Abstract
Introduction and importance
Bladder metastatic melanoma is a very uncommon condition.
Case presentation
On 62 reported cases, 55 studies have been done so far. We describe a 53-year-old woman with a hematuria who underwent transurethral resection of bladder lesions caused by metastatic melanoma for eight years ago after receiving her initial diagnosis of cutaneous malignant melanoma.
Clinical discussion
We also review the medical literature to determine the prognosis of bladder metastatic melanoma. Synchronous metastases with metastatic melanoma to the bladder also reduces the mean survival compared with patients with metachronous metastases.
Conclusion
Bladder metastatic melanoma combined with other factors, such as male, lymph node metastases, primary skin tumor, two or more bladder metastatic foci, and synchronous metastases are predictors of worse prognosis.
Keywords: Melanoma, Bladder metastatic melanoma, Synchronous or Metachronous metastases
Highlights
-
•
A 53 - year - old woman with hematuria who was found to have metastatic melanoma to the bladder, eight years after initial diagnosis of malignant melanoma of the skin.
-
•
The 62 cases of metastatic melanoma to the bladder have been described in the English literature to date.
-
•
This study demonstrated that bladder metastatic melanoma combined with other factors, such as male sex, lymph node metastasis, primary skin tumor, two or more than 2 bladder metastases, and synchronous metastases are predictors of worse prognosis.
1. Introduction
Melanoma is an aggressive cancer that typically metastasizes to the lungs, brain, liver, bones, and gut. It has a bad prognosis. <30 cases of metastatic melanoma to the bladder have been reported in the English literature to date; 5 of these cases were recognized, and 62 reported cases were combined to create the literature study [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. The presented case is the 63rd. The hematuria or symptoms of the lower urinary tract are the primary complaints when it happens [1,2,[5], [6], [7], [8], [9], [10], [11], [12]]. However, the metastases to the bladder were found in 18.0–37.0 % of the individuals with extraregional illness in the autopsy collection. Additionally, synchronous or metachronous bladder metastases of malignant melanoma has not been yet researched. We report the case of a 53-year-old woman with a hematuria who has presented for transurethral resection and was found to have metastatic melanoma to the bladder, for the previous eight years after initial diagnosis of cutaneous malignant melanoma. Here, we also present a literature review on the outcome of metastatic melanoma to the bladder.
2. Case presentation, material and methods
2.1. Case presentation
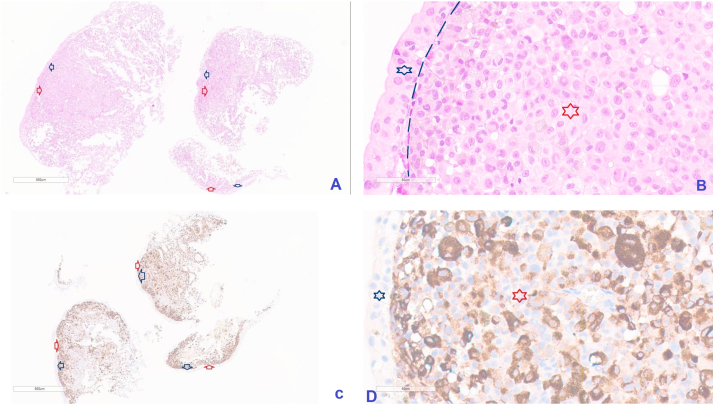
This case is an 53-year-old female patient with a history for malignant melanoma in the abdominal wall skin for previous 8 years, in 2015. Four months later, she was diagnosed with a metastatic melanoma of the left axillary nodes. The patient underwent axillary lymph node resection. Subsequently, the patient was treated with the 17 cycles by Pembrolizumab. The patient achieved a complete clinical response after the end of treatment. By March 2020 (5 year-period), the patient was diagnosed with metastatic melanoma in the inguinal lymph nodes without BRAF V600E mutation. The patient was treated with the additional 17 cycles of Pembrolizumab. Subsequently, the patient's disease stabilized. In April 2022, the patient was metastasized to the supraclavicular nodes by the confirmation of histopathological diagnosis. Chest computed tomography revealed many hilar and mediastinal lymph nodes with the largest size of 31 × 24 mm. Simultaeously, the bladder wall displayed abnormal nodules up to 14 × 10 mm in size. Metastatic lesions were not detected in other organs. Abnormal bladder nodules were biopsied by cystoscopy for histopathological diagnosis (Fig. 1A).
Fig. 1.
Illustrated pictures presented comprised entirely of malignant melanoma cells (Red star and Red arrows) underlying the begnin bladder mucosa (Blue star and Blue arrows) (Separated by basement membrane – the green discontinuous line) (A & B of HE stain, C & D of IHC stain with positive HMB-45, 40×). A and C images are the total specimens, manification of 3.5×. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The bladder wall was heavily engaged by an undifferentiated malignant tumor, according to a histopathological study. The increased vesicular and pleomorphic nuclei, as well as sporadic melanin pigments, were visible after hematoxylin and eosin staining, which underlie benign bladder mucosa (Fig. 1A & B). Immunohistochemical staining revealed that malignant cells were positive for HMB-45 (Fig. 1C & D). Based on the results of pathology and immunohistochemical staining, combined with the patient's history, the final diagnosis was metastatic malignant melanoma rather than primary malignant melanoma of the bladder. Adjuvant chemotherapy with darcabazine is recommended in this patient. However, owing to widespread and late-staged disease, the patient died in January 2023. The OS of the patient from the diagnosis of malignant melanoma that metastasized to the bladder, was 10 months, whereas the OS from the initial diagnosis was 86 months.
