Abstract
Introduction
The introduction of biological therapies has revolutionized the treatment of moderate-to-severe plaque psoriasis. In particular, ixekizumab, an inhibitor of interleukin-17A, has shown great results in terms of efficacy and safety in both clinical trials and real-world experiences. However, there is a lack of long-term real-world data available for ixekizumab.
Methods
We conducted a multicenter real-life study to evaluate the effectiveness and safety of ixekizumab in patients with moderate-to-severe plaque psoriasis. Psoriasis Area and Severity Index score (PASI) was collected at baseline and after 1, 2, 3, 4, and 5 years. The occurrence of any adverse events was recorded at each time point.
Results
We enrolled 1096 patients treated with ixekizumab for at least 1 year. At week 52, the percentages of PASI 90 and PASI 100 were 85.04% and 69.07%, respectively. After 5 years of treatment with ixekizumab, out of 145 patients, a PASI 90 response was achieved by 86.90% of patients, while complete skin clearance was reached by 68.28% of patients. We did not observe any new significant safety findings throughout the study period.
Conclusion
This study supports the long-term effectiveness and safety of ixekizumab in a real-world setting.
Keywords: Anti-IL-17, Ixekizumab, Psoriasis, Psoriasis treatment, Real-world
Key Summary Points
| The efficacy and safety of ixekizumab have been assessed in both clinical trials and real life, but data on long-term effectiveness are still limited. |
| We conducted a multicenter retrospective real-life study to assess the effectiveness and safety profiles of ixekizumab in patients affected by moderate-to-severe plaque psoriasis. |
| In our study, we observed higher rates of PASI 90 and PASI 100 responses compared with data from phase 3 clinical trials, and 78.97% of our patients were still on ixekizumab treatment after 5 years. |
| After 5 years of treatment with ixekizumab, the presence of PsA was associated with higher rates of PASI and PASI 100 in the multivariate analysis. |
Introduction
Psoriasis is an inflammatory skin disease that affects up to 2–3% of the worldwide population [1]. Psoriasis is considered a systemic disease that not only affects the skin but is often associated with several comorbidities, in particular, cardiometabolic disorders, joint involvement, anxiety, depression, and inflammatory bowel disease (IBD) [1].
Biological therapies have dramatically changed the approach to treating moderate-to-severe psoriasis, achieving excellent levels of skin clearance in a significant percentage of patients [2]. One of the most recent biological classes to enter the psoriasis armamentarium is the interleukin-17 (IL-17) inhibitors [2]. In particular, ixekizumab, an anti-IL-17A drug, was approved by the European Medicines Agency (EMA) in April 2016 for the treatment of moderate-to-severe plaque psoriasis and in December 2017 for the treatment of active psoriatic arthritis [3]. Data from clinical trials have shown great results in terms of rapidity and safety and impressive results in terms of long-term efficacy [4, 5]. However, as a result of its only recent introduction to the market, real-world data on long-term maintenance of skin clearance and safety are still lacking. For this reason, we conducted a retrospective, multicenter, real-life study of patients with moderate-to-severe plaque psoriasis treated with ixekizumab for at least 1 year.
Methods
A total of 1096 patients with moderate-severe plaque-type psoriasis were enrolled and treated with ixekizumab between January 2018 and January 2024. The databases of 14 Italian Dermatology units were analyzed for this study. All patients received ixekizumab in accordance with the Italian adaptation of EuroGuiDerm guidelines for the management of moderate-to-severe plaque psoriasis [6, 7]. Prior to receiving ixekizumab, all patients had been treated with at least one conventional systemic drug or had a contraindication to these treatments. Prior to initiating ixekizumab treatment, screening for hepatitis C, hepatitis B, tuberculosis, and HIV was conducted [6]. The administration of ixekizumab followed the Summary of Product Characteristics [3].
Patients’ characteristics, including gender, age, body mass index (BMI), cardiovascular comorbidities (arterial hypertension, obesity, type 2 diabetes mellitus, and hypercholesterolemia), concomitant psoriatic arthritis (PsA), previous exposure to other biological agents, and involvement of difficult-to-treat areas (scalp, palms/soles, genitalia, and nails) were obtained from electronic medical records.
During each dermatological visit, we recorded the Psoriasis Area and Severity Index (PASI) score and calculated the percentages of patients who achieved a 90% and 100% reduction in PASI compared to the baseline after 1, 2, 3, 4, and 5 years of follow-up. Additionally, we evaluated the proportion of patients who reached an absolute PASI of 2 or less at the same time points, following the Italian Guidelines [7].
