Abstract
The triterpene RPR103611 is an efficient inhibitor of membrane fusion mediated by the envelope proteins (Env, gp120-gp41) of CXCR4-dependent (X4) human immunodeficiency virus type 1 (HIV-1) strains, such as HIV-1LAI (LAI). Other X4 strains, such as HIV-1NDK (NDK), and CCR5-dependent (R5) HIV-1 strains, such as HIV-1ADA (ADA), were totally resistant to RPR103611. Analysis of chimeric LAI-NDK Env proteins identified a fragment of the NDK gp41 ectodomain determining drug resistance. A single difference at position 91, leucine in LAI and histidine in NDK, apparently accounted for their sensitivity or resistance to RPR103611. We had previously identified a mutation of isoleucine 84 to serine in a drug escape LAI variant. Both I84 and L91 are located in the “loop region” of gp41 separating the proximal and distal helix domains. Nonpolar residues in this region therefore appear to be important for the antiviral activity of RPR103611 and are possibly part of its target. However, another mechanism had to be envisaged to explain the drug resistance of ADA, since its gp41 loop region was almost identical to that of LAI. Fusion mediated by chimeric Env consisting of LAI gp120 and ADA gp41, or the reciprocal construct, was fully blocked by RPR103611. The gp120-gp41 complex of R5 strains is stable, relative to that of X4 strains, and this stability could play a role in their drug resistance. Indeed, when the postbinding steps of ADA infection were performed under mildly acidic conditions (pH 6.5 or 6.0), a treatment expected to favor dissociation of gp120, we achieved almost complete neutralization by RPR103611. The drug resistance of NDK was partially overcome by preincubating virus with soluble CD4, a gp120 ligand inducing conformational changes in the Env complex. The antiviral efficacy of RPR103611 therefore depends on the sequence of the gp41 loop and the stability of the gp120-gp41 complex, which could limit the accessibility of this target.
The human immunodeficiency virus type 1 (HIV-1) and HIV-2 envelope glycoproteins (Env) consist of noncovalent complexes of surface (gp120) and transmembrane (gp41) subunits, both derived from a gp160 precursor which is oligomerized and cleaved during its transport to the cell surface (reviewed in references 9, 26, and 46). The function of these proteins is to mediate virus entry by allowing binding of virions to the cell surface and fusion of their lipidic envelopes with the cell membrane. Our knowledge of the structure of HIV-1 Env and of the mechanism by which it fulfils its function has considerably improved over the last years, although a number of aspects remain to be elucidated. Schematically, the initial steps of virus entry (binding) are mediated by gp120, while gp41 is responsible for the membrane fusion process itself. By analogy with the influenza virus hemagglutinin model, gp41 is thought to become fusion competent after conformation changes in the gp120-gp41 complex (15, 38), which are not as yet understood at the molecular level. These events seem to be usually triggered by the interaction of gp120 with two classes of cell surface molecules, CD4 and chemokine receptors, in particular CCR5 or CXCR4, often viewed as HIV coreceptors (reviewed in references 2, 14, and 20). In vivo, strains using CXCR4 (termed X4 strains) or both CXCR4 and CCR5 (R5X4) are isolated at later stages of infection, while strains using CCR5 (R5) are predominant at the earlier stages. The X4 strains, in particular when adapted to replication in T-cell lines, are characterized by a relatively labile gp120-gp41 association, evidenced by the shedding of gp120, spontaneously or upon contact with soluble CD4 (sCD4) or anti-gp120 antibodies (24, 33, 36), while the gp120-gp41 complex of R5 strains seems comparatively stable (27, 30).
Like other retroviral transmembrane proteins, gp41 comprises an N-terminal extracellular domain (ectodomain), a membrane-spanning domain, and a C-terminal cytoplasmic domain, apparently dispensible for the fusion process (9). The main features of the ectodomain are a hydrophobic N-terminal sequence (“fusion peptide”), thought to insert in the target cell membrane, and two domains with a predicted α-helix conformation separated by a region containing a conserved dicysteine motif, representing a highly immunogenic determinant (11). Several residues in the proximal helix and the loop region of gp41 seem to be involved in interactions with gp120 (13). Peptides corresponding to the proximal (N) and distal (C) helix domains of HIV-1 gp41 spontaneously form highly stable coiled-coil structures with an inner core of three parallel N helices on which are stacked three C helices placed in an antiparallel orientation (4, 41, 42). Structural analysis of the gp41 ectodomain of the HIV-2-related simian immunodeficiency virus revealed the same organization (3). Whether the formation of this structure is the motive force driving the viral and target membranes to a closer apposition (4, 42) or whether this structure is already present in the native form of gp41 is not known (3).
Different strategies to block the HIV-1 infectious process at the cell entry step, either by targeting one of the cellular receptors or the envelope proteins themselves, are envisioned. To date, the vast majority of available compounds interfere with the initial steps of virus entry, i.e., the interaction of gp120 with cell surface components. The most promising compounds are bicyclams, which are nonpeptidic antagonists of CXCR4 (1). The extreme genetic variability of gp120 among isolates (29) and the ability of HIV-1 to switch from one type of receptor to another (e.g., CXCR4 to CCR5) by a few mutations in gp120 (39) are obvious limitations for these strategies. In contrast, gp41 is far more conserved and does not seem to require further interactions with cellular proteins to mediate membrane fusion. However, very few compounds targeting gp41 have been described to date. Peptides derived from the proximal or the distal α-helix regions of gp41 inhibit cell-cell fusion and HIV-1 entry (44, 45). These compounds could act as dominant-negative inhibitors of the interaction between the proximal and distal helices, thereby preventing gp41 from attaining its fusion-active conformation (5), but other mechanisms of action have been proposed (3, 28). Peptides from the distal helix display the most efficient antiviral activity, and one such peptide (T20) was recently found to reduce the virus load in HIV-1-infected individuals (17). However, cost and bioavailability issues seem to represent serious limitations to the use of such peptides on a larger scale.
To our knowledge, the triterpene RPR103611, a betulinic acid derivative (molecular weight, 712), is the only nonpeptidic antiviral compound thought to target gp41. It was isolated through random screening and was found to block cell-cell fusion and HIV-1 infection at a postbinding step (21). We have characterized a drug escape mutant of the X4 strain HIV-1LAI (LAI) and have found that its phenotype stemmed from a mutation in the gp41 ectodomain (19). In addition, the drug-resistant phenotype of an X4 strain of African origin, HIV-1NDK (NDK), was attributed to differences with LAI in gp41. Here we have further addressed the mechanism of the resistance of NDK to RPR103611 and have identified a gp41 residue apparently critical for its antiviral effect. Unexpectedly, HIV-1ADA (ADA), an R5 strain almost identical to LAI in its gp41 sequence, was fully resistant to neutralization by RPR103611. In this case, drug resistance seemed related to the relatively stable association of the gp120-gp41 complex, probably limiting access of the drug to its target.
