Abstract
Aim
Associations of the adipose tissue insulin resistance index (AT-IR, a product of fasting insulin and free fatty acid) with body fat distribution and the ratio of alanine to aspartate aminotransferase (ALT/AST), a marker of hepatosteatosis, were examined in the context of the metabolic syndrome.
Methods
Legs, the trunk and body fat by DXA, blood pressure (BP) and blood chemistry were measured in 284 young Japanese female university students and 148 middle-aged biological mothers whose BMI averaged <23 kg/m2.
Results
Young women had higher leg fat/body fat and lower trunk fat/body fat ratio (both p < 0.001) compared with middle-aged women but AT-IR did not differ between the two groups. We had multivariable linear regression analysis for AT-IR as a dependent variable including leg fat/body fat ratio, trunk fat/body fat ratio, fasting glucose, triglyceride, HDL cholesterol and systolic BP as independent variables. Leg fat/body fat ratio, fasting glucose and triglyceride (p = 0.013, 0.009 and 0.016, respectively) emerged as determinants of AT-IR in young women. Trunk fat/body fat ratio and fasting glucose (p = 0.003 and 0.019, respectively) emerged in middle-aged women. In a model which included ALT/AST as an additional independent variable, ALT/AST (p = 0.016) was the fourth independent determinant in young women and the single determinant of AT-IR in middle-aged women (p < 0.001).
Conclusion
In young Japanese women, adipose tissue insulin resistance was associated with reduced leg fat, a subtle partial lipodystrophy-like phenotype associated with reduced adipose tissue expandability. It was associated with elevated trunk (abdominal) fat in middle-aged women and with ALT/AST, a marker of hepatosteatosis, in two groups of Japanese women, suggesting ectopic fat deposition associated with reduced adipose tissue expandability.
Keywords: Metabolic syndrome, Adipose tissue insulin resistance, Gluteofemoral fat, Liver enzymes, Japanese women
1. Introduction
The metabolic syndrome is an important predictor of type 2 diabetes and cardiovascular disease and characterized by a core set of disorders, including visceral/abdominal obesity, impaired fasting glucose, high blood pressure (BP), high triglyceride (TG) and low high-density lipoprotein (HDL) cholesterol [1]. Insulin resistance associated with abdominal obesity is a pathophysiological basis of the metabolic syndrome. Studies employed computed tomography images show that visceral fat is higher in East Asians compared to Europeans [2,3].
The liver is well known to produce glucose and TG. Liver fat is highly significantly and linearly correlated with all components of the metabolic syndrome independent of obesity and fatty liver is considered as a component of the metabolic syndrome [4]. We showed that alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio, a marker of hepatosteatosis, was associated with all components of the metabolic syndrome in Japanese women [5].
In addition to excess of adipose tissue (e.g. overweight/obesity), the metabolic syndrome is associated with a deficiency of adipose tissue (e.g. lipodystrophies), relatively rare conditions [6]. Genome-wide association studies focusing on insulin resistance [7,8] revealed that elevated insulin resistance scores were associated with lower BMI and lower leg (gluteofemoral) subcutaneous fat mass, a subtle lipodystrophy-like phenotype. These observations suggest that the link may be associated with the impaired capacity to adequately expand the peripheral adipose tissue compartments. Han et al. [9] reported in a Korean population that low leg fat to total body fat ratio is the strongest independent predictor of cardiometabolic health in normal weight subjects whereas it is not a significant determinant of metabolic risk in obese subjects.
It appears that Asian people have lower fat in extremities compared to whites [[10], [11], [12], [13]]. We showed that lower birthweight [14] and positive family history of type 2 diabetes [15] were associated with low leg fat mass, a subtle lipodystrophy-like phenotype, in young Japanese women. In addition, a recent Mendelian randomization analysis [16] revealed that increased absolute and relative gluteofemoral adipose tissue were associated with a favorable cardiometabolic profile independently of BMI. There are two simple and validated indices to measure insulin resistance: homeostasis model assessment-insulin resistance (HOMA-IR) [17] and adipose tissue insulin resistance index (AT-IR) [18]. We reported that AT-IR appeared to be a simple and useful surrogate index of adipose tissue insulin resistance even in nonobese and nondiabetic Japanese women [19]. We also showed that AT-IR was associated with ALT/AST ratio, a marker of hepatosteatosis [20]. Therefore, we studied whether insulin resistance indices may be associated with gluteofemoral (leg) fat and ALT/AST ratio in young Japanese women relative to middle-aged women in a setting where overweight/obesity is not associated with socioeconomic and educational status.
