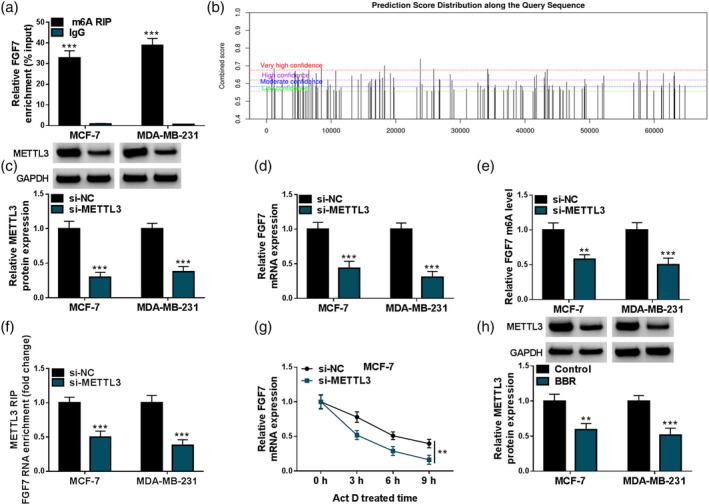
Fibroblast growth factor 7 (FGF7) was regulated by methyltransferase‐like 3 (METTL3)‐mediated m6A methylation. (a) Methylated RNA immunoprecipitation (MeRIP) assay was used to assess the m6A methylation level of FGF7 in MCF‐7 and MDA‐MB‐231 cells. (b) SRAMP software predicted that FGF7 mRNA had an m6A site. (c and d) METTL3 protein level and FGF7 mRNA level were examined using MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC or si‐METTL3 using real‐time quantitative polymerase chain reaction (RT‐qPCR) or western blot. (e) Changes in the m6A methylation level of FGF7 after METTL3 knockdown were assessed using MeRIP‐qPCR assay. (f) Their binding was verified using RNA immunoprecipitation (RIP) assay in MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC or si‐METTL3. (g) Influences of METTL3 downregulation on FGF7 mRNA stability after actinomycin D treatment was assessed using RT‐qPCR in MCF‐7 cells. (h) FGF7 protein levels were examined in MCF‐7 and MDA‐MB‐231 cells treated with berberine (BBR) or control using western blot. **p < 0.01, ***p < 0.001.
IGF2BP3 recognized the m6A modification of FGF7 mRNA and enhanced its expression
Previous studies have shown that the effects of m6A modifications on mRNA transcripts require that these modifications be recognized by m6A binding proteins called m6A readers. Therefore, we knocked down IGF2BP1, IGF2BP2, and IGF2BP3 in MCF‐7 cells. Among these reader proteins, our data displayed that the downregulation of IGF2BP3 might obviously decrease FGF7 mRNA levels in breast cancer cells (Figure 5a). Beyond that, the GSE85871 database determined that BBR treatment might remarkably decrease the IGF2BP3 mRNA level (Figure 5b). Moreover, we further validated that IGF2BP3 protein level was clearly repressed in BBR‐treated MCF‐7 and MDA‐MB‐231 cells versus the control group (Figure 5c). Apart from that, FGF7 RNA enrichment was significantly increased in the IGF2BP3 group compared with the IgG group when METTL3 knockdown in MCF‐7 cells (Figure 5d), suggesting that silencing METTL3 inhibited the binding of FGF7 RNA to IGF2BP3. In addition, the overexpression efficiency of IGF2BP3 in MCF‐7 and MDA‐MB‐231 cells was measured and displayed in Figure 5e. Then, western blot assay presented that the cotransfection of pcDNA‐IGF2BP3 might significantly abolish the repression of METTL3 deficiency on FGF7 protein level in MCF‐7 and MDA‐MB‐231 cells (Figure 5f). Overall, these results suggested that METTL3 might increase FGF7 expression by regulating the RNA m6A modification, which might be recognized by IGF2BP3 and is essential for mRNA stability.
FIGURE 5.