MATERIALS AND METHODS
Cell lines and viral strains.
Infections were performed in target cells derived from the U373MG-CD4 (12) or HeLa-P4 (6) cell line; both of these cell lines are CD4+ and bear the Escherichia coli β-galactosidase gene (lacZ) under transcriptional control of the HIV-1 long terminal repeat (LTR). The U373MG-CD4 cells stably expressing either CXCR4 or CCR5 (18) and the HeLa-P4 derivatives stably expressing CCR5 (HeLa-P5) (32) have been described. Cells were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), antibiotics (penicillin and streptomycin), and 2 mM glutamine. Virus stocks were supernatants of HeLa cells transfected with the cloned molecular genomes of the HIV-1 strains LAI (31), NDK (40), HIV-189.6 (89.6) (7), and ADA, actually a recombinant LAI provirus with env from ADA (32, 43). Infectious titers were determined in Hela-P4 cells, except for ADA, which was titrated in HeLa-P5 cells.
Antibodies, recombinant proteins, and other reagents.
The sheep anti-gp120 antibody D7324 raised against a peptide from the C terminus of gp120 (25) was obtained from Aalto BioReagents (Dublin, Ireland). Pooled sera from HIV-1-infected individuals were a gift from N. Sol (Hôpital St.-Louis, Paris, France). Peroxidase-conjugated goat anti-human immunoglobulin G was obtained from Jackson ImmunoResearch Laboratories (West Grove, Pa.). The betulinic acid derivative RPR103611 was obtained from Rhône-Poulenc Rorer Laboratories (Vitry-sur-Seine, France) as a dry powder and diluted in dimethylformamide (10 mM stock solution). Baculovirus-expressed gp120 from HIV-1IIIB (a LAI variant) and sCD4 were obtained from Neosystems Laboratories (Strasbourg, France).
Env expression vectors.
The vectors allowing expression of Env from LAI (NS105) (19) and ADA (32) have been described. Env LAI mutants were derived from NS105 by site-directed mutagenesis on a single-stranded template. Mutants were screened for the creation of restriction enzyme sites and checked by sequencing the gp41 ectodomain. The 89.6 Env expression vector was obtained by replacing the KpnI-BamHI fragment of NS105 (nucleotides [nt] 5925 to 8068 in LAI) by a KpnI-BamHI PCR fragment amplified from the 89.6 env. Sequences of oligonucleotides used for PCR and site-directed mutagenesis can be provided upon request. The LAI-ADA Env chimeras, LA-1 and LA-2, were obtained by ligating KpnI site-to-blunt end (gp120) and blunt end-to-BamHI site (gp41) PCR fragments amplified from ADA env or from LAI env. Blunt-end ligation created a PvuI site at the gp120-gp41 junction (see Fig. 5A). The resulting V2I substitution in gp41 had no apparent effect on its function. The NDK Env expression vector contained a portion of LAI gp41 corresponding to the membrane anchor and cytoplasmic domains. It was derived from the previously described LN-3 vector (19) by replacing a SalI-MluI fragment (nt 5320 to 6632) by a SalI-MluI PCR fragment amplified from NDK env. The LN-7, LN-8, and LN-9 expression vectors have been described (19). The XbaI site introduced in LAI env (nt 7669) facilitated construction of LN-10 by substituting the LAI env XbaI-BamHI fragment for the corresponding LN-8 fragment. LN-11 was obtained by replacing the ApaLI-BamHI fragment of the NDK Env expression vector by the corresponding fragment from LN-9. The HIV-2ROD (ROD) Env expression vector was a cloned ROD provirus (34) with nucleotides between HindIII sites (nt 1457 and 5782 located in gag and vpr, respectively) deleted.
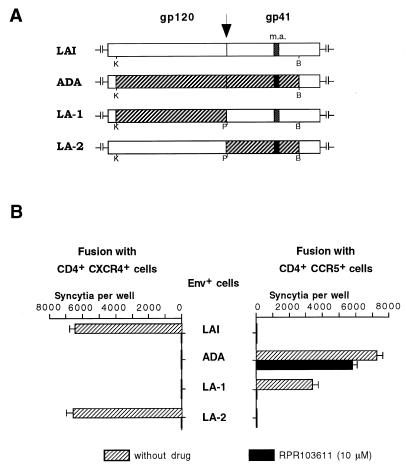
FIG. 5.
Inhibition of cell-cell fusion mediated by HIV-1 LAI-ADA Env chimeras. (A) Schematic representation of wild-type and chimeric Env. K, P, and B, KpnI, PvuI, and BamHI sites, respectively, used for construction of chimeric env (see Materials and Methods); m.a., membrane anchor domain. (B) Fusion of HeLa cells expressing indicated Env and U373MG-CD4 cells expressing either CXCR4 or CCR5, in the presence or absence of RPR103611. The experiment was performed and presented as described for Fig. 1.
Syncytium formation assay.
Subconfluent monolayers of HeLa cells in six-well trays (∼2 × 105 cells per well) were transfected with Env expression vectors by calcium phosphate precipitation. An equivalent number of LTR-lacZ target cells freshly detached by trypsinization were added 20 h after transfection. After overnight coculture in the absence or presence of RPR103611 (10 μM), adherent cells were fixed in 0.5% glutaraldehyde and stained with the β-galactosidase substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), as described previously (8). Blue-stained foci were scored under ×20 magnification.
Infectivity assays.