2. Material and methods
2.1. Study design and recruitment
We cross-sectionally studied 284 Japanese female university students (74 collegiate athletes and 210 non-athletes) and 148 their biological mothers, whose details have been reported elsewhere [[19], [20], [21], [22], [23]]. Young and middle-aged women participated the present study as volunteers.
2.2. Participants
Athletes were students of the Department of Health and Sports Sciences and nonathletes were students of the Department of Food Sciences and Nutrition. Although our previous study on AT-IR [19] excluded athletic students to avoid effects of endurance training on AT-IR, the present study included athletic students to obtain wider range of AT-IR [21]. Women with clinically diagnosed acute or chronic diseases, those on hormonal contraception and those on a diet to lose weight were excluded from the study. Nobody reported to receiving any medications or having regular supplements.
2.3. Ethics clearance and informed consent
The study was in accordance with the Helsinki declaration. All subjects were recruited as volunteers and gave written consent after the experimental procedure had been explained. The study was approved by the Ethics Committees of the Mukogawa Women's University (No. 07–28 on February 19, 2008).
2.4. Clinical assessments
After a 12-h overnight fast, participants underwent blood sampling and measurement of height, weight, blood pressure and body composition by whole-body dual-energy X-ray absorptiometry (DXA) as described later. Blood pressure was measured using an automated sphygmomanometer (BP-203RV II, Colin, Tokyo, Japan) after participants were seated at least for 5 min. Glucose was determined by the hexokinase/glucose-6-phosphate dehydrogenase method (inter-assay coefficient of variation (CV) < 2 %). Serum insulin was measured by an ELISA method with a narrow specificity excluding des-31, des-32, and intact proinsulin (interassay CV <6 %). Serum free fatty acid (FFA) concentrations were measured using enzymatic colorimetric methods (Wako, Tokyo, Japan). Serum cholesterol, TG, HDL cholesterol and liver enzymes were measured as previously reported [21,23]. ALT/AST ratio was used as a proxy of hepatosteatosis [24]. We showed that the ratio was superior to ALT alone in evaluating the relationship to cardiometabolic health in Japanese women [25]. HOMA-IR [17] was calculated as a product of fasting concentrations of insulin (μU/mL) and glucose (mg/dL)/405 and AT-IR [18] as a product of fasting concentrations of insulin (μU/mL) and FFA (mEq/L).
Fat mass for whole body, legs and trunk all in kilograms were measured using whole-body DXA (Hologic QDR-2000, software version 7.20D, Waltham, MA) as previously reported.22 The leg region included the entire hip, thigh and legs. General adiposity was assessed using height-adjusted and weight-adjusted body fat; fat mass index (FMI) and body fat (BF) percentage (%), respectively. Leg fat (LF) to BF ratio was calculated as LF divided by BF x 100 and trunk fat (TF) to BF ratio as TF divided by BF x 100.
2.5. Statistical analyses
Data were presented as mean ± SD. Due to deviation from normal distribution, gamma-glutamyl transferase (GGT) was logarithmically transformed for analyses. Bivariate correlations of two insulin resistance indices with other parameters were evaluated by Pearson's correlation analysis. In order to evaluate the most important determinants of AT-IR and HOMA-IR, a stepwise multivariate linear regression analysis was performed. In model A, fat distribution measures (trunk fat/body fat ratio and leg fat/body fat ratio and four core cardiometabolic variables (fasting glucose, TG, HDL cholesterol and systolic BP) were included as independent variables. ALT/AST was further included in model B. Differences between two groups were analyzed with t-test and χ2 test when appropriate. Differences among three groups were analyzed by analysis of variance and then Bonferroni's multiple comparison procedure. A two-tailed value of p < 0.05 was considered significant. Statistics were performed with SPSS system 23.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Basic demographics and characteristics
BMI averaged <23 kg/m2 both in young and middle-aged women and there was no difference in absolute leg fat, AT-IR and HOMA-IR between two groups of women (Table 1). HOMA-IR averaged 1.2 in two groups of women. Although young compared with middle-aged women had lower absolute arm fat, TF, TF/BF ratio, %arm fat, %TF, BMI, FMI and %BF, LF/BF ratio was higher in young women. Young women showed favorable ccardiometabolic profile except for HDL cholesterol, which showed no significant difference.