2.2. Search methods for identification of studies
Several Internet databases, including PubMed, Medline, Google Scholar, Google, EMBASE, Cochrane Library, ScienceDirect, and SpringerLink, were used to find reported cases of metastatic melanoma of the urinary bladder to document the presentation, investigation, management, and treatment outcome of the disease from inception to April 2023. The goal of this study was to assess the influence of bladder metastatic features on the outcome of patients with malignant melanoma. The search keywords used were “metastatic malignant melanoma AND bladder.” In addition, the authors checked all references in the articles. A total of 55 references were identified, and 62 reported cases were included in the literature review. This case belonged to 63rd. We wish to acknowledge fully the patient information reported in the literature review. However, out of the total of these reports, there were nine reports (nine cases) without clinical and information; 35 patients had survival information recorded from the time of detection of bladder metastatic melanoma, and the overall survival of 27 patients was reported from the time of the initial diagnosis. In total, 38 patients were recorded as alive or dead at the time of publication.
2.3. Variables of interest
Various Internet databases have been used to document the presentation, investigation, management, and treatment outcomes of the disease.
The computerized medical record database at our hospital as well as published literature were examined for clinicopathological traits and therapeutic approaches. Features of the patient include: (1) age at diagnosis and (2) male and female sex. Primary tumor data: (1) primary cutaneous and mucosal melanoma and, (2) tumor site. Metastatic features: (1) number of bladder metastatic foci; (2) synchronous and metachronous metastases; (3) nodular, extranodal, or widespread metastases; and, (4) gross hematuria (yes or no). Outcomes: (1) OS, (2) OS to bladder metastasis, and (3) survival and death.
Endpoint and definitions.
The endpoint of this study was to review all reports on metastatic bladder malignant melanoma up to April 2023. Double or multiple metastatic malignancies, such as synchronous and metachronous metastatic neoplasms, depending on the time between tumor diagnoses, can be split into two groups. Synchronous metastatic malignancies were secondary tumors that occurred either simultaneously, or within 6 months after the first metastatic malignancy, while metachronous metastatic malignancies were secondary metastatic tumors that developed 6 months, or more after the first metastatic malignancy. The time between the initial malignant melanoma diagnosis and the bladder metastases, the death date, or the published date of each report was used to define OS.
2.4. Statistical analysis
We employed the chi-square test to examine the differences in the associated factors between the groups with malignant melanoma and bladder metastases. According to cutaneous and mucosal melanoma, synchronous and metachronous metastases, node and extranodal metastases, and widespread status, the Kaplan-Meier model was used to examine OS. Log-rank tests were used to compare survival curves. If the p-value was <0.05, differences were deemed statistically significant. SPSS version 19.0 statistical software was used for all analyses. Her information is anonymous. The work has been reported in line with the SCARE criteria [23].
3. Results
Table 1 shows the baseline clinicopathological features and their correlations with the outcomes of patients with malignant melanoma and bladder metastases. There were more males in the study population (77.8 % vs 22.2 %). The median OS of females (91 months, IQR: 67–100.7), was higher than males (21 months, IQR: 12–124 months). The difference of the median survival between the two sexes was significant (p < 0.05 – Median test). Most participants were aged 70–79 years (40.7 %). The median overall survival (OS) increased with age at diagnosis. The highest OS was 83 months (IQR: 26–142.5) in the age group of 70–79. Nevertheless, 70.4 % of the patients had skin melanoma, and the median OS was 23 months (IQR: 12.5–81). Mucosal melanoma was less common, but the median OS was higher at 124.5 months (IQR: 67.5–137.25). Four patients (14.8 %) were missed their information, regarding the bladder tumors. Patients with one bladder metatastic focus were more common than those with equal or greater 2 bladder metatastic foci, and the median OS was also higher (71.5 months, IQR: 16.5–147.75 versus 16.5 months, IQR: 12.5–44.5). Nearly 34 % of the study population had synchronous metastases, and the median OS of this population was much lower than that of patients without synchronous metastases (24.5 months, IQR: 11–96.25 versus 103.5 months, IQR: 56.75–130). One-third of the patients had synchronous metastases and lymph nodes, and the median OS was lower than that of the other groups (12 months, IQR:6–28 versus 83 months, IQR:21–132). The difference in mean survival between the groups with positive and negative lymph node was significant (p < 0.05 - Mann-Whitney U test). Eleven patients (40.7 %) had synchronous metastases and extranodal nodes, and the median OS was higher than that of the other groups (60 months, IQR: 14.5–114 versus 38 months, IQR: 13.5–109). Four patients (14.8 %) had synchronous and widespread metastases, and the median OS was lower than that of the other group (29.5 months, IQR: 17.75–70.5 versus 54 months, IQR: 14.25–118.75). More half of the study population had metachronous metastases (14 cases, 51.9 %), and the median OS was higher than that of the others (85.5 months, IQR: 15–130 versus 24.5 months, IQR: 12.5–70.75). 1/3 of the patients had metachronous metastases and lymph nodes, and the median OS was higher than that of the other groups (48 months, IQR: 12–94 versus 38 months, IQR: 15–124). Eight patients (29.6 %) had metachronous metastases and extranodal nodes, and the median OS was higher than that of the other groups (109 months, IQR: 37.5–137.25 versus 24.5 months, IQR: 14.25–86.75). Three patients (11.1 %) had metachronous and widespread metastases, and the median OS was higher than that of the other groups (83 months; IQR: 49–194 versus 38 months; IQR: 13–113.5). Three patients (11.1 %) were missed their information, regarding the gross hematuria. Fourteen patients (51.0 %) had the gross hematuria, and the median overall survival (OS) was 38 months (IQR: 15.75–99.25). The median OS of patients with the gross hematuria was lower than that of those without the gross hematuria. However, no statistically significant differences were observed in the OS or other clinicopathological characteristics (p > 0.05).
