Abstract
We have previously shown that two inhibitors specific for cellular cyclin-dependent kinases (cdks), Roscovitine (Rosco) and Olomoucine (Olo), block the replication of herpes simplex virus (HSV). Based on these results, we demonstrated that HSV replication requires cellular cdks that are sensitive to these drugs (L. M. Schang, J. Phillips, and P. A. Schaffer. J. Virol. 72:5626–5637, 1998). We further established that at least two distinct steps in the viral replication cycle require cdks: transcription of immediate-early (IE) genes and transcription of early (E) genes (L. M. Schang, A. Rosenberg, and P. A. Schaffer, J. Virol. 73:2161–2172, 1999). Since Rosco inhibits HSV replication efficiently even when added to infected cells at 6 h postinfection, we postulated that cdks may also be required for viral functions that occur after E gene expression. In the study presented herein, we tested this hypothesis directly by measuring the efficiency of viral replication, viral DNA synthesis, and expression of several viral genes during infections in which Rosco was added after E proteins had already been synthesized. Rosco inhibited HSV replication, and specifically viral DNA synthesis, when the drug was added at the time of release from a 12-h phosphonoacetic acid (PAA)-induced block in viral DNA synthesis. Inhibition of DNA synthesis was not a consequence of inhibition of expression of IE or E genes in that Rosco had no effect on steady-state levels of two E transcripts under the same conditions in which it inhibited viral DNA synthesis. Moreover, viral DNA synthesis was inhibited by Rosco even in the absence of protein synthesis. In a second series of experiments, the replication of four HSV mutants harboring temperature-sensitive mutations in genes essential for viral DNA replication was inhibited when Rosco was added at the time of shift-down from the nonpermissive to the permissive temperature. Viral DNA synthesis was inhibited by Rosco under these conditions, whereas expression of viral E genes was not affected. We conclude that cellular Rosco-sensitive cdks are required for replication of viral DNA in the presence of viral E proteins. This requirement may indicate that HSV DNA synthesis is functionally linked to transcription, which requires cdks, or that both viral transcription and DNA replication, independently, require viral or cellular factors activated by Rosco-sensitive cdks.
Herpes simplex virus (HSV) replicates in both cycling and noncycling cells, including terminally differentiated neurons. HSV replication, however, requires cellular functions associated with cell cycle progression. Thus, HSV replicates more efficiently in actively dividing than growth-arrested cells. This difference in replication efficiency is especially prominent among viral mutants which lack specific viral functions such as those provided by the regulatory proteins ICP0 and VP16 or by enzymes involved in nucleotide metabolism, such as thymidine kinase (TK) or ribonucleotide reductase (9, 16, 25). The dependence on cell cycle-activated cellular functions is further evidenced by the fact that HSV cannot replicate in temperature-sensitive (ts) cells growth-arrested in G0/G1 at the nonpermissive temperature (78, 86). At the molecular level, several cellular proteins, DNA binding activities, and other cellular activities normally involved in cell cycle progression, including HCF, E2F, cdk-2, cyclin D3, and DNA polymerase (Pol) α, are (i) required for viral gene expression, (ii) activated during HSV infection, (iii) localize to the sites of viral replication, and/or (iv) interact physically with HSV proteins (26, 33, 37, 45, 80, 83, 84). Furthermore, we have recently shown that cellular cyclin-dependent kinases (cdks) are required, directly or indirectly, for the transcription of HSV immediate-early (IE) and early (E) genes and hence for viral replication (44, 70, 71).
Cellular cdks comprise a family of serine-threonine protein kinases which, at present, includes nine members. The activities of these enzymes are regulated by association with specific cyclin partners, phosphorylation, and interaction with cdk inhibitors (cdis) such as p21CIP, p27KIP1, p16INK4a, and p15INK4b, whose expression is itself tightly regulated. Thus, the most active form of a typical cdk occurs when it is associated with its appropriate cyclin partner, phosphorylated by the cdk-activating kinase at specific residues, dephosphorylated by Cdc25 at other specific residues, and not complexed with cdis (reviewed in references ;[31, 39, 54, and 65]). Of all known cdks, cdk-1, cdk-2, cdk-3, cdk-4, cdk-6, and cdk-7 are recognized as key cell cycle regulators in that they directly and indirectly regulate DNA synthesis, mitosis, and transcription of genes whose products are required for cell cycle progression. Moreover, inhibition of the activity of cdk-1, cdk-2, or cdk-3 results in cell cycle arrest (79). With regard to the involvement of cdks in transcription, cdk-7 and cdk-8 phosphorylate directly the components of the cellular transcriptional complex whereas cdk-1, cdk-2, cdk-4, and cdk-6 directly and indirectly regulate the activity of several transcription factors (4, 13, 19, 20, 24, 34, 47, 49, 52, 66, 67, 73, 74, 76, 79, 85). With regard to the involvement of cdks in DNA synthesis, cdk-2 is required for this process in that DNA synthesis does not occur if cdk-2 or one of its cyclin partners, cyclin A, is blocked with specific antibodies (10, 22, 62, 68, 88). Mechanistically, activation of cdk-2 is required for expression of many cellular DNA replication proteins and cdk-2 phosphorylates and activates several of the cellular proteins that are required for DNA synthesis (6, 10, 11, 40, 48).
In addition to their roles in cellular functions, cdks are known to be involved directly or indirectly in viral DNA synthesis. For example, simian virus 40 and polyomavirus large T antigens, which initiate viral DNA replication, are activated by cdk-2-mediated phosphorylation (12, 28, 51). cdks are also required for human cytomegalovirus HCMV DNA replication in that cdk inhibitors block HCMV DNA synthesis but do not inhibit the expression of IE or E proteins (7).
Our previous studies have shown that, like other DNA viruses, cdks are also required for HSV replication (44, 70, 71). Specifically, we have shown that HSV replication is blocked by two cdk inhibitors, roscovitine (Rosco) (17, 56) and olomoucine (Olo) (1, 8, 23, 72, 81). Rosco and Olo specifically inhibit a subset of cdks which includes cdk-1, cdk-2, and cdk-5 but not cdk-4 or cdk-6. In vivo, Rosco inhibits cell cycle progression at concentrations between 10 and 100 μM, depending on the cell line used (2, 38, 56). This inhibition of cell cycle progression has been determined to be mediated by inhibition of cdk-1 and cdk-2 activities (2, 56). Other inhibitors which are specific for protein kinases or cell cycle progression but which do not inhibit cdks had no significant effect on HSV replication (70). With respect to individual viral functions that require cdks, Rosco inhibited the transcription of IE and E genes as well as viral DNA synthesis (44, 70, 71). As noted in these previous studies, however, inhibition of viral DNA synthesis could have resulted exclusively from the block in expression of IE and E proteins. Alternatively, HSV DNA synthesis could have been inhibited as a result of inhibition of IE and E protein expression and inhibition of cdks required directly for viral DNA synthesis. Although we were unable to discriminate between these two alternatives in the previous studies, we noted that Rosco inhibited HSV replication even when added to infected cells at 6 h postinfection (p.i.), when E proteins should already have been synthesized (71). These unexpected results prompted us to ask whether Rosco also inhibits essential viral replication functions, such as DNA synthesis, that occur after E proteins have been expressed.
In the experiments described herein, we examined the effects of Rosco on HSV DNA synthesis under conditions in which IE and E proteins had already been synthesized. We found that Rosco inhibits HSV replication after release from a block in viral DNA synthesis induced by phosphonoacetic acid (PAA) or following a shift-down of cells infected with HSV-1 DNA− ts mutants from the nonpermissive to the permissive temperature. Under these conditions, Rosco-induced inhibition of viral replication was shown to occur at the level of viral DNA synthesis whereas expression of E proteins was not affected. Based on these observations, we conclude that HSV DNA synthesis, as well as transcription of IE and E genes, requires the activities of Rosco-sensitive cellular cdks.
MATERIALS AND METHODS
Cells, virus, plasmids, and drugs.
Methods used for the growth and maintenance of Vero cells and for the propagation of a low-passage (p9), plaque-purified, stock of HSV-1, strain KOS, have been described previously (70). DNA− ts mutants of HSV-1 KOS, tsA1, tsA15, tsD9, and tsP23 (41, 63, 69), and plasmids prpTK and prp8 have also been described (43).
Rosco, PAA, and cycloheximide (CHX) were prepared and diluted as described previously (70, 71). Final concentrations of drugs were 100 μM Rosco, 100 or 400 μg of PAA per ml, as indicated in the text and figure legends, and 50 μg of CHX per ml in all experiments.
Infections.
Vero cells (105) were infected at the indicated multiplicities with HSV-1 KOS as previously described (70, 71). Where indicated, medium overlying infected cells was replaced with fresh drug-containing or control (drug-free) medium. Viral titers at specific times p.i. were determined by standard plaque assays (70, 71).
For experiments in which the time of addition of Rosco was varied (see Fig. 1), drug-free medium was removed from infected cells at the indicated times p.i. and replaced with 2 volumes of drug-containing medium. Two volumes of medium was used to dilute any residual drug-free medium remaining on the monolayers after the washes.
FIG. 1.
Effect of Rosco on HSV replication when added at 3-h intervals after infection. Vero cells were infected with 2.5 PFU of HSV-1 per cell. After 1 h of adsorption, the cells were washed and overlaid with medium containing no drug (control) or 100 μM Rosco. At 3, 6, 9, 12, 15, 18, and 21 h p.i., medium was removed and replaced with medium containing 100 μM Rosco (dotted lines, solid triangles). Changes from drug-free to Rosco-containing medium are indicated by the arrows. A second set of infected monolayers was left in drug-free medium (solid line, solid squares). At 1, 3, 6, 9, 12, 18, 21, and 24 h p.i., cultures were harvested and viral titers were determined by a standard plaque assay. Viral titers are plotted as a function of time p.i. Each time point indicates the average of two independent experiments.