Table 1.
Anthropometric and adiposity measures and cardiometabolic traits in young and middle-aged Japanese women.
| Young |
Middle-aged |
||||||
|---|---|---|---|---|---|---|---|
| n = 284 | n = 148 | p values | |||||
| Age (years) | 20.7 | ± | 1.2 | 49.8 | ± | 3.6 | <0.001 |
| Body mass index (kg/m2) | 20.7 | ± | 2.2 | 22.0 | ± | 2.8 | <0.001 |
| Arm fat (kg) | 1.1 | ± | 0.6 | 1.5 | ± | 0.7 | <0.001 |
| Leg fat (kg) | 5.5 | ± | 1.6 | 5.4 | ± | 1.7 | 0.465 |
| Trunk fat (kg) | 6.7 | ± | 2.3 | 8.8 | ± | 3.4 | <0.001 |
| Body fat (kg) | 13.9 | ± | 4.3 | 16.3 | ± | 5.7 | <0.001 |
| Leg fat/body fat (%) | 40.0 | ± | 4.4 | 33.6 | ± | 5.0 | <0.001 |
| Trunk fat/body fat (%) | 48.1 | ± | 4.0 | 53.3 | ± | 5.0 | <0.001 |
| %Arm fat (%) | 22.2 | ± | 8.4 | 27.8 | ± | 8.8 | <0.001 |
| %Leg fat (%) | 28.7 | ± | 5.7 | 30.3 | ± | 6.7 | 0.010 |
| %Trunk fat (%) | 26.7 | ± | 6.8 | 32.9 | ± | 8.7 | <0.001 |
| %Body fat (%) | 25.9 | ± | 5.9 | 30.1 | ± | 7.3 | <0.001 |
| FMI (kg/m2) | 5.4 | ± | 1.7 | 6.7 | ± | 2.4 | <0.001 |
| Fasting glucose (mg/dL) | 83 | ± | 7 | 89 | ± | 14 | <0.001 |
| Triglyceride (mg/dL) | 58 | ± | 34 | 81 | ± | 36 | <0.001 |
| HDL cholesterol (mg/dL) | 75 | ± | 13 | 77 | ± | 16 | 0.082 |
| Systolic BP (mmHg) | 107 | ± | 11 | 121 | ± | 16 | <0.001 |
| Diastolic BP (mmHg) | 63 | ± | 7 | 74 | ± | 11 | <0.001 |
| Insulin (μU/mL) | 5.9 | ± | 3.2 | 5.4 | ± | 2.8 | 0.143 |
| FFA (mEq/L) | 0.52 | ± | 0.22 | 0.60 | ± | 0.23 | 0.001 |
| HOMA-IR | 1.2 | ± | 0.7 | 1.2 | ± | 0.7 | 0.985 |
| AT-IR | 3.1 | ± | 2.5 | 3.2 | ± | 2.1 | 0.578 |
| GGT (U/L) | 14 | ± | 4 | 22 | ± | 20 | <0.001 |
| AST (U/L) | 19 | ± | 6 | 21 | ± | 13 | 0.003 |
| ALT (U/L) | 14 | ± | 6 | 20 | ± | 18 | <0.001 |
| ALT/AST | 0.74 | ± | 0.22 | 0.91 | ± | 0.26 | <0.001 |
Mean ± SD. AST: aspartate aminotransferase, ALT: alanine aminotransferase, AT-IR: adipose tissue-insulin resistance index, HOMA-IR: homeostasis model assessment-insulin resistance, BP: blood pressure, FFA: free fatty acid, FMI: fat mass index, GGT: gamma-glutamyl transferase.