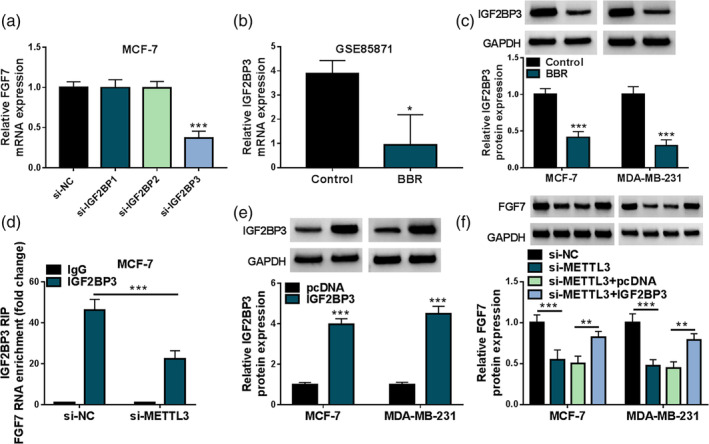
IGF2BP3 mediated the M6A methylation of fibroblast growth factor 7 (FGF7). (a) FGF7 mRNA level was determined in MCF‐7 cells transfected with si‐NC, si‐IGF2BP1, si‐IGF2BP2, or si‐IGF2BP3 using real‐time quantitative polymerase chain reaction (RT‐qPCR). (b) The GSE85871 database was used to analyze the expression level of IGF2BP3 in the control and BBR groups. (c) Western blot analysis of IGF2BP3 protein level in MCF‐7 and MDA‐MB‐231 cells treated with berberine (BBR) or control. (d) Interaction between FGF7 and IGF2BP3 was assessed in MCF‐7 cells transfected with si‐NC or si‐METTL3 using RNA immunoprecipitation (RIP) assay. (e) IGF2BP3 protein level was determined in MCF‐7 and MDA‐MB‐231 cells transfected with pcDNA or IGF2BP3 using western blot. (f) Western blot analysis of FGF7 protein level in MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC, si‐METTL3, si‐METTL3 + pcDNA, or si‐METTL3 + IGF2BP3. *p < 0.05, **p < 0.01, ***p < 0.001.
Knockdown of METTL3 might block breast cancer cell malignant phenotypes by targeting FGF7
Next, to further explore whether the influence of METTL3 on breast cancer cell development was mediated by regulating FGF7, rescue experiments were performed in MCF‐7 and MDA‐MB‐231 cells. Western blot results exhibited that the lack of METTL3 might strikingly dampen FGF7 protein level in MCF‐7 and MDA‐MB‐231 cells, which were partly overturned after pcDNA‐FGF7 co‐transfection (Figure 6a). Functionally, reduced cell viability and proliferation were observed due to the downregulation of METTL3, whereas these effects were ameliorated by FGF7 overexpression (Figure 6b, c). Beyond that, METTL3 silencing‐mediated apoptosis rate promotion was partly mitigated through the upregulation of FGF7 in MCF‐7 and MDA‐MB‐231 cells (Figure 6d, e). In addition, the forced expression of FGF7 might markedly abrogate the repression of METTL3 knockdown on MCF‐7 and MDA‐MB‐231 cell invasion and migration (Figure 6f, g). Altogether, the above‐mentioned results indicated that the METTL3 downregulation might impede breast cancer cell malignancy by modulating FGF7 in vitro.
FIGURE 6.
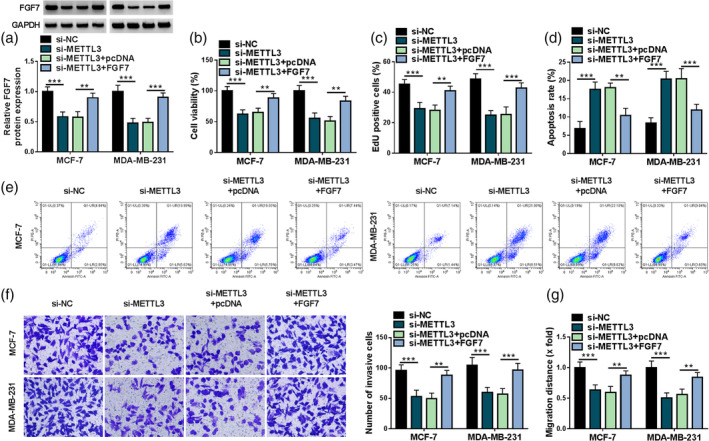
Effects of methyltransferase‐like 3 (METTL3) and fibroblast growth factor 7 (FGF7) on breast cancer progression. MCF‐7 and MDA‐MB‐231 cells transfected with si‐NC, si‐METTL3, si‐METTL3 + pcDNA, or si‐METTL3+ FGF7. (a) FGF7 protein level was determined using western blot in transfected MCF‐7 and MDA‐MB‐231 cells. (b and c) Cell viability and proliferation were examined using 5‐diphenyl‐2H‐tetrazolium bromide (MTT) and 5‐ethynyl‐2′‐deoxyuridine (EdU) assays. (d and e) Cell apoptosis rate was examined using flow cytometry assay. (f and g) Cell invasion and migration were measured using transwell and wound healing assays. **p < 0.01, ***p < 0.001.