For drug replacement and drug release experiments (see Fig. 2 to 5), drug-containing medium was removed from infected monolayers at the indicated times p.i. and infected cells were then washed twice with phosphate-buffered saline containing the same concentration of drug to be added to the cultures after washing. Afterwards, 2 volumes of drug-free medium or medium containing CHX, PAA, and/or Rosco was added to each monolayer. Two volumes of medium was used to dilute any residual drug remaining on the monolayers after the washes.
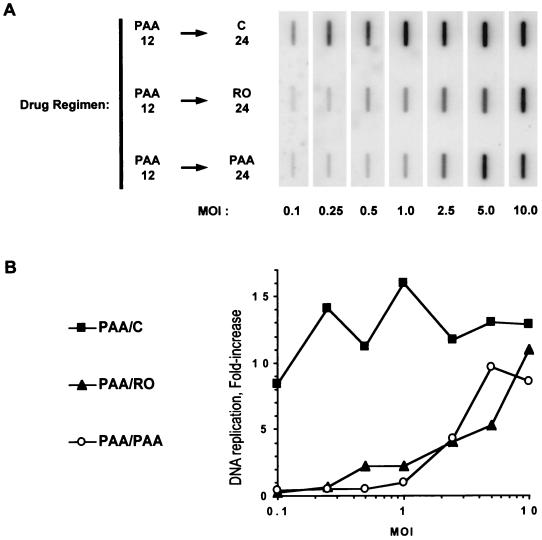
FIG. 2.
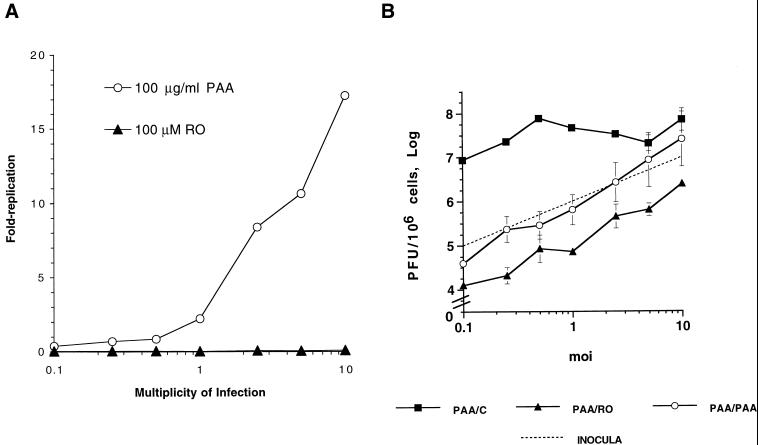
The inhibition of HSV replication by Rosco added at the time of release from a 12-h PAA block is multiplicity dependent. (A) Vero cells were infected with HSV-1 at the indicated multiplicities of infection, washed, and overlaid with medium containing 100 μg of PAA per ml or 100 μM Rosco. Infected monolayers were harvested at 24 h p.i., and viral replication was calculated by dividing the viral titer at 24 h p.i. by the adsorbed PFU. (B) Vero cells were infected with HSV-1 at the indicated multiplicities, washed, and overlaid with medium containing 100 μg of PAA per ml. At 12 h p.i., medium was removed from infected monolayers and replaced with fresh medium containing no drug (PAA/C), 100 μg of PAA per ml (PAA/PAA), or 100 μM Rosco (PAA/RO). At 24 h after the change of medium, cells were harvested and viral titers were determined by a standard plaque assay. Viral yields at 24 h p.i. are plotted against the multiplicities of infection (moi). For reference, the dotted line indicates the number of PFU/106 cells in inocula at each multiplicity. The average and range of two independent experiments are shown for each time point.
FIG. 5.
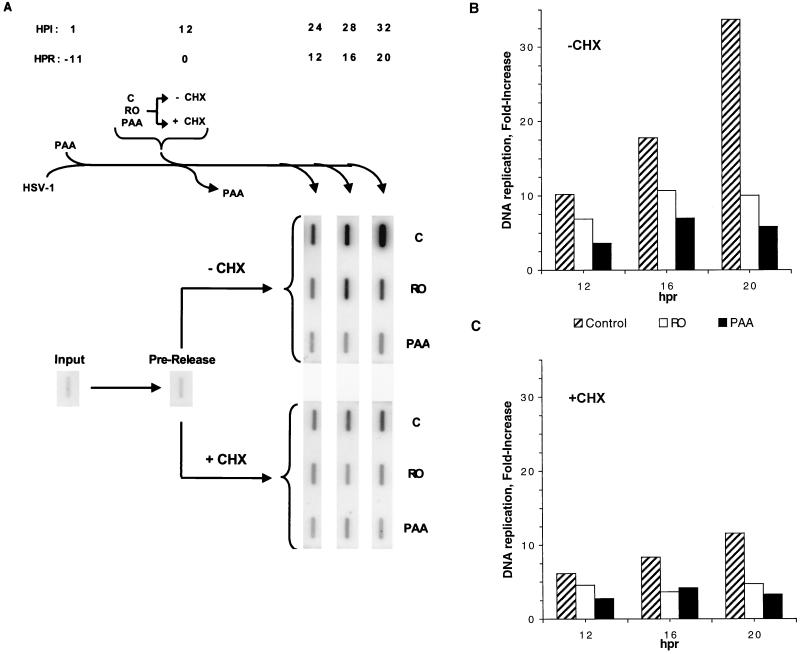
Inhibition of HSV DNA synthesis by Rosco in the absence or presence of CHX added at the time of release from a 12-h PAA block. (A) Vero cells were infected with 2 PFU of HSV per cell, incubated in the presence of 100 μg of PAA per ml for 12 h, and released from the block as described in the legend to Fig. 3A, except that CHX alone or together with the secondary drug was added to one set of infected monolayers at the time of release (+ CHX). At 1 h p.i. (HPI) (−11 h postrelease [HPR]) immediately before release (0 h postrelease; 12 h p.i.), and at 12, 16, and 20 h postrelease (24, 28, and 32 h p.i., respectively), cells were harvested and total DNA was extracted. Levels of viral DNA were determined by slot blot hybridization at the indicated times. (B and C) Viral DNA in the blots in panel A were quantitated using a Molecular Dynamics PhosphorImager system, and the fold increase in viral DNA synthesis after release from the PAA block in the absence (B) (−CHX) or presence (C) (+CHX) of CHX was calculated by dividing the amounts of viral DNA at the indicated times postrelease by the amounts of viral DNA before release and subtracting 1, such that total inhibition of DNA replication by the secondary drug would be represented by 0-fold increase. The fold increase in DNA synthesis is plotted against hours postrelease (hpr). Data from one of two repeat experiments is presented.
For temperature shift-down experiments (see Fig. 6 to 8), Vero cells were infected with the indicated ts mutants at the nonpermissive temperature (39.5°C) with the viral inoculum prewarmed to 39.5°C immediately before addition to cells. After a 1-h adsorption at 39.5°C, the inoculum was removed and prewarmed medium was added to infected monolayers. For shift-down in the presence of Rosco or PAA, the medium overlying infected monolayers was replaced at 5 h p.i. with fresh medium containing the indicated drug and prewarmed to 39.5°C immediately prior to use. After 1 h at the nonpermissive temperature in the presence of drug, infected monolayers were transferred to the permissive temperature (34°C) and maintained at this temperature until harvested.
FIG. 6.
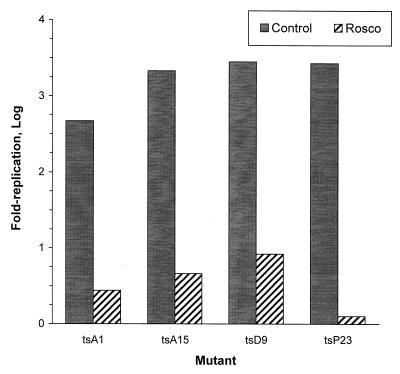
Replication of four HSV ts mutants after the shift-down from the nonpermissive to the permissive temperature in the presence of Rosco. Vero cells were infected at the nonpermissive temperature with 2.5 PFU of the indicated HSV ts mutants per cell. At 6 h p.i., infected cultures were transferred to the permissive temperature in the absence of drug (Control) or in the presence of 100 μM Rosco. One culture infected with each ts mutant was harvested immediately before the shift-down. At 24 h after the shift-down, the remaining infected monolayers were harvested and viral replication was monitored by a standard plaque assay. Fold viral replication after release was determined by dividing the titers at 24 h postrelease by the titers measured before release and subtracting 1.
FIG. 8.
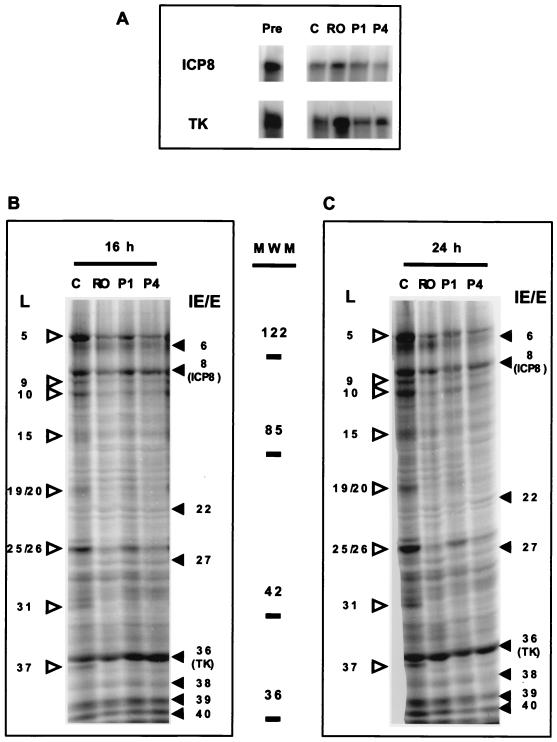
Expression of E gene products by tsA15 in the presence of Rosco added at the time of the shift-down. (A) Vero cells were infected with tsA15 at the nonpermissive temperature and shifted down to the permissive temperature as described in the legend to Fig. 7. Immediately before release (Pre) or 16 h after the shift-down in the absence of drug or in the presence of 100 μM Rosco or 100 or 400 μg of PAA per ml (C, RO, P1, and P4, respectively), cells were harvested and RNA was extracted. Steady-state levels of the transcripts of the genes encoding ICP8 or TK were evaluated by RNase protection assays. (B) Vero cells were infected with tsA15 at the nonpermissive temperature and shifted down to the permissive temperature as described for panel A, except that methionine-free medium supplemented with 50 μCi of [35S]methionine per ml and the indicated drugs (C, RO, P1, or P4) were added at the time of the shift-down. Infected cells were harvested at 16 h p.i., and viral proteins were resolved in a sodium dodecyl sulfate-polyacrylamide gel. Molecular weights (in thousands) are indicated on the right. The ICP nomenclature was used, such that VP16 is designated ICP25/26. HSV late proteins (γ1 and γ2) are indicated on the left of the gel by open arrowheads. Viral IE and E proteins are indicated on the right of the gel by solid arrowheads. (C) Infected cells were infected at the nonpermissive temperature, shifted down, and labeled with [5S]methionine as described in the legend to panel B, except that the cells were harvested at 24 h after the shift-down. Molecular weights, ICP nomenclature, and HSV IE, E, and L proteins (γ1 and γ2) are labeled as in panel B.