3.2. Associations of adipose tissue-insulin resistance index
Among twelve anthropometric and fat distribution measures studied, only LF/BF ratio showed significant association with AT-IR (inverse) in young women (Table 2). In contrast, only absolute leg fat did not show significant association with AT-IR in middle-aged women. AT-IR was inversely associated with LF/BF ratio and positively with TF/BF ratio. AT-IR showed positive associations with fasting glucose, TG, HOMA-IR, log GGT and ALT/AST ratio in both young and middle-aged women. An association with blood pressure was significant in middle-aged women only and there was no association with HDL cholesterol in both young and middle-aged women. Associations of AT-IR with HOMA-IR (p < 0.001) and with ALT/AST (p = 0.003) were stronger in middle-aged compared with young women.
Table 2.
Correlation coefficients (r) of adipose tissue-insulin resistance index with anthropometric and cardiometabolic variables in young and middle-aged Japanese women.
| Young |
Middle-aged |
|||
|---|---|---|---|---|
| r | p values | r | p values | |
| Body mass index | −0.097 | 0.104 | 0.206 | 0.012 |
| Arm fat | 0.023 | 0.705 | 0.202 | <0.001 |
| Leg fat | −0.100 | 0.097 | 0.155 | 0.064 |
| Trunk fat | −0.003 | 0.962 | 0.291 | <0.001 |
| Body fat | −0.034 | 0.566 | 0.256 | 0.002 |
| Leg fat/body fat | −0.159 | 0.008 | −0.271 | 0.001 |
| Trunk fat/body fat | 0.104 | 0.083 | 0.275 | 0.001 |
| %Arm fat | 0.092 | 0.124 | 0.233 | 0.005 |
| %Leg fat | 0.034 | 0.569 | 0.194 | 0.020 |
| %Trunk fat | 0.078 | 0.195 | 0.299 | <0.001 |
| %Body fat | 0.065 | 0.277 | 0.279 | 0.001 |
| Fat mass index | 0.012 | 0.847 | 0.264 | 0.001 |
| Fasting glucose | 0.149 | 0.012 | 0.213 | 0.009 |
| Triglyceride | 0.178 | 0.003 | 0.215 | 0.009 |
| HDL cholesterol | 0.022 | 0.707 | −0.072 | 0.385 |
| Systolic BP | 0.090 | 0.131 | 0.228 | 0.005 |
| Diastolic BP | 0.025 | 0.678 | 0.193 | 0.019 |
| HOMA-IR | 0.475 | <0.001 | 0.739 | <0.001 |
| log GGT |
0.126 |
0.034 |
0.264 |
0.001 |
| ALT/AST | 0.181 | 0.002 | 0.454 | <0.001 |
Bold letters indicate statistically significant associations. Abbreviations are the same as in Table 1.
3.3. Multivariate linear regression analysis for adipose tissue-insulin resistance index
In multivariate linear regression analysis for AT-IR (Table 3, model A), AT-IR was independently associated with LF/BF ratio, fasting glucose and TG in young women and TF/BF ratio and fasting glucose in middle-aged women. However, these variables explained only 6.2 % and 9.9 % of AT-IR variability in young and middle-aged women, respectively. When ALT/AST was further included (model B), ALT/AST was the fourth independent determinant in young women and ALT/AST was the single determinant of AT-IR in middle-aged women. Results did not change when log GGT was included instead of and in addition to ALT/AST.
Table 3.