Table 1.
Associations between the baseline clinicopathological features with OS in bladder metastatic malignant melanoma.
| Characteristics | N | % | OS (months) |
p Value (Mann-Whitney U test) |
p Value (Median test) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Compare median (Median test) | IQR | ||||||
| Gender | Female | 6 | 22.2 | 86.3 | 28.3 | 91.0 | 67.0–100.8 | 0.137 | 0.016 |
| Male | 21 | 77.8 | 66.8 | 84.9 | 21.0 | 12.0–124.0 | |||
| Age group | <40 | 1 | 3.7 | 6.0 | N/A | 6.0 | N/A | N/A | |
| 40–49 | 2 | 7.4 | 8.0 | 9.9 | 8.0 | 4.5–11.5 | |||
| 50–59 | 4 | 14.8 | 48.5 | 53.9 | 34.5 | 16.0–67.0 | |||
| 60–69 | 9 | 33.3 | 64.0 | 59.0 | 38.0 | 12.0–103.0 | |||
| 70–79 | 11 | 40.7 | 102.6 | 93.7 | 83.0 | 26.0–142.5 | |||
| Primary site | Skin | 19 | 70.4 | 52.3 | 61.3 | 23.0 | 12.5–81.0 | 0.071 | 0.164 |
| Mucosal | 8 | 29.6 | 118.0 | 93.2 | 124.5 | 67.5–137.3 | |||
| Bladder tumor No | 1 | 14 | 51.9 | 93.7 | 91.5 | 71.5 | 16.5–147.8 | 0.175 | 0.123 |
| ≥ 2 | 9 | 33.3 | 37.6 | 45.4 | 16.5 | 12.5–44.5 | |||
| Missing | 4 | 14.8 | N/A | N/A | N/A | N/A | |||
| Synchronous metastasis | |||||||||
| Synchronous metastasis | No | 7 | 25.9 | 117.8 | 101.9 | 103.5 | 56.8–130.0 | 0.15 | 0.321 |
| Yes | 20 | 74.1 | 58.9 | 65.2 | 24.5 | 11.0–96.3 | |||
| Lymph node metastasis | Absent | 18 | 66.7 | 95.1 | 83.2 | 83.0 | 21.0–132.0 | 0.013 | 0.134 |
| Present | 9 | 33.3 | 29.9 | 39.9 | 12.0 | 6.0–28.0 | |||
| Extranodal metastasis | Absent | 16 | 59.3 | 72.2 | 83.3 | 38.0 | 13.5–109.0 | 0.921 | 0.873 |
| Present | 11 | 40.7 | 73.0 | 71.8 | 60.0 | 14.5–114.0 | |||
| Widespread | Absent | 23 | 85.2 | 75.1 | 79.08 | 54.0 | 14.3–118.8 | 0.973 | 0.644 |
| Present | 4 | 14.8 | 58.8 | 73.86 | 29.5 | 17.8–70.5 | |||
| Metachronous metastasis | |||||||||
| Metachronous metastasis | No | 13 | 48.1 | 53.1 | 65.83 | 24.5 | 12.5–70.8 | 0.275 | 0.175 |
| Yes | 14 | 51.9 | 89.2 | 84.34 | 85.5 | 15.0–130.0 | |||
| Lymph node metastasis | Absent | 18 | 66.7 | 77.5 | 86.02 | 38.0 | 15.0–124.0 | 0.837 | 0.892 |
| Present | 9 | 33.3 | 63.2 | 60.21 | 48.0 | 12.0–94.0 | |||
| Extranodal metastasis | Absent | 19 | 70.4 | 64.2 | 82.47 | 24.5 | 14.3–86.8 | 0.253 | 0.164 |
| Present | 8 | 29.6 | 91.4 | 64.2 | 109.0 | 37.5–137.3 | |||
| Widespread | Absent | 24 | 88.9 | 64.5 | 63.83 | 38.0 | 13.0–113.5 | 0.44 | 0.946 |
| Present | 3 | 11.1 | 134.3 | 151.66 | 83.0 | 49.0–194.0 | |||
