Abstract
Enwrapment by membrane cisternae has emerged recently as a mechanism of envelopment for large enveloped DNA viruses, such as herpesviruses, poxviruses, and African swine fever (ASF) virus. For both ASF virus and the poxviruses, wrapping is a multistage process initiated by the recruitment of capsid proteins onto membrane cisternae of the endoplasmic reticulum (ER) or associated ER-Golgi intermediate membrane compartments. Capsid assembly induces progressive bending of membrane cisternae into the characteristic shape of viral particles, and envelopment provides virions with two membranes in one step. We have used biochemical assays for ASF virus capsid recruitment, assembly, and envelopment to define the cellular processes important for the enwrapment of viruses by membrane cisternae. Capsid assembly on the ER membrane, and envelopment by ER cisternae, were inhibited when cells were depleted of ATP or depleted of calcium by incubation with A23187 and EDTA or the ER calcium ATPase inhibitor, thapsigargin. Electron microscopy analysis showed that cells depleted of calcium were unable to assemble icosahedral particles. Instead, assembly sites contained crescent-shaped and bulbous structures and, in rare cases, empty closed five-sided particles. Interestingly, recruitment of the capsid protein from the cytosol onto the ER membrane did not require ATP or an intact ER calcium store. The results show that following recruitment of the virus capsid protein onto the ER membrane, subsequent stages of capsid assembly and enwrapment are dependent on ATP and are regulated by the calcium gradients present across the ER membrane cisternae.
Most enveloped viruses acquire a single envelope from the host cell by budding into intracellular membrane compartments or by budding from the plasma membrane (9, 19, 36). A second pathway of envelopment, termed wrapping, has been described recently for the large enveloped DNA viruses, such as poxviruses (35), herpesviruses (18, 38), and African swine fever (ASF) virus (2, 30). These viruses are wrapped by membrane cisternae of the secretory pathway and gain two envelopes in one step. It appears, therefore, that enwrapment is emerging as a general mechanism for the envelopment of large DNA viruses. Even so, very little is known about the underlying biochemical and cell biological control of this process.
Thus far, the endoplasmic reticulum (ER), the closely associated intermediate compartment between the ER and the Golgi (ERGIC), and the trans-Golgi network have been identified as sites of virus wrapping within cells. The trans-Golgi network wraps herpesviruses (8, 17, 38, 42) and poxviruses (33, 38) at a relatively late stage of assembly, when viral particles have adopted the characteristic shape of the mature virion and have packaged genomes. Wrapping by the ER and possibly the ERGIC takes place early during morphogenesis of ASF virus and provides two inner-membrane envelopes (2, 30). The precise origin and number of the inner envelopes of vaccinia virus remain controversial. Membrane cisternae labeled with markers for the ERGIC are present in virus assembly sites. In some cases (33, 35), these cisternae are seen in continuity with the viral crescents which form during the earliest stages of morphogenesis, suggesting that wrapping by ERGIC cisternae provides the envelopes for the intracellular mature virion (IMV). Interestingly, high-resolution electron microscopy detects a single rather than double membrane envelope in IMVs (20). The mechanism of loss of the second membrane from the ERGIC cisternae, or possible involvement of other membrane systems during the acquisition of envelopes by the IMV, remains to be determined (20, 33, 35).
Interestingly, there is little evidence for the wrapping of preformed viral particles by ER or ERGIC membrane cisternae; rather, virus assembly takes place on the cytoplasmic face of the organelle and proceeds via a series of well-defined structural intermediates. For vaccinia virus, the membranes bend into crescents of uniform size and condensation of the nucleoprotein core of the virus on the inner face of the crescent produces spherical intermediates. The spherical intermediates then mature into IMVs with the brick shape characteristic of poxviruses. The assembly of ASF virus is initiated by the recruitment of the major capsid protein, p73, from the cytosol onto ER cisternae (12). After a lag period of 60 min, the membrane-bound capsid protein is assembled into a large oligomeric complex indicative of a viral capsid, with kinetics which closely match the envelopment of the virus by ER cisternae (12, 13). Early structural intermediates of ASF virus appear as angular forms with one to six sides (4, 5, 11, 25). It has been postulated that protein-protein interactions between the capsid protein and possibly other viral proteins targeted to the ER membrane cause ER cisternae to bend through an ordered series of one- to six-sided structural intermediates, eventually forming icosahedral particles (2, 30).
The identification of discrete structural intermediates for vaccinia virus and ASF virus implies that assembly and envelopment are carefully controlled. For both viruses, the acquisition of inner-membrane envelopes and assembly of core particles take place in specialized domains of the cell called viral factories. The factories coordinate the complex processes of particle assembly, genome packaging and envelopment, and finally export of wrapped particles into the cytoplasm. Even though these functions of viral factories are crucial for virus maturation, the mechanisms of recruitment of structural proteins and cellular membranes into virus assembly sites, and the subsequent processing of membrane cisternae into rigid symmetrical structures, remain largely unknown. Previous studies of the wrapping of virions by membrane cisternae have relied heavily on morphological analysis (2, 6, 30, 33, 35, 38). While these have given important insights into the shapes of assembly intermediates and the natures of the membrane compartments providing envelopes, they have not identified the host factors required for assembly. In this study we have developed biochemical assays for ASF virus assembly and envelopment and used them to define the cellular processes important for the wrapping of ASF virus by the ER. The wrapping of ASF virus was highly sensitive to reagents that depleted cells of ATP and was blocked following dissipation of the lumenal ER calcium store. The results suggest that ASF assembly is an energy-dependent process and that the calcium gradients that exist across the ER membrane may be important regulators of the wrapping of ASF virus by ER cisternae.
MATERIALS AND METHODS
Reagents, cells, viruses, and antibodies.
A23187 and thapsigargin were purchased from Calbiochem-Novabiochem (Beeston, United Kingdom). Brij 35, 2-deoxy-d-glucose, hen egg white trypsin inhibitor, and trypsin were purchased from Sigma (Poole, United Kingdom). Vero cells (ECACC 84113001) were grown at 37°C in HEPES-buffered Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, l-glutamine (20 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The tissue culture-adapted BA71v isolate of ASF virus has been described previously (11). The monoclonal antibody 4H3, specific for p73, was characterized by Cobbold et al. (12).
Metabolic labeling and immunoprecipitation.
Metabolic labeling and immunoprecipitation were carried out as described previously (12). Briefly, infected cells were starved in methionine- and cysteine-free Eagle's medium for at least 10 min and then labeled by incubation with 0.75 MBq of 35S-Express (New England Nuclear, Boston, Mass.) per ml in the same medium. The cells were chased by replacing the labeling medium with normal culture medium supplemented with methionine and cysteine. At appropriate times after incubation at 37°C, the cells were washed and lysed on ice in immunoprecipitation buffer (10 mM Tris [pH 7.8]; 150 mM NaCl; 10 mM iodoacetamide; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 μg each of leupeptin, chymostatin, and antipain [Boehringer Mannheim] per ml) containing 1% Brij 35. Antigens were immunoprecipitated with antibodies immobilized on protein-G Sepharose. Proteins were resolved by sodium dodecyl sulfate (SDS) polyacrylamide gels and detected by autoradiography. Protein bands were quantitated using a Bio-Rad 620 video densitometer or Image Tool software.