Standard infectivity assays were performed with HeLa-P4 target cells using in situ detection of β-galactosidase activity as the readout. Briefly ∼2 × 105 cells were infected with about 103 infectious units (i.u.) in 12-well plates in the absence or presence of RPR103611 (10 μM), fixed, and stained with X-Gal 20 h later. Infections of HeLa-P4 cells with HIV-1 stocks pretreated with sCD4 were performed in 96-well flat-bottom microtiter plates. LAI and NDK stocks were preincubated with concentrations of sCD4 ranging from 1 to 1,000 μg/ml, or were mock-treated, for 1 h at 37°C and then added to cells (5 × 102 i.u. per well). After overnight incubation, cells were lysed in 0.5% Nonidet P-40 (50 μl per well) and β-galactosidase activity was quantified using the chromogenic substrate chlorophenol red-β-d-galactopyranoside (CPRG) by measuring absorbance at 575 nm as described previously (8). To study the effect of pH on HIV-1 infectivity, HeLa-P5 cells seeded in 48-well plates were left in contact with ADA or LAI (∼103 i.u. per well) for 2 h at 4°C, in order to allow virus adsorption but not fusion. Unbound virus was removed by two washes in phosphate-buffered saline (PBS), and plates were shifted to 37°C. After 30 min, the supernatant was replaced by culture medium (DMEM–10% FCS) buffered at pH 6, 6.5, 7, or 7.5 with acetic acid and containing or not containing RPR103611 (10 μM). After 90 min at 37°C, cells were washed and cultured in standard medium (pH 7.4) with or without RPR103611 (10 μM) for 20 h before being stained with X-Gal.
Assay for gp120.
Concentrations of gp120 (and gp160) in cells and supernatants were measured by a previously described sandwich enzyme-linked immunosorbent assay (ELISA) technique (22). Briefly, HeLa cells were transfected in 100-mm-diameter petri dishes with Env expression vectors, and supernatants were replaced 24 h posttransfection by fresh medium (4 ml). One day later, cell-free supernatants were harvested and treated with 0.5% Empigen BB (Calbiochem), while cells were lysed in 0.5 ml of PBS–0.5% Empigen BB. Quadruplicate samples (100 μl) of supernatants and cell lysates, diluted in DMEM containing (10% FCS and 0.5% Empigen BB, were added to microtiter plates (Immulen 2; Dynex Technologies, Guyancourt, France) coated with affinity-purified sheep anti-HIV-1 gp120 antibody D7324 (0.5 μg per well). After overnight incubation at 4°C and five washes in PBS–0.1% Tween 20, 100 μl per well of a 1/500 dilution of pooled sera from HIV-1-infected individuals in PBS–4% nonfat milk–0.1% Tween 20 was added. After 1 h at room temperature, plates were washed five times in PBS–0.1% Tween 20, and 100 μl per well of a 1/7,500 dilution of peroxidase-conjugated goat anti-human immunoglobulin G (Calbiochem) in PBS–4% nonfat milk–0.5% Tween 20 was added. After 1 h at room temperature and five washes in PBS–0.5% Tween 20, 100 μl of a 0.4-mg/ml solution of 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS; Sigma, St. Louis, Mo.) in 0.6% acetic acid–0.03% H2O2 was added. Absorbance at 405 nm (A405) was read 30 min later. Concentrations of gp120 in samples were deduced by comparison with the A405 in a series of samples containing known amounts of recombinant gp120 (ranging from 0.2 to 50 ng/ml) processed in parallel. The threshold of detection of gp120 was 3 ng/ml (0.3 ng per well). The gp120-gp41 association index for a given Env was calculated, as described by Helseth et al. (13), as the ratio of the total amount of gp120 (plus gp160) in cell lysate to the total amount of gp120 in supernatant.
RESULTS
Activity of RPR103611 on virus entry via CXCR4 or CCR5.
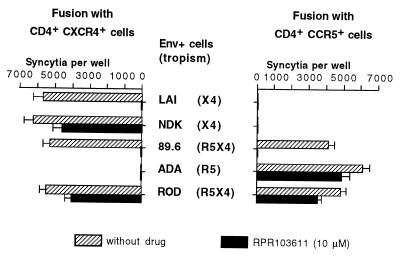
In an initial study performed before the discovery of the role of chemokine receptors in HIV entry and cell tropism, two primary HIV-1 isolates of European origin and three cell line-adapted HIV-1 strains from clade B (LAI, MN, and RF) were neutralized by RPR103611 with 50% inhibitory concentrations ranging from 0.05 to 0.75 μM, while cell line-adapted HIV-1 of African origin (ELI and NDK, both from clade D) and HIV-2 strains (ROD and EHO) were fully resistant to concentrations exceeding 10 μM (21). To directly address the possible importance of the type of chemokine receptor used by HIV-1 or HIV-2 for their sensitivity to RPR103611, we have performed syncytium formation assays with cells expressing different types of Env from HIV-1 (LAI, NDK, ADA, 89.6) or from HIV-2 (ROD) and target cells (U373MG-CD4) expressing either CXCR4 or CCR5. As expected, LAI and NDK Env only induced fusion with CXCR4+ cells and ADA Env only induced fusion with CCR5+ cells. The 89.6 and ROD Env apparently used both types of receptors with similar efficacies (Fig. 1). Fusion mediated by the LAI Env was fully blocked by 10 μM RPR103611, while fusion mediated by the NDK Env was only reduced by ∼20% (Fig. 1). Fusion mediated by the 89.6 Env with the CXCR4+ or the CCR5+ cells was completely blocked by RPR103611, while RPR103611 reduced the efficiency of fusion mediated by the ROD Env by ∼25% for both target cells. Therefore, the drug sensitivity or resistance of the 89.6 and ROD Env appeared to be independent of the chemokine receptors CXCR4 and CCR5. The drug resistance of cell fusion mediated by Env from ADA was unexpected, since this strain belongs to clade B (like LAI and 89.6). Resistance to RPR103611 was also observed for fusion mediated by Env from other clade B R5 strains (BaL, Jr-CSF, and YU-2; data not shown).
FIG. 1.
Effect of RPR103611 on syncytium formation between HIV-1 or HIV-2 Env+ cells and CXCR4+ or CCR5+ target cells. HeLa cells transfected with Env expression vectors corresponding to the HIV-1 strains LAI, NDK, 89.6, ADA, and ROD were cocultured overnight with an equivalent number (∼2 × 105 cells per well) of U373MG-CD4 cells stably expressing CXCR4 or CCR5, in the presence or absence of RPR103611 (10 μM). Fusion with Env+ cells (also expressing the HIV-1 or HIV-2 transactivator Tat) activates the LTR-lacZ transgenes of target cells, allowing detection of syncytia by their blue staining in the presence of the X-Gal substrate. Bars, mean numbers of blue foci in three independent transfections with standard error.
Role of gp41 in resistance to RPR103611.