Multivariable linear regression analysis for adipose tissue-insulin resistance index.
| Young | Middle-aged | |||
|---|---|---|---|---|
| Model A | Standardizedβ | p values | Standardizedβ | p values |
| Leg fat/body fat | −0.148 | 0.013 | ||
| Trunk fat/body fat | 0.244 | 0.003 | ||
| Fasting glucose | 0.154 | 0.009 | 0.191 | 0.019 |
| Triglycerides |
0.143 |
0.016 |
||
| Cumulative R2 |
0.062 |
0.099 |
||
|
Model B | ||||
| Leg fat/body fat | −0.127 | 0.034 | ||
| Trunk fat/body fat | ||||
| Fasting glucose | 0.137 | 0.020 | ||
| Triglycerides | 0.136 | 0.021 | ||
| ALT/AST |
0.142 |
0.016 |
0.445 |
<0.001 |
| Cumulative R2 | 0.078 | 0.193 | ||
All models included the same six independent variables: trunk fat/body fat ratio, leg fat/body fat ratio, fasting glucose, TG, HDL cholesterol and systolic BP (model A). ALT/AST was further included in model B. Abbreviations are the same as in Table 1.
3.4. Features of adipose insulin-resistant young women
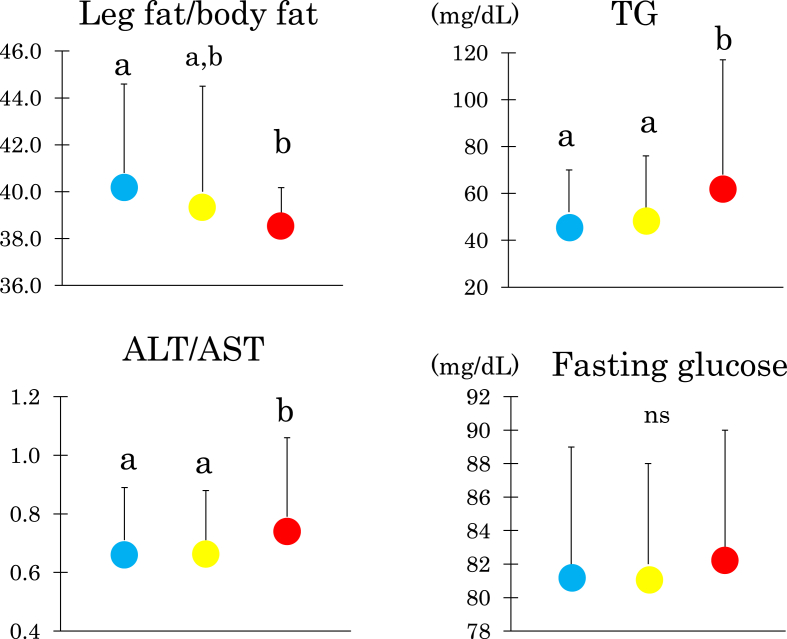
We divided 284 young women into three groups according to AT-IR tertile (Fig. 1). Women with the high compared with the low tertile had lower LF/BF ratio and higher serum TG and ALT/AST ratio. However, fasting glucose did not differ.
Fig. 1.
Leg fat/body fat ratio, fasting triglyceride, the ratio of alanine aminotransferase to aspartate aminotransferase (ALT/AST), fasting glucose in young Japanese women with the low, median and high tertile (blue, yellow and red circles, respectively) of the adipose tissue insulin resistance index. Mean ± SD. Means not sharing common alphabetical letters are significantly different with each other at p < 0.05 or less by Bonferroni's multiple comparison procedure. ns: not significant.
4. Discussion
The present study has shown that in young Japanese women, adipose tissue insulin resistance (measured by AT-IR) was associated positively with fasting glucose and TG, and inversely with leg fat (evaluated by LF/BF ratio), the largest and best storage site as fat [26]. Positive association of adipose tissue insulin resistance with trunk (abdominal) fat (evaluated by TF/BF ratio), a core component of the metabolic syndrome and a marker of ectopic fat accumulation [27], was confirmed in middle-aged Japanese women. Further, ALT/AST ratio, a proxy of hepatosteatosis [24], was a determinant of adipose tissue insulin resistance independent of leg fat in young Japanese women. However, in middle-aged women, ALT/AST ratio was a single determinant of adipose tissue insulin resistance. It is to be noted that these observations were found in women whose BMI and ALT averaged <23 kg/m2 and <30 U/L, respectively.