BBR treatment might inhibit FGF7 expression by regulating METTL3
As previously mentioned, we speculated that BBR treatment might repress breast cancer progression partly by the METTL3/FGF7 regulation pathway. To prove the assumption, we further investigated whether BBR exposure might affect the expression of FGF7 by METTL3. First, western blot results exhibited that METTL3 protein level was clearly improved in pcDNA‐METTL3‐transfected MCF‐7 and MDA‐MB‐231 cells compared with the pcDNA group (Figure 7a), indicating that the overexpression efficiency is successful. After that, BBR treatment might significantly dwindle FGF7 protein level in MCF‐7 and MDA‐MB‐231 cells, while these effects were partly abolished by METTL3 overexpression (Figure 7b, c). Collectively, these findings implied that BBR might exert its function by regulating the METTL3/FGF7 pathway.
FIGURE 7.
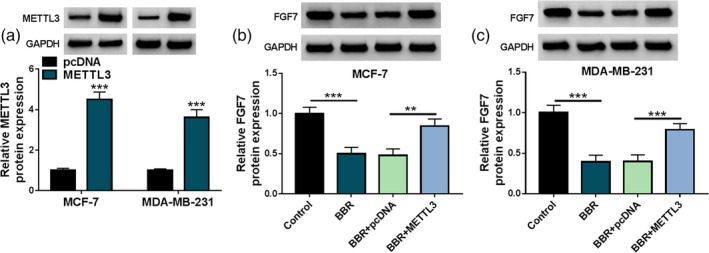
Berberine (BBR) affected fibroblast growth factor 7 (FGF7) expression by regulating methyltransferase‐like 3 (METTL3). (a) METTL3 protein level was detected in MCF‐7 and MDA‐MB‐231 cells transfected with pcDNA or METTL3 using western blot. (b and c) FGF7 protein level was in MCF‐7 and MDA‐MB‐231 cells treated with control, BBR, BBR + pcDNA, or BBR + METTL3 using western blot. **p < 0.01, ***p < 0.001.
BBR might hinder breast cancer cell growth in vivo
Additionally, mice xenograft models of breast cancer were established to explore the influence of BBR treatment on tumor growth. As shown in Figure 8a, b, BBR treatment significantly blocked tumor volume and weight, implying the repression of BBR exposure on tumor growth. Beyond that, western blot results presented that METTL3 and FGF7 protein levels were obviously lower in tumor tissues from the BBR group than in the control group (Figure 8c). In addition, IHC staining also uncovered that the positive expression rate of METTL3 and FGF7 was apparently decreased in the BBR group compared with the control group (Figure 8d). Collectively, these data illuminated that BBR treatment diminished breast cancer xenograft tumor growth in vivo.
FIGURE 8.