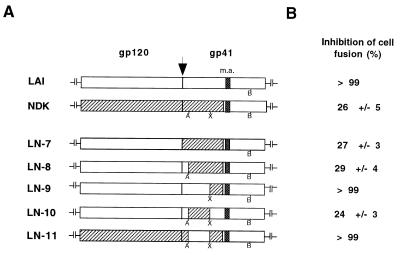
By testing the effect of RPR103611 on cell-cell fusion and HIV-1 infection mediated by a chimeric LAI-NDK Env, we had previously found that the NDK gp41 was associated with drug resistance and that the LAI gp41 was associated with drug sensitivity (19). Here we have refined this approach by using chimeric LAI-NDK Env in which fragments of the gp41 ectodomain were exchanged (Fig. 2A). All chimeric Env tested (LN-7 to LN-11) mediated fusion with HeLa-P4 cells with efficiencies similar to that of the parental Env. As previously observed, the efficiency of fusion mediated by the LN-7 and LN-8 Env was only reduced by ∼30% in the presence of 10 μM RPR103611 (Fig. 2B), indicating that the first 38 residues of NDK gp41 were dispensible for the drug-resistant phenotype. Introducing residues 109 to 163 of the NDK gp41 ectodomain into LAI Env (LN-9) did not modify its sensitivity to RPR103611. In contrast, residues 39 to 108 of the NDK gp41 were apparently sufficient to confer drug resistance in the LAI Env context (LN-10). When this fragment of NDK gp41 was replaced by the corresponding LAI gp41 fragment (LN-11 chimera), cell fusion was totally blocked by RPR103611. Exchanging a 70-amino-acid gp41 fragment was therefore sufficient to revert the phenotype of LAI or NDK Env with regard to inhibition by RPR103611. This fragment contains the proximal helix domain and most of the loop region of gp41, including the dicysteine motif (Fig. 3).
FIG. 2.
Inhibition of cell-cell fusion mediated by chimeric LAI-NDK Env. (A) Schematic representation of wild-type and chimeric Env. A, B, and X, ApaLI, BamHI, and XbaI sites, respectively, used to exchange domains of gp41 (see Materials and Methods for details); m.a., membrane anchor domain of gp41. Hatched bars, NDK sequences; arrow, cleavage site. (B) Extent of inhibition of fusion between HeLa cells expressing the indicated Env and HeLa-P4 cells (CD4+, CXCR4+) by RPR103611 (10 μM). The experiment was performed as described for Fig. 1. For all types of Envs, 5,000 to 10,000 syncytia per well were scored in the absence of the drug. Numbers represent the means of three independent wells.
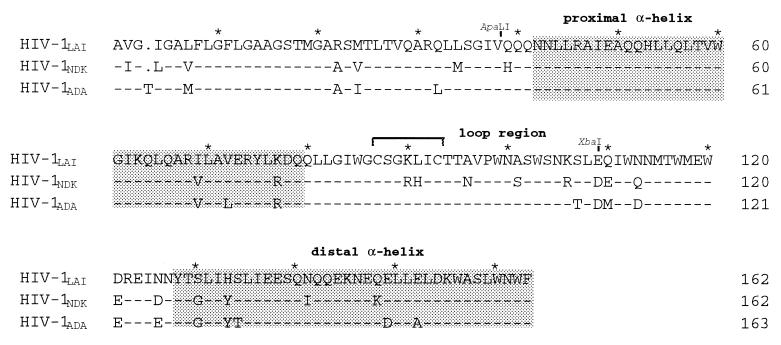
FIG. 3.
Amino acid sequence of the gp41 extracellular domain of the HIV-1 strains LAI, NDK, and ADA. The proximal and distal α-helical domains are shaded. Also shown are the dicysteine motif of the loop region and the positions of the ApaLI and XbaI sites used to construct LN chimeras.
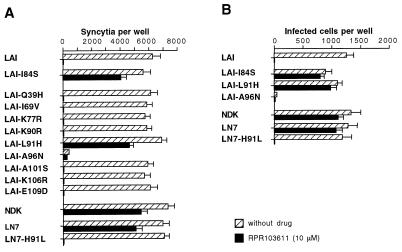
In this region of gp41, LAI and NDK Env differ at nine positions (Fig. 3). Each of these nine residues of LAI gp41 was replaced by its NDK counterpart, and the resulting mutant LAI Env were tested for their ability to mediate fusion with HeLa-P4 target cells in the presence or absence of RPR103611 (10 μM). All mutations, except A96N, were apparently compatible with the processing and function of Env, since the number of syncytia was similar to that obtained with wild-type LAI Env (Fig. 4A). As will be seen later, the A96N mutation seems to affect the stability of the gp120-gp41 complex. The low level of fusion mediated by the A96N mutant was apparently resistant to RPR103611. Among the fully functional Env LAI mutants, only the leucine 91-to-histidine (L91H) substitution was associated with drug resistance. Fusion mediated by other mutants was completely blocked in the presence of the drug. This experiment included the previously characterized drug escape LAI mutant carrying the isoleucine 84-to-serine (I84S) mutation.
FIG. 4.
Inhibition of cell-cell fusion and infection mediated by wild-type and mutant HIV-1 Env. Assays of syncytium formation (A) between HeLa cells transfected with Env expression vectors and HeLa-P4 cells were performed as described for Fig. 1. Infections of HeLa-P4 cells (B) in the presence or absence of RPR103611 (10 μM) were performed in six-well trays using wild-type or mutant LAI or NDK. Cells were stained with X-Gal 20 h after infection. Viral stocks were supernatants of transiently transfected HeLa cells. The inocula were adjusted to yield ∼1,000 blue foci per well in the absence of the drug, except for the A96N LAI mutant, for which undiluted stock was used. Bars, means of three independent wells with standard error.
The effects of the I84S, L91H, and A96N mutations on HIV-1 infection were tested by subcloning the corresponding mutant env genes into a LAI provirus. The A96N mutation abolished HIV-1 infectivity, and the effect of RPR103611 could not therefore be tested. Infection mediated by virus carrying the I84S and L91H mutations was only slightly reduced in the presence of 10 μM RPR103611 (Fig. 4B). Parallel results were therefore obtained in infection and cell fusion assays.
Since the L91H mutation was sufficient to revert the phenotype of LAI Env, we have tested the effect of the reciprocal mutation (H91L) in the context of NDK gp41. Cell fusion and HIV-1 infection mediated by the LN-7 chimeric Env (NDK gp41 ectodomain) bearing this H91L mutation were totally blocked by 10 μM RPR103611 (Fig. 4). These experiments showed that the drug resistance of NDK could be explained by a single amino acid difference with respect to LAI in gp41. The L91H mutation and the previously characterized drug escape mutation I84S both replace a hydrophobic residue of the loop region of gp41 by a polar residue. The L91 and I84 residues are conserved among clade B HIV-1 strains (29) but not among clade D HIV-1 strains or among HIV-2 strains, which may explain their drug resistance.