Protease protection assay for viral envelopment.
The details of the protease protection assay have been described previously (12, 13). Briefly, Vero cells infected with ASF virus were pulse-labeled for 15 min at 37°C and chased for increasing time intervals. The cells were then homogenized in sucrose (0.25 M sucrose, 50 mM Tris, 1 mM EDTA [pH 7.4]) by 15 passages through a 25-gauge syringe needle. Whole cells and nuclei were removed by pelleting at 2,000 rpm for 10 min at 4°C in an Eppendorf 5402 centrifuge. Crude postnuclear membrane fractions were prepared by pelleting them at 14,000 rpm for 20 min at 4°C in an Eppendorf 5402 centrifuge. The membrane fractions were incubated with or without trypsin (0.4 mg/ml) in HEPES-acetate buffer (50 mM potassium acetate, 2.5 mM magnesium acetate, 25 mM HEPES [pH 7.2], 20 mM CaCl2), pH 7.2, for 30 min at 37°C. Proteolysis was stopped by the addition of a 10-mg/ml hen egg white trypsin inhibitor. Reaction mixtures were diluted with 3 volumes of immunoprecipitation buffer containing 5 mM phenylmethylsulfonyl fluoride, 3% fetal calf serum, and 10 mg of hen egg white trypsin inhibitor/ml, and the levels of p73 remaining were determined by immunoprecipitation and SDS-polyacrylamide gel electrophoresis (PAGE) as described above.
Sucrose density sedimentation analysis.
All gradients contained sucrose dissolved in 1% Brij 35 in immunoprecipitation buffer. Step gradients contained 2 ml of 10, 20, 30, 35, and 40% sucrose layered over a 70% cushion and were left overnight at 4°C to equilibrate. Postnuclear membrane fractions were dissolved in 2 ml of immunoprecipitation buffer containing 1% Brij 35 and layered over the gradient. After centrifugation at 40,000 rpm for 20 h at 4°C in a Beckman SW40 rotor, the gradients were separated into 1.2-ml fractions. The migration of p73 was monitored by immunoprecipitation followed by SDS-PAGE and autoradiography. The gradients were calibrated by following the migration of bovine serum albumin (66 kDa) and beta-amylase (200 kDa).
Electron microscopy.
Ultrathin resin sections were prepared and processed as described by Rouiller et al. (30). Briefly, infected cells growing in tissue culture flasks were fixed with 2.5% glutaraldehyde in 100 mM cacodylate buffer (pH 7.2) and harvested by scraping. Samples were intensified by incubation in 1% osmium tetroxide in 100 mM cacodylate and then progressively dehydrated in ethanol prior to being embedded in Spurr resin (Agar Scientific Ltd., Stanstead, United Kingdom). Sections were cut using a Reichert (Vienna, Austria) OmU3 microtome with glass knives and were stained in uranyl acetate and Reynold's lead citrate. The sections were examined using a Jeol 1200 EX electron microscope.
RESULTS
A protease protection assay and sucrose gradient sedimentation can be used to follow the kinetics of virus assembly and envelopment.
We have described the use of a protease protection assay to follow the kinetics of envelopment of the major capsid protein of ASF virus, p73, by the ER membrane (12, 13). During the assay, trypsin is added to membrane fractions isolated from infected cells. The nonenveloped capsid protein is accessible to the protease and is degraded, while the enveloped capsid is protected from trypsin and survives for subsequent immunoprecipitation and quantification by SDS-PAGE. By using a pulse-chase metabolic-labeling protocol we have demonstrated that p73 binds the ER membrane approximately 15 min after synthesis in the cytosol. Envelopment of p73 starts 1 h later and is completed during the next 60 to 90 min. During this period, p73 is incorporated into a large oligomeric complex, which migrates in the bottom fractions of a 10 to 40% sucrose gradient, indicative of assembly into a viral capsid. In the experiments described below we used the protease protection assay and sucrose density centrifugation to determine the cellular factors that are required for the wrapping of ASF virus by ER cisternae and the assembly of p73 into the viral capsid.
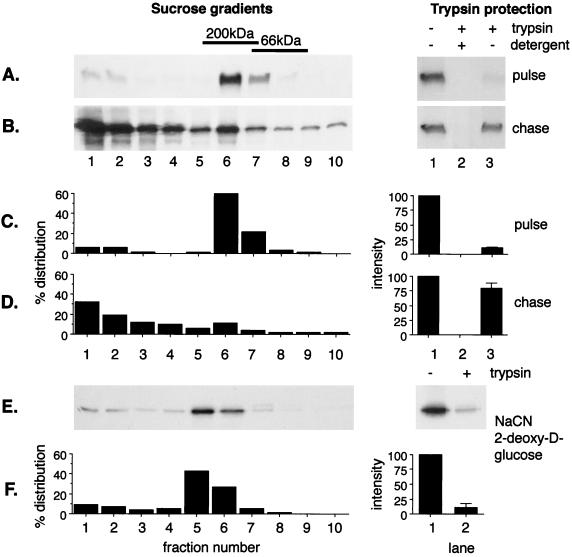
The results of this type of analysis are shown in Fig. 1A and B. Vero cells infected with ASF virus were pulse-labeled for 15 min and either placed on ice or chased for 2 h in complete medium. Postnuclear membrane fractions were divided into two aliquots; one was incubated with trypsin to test for envelopment, while the other was solubilized in Brij 35 and centrifuged on a 10 to 40% sucrose gradient to test for capsid assembly. The autoradiographs on the right in Fig. 1A and B show the results of the trypsin protection assay. A comparison of lanes 1 and 3 shows that in pulse-labeled cells very little membrane-associated p73 survived the trypsin incubation, suggesting that the protein was not enveloped at this time point. The autoradiographs on the left in Fig. 1A show the distribution of pulse-labeled p73 across the sucrose gradient. The p73 within the postnuclear membrane fraction, and therefore membrane-associated before solubilization, migrated in the central fractions of the gradient, indicating a mass of 150 to 200 kDa. This is in agreement with our previous work showing that p73 forms a dimer and/or trimer immediately after synthesis (13). The experiment was repeated for cells chased for 2 h. A comparison of lanes 1 and 3 of the trypsin protection assay presented in Fig. 1B shows that the majority of the membrane-associated p73 was now resistant to trypsin, indicating envelopment by the ER membrane. When the size of solubilized membrane-associated p73 was analyzed by sucrose density sedimentation, the bulk of the protein migrated at the bottom of the sucrose gradient, indicating incorporation into a large oligomer indicative of assembly into the viral capsid and/or matrix. We have shown previously that the fast-migrating material is recovered from the postnuclear membrane fraction because it is membrane associated (13). The complex does not pellet under the conditions used to prepare a membrane pellet if the membranes are first solubilized in mild detergent. The fast-migrating material was examined by electron microscopy for structures resembling virus or assembly intermediates. The pellets contained amorphous material, but no convincing viral structures were observed. We have shown previously that the largest oligomeric complex formed in cells migrates at approximately 50,000 kDa on sucrose gradients following solubilization, and structures of similar size are present in virions secreted from cells (13). The complex is, however, only 1/10 the size of a fully assembled virion, suggesting that the virus is partially disrupted by detergent lysis and centrifugation. This partial disruption, and the inability to predict the effects of homogenization and centrifugation on the morphology of assembly intermediates, may explain why assembly intermediates were difficult to detect among large quantities of host protein sedimenting in the same fractions. Importantly, a comparison of Fig. 1B, lanes 2 and 3, of the trypsin protection assay showed that the trypsin-resistant p73 seen bound to intact membranes (lane 3) was not observed if the membranes were solubilized in 1% Triton X-100 prior to the addition of trypsin (lane 2). This control indicated that protection from trypsin resulted from envelopment by a membrane and not from steric hindrance of proteolysis caused by the assembly of p73 into a large oligomer.