| Gross hematuria | No | 10 | 37.0 | 78.4 | 70.54 | 60.5 | 19.8–117.8 | 0.558 | 1.0 |
| Yes | 14 | 51.9 | 59.2 | 54.2 | 38.0 | 15.8–99.8 | |||
| Missing | 3 | 11.1 | N/A | N/A | N/A | N/A | |||
3.1. Survival estimates
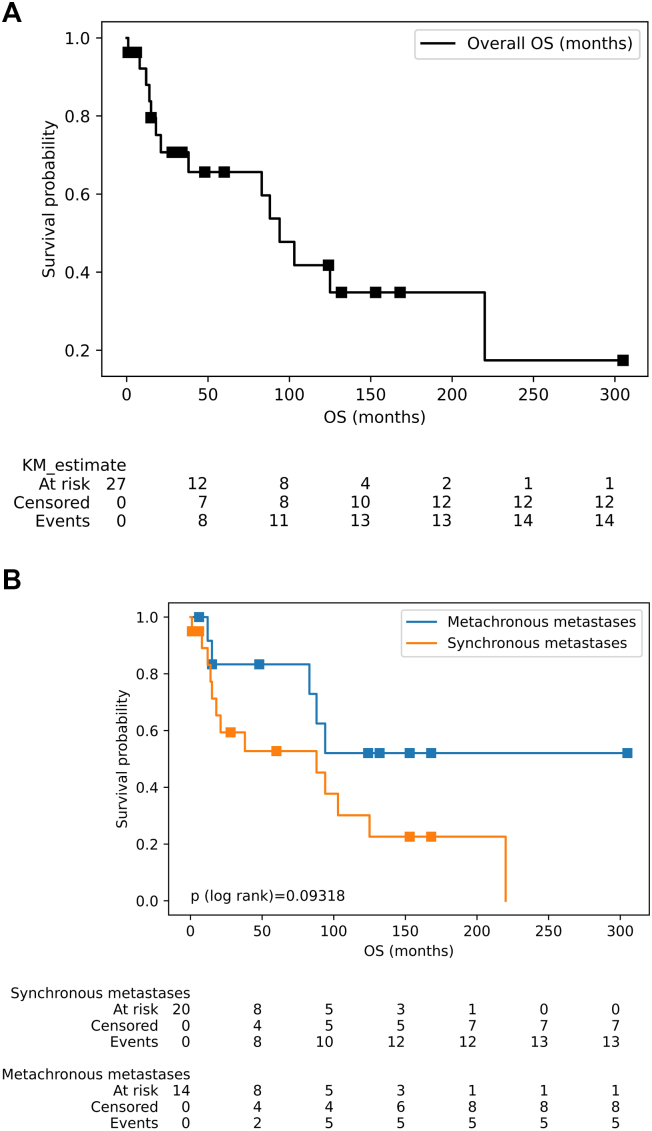
The OS curves of patients with malignant melanoma and bladder metastasis are summarized in Fig. 2. The median OS was 94 months (95 % confidence interval [CI]: 21–220 months) (Fig. 2A). According to the primary tumor site, the median OS of patients with cutaneous melanoma was 88 months (95 % CI: 15–220), and the mean survival was 51.37 ± 60 months. While the median survival among patients with mucosal melanoma was 125 months (95 % CI: 1 – inf), the mean survival was 118.0 ± 93 months. This difference was not statistically significant (p > 0.05). Notably, no events were observed in the mucosal melanoma group; therefore, the upper value of the confidence interval was infinity. The median survival among patients with one bladder metastatic focus was 88 months (95 % CI: 15 – inf), mean of 93.71 ± 92 months. The median survival among patients with two or more bladder metastatic foci was 94 months (95 % CI: 8 – inf), mean of 39.75 ± 45 months. The survival difference of the groups was not significant, and the p-value of the log-rank test was 0.86997. Note that, because of the right-censor in both groups, the upper value of the confidence interval is infinite. Fig. 2B shows the median survival among the synchronous metastatic group illustrated for 88 months (95 % CI: 14–125), mean of 58.95 ± 65 months; compared to the metachronous metastases, the 100th months, 36 % of events were observed, mean of 89.21 ± 84 months. The survival difference of the groups was not significant, too (p > 0.05).
Fig. 2.
Survival curves (log rank) of patients with metastatic melanoma. (A) OS of patients with the metastatic melanoma to bladder. (B) OS of patients with synchronous vs metachronous metastases. The differences between groups are not significant, the p value of the log rank test is up 0.05.
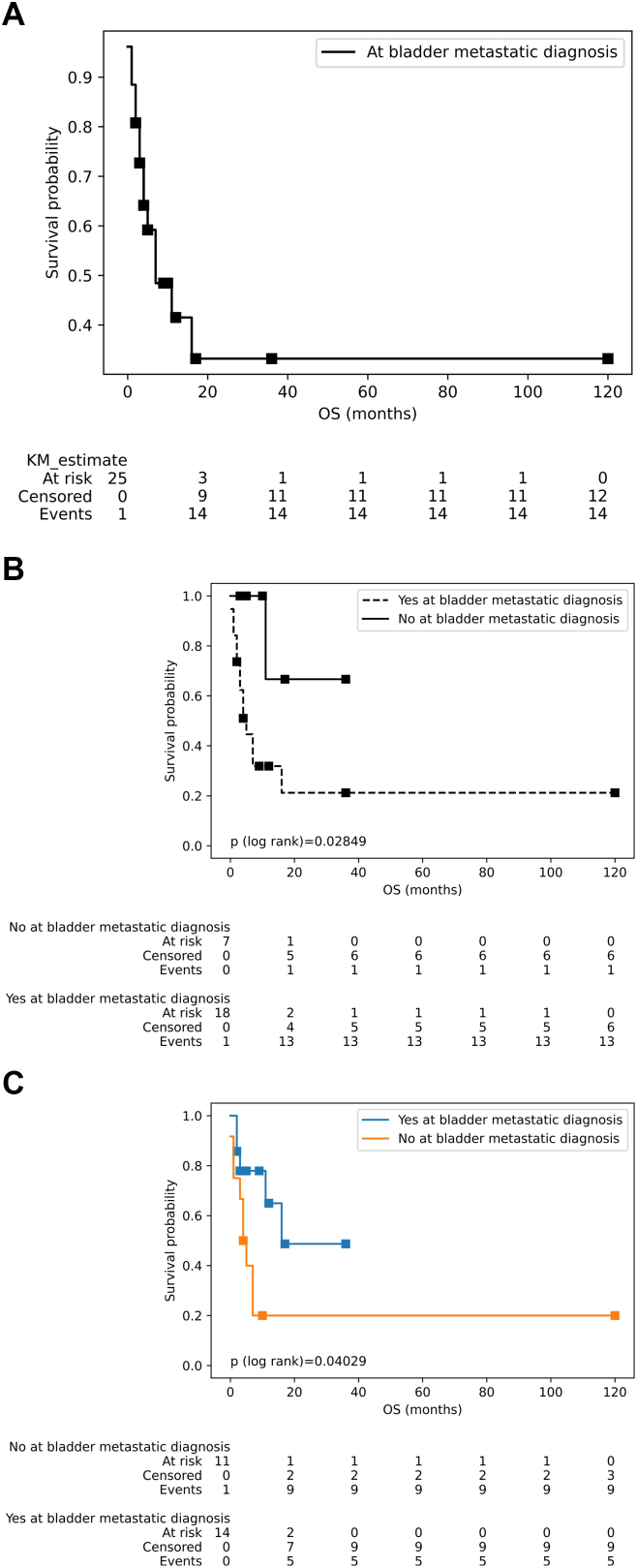