Probes, RNase protection assays, and viral DNA synthesis assays.
The synthesis of riboprobes, RNase protection assays, and quantitation of viral DNA by slot blot analysis were performed as previously described (70, 71).
Metabolic labeling.
For metabolic labeling experiments (see Fig. 8), cells were infected with 5 PFU of tsA15 per cell at the nonpermissive temperature and shifted down to the permissive temperature in methionine-free medium containing no drug, PAA (100 or 400 μg/ml, as indicated) or Rosco (100 μM), and 50 μCi of [35S]methionine per ml.
RESULTS
Rosco inhibits HSV replication when added to infected cells at 3, 6, 9, or 12 h p.i.
As a first step in determining whether Rosco-sensitive cdks are required for essential viral functions that occur after E protein synthesis, we asked whether addition of Rosco at different times after infection inhibited HSV replication. If Rosco inhibits only expression of IE and E gene products, addition of the drug after 6 h p.i., when IE and E gene products have already been synthesized, should have little effect on viral replication. In contrast, if the drug inhibits an essential viral function that occurs after synthesis of E gene products, Rosco should inhibit HSV replication even when the drug is added after 6 h p.i. To address this question, Vero cells were infected with 2.5 PFU of HSV-1 per cell and one infected monolayer was harvested every 3 h while medium from another infected monolayer was replaced with medium containing 100 μM Rosco. We selected this concentration because we have previously determined that 100 μM Rosco is required to block cell cycle progression completely and HSV replication in Vero cells (70). All monolayers in Rosco-containing medium and the single monolayer remaining in control medium were harvested at 24 h p.i. Levels of total infectious virus were determined by plaque assays.
As shown previously (70, 71), addition of Rosco at 6 h p.i. or earlier resulted in significant inhibition of HSV replication (Fig. 1). Addition of Rosco at any time after 6 h p.i. but before peak titers were attained in a single-step growth cycle (15 to 18 h p.i.) also inhibited HSV replication significantly (Fig. 1). Inhibition of viral replication was nearly complete when Rosco was added to infected monolayers at 1 or 3 h p.i., whereas inhibition was only partial when the drug was added at 6 to 12 h p.i. Nonetheless, when Rosco was added at 6, 9, or 12 h p.i., HSV titers at 24 h p.i. were approximately 2.5 orders of magnitude lower than titers in untreated cultures. Thus, Rosco inhibited HSV replication even when added under conditions in which E proteins should have been present (i.e., at 6 to 12 h p.i.).
Rosco inhibits HSV replication after release from a 12-h PAA block.
Based on previously published observations (71) and on the findings shown in Fig. 1, we hypothesized that cellular cdks are required for essential viral functions that occur after E gene expression. Because viral DNA synthesis occurs immediately after, or concomitant with, E gene expression, we hypothesized that viral DNA synthesis may be an essential viral function that requires cdks. As a first test of this hypothesis, we asked whether Rosco can inhibit HSV replication after release from a PAA block. PAA is a well-characterized inhibitor of HSV DNA polymerase (3, 29, 36, 42, 53, 61). In the presence of PAA, viral IE and E proteins are synthesized but the activity of the viral DNA Pol (an essential E protein) is directly inhibited by the drug. Consequently, E proteins are expressed but viral DNA is not synthesized.
Because high doses of PAA cause irreversible inhibition of viral DNA synthesis (36), relatively low doses (e.g., 100 μg/ml) must be used for the block to be reversed (61). In addition, the efficiency of inhibition of HSV synthesis by PAA is multiplicity dependent (36, 61). This multiplicity dependence is based on the fact that the number of viral gene copies which direct the synthesis of viral proteins increases with increasing multiplicity. Consequently, the level of the molecular target of PAA inhibition, viral DNA Pol, increases as a function of multiplicity. We therefore first determined the range of multiplicities that can be used in PAA (100 μM) reversal experiments. Rosco was included in these experiments for comparison because Rosco targets cellular proteins (56, 70, 71), whose levels of expression should not be affected as grossly by multiplicity as viral proteins are. Thus, in contrast to the inhibition of HSV replication by PAA, inhibition by Rosco was expected to be relatively independent of the multiplicity of infection.
For these tests, Vero cells were infected with 0.1 to 10 PFU of HSV-1 per cell. After adsorption, infected cells were overlaid with medium containing 100 μg of PAA per ml or 100 μM Rosco. At 24 h after infection, cells were harvested and viral titers were measured by a standard plaque assay. As shown in Fig. 2A, 100 μg of PAA per ml inhibited HSV replication efficiently at multiplicities of 1 PFU/cell or lower but considerably less efficiently at multiplicities of 2.5 PFU/cell or higher. In contrast, 100 μM Rosco inhibited HSV replication efficiently at all multiplicities tested (Fig. 2A).
Using the same range of multiplicities, we next determined whether Rosco inhibits viral replication after release from a PAA block lasting 12 h. A 12-h block was chosen for these tests to ensure that maximum levels of E transcripts and proteins had been synthesized before release of the block. For these tests, Vero cells were infected at multiplicities ranging from 0.1 to 10 PFU/cell and infected monolayers were overlaid with medium containing 100 μg of PAA per ml. At 12 h p.i., PAA-containing medium was replaced with fresh medium containing the secondary drug (PAA or Rosco) or no drug (control) and 24 h later (36 h p.i.) the cells were harvested and infectious virus was measured. As shown in Fig. 2B, Rosco added at the time of release from a 12-h PAA block inhibited viral replication slightly more efficiently than did readdition of PAA itself at all multiplicities tested.
Inhibition of HSV replication by either Rosco or PAA added after release from a 12-h block in PAA was multiplicity dependent (Fig. 2B). In contrast, when added immediately after infection, Rosco and PAA differed in their ability to inhibit viral replication. Under these last conditions, Rosco inhibited HSV replication in a multiplicity-independent manner whereas PAA inhibited HSV replication in a multiplicity-dependent manner (Fig. 2A). We concluded from the experiments in Fig. 2 that the multiplicity dependence of the block in the secondary drug (PAA or Rosco) is due, at least in part, to the multiplicity dependence of the 12-h primary PAA block.
Collectively, these results suggest that cellular cdks whose activities are inhibited by Rosco are required for a viral replication function(s) that occurs after E proteins are synthesized.
Inhibition of HSV DNA synthesis by Rosco added at the time of release from a 12-h PAA block is multiplicity dependent.
We next determined the level of HSV DNA synthesis that occurs after release from a 12-h PAA block into Rosco-containing medium. As controls, we also measured the efficiency of DNA synthesis after release from a 12-h PAA block in drug-free medium (control) or in the presence of PAA. For these experiments, we used the same range of multiplicities used in the experiments in Fig. 2. Vero cells were thus infected with 0.1 to 10 PFU of HSV-1 per cell, and infected cells were treated with 100 μg of PAA per ml for 12 h as described above. Infected cells were then released from the PAA block in the absence of drug or in the presence of PAA or Rosco. Immediately before or 24 h after the change of medium, cells were harvested, DNA was extracted, and viral DNA was quantitated as previously described (70, 71).
After release from the 12-h PAA block in control medium containing no drug, HSV DNA was synthesized efficiently, independent of the multiplicity of infection (Fig. 3). Since 100 μg of PAA per ml blocks HSV DNA replication in a multiplicity-dependent manner (Fig. 2), addition of any secondary drug (PAA or Rosco) after release of a 12-h, multiplicity-dependent block in 100 μg of PAA per ml was also expected to block DNA replication in a multiplicity-dependent manner. As anticipated, when the PAA-containing medium was changed at 12 h p.i. to fresh PAA-containing medium, the extent of inhibition of HSV DNA synthesis was dependent upon the multiplicity of infection (Fig. 3). Similarly, Rosco inhibited HSV DNA synthesis efficiently after release from the 12-h PAA block at multiplicities of 1 PFU/cell or lower. In contrast, when the multiplicity was 2.5 PFU/cell or higher, Rosco inhibited viral DNA replication less efficiently after release from the 12-h PAA block. Importantly, at all multiplicities tested, the extent of inhibition of HSV DNA synthesis by Rosco was similar to that achieved by PAA, a well-characterized inhibitor of HSV DNA synthesis. These results suggest that Rosco also inhibits the activities of proteins required for viral DNA synthesis, either directly or indirectly.
FIG. 3.
The inhibition of HSV DNA synthesis by Rosco added at the time of release from a 12-h PAA block is multiplicity dependent. (A) Vero cells were infected at the indicated multiplicities (MOI) with HSV, washed, and overlaid with medium containing 100 μg of PAA per ml. At 12 h p.i. medium was removed and fresh medium containing no drug (PAA 12 → C 24), 100 μg of PAA per ml (PAA 12 → PAA 24), or 100 μM Rosco (PAA 12 → Ro 24) was added. At 24 h after the change of medium, cells were harvested and the amounts of viral DNA were determined by slot blot analysis. (B) After quantitation of the blots presented in panel A using a Molecular Dynamics PhosphorImager system, the fold increase in viral DNA replication was calculated by dividing the amount of viral DNA detected at 12 h postrelease by the amount detected immediately after infection and subtracting 1, such that a total block in viral DNA replication by the secondary drug would be indicated by 0-fold increase. The fold increase in DNA replication was then plotted against the multiplicity (MOI). The results from one of two repeat experiments are presented.