FIG. 1.
Assembly and envelopment of ASF virus require ATP. Sixteen hours after infection with the BA71v strain of ASF virus, Vero cells were pulse-labeled with [35S]methionine and cysteine for 15 min at 37°C and then placed on ice (A) or chased for 2 h in complete medium (B). The cells were homogenized, and postnuclear membrane fractions were split into two aliquots. One was solubilized in 1% Brij 35 and centrifuged on a 10 to 40% sucrose gradient (left); the other was incubated with trypsin and 1% Triton X-100 as indicated (right). The levels of p73 present were estimated by immunoprecipitation followed by SDS-PAGE under reducing conditions. The migration of the molecular mass markers bovine serum albumin (66 kDa) and beta-amylase (200 kDa) on the sucrose gradient are indicated. (E) Cells were chased for 2 h in the presence of 1 mM NaCN and 50 mM 2-deoxy-d-glucose to deplete ATP. Membrane fractions were prepared as described above, but incubation with detergent during the trypsin protection assay was omitted. (C, D, and F) Relative protein levels calculated from densitometric analysis of autoradiographs. For the trypsin protection assays, the averages of at least four estimations are shown with standard errors. +, present; −, absent.
A semiquantitative assessment of capsid assembly and envelopment was made by densitometric analysis of autoradiographs (Fig. 1C and D). The extent of capsid assembly was determined by calculating the percentage distribution of p73 across the sucrose gradients and determining the proportion that moved from fractions 6 and 7 in the center of the gradient to fractions 1 to 3 at the bottom of the gradient during the 2-h chase. The results showed that on average for control cells, 60 to 65% of the membrane-bound pool of p73 was incorporated into a large complex during the chase. The degree of envelopment during this period was calculated as the proportion of the membrane-bound pool of p73 that became protected from trypsin. For control cells, this varied between 70 and 80%.
Envelopment and assembly of ASF virus requires ATP.
The above-mentioned assays allowed us to test whether cellular ATP was required during the wrapping of ASF virus. The effects of depleting cellular ATP are shown in Fig. 1E and F. Cells were pulse-labeled and chased in the presence of 2-deoxy-d-glucose and sodium cyanide to block oxidative phosphorylation. Analysis of ATP using a coupled luciferase-luciferin assay showed that 2-deoxy-d-glucose and sodium cyanide reduced cellular ATP levels to 5% of control within 5 min (data not shown). Comparison of the trypsin protection assays performed on control cells (Fig. 1B) or cells depleted of ATP (Fig. 1E) showed that ATP depletion markedly reduced the quantity of p73 protected from trypsin at the end of a 2-h chase. A densitometric analysis of autoradiographs (Fig. 1F) showed that the percentage of the membrane-bound pool of p73 enveloped during the chase was reduced from 75 to 15% following ATP depletion. Sucrose density sedimentation was used to test for the effects of ATP depletion on oligomerization of p73. Unlike the control experiment (Fig. 1B and D), in which 60% of the membrane-bound p73 formed a large oligomer during the chase, only 20% moved to fractions 1 to 3 of the sucrose gradient after ATP depletion (Fig. 1E and F).
Assembly and envelopment require an intact ER Ca2+ store.
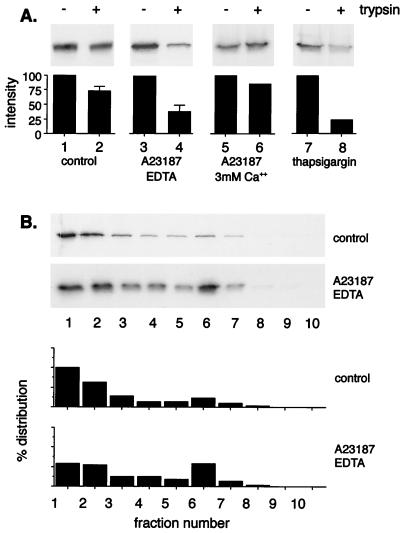
Most of the calcium in cells is sequestered in the lumen of the ER and is subject to release in response to many agents, including inositol phosphates, calcium ionophores, and arachidonate-related fatty acids. The release of ER calcium increases intracellular calcium concentrations and stimulates Ca2+-dependent enzymes, such as phosphatases and kinases, that can modulate many regulatory proteins in the cytosol. We (30) and others (2) have provided evidence that the capsid of ASF virus is assembled on the cytosolic face of the ER during the wrapping of virions by ER cisternae. During this process, the lumen of the ER is in continuity with the membranes that form the two inner envelopes of ASF virus, while the major structural proteins are exposed to the cytosol. The next experiments were designed to test whether changes in the Ca2+ store maintained within the lumen of the ER would affect the wrapping of ASF virus by the ER cisternae. To do this, monkey kidney Vero cells infected with ASF virus were pulse-labeled for 15 min and depleted of cellular calcium by incubation for a further 2 h in calcium-free medium containing the calcium ionophore A23187 and EDTA. A23187 was used at 1 μM, a concentration shown to inhibit rotavirus assembly in the ER of monkey kidney cells (28). Figure 2A shows that in untreated cells greater than 70% of the membrane-bound pool of p73 became resistant to trypsin during the chase (lanes 1 and 2), indicating envelopment by the ER. The levels protected from trypsin were reduced to 35% when the cells were depleted of Ca2+ with A23187 and EDTA (lanes 3 and 4). Figure 2B shows that depletion of cellular calcium during the chase also reduced the recovery of oligomeric complexes from the bottom of the sucrose gradient; the levels assembled at the end of the chase were decreased from the 60 to 70% routinely seen for control cells to between 30 and 40% after depletion of ER calcium stores.
FIG. 2.