During the period of malignant melanoma metastasizing to the bladder, at that time, the median survival was 7 months (95 % CI: 4 – inf) (20.13 ± 4.1 months) (Fig. 3A). Patients with metachronous (11.29 ± 12 months) or non-synchronous metastasis (12.29 ± 12 months) achieved a higher median survival compared with the group without metachronous metastases (13.83 ± 34 months) or presenting synchronous metastases (12.53 ± 27 months) with bladder metastases of malignant melanoma, (p < 0.05) (Fig. 3B and C, respectively). However, no statistically significant difference was observed in the median survival of the malignant melanoma patients at the diagnosis of bladder metastases in relation to clinical features such as primary tumor location (skin: 13.89 ± 28 vs mucosa:9.25 ± 11 months) and number of bladder metastatic foci (1 focus: 11.92 ± 12 vs ≥2 foci: 18.75 ± 41 months) (p > 0.05).
Fig. 3.
Survival curves (log rank) of patients at the period of malignant melanoma metastasizes to the bladder. (A) Survival curve of period of the metastatic melanoma to bladder. (B) Survival of patients with synchronous vs without synchronous metastases. (B) Patients with metachronous vs non-metachronous metastases. The Log-rank test of Figs. B & C indicate that there were the significant differences between the couple of survival curves (p < 0.05).
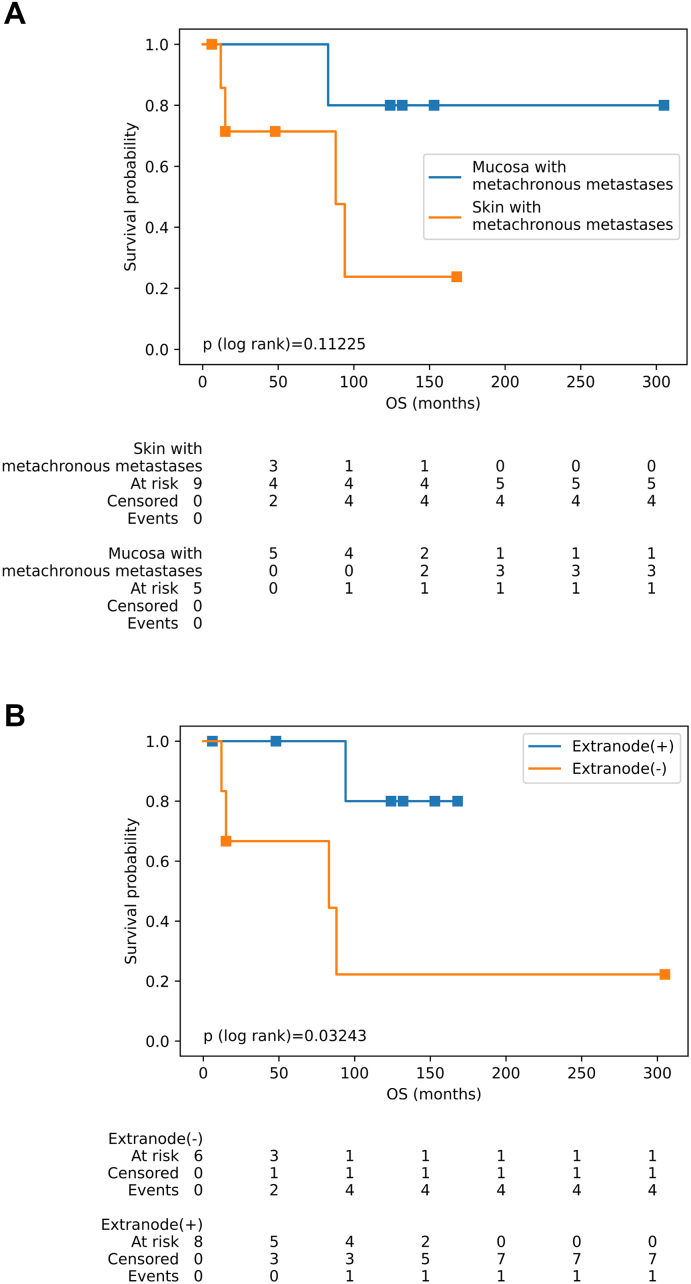
Figs. 4 illustrates the overall survival rates of the patients with metachronous metastases. At the 100th month, 36.0 % of events were observed, and the mean OS was 188.71 ± 38 months. When considering the combined characteristics of patients with metachronous metastases,the median survival of patients with mucosal melanomas are 159.4 ± 85 months, the median survival of unifocal bladder metastases are 101.0 ± 93 months, the median survival of patients without lymph node metastases are 136.0 ± 108 months, and the median survival of patients without disseminated metastases are 76.91 ± 62 months. The median survival of patients with these characteristics were all respectively higher than cases presenting the primary cutaneous tumors (50.22 ± 56 months), bladder metastases from 2 or more foci (77.67 ± 56 months), with lymph node metastases (63.22 ± 60 months) or widespread metastases (134.33 ± 152 months). Fig. 4A shows that these differences were not statistically significant (p > 0.05). For patients with metachronous metastases, with median survival of the extranodal metastatic groups was higher than those of the group without extranodal metastases (94 vs 88 months) (mean: 91.38 ± 64 vs 86.33 ± 113 months) (Fig. 4B). The observation was statistically significant (p < 0.05).