Drug resistance of R5 strains.
In the region of gp41 that we found important for sensitivity or resistance to RPR103611 (residues 38 to 109), the HIV-1 strain ADA differs from LAI by five conservative substitutions (Fig. 3). Three of these ADA residues (V69, R77, and D109) are also found in the NDK sequence, with no apparent role in the drug resistance phenotype. We have addressed the possible role of the two other ADA residues (L72 and T107) by site-directed mutagenesis in the LAI gp41 context. The V72L and S107T mutations did not affect the efficiency and drug sensitivity of fusion with HeLa-P4 cells (data not shown).
To define whether the gp120 or gp41 of ADA was responsible for the drug-resistant phenotype, each one was replaced by the corresponding Env subunit of LAI, thus yielding the LA-1 and LA-2 chimeric Env (Fig. 5A). As expected, fusion mediated by LA-1 (ADA gp120) was CCR5 dependent, while fusion mediated by LA-2 (LAI gp120) was CXCR4 dependent (Fig. 5B). Fusion mediated by LA-1 was less efficient than fusion mediated by the parental Env (about 50%), although the number of syncytia remained high, indicating relatively efficient processing and expression of this chimeric Env. Fusion mediated by LA-1 and LA-2 was fully blocked in the presence of RPR103611, which confirmed that the minor sequence differences in gp41 did not determine the phenotypic differences between LAI and ADA. This ruled out a direct role for gp120 in the drug resistance of ADA. Since the gp120 and gp41 of ADA only expressed a drug-resistant phenotype when they were associated, we were led to envisage a potential role for the stability of the gp120-gp41 complex of R5 strains in their resistance to RPR103611.
Role of gp120-gp41 stability.
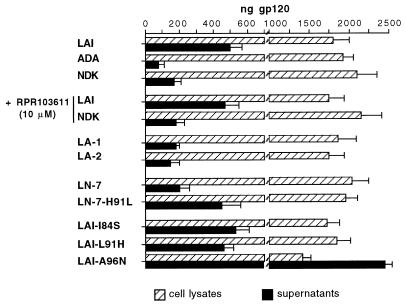
The stability of the gp120-gp41 association can be indirectly assessed by measuring the amount of gp120 shed in the supernatant of Env+ cells (13). When HeLa cells were transfected with different Env expression vectors, similar amounts of cell-associated gp120 (and gp160) were measured by quantitative ELISA (Fig. 6). As expected, measurements made in parallel in cell-free supernatants revealed a markedly higher level of gp120 in cells transfected with LAI Env than in cells transfected with ADA Env (Fig. 6). Assuming an association index (cell-associated/cell-free gp120) of 1 for LAI Env, we obtained a value of 6.7 for ADA Env (Table 1). The LA-1 and LA-2 chimeras formed apparently weaker gp120-gp41 complexes, with relative association indexes of 2.8 and 3.5, respectively (Table 1 and Fig. 6).
FIG. 6.
Shedding of gp120 in supernatants of cells expressing HIV-1 Env. About 106 HeLa cells were transfected in a 100-mm-diameter plate with the indicated Env expression vector. The supernatant was replaced 24 h later by 4 ml of culture medium containing or not containing RPR103611 (10 μM). After another 24 h, supernatant and adherent cells were independently harvested, and the concentrations of gp120 were determined by ELISA (see Materials and Methods) by comparison with samples containing known amounts of recombinant gp120 processed in parallel. Bars, total amounts of gp120 in the supernatant and in the cells for a given transfected plate (means of at least four independent transfections).
TABLE 1.
Effect of RPR103611 on fusion and stability of different HIV-1 Env
| Env | RPR103611 phenotypea | gp120-gp41 association indexb |
|---|---|---|
| Wild types | ||
| LAI | Sensitive | 1.0 |
| ADA | Resistant | 6.7 |
| NDK | Resistant | 3.5 |
| LAI-ADA chimeras | ||
| LA-1 | Sensitive | 2.8 |
| LA-2 | Sensitive | 3.3 |
| LAI mutants | ||
| I84S | Resistant | 0.9 |
| L91H | Resistant | 1.1 |
| LAI-NDK chimeras | ||
| LN-7 | Resistant | 2.8 |
| LN-7–H91L | Sensitive | 1.2 |
Effect of 10 μM RPR103611 on fusion between HeLa cells expressing Env and CXCR4+ cells (or CCR5+ cells for ADA and LA-1).
Ratio of the total amount of gp120 (plus gp160) in cell lysates to the total amount of gp120 in supernatants of HeLa cells expressing Env (data from Fig. 6).
The spontaneous gp120 shedding was lower in cells expressing Env from NDK than in cells expressing LAI Env (association index of 3.5; Table 1), in agreement with a previous report (35). This indicated a stabler gp120-gp41 association for NDK Env. The amounts of shed gp120 for wild-type NDK Env and the LN-7 chimeric Env (LAI gp120-NDK gp41) were similar, suggesting that gp41 rather than gp120 determined the relative stabilities of the LAI and NDK Env complexes. In the LN-7 Env context, the H91L mutation markedly reduced the stability of the gp120-gp41 complex while point mutations I84S and L91H did not seem to modify the stability of the LAI gp120-gp41 complex (Fig. 6, Table 1). The A96N mutation was associated with a very large amount of gp120 shedding (Fig. 6). Instability of the Env complex probably explained the lack of activity of this mutant. Finally, incubating cells with 10 μM RPR103611 did not detectably increase shedding of the NDK or LAI gp120 (Fig. 6), which represented another argument against the activity of this compound on gp120.
Drug sensitivity of ADA at low pH.
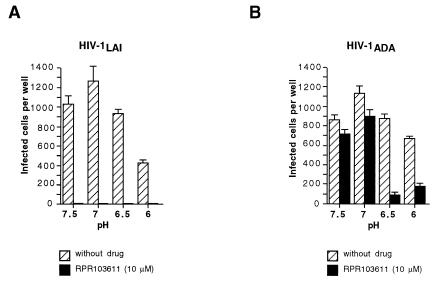
For R5 strains, it is not usually possible to induce dissociation of the gp120-gp41 complex and gp120 shedding upon incubation of virions and gp120 ligands such as sCD4 (27, 30) incubation in acidic pH conditions was previously shown to enhance the effect of sCD4 on X4 HIV-1 strains (10). We have therefore sought to use low-pH conditions as a means to reduce the stability of the gp120-gp41 association and to possibly facilitate the effect of RPR103611. Since this treatment was likely to be detrimental to the initial steps of HIV-1 infection (binding), and possibly to early postbinding steps if they require a tight gp120-gp41 association, it was omitted during the binding step performed at 4°C and during the first 30 min following the shift of temperature to 37°C. At this time, the culture medium was replaced by medium at pH 6, 6.5, 7, or 7.5, containing or not containing RPR103611 (10 μM) and incubation was pursued for 90 min at 37°C. Cells were then grown in standard culture medium, with or without the drug, and then fixed and stained to reveal HIV-1 infection. Usage of HeLa-P5 cells, which express both CCR5 and CXCR4, allowed a comparison of the effects of RPR103611 on LAI and ADA in the same target cells.