Levels of E transcripts are unaffected when Rosco is added after release from a 12-h PAA block.
The inhibition of HSV DNA synthesis induced by Rosco after release from a 12-h PAA block might have been secondary to a decrease in the levels of the viral E proteins required for viral DNA synthesis. Because metabolically labeled viral proteins were not detected in amounts that allowed reliable quantitation at low multiplicities (<5 PFU/cell) (reference ;[59] and data not shown), we evaluated levels of viral transcripts as an indirect measure of levels of viral proteins. Notably, we have shown previously that Rosco does not inhibit the translation of viral proteins after their mRNAs have been synthesized (71). Vero cells were infected with 2 PFU of HSV-1 per cell, and infected cells were incubated in the presence of 100 μg of PAA per ml and released from the PAA block into no drug, PAA, or Rosco (Fig. 4A), as described above. Immediately before or 4, 8, or 16 h after the change of medium, cells were harvested and RNA was extracted and quantitated by RNase protection assays. A multiplicity of 2 PFU/cell was selected because it was the highest multiplicity at which Rosco inhibits viral DNA synthesis efficiently after release from a 12-h PAA block (Fig. 3).
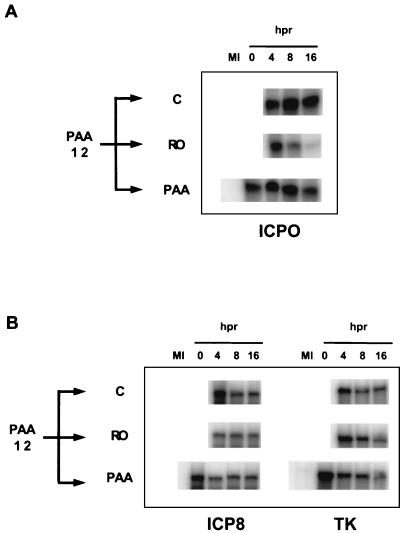
FIG. 4.
Effect of Rosco and PAA on the steady-state levels of selected viral transcripts when drug is added at the time of release from a 12-h PAA block. Vero cells were infected with 2 PFU of HSV per cell, and medium was replaced as described in the legend to Fig. 3B. Immediately before release (0) and at 4, 8, and 16 h after release (hpr) from the PAA block and addition of the secondary drug, cells were harvested and total RNA was extracted. RNA extracted from mock-infected cells served as a negative control (MI). Levels of ICP0 (IE), ICP8 (E), and TK (E) transcripts were determined by RNase protection assays.
After release from a 12-h PAA block (100 μg/ml), steady-state levels of transcripts of the IE gene encoding ICP0 were maintained at approximately the same levels if infected cells were incubated in the presence of PAA or increased slightly if cells were released into control, drug-free medium (Fig. 4A). In contrast, and as shown previously (71), steady-state levels of the ICP0 transcripts decreased after addition of Rosco (Fig. 4A), exhibiting a half-life consistent with (i) the recognized half-life of the ICP0 transcript (between 1.5 and 4 h [32, 60] and (ii) the half-life observed when Rosco was added at the time of release from a 6-h CHX block (4.5 h [71]). Steady-state levels of the transcripts of two E genes (those encoding ICP8 and TK) did not change dramatically after release from the 12-h PAA block into PAA, Rosco, or drug-free control medium (Fig. 4). Thus, Rosco added after release from a PAA block had only a minor effect on steady-state levels of the ICP8 transcript and no effect on the levels of the TK transcript, consistent with the lack of effect of Rosco on E transcripts when the drug is added at 6 h p.i. (Fig. 3 of reference ;[71]). Rosco, however, dramatically inhibited accumulation of the same two transcripts when the drug was added after release from a 6-h CHX block (Fig. 7 of reference ;[71]). A significant difference is that in this last experiment, Rosco was added before E transcripts accumulated, whereas in the experiments presented in this paper and in Fig. 3 of reference ;[71], Rosco was added after high levels of E gene transcripts had already accumulated. The significance of these differences in the experimental design is considered in detail in Discussion.
Rosco inhibits HSV DNA synthesis when added after release from a 12-h PAA block, even in the absence of new protein synthesis.
Because a reversible PAA block can be achieved only at relatively low multiplicities (36, 61) and because most of the essential viral DNA synthesis proteins are expressed at very low levels (e.g., UL5, UL8, UL9-OBP-, and UL52 [59]), it was not possible to measure accurately the levels of all viral DNA replication proteins synthesized in cells infected and released from a PAA block. As an alternative, we evaluated HSV DNA synthesis after release from a PAA block in the presence of 50 μg of CHX per ml and either 100 μg of PAA per ml or 100 μM Rosco. Addition of CHX at the time of release from a PAA block results in inhibition of new viral protein synthesis. Therefore, in these experiments, viral DNA is synthesized after release from the PAA block by viral proteins synthesized before release. Hence, Rosco could have no effect on viral gene expression, which would have occurred before addition of the drug. If the inhibition of viral DNA synthesis produced by Rosco in the PAA release experiments was exclusively a consequence of inhibition of E gene expression, Rosco should not inhibit viral DNA synthesis beyond the inhibition resulting from the addition of CHX at the time of release. In contrast, if inhibition of viral DNA synthesis was at least partially due to a requirement for enzymes that are sensitive to inhibition by Rosco, CHX and Rosco together should inhibit viral DNA synthesis to a greater extent that should CHX alone. We therefore performed experiments in which no drug, Rosco, CHX alone, Rosco and CHX, or PAA and CHX were added to infected cells at the time of release from the primary 12-h PAA block.
In these tests, two sets of Vero cells were infected with 2 PFU of HSV-1 per cell, treated with PAA, and released from the 12-h PAA block into drug-free or drug-containing medium as described above. The first set of infected monolayers was released into medium containing no drug, 100 μM Rosco, or 100 μg of PAA per ml but no CHX (Fig. 5A). In contrast, the medium added to the second set of infected monolayers at the time of release contained 50 μg of CHX per ml alone or together with the secondary drug (100 μM Rosco or 100 μg of PAA per ml) (Fig. 5). One infected monolayer was harvested at 1 h p.i. to measure the input level of viral DNA, and another was harvested immediately before release from the PAA block to analyze the level of viral DNA at the time of release. Individual monolayers in each of the two sets of dishes were then harvested at 12, 16, or 20 h after release (24, 28, and 32 h p.i.) and the levels of viral DNA synthesis that occurred after the release from the PAA block were determined by slot blot analysis. The blots are shown in Fig. 5A, and quantitation of these data is shown in Fig. 5B and C.
After release from the PAA block into medium containing no drug, viral DNA replicated ∼10-fold within the first 12 h and replication continued through 20 h postrelease, when viral DNA levels were approximately 35-fold higher than the levels attained during the PAA block (Fig. 5). As expected, when infected monolayers were maintained in PAA, only low levels of viral DNA synthesis (∼sevenfold) were observed within the 20 h period after the change from the primary to the secondary PAA block. When infected monolayers were released from the PAA block into Rosco alone, viral DNA synthesis was only slightly more efficient than in the presence of PAA during the 20-h period (12 to 32 h p.i.) of the secondary block (∼10 and ∼7-fold, respectively). Notably, most DNA synthesis in the presence of Rosco (or PAA) alone occurred during the first 12 h postrelease (Fig. 5).
When the release from the PAA block was performed in the presence of CHX alone (Fig. 5A and C), only low levels of viral DNA synthesis were achieved in the absence of new protein synthesis. Thus, viral DNA replicated only ∼6-fold in the first 12 h postrelease and ∼12-fold by 20 h postrelease. Viral DNA synthesis was inhibited even further, however, if PAA was added together with CHX at the time of release from the primary PAA block. Thus, viral DNA levels were only ca. fivefold greater within the 20-h period after the change of medium than before release. Interestingly, when infected cells were released into Rosco and CHX, viral DNA synthesis was inhibited to a similar level (ca. fivefold in 20 h postrelease) as when the release occurred in the presence of CHX and PAA, a drug known to inhibit the activity of viral DNA polymerase directly.
We conclude from the data shown in Fig. 2 through 5 that Rosco inhibits viral DNA synthesis after release from a PAA block and that this inhibition is not mediated exclusively by inhibition of viral gene expression, as shown by the inhibition of HSV DNA synthesis by Rosco in the presence of CHX (Fig. 5B).
Replication of DNA− HSV ts mutants is inhibited by Rosco added at the time of shift-down from the nonpermissive to the permissive temperature at 6 h p.i.
To obtain independent evidence to support or refute the hypothesis that Rosco inhibits viral DNA replication in the presence of E proteins, we asked whether Rosco added at the time of shift-down from the nonpermissive to the permissive temperature inhibits replication of HSV mutants with ts mutations in genes encoding E proteins required for viral DNA synthesis. For this purpose, we tested four mutants, tsA1, tsA15, tsD9, and tsP23. The mutations in tsA1 and tsA15 have been mapped to the ICP8 gene, and the mutation in tsD9 has been mapped to the catalytic subunit of the viral DNA polymerase (63, 69). The mutation in tsP23 has not been mapped definitely, but available evidence indicates that it, too, maps to the viral DNA polymerase gene (42).