Assembly and envelopment of ASF virus require an intact ER Ca2+ store. Sixteen hours after infection with the BA71v strain of ASF virus, Vero cells were pulse-labeled with [35S]methionine and cysteine for 15 min at 37°C and chased for 2 h in complete medium, in the presence of A23187 (1 μM), EDTA (3 mM), or thapsigargin (500 nM) as indicated. (A) The degree of envelopment of p73 was determined by the trypsin protection assay followed by immunoprecipitation and autoradiography. The bar graphs show relative protein levels calculated by densitometry. (B) The levels of membrane-bound p73 assembled into oligomers after incubation of cells alone or with A23187 (1 μM) and EDTA (3 mM) were determined by sucrose density sedimentation. The levels of p73 present were estimated by immunoprecipitation followed by SDS-PAGE under reducing conditions. The percentage distribution of p73 across the gradients was estimated by densitometry of autoradiographs. For the trypsin protection assays in the presence or absence of A23187, the averages of at least four estimations are shown with standard errors. The other experiments were performed twice, and the averages are shown.
The incubation of cells with A23187 alone leads to a loss of calcium from the ER lumen and a transient rise in cytosolic calcium concentrations. To test the effects of raising cytosolic calcium levels directly, the experiment was repeated but the cells were incubated with A23187 in medium supplemented with 3 mM calcium during the chase. The trypsin protection assays presented in Fig. 2A (lanes 5 and 6) showed that under these conditions the quantity of p73 protected from trypsin was similar to those observed in the control experiment (lanes 1 and 2). Raised cytosolic calcium levels were not, therefore, able to inhibit ASF envelopment directly. Thus, it was likely that the inhibition of wrapping and virus assembly seen in the presence of A23187 and EDTA was caused by depletion of the ER Ca2+ store. The ER Ca2+ store is maintained by a calcium ATPase that spans the ER membrane. The incubation of cells with thapsigargin, a drug that specifically inhibits the ER calcium ATPase (37), provided a further means of testing the role played by ER calcium during the wrapping of ASF virus. Thapsigargin was used at 500 nM, a nontoxic concentration which perturbs ER protein degradation in CHO cells (40) and is four times the reported 50% effective dose for inhibition of the ER Ca2+ ATPase of hepatocytes (37). A comparison of Fig. 2A, lanes 7 and 8 shows that incubation of cells with thapsigargin reduced the levels of p73 protected from trypsin during the chase from 73 to 38%. The results again suggested that an intact ER calcium store was required for the wrapping of ASF virus by the ER cisternae.
Reversal of ATP and ER Ca2+ depletion allows partial recovery of assembly and envelopment.
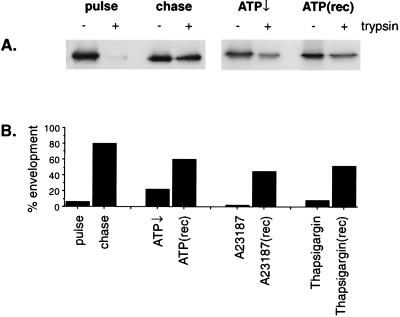
It was important to establish that the ATP depletion cocktail and the reagents used to deplete cellular calcium were having specific effects on ASF assembly and envelopment rather than being generally toxic to cells. Trypan blue exclusion was used as a preliminary screen for toxicity and, at the concentrations of drugs used, the cells remained impermeable to the dye. For cells depleted of calcium, ATP was measured using a luciferase-luciferin coupled assay, and little effect on ATP was observed. In the next experiments, the ability of the virus assembly pathway to recover after removal of the drugs was tested. Cells infected with ASF virus were pulse-labeled and then chased for 2 h in the presence of inhibitor. Half the cells were placed on ice, and the other half were washed and incubated for a further 2 h in cell culture medium. The inhibition and recovery of envelopment were assessed using the trypsin protection assay. Fig. 3A shows that depletion of ATP caused a marked reduction in the levels of capsid protein protected from trypsin after a 2-h chase. When the cells were washed and allowed to recover for 2 h, trypsin-resistant p73 was again recovered from the membrane fraction. A densitometric analysis of autoradiographs (Fig. 3B) indicated that the percentage of the membrane-bound pool of p73 enveloped after 2 h fell from 80 to 22% after depletion of ATP but rose to 60% during recovery. Figure 3B also shows analysis of similar experiments following recovery after removal of thapsigargin or A23187. Again, substantial levels of capsid were enveloped. On the basis of these results, it was concluded that the inhibitors were acting specifically on ASF virus envelopment and were not generally toxic to cells.
FIG. 3.
Recovery of envelopment following depletion of ATP or calcium. Sixteen hours after infection with the BA71v strain of ASF virus, Vero cells were pulse-labeled with [35S]methionine and cysteine for 15 min at 37°C and chased for 2 h in complete medium in the presence of 1 mM NaCN and 50 mM 2-deoxy-d-glucose to deplete ATP↓, or depleted of Ca2+ using A23187 (1 μM) and EDTA (3 mM) or thapsigargin (500 nM). Half of the cells were placed on ice, while the remainder were washed and incubated in complete culture medium for a further 2 hours at 37°C [ATP(rec)]. Membrane fractions prepared from the cells were incubated with trypsin to test for capsid envelopment as described in the legend to Fig. 1. (A) The levels of p73 remaining were determined by immunoprecipitation and SDS-PAGE. (B) The levels of p73 protected from trypsin were determined by densitometry of autoradiographs and are expressed as percentages of the membrane-bound pool of p73 that is protected from trypsin.
The assembly of angular structural intermediates is disrupted when cells are depleted of Ca2+.
The experiments described above showed that the levels of p73 protected from trypsin, and the assembly of p73 into oligomeric structures, were inhibited when the cells were incubated with A23187 and EDTA or thapsigargin. If this were the case, then it seemed likely that Ca2+ depletion would alter the morphology of virions and structural intermediates formed at virus assembly sites. Many electron microscopy studies have shown that virus assembly sites contain fully assembled virions appearing as hexagons and an ordered series of one- to six-sided structural intermediates (2, 6, 11, 25, 30). In the next experiment, the effects of calcium depletion on the morphologies of viral particles was examined by electron microscopy. Virus factories are first seen in cells between 8 and 10 h postinfection and increase in size and complexity for a further 10 to 12 h. For morphological studies, it was important that there be very few preexisting virus structures at the start of the study. The cells were therefore incubated with inhibitors at the start of factory morphogenesis at 10 h and fixed for electron microscopy 2 h later.