Finally, a safety evaluation was conducted and based on reported adverse events (AEs) at each visit, including AEs that led to the discontinuation of ixekizumab and severe AEs.
As the study’s procedures did not deviate from good clinical practice, no institutional review board was required. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. All patients included in the study provided written informed consent for the retrospective analysis of their clinical data.
The categorical data were described as absolute frequencies and percentages, while continuous variables were reported as mean and standard deviation (SD). To evaluate the impact of multiple factors on the dependent variables PASI 90, PASI 100, and PASI ≤ 2, we performed multivariate logistic regression analysis. The independent variables considered were BMI, cardiometabolic comorbidities (yes or no), previous exposure to other biologics (yes or no), involvement of at least one difficult-to-treat areas (yes or no). Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated, and a p value < 0.05 was considered statistically significant. All patients treated with ixekizumab were included in the drug survival analysis using the Kaplan–Meier curve. The date the patient discontinued treatment for any reason was selected as the event date. Time data was censored for patients still on treatment when the study was conducted and for patients lost to follow-up.
The statistical analysis was performed using Stata/SE 17.0 software. Figures and tables were generated using Microsoft Excel.
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received ixekizumab as part of routine clinical practice in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Results
A total of 1096 patients were enrolled in the study. All of them reached at least 52 weeks of treatment, while 848 patients reached 2 years of follow-up. After 3, 4, and 5 years, the study population was composed of 636, 359, and 145 patients, respectively. Seven hundred and thirty-five patients were male (67.06%). The mean age was 53.03 (SD 14.74). Regarding the previous exposure to biologics, 622 patients were bio-experienced (56.57%), meaning that more than half of the analyzed sample was already treated with at least one biological drug before switching to ixekizumab. The mean BMI at baseline was 27.14 kg/m2 (SD 5.25). A total of 374 patients (34.12%) previously received a diagnosis of PsA, while 671 patients (61.22%) had the involvement of at least one difficult-to-treat area (including palms/soles, scalp, genitalia, and nails).
Additional demographic characteristics of our population at baseline are shown in Table 1.
Table 1.
Baseline characteristics of the study population
| Mean (SD) | |
|---|---|
| Age, years | 53.03 (14.74) |
| BMI, kg/m2 | 27.14 (5.25) |
| PASI at baseline | 15.75 (7.29) |
| N (%) | |
| Male | 735 (67.06) |
| PsA | 374 (34.12) |
| Difficult-to-treat areas | 671 (61.22) |
| Bio-naïve | 476 (43.43) |
| Obese | 224 (20.44) |
| At least one CMD | 490 (44.71) |
| Hepatitis C | 1 (0.09) |
| Latent tuberculosis | 1 (0.09) |
| Past history of cancer | 2 (0.18) |
The mean PASI at baseline was 15.75 (SD 7.29), and during the treatment with ixekizumab, it decreased to 0.69 (1.65) at week 52 and 0.83 (1.88) after 2 years of treatment. Mean PASI was similar after 3, 4, and 5 years of follow-up, being 0.73 (2.07), 1.08 (2.79), and 0.82 (2.13), respectively.
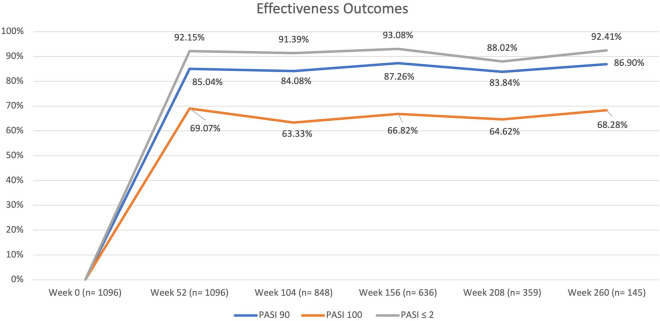
During the first year of treatment, 85.04% of the patients reached PASI 90, and 69.07% reached PASI 100. The effectiveness of ixekizumab was maintained throughout the study period, with 84.08%, 87.26%, 83.84%, and 86.90% of patients achieving PASI 90 after 2, 3, 4, and 5 years of follow-up, respectively. Complete skin clearance (PASI 100) was reached by 63.33%, 66.82%, 64.62%, and 68.28% of patients after 2, 3, 4, and 5 years of treatment with ixekizumab.