Vero cells were infected at 39.5°C with each of the ts mutants, and infected cells were maintained at the nonpermissive temperature for 6 h. At this time, viral IE and E proteins have been expressed but viral DNA has not been synthesized because one of the proteins required for viral DNA synthesis (ICP8 or DNA Pol, for the mutants used) is nonfunctional at the nonpermissive temperature (63, 69). At 6 h p.i., one set of monolayers infected with each of the mutants was harvested and two other sets of monolayers were shifted-down to the permissive temperature (34°C). Of the latter two sets of monolayers, one was shifted down in control medium containing no drug and the other was shifted down in Rosco-containing medium. Viral replication was evaluated 18 h after shift-down by standard plaque assay. Infected cells were maintained at the nonpermissive temperature for 6 h because this is the longest time that permits efficient initiation of viral DNA synthesis after the temperature shift-down (42, 63).
Figure 6 shows that Rosco inhibited the replication of the four ts mutants efficiently following the temperature shift-down. Thus, when cells infected with tsA15, tsD9, or tsP23 were shifted down to the permissive temperature at 6 h p.i. in the presence of Rosco, viral replication 18 h later (24 h p.i.) was approximately 3 orders of magnitude lower than when infected cells were shifted down in the absence of drug. During infections with tsA1, the difference in viral titers at 24 h p.i. in infected monolayers shifted down in the presence or absence of Rosco was only 2 orders of magnitude, primarily because replication of this mutant after the temperature shift-down was not very efficient (Fig. 6).
Rosco inhibits DNA synthesis by tsA15 following the temperature shift-down.
We next asked whether the inhibition of viral replication observed in the experiments described above was a result of inhibition of viral DNA synthesis. In these experiments, we chose to study mutant tsA15 because (i) the mutation in this virus has been mapped, (ii) reversal of the block in viral replication is efficient following the shift-down, and (iii) Rosco inhibited its replication efficiently after the shift-down (Fig. 6). We used a multiplicity of 5 PFU/cell because expression of E gene products and of viral DNA synthesis were to be examined in parallel. We had determined in preliminary experiments that 5 PFU/cell is the lowest multiplicity that permits reliable quantitation of E protein synthesis by metabolic labeling in our hands (data not shown).
Vero cells were infected with tsA15 at the nonpermissive temperature, and infected cells were shifted down to 34°C at 6 h p.i. as described above. At the indicated times before and after the shift-down, infected monolayers were harvested, DNA was extracted, and the extent of viral DNA replication was evaluated by slot blot hybridization. For comparison, a set of monolayers was infected and maintained at the permissive temperature (34°C) throughout the experiment (Fig. 7A). The data shown in Fig. 7A are quantitated in Fig. 7B.
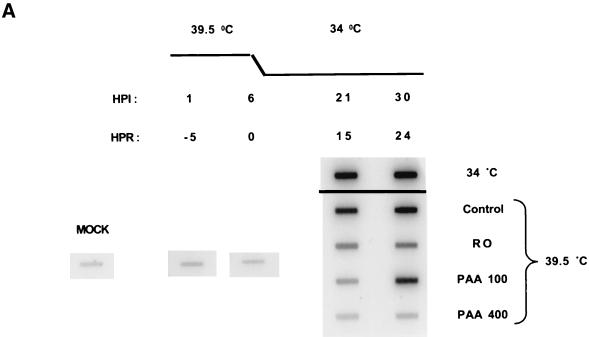
FIG. 7.
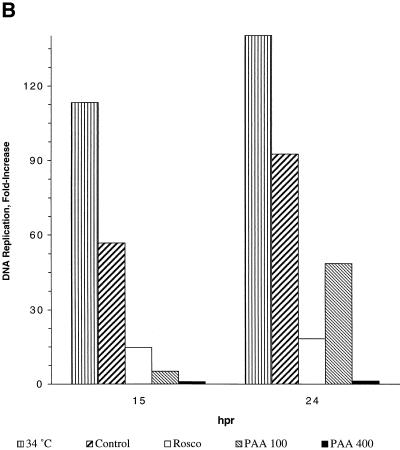
HSV DNA synthesis after the shift-down from the nonpermissive to the permissive temperature in the presence of Rosco added at the time of the shift-down. (A) Vero cells were infected with HSV tsA15 at the nonpermissive temperature and shifted down in the absence of drug or in the presence of 100 μM Rosco or 100 or 400 μg of PAA per ml (Control, RO, PAA100, and PAA400, respectively), as described in the text. At 1 h p.i. (HPI) (−5 h postrelease [HPR]), immediately before release (6 h pi/0 hpr) and at 15 and 24 h after release (21 or 30 h p.i., respectively), cells were harvested and DNA was extracted. DNA was also extracted from mock-infected cells for comparison (MOCK). Levels of viral DNA synthesis were determined by slot blot analysis. For comparison, a set of infected monolayers was incubated at the permissive temperature throughout the experiment. (B) The slot blots shown in panel A were quantitated using the ImageQuant software package (Molecular Dynamics), and the fold increase in DNA synthesis was calculated by dividing the amount of viral DNA at a given time point by the amount of viral DNA detected immediately before release and subtracting 1, such that complete inhibition of viral DNA replication after release would be indicated by 0-fold increase. The fold increase in DNA replication is plotted against hours postrelease (hpr). Data from one of two repeat experiments are presented.
Viral DNA synthesis by tsA15 increased ∼115-fold by 21 h p.i. (equivalent to 16 h postrelease) and ∼140-fold by 30 h p.i. (equivalent to 24 h postrelease) in cells infected and maintained at the permissive temperature throughout the experiment (Fig. 7). When infected cells were maintained at the nonpermissive temperature (39.5°C) for 6 h and were then shifted down to 34°C in the absence of drug, viral DNA synthesis increased 60-fold in the first 15 h and ∼95-fold by 24 h postrelease (Fig. 7). At 24 h after the shift-down, levels of viral DNA in cells infected at the nonpermissive temperature and shifted to 34.5°C in control medium had reached ∼70% of the levels detected in cells infected and maintained at the permissive temperature throughout the 30-h experiment. In contrast, when cells were shifted down in the presence of 400 μg of PAA per ml, viral DNA replication was totally inhibited during the 24-h period following the shift-down. When infected monolayers were shifted down in the presence of 100 μg of PAA per ml, viral DNA replication was significantly delayed but not totally blocked relative to the no-drug control. Thus, in 100 μg of PAA per ml, viral DNA replicated only ∼5-fold in the first 15 h after the shift-down but almost 50-fold by 24 h after the shift-down. When the shift-down was performed in the presence of Rosco, viral DNA replicated only ∼15-fold in the first 15 h postrelease and ∼18-fold by 24 h postrelease. As observed in the PAA release experiments, most of the viral DNA synthesis that occurred in the presence of Rosco occurred during the first 15 h after the shift-down.
From these experiments, we conclude that Rosco inhibited viral DNA synthesis by tsA15 efficiently when infected cells were shifted down from the nonpermissive to the permissive temperature.
tsA15 gene expression when shifted down to 34°C in the presence of Rosco after a 6-h incubation at 39.5°C.
If inhibition of viral DNA synthesis by Rosco after the shift-down was exclusively due to inhibition of E gene expression, levels of E transcripts and proteins should be significantly reduced under the same conditions in which viral DNA synthesis is inhibited. If inhibition of viral DNA synthesis was mediated at least partially by a posttranslational mechanism, levels of E transcripts and proteins might not be significantly inhibited under conditions in which viral DNA synthesis was. To test these hypotheses, we evaluated the levels of two E transcripts and a number of E proteins following a shift-down of tsA15-infected cells from 39.5 to 34°C. Specifically, we choose to examine transcripts of the gene encoding ICP8, because it is the gene mutated in tsA15, and the gene encoding TK, because it is an E protein involved in an aspect of viral DNA replication distinct from the function of ICP8 (55).
Vero cells were infected at 39.5°C with 5 PFU of tsA15 per cell and incubated at the nonpermissive temperature for 6 h. At 1 h before the shift-down to the permissive temperature, 100 μM Rosco or 100 or 400 μg of PAA per ml was added to three sets of infected monolayers, whereas a fourth set of infected monolayers was maintained in drug-free medium (control). Infected cells in one dish were harvested at the time of the shift-down, to establish the baseline levels of ICP8 and TK RNAs (Fig. 8A). At 16 h after the shift-down, cells were harvested and levels of ICP8 and TK transcripts were measured by RNase protection assays.
As expected for the kinetics of expression of E genes (35), levels of ICP8 and TK mRNA at 16 h after the shift-down in control medium containing no drug were lower than the levels detected immediately before the shift-down (6 h p.i.) (Fig. 8A). Also as expected, addition of 100 or 400 μg of PAA per ml did not lead to lower levels of transcripts of these two E genes (Fig. 8A). Interestingly, addition of 100 μM Rosco at the time of the shift-down did not result in reduced levels of the transcripts of these two E genes at 16 h after the shift-down either, consistent with the lack of effect of Rosco on E transcripts when the drug was added at the time of release from a 12-h block in PAA (Fig. 4). Similarly, we had previously shown that levels of E transcripts do not change significantly after addition of Rosco at 6 h p.i. (Fig. 3 of reference ;[71]). In contrast, addition of Rosco before the start of E transcription results in a significant decrease in the levels of accumulation of E transcripts (Fig. 7 of reference ;[71]). Based on the analysis of the data presented in Fig. 8A and our previous publication (71), we conclude that addition of Rosco has no significant effect on the levels of previously synthesized transcripts but inhibits de novo transcription of E genes significantly.
The results of the RNase protection assays in Fig. 8A do not support the hypothesis that inhibition of viral DNA synthesis by Rosco added at the time of the shift-down (Fig. 7) was mediated exclusively by inhibition of accumulation of E transcripts.
We next analyzed the expression of viral proteins after the shift-down to the permissive temperature. For this purpose, Vero cells were infected with 5 PFU of tsA15 per cell, maintained at the nonpermissive temperature for 6 h, and shifted down in the presence or absence of drugs as described previously. In these tests, however, the culture medium added at the time of the shift-down contained [35S]methionine to evaluate viral protein synthesis. Infected cells were harvested at 16 and 24 h after the shift-down, and levels of viral proteins were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Metabolic labeling was chosen over Western blot analysis or immunoprecipitation because it allows the simultaneous observation of many viral proteins of different kinetic classes. Long-term labeling was selected over short-term labeling to determine steady-state levels of viral proteins during the period in which viral DNA synthesis was evaluated in the experiments in Fig. 7. Steady-state levels of E proteins are more relevant to determining whether the observed inhibition of viral DNA synthesis was mediated by the presence of limiting amounts of the required E proteins.