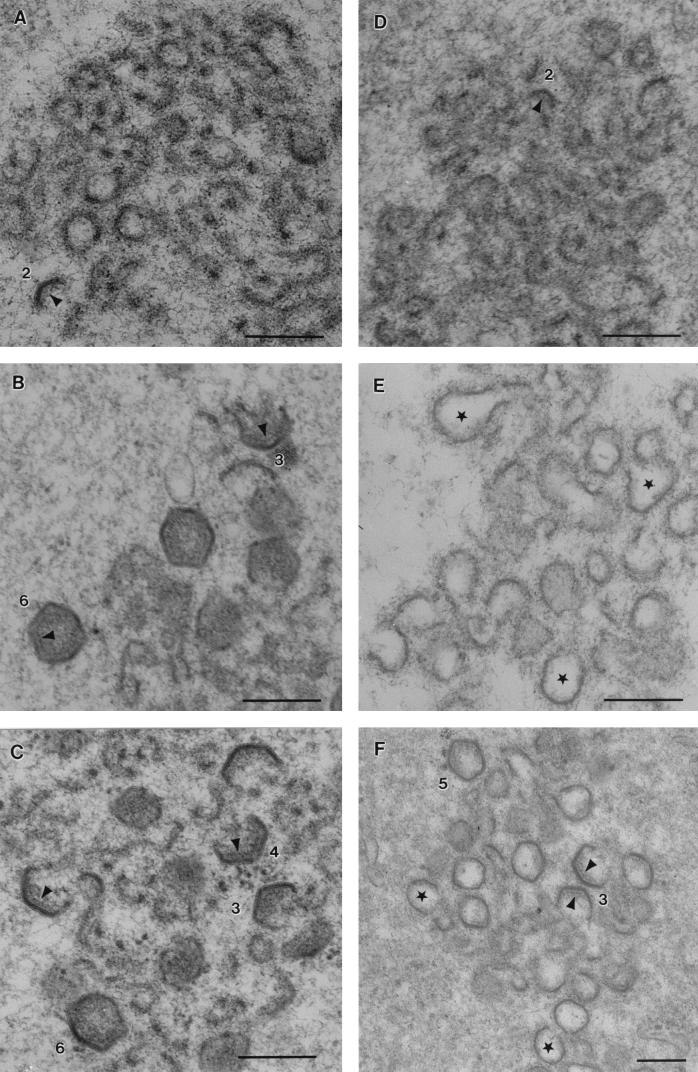
Figure 4A and B shows early stages of virus assembly observed in the control cells. Viral assembly sites examined 10 h postinfection (Fig. 4A) contained membranous material but very few virions. Angular structures representing virions at different stages of assembly were, however, visible at 12 h (Fig. 4B). Several viral factories were examined at each time point and scored according to the numbers of one- to six-sided structures present (Table 1). At 10 h very few ordered structures were detected. At 12 h postinfection, the bulk of the particles were two or three sided, but the factories also contained considerable numbers of five- and six-sided structures. Importantly, the only closed structures observed at assembly sites at this time point were hexagons. Electron-dense material was visible on the concave faces of the more complex particles, suggestive of the assembly of a matrix or core shell, as has been reported previously (2, 30).
FIG. 4.
Effect of depletion of cellular Ca2+ on the morphology of ASF virus. Ten hours after infection with the BA71v strain of ASF virus, Vero cells were fixed or were incubated for 2 h either under control conditions or depleted of Ca2+ and then fixed and processed for electron microscopy. The micrographs show sections taken through virus assembly sites (bar = 200 nm). (A) Control cells fixed at 10 h. Viral factories contain membranous material but few angular structures. (B) Control cells fixed 12 h after infection. Open geometric viral assembly intermediates and sealed hexagons are indicated numerically. (C) Cells were fixed after incubation in calcium-free medium containing EDTA (3 mM) for 2 h. Three-, four-, and six-sided structures are indicated. (D) Cells were fixed 2 h after incubation with thapsigargin (500 μM). Viral factories contain membranous material but few angular structures. (E and F) Cells were fixed 2 h after incubation in calcium-free medium containing A23187 (1 μM) and EDTA (3 mM). The factories contained crescent-shaped and bulbous structures. The centers of many bulbous particles lacked electron density (∗). (F) In rare cases, bulbous structures attached to three angular faces were detected, as were closed five-sided structures (e.g., those labeled 3 and 5). In some cases protein coats were visible on the concave sides of angular particles (arrowheads).
TABLE 1.
Effect of calcium depletion on morphogenesis of ASF virusa
| No. of sides | No. of particles
|
||||
|---|---|---|---|---|---|
| Control
|
EDTA 12 h | Thapsigargin 12 h | EDTA and A23187 12 h | ||
| 10 h | 12 h | ||||
| 1 | 0 | 33 | 28 | 10 | 0 |
| 2 | 0 | 36 | 15 | 3 | 0 |
| 3 | 0 | 14 | 14 | 3 | 0 |
| 4 | 0 | 8 | 2 | 2 | 0 |
| 5 | 0 | 13 | 4 | 6 | 0 |
| 6 | 0 | 16 | 3 | 3 | 0 |
| Total | 0 | 120 | 66 | 27 | 0 |
| % Control | 0 | 100 | 55 | 23 | 0 |
Cells were infected with ASF virus and processed for electron microscopy as described in the legend to Fig. 4. For each set of conditions, 20 viral-replication areas were identified, and the particles with one to six sides were counted. For cells treated with A23187 and EDTA, it was not possible to distinguish angular forms and factories; therefore, they are scored zero.
Figure 4C shows a section taken through a factory assembled within a cell incubated in calcium-free medium and EDTA 10 h after infection and fixed 2 h later. As observed for the control cells (Fig. 4B), the viral factory contained angular assembly intermediates and six-sided virions. The quantitation presented in Table 1 showed that the number of angular structures present was reduced by approximately one-half overall. Since a greater proportion of the structures were one to three sided rather than more complex, it appeared that incubation of cells in calcium-free medium slowed morphogenesis. Importantly, however, calcium-free conditions did not cause gross changes in the structures of the virions formed. When cells were incubated with thapsigargin for 2 h, the numbers of angular viral structures in viral factories were greatly reduced (Fig. 4D) and virus assembly sites appeared very similar to those seen in control cells fixed 10 h postinfection (Fig. 4A). The quantitation presented in Table 1 showed that thapsigargin reduced the number of angular intermediates to 20% of control levels. Thapsigargin, therefore, had a greater effect than incubating cells in calcium-free medium alone; however, the cells were still able to assemble angular structures, albeit at low levels. Fig. 4E and F shows cells incubated with calcium ionophore and EDTA. The viral factory contained membranous material, but the structures were different from those seen after incubation with thapsigargin. Most striking was the presence of crescent-shaped or bulbous particles and an absence of complete six-sided structures. In most factories (Fig. 4E and Table 1), virions lacked all angular structure. Interestingly, in rare instances, closed particles with five angular sides were seen and other particles retained one or two angular faces, but the remaining sides were crescent shaped (Fig. 4F). The residual angularity seen in these particles provided strong evidence that the clusters of crescent-shaped particles shown in Fig. 4E represent perturbed virion assembly sites rather than areas of membrane proliferation caused by the calcium ionophore. Interestingly, the centers of the aberrant particles were noticeably lacking in electron-dense material, suggesting an absence of protein. For particles with residual angularity (Fig. 4F), the remnants of the electron-dense matrix or core shell were seen attached to the concave sides of angular faces but were absent from the crescent-shaped or bulbous sides of the virions that lacked structure.