Complete data regarding the effectiveness of ixekizumab in terms of PASI 90, PASI 100, and PASI ≤ 2 are shown in Fig. 1.
Fig. 1.
Percentages of patients achieving PASI 90, PASI 100, and absolute PASI ≤ 2 throughout the study period. PASI Psoriasis Area and Severity Index
The effectiveness of ixekizumab was analyzed in relation to different variables, including age, gender, the presence of concomitant PsA, BMI, PASI score at baseline, involvement of difficult sites, and diagnosis of at least one cardiometabolic disease (CMD).
The complete results of the multivariate analysis are reported in Table 2. After 1 year of treatment, bio-naïve patients were significantly more likely to achieve PASI 90 (OR 0.38 [95% CI 0.26–0.56], p < 0.001), PASI 100 (OR 0.57 [95% CI 0.43–0.75, p < 0.001), and PASI ≤ 2 (OR 0.30 [95% CI 0.17–0.51], p < 0.001). At the same time point, patients with the involvement of at least one difficult-to-treat area had a lower probability of achieving a complete skin clearance (OR 0.67 [95% CI 0.50–0.90], p < 0.01). Regarding the long-term effectiveness of ixekizumab at week 208, none of the variables analyzed were associated with better rates of PASI 90, PASI 100, and PASI ≤ 2. After 5 years of treatment, patients with concomitant PsA were more likely to achieve PASI 90 (OR 10.04 [95% CI 1.20–83.70], p < 0.05) and PASI 100 (OR 2.79 [95% CI 1.03–7.57], p < 0.05). At the same time point, the status of bio-experienced was associated with higher rates of PASI ≤ 2 (OR 0.21 [95% CI 0.47–0.96], p < 0.05). To assess the maintenance of ixekizumab treatment, we evaluated the drug survival after 5 years using the Kaplan–Meier curve. At 36, 48, and 60 months of treatment, 88.59%, 84.23%, and 78.97% of our cohort were still on treatment with ixekizumab, respectively (Fig. 2).
Table 2.
Summary of the multivariate analysis results
| PsA OR [95% CI]; p value |
Difficult-to-treat areas OR [95% CI]; p value |
CMD OR [95% CI]; p value |
Bio-experienced OR [95% CI]; p value |
BMI OR [95% CI]; p value |
|
|---|---|---|---|---|---|
| PASI 90 week 52 | 1.10 [0.75–1.62]; 0.635 | 0.70 [0.47–1.04]; 0.078 | 1.05 [0.56–1.99]; 0.873 | 0.38 [0.47–0.96]; 0.044 | 1.26 [0.96–1.64]; 0.091 |
| PASI 100 week 52 | 1.32 [0.98–1.79]; 0.067 | 0.67 [0.50–0.91]; 0.09 | 0.86 [0.53–1.41]; 0.551 | 0.56 [0.42–0.74]; < 0.001 | 0.98 [0.94–1.02]; 0.273 |
| PASI ≤ 2 week 52 | 1.05 [0.63–1.76]; 0.854 | 1.03 [0.62–1.72]; 0.901 | 1.25 [0.62–1.72]; 0.617 | 0.29 [0.52–3.00]; < 0.001 | 0.97 [0.90–1.04]; 0.392 |
| PASI 90 week 104 | 1.19 [0.78–1.82]; 0.429 | 1.16 [0.78–1.72]; 0.460 | 0.58 [0.30–1.10]; 0.095 | 0.75 [0.51–1.10]; 0.139 | 1.01 [0.96–1.07]; 0.623 |