For LAI, incubation at pH 6 resulted in a twofold reduction of the infectious titer relative to incubations at pH 6.5 or higher. At all pH conditions tested, infection was totally blocked by RPR103611 (Fig. 7A), showing that the drug was fully active in this pH range and could block infection when added after adsorption, as we previously observed (19). A different situation for infections with ADA was observed. There was a very limited inhibitor effect at neutral pH, like that in the previous cell fusion experiments, while infection was reduced by approximately 90 and 70% at pHs 6.5 and 6, respectively (Fig. 7B). In acidic conditions, we could therefore achieve partial neutralization of an otherwise fully resistant HIV-1 strain.
FIG. 7.
Effect of pH on neutralization of LAI (A) and ADA (B) by RPR103611. HeLa-P5 cells (CXCR4+, CCR5+) in 48-well plates were left in contact with virus for 2 h at 4°C to allow binding but not the subsequent steps of virus entry. After unbound virus was washed off, plates were shifted to 37°C to initiate fusion (30 min), and then supernatant was replaced by culture medium buffered at the indicated pH (7.5 to 6.0) and containing or not containing RPR103611 (10 μM). After another 90 min, supernatant was replaced by standard cell culture medium containing or not containing RPR103611, and cells were stained with X-Gal 20 h later. Bars, means of three independent wells.
Relative drug sensitivity of NDK in the presence of sCD4.
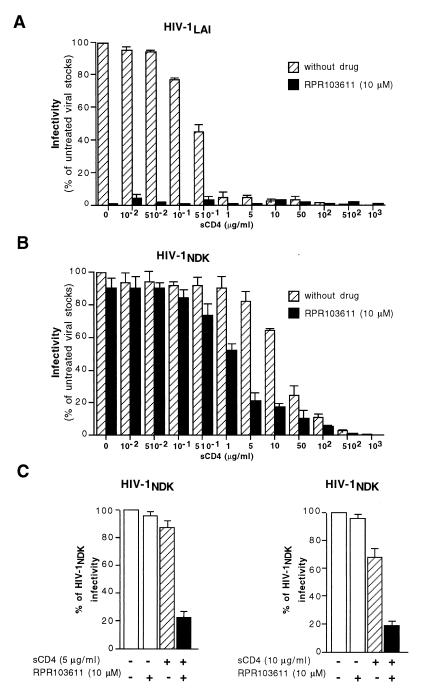
The acidic treatment could also confer sensitivity to RPR103611 on NDK (data not shown). To address the possible influence of the stability of the gp120-gp41 complex, we took advantage of the ability of sCD4 to induce conformational changes in the gp120-gp41 complex of X4 HIV-1 strains. Incubation of LAI with 1-μg/ml or higher sCD4 concentrations abolished infectivity (Fig. 8A), while NDK was more resistant to the antiviral effect of sCD4 (Fig. 8B), in agreement with a previous report (35). When infections were performed in the presence of 10 μM RPR103611, we observed a reduced infectivity when NDK had been in contact with sCD4 concentrations higher than 1 μg/ml (Fig. 8B). As expected, RPR103611 had no significant effect on untreated virus. Therefore, contact with sCD4 concentrations apparently too low to reduce NDK infectivity (1 and 5 μg/ml) or only reducing it by 10% (10 μg/ml) could result in a partial sensitivity to RPR103611 (Fig. 8B and C). As previously observed with acidic treatment of ADA, we were able to achieve partial neutralization of a drug-resistant strain by applying a treatment expected to dissociate the gp120-gp41 complex. However, in both cases, we did not observe complete neutralization, as is achieved for drug-sensitive HIV-1 strains.
FIG. 8.
Effect of pretreatment of LAI and NDK with sCD4 on neutralization by RPR103611. HeLa-P4 cells were infected in 96-well trays (∼1.25 × 104 per well) with LAI (A) or NDK (B and C) stocks preincubated for 60 min at 37°C with the indicated concentrations of sCD4. RPR103611 (10 μM) was present during virus-cell contact and subsequent steps when indicated but not during the pretreatment. Cells were washed and lysed in situ 20 h postinfection, and β-galactosidase activity was measured with the chromogenic CRPG substrate by determining the A575 (A). Bars, percentages of infectivity of sCD4-treated viral stocks relative to untreated stocks. In panel C, the effect of RPR103611 on untreated viral stocks is also shown. Results are the means of three independent experiments.
DISCUSSION
The betulinic acid derivative RPR103611 is a potent inhibitor of HIV-1 entry and fusion, but its activity is clearly strain dependent. Antiviral activity of RPR103611 against LAI and other cell line-adapted HIV-1 strains belonging to clade B was initially reported, while HIV-2 strains (ROD, EHO) and two clade D HIV-1 strains (ELI and NDK) were fully resistant (19, 21). By exchanging env gene domains between LAI and NDK, we found that the ectodomain of gp41 was apparently determining their sensitivity or resistance to RPR103611 (19). Here we have refined this approach and found that a discrete domain of 70 residues from the NDK gp41 ectodomain was sufficient to confer drug resistance in the LAI Env context. Reciprocally, the same fragment from LAI gp41 conferred sensitivity in an NDK Env context. This fragment contains part of the proximal helix and the loop region located between the proximal and distal helices of gp41. We had previously mapped a drug escape mutation in LAI in the same region of gp41 at isoleucine 84 (19). Here we found that a single amino acid difference at position 91, a leucine for LAI and a histidine for NDK, was sufficient to account for their distinct phenotypes. Indeed, fusion mediated by the LAI Env mutant carrying the L91H mutation was resistant to RPR103611, while the H91L mutation caused reversion of the phenotype of a chimeric construct bearing the ectodomain of NDK gp41. Other amino acid differences between LAI and NDK had no apparent role, with the possible exception of the replacement of alanine 96 of LAI by asparagine in NDK. The effect of the A96N mutation was difficult to appreciate since it markedly reduced fusion efficiency and abolished HIV-1 infectivity. Since the H91L mutation was sufficient to revert the NDK phenotype, it seems that the N96 residue does not have a major role.