Requirement for ATP and ER Ca2+ for binding of p73 to the ER membrane.
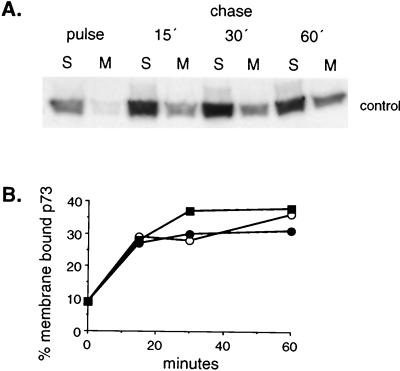
One of the first steps in the assembly of ASF virus is the recruitment of approximately 40% of the newly synthesized pool of p73 from the cytosol onto the cytosolic face of ER cisternae (12). The next experiments tested whether any of the inhibitors of viral wrapping described above acted by preventing the binding of p73 to the ER membrane. Cells infected with ASF virus were pulse labeled for 5 min and then chased for increasing times in the presence or absence of inhibitor. Crude postnuclear membrane and cytosol fractions were prepared at each time point, and the levels of p73 present were determined by lysis, immunoprecipitation, and autoradiography. We have shown previously that p73 recovered in the postnuclear membrane fraction fails to pellet if the membranes are first lysed in mild detergent (13). The protein therefore pellets because it is membrane associated and not as a result of oligomerization. Figure 5A shows the results obtained from control cells. In pulse-labeled cells, the bulk of the capsid protein was recovered from the cytosolic fraction. A rapid increase in the level of p73 detected in the membrane fraction occurred during the first 15 min of the chase and reached a maximum at 30 min. Densitometric analysis of autoradiographs (Fig. 5B) showed that between 30 and 40% of the newly synthesized pool of p73 was recovered from the membrane fraction at 30 min, and this level was maintained at 60 min. The experiment was repeated with cells incubated with inhibitors, and the graph presented in Fig. 5B shows that recruitment of p73 into the membrane fraction was largely unaffected when the cells were depleted of ATP or ER calcium. Taken together, the results showed that the inhibition of assembly observed in cells depleted of ATP or ER Ca2+ occurred at a stage after recruitment of the capsid onto the ER membrane. ATP and ER Ca2+ were required for oligomerization and envelopment rather than membrane binding.
FIG. 5.
Biochemical requirements for recruitment of p73 onto the ER membrane. Sixteen hours after infection with the BA71v strain of ASF virus, Vero cells were pulse-labeled with [35S]methionine and cysteine for 5 min at 37°C and then chased for increasing times under the conditions described in the legends to Fig. 3 and 4. Cells taken at each time point were homogenized, and postnuclear-membrane and cytosolic fractions were lysed in immunoprecipitation buffer. The levels of p73 present were estimated by immunoprecipitation followed by SDS-PAGE and autoradiography. (A) The distribution of p73 between membrane (M) and soluble (S) fractions was determined for control cells by immunoprecipitation followed by SDS-PAGE and autoradiography. (B) The levels of p73 present in the membrane fraction were estimated by densitometry, and the percentage of the total p73 recovered at each time point is presented for control cells (■) and cells incubated with 50 mM 2-deoxy-d-glucose and 1 mM sodium cyanide (○) or 1 μM A23187 and 3 mM EDTA (●).
Wrapped intracellular ASF virions are stable when depleted of ATP or Ca2+.
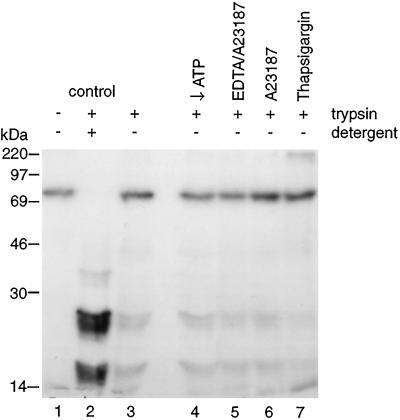
The above-mentioned experiments demonstrated that the envelopment and assembly of ASF virus required ATP and ER Ca2+ stores. Experiments were designed to investigate whether envelopment was a reversible process. Infected cells were pulse-labeled and chased for 2 h to allow recruitment of p73 onto the ER and subsequent oligomerization and envelopment. The cells were then homogenized, and crude postnuclear membrane fractions were incubated with either a mixture of hexokinase and glucose to deplete ATP or combinations of A23187, EDTA, or thapsigargin to deplete Ca2+. Trypsin was then added to test for loss of membrane envelopes. Figure 6 shows that for membranes incubated under control conditions the capsid protein was protected from trypsin unless mild detergent was added (lanes 1 to 3), as indicated by the tryptic fragments migrating between 14 and 30 kDa in lane 3. The rest of the lanes show that there was no increase in the sensitivity of p73 to trypsin under any of the conditions tested. Wrapped particles were therefore not destabilized when depleted of ATP or Ca2+.
FIG. 6.
Intracellular ASF virions are stable when depleted of ATP or Ca2+. Sixteen hours after infection with the BA71v strain of ASF virus, Vero cells were pulse-labeled with [35S]methionine and cysteine for 15 min at 37°C and then chased for 2 h. The cells were homogenized, and postnuclear membranes were prepared. These were incubated under control conditions or in the presence of hexokinase (50 U/ml) and glucose (5 mM) to deplete ATP (↓ATP), A23187 (1 μM) with and without 3 mM EDTA, or thapsigargin (500 nM) as indicated. Trypsin was added to membrane fractions to test for envelopment as described in the legend to Fig. 1. The levels of p73 remaining were assessed by immunoprecipitation and SDS-PAGE.
DISCUSSION
The aim of this study has been to identify host cell processes involved in the wrapping of ASF virus by the ER. The experiments depleting cells of ATP using sodium cyanide and 2-deoxy-d-glucose showed that the wrapping of ASF virus required ATP. Interestingly, ATP was not required for the initial recruitment of p73 from the cytosol onto the ER membrane but was required for the subsequent assembly of the capsid and the simultaneous envelopment of the virus by ER cisternae. Importantly, these studies demonstrated that the assembly of ASF virus was an active process and not simply driven by an accumulation of viral components in the cytosol. ASF virus differs in this respect from several icosahedral viruses which will assemble spontaneously in vitro in the absence of cellular components (43; reviewed in reference 22). Recent studies of type D retroviruses (39) and human immunodeficiency virus (23, 31) have shown that the assembly of viral procapsids in the cytosol and their transport to the plasma membrane for envelopment both require ATP. For these retroviruses, as well as hepadnaviruses and duck hepatitis B virus (21), it has been postulated that the ATP is hydrolyzed by cellular chaperones during the assembly of the procapsids. While it is possible that the assembly and envelopment of the ASF virus capsid may also require cellular chaperones, the virus itself encodes several proteins with nucleotide binding motifs (41) that may utilize ATP during morphogenesis. Herpes simplex virus capsids assemble spontaneously in vitro in the absence of ATP (26); interestingly, recent studies (14) show that the final maturation of the viral capsid requires ATP in vivo. This may again reflect a role played by cellular chaperones in herpesvirus assembly in vivo or indicate that capsid maturation has to be coordinated with DNA packaging, a process which is itself ATP dependent. The energy requirements of vaccinia virus assembly have also been probed recently (15). ATP is required early in the assembly of vaccinia virus particles, during the maturation of viral crescents into spherical immature virions.