| PASI 100 week 104 | 1.80 [1.29–2.53]; 0.001 | 0.99 [0.72–1.35]; 0.927 | 0.59 [0.35–1.02]; 0.056 | 0.80 [0.59–1.08]; 0.144 | 1.02 [0.98–1.07]; 0.270 |
| PASI ≤ 2 week 104 | 1.22 [0.69–2.15]; 0.499 | 1.11 [0.66–1.88]; 0.696 | 0.56 [0.25–1.28]; 0.173 | 0.84 [0.50–1.40]; 0.504 | 0.97[0.91–1.04]; 0.443 |
| PASI 90 week 156 | 3.75 [1.79–7.82]; < 0.001 | 0.69 [0.40–1.18]; 0.178 | 0.79 [0.32–1.96]; 0.617 | 0.70 [0.42–1.18]; 0.185 | 1.02 [0.94–1.10]; 0.618 |
| PASI 100 week 156 | 2.22 [1.45–3.38]; < 0.001 | 0.87 [0.60–1.26]; 0.461 | 0.61 [0.32–1.14]; 0.122 | 0.76 [0.53–1.10]; 0.144 | 1.02 [0.96–1.08]; 0.530 |
| PASI ≤ 2 week 156 | 3.52 [1.34–9.23]; 0.011 | 1.04 [0.53–2.04]; 0.910 | 0.88 [0.27–2.86]; 0.838 | 0.59 [0.30–1.15]; 0.120 | 1 [0.90–1.11]; 0.975 |
| PASI 90 week 208 | 1.54 [0.76–3.10]; 0.228 | 1.22 [0.66–2.24]; 0.523 | 0.69 [0.23–2.05]; 0.505 | 0.77 [0.43–1.40]; 0.398 | 1 [0.91–1.09]; 0.951 |
| PASI 100 week 208 | 1.43 [0.85–2.43]; 0.181 | 1.20 [0.74–1.95]; 0.469 | 0.87 [0.36–2.08]; 0.752 | 0.61 [0.38–0.97]; 0.037 | 0.97 [0.90–1.04]; 0.376 |
| PASI ≤ 2 week 208 | 0.88 [0.42–1.84]; 0.731 | 1.24 [0.62–2.50]; 0.541 | 0.79 [0.23–02.72]; 0.707 | 0.75 [0.38–1.49]; 0.411 | 0.98 [0.88–1.09]; 0.678 |
| PASI 90 week 260 | 10.04 [1.20–83.70]; 0.033 | 1.76 [0.57–5.49]; 0.329 | 0.25 [0.04–1.67]; 0.153 | 0.49 [0.17–1.42]; 0.191 | 1.12 [0.93–1.34]; 0.231 |
| PASI 100 week 260 | 2.79 [1.03–7.57]; 0.043 | 1.05 [0.44–2.48]; 0.916 | 0.34 [0.08–1.41]; 0.138 | 0.34 [0.15–0.75]; 0.008 | 1.06 [0.93–1.20]; 0.387 |
| PASI ≤ 2 week 260 | 1 | 2.13 [0.43–10.50]; 0.353 | 0.04 [0–0.55]; 0.017 | 0.21 [0.05–0.96]; 0.044 | 1.26 [0.96–1.64]; 0.091 |
Fig. 2.
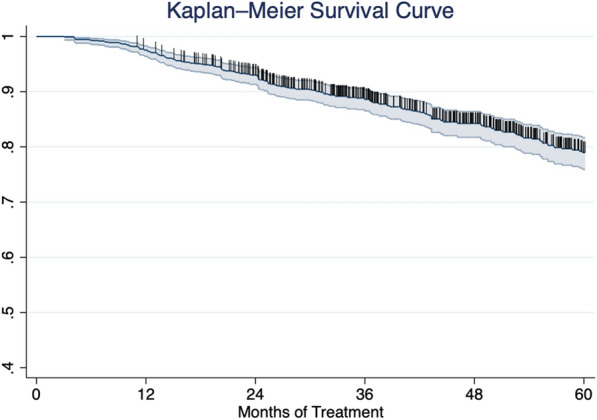
Kaplan–Meier curve of all-cause therapy discontinuation in all patients treated with ixekizumab up to 60 months
Discussion
Ixekizumab is an anti-IL17A humanized monoclonal antibody that was approved in 2016 for moderate-to-severe plaque-type psoriasis [3]. This study supports the long-term effectiveness of ixekizumab for 5 years of continuous treatment in accordance with data from open-label extensions of phase 3 clinical trials [4, 5]. To date, this is the largest real-life study with a follow-up reaching 5 years of treatment with ixekizumab.
Compared to clinical trials UNCOVER-1, UNCOVER-2, and UNCOVER-3, our cohort had a few different demographic characteristics at baseline. In particular, we reported a higher mean age, which is expected because of the real-life nature of our experience, and a lower mean PASI, probably due to the strict inclusion criteria of clinical trials [8]. Moreover, a higher proportion of our patients was previously exposed to other biological treatments (more than 50% compared with 26.4% in clinical trials).