When the shift-down from the nonpermissive to the permissive temperature was performed in control medium containing no drug, high levels of E and L proteins accumulated during the first 16 h (Fig. 8B). As expected, addition of 400 μg of PAA per ml at the time of the shift-down resulted in a major inhibition of L protein accumulation during the same period but had a more modest effect on the levels of E proteins (Fig. 8B). Also as expected, 100 μg of PAA per ml was not as effective an inhibitor of L protein expression as was 400 μg of PAA per ml. When Rosco was added at the time of the shift-down, significantly lower levels of L proteins accumulated during the first 16 h (Fig. 8B), consistent with the observed inhibition of viral DNA replication (Fig. 7). In contrast, and consistent with the results of the RNase protection assays in Fig. 8A, Rosco had only a minor inhibitory effect on the accumulation of E proteins, including ICP8 and TK, during the first 16 h after the shift-down (Fig. 8B). These results are also inconsistent with the hypothesis that Rosco-mediated inhibition of viral DNA synthesis after the shift-down is mediated exclusively by inhibition of E protein synthesis.
As expected, when viral protein synthesis was evaluated for a 24-h period after the shift-down in control medium containing no drug, the levels of L proteins were significantly higher than when protein synthesis was evaluated for a period of 16 h after the shift-down (Fig. 8B and C). Moreover, because significant viral DNA replication occurred between 16 and 24 h after the shift-down in control medium (Fig. 7), steady-state levels of viral E proteins were also higher at 24 h than at 16 h after the shift-down (Fig. 8B and C). The increases in the levels of E proteins, however, were clearly less pronounced than were the increases in levels of L proteins. Addition of PAA or Rosco at the time of the shift-down resulted in a nearly complete block in the increase in levels of L proteins between 16 and 24 h after the shift-down (Fig. 8B and C), consistent with the previously observed inhibition of viral DNA replication (Fig. 7). The changes in the levels of viral E proteins, in contrast, were only marginally affected by either 100 μM Rosco or 400 μg of PAA per ml (Fig. 8B and C). For example, the levels of the E proteins ICP6, ICP8, and TK increased between 16 and 24 h after the shift-down in the presence of either Rosco or PAA, whereas the levels of the L proteins ICP5, ICP9, ICP10, and VP16 (ICP25/26), did not change markedly during the same period.
DISCUSSION
Collectively, the results of these studies demonstrate that in addition to transcription of IE and E genes, HSV DNA synthesis requires cellular cdks or perhaps some other cellular protein(s) not yet known to be sensitive Rosco. Four points are critical to the interpretation of the results described herein. First, a structural isomer of Rosco, which does not inhibit cdks, does not inhibit HSV replication (70). Thus, the inhibition of viral DNA synthesis by Rosco observed in the experiments reported herein is not mediated by the core structure of the compound but, rather, by its kinase-inhibitory activity. Second, the concentration of PAA used in the PAA reversal experiments, 100 μg/ml, is acknowledged to be below the concentration required to achieve a complete block in HSV DNA synthesis (200 to 400 μg/ml) (3, 36, 61); (Fig. 3, 5, and 7). However, concentrations of PAA that completely inhibit HSV DNA synthesis will block viral replication irreversibly (36, 61]; L. Schang and P. Schaffer, unpublished data), and hence could not be used in the PAA reversal experiments. Since Rosco inhibited HSV DNA synthesis when added at the time of release from a partial PAA block (Fig. 3 and 5), we are confident that the inhibition observed in these experiments is an underestimate of the actual inhibitory effect of Rosco on viral DNA synthesis. Third, the three known or putative viral kinases are all nonessential in vitro (64), and we have yet to detected any HSV Rosco-resistant mutant (;[70]; Schang and Schaffer, unpublished). Thus, the inhibitory effects of Rosco observed in the experiments described in this paper are not likely to be the result of inhibition of one or more of the three virally encoded kinases. Finally, it must be emphasized that the design of the experiments presented in this paper required rapid inhibition of cellular cdk activities at specific times p.i. Only chemical cdk inhibitors act with the required speed, and consequently only chemical (as opposed to protein) cdk inhibitors could be used in the experiments presented in this paper.
Effects of Rosco on the levels of HSV-1 E gene transcription.
We had observed previously that Rosco inhibits accumulation of transcripts of viral E genes after release from a 6-h CHX block but not when added at 6 h p.i. in the absence of a previous block (71). Moreover, steady-state levels of IE transcripts were lower in the presence of Rosco (70, 71). In the experiments presented herein, the levels of two E transcripts remained nearly constant when Rosco was added after release from a PAA block (Fig. 4) or after a shift-down from the nonpermissive to the permissive temperature (Fig. 8). We observed, however, that the levels of ICP8 transcripts were somewhat lower in the presence than in the absence of Rosco. The nearly constant levels of E transcripts following the addition of Rosco may be due to the long intrinsic half-lives of E transcripts (32), especially since E transcripts are stabilized even further in the absence of vhs (60), a late gene not expressed in the absence of DNA synthesis. Notably, in the experiments in which Rosco inhibited accumulation of E transcripts (Fig. 7 of reference ;[71]), Rosco was added before initiation of E gene transcription. In contrast, in the experiments in which the levels of E transcripts remained constant in the presence of Rosco (Fig. 4 and 8; Fig. 3 of reference ;[71]), Rosco was added several hours after transcription of E genes had begun. It is thus possible that only initiation of E gene transcription is sensitive to inhibition by Rosco. In this scenario, the constant levels of E transcripts observed in the experiments in Fig. 4 and 8 would be the result of continuation of transcription initiated before addition of the drug. Experiments are in progress to determine whether the constant levels of E transcripts in the presence of Rosco added at 6 h p.i. (71) or at the time of release from blocks in viral DNA synthesis are due to (i) the extended half-lives of these transcripts or (ii) continuous transcription in the presence of the drug. The reduced steady-state levels of ICP0 transcripts detected in the presence of Rosco could be explained by destabilization of these transcripts or by inhibition of transcription in the presence of the drug. Because the levels of ICP0 mRNA decreased in the presence of Rosco with a half-life consistent with the half-life of ICP0 transcripts when transcription is inhibited with amantadine (32, 60), we conclude that inhibition of transcription rather than destabilization is the most likely explanation for the reduced levels of ICP0 mRNA in the presence of Rosco.
Effects of Rosco on HSV DNA synthesis.
Based on a comparison of the findings presented in Fig. 5, and 7, Rosco appeared to inhibit DNA synthesis more efficiently in ts mutant-infected cells after the shift-down to the permissive temperature than in wild-type-virus-infected cells after reversal of a PAA block (compare Fig. 5 and 7). Several differences between the two experimental systems could explain these apparent differences in efficiency. First, the block in DNA synthesis (for tsA15) at 39.5°C is clearly tighter than the block in DNA synthesis achieved by 100 μg of PAA per ml for wild-type KOS. Consequently, if Rosco inhibited DNA synthesis to the same extent after release from the blocks induced by PAA or by incubation at 39.5°C (for instance, if the efficiency of viral DNA synthesis after release were only onefold above the efficiency of viral DNA synthesis before release), the observed inhibition would be greater in the shift-down than in the PAA release experiments. Moreover, reversal of the block in DNA synthesis after the shift-down is significantly more efficient than the reversal which followed removal of PAA (compare Control in Fig. 5 and 7). Consequently, if viral DNA replicated to the same extent in the presence of Rosco added after the shift-down from the nonpermissive temperature as after release from the PAA block, the inhibition of DNA synthesis measured as a fraction of DNA replication after release in the absence of secondary drug would be significantly different in the two experimental models.
DNA replication proteins expressed during the blocks induced either by PAA (wild-type virus) or high temperature (ts mutants), whether viral or cellular, should have been phosphorylated during the block. Moreover, these phosphorylated proteins should have retained the capacity to synthesize viral DNA in the presence of kinase inhibitors until the phosphorylated proteins were either degraded or dephosphorylated. If Rosco inhibits DNA synthesis by inhibiting new phosphorylation of DNA replication proteins, most of the viral DNA synthesis which occurred after release of the blocks in the presence of Rosco should have occurred at relatively early times after release. It is clear from the results presented in Fig. 5 and 8 that the majority of the viral DNA synthesis that occurred in the presence of Rosco did, in fact, occur during the first 12 h after release from the PAA block (Fig. 5) or during the first 15 h after the shift-down to the permissive temperature (Fig. 7). In contrast, significant viral DNA synthesis occurred between 12 and 20 h postrelease (Fig. 5) or 15 and 24 h after the shift-down (Fig. 7) in the absence of a secondary drug.
Potential roles of cdks in HSV DNA synthesis.
The observed requirement for cdks in HSV DNA synthesis can be explained by three potential mechanisms of action: (i) cdks may be required to activate viral DNA replication proteins, (ii) HSV DNA synthesis may require cellular DNA replication proteins (several of which are known to be activated by cdks), or (iii) HSV DNA synthesis may be mechanistically linked to viral transcription (which is also known to require cdks).
(i) HSV encodes seven proteins required for viral DNA synthesis (55, 59), any or all of which may require activation by cdk-mediated phosphorylation. Although the requirement for phosphorylation for activation of one or more of these proteins has not yet been examined, DNA replication proteins of other viruses are known to be activated by cdks. Thus, for example, large T antigens of simian virus 40 and polyomavirus and the E1 protein of adenovirus are activated by cdk phosphorylation (28, 30, 77). Moreover, 15 μM Rosco, as well as dominant-negative forms of cdk-2, inhibit HCMV DNA synthesis of in the presence of E proteins (7). By analogy, it seems likely that HSV DNA replication proteins may also be activated by cdk-mediated phosphorylation.