Capsid assembly and envelopment were both slowed when cells were incubated with A23187 and EDTA or the ER calcium pump inhibitor, thapsigargin. The results suggested that wrapping of ASF virus required an intact ER calcium store. The block in assembly may also have been caused by the transient rise in cytosolic Ca2+ that occurs as Ca2+ leaves the ER in response to these drugs. This is unlikely because direct introduction of Ca2+ into the cytosol using A23187 and 3 mM Ca2+ had little effect on ASF virus envelopment. Interestingly, the rate and extent of binding of p73 to the ER was unaffected when A23187 and EDTA were added to cell cultures. The ER Ca2+ store was not, therefore, needed for the recruitment of the capsid protein onto the ER membrane but, as seen for ATP, was required for the subsequent assembly of p73 into the capsid complex. These results were consistent with the electron microscopy study showing that the calcium ionophore had a profound effect on virus morphology. Incubation with A23187 reduced the number of ordered one- to six-sided assembly intermediates present in assembly sites and prevented the appearance of mature virions. Instead, assembly sites contained many crescent-shaped and bulbous structures and, in rare instances, closed five-sided particles and bulbous structures with residual angularity. These images are consistent with the observation that calcium depletion prevented oligomerization of p73 on the ER membrane, and they support recent models predicting that the production of angular assembly intermediates is driven by the assembly of the capsid on ER cisternae (2, 13, 16, 30). Surprisingly, thapsigargin had a different effect on virus morphology. The drug markedly reduced the number of assembly intermediates but did not cause gross distortion of the few particles that were able to form. The precise reason for this difference is unknown but may reflect the different pharmacological properties of the two drugs. A23187 is a calcium ionophore, while thapsigargin inhibits the calcium ATPase that maintains the ER calcium store. The release of Ca2+ from microsomes by thapsigargin is slower than the release induced by inositol trisphosphate or hormonal stimulation of cells. Similarly, thapsigargin may be slower acting than the combination of A23187 and EDTA. Incubation of cells in calcium-free medium also reduced the number of virions seen at assembly sites, but the effect was much less than that seen for thapsigargin. The precise mechanism is unknown, but it is possible that the 2-h incubation conditions ultimately lead to a loss of Ca2+ from cells and consequent depletion of the ER Ca2+ store.
The lumen of the ER contains a concentrated matrix of chaperones and calcium binding proteins, most notably calreticulin, immunoglobulin binding protein (BiP), protein disulfide isomerase, calnexin, and glucose-regulated protein (27, 34). Calcium ions held within the matrix are thought to be important for the chaperone functions of the ER that catalyze the folding of newly synthesized proteins after delivery into the secretory pathway (24, 32). Depletion of ER Ca2+ may, therefore, block ASF virus assembly by perturbing the folding and/or assembly of viral proteins targeted to the ER to coordinate capsid assembly. A direct effect of calcium depletion on the folding of p73 seems unlikely. Under normal conditions, p73 folds in the cytosol, which maintains a very low level of free Ca2+; furthermore, A23187 and EDTA had little effect on the recruitment of p73 onto the ER membrane. Such results would not be expected if depletion of calcium leads to substantial misfolding of the capsid protein. Assembly of p73 capsids takes place on the cytosolic face of the ER. The sensitivity of capsid assembly to loss of the ER Ca2+ store raises the interesting possibility that the capsid protein can in some way sense the Ca2+ content of the ER lumen. This would require interaction with proteins with a transmembrane topology able to sense both the ER lumen and the cytosol. ASF virus encodes some 26 proteins with membrane-targeting sequences; of these, there are three predicted to be type 1 membrane proteins and eight with type 2 membrane topology (41). Few of these proteins have been studied in detail; however, the E183L (29), H108R (7), D117L (30), and O61R (3) gene products have been shown to localize to viral factories and may communicate with p73 during capsid recruitment and virus assembly. Interestingly, members of the ASF virus 110 multigene family contain sequences that target them to the lumen of the ER (1, 41), and one member, pXP124L, is found in viral factories and virions (30). Significantly, all members of the family share cysteine repeat domains that may bind divalent cations (1), and given their localization in the lumen of the ER, these proteins may be involved in sensing the lumenal ER calcium store during ASF virus capsid assembly.
Mechanistically, the assembly of the ASF virus capsid on the cytoplasmic face of the ER during the recruitment of the membrane, and the dependence of this process on ATP, resembles the assembly of coatamer or clathrin coats during the budding of transport vesicles. For transport vesicles, coat assembly is reversible and is regulated by cycles of GTP and ATP hydrolysis. With this in mind, we tested whether the assembly and envelopment of ASF virions could also be reversed. The results showed that once formed, enveloped particles could not be destabilized by the depletion of nucleotides or Ca2+. This may reflect the life cycle of the virion. Following assembly in viral factories, individual virions enter the cytosol, which maintains low levels of free Ca2+. At this point, the connection between the virion and the ER lumen would be severed and the integrity of the virus would be expected to be independent of the Ca2+ concentration. Moreover, once released from cells, virions enter unpredictable environments, possibly low in nucleotides, before initiating further rounds of infection.
In summary, we have used novel assay systems to study the biochemistry of ASF virus assembly and envelopment by the ER. The results show a strong correlation between capsid assembly and virus envelopment, suggesting that they are linked processes. Such observations are consistent with a model in which capsid assembly on the ER membrane proceeds through the ordered series of angular intermediates seen within virus assembly sites, eventually forming icosahedral particles. Our present data take this model further and show that both the assembly of the capsid into angular structures and the subsequent envelopment of the virion require ATP and are dependent on the ER Ca2+ store.