Compared to data from UNCOVER-1 and UNCOVER-2 studies, our real-life experience showed better results in terms of PASI90 and PASI100 at each time point throughout the 5 years of follow-up [4]. In particular, PASI 90 and PASI 100 were achieved by 71.3% and 46.3% of patients after 264 weeks of treatment in clinical trials, while we observed them in 86.90% and 68.28% of our patients at week 260 [4] (Fig. 3). Also, in terms of absolute PASI, our study highlighted a higher percentage of patients who achieved a PASI of 2 or less after 5 years of treatment compared to data from clinical trials (92.41% compared with 73.6%) [4]. Our findings are very similar to those of Mastorino et al., who observed PASI 90 and PASI 100 in 81.8% and 59.1% of the 22 patients who completed 5 years of treatment [9].
Fig. 3.
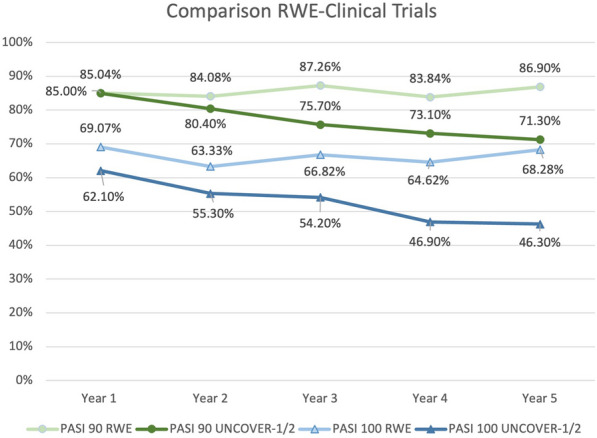
Comparison between phase 3 clinical trials (modified nonresponder imputation analysis) and our real-world experience. PASI Psoriasis Area and Severity Index
In this study, ixekizumab showed better clinical outcomes at week 52 in bio-naïve patients at the multivariate analysis compared with the bio-experienced subgroups. Similar data have also been observed regarding other IL-17 inhibitors for the treatment of psoriasis [10, 11].
In our experience, ixekizumab proved to be equally effective in patients with and without the involvement of difficult-to-treat areas across almost all the endpoints, consistent with available data on IL-17 inhibitors in patients with palmoplantar psoriasis and genital psoriasis [12–14].
Current European and Italian guidelines for the treatment of plaque psoriasis do not differentiate between the use of anti-IL-23 and anti-IL-17 agents as first-line therapies. Our multivariate analysis results support the use of ixekizumab in the first-line setting in all subpopulations, especially in patients with concomitant PsA and involvement of difficult-to-treat areas.
Ixekizumab did not show any new significant safety findings during our 5-year follow-up. The most common adverse events were mild-to-moderate upper respiratory tract infections, injection-site reactions, and Candida infections.
Our study has several limitations, which are primarily due to its retrospective nature. These include the heterogeneity of clinical assessment among different clinicians throughout the study period. Moreover, as expected in a real-world study, the prevalence of adverse events is probably underestimated, as both patients and clinicians tend not to report mild AEs outside of clinical trials.
Conclusion
Our multicenter study supports data from both clinical trials and other real-life experiences, highlighting the long-term effectiveness and safety of ixekizumab throughout a 5-year follow-up.
Acknowledgements
We thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
The authors did not use any medical writing and editorial assistance for this article.
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Mario Valenti, Luigi Gargiulo and Luciano Ibba. Data collection was performed by Mario Valenti, Luigi Gargiulo and Luciano Ibba, Fabrizio Amoruso, Anna Balato, Federico Bardazzi, Martina Burlando, Carlo G. Carrera, Paolo Dapavo, Valentina Dini, Francesca M. Gaiani, Giampiero Girolomoni, Claudio Guarneri, Claudia Lasagni, Francesco Loconsole, Angelo V. Marzano, Martina Maurelli, Matteo Megna, Diego Orsini and Massimo Travaglini. All authors commented on previous versions of the manuscript. Resources: Antonio Costanzo. Supervision: Alessandra Narcisi, Antonio Costanzo and Piergiorgio Malagoli. All authors read and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Mario Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. Luigi Gargiulo has been a consultant for Almirall, Pfizer and UCB. Luciano Ibba has been a consultant for Almirall. Piergiorgio Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. Anna Balato has received honoraria for participation in advisory boards, meetings, or as speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. Federico Bardazzi has been a consultant advisor and clinical study investigator for Eli Lilly, AbbVie, Novartis, Leo Pharma, Sandoz, Bristol Myers, Abiogen-Pharma, Celgene and Janssen. Martina Burlando has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, UCB Pharma. Carlo G. Carrera has served as a board participant or speaker for AbbVie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. Francesca M. Gaiani acted as a speaker or