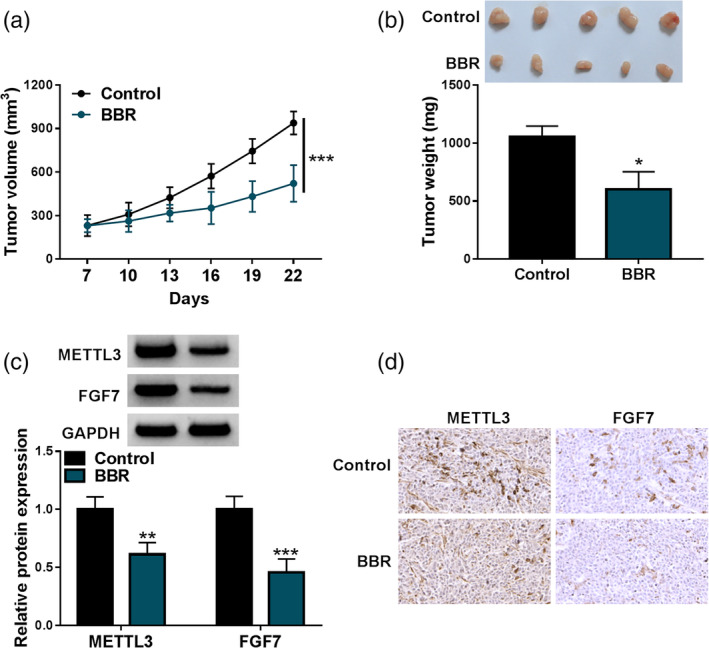
Berberine (BBR) blocked tumor growth in a xenograft model. MCF‐7 cells were inoculated subcutaneously into the nude mice. Then, mice with tumor formation were given 10 mg/kg BBR. (a) Growth curve of xenografted tumors was presented. (b) Weight of resected tumor masses was measured. (c) Methyltransferase‐like 3 (METTL3) and fibroblast growth factor 7 (FGF7) protein levels were assessed using western blot in the xenografts. (d) The positive expression rate of METTL3 and FGF7 was gauged in xenografts using immunohistochemical (IHC) staining. *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
In recent years, natural herbal extracts from TCM have attracted extensive attention in the field of tumor research for their distinctive benefits of multitargeting and minimal side impacts. 28 , 29 As a natural bioactive alkaloid mainly found in the Chinese herb Coptis chinensis, BBR possesses a broad range of pharmacological effects, containing antiapoptosis, antioxidative, antimicrobial, and anticancer activities. 9 , 30 Lately, it has become evident that BBR displays anticancer effects on various cancers, including breast cancer. 31 Herein, our data verified that BBR treatment might suppress breast cancer cell proliferation, invasion, migration, and induce apoptosis in vitro, in agreement with previous studies. 32 , 33 Consistently, the repression of BBR exposure on breast cancer growth has been validated in vivo. In total, the above findings demonstrated the anticancer activity of BBR in breast cancer.
Regarding the molecular mechanism, numerous studies have validated the antitumor effects of BBR occur in breast cancer via different biochemical mechanisms, containing regulating gene expression and signal pathways. 13 , 34 , 35 Herein, we used the GSE85871 database to screen FGF7, which clearly differs in BBR‐treated breast cancer. Beyond that, our data further verified that FGF7 content was obviously reduced in BBR‐triggered breast cancer cells. Of interest, FGF7 level was identified to be upregulated in breast cancer, consistent with a former study. 15 As a mesenchyme‐specific heparin‐binding growth factor, FGF7 was reported to bind FGF receptor 2 to affect various cellular processes. 36 , 37 It has been confirmed that the knockdown of FGF7 might hinder breast cancer cell growth and metastasis. 16 , 17 Therefore, we inferred that FGF7 might be implicated in BBR‐mediated antitumor activity. In the present study, our data confirmed that BBR‐triggered breast cancer growth and metastasis inhibition were partly overturned by FGF7 upregulation. These observations provide first‐hand evidence of the antitumor effects of BBR through the regulation of FGF7.