REFERENCES
- 1.Almendral J M, Almazan F, Blasco R, Vinuela E. Multigene families in African swine fever virus: family 110. J Virol. 1990;64:2064–2072. doi: 10.1128/jvi.64.5.2064-2072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres G, Garcia-Escudero R, Simon-Mateo C, Vinuela E. African swine fever virus is enveloped by a two-membraned collapsed cisterna derived from the endoplasmic reticulum. J Virol. 1998;72:8988–9001. doi: 10.1128/jvi.72.11.8988-9001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo A, Vinuela E, Alcami A. Inhibition of African swine fever virus binding and infectivity by recombinant virus attachment protein p12. J Virol. 1993;67:5463–5471. doi: 10.1128/jvi.67.9.5463-5471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arzuza O, Urzainqui A, Diazzruiz J R, Tabares E. Morphogenesis of African Swine Fever virus in monkey kidney cells after reversible inhibition of replication by cycloheximide. Arch Virol. 1992;124:343–354. doi: 10.1007/BF01309814. [DOI] [PubMed] [Google Scholar]
- 5.Breese S S, Jr, De Boer C J. Electron microscopic observation of African Swine Fever virus in tissue culture cells. Virology. 1966;28:420–428. doi: 10.1016/0042-6822(66)90054-7. [DOI] [PubMed] [Google Scholar]
- 6.Brookes S M, Dixon L K, Parkhouse R M E. Assembly of African swine fever virus: quantitative ultrastructural analysis in vitro and in vivo. Virology. 1996;224:84–92. doi: 10.1006/viro.1996.0509. [DOI] [PubMed] [Google Scholar]
- 7.Brookes S M, Sun H, Dixon L K, Parkhouse R M E. Characterization of African swine fever virus proteins j5R and j13L: immuno-localization in virus particles and assembly sites. J Gen Virol. 1998;79:1179–1188. doi: 10.1099/0022-1317-79-5-1179. [DOI] [PubMed] [Google Scholar]
- 8.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrascosa A L, del Val M, Santaren J F, Vinuela E. Purification and properties of African swine fever virus. J Virol. 1985;54:337–344. doi: 10.1128/jvi.54.2.337-344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrascosa J L, Gonzalez P, Carrascosa A L, Garcia-Barreno B, Enjuanes L, Vinuela E. Localization of structural protein of African swine fever virus particles by immunoelectron microscopy. J Virol. 1986;58:377–384. doi: 10.1128/jvi.58.2.377-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobbold C, Whittle J T, Wileman T. Involvement of the endoplasmic reticulum in the assembly and envelopment of African swine fever virus. J Virol. 1996;70:8382–8390. doi: 10.1128/jvi.70.12.8382-8390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobbold C, Wileman T. The major structural protein of African swine fever virus, p73, is packaged into large structures, indicative of viral capsid or matrix precursors, on the endoplasmic reticulum. J Virol. 1998;72:5215–5223. doi: 10.1128/jvi.72.6.5215-5223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasgupta A, Wilson D. ATP depletion blocks herpesvirus DNA packaging and capsid maturation. J Virol. 1999;73:2006–2015. doi: 10.1128/jvi.73.3.2006-2015.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ericsson M, Sodeik B, Krinse Locker J, Griffiths G. In vitro reconstitution of intermediate assembly stage of Vaccinia virus. Virology. 1997;235:218–227. doi: 10.1006/viro.1997.8683. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Escudero R, Andres G, Almazan F, Vinuela E. Inducible expression from African swine fever recombinants: analysis of the major capsid protein p73. J Virol. 1998;72:3185–3195. doi: 10.1128/jvi.72.4.3185-3195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon A, Sherman A D L, Zhu Z, Gabel C, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment by the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granzow H, Weiland F, Jons A, Klupp B G, Karger A, Mettenleiter T C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71:2072–2082. doi: 10.1128/jvi.71.3.2072-2082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths G, Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992;3:367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollinshead M, Vanderplasschen A, Smith G L, Vaux D. Vaccinia virus intracellular mature virions contain only one lipid membrane. J Virol. 1999;73:1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Toft D, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J E, Speir J A. Quasi-equivalent viruses: a paradigm for protein assemblies. J Mol Biol. 1997;269:665–675. doi: 10.1006/jmbi.1997.1068. [DOI] [PubMed] [Google Scholar]
- 23.Lingappa J R, Hill R L, Wong M L, Hedge R S. A multistep ATP dependent pathway for the assembly of human immunodeficiency virus capsids in a cell free system. J Cell Biol. 1997;136:567–581. doi: 10.1083/jcb.136.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lodish H F, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990;265:10893–10899. [PubMed] [Google Scholar]
- 25.Moura Nunes J F, Vigario J D, Terrinha A M. Ultrastructural study of African swine fever virus replication in cultures of swine bone marrow cells. Arch Virol. 1975;49:59–66. doi: 10.1007/BF02175596. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb W W, Homa F L, Thomsen D R, Ye Z, Brown J C. Cell-free assembly of the herpes simplex virus capsid. J Virol. 1994;68:6059–6063. doi: 10.1128/jvi.68.9.6059-6063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigam S K, Goldberg A L, Ho S, Rohde M F, Bush K T, Sherman M Y. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes calcium binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- 28.Poruchynsky M S, Maass D R, Atkinson P. Calcium depletion blocks the maturation of rotavirus by altering oligomerization of virus encoded proteins in the ER. J Cell Biol. 1991;114:651–661. doi: 10.1083/jcb.114.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez F, Alcaraz C, Eiras E, Yanez R J, Rodriguez J M, Alonso C, Rodriguez J F, Escribano J M. Characterization and molecular basis for heterogeneity of the African swine fever virus envelope protein p54. J Virol. 1994;68:7244–7252. doi: 10.1128/jvi.68.11.7244-7252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouiller I, Brookes S M, Hyatt A D, Windsor M, Wileman T. African swine fever virus is wrapped by the endoplasmic reticulum. J Virol. 1998;72:2327–2387. doi: 10.1128/jvi.72.3.2373-2387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakalian M, Parker S D, Weldon R A, Hunter E. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J Virol. 1996;70:3706–3715. doi: 10.1128/jvi.70.6.3706-3715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J F. The involvement of calcium in the transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- 33.Schmelz M B, Ericsson M, Hiller G, Machamer C E, van't Hof W, van Meer G, Griffiths G. Assembly of vaccinia virus; the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith M J, Koch G L E. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J. 1989;8:3581–3586. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodiek B, Doms R W, Ericsson M, Hiller G, Machamer C E, van't Hof W, van Meer G, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartments between the endoplasmic reticulum and the Golgi apparatus. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens E B, Compans R W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- 37.Thastrup O, Cullen P, Drobak B K, Hanley M R, Dawson A. Thapsigargin, a tumor promoter, discharges intracellular Ca stores by a specific inhibition of the endoplasmic reticulum Ca-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 39.Weldon R A, Parker W B, Sakalian M, Hunter E. Type D retrovirus capsid assembly and release are active events requiring ATP. J Virol. 1998;72:3098–3106. doi: 10.1128/jvi.72.4.3098-3106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wileman T, Kane L P, Carson G R, Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991;266:4500–4507. [PubMed] [Google Scholar]
- 41.Yanez R J, Rodriguez J M, Nogal M L, Yuste L, Enriquez C, Rodriguez J F, Vinuela E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995;208:249–278. doi: 10.1006/viro.1995.1149. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zlotnic A. To build a virus capsid. An equilibrium model of the self assembly of polyhedral protein complexes. J Mol Biol. 1994;241:59–67. doi: 10.1006/jmbi.1994.1473. [DOI] [PubMed] [Google Scholar]