consultant for Novartis, AbbVie, Eli Lilly, Celgene, LeoPharma, and Almirall. Paolo Dapavo has been a speaker for Novartis, AbbVie, Sanofi, UCB, Janssen, Lilly, and LeoPharma. Giampiero Girolomoni served as consultant and/or speaker for AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Eli-Lilly, LeoPharma, Novartis, Pfizer, Samsung, Sanofi and UCB. Claudio Guarneri has been a scientific consultant/speaker/clinical study investigator for AbbVie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Almirall, LEO Pharma. Claudia Lasagni declares a conflict of interest with AbbVie, Novartis, Lilly and Almirall. Francesco Loconsole served on advisory boards and/or received honoraria for lectures from AbbVie, Janssen-Cilag, Novartis, Lilly, Sanofi. Angelo V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. Matteo Megna acted as a speaker or consultant for AbbVie, Eli Lilly, Janssen, Leo-Pharma, UCB and Novartis. Diego Orsini has been a speaker and/or consultant for AbbVie, LeoPharma, UCB, Bristol-Meyer-Squibb and Boehringer-Ingelheim. Antonio Costanzo has been a consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Galderma, Boehringer, Novartis, Pfizer, Sandoz, and UCB. Alessandra Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, AbbVie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. Fabrizio Amoruso, Valentina Dini, Martina Maurelli and Massimo Travaglini have nothing to disclose.
Ethical Approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received ixekizumab as part of routine clinical practice in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
References
- 1.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 2.Dave R, Alkeswani A. An overview of biologics for psoriasis. J Drugs Dermatol. 2021;20(11):1246–1247. doi: 10.36849/jdd.6040. [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency. Taltz (ixekizumab): summary of product characteristics. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/taltz. Accessed 15 Mar 2024.
- 4.Leonardi C, Reich K, Foley P, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther (Heidelb) 2020;10(3):431–447. doi: 10.1007/s13555-020-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blauvelt A, Lebwohl MG, Mabuchi T, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 2021;85(2):360–368. doi: 10.1016/j.jaad.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498. doi: 10.1111/jdv.16915. [DOI] [PubMed] [Google Scholar]
- 7.Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl 1):1–78. doi: 10.23736/S2784-8671.21.07132-2. [DOI] [PubMed] [Google Scholar]
- 8.Papp KA, Leonardi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3) Br J Dermatol. 2018;178(3):674–681. doi: 10.1111/bjd.16050. [DOI] [PubMed] [Google Scholar]
- 9.Mastorino L, Dapavo P, Burzi L, et al. Drug survival, effectiveness and safety of ixekizumab for moderate-to-severe psoriasis up to 5 years. J Eur Acad Dermatol Venereol. 2024;38(3):568–575. doi: 10.1111/jdv.19682. [DOI] [PubMed] [Google Scholar]
- 10.Gargiulo L, Ibba L, Malagoli P, et al. Brodalumab for the treatment of plaque psoriasis in a real-life setting: a 3 years multicenter retrospective study-IL PSO (Italian landscape psoriasis) Front Med (Lausanne) 2023;10:1196966. doi: 10.3389/fmed.2023.1196966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiricozzi A, Balato A, Conrad C, et al. Secukinumab demonstrates improvements in absolute and relative psoriasis area severity indices in moderate-to-severe plaque psoriasis: results from a European, multicentric, retrospective, real-world study. J Dermatol Treat. 2020;31(5):476–483. doi: 10.1080/09546634.2019.1671577. [DOI] [PubMed] [Google Scholar]
- 12.Ibba L, Gargiulo L, Alfano A, et al. Anti-IL-23 and anti-IL-17 drugs for the treatment of non-pustular palmoplantar psoriasis: a real-life retrospective study. J Dermatol Treat. 2023;34(1):2199108. doi: 10.1080/09546634.2023.2199108. [DOI] [PubMed] [Google Scholar]
- 13.Valenti M, Gargiulo L, Ibba L, et al. Effectiveness of ixekizumab for the treatment of moderate-to-severe plaque psoriasis with involvement of difficult-to-treat areas: a 52-week multicenter retrospective study. J Dermatol. 2024 doi: 10.1111/1346-8138.17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piaserico S, Riedl E, Pavlovsky L, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational Psoriasis Study of Health Outcomes (PSoHO) Front Med (Lausanne) 2023;10:1185523. doi: 10.3389/fmed.2023.1185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.