Subcutaneous adipose tissue, mainly located in gluteofemoral or leg region, is the largest and best storage site [26]. It was suggested that it is not the absolute amount of adipose tissue but rather the capacity of adipose tissue to expand that affects metabolic homeostasis [28,29]. An inverse association of adipose tissue insulin resistance with leg fat in young Japanese women in the present study may be consistent with the large genome-wide association study that high insulin resistance scores were associated with lower BMI and lower leg fat, a subtle lipodystrophy-like phenotype [7]. Associations of adipose tissue insulin resistance with leg fat and ALT/AST in the present study may be related to the observation that low leg fat mass (evaluated by leg fat/body fat ratio), followed by fatty liver, was the strongest independent predictor of metabolic risk in normal-weight middle-aged Caucasians, but not in obese subjects [30] Among postmenopausal US women, both elevated trunk fat and reduced leg fat are associated with increased risk of cardiovascular disease and lower-extremity arterial disease [31,32]. Our results provide a clue that reduced adipose expandability may be one mechanism that links adipose insulin resistance with the other metabolic traits of the metabolic syndrome in the Japanese population at least in women.
We reported that in another set of daughter-mother pairs, a mean BMI of mothers increased from 20.0 kg/m2 at 18 years old to 21.8 kg/m2 at 48 years old [33]. The BMI of 18-year-old daughters (20.2 kg/m2) was associated not only with their mothers' BMI when they were 18 years old but with the current BMI of their mothers. In middle-aged compared with young Japanese women in the present study, higher body fat resulted from higher trunk fat with similar leg fat. As 148 out of 284 young women were daughters of 148 middle-aged women in the present study, these findings suggest that increases in body fat from young to middle-aged Japanese women resulted mainly from increases in trunk fat, supporting reduced leg fat expandability in Japanese women.
Studies from University of Hawaii quantified fat mass in arms, legs, the trunk and total body by DXA in Asian and White women [[10], [11], [12]]. Leg fat/body fat ratio calculated using these mean values was consistently lower in Asian compared with white adolescent girls (39 vs. 44 %) [11] and elderly Japanese American compared with Caucasian (30 vs. 36 %) [12]. Elevated trunk/peripheral (arms and legs) fat ratio was repeatedly confirmed in adolescent, middle-aged and elderly Asian compared with White women [[10], [11], [12]]. However, elevated trunk/peripheral fat ratio was not due to elevated trunk fat rather reduced peripheral fat. For example, elderly Japanese American compared with White women had comparable BMI, total body and trunk fat (14.3 and 14.9 kg, p = 0.68) but reduced leg fat (7.7 and 10.4 kg, p = 0.0007) [12]. These observations may indicate reduced leg fat expandability, a subtle lipodystrophy-like phenotype, in women of Asian ancestry.
The strength of the present study includes homogeneous study population with few confounding factors [22], and accurate and reliable measures of body composition by DXA. Several limitations of this study warrant consideration. The cross-sectional design complicates the drawing of causal inferences, and a single measurement of biochemical variables may be susceptible to short-term variation, which would bias the results toward the null. We used crude measures of insulin sensitivity/IR, which may be less accurate. Statistical power was not calculated. As we studied Japanese women only, results may not be generalized to other gender, races or ethnicities.
5. Conclusions
In young lean Japanese women, adipose tissue insulin resistance was associated with reduced leg fat, a subtle partial lipodystrophy-like phenotype associated with reduced adipose tissue expandability [7]. In middle-aged Japanese women, it was associated with elevated trunk (abdominal) fat, a proxy of ectopic fat deposition [27]. Results may be consistent with the theory that a lower amount of lower-body (leg) fat mass is equally important to a high amount of visceral fat mass as a determinant of cardiometabolic diseases [34]. Further, because nearly half of people with type 2 diabetes live in Asia, mainly in India and China [35] and because Asian people and people of Asian descent tend to develop type 2 diabetes at much lower levels of BMI than do members of other race/ethnic groups [36], adipose tissue insulin resistance associated with reduced leg fat may be an important determinant of cardiometabolic health in young Asian people. Consequently, and finally, weight gain since young adulthood should be minimized to avoid ectopic fat accumulation in Asian people, those with positive family history of diabetes [15] and those with lower birthweight [14] in particular.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Compliance with ethical standards
The study was approved by the Ethics Committees of the Mukogawa Women's University (No. 07–28 on February 19, 2008) and followed the tenets of the Declaration of Helsinki. All participants gave written informed consent after the experimental procedure had been explained. The authors declare that there is no duality of interest associated with this manuscript.