Fig. 4.
Figures illustrated the survival rate of patients with metachronous metastases and association of clinical characteristics. (A) Patients with skin versus mucosal melanoma. The Log-rank test of Fig. B indicates that there was a significant difference between positive vs negative extranode curves (p < 0.05). The differences of the remaining figure between groups are not significant (p > 0.05).
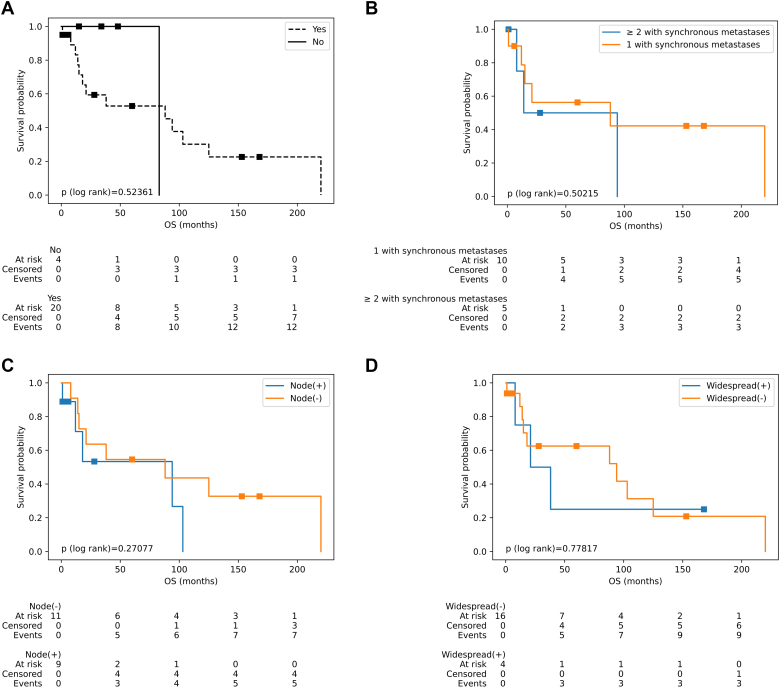
Fig. 5 illustrates the overall survival of patients with synchronous metastases, and the impact of other factors and metastatic status on the overall survival of patients with metastatic melanoma of the bladder. Fig. 5A, it can be seen that the average OS of patients with synchronous metastases was 88 months (95 % CI: 14–125) (58.95 ± 65 months), while patients without synchronous metastases have an average OS of 83 months (45.0 ± 29 months) (p = 0.524). When combining the primary tumor location with synchronous metastasis, it is evident that patients with primary cutaneous melanomas have a median OS of 88 months (95 % CI: 14–103) (mean: 54.94 ± 64 months), while in the group of mucosal melanomas, there was a marked reduction of 21 months (95 % CI: 1 – inf) (mean: 75.0 ± 75 months). Fig. 5B displays the relationship between the number of bladder metastatic foci and synchronous metastases, revealing that patients of synchronous metastases with 1 bladder metastatic focus have a median OS of 88 months (95 % CI: 1–220) (mean: 74.4 ± 79 months), whereas those with 2 or more bladder metastatic foci have a significantly lower median OS of 14 months (95 % CI: 8–94) (p = 0.845) (mean: 29.0 ± 38 months). This median OS was similar in patients with synchronous metastases and lymph node metastases (mean: 29.89 ± 40 months) and without lymph node metastases (mean: 82.73 ± 74 months) (Fig. 5C) (p = 0.271). In terms of the association of synchronous metastases with the status of extranodal metastases, the results indicate that patients with both synchronous metastases and extranodal metastases have a median OS of 38 months (95 % CI: 1 – inf) (mean: 73.0 ± 72 months). On the other hands, patients without extranodal metastases have a significantly higher median OS of 103 months (95 % CI: 14–220) (mean: 41.78 ± 55 months) (p = 0.301). The data for patients with synchronous metastases, with or without widespread metastases, is presented in Fig. 5D. The median OS for patients with widespread metastasis was only 21 months (95 % CI: 12 – inf) (mean: 58.75 ± 74 months), while the group without widespread metastases had a median OS of 94 months (95 % CI: 14–125) (mean: 59.0 ± 65 months) (p = 0.778).