The L91 residue is part of the dicysteine motif, which is a hallmark of the transmembrane proteins of retroviruses (11). Its sequence is highly conserved among HIV-1 strains, with a leucine at position 91 except in clade D strains, which have a histidine (29). This may explain the drug resistance of the clade D strains ELI (21) and MAL (data not shown). The sequence of the gp41 dicysteine motif is different in HIV-2; in particular, there is a conserved glutamine in place of the LAI L91 residue (29). Also, in the gp41 of ROD and most HIV-2 strains, the residue corresponding to the I84 of LAI is a serine. Both features could contribute to the drug resistance of the HIV-2 strains tested (19, 21).
The I84S and L91H mutations conferring drug resistance to HIV-1 represent replacements of nonpolar by polar amino acids. These residues are not part of the available HIV-1 gp41 crystal structure (4, 41, 42) but are expected to be in close proximity, according to the simian immunodeficiency virus gp41 structure (3). It is therefore possible that both I84 and L91 contribute to the formation of a pocket binding RPR103611 through hydrophobic interactions. Analysis of the effect of other mutations at these positions would be necessary to validate this model.
We sought to obtain evidence for the interaction of the loop region of gp41 with RPR103611 by testing its ability to compete with the binding of the anti-gp41 monoclonal antibodies (MAbs) 50-69 and 3D6 (47). Their epitopes correspond to a region of gp41 masked in the gp120-gp41 complex and become exposed upon treatment with sCD4 (36). Flow cytometry experiments were performed with LAI-infected cells expressing relatively high levels of Env, judging by staining with anti-gp120 antibodies. Preincubating cells with a fully active antiviral RPR103611 concentration (10 μM) did not significantly decrease reactivity with the 50-69 and 3D6 MAbs when either fractions of stained cells or mean fluorescence intensities were considered (data not shown). However, these results did not rule out the possibility of a direct interaction of RPR103611 with the loop region of gp41 if, for example, the binding sites of the drug and antibodies tested are distinct or the affinity of gp41 for RPR103611 is lower. It is even conceivable that RPR103611 only interacts with a transient conformational state of gp41.
The fact that the clade B HIV-1 strain ADA was fully resistant to RPR103611, despite being almost identical to LAI in the gp41 loop region and in particular at the I84 and L91 residues, led us to envisage another possible mechanism for HIV-1 drug resistance. Like other macrophage-tropic HIV-1 strains, ADA is strictly dependent on the CCR5 receptor pathway. Usage of this receptor did not seem to be the direct cause of drug resistance, since infection by the R5X4 HIV-1 strain 89.6 was blocked by RPR103611 in target cells expressing CCR5 but not CXCR4. Fusion mediated by chimeric Env consisting of gp120 from ADA and gp41 from LAI or the reciprocal construction was also fully blocked by RPR103611. The drug resistance phenotype of ADA therefore seemed to result from the combination of determinants in the two envelope subunits. A feature of R5 strains that could account for these observations is the higher stability of the gp120-gp41 complex relative to that of X4 strains (27, 30). This might limit the access of compounds to regions of gp41 that are masked by gp120 in the native complex (37). For R5 strains, these regions of gp41 may not become accessible, or may become so too late for compounds to exert their antiviral activity. This issue was addressed by performing part of the cell entry process in mildly acidic conditions (pH 6 or 6.5), a treatment expected to favor dissociation of gp120 and gp41. By this method, we observed a marked reduction (up to 90%) in the infectious titer of ADA in the presence of RPR103611, while LAI infection was totally blocked. It is noteworthy that RPR103611 was added to cells 30 min after cells were shifted from 4 to 37°C in order to initiate virus-cell fusion. This confirmed that the antiviral activity of RPR103611 is exerted at a postbinding step of the HIV-1 entry process. The drug resistance of ADA and other R5 strains was not therefore due to the lack of a target sequence but rather was probably due to constraints on its accessibility. Besides differences in the stability of the gp120-gp41 complex, there could be differences between R5 and X4 strains in the nature or the kinetics of the conformational changes eventually allowing gp41 to mediate fusion (16).
Contrary to LAI, the gp120-gp41 complex of NDK is also relatively stable (35), which could have contributed to its resistance to RPR103611. Indeed, when NDK was preincubated with sCD4 concentrations too low to reduce infectivity, we observed partial neutralization by RPR103611. Like the low-pH conditions for ADA, a treatment expected to favor dissociation of the gp120-gp41 complex conferred RPR103611 sensitivity on an otherwise fully resistant HIV-1 strain.
The stability of the gp120-gp41 complex can be indirectly quantified by measuring gp120 shed in the supernatant of Env+ cells. This amount is markedly higher for LAI than for ADA, resulting in relative gp120-gp41 association indexes of 1 and 6.7, respectively. The index was markedly reduced for the two LAI-ADA gp120-gp41 chimeras (2.8 and 3.3), consistent with their drug sensitivity. It was on the same order of magnitude (3.5) for NDK, suggesting that parameters other than Env stability contributed to the phenotype. While the H91L mutation in NDK gp41 reduced the stability of Env, the I84S and L91H mutations conferring drug resistance to LAI did not modify the association index. The observation that the drug resistance of ADA and NDK could be counteracted by treatments expected to improve gp41 access does not necessarily exclude a direct role for the gp41 sequence (in particular the presence of hydrophobic residues I84 and L91) in neutralization by RPR103611. Furthermore, low pH (for ADA) and sCD4 (for NDK) treatments only permitted partial neutralization by 10 μM RPR103611, while the 50% inhibitory concentration was 2 orders of magnitude lower for the fully sensitive HIV-1 strains (21).