(ii) The seven HSV-encoded DNA replication proteins are necessary but not sufficient to replicate DNA templates which do or do not contain an HSV origin of replication (5, 75). Consequently, cellular proteins have long been hypothesized to be required for HSV DNA synthesis (5, 15, 57, 75), and recent evidence has implicated several cellular proteins in this essential process. Thus, Sp-1, Sp-3, and other as yet uncharacterized cellular proteins bind to HSV origins of DNA replication and are necessary for efficient viral DNA synthesis (15, 57). Furthermore, many cellular proteins involved in cellular DNA synthesis, including proliferating-cell nuclear antigen, RP-A, DNA Pol α, DNA ligase 1, and p170/topoisomerase II, are known to localize to HSV replication compartments during HSV infections (21, 83). Of these proteins, DNA ligase 1 may serve to circularize the viral genome, an event required for viral DNA synthesis (5). Cellular DNA Pol α interacts with, and is activated by, the HSV origin binding protein (50). Topoisomerase II (p170) has been reported to be involved in viral DNA replication, since inhibition of this cellular enzyme results in some inhibition of HSV DNA synthesis (27). Most cellular proteins involved in cellular DNA synthesis, including those just mentioned, are thought to be activated by, or their expression is thought to be induced by, Rosco-sensitive cdks. Thus, the observed inhibition of viral DNA synthesis by Rosco could result from inhibition of expression or phosphorylation of cellular proteins required for viral DNA synthesis.
(iii) A third potential mechanism of DNA synthesis involving cdks hypothesizes that the requirements for cdks in HSV transcription and DNA synthesis may be linked. This hypothesis is based on the fact that certain transcription factors, including cellular oct-1 and HSV VP16, are known to stimulate DNA synthesis (14, 57). Reciprocally, HSV DNA synthesis may well have stimulatory effects on transcription of specific HSV IE and E genes (58). Thus, transcription and DNA synthesis may be tightly linked during HSV replication, and, consequently, inhibition of viral DNA synthesis and inhibition of transcription by Rosco may be two distinct manifestations of the same phenomenon.
cdks that may be required for viral DNA synthesis.
From the results of the experiments presented in this and previous papers (70, 71), we conclude that transcription of HSV IE and E genes and viral DNA replication all require Rosco-sensitive enzymes. Given that Rosco inhibits cdk-1, cdk-2, cdk-5 (56), and cdk-7 (L. Schang, R. Shiekhattor, and P. Schaffer, unpublished observations) and probably also cdk-3 (70), different cdks may be required for different viral functions. Moreover, other as yet unknown cellular targets of Rosco may exist. Therefore, we have yet to determine which one(s) of the Rosco-sensitive cdks, or other kinases, is specifically required for each of the Rosco-sensitive HSV replication functions. With respect to viral DNA replication, however, we hypothesize that cdk-2 may be the cellular kinase that is (i) required for this viral function and (ii) sensitive to inhibition by Rosco. This hypothesis is based on the fact that cdk-2 is considered a central player in cellular DNA replication. Thus, cdk-2 localizes to cellular DNA replication forks (10) and its activity is required to initiate replication of cellular DNA (48). At the molecular level, cdk-2 phosphorylates and activates a number of DNA replication proteins, including cellular ribonucleotide reductase, RF-A, DNA Pol α, Pol δ, proliferating-cell nuclear antigen, and/or topoisomerase I and II (18, 82). Moreover, cdk-2-mediated phosphorylation of proteins that regulate DNA replication, such as HsCdc6, is also required for cellular DNA replication (40). Finally, the expression of many enzymes required for cellular DNA replication (including DNA ligase 1 and DNA Pol α) is dependent on cdk-2 activity (18, 52), and, as noted above, some of these cellular proteins may also be required for HSV DNA synthesis (27, 46, 50, 75).
It is possible that the activities of some cdks are redundant during HSV infection, such that more than one cdk may be able to provide the function(s) required for viral replication. If this were the case, inhibition of only one cdk may not be sufficient to inhibit Rosco-sensitive HSV replication functions. Moreover, given that Rosco-sensitive cdks are required for HSV replication, these cdks might be expected to be induced during HSV infection. This hypothesis is currently being tested.
HSV replication functions that occur after DNA synthesis are likely to be sensitive to inhibition by cdk inhibitors.
The fact that Rosco also inhibits HSV replication when added at times after the time when the majority of viral DNA should already have been synthesized (i.e., 12 h p.i.) has not escaped our notice. Considering that three viral processes (transcription of IE genes, transcription of E genes, and viral DNA synthesis) require enzymes sensitive to inhibition by Rosco, it seems likely that viral functions that occur after DNA synthesis may also require Rosco-sensitive enzymes. Interestingly, a structural L protein of another alphaherpesvirus, varicella-zoster virus, was recently shown to be phosphorylated by cellular cdk-1, which is sensitive to Rosco inhibition (87). Although cdk-mediated phosphorylation of L proteins may well occur during HSV infection, this hypothesis remains to be tested.
Since at least three viral processes are inhibited by cdk inhibitors, any drug such as Rosco that inhibits selected cdks may be considered for possible use as an antiviral compound in vivo. It should be emphasized, however, that cdk inhibitors block multiple cellular activities, such as cell division (38, 56), which are essential in healthy animals or humans. Thus, viral diseases that may be treated with these drugs must be identified with great care. For instance, cdk inhibitors may be used for the treatment of diseases caused by a virus that is sensitive to these drugs and whose pathology involves activation of cellular cdks. Having identified the most prominent effects that cdk inhibitors have on HSV replication, we are now embarking on the characterization of the mechanisms underlying these effects.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R07CA20260 from the National Cancer Institute and PO1NS35138 from the National Institute of Neurological Disorders and Stroke.
We thank Robert Jordan for very helpful discussions and ideas, Drew Bantly for excellent technical assistance, and members of the Schaffer laboratory for critical evaluation of the manuscript.
ADDENDUM IN PROOF
While the article was in press, Advani and colleagues reported independently that HSV infection of HeLa cells induces cdk-1 (also known as cdc2) kinase activity (S. J. Advani, R. Brandimarti, R. R. Weichselbaum, and B. Roizman, J. Virol. 74:8–15, 2000). Since cdk-1 is among the kinases known to be inhibited by Rosco, it may be one of the targets of this drug that is also required for HSV replication.
REFERENCES
- 1.Abraham R T, Acquarone M, Andersen A, Asensi A, Belle R, Berger F, Bergounioux C, Brunn G, Buquet-Fagot C, Fagot D, et al. Cellular effects of olomoucine, an inhibitor of cyclin-dependent kinases. Biol Cell. 1995;83:105–120. doi: 10.1016/0248-4900(96)81298-6. [DOI] [PubMed] [Google Scholar]
- 2.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala L, Scovassi A, Meijer L, Prosperi E. The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 3.Becker Y, Asher Y, Cohen Y, Weinberg-Zahlering E, Shlomai J. Phosphonoacetic acid-resistant mutants of herpes simplex virus: effect of phosphonoacetic acid on virus replication and in vitro deoxyribonucleic acid synthesis in isolated nuclei. Antimicrob Agents Chemother. 1997;11:919–922. doi: 10.1128/aac.11.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 5.Bohemer P E, Lehman I. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 6.Boulikas T. Control of DNA replication by protein phosphorylation. Anticancer Res. 1994;14:2465–2472. [PubMed] [Google Scholar]
- 7.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 8.Buquet-Fagot C, Lallemand F, Montagne M N, Mester J. Effects of olomoucine, a selective inhibitor of cyclin-dependent kinases, on cell cycle progression in human cancer cell lines. Anti-Cancer Drugs. 1997;8:623–631. doi: 10.1097/00001813-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso M C, Leonhart H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- 11.Chan A K, Litchfield D W, Wright J A. Phosphorylation of ribonucleotide reductase R2 protein: in vivo and in vitro evidence of a role for p34cdc2 and CDK2 protein kinases. Biochemistry. 1993;32:12835–12840. doi: 10.1021/bi00210a036. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee A, Bockus B J, Gjørup O V, Schaffhausen B S. Phosphorylation sites in polyomavirus large T antigen that regulate its function in viral, but not cellular DNA synthesis. J Virol. 1997;71:6472–6478. doi: 10.1128/jvi.71.9.6472-6478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobrinik D, Whyte P, Peeper D S, Jacks T, Weinberg R A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- 14.Coenjaerts F E, vanOosterhout J A W M, van der Vliet P C. The Oct-1 POU domain stimulates adenovirus DNA replication by a direct interaction between the viral precursor terminal protein-DNA polymerase complex and the POU domain. J Virol. 1994;13:5401–5409. doi: 10.1002/j.1460-2075.1994.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabrowski C E, Schaffer P A. Herpes simplex virus type 1 origin-specific binding protein: oriS-binding properties and effects of cellular proteins. J Virol. 1991;65:3140–3150. doi: 10.1128/jvi.65.6.3140-3150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daksis J I, Preston C M. Herpes simplex virus immediate early gene expression in the absence of transinduction by Vmw65 varies during the cell cycle. Virology. 1992;189:196–202. doi: 10.1016/0042-6822(92)90695-l. [DOI] [PubMed] [Google Scholar]
- 17.De Azevedo W F, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim S H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 18.DePamphilis M L. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 19.Dynlacht B. Regulation of transcription by proteins that control the cell cycle. Nature. 1997;389:149–152. doi: 10.1038/38225. [DOI] [PubMed] [Google Scholar]
- 20.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 21.Ebert S, Dubramanian D, Shtrom S, Chung I, Parris D, Muller M. Association between the p170 form of human topoisomerase II and progeny viral DNA in cells infected with herpes simplex virus type 1. J Virol. 1994;68(2):1010–1020. doi: 10.1128/jvi.68.2.1010-1020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 23.Glab N, Labidi B, Qin L X, Trehin C, Bergounioux C, Meijer L. Olomoucine, an inhibitor of the cdc2/cdk2 kinases activity, blocks plant cells at the G1 to S and G2 to M cell cycle transitions. FEBS Lett. 1994;353:207–211. doi: 10.1016/0014-5793(94)01035-8. [DOI] [PubMed] [Google Scholar]