As a novel layer of epigenetic modulation, m6A is considered an abundant cotranscriptional modification in mRNA and participates in diverse aspects of post‐transcriptional mRNA metabolism. 38 , 39 Herein, our data identify for the first time an important role for METTL3 in mediating the m6A modification of FGF7 mRNA. Moreover, it has been confirmed that IGF2BPs might affect target mRNA expression by regulating the stability of target mRNA. 40 Meanwhile, IGF2BP3 might facilitate breast cancer cell proliferation through destabilizing NF1 mRNA via an m6A‐dependent way. 41 In the current study, this modification is recognized by IGF2BP3, which is responsible for maintaining the mRNA stability and expression of FGF7. In addition, METTL3 might increase PD‐L1 mRNA stability and its protein expression in an m6A‐IGF2BP3‐dependent manner, thereby repressing tumor immune surveillance in breast cancer. 26 Herein, our study showed that FGF7 overexpression might partially abolish the repression of METTL3 deficiency in breast cancer development. Some studies have indicated that METTL3 might be implicated in BBR‐or allocryptopine (an isoquinoline alkaloid extracted from Macleaya cordata)‐mediated anti‐ischemia–reperfusion injury or antitumor activity. 27 , 42 As expected, our data verified that METTL3 upregulation might partly abrogate BBR‐mediated FGF7 reduction in breast cancer cells, further supporting the regulatory mechanism of BBR/METTL3/FGF7 in breast cancer. In addition, previous studies have indicated that aberrant activation of the Wnt/β‐catenin signaling pathway is one of the main reasons for breast tumorigenesis and metastasis. 43 , 44 Meanwhile, it has been reported that BBR might suppress tumor growth in various human cancers by repressing Wnt/β‐catenin signaling, 45 , 46 containing breast cancer. 47 Furthermore, FGF7 might induce the accumulation of cytoplasmic β‐catenin. 48 Therefore, we will explore whether BBR treatment impedes the growth and metastasis of breast cancer cells through the METTL3/FGF7‐mediated Wnt/β‐catenin signaling pathway in further studies. In addition, the subcutaneous xenograft model used in this study may not fully mimic the natural breast cancer microenvironment. Hence, some of our findings may not be reproducible in the natural disease state, and the patient‐derived xenograft (PDX) model needs to be performed in future studies.
In conclusion, our results provided compelling evidence that BBR might hinder breast cancer cell growth and metastasis by regulating the METTL3/FGF7 axis, which provides novel strategies for the design of new METTL3/FGF7‐based antitumor agents and drug combinations.
AUTHOR CONTRIBUTIONS
Wei Fu conceived, designed and revised the current study. Wei Fu wrote the manuscript. Lixin Liu and Suiju Tong analyzed the data. All authors read and approved the final manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Supporting information
Figure S1. Upregulation of FGF7 might abolished the effects of BBR on breast cancer cell malignant phenotypes. (a) FGF7 protein level was measured in BT‐474 and BT‐549 cells transfected with pcDNA or FGF7 using western blot. (b–g) MCF‐7 and MDA‐MB‐231 cells were treated with control, BBR, BBR + pcDNA, and BBR + FGF7. (b and c) Cell viability and proliferation were assessed using MTT assay and EdU assay. (d and e) Cell apoptosis rate was analyzed using flow cytometry assay. (f and g) Cell invasion and migration were measured using transwell and wound healing assays. *p < 0.05, **p < 0.01, ***p < 0.001.
Fu W, Liu L, Tong S. Berberine inhibits the progression of breast cancer by regulating METTL3‐mediated m6A modification of FGF7 mRNA . Thorac Cancer. 2024;15(17):1357–1368. 10.1111/1759-7714.15321
Wei Fu and Lixin Liu contributed equally to this study.
REFERENCES
- 1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu JW, Charkhchi P, Adekunte S, Akbari MR. What is known about breast cancer in young women? Cancers (Basel). 2023;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zannetti A. Breast cancer: from pathophysiology to novel therapeutic approaches 2.0. Int J Mol Sci. 2023;24:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Courtney D, Davey MG, Moloney BM, Barry MK, Sweeney K, McLaughlin RP, et al. Breast cancer recurrence: factors impacting occurrence and survival. Ir J Med Sci. 2022;191:2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, et al. Traditional Chinese medicine (TCM) in the treatment of COVID‐19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang SQ, Jiang XX, Li JC. Traditional Chinese medicine in human diseases treatment: new insights of their potential mechanisms. Anat Rec (Hoboken). 2023;306:2920–2926. [DOI] [PubMed] [Google Scholar]