The lack of activity of RPR103611 on R5 HIV-1 strains certainly limits its therapeutic value, although this class of compounds could be of interest at late stages of infection. They may nevertheless represent valuable tools to address the mechanism by which the transmembrane protein mediates fusion of the virus envelope with the cell membrane and possibly contributes to subsequent steps. Although these postfusion steps are not as well known, it appears that virus entry requires the formation of a fusion pore and its dilatation to a diameter allowing passage of the viral capsid (23). It will be of interest to compare the activity of RPR103611 with that of antiviral peptides derived from the gp41 helix domains. Our preliminary studies suggested similar kinetics for the antiviral activity of these compounds (data not shown). Their antiviral effects were additive, suggesting that the targets were not identical. This is consistent with our finding that the target of gp41 was the loop region, while peptides are expected to act on the helical regions. For peptides of the gp41 helices, high concentrations were required to block lipid exchange between fusion effector and target cells, while markedly lower concentrations blocked both the formation of fusion pores and HIV-1 entry (28). This led to the proposal that the antiviral activity of these peptides at low concentrations was not due to the disruption of the gp41 coiled-coil structure or to competition with its formation but rather to the blocking of a step following lipid mixing. This step should therefore be posterior to the interaction of the fusion peptide with the target membrane and the close apposition with the virus envelope. Peptides could, for example, prevent the clustering of gp41 trimers to form a proteinaceous pore or, conversely, could prevent their dissociation to allow dilatation of the pore. Testing the effect of RPR103611 on the exchange of lipids between cells should provide further information and should contribute to the understanding of the mechanism by which gp41 mediates HIV-1 entry.
ACKNOWLEDGMENTS
We thank J. Richardson and N. Heveker (ICGM) for comments on the manuscript, Y. Hénin (Rhône-Poulenc Rorer) for the gift of RPR103611, N. Sol (Hôpital Saint-Louis) for the gift of anti-HIV sera, and T. Teste-Lasserre and F. Letourneur (ICGM) for help with gp120 ELISA and DNA sequencing.
This work was supported by the Agence Nationale de Recherches sur le SIDA.
REFERENCES
- 1.Baggiolini M. Blocking chemokine receptors. J Exp Med. 1997;186:1189–1191. doi: 10.1084/jem.186.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Caffrey M, Cai M, Kaufman J, Stahl S J, Wingfield P T, Covell D G, Gronenborn A M, Clore G M. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 5.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 6.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collman R, Balliet J, Gregory S, Friedman H, Kolson D, Nathanson N, Srinivasan A. An infectious clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dragic T, Hazan U, Alizon M. Detection of cell fusion mediated by the envelopes of human retroviruses by transactivation of a reporter gene. Methods Mol Genet. 1995;7:218–236. [Google Scholar]
- 9.Freed E O, Martin M A. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J Biol Chem. 1995;270:23883–23886. doi: 10.1074/jbc.270.41.23883. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y K, Hart T K, Jonak Z L, Bugelski P J. Physicochemical dissociation of CD4-mediated syncytium formation and shedding of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:3818–3825. doi: 10.1128/jvi.67.7.3818-3825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallaher W R, Ball J M, Garry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 12.Harrington R D, Geballe A P. Cofactor requirements for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991;65:2119–2125. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman T L, Doms R W. Chemokines and coreceptors in HIV/SIV-host interactions. AIDS. 1998;12(Suppl. A):S17–S26. [PubMed] [Google Scholar]
- 15.Hughson F M. Enveloped viruses: a common mode of membrane fusion? Curr Biol. 1997;7:R565–R569. doi: 10.1016/s0960-9822(06)00283-1. [DOI] [PubMed] [Google Scholar]
- 16.Jones P L S J, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 17.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldrege L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 18.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, De Clercq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labrosse B, Pleskoff O, Sol N, Jones C, Hénin Y, Alizon M. Resistance to a drug blocking human immunodeficiency virus type 1 entry (RPR103611) is conferred by mutations in gp41. J Virol. 1997;71:8230–8236. doi: 10.1128/jvi.71.11.8230-8236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littman D R. Chemokine receptors: keys to AIDS pathogenesis. Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 21.Mayaux J-F, Bousseau A, Pauwels R, Huet T, Hénin Y, Dereu N, Evers M, Soler F, Poujade C, De Clercq E, Le Pecq J-B. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc Natl Acad Sci USA. 1994;91:3564–3568. doi: 10.1073/pnas.91.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merat R, Raoul H, Leste-Lasserre T, Sonigo P, Pancino G. Variable constraints on the principal immunodominant domain of the transmembrane glycoprotein of human immunodeficiency virus type 1. J Virol. 1999;73:5698–5706. doi: 10.1128/jvi.73.7.5698-5706.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monck J R, Fernandez J M. The fusion pore and mechanisms of biological membrane fusion. Curr Opin Cell Biol. 1996;8:524–533. doi: 10.1016/s0955-0674(96)80031-7. [DOI] [PubMed] [Google Scholar]
- 24.Moore J, McKeating J, Weiss R, Sattentau Q. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 25.Moore J P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to sCD4 by ELISA: HIV-2 has a 25-fold lower binding affinity than HIV-1 for sCD4. AIDS. 1990;3:297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Moore J P, Jameson B A, Weiss R A, Sattentau Q J. The HIV-cell fusion reaction. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press Inc.; 1993. pp. 233–289. [Google Scholar]
- 27.Moore J P, McKeating J A, Huang Y, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilatation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers G, Korber B, Wain-Hobson S, Jeang K-T, Henderson L E, Pavlakis G N, editors. Human retroviruses and AIDS. A compilation of nucleic and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1994. [Google Scholar]
- 30.Orloff S L, Kennedy M S, Belperron A A, Maddon P J, McDougal J S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993;67:1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 32.Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 33.Poignard P, Fouts T, Naniche D, Moore J P, Sattentau Q J. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J Exp Med. 1996;183:473–484. doi: 10.1084/jem.183.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan-Graham M A, Peden K. Both virus and host components are important for the manifestation of a Nef− phenotype in HIV-1 and HIV-2. Virology. 1995;213:158–168. doi: 10.1006/viro.1995.1556. [DOI] [PubMed] [Google Scholar]
- 35.Sattentau Q, Moore J, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattentau Q J, Zolla-Pazner S, Poignard P. Epitope exposure on functional oligomeric HIV-1 gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 38.Skehel J J, Wiley D C. Coiled coils in both extracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- 39.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spire B, Sire J, Zachar V, Rey F, Barré-Sinoussi F, Galibert F, Hampe A, Chermann J-C. Nucleotide sequence of HIV1-NDK, a highly cytopathic strain of the human immunodeficiency virus, HIV1. Gene. 1989;81:275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 41.Tan K, Liu J H, Wang J H, Shens S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 43.Westervelt P, Gendelman H E, Ratner L. Identification of a determinant within the human immunodeficiency virus type 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 47.Xu J Y, Gorny M K, Palker T, Karwowska S, Zolla-Pazner S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J Virol. 1991;65:4832–4838. doi: 10.1128/jvi.65.9.4832-4838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]