- 24.Gold M O, Tassan J P, Nigg E A, Rice A P, Herrmann C H. Viral transactivators E1A and VP16 interact with a large complex that is associated with CTD kinase activity and contains CDK8. Nucleic Acids Res. 1996;24:3771–3777. doi: 10.1093/nar/24.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 26.Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 27.Hammersten O, Xiaodan Y, Elias P. Inhibition of topoisomerase II by ICRF-193 prevents efficient replication of herpes simplex virus type 1. J Virol. 1996;70:4523–4529. doi: 10.1128/jvi.70.7.4523-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassell J A, Brinton B. SV40 and polyomavirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 639–677. [Google Scholar]
- 29.Hay J, Subak-Sharpe J H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activity. J Gen Virol. 1976;31:145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- 30.Hay R. Adenovirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 699–719. [Google Scholar]
- 31.Hengst L, Reed S I. Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol. 1998;227:25–41. doi: 10.1007/978-3-642-71941-7_2. [DOI] [PubMed] [Google Scholar]
- 32.Henley D C, Weir J P. The relative stability of selected herpes simplex virus type 1 mRNAs. Virus Res. 1991;20:121–132. doi: 10.1016/0168-1702(91)90104-4. [DOI] [PubMed] [Google Scholar]
- 33.Hilton M J, Mounghane D, McLean T, Contractor N V, O'Neil J, Carpenter K, Bachenheimer S L. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1995;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann F, Livingston D M. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996;10:851–861. doi: 10.1101/gad.10.7.851. [DOI] [PubMed] [Google Scholar]
- 35.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honess R W, Watson D H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977;21:584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997;78:3341–3348. doi: 10.1099/0022-1317-78-12-3341. [DOI] [PubMed] [Google Scholar]
- 38.Iseki H, Ko T C, Xue X Y, Seapan A, Hellmich M R, Townsend C M., Jr Cyclin-dependent kinase inhibitors block proliferation of human gastric cancer cells. Surgery. 1997;122:187–194. doi: 10.1016/s0039-6060(97)90008-8. [DOI] [PubMed] [Google Scholar]
- 39.Jackman M R, Pines J N. Cyclins and the G2/M transition. Cancer Surv. 1997;29:47–73. [PubMed] [Google Scholar]
- 40.Jiang W, Wells N J, Hunter T. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Nat Acad Sci USA. 1999;96:6193–6198. doi: 10.1073/pnas.96.11.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jofre J T, Courtney R J, Schaffer P A. A dominant lethal temperature-sensitive mutant of herpes simplex virus type 1. Virology. 1981;111:173–190. doi: 10.1016/0042-6822(81)90663-2. [DOI] [PubMed] [Google Scholar]
- 42.Jofre J T, Schaffer P A, Parris D S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977;23:833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan R, Schang L M, Schaffer P A. Transactivation of HSV-1 IE gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol. 1999;73:843–847. doi: 10.1128/jvi.73.10.8843-8847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim C G, Shim E Y, Lee J E, Jang Y K, Lee C G, Park S D. Allosteric interaction of a herpes simplex viral thymidine kinase with host DNA polymerase alpha in mouse LP1-1 cells. Biochem Mol Biol Int. 1994;32:651–657. [PubMed] [Google Scholar]
- 47.Kitagawa M, Higashi H, Suzuki-Takahashi I, Segawa K, Hanks S K, Taya Y, Nishimura S, Okuyama A. Phosphorylation of E2F-1 by cyclin A-cdk2. Oncogene. 1995;10:229–236. [PubMed] [Google Scholar]
- 48.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 49.Leclerc V, Tassan J P, O'Farrell P H, Nigg E A, Leopold P. Drosophila Cdk8, a kinase partner of cyclin C that interacts with the large subunit of RNA polymerase II. Mol Biol Cell. 1996;7:505–513. doi: 10.1091/mbc.7.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S-K, Dong Q, Lehman I. Interaction of herpes simplex virus 1 origin-binding protein with DNA polymerase α. Proc Natl Acad Sci USA. 1995;92:7882–7886. doi: 10.1073/pnas.92.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Bhattacharye S, Prives C. Cyclin-dependent kinase regulation of the replication functions of polyomavirus large T antigen. J Virol. 1997;71:6479–6485. doi: 10.1128/jvi.71.9.6479-6485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L-J, Naeve G S, Lee A S. Temporal regulation of cyclin A-p107 and p33cdk2 complexes binding to a human thymidine kinase promoter element important for G1-S phase transcriptional regulation. Proc Nat Acad Sci USA. 1993;90:3554–3558. doi: 10.1073/pnas.90.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao J G-H, Robinshaw E E, Overby L R. Inhibition of DNA polymerase from herpes simplex virus-infected Wi-38 cells by phosphonoacetic acid. J Virol. 1975;15:1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martín-Castellanos C, Moreno S. Recent advances on cyclins, CDKs and CDK inhibitors. Trends Cell Biol. 1997;7:95–98. doi: 10.1016/S0962-8924(96)10055-6. [DOI] [PubMed] [Google Scholar]
- 55.McGeoch D, Barnett B, McLean C. Emerging functions of alphaherpesvirus genes. Semin Virol. 1993;4:125–134. [Google Scholar]
- 56.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen-Huynh A T, Schaffer P A. Cellular transcription factors enhance herpes simplex virus type 1 oriS-dependent DNA replication. J Virol. 1998;72:3635–3645. doi: 10.1128/jvi.72.5.3635-3645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichol P F, Chang J Y, Johnson E M, Jr, Olivo P D. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J Virol. 1996;70:5476–5486. doi: 10.1128/jvi.70.8.5476-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivo P D, Nelson N J, Challberg M D. herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J Virol. 1989;63:196–204. doi: 10.1128/jvi.63.1.196-204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oroskar A A, Read S G. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989;63:1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Overby L, Robishaw E, Schleicher J, Reuter A, Shipkowitz N, Mao J-H. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974;6:360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parris D S, Courtney R J, Schaffer P A. Temperature-sensitive mutants of herpes simplex virus type 1 defective in transcriptional and post-transcriptional functions required for viral DNA synthesis. Virology. 1978;90:177–186. doi: 10.1016/0042-6822(78)90301-x. [DOI] [PubMed] [Google Scholar]
- 64.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed S I. G1/S regulatory mechanisms from yeast to man. Prog Cell Cycle Res. 1996;2:15–27. doi: 10.1007/978-1-4615-5873-6_2. [DOI] [PubMed] [Google Scholar]
- 66.Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene. 1996;12:2631–2640. [PubMed] [Google Scholar]
- 67.Rieber M, Rieber M S. Cyclin-dependent kinase 2 and cyclin A interaction with E2F are targets for tyrosine induction of B16 melanoma terminal differentiation. Cell Growth Differ. 1994;5:1339–1346. [PubMed] [Google Scholar]
- 68.Rosenberg A R, Zindy F, Le Deist F, Mouly H, Metezeau P, Brechot C, Lamas E. Overexpression of human cyclin A advances entry into S phase. Oncogene. 1995;10:1501–1509. [PubMed] [Google Scholar]
- 69.Schaffer P A, Aron G M, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52:57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- 70.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schang L M, Rosenberg A, Schaffer P A. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schulze-Gahmen U, Brandsen J, Jones H D, Morgan D O, Meijer L, Vesely J, Kim S H. Multiple modes of ligand recognition: crystal structures of cyclin-dependent protein kinase 2 in complex with ATP and two inhibitors, olomoucine and isopentenyladenine. Proteins. 1995;22:378–391. doi: 10.1002/prot.340220408. [DOI] [PubMed] [Google Scholar]
- 73.Serizawa H, Makela T P, Conaway J W, Conaway R C, Weinberg R A, Young R A. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature. 1995;374:280–282. doi: 10.1038/374280a0. [DOI] [PubMed] [Google Scholar]
- 74.Shiekhattar R, Mermelstein F, Fisher R P, Drapkin R, Dynlacht B, Wessling H C, Morgan D O, Reinberg D. Cdk-activating kinase complex is a component of human transcription factor. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- 75.Skaliter R, Lehman I. Rolling circle DNA replication in vitro by a complex of herpes simplex virus type 1-encoded enzymes. Proc Natl Acad Sci USA. 1994;91:10665–10669. doi: 10.1073/pnas.91.22.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith E J, Nevins J R. The Rb-related p107 protein can suppress E2F function independently of binding to cyclin A/cdk2. Mol Cell Biol. 1995;15:338–344. doi: 10.1128/mcb.15.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stenlund A. Papillomavirus DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 679–697. [Google Scholar]
- 78.Umene K, Nishimoto T. Inhibition of generation of authentic genomic termini of herpes simplex virus type 1 DNA in temperature-sensitive mutant BHK-21 cells with a mutated CCG1/TAF(II)250 gene. J Virol. 1996;70:9008–9012. doi: 10.1128/jvi.70.12.9008-9012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 80.Van Sant C, Kawaguchi Y, Roizman B. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc Natl Acad Sci USA. 1999;96:8184–8189. doi: 10.1073/pnas.96.14.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vesely J, Havlicek L, Strnad M, Blow J J, Donella-Deana A, Pinna L, Letham D S, Kato J, Detivaud L, Leclerc S, Meijer L. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 82.Weisshart K, Fanning E. Roles of phosphorylation in DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor Laboratory; 1996. pp. 295–329. press, Cold Spring Harbor, N.Y. [Google Scholar]
- 83.Wilcock D, Lane D P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991;349:429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- 84.Wilson A C, Freiman R N, Goto H, Nishimoto T, Herr W. VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol. 1997;17:6139–6146. doi: 10.1128/mcb.17.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu M, Sheppard K A, Peng C Y, Yee A S, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yanagi K, Talavera A, Nishimoto T, Rush M G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978;25:42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye M, Duus K M, Peng J, Price D H, Grose C. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J Virol. 1999;73:1320–1330. doi: 10.1128/jvi.73.2.1320-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zindy F, Lamas E, Chenivesse X, Sobczak J, Wang J, Fesquet D, Henglein B, Brechot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]