Fig. 5.
Figures illustrated the survival rate of patients with synchronous metastases and association of clinical characteristics. (A) Survival curve of patients with or without synchronous metastases. (B) Patients with one versus two or more metastatic bladder tumors. (C) Survival of patients with positive vs negative lymph node. (D) Survival of widespread metastases or not. The Log-rank test of figures indicate that there were not the significant differences between groups of curves (p > 0.05).
4. Discussion
Thirty percent of melanoma cases are thought to develop metastases in the end, with the majority spreading to the lungs, skin, brain, and liver. Bladder metastases is uncommon and frequently goes undetected [9]. The gross hematuria is typically the first symptom present in clinical cases [1,2,[5], [6], [7], [8], [9], [10], [11], [12]], although urinary retention, dysuria, increased urination frequency, flank pain, and suprapubic pain have been documented in rare circumstances. However, some melanoma cases that metastasize to the bladder are not clinically present. The metastatic tumors were only discovered incidentally during systemic assessment at patient follow-up with imaging tests such as abdominal CT scans, as in our case and some previous reports [9,13,16,22].
Bladder metastatic malignant melanoma is treatable in a number of ways. The patient's performance status, anatomical site of metastases, presence of bladder symptoms, and life expectancy must all be taken into account. The severe treatment option of radical cystectomy should only be suggested in the case of certain metastatic illnesses and good prognostic indicators. Conservative transurethral resection of the bladder or partial cystectomy are options for treating bladder problems. As a supplement to endoscopic resection, systemic chemotherapy has been documented; however, this should only be used in patients with good performance status [16]. Patients who have the BRAF V600E mutation should also receive special consideration because there are two workable treatment options to take into account: targeted therapy (anti-BRAF/MEK) versus immunotherapy (anti-CTLA4 and anti-PD-1) and sequential versus combination regimens. Despite a higher risk of systemic toxicity, these therapies are associated with a greater OS and progression-free survival than chemotherapy or interleukin 2 [2].
Malignant melanoma has a usually dismal prognosis once it has metastasized. Surgery is typically performed to relieve a patient's symptoms. As a result, when cases of melanoma metastatic to the bladder are found, therapy is frequently palliative in character and focused on symptom relief. The 5-year overall survival rate following treatment for metastatic melanoma has historically been <10 % [24]. With a five-year survival rate of 5–19 % and a median survival of 12 months after melanoma has metastasized, metastatic bladder melanoma poses the highest risk of mortality [25,26].
The patient discussed in this report underwent her first wide local excision of melanoma on her abdominal wall 86 months ago, and 10 months ago was diagnosed with metastatic melanoma to her bladder, and received chemotherapy. Unfortunately, she died of the advanced and widespread nature of disease.
Overall, malignant mucosal melanomas have a longer mean survival than that of skin-derived melanomas. This result was also supported by the presence of synchronous or metachronous metastases of malignant melanoma to the bladder, except that the mean survival of patients with skin melanoma was longer at the time of bladder metastases. This finding is consistent with bladder metastases, with 1 mass having a longer mean survival compared to two or more masses. Additionally, synchronous metastases with metastatic melanoma to the bladder was found to reduce the mean survival compared with patients with metachronous metastases. Furthermore, malignant melanoma with bladder metastases associated with lymph node metastases generally has a shorter mean OS than patients without lymph node metastasis. Similarly, male patients with bladder metastases had a lower median OS than did female patients.
5. Conclusion
Bladder metastatic melanomas have only seldom been reported. Bladder metastatic melanoma combined with other factors such as male sex, lymph node metastases, skin primary tumor, 2 bladder metastases, and synchronous metastases are predictors of worse prognosis.
CRediT authorship contribution statement
Tu Anh Do completed a literature research, assisted in writing the case report's parts, and helped structure the information that was provided. Do also personally engaged in the patient's diagnosis, treatment, and assessment. He ought to be regarded as a notable author.
Chu Van Nguyen worked as a diagnostic consultant for the HE and immunohistochemical staining, conducted a literature study, helped to construct the case report's components, and formatted the material that was given.
Nhung Thi Mai and Duong Ngoc Nguyen participated in the pathological techniques of the patient, reviewed the patient charts, and assisted in the literature review.
Uyen Thi Le performed follow-up of the patient, reviewed the literature, and assisted in drafting the components of the study.
Phuong Thanh Vu participated diagnostic and treatment consultants and assisted in the literature review.
Consent
Written informed consent was obtained from the patient's next of kin for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Guarantor
Tu Anh Do, MD, PhD doanhtu@bvk.org.vn.
Statement of ethics
The study protocol was approved by the Science and Ethical Committee.
This study was performed in accordance with the Helsinki Declaration of 1964 and its amendments.
Written informed consent was obtained from next of kin for the publication of their details and any accompanying images.
Funding sources
The research, writing, and publication of this work were all done without any financial assistance from the authors.
Declaration of competing interest
Regarding the research, writing, or publication of this paper, the authors declare that they have no potential conflicts of interest.