- 8. Samadi P, Sarvarian P, Gholipour E, Asenjan KS, Aghebati‐Maleki L, Motavalli R, et al. Berberine: a novel therapeutic strategy for cancer. IUBMB Life. 2020;72:2065–2079. [DOI] [PubMed] [Google Scholar]
- 9. Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14:564–582. [DOI] [PubMed] [Google Scholar]
- 10. He J, Wei Q, Jiang R, Luan T, He S, Lu R, et al. The Core‐targeted RRM2 gene of berberine hydrochloride promotes breast cancer cell migration and invasion via the epithelial‐mesenchymal transition. Pharmaceuticals (Basel). 2022;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu L, Sun L, Zheng J, Cui L. Berberine modulates keratin 17 to inhibit cervical cancer cell viability and metastasis. J Recept Signal Transduct Res. 2021;41:521–531. [DOI] [PubMed] [Google Scholar]
- 12. Zhong XD, Chen LJ, Xu XY, Liu YJ, Tao F, Zhu MH, et al. Berberine as a potential agent for breast cancer therapy. Front Oncol. 2022;12:993775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun Y, Wang W, Tong Y. Berberine inhibits proliferative ability of breast cancer cells by reducing Metadherin. Med Sci Monit. 2019;25:9058–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yen TT, Thao DT, Thuoc TL. An overview on keratinocyte growth factor: from the molecular properties to clinical applications. Protein Pept Lett. 2014;21:306–317. [DOI] [PubMed] [Google Scholar]
- 15. Penault‐Llorca F, Bertucci F, Adélaïde J, Parc P, Coulier F, Jacquemier J, et al. Expression of FGF and FGF receptor genes in human breast cancer. Int J Cancer. 1995;61:170–176. [DOI] [PubMed] [Google Scholar]
- 16. Zhu Y, Yang L, Chong QY, Yan H, Zhang W, Qian W, et al. Long noncoding RNA Linc00460 promotes breast cancer progression by regulating the miR‐489‐5p/FGF7/AKT axis. Cancer Manag Res. 2019;11:5983–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang B, Zhang Y, Sun P, Lin J, Wang C. Knockdown of circADAM9 inhibits cell progression and glycolysis by targeting the miR‐1236‐3p/FGF7 axis in breast cancer. Thorac Cancer. 2023;14:2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan M, Pan D, Zhang M, Leng X, Yao B. The aqueous extract of Eucommia leaves promotes proliferation, differentiation, and mineralization of osteoblast‐like MC3T3‐E1 cells. Evid Based Complement Alternat Med. 2021;2021:3641317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Harada BT, He C. Regulation of gene expression by N(6)‐methyladenosine in cancer. Trends Cell Biol. 2019;29:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun T, Wu R, Ming L. The role of m6A RNA methylation in cancer. Biomed Pharmacother. 2019;112:108613. [DOI] [PubMed] [Google Scholar]
- 21. Petri BJ, Klinge CM. m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer. J Mol Endocrinol. 2023;70:e220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–624. [DOI] [PubMed] [Google Scholar]
- 24. Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)‐methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai Y, Yang C, Wu R, Huang L, Song S, Li W, et al. YTHDF1 regulates tumorigenicity and cancer stem cell‐like activity in human colorectal carcinoma. Front Oncol. 2019;9:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan W, Ao X, Chen Q, Yu Y, Ao L, Xing W, et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)‐methyladenosine modification of PD‐L1 mRNA in breast cancer. Mol Cancer. 2022;21:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu J, Duan H, Zou J, Ding W, Wei Z, Peng Q, et al. METTL3‐dependent N6‐methyladenosine modification is involved in berberine‐mediated neuroprotection in ischemic stroke by enhancing the stability of NEAT1 in astrocytes. Aging (Albany NY). 2024;16:299–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liauw W. Western medicine and traditional Chinese medicine: encouraging the twain to meet. Intern Med J. 2021;51:833–834. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Wong YK, Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20:e4. [DOI] [PubMed] [Google Scholar]
- 30. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33:504–523. [DOI] [PubMed] [Google Scholar]