CRediT authorship contribution statement
Satomi Minato-Inokawa: Formal analysis. Mari Honda: Visualization, Data curation. Ayaka Tsuboi-Kaji: Investigation, Data curation. Mika Takeuchi: Data curation. Kaori Kitaoka: Data curation. Miki Kurata: Data curation. Bin Wu: Methodology, Investigation. Tsutomu Kazumi: Writing – original draft, Methodology, Conceptualization. Keisuke Fukuo: Writing – review & editing.
Acknowledgments
We thank all participants for their dedicated and conscientious collaboration.
References
- 1.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T., Sekikawa A., Murata K., Maegawa H., Takamiya T., Okamura T., et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes. 2006;30:1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 3.Lear S.A., Humphries K.H., Kohli S., Chockalingam A., Frohlich J.J., Birmingham C.L. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 4.Nafld Kotronen A., Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 5.Minato-Inokawa S., Tsuboi-Kaji A., Honda M., Takeuchi M., Kitaoka K., Kurata M., et al. Associations of ALT/AST, a marker of hepatosteatosis, with pulse rate in young women and with blood pressure in middle-aged women independently of abdominal fat accumulation and insulin resistance. Diabetol Int. 2024;15:270–277. doi: 10.1007/s13340-023-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann J.P., Savage D.B. What lipodystrophies teach us about the metabolic syndrome. J Clin Invest. 2019;129:4009–4021. doi: 10.1172/JCI129190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotta L.A., Gulati P., Day F.R., Payne F., Ongen H., van de Bunt M., et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotta L.A., Wittemans L.B.L., Zuber V., Stewart I.D., Sharp S.J., Luan J., et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. 2018;320:2553–2563. doi: 10.1001/jama.2018.19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han E., Lee Y.H., Lee B.W., Kang E.S., Lee I.K., Cha B.S. Anatomic fat depots and cardiovascular risk: a focus on the leg fat using nationwide surveys (KNHANES 2008-2011) Cardiovasc Diabetol. 2017;16:54. doi: 10.1186/s12933-017-0536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto Y., Maskarinec G., Conroy S.M., Lim U., Shepherd J., Novotny R. Asian ethnicity is associated with a higher trunk/peripheral fat ratio in women and adolescent girls. J Epidemiol. 2012;22:130–135. doi: 10.2188/jea.je20110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novotny R., Daida Y.G., Grove J.S., Le Marchand L., Vijayadeva V. Asian adolescents have a higher trunk:peripheral fat ratio than Whites. J Nutr. 2006;136:642–647. doi: 10.1093/jn/136.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim U., Ernst T., Buchthal S.D., Latch M., Albright C.L., Wilkens L.R., et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1(5) doi: 10.1038/nutd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J., Hendryx M., Laddu D., Phillips L.S., Chlebowski R., LeBlanc E.S., et al. Racial and ethnic differences in anthropometric measures as risk factors for diabetes. Diabetes Care. 2019;42:126–133. doi: 10.2337/dc18-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda M., Tsuboi A., Minato-Inokawa S., Takeuchi M., Yano M., Kurata M., et al. Birth weight was associated positively with gluteofemoral fat mass and inversely with 2-h postglucose insulin concentrations, a marker of insulin resistance, in young normal-weight Japanese women. Diabetol Int. 2021;13:375–380. doi: 10.1007/s13340-021-00543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honda M., Tsuboi A., Minato-Inokawa S., Takeuchi M., Kurata M., Wu B., et al. Reduced gluteofemoral (subcutaneous) fat mass in young Japanese women with family history of type 2 diabetes: an exploratory analysis. Sci Rep. 2022;12 doi: 10.1038/s41598-022-16890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B., DePaolo J., Judy R.L., Shakt G., Witschey W.R., Levin M.G., et al. Relationships between body fat distribution and metabolic syndrome traits and outcomes: a Mendelian randomization study. PLoS One. 2023;18(10) doi: 10.1371/journal.pone.0293017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Søndergaard E., Espinosa De Ycaza A.E., Morgan-Bathke M., Jensen M.D. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102:1193–1199. doi: 10.1210/jc.2017-00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitaoka K., Tsuboi A., Minato-Inokawa S., Honda M., Takeuchi M., Yano M., et al. Determinants and correlates of adipose tissue insulin resistance index in Japanese women without diabetes and obesity. BMJ Open Diabetes Res Care. 2020 Sep;8(1) doi: 10.1136/bmjdrc-2020-001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minato-Inokawa S., Tsuboi-Kaji A., Honda M., Takeuchi M., Kitaoka K., Kurata M., et al. Associations of alanine aminotransferase/aspartate aminotransferase, a marker of hepatosteatosis, with adipose tissue insulin resistance index and leptin/adiponectin ratio in Japanese women. Metab Syndr Relat Disord. 2023;21:590–595. doi: 10.1089/met.2023.0118. [DOI] [PubMed] [Google Scholar]
- 21.Kitaoka K., Takeuchi M., Tsuboi A., Minato S., Kurata M., Tanaka S., et al. Increased adipose and muscle insulin sensitivity without changes in serum adiponectin in young female collegiate athletes. Metab Syndr Relat Disord. 2017;15:246–251. doi: 10.1089/met.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M., Yoshida T., Bin W., Fukuo K., Kazumi T. FTO, abdominal adiposity, fasting hyperglycemia associated with elevated HbA1c in Japanese middle-aged women. J Atherosclerosis Thromb. 2012;19:633–642. doi: 10.5551/jat.11940. [DOI] [PubMed] [Google Scholar]
- 23.Wu B., Huang J., Fukuo K., Suzuki K., Yoshino G., Kazumi T. Different associations of trunk and lower-body fat mass distribution with cardiometabolic risk factors between healthy middle-aged men and women. Internet J Endocrinol. 2018 doi: 10.1155/2018/1289485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long M.T., Pedley A., Colantonio L.D., Massaro J.M., Hoffmann U., Muntner P., et al. Development and validation of the Framingham steatosis index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol. 2016;14(8):1172–1180.e2. doi: 10.1016/j.cgh.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minato-Inokawa S., Tsuboi-Kaji A., Honda M., Takeuchi M., Kitaoka K., Kurata M., et al. Associations of alanine aminotransferase/aspartate aminotransferase with insulin resistance and β-cell function in women. Sci Rep. 2023;13:7853. doi: 10.1038/s41598-023-35001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolopoulos K.N., Karpe F., Frayn K.N. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 27.Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125:1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith U., Kahn B.B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virtue S., Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Stefan N., Schick F., Häring H.U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metabol. 2017;26:292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Chen G.C., Arthur R., Iyengar N.M., Kamensky V., Xue X., Wassertheil-Smoller S., et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40:2849–2855. doi: 10.1093/eurheartj/ehz391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G.C., Arthur R., Kamensky V., Chai J.C., Yu B., Shadyab A.H., et al. Body fat distribution, cardiometabolic traits, and risk of major lower-extremity arterial disease in postmenopausal women. Diabetes Care. 2022;45:222–231. doi: 10.2337/dc21-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka S., Bin W., Honda M., Nanbu S., Suzuki K., Fukuo K., et al. Associations of 18-year-old daughters' and mothers' serum leptin, body mass index and DXA-derived fat mass. J Atherosclerosis Thromb. 2010;17:1077–1081. doi: 10.5551/jat.5371. [DOI] [PubMed] [Google Scholar]
- 34.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 35.Ke C., Narayan K.M.V., Chan J.C.N., Jha P., Shah B.R. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18:413–432. doi: 10.1038/s41574-022-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gujral U.P., Weber M.B., Staimez L.R., Narayan K.M.V. Diabetes among non-overweight individuals: an emerging public health challenge. Curr Diabetes Rep. 2018;18:60. doi: 10.1007/s11892-018-1017-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.