Data availability
All the data analyzed in this case report are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Abdou C.A., et al. Metastatic melanoma to the bladder presenting as autonomic dysreflexia in a patient with paraplegia. Urol. Case Reports. 2021;39 doi: 10.1016/j.eucr.2021.101820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitton J., et al. Metastatic melanoma of the bladder: a case-report and literature review. J. Urol. Neph. St. 2019;2:169–171. [Google Scholar]
- 3.Caputo A., et al. Urinary bladder metastasis from malignant melanoma. J. Clin. Urol. 2022;15(1):63–65. [Google Scholar]
- 4.Charfi S., et al. Plasmacytoid melanoma of the urinary bladder and lymph nodes with immunohistochemical expression of plasma cell markers revealing primary esophageal melanoma. Case Rep. Pathol. 2012;2012:916256. doi: 10.1155/2012/916256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efesoy O., Cayan S. Bladder metastasis of malignant melanoma: a case report and review of literature. Med. Oncol. 2011;28 doi: 10.1007/s12032-010-9730-x. (p. S667–S9) [DOI] [PubMed] [Google Scholar]
- 6.Fink W., Zimpfer A., Ugurel S. Mucosal metastases in malignant melanoma. Onkologie. 2003;26:249–251. doi: 10.1159/000071620. [DOI] [PubMed] [Google Scholar]
- 7.Grunhut J., et al. Recognising immunotherapy-induced meningoencephalitis: a case during treatment for primary metastatic melanoma of the bladder neck. BMJ Case Rep. 2022 Jul 20;(15):e249411. doi: 10.1136/bcr-2022-249411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda A., et al. A case of metastatic malignant melanoma of the urinary bladder. Hinyokika Kiyo. 2013;59:579–582. [PubMed] [Google Scholar]
- 9.Kumar R.A., et al. Melanoma metastasis to the bladder: a case report. Urol. Case Rep. 2022;40:101941. doi: 10.1016/j.eucr.2021.101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C.S.D., et al. Management of metastatic malignant melanoma of the bladder. Urol. Case Reports. 2003;62:351. doi: 10.1016/s0090-4295(03)00354-6. [DOI] [PubMed] [Google Scholar]
- 11.Machuca-Aguado J., et al. Metastasis of uveal melanoma in bladder: presentation of two cases and review of the literature. Arch. Esp. Urol. 2022;75:873–877. doi: 10.56434/j.arch.esp.urol.20227510.127. [DOI] [PubMed] [Google Scholar]
- 12.Meunier R., Pareek G., Amin A. Metastasis of malignant melanoma to urinary bladder: a case report and review of the literature. Case Reports Pathol. 2015;2015:173870. doi: 10.1155/2015/173870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore N.A., et al. Malignant melanoma metastasis to the urinary bladder: a rare cystoscopic finding. J. Clin. Urol. 2015;8:143–146. [Google Scholar]
- 14.Nair B.C.J., et al. Conjunctival melanoma: bladder and upper urinary tract metastases. J. Clin. Oncol. 2011;29 doi: 10.1200/JCO.2010.32.3584. (p. e216–e9) [DOI] [PubMed] [Google Scholar]
- 15.Nohara T., et al. Metastatic malignant melanoma of the urinary bladder : a case report. Nihon Hinyokika Gakkai Zasshi. 2009;100:707–711. doi: 10.5980/jpnjurol.100.707. [DOI] [PubMed] [Google Scholar]
- 16.Paterson A., et al. Metastatic malignant melanoma of the urinary bladder: case report and literature review. Central Eur. J Urol. 2012;65:232–234. doi: 10.5173/ceju.2012.04.art13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rishi A., et al. Metastatic malignant melanoma to urinary bladder: a potential pitfall for high-grade urothelial carcinoma. Int. J. Surg. Pathol. 2014;22:347–351. doi: 10.1177/1066896913492199. [DOI] [PubMed] [Google Scholar]
- 18.Sandru A., et al. Survival rates of patients with metastatic malignant melanoma. J. Med. Life. 2014;7:572–576. [PMC free article] [PubMed] [Google Scholar]
- 19.Șereș R., et al. Synchronous urinary bladder and gluteal muscle metastases of malignant melanoma: a case report and review of the literature. J. Medic Radiat. Oncol. July 2022;11:63–69. [Google Scholar]
- 20.Shukla A., et al. Hexaminolevulinate blue-light cystoscopy in a patient with metastatic melanoma of the bladder. J. Endourol. Case Reports. 2016;2:68–70. doi: 10.1089/cren.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theocharides C., et al. Metastatic melanoma to the urinary bladder of ocular origin accompanied with primary cutaneous melanoma: diagnostic challenge—a report of a case. Case Reports Pathol. 2017;2017:5. doi: 10.1155/2017/4818537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisenbaugh E.S., et al. Metastatic malignant melanoma to the bladder: a case series. Curr. Urol. 2012;6:53–56. doi: 10.1159/000338872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohrabi C., et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(5):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmberg C.J., et al. Surgery of metastatic melanoma after systemic therapy - the SUMMIST trial: study protocol for a randomized controlled trial. Acta Oncol. 2021;60(1):52–55. doi: 10.1080/0284186X.2020.1846213. [DOI] [PubMed] [Google Scholar]
- 25.Venyo A.K.-G. Melanoma of the urinary bladder: a review of the literature. Surg. Res. Pract. 2014;2014:13. doi: 10.1155/2014/605802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandru A., et al. Survival rates of patients with metastatic malignant melanoma. J. Med. Life. 2014;7(4):572–576. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data analyzed in this case report are included in this article. Further inquiries can be directed to the corresponding author.