- 31. Xiong RG, Huang SY, Wu SX, Zhou DD, Yang ZJ, Saimaiti A, et al. Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules. 2022;27:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Zhou Q, Chen F, Gao X, Yang L, Jin X, et al. Berberine inhibits breast carcinoma proliferation and metastasis under hypoxic microenvironment involving gut microbiota and endogenous metabolites. Pharmacol Res. 2023;193:106817. [DOI] [PubMed] [Google Scholar]
- 33. Lin YS, Chiu YC, Tsai YH, Tsai YF, Wang JY, Tseng LM, et al. Different mechanisms involved in the berberine‐induced antiproliferation effects in triple‐negative breast cancer cell lines. J Cell Biochem. 2019;120:13531–13544. [DOI] [PubMed] [Google Scholar]
- 34. Ponnusamy L, Kothandan G, Manoharan R. Berberine and emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3‐induced mTOR and Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165897. [DOI] [PubMed] [Google Scholar]
- 35. El Khalki L, Maire V, Dubois T, et al. Berberine impairs the survival of triple negative breast cancer cells: cellular and molecular analyses. Molecules. 2020;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang T, Wang L, Liu D, Li P, Xiong H, Zhuang L, et al. FGF7/FGFR2 signal promotes invasion and migration in human gastric cancer through upregulation of thrombospondin‐1. Int J Oncol. 2017;50:1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mieczkowski K, Kitowska K, Braun M, Galikowska‐Bogut B, Gorska‐Arcisz M, Piasecka D, et al. FGF7/FGFR2‐JunB signalling counteracts the effect of progesterone in luminal breast cancer. Mol Oncol. 2022;16:2823–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gilbert WV, Bell TA, Schaening C. Messenger RNA modifications: form, distribution, and function. Science. 2016;352:1408–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu TY, Hong LL, Ling ZQ. Oncofetal protein IGF2BPs in human cancer: functions, mechanisms and therapeutic potential. Biomark Res. 2023;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, Shi L, Sun HD, Wang ZW, Xu F, Wei JF, et al. IGF2BP3 mediates the mRNA degradation of NF1 to promote triple‐negative breast cancer progression via an m6A‐dependent manner. Clin Transl Med. 2023;13:e1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gong J, Wang C, Zhang F, Lan W. Effects of Allocryptopine on the proliferation and epithelial‐mesenchymal transition of oral squamous cell carcinoma through m6A mediated hedgehog signaling pathway. J Environ Pathol Toxicol Oncol. 2022;41:15–24. [DOI] [PubMed] [Google Scholar]
- 43. Zhu L, Tian Q, Gao H, Wu K, Wang B, Ge G, et al. PROX1 promotes breast cancer invasion and metastasis through WNT/β‐catenin pathway via interacting with hnRNPK. Int J Biol Sci. 2022;18:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen ZH, Tian Y, Zhou GL, Yue HR, Zhou XJ, Ma HY, et al. CMTM7 inhibits breast cancer progression by regulating Wnt/β‐catenin signaling. Breast Cancer Res. 2023;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li SY, Shi CJ, Fu WM, Zhang JF. Berberine inhibits tumour growth in vivo and in vitro through suppressing the lincROR‐Wnt/β‐catenin regulatory axis in colorectal cancer. J Pharm Pharmacol. 2023;75:129–138. [DOI] [PubMed] [Google Scholar]
- 46. Vishnoi K, Ke R, Saini KS, Viswakarma N, Nair RS, das S, et al. Berberine represses β‐catenin translation involving 4E‐BPs in hepatocellular carcinoma cells. Mol Pharmacol. 2021;99:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dian L, Xu Z, Sun Y, Li J, Lu H, Zheng M, et al. Berberine alkaloids inhibit the proliferation and metastasis of breast carcinoma cells involving Wnt/β‐catenin signaling and EMT. Phytochemistry. 2022;200:113217. [DOI] [PubMed] [Google Scholar]
- 48. Liu XY, Li X, Bai MR, Chen X, Wang CL, Xie J, et al. FGF‐7 dictates osteocyte cell processes through Beta‐catenin transduction. Sci Rep. 2018;8:14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Upregulation of FGF7 might abolished the effects of BBR on breast cancer cell malignant phenotypes. (a) FGF7 protein level was measured in BT‐474 and BT‐549 cells transfected with pcDNA or FGF7 using western blot. (b–g) MCF‐7 and MDA‐MB‐231 cells were treated with control, BBR, BBR + pcDNA, and BBR + FGF7. (b and c) Cell viability and proliferation were assessed using MTT assay and EdU assay. (d and e) Cell apoptosis rate was analyzed using flow cytometry assay. (f and g) Cell invasion and migration were measured using transwell and wound healing assays. *p < 0.05, **p < 0.01, ***p < 0.001.


